Does Generalised Reciprocity Explain the Evolution of Cooperation in the Trinidadian Guppy?
Info: 8388 words (34 pages) Dissertation
Published: 9th Dec 2019
Tagged: Biology
Does generalised reciprocity explain the evolution of cooperation in the Trinidadian guppy (Poecilia reticulata)?
Abstract
Generalised reciprocity is a cognitively simple cooperation mechanism whereby individuals cooperate with a partner based solely on the fact that they have received help previously: “A helps B because A was helped”. However, this type of reciprocity has only been recognised to occur in humans and rats. The current research, therefore, aimed to provide evidence for generalised reciprocity in a different species – the Trinidadian guppy (Poecilia reticulata). To investigate this, we manipulated the conditions under which female guppies performed predator inspections (a social behaviour which requires cooperation in fish). A cooperative social environment was simulated using a mirror. Significant evidence was found for the use of generalised reciprocity. Guppies who had previous experience of cooperation were significantly more likely to behave cooperatively in subsequent interactions, irrespective of the identity of the partner. To our knowledge, this is the second study providing experimental evidence for the use of generalised reciprocity in non-human animals.
Organisms are inherently competitive, yet cooperation has been observed across the entire Animal kingdom. Cooperation can be defined as a behaviour whereby two or more individuals engage in a mutually beneficial exchange instead of competing (West et al, 2007). Cooperation has been documented across a range of organisms including humans, mammals, insects, birds, and even simple organisms such as amoebas (Rubenstein, 2010). Cooperation has also been documented across a range of behaviours, including foraging, avoidance, defending resources, and hunting (Dugaatkin, 1992). For example, in insects such as bees (Apis) and ants (Formicidae), workers collectively rear eggs laid by their queen, and in mammals, wolves (Canis lupus) hunt in packs to secure food for the entire pack (Wilson, 2000). In cases like these, cooperation between individuals can be mutually beneficial and may result in advances/benefits in fitness. That being said, it is widely misunderstood why one individual would pay a cost to help another individual. According to Darwin’s (1859) theory of evolution and natural selection, individuals should favour selfish behaviour in order to increase their own fitness, not selfless behaviour. This is known as altruism; behaviour that benefits the fitness of others at a personal cost to the altruistic individual (Kerr et al, 2004). According to Darwin (1859), altruism can lead to decrease in fitness to the altruistic individual and thus, should be eliminated by natural selection. This evolutionary conundrum has been widely researched resulting in an abundance amount of research exploring different underlying mechanisms for the reason why organisms cooperate.
One prominent solution to the evolution of cooperation conundrum was formally established by W.D. Hamilton (1964), and is referred to as ‘kin selection’. Hamilton believed that individuals cooperate to gain indirect fitness advantages by increasing the reproductive success of their kinsfolk. Kin selection is widely documented across non-human animals. For example, Florida scrub jays (Aphelocoma coerulescens) have demonstrated altruistic behaviour favouring their close blood relatives over strangers. They are one example of a species of bird that employs ‘helpers’. Instead of pairing with their own mates, the ‘helper’ jays abstain from reproduction to assist other breeding pairs with nest defence, mobbing predators and rearing young. In one study, monitoring a flock of Florida scrub jays over several generations, they found that almost all ‘helpers’ (73/74) were assisting biological relatives (Rausher, 2015). On the surface, ‘kin selection’ seems like a plausible explanation for cooperation. However, occasions have been found where cooperation occurs between non-kin individuals.
Altruistic behaviour such as defending another birds nest (Rausher, 2015), or collecting food for the queen bee (Wilson. 2000) may reduce the reproductive fitness of the altruistic individual, but it may raise the net fitness of the entire group. The first idea of group selection was hypothesised by Darwin himself in 1871’s “The Descent of Man”. He proposed that altruistic traits are passed on if they increase the reproductive capacity of the entire group. Examples of behaviours demonstrating group selection include cooperative hunting among lions (Panthera leo) (Scheel & Packer, 1991), and cooperative rearing of offspring in elephants (Loxodonta) (Burkart et al, 2009). The basic idea is that cooperative individuals will interact with other cooperative individuals, which will, in turn, create a group of cooperative individuals, resulting in higher benefits of fitness to the overall group. However, occasions have been documented where individuals continue to cooperate with defectors (Study last year, 2017)
Reciprocity is one of the most well-known evolutionary explanations for cooperation. Reciprocity occurs when the cost that an individual suffers from assisting another individual are repaid by receiving assistance from others in the future (Trivers, 1971). There are different types of reciprocity, all of which provide key mechanisms for the evolution of cooperative behaviour in non-human animals. Direct reciprocity occurs during repeated encounters between the same two individuals; it involves the sequential performance of costly fitness behaviours that produce benefits to the interacting individuals (Freidin et al, 2015). In simpler terms: ‘A’ helps ‘B’ because ‘B’ helps ‘A’ (Carter, 2014). Direct reciprocity has been documented across a wide range of non-human animals. For example, Wilkinson (1984) discovered that unrelated vampire bats (Desmodus rotundus) exchange food by the regurgitation of blood amongst other vampire bats that have shared food with them in the past, rather than those who have not shared food in the past. It has been questioned whether animals which are assumed to lack complex social and cognitive skills are able to engage in direct reciprocity (Connor, 2010). Despite this, there has been experimental evidence for direct reciprocity derived from a range of non-human animals such as fish (Dugatkin, 1997; Croft et al, 2006), birds (Karma et al, 2012), and primates (de Waal & Suchak, 2010). However, there are instances where direct reciprocity cannot take place. For example, when donating to charity there is no opportunity to have repeated encounters between the same two individuals (Nowak, 2006).
Another form of reciprocity is indirect reciprocity. The evolution of cooperation by indirect reciprocity is based on reputation (Nowak & Sigmund, 2005). Defective acts reduce an individual’s reputation, while cooperative acts increase an individual’s reputation, resulting in cooperative individuals being more likely to get helped by others than defective individuals. (Roberts, 2008). In simpler terms, ‘A’ helps ‘B’ because ‘B’ helps ‘C’ (Carter, 2014). An additional form of indirect reciprocity is generalised reciprocity, a cognitively simple conditional cooperation mechanism whereby individuals cooperate with a partner based solely on the fact that they have received help previously, irrespective of who has helped them in the past (Nowak & Roch, 2007). In simpler terms, ‘A’ helps ‘B’ because ‘A’ was helped (Carter, 2014). It makes sense that this would be a primary model to explain the evolution of cooperation in non-human animals that lack the cognitive skills needed for other types of reciprocity, as generalised reciprocity requires minimal cognitive abilities (van Doorn & Taborsky, 2011). However, there is very little evidence demonstrating generalised reciprocity in non-human animals. One of the only studies providing evidence for generalised reciprocity is Rutte and Taborsky’s (2007) experiment using rats. They showed how individuals were more cooperative when they had previously received help, irrespective of the identity of the partner, suggesting that generalised reciprocity could be a mechanism underlying cooperative behaviour amongst non-human animals.
Axelrod & Hamilton (1981) modelled generalised reciprocity using the “TIT FOR TAT” (TFT) evolutionary game theory. The TFT strategy proposes that an individual cooperates during their first interaction with another individual and then they subsequently copy their partner’s last move (that is, cooperate unless your partner did not cooperate in the previous interaction). Thus, with the exception of the first move, a TFT individual cooperates with other cooperative individuals and defects with other defecting individuals. There has been a debate about whether non-human animals are capable of adopting TFT strategy due to the sophisticated cognitive abilities needed for this to occur. To play TFT an individual must be able to identify its current partner, remember what that individual did in their last encounter, and then base its current behaviour on this information.
