Protein Stabilisation by Small Molecules
Info: 8708 words (35 pages) Dissertation
Published: 9th Dec 2019
Tagged: Biology
Abstract
Proteins are increasingly employed in pharmaceutical formulations, detergents, industrial processes and as analytical aids. A major obstacle to their development however, is the restricted stability of proteins in aqueous formulations, a feature which severely impacts the commercial and biological success of products. The principal method used to achieve stabilisation of proteins in liquid formulations is the addition of excipients. However, regulatory barriers and ambiguity surrounding the mechanisms by which excipients influence stability of active ingredients have resulted in a limited repertoire of molecules to aid formulation development. In view of the emerging prevalence of protein and peptides, especially within the therapeutic sphere, there is a significant requirement for the development of novel excipients. To this end, this research aims to characterise the performance of the polyphenol catechin as a protein stabiliser for industrial use. The interactions between proteins and small polyphenolic compounds has occupied considerable research efforts over the last three decades, owing to the role they play in food and beverage astringency, as well as the antioxidant potential of these compounds and their role in human health. Analysis of the influence of catechin on thermal protein stability will be carried out using differential scanning calorimetry.
Table of Contents
Protein stabilisation by small molecules
Protein unfolding and stability of proteins in liquid formulations
Differential scanning calorimetry
Interactions between proteins and small polyphenolics
Predictions on the stabilizing capacity of Catechin/Epicatechin on proteins for therapeutic use
1 Introduction
Protein biopharmaceuticals represent an ever-increasing portion of the global pharmaceutical market [Moorkens et al. 2017]. They include monoclonal antibodies, recombinant proteins, peptides and a range of vaccines. Their ability to act with high specificity at low concentrations, in comparison with synthetic drugs have made them a requisite in fighting the world’s biggest health challenges. However, the process of formulation development of protein products can often be met with significant complications, not encountered by small molecule drugs [Razinkov et al. 2015]. One of the primary goals and critical bottlenecks in the formulation of these biopharmaceuticals is the stabilisation of proteins and peptides during development, manufacture and long-term storage. Preserving the three-dimensional structure of therapeutic proteins is crucial, not only to maintaining biological activity, but for preventing adverse immunogenic effects and ensuring controlled delivery following parenteral administration [Jorgensen 2009]. Proteins have not evolved to be remarkably stable. That is, there is no evolutionary pressure to drive a protein’s stability further than that which is ‘stable enough’ [Williamson 2012]. Designing therapeutic formulations containing proteins at high concentrations, stable over lengthy periods of time and insusceptible to temperature fluctuations and agitation presents a substantial challenge. Ideally, the formulation would be an aqueous liquid with 18-24 months shelf-life and minimal aggregation. Introduction of suitable excipients serves as one of the main routes to meeting these requirements and enhancing protein stability in liquid formulations [Wang 2015].
Upon protein unfolding, non-covalent bonds are broken and hydration of the now exposed hydrophobic core occurs. This can be observed and measured as an increase in heat capacity using a thermal analytical technique known as differential scanning calorimetry (DSC). Stabilisation of proteins by excipients is achieved by adjusting the free energy associated with this hydration or by direct binding to the protein and subsequent alteration of the strength of the protein structure [Bye et. al 2014]. Amongst the molecules that have centered protein formulation are amino acids, polyols, salts and sugars [Arakawa et al. 2007a] [Jorgensen 2009]. The importance of excipients also extends further than therapeutic applications, encompassing industrial enzymes, as well as the composition of liquid detergents [Iyer & Ananthanarayan 2008]. The limited repertoire of excipients available for use in industrial and parenteral formulations however, presents a major challenge for formulation scientists and undoubtedly, there is a demand for the characterisation of novel excipients.
1.1 Focus of work
The main objective of this thesis is to elucidate the efficacy of the small polyphenolic compound catechin as a protein stabiliser for industrial applications. A substantial volume of studies exist that describe protein-polyphenol interactions, centering around the influence of polyphenol structure on these interactions, in addition to the impact on protein structure, digestibility, enzymatic activity and astringency [Jakobek 2015]. The driving forces are predominantly hydrogen bonds, arising from the extensive hydroxyl groups of polyphenols and hydrophobic interactions between polyphenolic aromatic rings and protein hydrophobic patches [Oh et al. 1980; Hagerman et al. 1998].