Evidence for non-human animals adopting the TFT strategy has been found in more intelligent animals that live in complex social societies. For example, Vervet monkeys (Chlorocebus pygerythrus) are more likely to cooperate with an animal that has helped them in the past (Packer, 1977). However, many believe less sociable and much less intelligent animals may not have this cognitive ability (Raihani & Bshary, 2011). This raises the question whether less intelligent non-human animals are capable of these cognitively complex strategies. Scientific evidence derived from observing fish when they perform predator inspections suggest they are. During predator inspection, teams of fish break away from the main shoal to get a closer look at the predator, gaining information regarding its identity, location, and readiness to attack (Magurran & Higham, 1988). Fish can either swim closer to the predator with its companion (cooperate) or consistently drop back behind it (defect). A well-known demonstration of this is Milinski’s (1987) experiments on sticklebacks. During his experiments, sticklebacks were placed in an experimental tank whereby a predator was visible. He manipulated the conditions in which sticklebacks experienced cooperation with the use of a mirror. Fish with a cooperating partner (mirror present) ventured closer to the predator and stayed there longer than those who experienced defection (mirror absent). Milinski believed sticklebacks acted on how their partner was behaving and thus, thought to have found experimental evidence of the stickleback using TFT strategies to maintain cooperation. Support for this finding comes from Dugatkin & Alfieri (1991) who discovered that guppies are capable of identifying and remembering their partner’s behaviour, suggesting they are capable of using TFT strategies. Although it is still not widely understood why fish pay a cost to help others.
A study conducted by an undergraduate at the University of Exeter (Reference, 2017) set out to further investigate behaviour during predator inspections by manipulating the conditions under which guppies performed predator inspections. If guppies were using TFT strategies to maintain cooperation then one would expect guppies that had previously experienced cooperation to cooperate with unfamiliar fish in subsequent interactions, and fish that had previously experienced defection to defect with unfamiliar fish in subsequent interactions. However, this is not what they found. Surprisingly they discovered that guppies with previous experience of defection showed a tendency to cooperate more with unfamiliar fish in subsequent interactions compared to guppies with previous experience of cooperation. This unexpected result could be due to a flaw in the paradigm that does not consider expectancy violation. The guppies firstly experiencing defection in this particular study are coming into the experimental tank the second time expecting defection, but then suddenly experiencing cooperation, which means their expectations are violated. Perhaps if experimenters do not violate what they are expecting, we could expect guppies to copy their partner’s last move just like TFT proposes.
The current study aims to replicate (2017)’s study with a slightly different paradigm that does not violate the expectations of the guppies, in order to investigate the research question: ‘Do Trinidadian guppies maintain cooperation through generalised reciprocity?’. We hypothesise that guppies will engage in generalised reciprocity. More specifically we hypothesise that individuals who experience cooperation will behave cooperatively in subsequent interactions and that individuals who experience defection should behave less cooperatively in subsequent interactions. We tested this by manipulating the conditions under which 96 female Trinidadian guppies performed predator inspections. We then observed and compared their behaviour as they performed these predator inspections. If expectancy violation is the cause of the unexpected result seen in (REFERENCE’s) study, then we would expect individuals who experience defection to be less cooperative in subsequent interactions than individuals who had previously experienced cooperation.
Methods
Subjects
The current study was executed using 96 laboratory-reared female Trinidadian guppies (Poecilia reticulata) as subjects. Only females were used due to the lack of significant difference between sexes previously documented (study last year, 2017). Focal fish were housed in mixed-sex holding tanks prior to testing (each 17.5cm x 10cm x 19cm; each tank contained three males and ten females). They were fed daily on a diet of flake and shrimp. An additional 800 stimulus shoal fish were accumulated from stock pools and were housed equally across four different stimulus population tanks (each 80cm x 40.5cm x 29.5cm) with a 4:1 (M/F) sex ratio. Stimulus shoal fish were fed a diet that was novel to our focal fish in order to generate different odour cues; either bloodworm (B/W) or daphnia and mosquito larvae (M/D). This allowed focal fish to differentiate between individuals they had previously interacted with.
Design
The current study is a between-subjects design. The experiment has two phases, with three independent variables (IVs) in the first phase, and four IVs in the second phase. Each IV has two levels; these are demonstrated in Table 1.
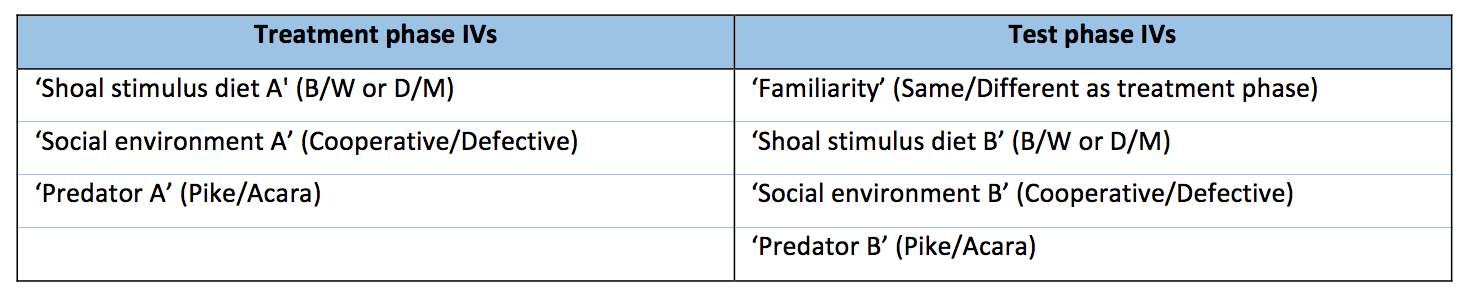 Table 1: demonstrating the IVs in each phase of the experiment: (a) treatment phase, (b) test phase.
Table 1: demonstrating the IVs in each phase of the experiment: (a) treatment phase, (b) test phase.
Materials
The Inspection tank (81cm x 28cm x 30cm) was divided into six separate compartments: two predator compartments (each 22cm x 14.5cm) behind a removable opaque barrier, two inspection lanes (each 51cm x 14.5 cm), containing an artificial plant as refuge, and two stimulus shoal compartments (each 10cm x 14.5cm) behind a ‘Perspex’ barrier with small holes in. This allows for the stimulus shoal fish to be visible, and their odour cues to accumulate throughout the tank. Water was filled up to a depth of 10cm. The presence of a mirror was used to give the illusion of a cooperative social environment, whereas the absence of a mirror was used to stimulate a defective social environment during predator inspection. Two types of models were used to replicate two of the Trinidadian guppy’s real-life predators (Endler, 1978); the Pike (Crenicichla alta) and the Acara (Aquidens pulcher). Inspection lanes were split into ten (5cm x 15cm) zones, ranging from zone one (nearest to the stimulus shoal fish) to zone ten (nearest to the predator). After the treatment phase, focal fish were individually kept in holding tanks (20.5cm x 12.5cm) overnight until the test phase was ready to begin. A diagram showing every aspect of the inspection tank is shown below (Figure 1; Figure 2)
Figure 1: Top view of the experimental tank: (a) the two stimulus shoal compartments (b) the two inspection lane compartments), (c) the two predator compartment. Width and lengths are indicated.
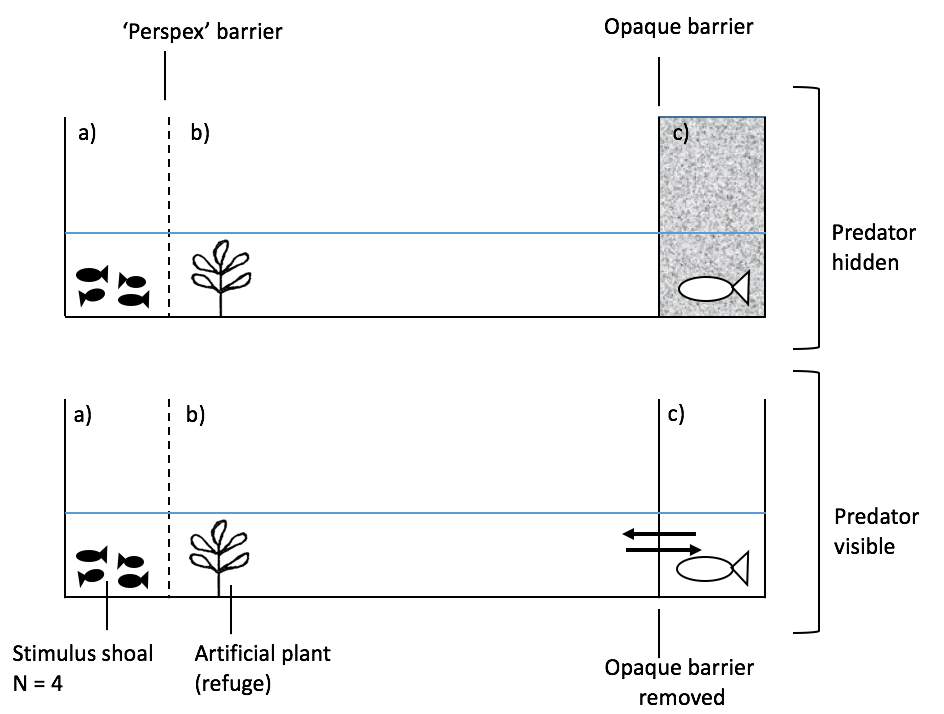
Figure 2: Side view of the experimental tank: a) stimulus shoal compartment, b) inspection lane, c) predator compartment. The direction of the arrows between b) and c) highlight the fish viewing directions. Predator is hidden when the opaque barrier is inserted and revealed with the opaque barrier is removed.
Experimental procedure
Overview. Each focal fish was assessed in two phases separated by 24 hours. In both phases, focal fish were exposed to a predator and given the opportunity to carry out a predator inspection for five minutes. In the treatment phase, a focal fish experienced either a cooperative or defective social environment, manipulated by either the absence (defection) or presence (cooperation) of a mirror in the inspection lane. In the test phase, focal fish were either exposed to familiar individuals (stimulus shoal diet the same as that in the treatment phase), or unfamiliar ones (stimulus shoal diet different to that in the treatment phase). Due to the fact that exposure to vibration may elicit behavioural changes in fish (Roberts & Elliot, 2017), polystyrene was placed under the tank to reduce vibrations caused by external sounds and movement. Each focal fish was exposed to a different predator model in each phase to ensure that the predator model exposed in the test phase was new to the focal fish and thus, would be worth inspecting to gathering information on. Study conditions are shown in Table 2.
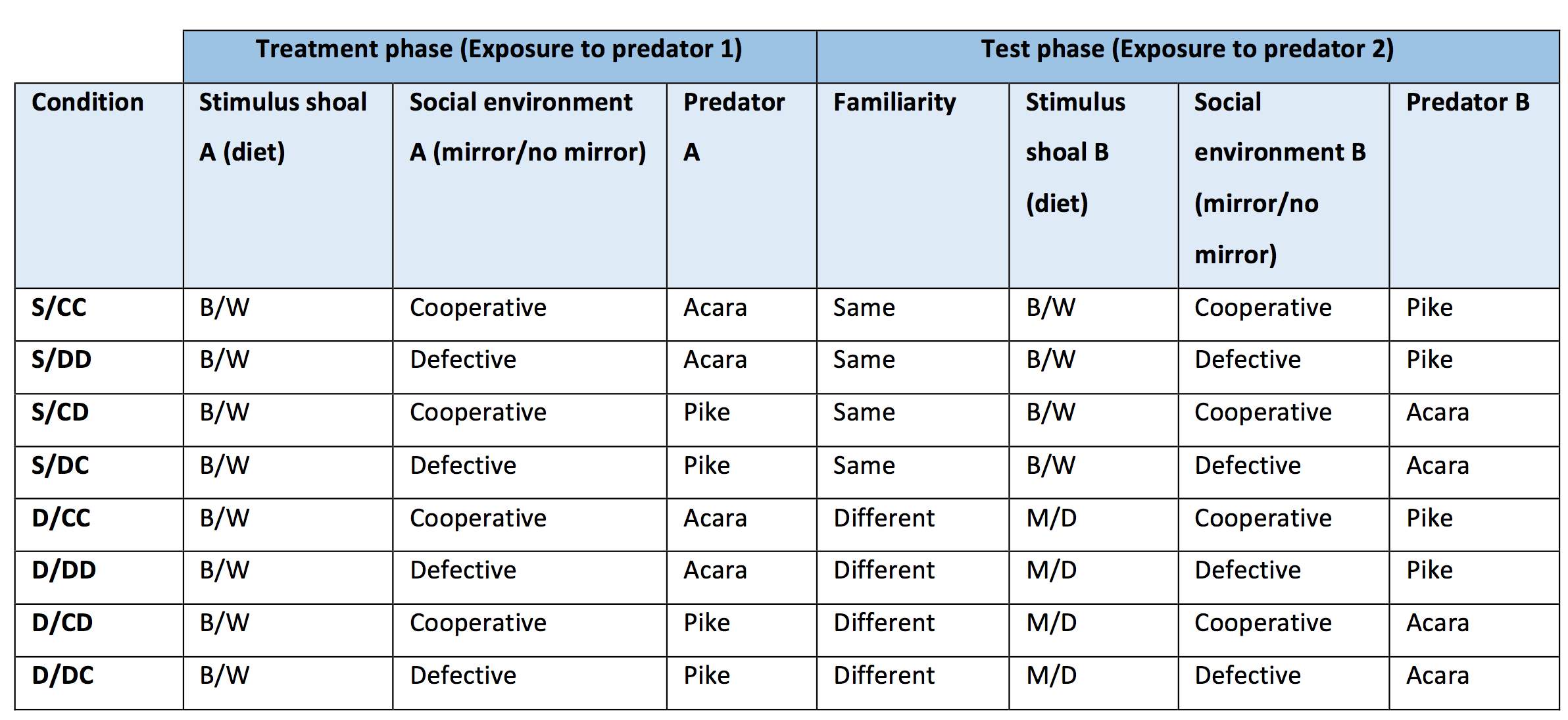
Table 2: demonstrating each condition used in the study, and examples of how each IV was manipulated for each condition.
Treatment Phase.Groups of four female guppies matched to size were selected at random from the stimulus population. They were placed in the stimulus shoal compartment and were left undisturbed for 20 minutes, allowing time for them to familiarise themselves with their new environment and allow their odour cues to accumulate. After 20 minutes, focal fish were allocated individually to one of the inspection lanes. Here they were left undisturbed for ten minutes while they familiarised themselves in their new environment. After ten-minutes the opaque barrier was removed, exposing the predator models, and focal fish were able to carry out predator inspections for five minutes. Behaviour was recorded from a bird’s eye point of view on a ‘GoPro Hero 4’ camera. After five minutes had passed focal fish were removed from the tank and placed into individual holding tanks. Because social deprivation has been proven to affect cooperativeness during predator inspections (Hesse et al, 2015), individual holding tanks were placed beside one another in a larger tank filled with water. This allowed fish to be physically but not visually isolated, which means we can be sure social deprivation did not affect our results. The stimulus shoal fish were placed back into the stimulus population tank they came from. The inspection tank was then drained and refilled with clean water to ensure the removal of any odour cues.