Differential scanning calorimetry will be used to provide detailed measurements on the thermal stability of the model protein lysozyme after addition of catechin. From this data, we can then attempt to postulate the mechanisms behind any observed effects on protein stability. If the desired effect is not observed with catechin, the diastereoisomer epicatechin may be explored. Further investigations will be aimed at elucidating the suitability of catechin in commercial products and will include thermal stability studies on industrially relevant proteins, such as monoclonal antibodies and phytase.
1.2 Objectives
- Study the effect of catechin on the thermal stability of lysozyme using differential scanning calorimetry. pH will be controlled by the buffering capacity of the protein itself.
- Analyse the trends from these experiments to theorise the mechanisms behind the observed effects of catechin on the stabilisation (or destabilisation) of lysozyme.
- Explore the utility of catechin to biopharmaceutical and industrial formulations through thermal stability experiemnts on monoclonal antibodies and enzymes, such as phytase.
2 Protein stabilisation by small molecules
The impact of small molecules, ranging from salts to naturally occurring organic molecules, or osmolytes, on protein stability has been well documented. General trends have been reported, the most widely recognised being those of the Hofmeister salts, described further in this section. The means by which osmolytes exert their effects are more complex. Osmolytes find extensive utility in therapeutic protein formulations owing to their ability to stabilise macromolecules in cellular environments subject to osmotic stress [Yancey 2005]. They fall into several broad categories, including polyols and sugars (e.g. sorbitol and trehalose), amino acids (e.g. proline) and methylamines (e.g. TMAO). Recent collaborative attempts have aimed at elucidating the mechanisms behind small molecule effects on protein stability and solubility. Although highly complex, a better understanding will translate to more a predictable protein stability performance of these molecules in industrial settings, including biopharmaceutical formulation.
2.1 The effect of salts on protein stability
The specific interactions between salts and proteins have been well studied since protein precipitation experiments performed over a century ago [Mittheilung & Hofmeister 1888]. Hofmeister was the first to quantitatively characterise salting out effects of an entire set of salts. Known as the Hofmeister series, it orders the relative influence of ions on physical processes in aqueous solutions, ranging from protein stability to bacterial growth [Zhang & Cremer 2006] (Figure 1).

Figure 1. The Hofmeister series detailing the effects of ions in the series on the behavior of proteins.
Ions at one end of the series, known as kosmotropes, tend to contribute to precipitation and increase the structural stability of proteins [von Hippel & Schleich 1969]. On the other hand, chaotropes, residing at the opposite end, increase solubility and destabilise folded proteins. These effects are observed to be more pronounced for anions than for cations [Zhang et al. 2005]. Despite the relevance of these phenomena to a wide range of fields, the molecular mechanisms underlying the effect of the Hofmeister series on protein stability and solubility are yet to be elucidated. According to early theories, kosmotropic and chaotropic ions exerted their effects by strengthening and breaking the hydrogen-bond network of bulk water [Collins & Washabaugh 1985; Collins 2012]. Subsequent studies lent a significant challenge to the bulk water structure theory, whereby techniques such as femtosecond pump probe spectroscopy and pressure perturbation calorimetry revealed no significant changes to the hydrogen-bond network in the presence of ions [Omta et al. 2003, Omta et al. 2003b, Batchelor et al. 2004]. The high salt concentrations used in these studies have however been criticised owing to the absence of an extended hydration shell [Marcus 2009]. Adding more complexity to the subject was the identification of an inverse Hofmeister effect [Zhang & Cremer 2009]. Here, ions at lower salt concentrations are observed to have the opposite effects to those observed by Hofmeister originally. Further models include those of preferential interaction and excluded volume. Here, kosmotropes are thought to induce stabilisation of proteins by being preferentially excluded from the surface of proteins and interacting preferentially with water [Timasheff et al. 1993; Timasheff et al. 2002]. Chaotropes, on the other hand, cause destabilising effects by preferentially interacting with the protein surface. Excluded volume builds upon this theory, suggesting exclusion of kosmotropic solutes from the protein forms a cavity within the water, resulting in a rise in osmotic pressure and pushing the protein into a more compact and stable state [Knowles et al. 2011]. Experimental data reinforcing these models has led to their adoption as the more popular mechanisms behind the Hofmeister series and the effect on protein stability [Jungwirth & Cremer 2014].