Test Phase. After 24 hours had passed, four new female guppies were placed into the stimulus shoal compartment; again, they were left undisturbed for 20 minutes allowing time to familiarise themselves with their surroundings and allow for odour cue to accumulate. Focal fish in the familiar condition were given a stimulus shoal with the same diet used in the treatment phase so that the focal fish would ‘recognise’ these individuals to decipher whether they had cooperated or defected in previous experiences. Fish in the unfamiliar condition were given a stimulus shoal on a different diet than the stimulus shoal fish used in the treatment phase so that the focal fish would think they had no previous experience with them. After twenty minutes, the focal fish were individually placed into the inspection lanes and left to familiarise themselves with their surroundings for ten-minutes. Again, mirrors were either present to create the illusion of a cooperative social environment or absent to stimulate defection. After ten minutes, the opaque barrier was removed to expose the model predator, giving focal fish the opportunity to perform predator inspections for five minutes. Again, the behaviour of each guppy was video recorded. After five minutes had passed, the predators were hidden, and fish were removed from the inspection tank.
Behavioural Scoring
Behavioural Observation Research Interactive Software (BORIS) v.6.2.1 was used to score the behaviour of each individual focal fish. The time that a focal fish spent in each zone during the five-minute predator inspection period was recorded. This produced ten scores of duration spent in each zone, which was used to calculate an ‘average zone’. The ‘average zone’ was calculated for both the treatment phase and test phase for each individual fish, and represented cooperativeness. The higher the average zone, the more predator inspections were carried out. Thus, higher numbers reflected higher cooperative behaviour and lower numbers reflected less cooperative behaviour.
Exclusion Criteria
Three trials were excluded in the analysis due to an error involving the camera footage, and two trials were excluded from the analysis because focal fish did not begin the trial in the standard condition (refuge). Therefore, a total of 92 focal fish were included in the analysis for the treatment phase, and 93 focal fish were included in the analysis for the test phase.
Statistical Analysis
Before analysing our data, we tested for outliers and tested whether or not data was normally distributed. Upon inspection of the box-plot, we noticed a single outlier in our treatment phase data, and a single outlier in our test phase data. The tests of normality revealed a value of W = 0.001 on the Shapiro-Wilk test, meaning the data significantly deviated from a normal distribution. To deal with this, a log transformation was conducted on our data to provide new transformed data that approximated a normal distribution. The analysis was divided into two parts, firstly to test how focal fish respond to immediate social environment in the treatment phase (analysis a), and secondly, to test how focal fish respond to the immediate social environment as a function of their previous experience (analysis b). Illustrated below in table 3.
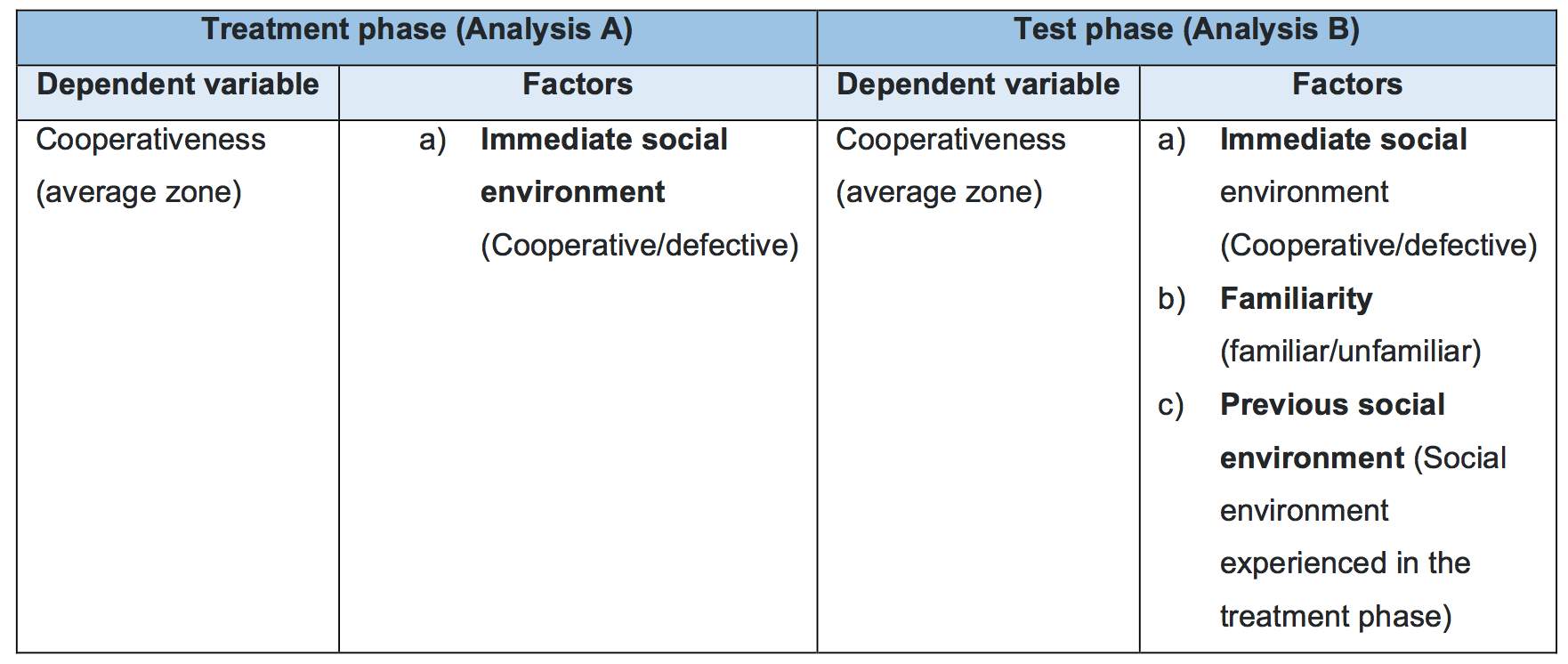
Table 3: Table demonstrating the dependent variable and the independent variable used as factors during the analysis.
Ethical Statement
No fish were harmed during the course of the study. Health checks were completed each day to ensure that all fish used in the study were physically well. Appropriate measures were taken to minimise the stress levels of every individual used in the experiment, for example, focal fish were never visually isolated, and predator models were used rather than live predators. Additionally, fish were only exposed to two predator models for a maximum of five minutes each. The Psychology Ethics Committee approved the current study at the University of Exeter.
Results
Treatment Phase (Analysis A)
To investigate whether there was an effect of our manipulation in the treatment phase on the cooperativeness of the focal fish during predator inspection, an ANOVA was conducted on the cooperativeness (average zone) in relation to the immediate social environment (mirror absent/present) they experienced (see Table 1 in methods). The analysis revealed a significant main effect, F(1, 90) = 3.50, p = 0.001. Given this significant effect, we can conclude that fish who experienced cooperation (mirror present) during predator inspection behaved more cooperatively than fish who experienced defection in this phase (Figure 3)
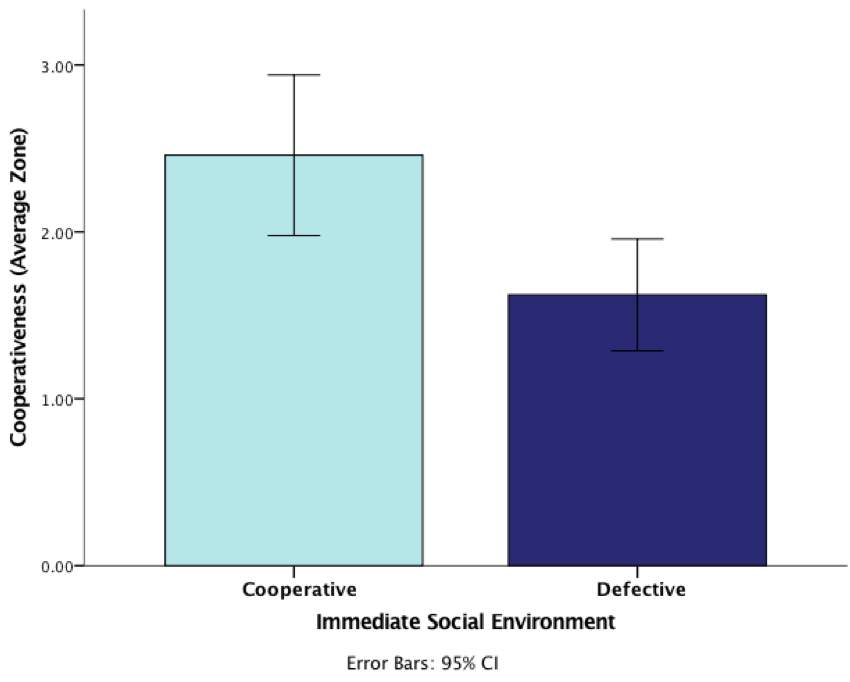
Figure 3: Graph demonstrating the effect of social environment (cooperative/defective) on cooperativeness (average zone) when inspecting a predator during the treatment phase (error bars are 95% CI)
Test Phase (Analysis B)
Here, we conducted an analysis to examine how focal fish respond to an immediate social environment (social environment during test phase & familiarity) as a function of previous experience (social environment previously experienced during the treatment phase) using an ANOVA (see Table 1 in methods).
Firstly, this analysis revealed a significant univariate main effect at the p<.05 level for immediate social environment, F(1,86) = 1.55, P = 0.006, again demonstrating how this factor affects inspection behaviour. This provides stronger evidence demonstrating how focal fish who were in an immediate cooperative environment during predator inspection in the test phase engaged in more inspections than the fish who had experienced defection in this phase. These results are illustrated in Figure 4.
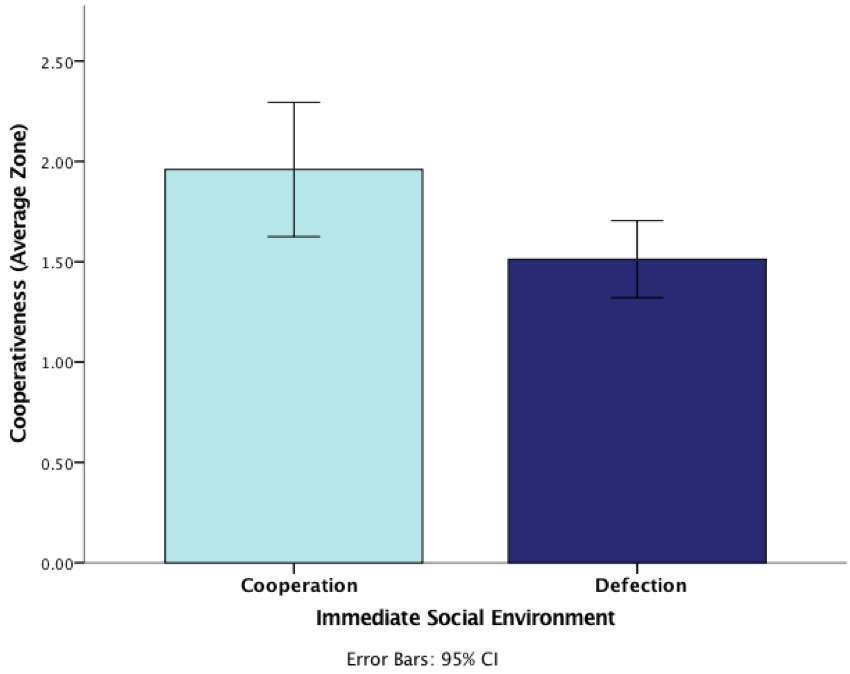
Figure 4: Graph demonstrating the effect of immediate social environment (cooperative/defective) on average zone (cooperativeness) during the test phase (error bars are 95% CI)
Secondly, this analysis revealed a significant main effect at the p<.05 level for previous social environment, F(1,86) = 1.05, P = 0.023, demonstrating how previous experience is a factor that affects cooperativeness during predator inspection behaviour. Given these significant results, we conclude fish which had a previous cooperative environment (cooperative social environment in the treatment phase) during predator inspection engaged in more inspections than the fish who had previously experienced defection (Figure 3). This suggests that guppies consider their previous experience when deciding whether or not to cooperate. These results are illustrated in Figure 5.
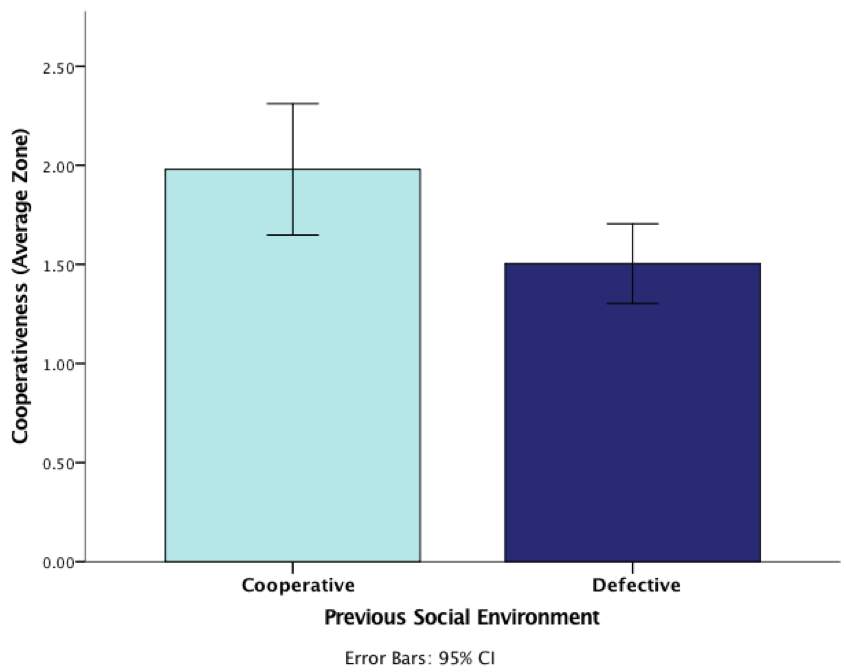
Figure 5: Graph demonstrating the effect of previous social experience (cooperative or defective social environment in the treatment phase) on cooperativeness (average zone) during the test phase (error bars are 95% CI)
Thirdly, the effect of inspecting with familiar (stimulus shoal fed the same diet as in the treatment phase) or unfamiliar (stimulus shoal fed a different diet to that in the treatment phase) individuals during predator inspection on levels of cooperativeness during the test phase was not significant (F(1,86) = 1.552, P= .740, Figure 6).