In more recent studies, DSC was used to suggest an alternative, albeit similar model for modulation of protein stability by Hofmeister ions to those concerning preferential interaction and exlucded volume [Bye & Falconer 2013]. Findings indicate that competition for water between with the unfolding protein by kosmotropic anions is responsible for protein stabilisation. In doing so, the free energy associated with hydrating the newly exposed core increases, resulting in an increase in protein stability. Conversely, chaotropic anions directly interact with proteins through association with apolar residues, allowing them to be more efficiently hydrated, thus reducing the free energy associated with protein unfolding and destabilising the protein. The same group proposed a refinement to this model in studies performed on barnase using DSC (Bye et al .2016). Hofmeister effects were reported at high anion concentrations and it has been proposed that kosmotropes, which are effective at ordering water molecules, specifically their dipoles, about themselves, adjust the properties of water surrounding the protein. The protein is solvated to a lesser extent, increasing the strength of intramolecular interactions and subsequently improving their stability. Consequently, instead of preferential interaction, hydration or excluded volume, modulation of protein solvation is suggested to effect stability of proteins by Hofmeister anions.
In the future, it appears research will aim to address the approximation that ion effects exist within clear-cut anionic and cationic series [Okur et al. 2017]. Schneider et al. [2011] showed that guandinium-anion interactions diminish guanidinium-protein binding. This indicates that interactions between ions of opposite polarities both at the surface of proteins and in bulk solution for specific salts have significant consequences on the interaction of these salts with proteins and consequently, may require consideration in the rationalisation of Hofmeister salt’s effects on protein stability.
2.2 Amino acids
Amino acids, including arginine, proline and histidine are commonly employed as stabilising excipients in liquid biopharmaceutical formulations [Jorgensen et al. 2009]. For the design of therapeutic formulations, an understanding of the mechanisms underpinning the stabilisation of proteins can provide an accurate prediction of the performance of excipients and establish a foundation for the design of formulations with a higher chance of success [Bye et al. 2014]. The modes of action the protein stabilisation of most amino acids is reasonably well understood [Arakawa et al. 2007a]. Arginine prevents aggregation of and solubilizes proteins without impacting their native structure or for the most part their stability, but contrary to other amino acid excipients, the mechanism of these effects offers less clarity [Arakawa & Tsumoto 2003; Reddy et al. 2005; Lange & Rudolph 2009]. Several mechanisms have been proposed from different perspectives, including direct interactions [Shukla & Trout 2010], preferential interactions and interactions with the peptide backbone [Arakawa et al. 2007b]. The formation of large arginine clusters is one suggestion for its involvement in protein stability [Shukla & Trout 2010; Vagenende et al. 2013]. These observations were made by molecular dynamics simulation and therefore suffer from an absence of supplemental experimental support. Discrepancies surrounding the nature of the interactions involved in the clusters further adds to the uncertainty of the significance of self-association of arginine in its behaviour with proteins. Separate efforts were also made to establish a relationship between the effects of arginine and its charged side chain, which shares structural similarity with the chaotropic salt, guanidinium chloride [Xie et al. 2004; Arakawa et al. 2007b]. Guanidinium triggers thermal destabilization of proteins at low concentrations and has the capacity to induce complete denaturation at high enough concentrations. In comparison to guanidinium, however, the destabilisation effects of arginine are much less pronounced [Ishibashi et al. 2005], prompting the exploration of alternative theories. Work performed by Platts & Falconer [2015] suggests the mechanisms influencing the stability of proteins by arginine arise from the sum of its functional parts, both the glycine moiety and guanidinium side chain. Thermal stability of 3 proteins (bovine serum albumin, myoglobin and lysozyme) in the presence of glycine and guanidinium chloride was compared to that with arginine by using DSC. At lower concentrations (<100 mM), the effects of arginine were observed to be protein-specific, directed by weak interactions between the glycine moiety and both the peptide backbone and charged side chains. At concentrations over 100 mM, the destabilisation of proteins by arginine reflected that of guanidinium chloride, albeit more weakly, due to a simultaneous stabilisation by glycine operating at these concentrations. Competition for water between the cosolute and the unfolding protein was used to describe the stabilisation by the glycine moiety. This mechanism is similar to that assigned to high charge density anions [Bye & Falconer 2014]. The destabilisation observed from the guanidinium side chain occurs due to direct interaction with the apolar core of the unfolding protein, responsible also for lowering the free energy associated with the hydration of the exposed protein interior [Platts & Falconer 2015]. Similar results have been demonstrated for lysine and histidine [Platts et al. 2016]. Particularly at higher concentrations, these amino acids exerted protein stabilisation effects as a result of the sum of their glycine moieties and their respective side chains, methylamine HCl and imidazole. These findings have important implications for the development of biopharmaceutical formulations. The additive behavior of positively charged amino acids as a result of their constituent groups indicate that bespoke excipients may be designed where the ratio of these groups in solution have been altered. Still, owing to the protein and concentration specific effects observed for positively charged amino acids, the predictability of undesirable effects in formulations remains inaccurate [Platts et al. 2016]. As insight into the mechanisms governing protein stability deepens, the prediction of performance and safety of excipients will likely improve too.
3 Protein unfolding and stability of proteins in liquid formulations
The main forces that govern properly folded proteins include hydrophobic and electrostatic interactions, van der Waals forces and hydrogen bonding. Physical instability of proteins arises due to changes in these non-covalent interactions and subsequent alterations to their secondary, tertiary and quaternary structures. The free energy change (∆G) for the unfolding reaction under ambient conditions defines the conformational stability of a proteins folded state [Pace 1990]. For a majority of globular proteins, the folded state is only slightly more stable than that of the unfolded state, represented by a small ∆G equivalent to a few hydrogen bonds. This modest free energy change arises from the fact that the entropic and enthalpic components of folding nearly cancel [Scharnagl et al. 2005]. A reduction in entropy occurs as the protein folds, contributing positively to the total free energy change. Conversely, there is negative contribution with regard to enthalpy, predominantly due to hydrogen bond formation. The potential for conformational alterations or unfolding of proteins means that folded proteins in solution are not infinitely stable [Shortle 1996]. Protein unfolding can be described simply by a single transition step between native (N) and unfolded (U) states:
N⇌U
This transition from the native state to unfolded arises from the breaking of salt bridges, hydrogen bonds and van der Waals forces and the subsequent hydration of the newly exposed hydrophobic protein core [Jaenicke 1991]. A critical thermodynamic parameterrelated to this transition is the heat capacity (∆Cp), which increases upon unfolding owing to the larger heat capacity of water associated with nonpolar groups of the protein core compared to bulk water [Pace et al. 1996]. This change can be captured and measured by DSC, discussed in more detail in the following section, and has consequently found practical use by companies to assess the thermal stability of their therapeutic or industrial products.
The stability of proteins in aqueous formulations is influenced by a range of factors, including temperature, pH, mechanical stress and presence of cosolutes [Scharnagl et al. 2005] (Figure 2). Temperature is arguably the most important factor influencing therapeutic and industrial protein stability. Elevated temperatures during manufacture,
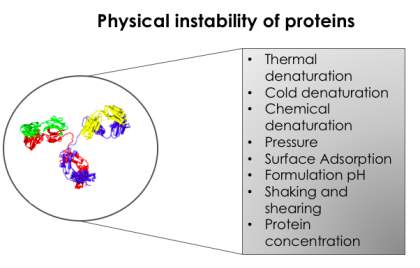
Figure 2. Examples of the physical instabilities of proteins. Physical instability defines any process by which proteins change their physical state with no alteration to their chemical composition. These contribute to the difficulty in developing liquid protein formulations.