Figure 6: Graph demonstrating the effect of familiarity (same diet as the treatment phase or different diet as the treatment phase) on cooperativeness (average zone) during the test phase (error bars are 95% CI)
Lastly, none of the interaction effects was significant (although see below; Table 4), suggesting that behavioural responses to experiences of cooperation or defection were globally applied, irrespective of who the partners were (Figures 7 and 8)
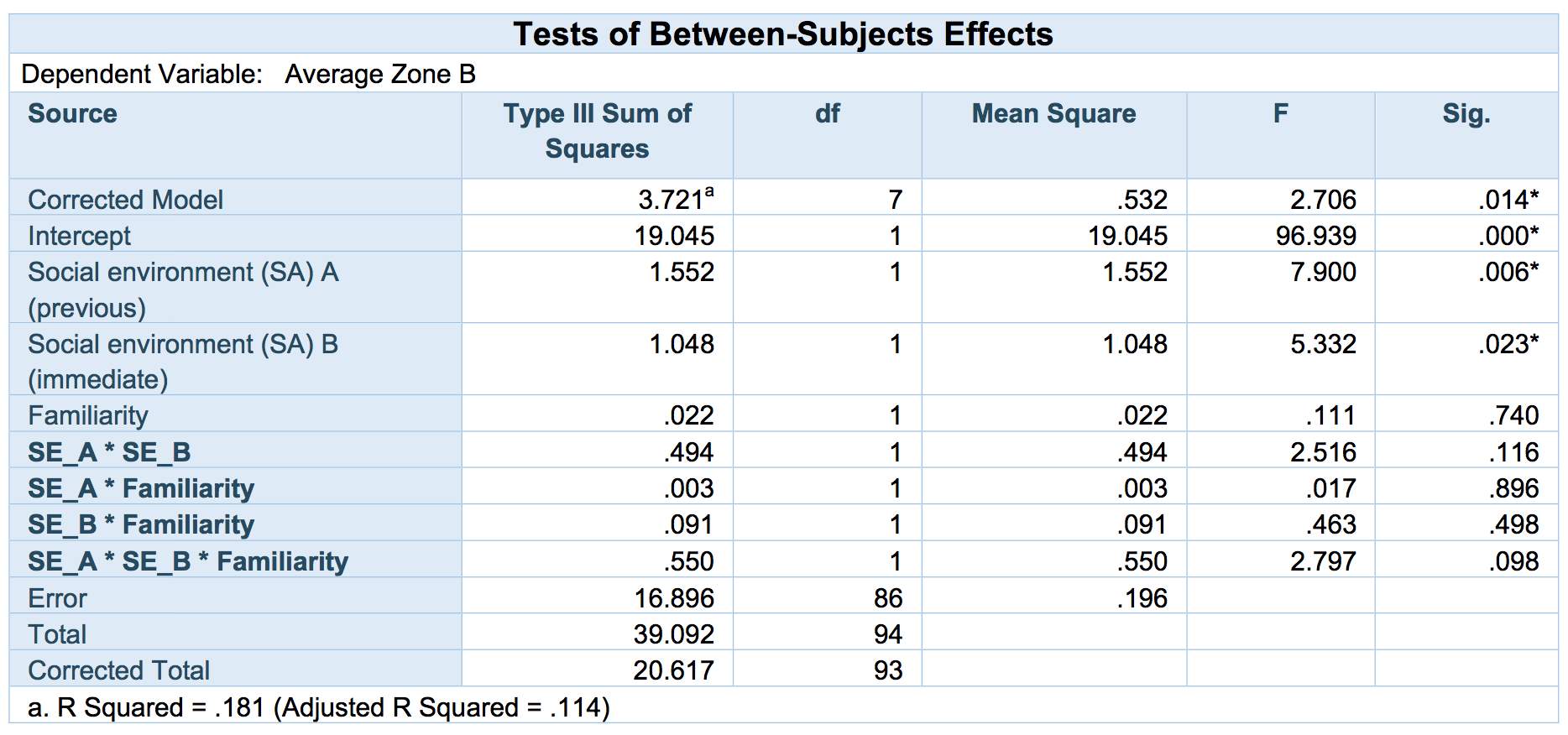
Table 4: F and P-values for the univariate main and interaction effects of immediate social environment (Social environment B) as a function of their previous experience (Social environment A, Familiarity) on cooperativeness in the test phase. (*results which are significant at the p<.05 level)
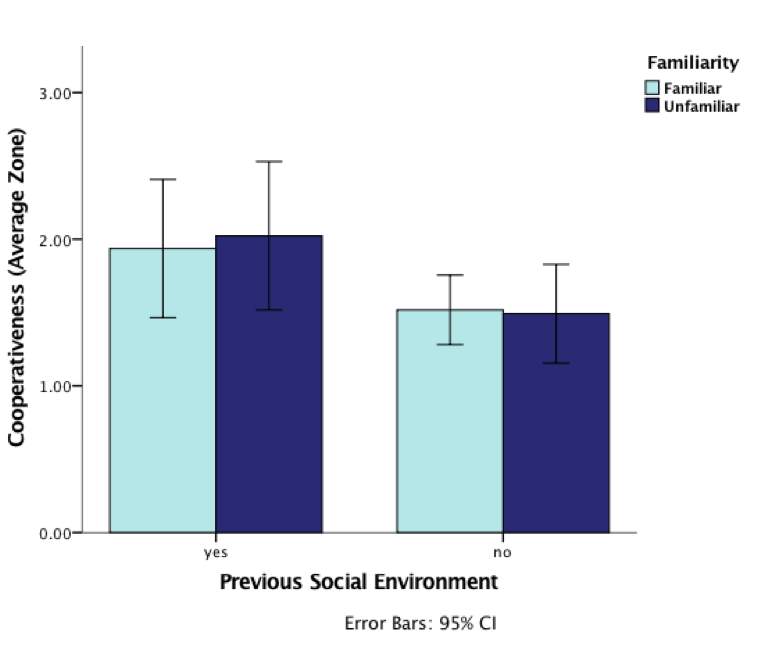
Figure 7: Graph demonstrating the effect of inspecting with familiar (same diet as the treatment phase) or unfamiliar (different diet as in the treatment phase) individuals and previous social experience (mirror present/absent) during predator inspection in the treatment phase on levels of cooperativeness (average zone) during the test phase (error bars are 95 CI%)
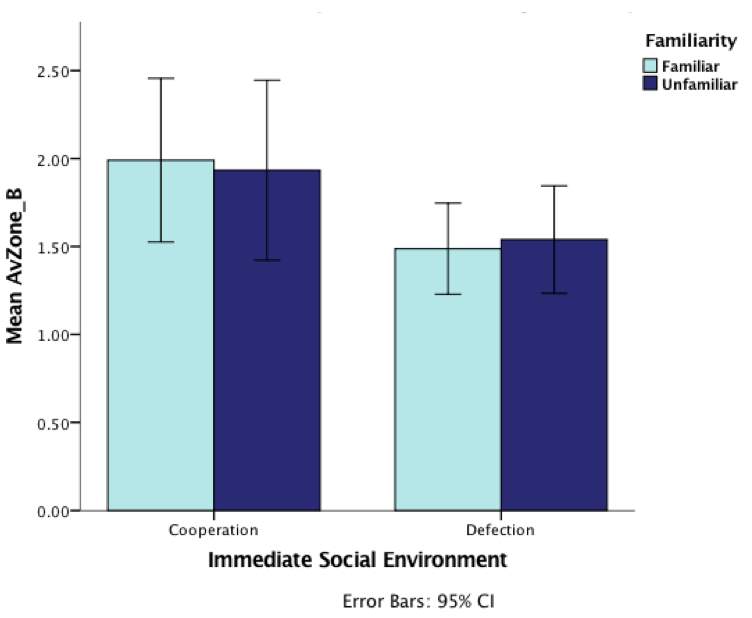
Figure 8: Graph demonstrating the effect of inspecting with familiar (same diet as the treatment phase) or unfamiliar (Different diet as the treatment phase) individuals and immediate social environment (cooperative/defective) during predator inspection on levels of cooperativeness (average zone) during the test-phase (error bars are 95% CI)
The current study aimed to investigate whether guppies engage in generalised reciprocity during predator inspection. We hypothesised that guppies would engage in general reciprocity during predator inspections. More specifically we hypothesised that a) social environment would affect cooperative behaviour during predator inspections, and b) familiarity will affect cooperative behaviour during predator inspections. The prediction that a) individuals who experience cooperation will behave more cooperatively in subsequent interactions than those who experienced defection was supported. Guppies that experienced a cooperative social environment were significantly more cooperative in subsequent interactions than those who experienced a defective environment in both the treatment and test phase. The prediction that b) individuals will behave more cooperatively to familiar groups of shoals compared to unfamiliar ones was not supported. Familiarity did not significantly affect cooperativeness during predator inspection in the test phase, indicating that guppies base their cooperativeness on their prior experience of cooperation, not on specific knowledge about the partner they are interacting with.
Evidence for direct reciprocity has been documented widely in previous research (Griffiths & Magurran, 1997; study last year, 2017; Wilkinson, 1984). In contrast to this previous literature, we found that manipulating the stimulus shoal diet to stimulate familiar or unfamiliar members was not significant in predicting cooperativeness in guppies during predator inspection. This suggests that guppies do not have the cognitive ability needed to engage in direct reciprocity after all. However, this is not completely surprising considering a number of studies demonstrating how non-human animals (Stevens et al, 2005), and even some humans (Wedekind, 2000) do not have the cognitive abilities, information retention and retrieval needed to engage in direct reciprocity (Tabosky, 2011). The conflicting results regarding whether guppies have the cognitive abilities to engage in direct reciprocity emphasises the need for further research to increase our understanding of their abilities.
Secondly, our results are consistent to those found by Rutte and Taborsky (2007) whereby rats were more cooperative when they had previously received help, irrespective of the identity of the partner. This is exactly what we observed in our experiment. Individuals who had experienced cooperation in the treatment phase were significantly more likely to behave cooperatively in the test phase, irrespective of the identity of the partner. This suggests that guppies may maintain cooperation through general reciprocity. This would make the most sense as a mechanism to explain the evolution of cooperation in guppies because minimal cognitive abilities are needed to engage in general reciprocity compared to direct reciprocity. More research is needed to strengthen the validity of this finding considering that this finding, to our knowledge is only the second experiment providing evidence for generalised reciprocity in non-human animals.
Strengths of the current study suggest that the method and measures used are an effective and well-established way to investigate whether guppies engage in general reciprocity and therefore could be replicated for future research. For example, guppies are known to approach live and model fish predators in both the laboratory (Dugatkin & Godin, 1992) and field (Magurran & Seghers, 1994) in the same way. This implies that using predator models are representative of a real live predator a guppy would come across in its natural environment. Guppies have also been proven to use olfactory sensory information in their natural environment (Bisazza et al, 1999), suggesting that changing the stimulus shoal diet in the current study was a valid way to differentiate between familiar and unfamiliar individuals. Another strength of this study was that recordings were taken from a bird’s eye view which meant there was no way for the experimenter to tell which way the mirror was facing (blind experiment), thus, errors arising from bias were avoided.
Furthermore, the fact that using mirrors to manipulate social environments is widely used in experimental procedures (Dugatkin, 1991; Milinsky, 1987) suggests that this is a reliable method to simulate cooperation or defection during predator inspection. In a study by Tinbergen (1951), male sticklebacks displayed aggressive behaviour towards mirror images, indicating that fish treat mirror images as a different individual. Additionally, Reiss and Marino (2001) note how most vertebrates fail the mirror awareness test (Gallup, 1970), and that the ability to recognise a mirror image of oneself seems to be limited to humans, great apes, and dolphins only. This allows us to reliably assume that guppies believe their reflection is another cooperating fish rather than them recognising their own reflection. In a study conducted by Bisazza et al (1999) predator inspections were more likely to occur when the cooperative partner was visible on the left rather than the right side of a fish. We dealt with this problem by alternating the side the mirror appeared to the focal fish at random.
However, some limitations to the current study were noted. The small sample size limited the study’s power of identifying significant results. The current study found a few insignificant results, however the, three-way interaction effect was approaching significance (see table 4 in results). Perhaps an increase in sample size would raise the possibility of detecting more significant effects. To overcome this issue and possibly detect some more significant results one could replicate the study with a larger sample size to see if those results approaching significance develops into a significant result. Also, the research could be repeated in the fish’s natural habitat with the recording of the behaviour of the fish being carried out whilst scuba diving. This method has proven to be successful in measuring cooperative behaviour in fish (Brandi & Bellwood, 2015). This would produce results with high ecological validity, although the observer may also be seen as a potential predator which might affect the results.
Another limitation to our study may be the fact that alternative mechanisms other than generalised reciprocity might explain the behaviour of the guppies on our experiment. For example, Godin et al (1995) suggest the reason why guppies engage in predator inspection is due to predator-deterrent signals. They demonstrated using female guppies that predator inspection behaviour actually deters predators and that inspectors actually experience on average a lower risk of predation than non-inspectors. They propose this is because prey benefit from signalling their alertness and physical condition (for example, escape ability) to the predator. Inspection behaviour also informs the predator that it has been detected and therefore the element of surprise has been lost (Hasson, 1994), thus, causing the predator to divert its attention to less vigilant prey (the non-inspecting fish). If this is correct, it provides evidence suggesting Darwin’s (1859) natural selection theory is valid and resolves the conundrum regarding why fish inspect a potential predator (pay a cost) when it may be able to obtain the associated benefits indirectly from other inspectors. Guppies may, therefore, inspect predators to increase their chances of survival rather than cooperating for the benefit of the group. Although, this raises the question of why every individual did not inspect the predator. A large number of trials were recorded where fish did not leave the refuge area to inspect the predator (N = 41). Due to this, it is unlikely that predator-deterrent signals are the reason for our results.
Another plausible mechanism that may explain the behaviour of the guppies in our experiment is prey’s ability to escape predators. More heavily armoured sticklebacks in good condition have been found to be more likely to inspect predators and approach them more closely than smaller and less armoured conspecifics (Kulling & Milinsky, 1992). If this is correct, it may suggest that size is a factor affecting cooperativeness in our study that we did not account for. In light of this, it would be interesting to replicate the study, taking into account the size and health condition of each guppy to see whether larger guppies in good condition perform more predator inspections than smaller guppies that are not in such good condition. If this is the case, size and physical fitness may be a strong factor when it comes to cooperative behaviour. Further investigation is needed to rule out alternative explanations for the results that have been observed in the current study.
Regardless of the limitations mentioned above, the current study provides new insights into the mechanism underlying cooperation among guppies during predator inspection. To our knowledge, this is only the second study to provide experimental evidence that non-human animals engage in generalised reciprocity. This provides contemporary evidence indicating that reciprocal cooperation is not based on specific knowledge about the partner they are cooperating with (demonstrated previously through direct reciprocity), but that any prior experience of cooperation can be used. Furthermore, our findings provide insight into why guppies have previously been shown to cooperate with previous defectors (study last year, 2017). They do not have the cognitive abilities needed to recall that individual and recall how that individual behaved previously to base its current behaviour on.
In conclusion, the current study demonstrates that individuals who had previously experienced cooperation were significantly more likely to behave cooperatively in future interactions, irrespective of the identity of the partner. This suggests that reciprocal cooperation in integrated encounters is not necessarily based on specific knowledge about the partner (familiarity), but that any prior experience of cooperation can be used. This provides evidence to support our first hypothesis that a) social environment will affect cooperative behaviour during predator inspections but does not support our second hypothesis that b) familiarity will affect cooperative behaviour during predator inspections. The strengths of the current study make it an effective way to test for generalised reciprocity. Thus, future research could replicate our work using a larger sample size, monitoring the size/physical condition of the fish, and comparing different species of fish with different levels of intelligence to see whether different results are found. Interestingly we found results that are in contrast to a vast amount of previous research suggesting non-human animals maintain cooperation through direct reciprocity. We also found evidence to support for the only (to our knowledge) demonstrating how non-human animals may maintain cooperation through generalised reciprocity. This is compatible with an ‘anonymous generous TFT’ like strategy whereby individuals copy the last type of behaviour (cooperative or defective) irrespective of the identity of their partner. The mechanisms underlying cooperation are therefore complex, and further research is needed to support the findings of behaviour observed in the current study, and to increase our understanding of social influences on the Trinidadian guppy’s behaviour.
References
Axelrod, R., & Hamilton, W. D. (1981). The evolution of cooperation. Science, 211, 1390-1396. Bateson, M., Nettle, D., & Roberts, G. (2006). Cues of being watched enhance cooperation in a real-world setting. Biology Letters, 2, 412-414.
Bisazza, A., De Santi, A., & Vallortigara, G. (1999). Laterality and cooperation: mosquitofish move closer to a predator when the companion is on their left side. Animal Behaviour, 57(5), 1145-1149.
Brandl, S., & Bellwood, D. (2015). Coordinated vigilance provides evidence for direct reciprocity in coral reef fishes. Scientific Reports, 5(1).
Burkart, J., Hrdy, S., & Van Schaik, C. (2009). Cooperative breeding and human cognitive evolution. Evolutionary Anthropology: Issues, News, And Reviews, 18(5), 175-186.
Carter, G. (2014). The reciprocity controversy. Animal Behavior and Cognition, 1(3), 368–386. doi:10.12966/abc. 08.11.2014.
Connor, R. (2010). Cooperation beyond the dyad: on simple models and a complex society. Philosophical Transactions Of The Royal Society B: Biological Sciences, 365(1553), 2687-2697.
Croft, D. P., James, R., Thomas, P. O. R., Hathaway, C., Mawdsley, C., Laland, K. N., & Krause, J. (2006). Social structure and cooperative interactions in a wild population of guppies (Poecilia reticulata). Behavioural Ecology and Sociobiology, 59, 644-650.
Darwin, C. (1859). On the origin of the species by natural selection.
de Waal, F. B., & Suchak, M. (2010). Prosocial primates: Selfish and unselfish motivations. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 2711-2722.
Dugatkin, L. (1992). Tendency to inspect predators predicts mortality risk in the guppy (Poecilia reticulata). Behavioral Ecology, 3(2), 124-127.
Dugatkin, L. A. (1997). Cooperation among animals: An evolutionary perspective.. Oxford, UK: Oxford University Press.
Dugatkin, L., & Alfieri, M. (1991). Tit-For-Tat in guppies (Poecilia reticulata): the relative nature of cooperation and defection during predator inspection. Evolutionary Ecology, 5(3), 300-309.
Dugatkin, L., & Alfieri, M. (1991). Tit-For-Tat in guppies (Poecilia reticulata): the relative nature of cooperation and defection during predator inspection. Evolutionary Ecology, 5(3), 300-309.
Dugatkin, L., & Godin, J. (1992). Reversal of Female Mate Choice by Copying in the Guppy (Poecilia reticulata). Proceedings Of The Royal Society B: Biological Sciences, 249(1325), 179-184.
Endler, J. A. (1978). A predator’s view of animal color patterns. In Evolutionary biology (pp. 319-364). Springer US.
Freidin, E., Carballo, F., & Bentosela, M. (2015). Direct reciprocity in animals: The roles of bonding and affective processes. International Journal Of Psychology, 52(2), 163-170.
Gallup, G. G., Jr. 1970. Chimpanzees: self-recognition. Science, 167, 86–87.
Godin, J., & Davis, S. (1995). Who Dares, Benefits: Predator Approach Behaviour in the Guppy (Poecilia reticulata) Deters Predator Pursuit. Proceedings Of The Royal Society B: Biological Sciences, 259(1355), 193-200. http://dx.doi.org/10.1098/rspb.1995.0028
Griffiths, S., & Magurran, A. (1997). Schooling preferences for familiar fish vary with group size in a wild guppy population. Proceedings Of The Royal Society B: Biological Sciences, 264(1381), 547-551.
Hamilton, W. D. (1964). The genetical evolution of social behaviour. Journal of Theoretical Biology, 7, 1-16.
Hasson, O. (1994). Cheating Signals. Journal Of Theoretical Biology, 167(3), 223-238.
Hesse, S., Anaya-Rojas, J. M., Frommen, J. G., & Thünken, T. (2015). Social deprivation affects cooperative predator inspection in a cichlid fish. Royal Society Open Science, 2(3), 140451.
Kerr, B., Godfrey-Smith, P., & Feldman, M. (2004). What is altruism?. Trends In Ecology & Evolution, 19(3), 135-140.
Krama, T., Vrublevska, J., Freeberg, T., Kullberg, C., Rantala, M., & Krams, I. (2012). You mob my owl, I’ll mob yours: birds play tit-for-tat game. Scientific Reports, 2(1). doi: 10.1038/srep00800
Külling, D., & Milinski, M. (1992). Size-dependent predation risk and partner quality in predator inspection of sticklebacks. Animal Behaviour, 44(5), 949-955.
Magurran, A. E. & Higham, A. (1988). Information transfer across fish shoals under predator threat. Ethology, 78, 153—158.
Magurran, A., & Seghers, B. (1994). Sexual Conflict as a Consequence of Ecology: Evidence from Guppy, Poecilia reticulata, Populations in Trinidad. Proceedings Of The Royal Society B: Biological Sciences, 255(1342), 31-36.
Milinski, M. (1987). TIT FOR TAT in sticklebacks and the evolution of cooperation. Nature, 325(6103), 433-435.
Milinski, M. (1987). TIT FOR TAT in sticklebacks and the evolution of cooperation. Nature, 325(6103), 433-435.
Nowak MA, Roch S (2007) Upstream reciprocity and the evolution of gratitude. Proceedings of the Royal Society B: Biological Sciences 274:605-61
Nowak, M. A. (2006). Five rules for the evolution of cooperation. Science, 314, 1560-1563.
Nowak, M., & Sigmund, K. (2005). Evolution of indirect reciprocity. Nature, 437(7063), 1291-1298.
Packer, C. (1977). Reciprocal altruism in Papio anubis. Nature, 265(5593), 441-443.
Raihani, N., & Bshary, R. (2011). Resolving the iterated prisoner’s dilemma: theory and reality. Journal Of Evolutionary Biology, 24(8), 1628-1639.
Rausher, Mark D. “Principles of Evolution, Lectures 11 & 12: Altruism and Kin Selection.” Duke University. (April 26, 2015)
Reiss, D., & Marino, L. (2001). Mirror self-recognition in the bottlenose dolphin: A case of cognitive convergence. Proceedings Of The National Academy Of Sciences, 98(10), 5937-5942. doi: 10.1073/pnas.101086398
Roberts, G. (2008). Evolution of direct and indirect reciprocity. Proceedings Of The Royal Society B: Biological Sciences, 275(1631), 173-179.
Roberts, L., & Elliott, M. (2017). Good or bad vibrations? Impacts of anthropogenic vibration on the marine epibenthos. Science Of The Total Environment, 595, 255-268.
Rubenstein, D. & Kealey, J. (2010) Cooperation, Conflict, and the Evolution of Complex Animal Societies. Nature Education Knowledge 3(10):78
Rutte, C., & Taborsky, M. (2007). Generalized Reciprocity in Rats. Plos Biology, 5(7), e196.
Scheel, D., & Packer, C. (1991). Group hunting behaviour of lions: a search for cooperation. Animal Behaviour, 41(4), 697-709.
Stevens, J. R., F. A. Cushman, and M. D. Hauser. 2005. Evolving the psychological mechanisms for cooperation. Annu. Rev. Ecol. Evol. Syst. 36:499–518.
Tinbergen N. (1951). The study of instinct. Oxford, UK: Clarendon Press
Trivers, R. L. (1971). The evolution of reciprocal altruism. Quarterly Review of Biology, 46, 35-57.
van Doorn, G., & Taborsky, M. (2011). The Evolution of Generalised Reciprocity on Social Interaction Networks. Evolution, 66(3), 651-664.
Wedekind, C. (2000). Cooperation Through Image Scoring in Humans. Science, 288(5467), 850-852.
West, S., Griffin, A., & Gardner, A. (2007). Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. Journal Of Evolutionary Biology, 20(2), 415-432.
Wilkinson, G. (1984). Reciprocal food sharing in the vampire bat. Nature, 308(5955), 181-184.
Wilson, E. (2000). Sociobiology the New Synthesis. Belknap Press of Harvard University Press.
(Study last year, 2017)
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