as well as unexpected temperature fluctuations during storage have a marked impact on the performance and quality of protein products. Generally, the higher the temperature, the higher the effect of destabilisation on proteins. This is mainly driven by increased entropy resulting in a decrease in the free energy of the denatured state [Shortle 1996]. Extreme pH also plays a significant role in protein unfolding, arising from an increase in electrostatic repulsions from the protein interior [Goto & Fink 1989]. Different formulation pHs also have the propensity to promote different routes of chemical degradation, including hydrolysis and deamidation [Wang 1999]. Shaking or shearing during commercial production exposes proteins to mechanical stress. These perturbations lead to the formation of an air/water interface, leading to exposure of hydrophobic regions [Bee et al. 2009]. Besides stability in a conformational sense, aggregation also plays a significant role in the challenges faced by formulation scientists and has a huge impact on the therapeutic success of protein formulations. Aggregation is the process by which proteins as monomers associate with one another into complexes. Held together predominantly by non-covalent interactions, aggregates typically require a degree of unfolding to form. For example, the hydrophobic regions exposed during the formation of an air/water interface from mechanical stresses initiate aggregation amongst proteins [Bee et al. 2009]. Besides altering the conformational stability of therapeutic proteins, excipients also have the ability to deter aggregation in therapeutic and industrial formulations [Wang 1999].
4 Differential scanning calorimetry
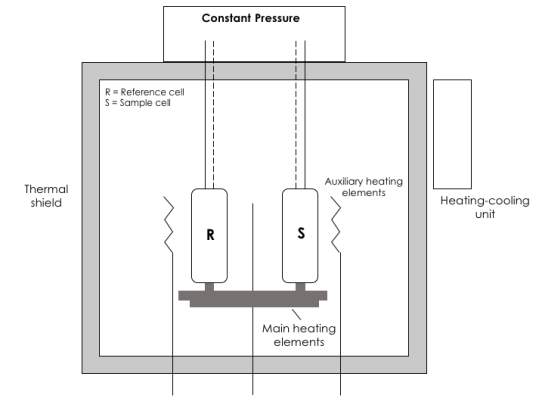
Differential scanning calorimetry is a technique used to study thermally induced transitions and both quantitatively and qualitatively determine the stability of the folded state of a protein. The calorimeters operate in a two-pan configuration, consisting of a sample cell and a reference cell (Figure 3). Separate heating jackets surround the cells,
Figure 3. Schematic cross-sectional view of a differential scanning calorimeter with a capillary cell design. The equipment consists of matching sample and reference cells, capillary tubes for solution introduction, main heating elements, a thermal adiabatic shield, a central sensor, temperature sensors (dashed lines), auxiliary feedback heaters and a heating-cooling unit.
and both sample cell and a reference cell. Separate heating jackets surround the cells, and both are contained within an adiabatic shield which controls the scanning temperature. The temperature of the cells in monitored by sensors to determine if there is a difference in temperature between the two. If so, compensating power is applied to keep the cells at the same temperature. The power value is used to determine the difference in heat capacity
(∆Cp)assigned to the analyte within the sample cell.
The measurements that can be obtained from a DSC scan when a protein is exposed to a temperature change, including heat capacity, enthalpy, entropy and free energy make this a valuable technique for investigating the thermodynamic properties of proteins. Upon protein unfolding, a change in heat capacity is observed. DSC calculates the excess heat capacity
(∆Cp)of a sample of a molecule of interest relative to a reference sample as a function of temperature. Consequently, the pressure in the cells of the calorimeter is kept constant. The heat required to raise the temperature of a solution of unfolded protein is larger than that of a solution of folded protein, occurring as a result of hydration of the newly exposed protein core and its apolar residues [Privalov & Makhatadze 1992; Bruylants et al. 2005]. The endothermic unfolding of a protein manifests as a peak on a DSC thermogram (Figure 4). The melting temperature (
Tm), 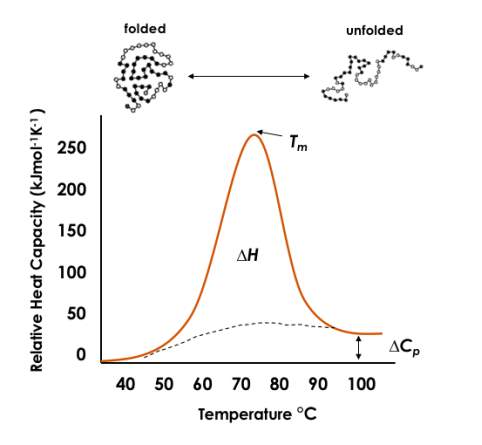
which centres the peak, is the point at which the excess heat capacity is maximal.
Figure 4. A schematic example of a DSC thermogram. Thermodynamic parameters are labelled; melting temperature (Tm); enthalpy change of reaction (∆H) and change in heat capacity (∆Cp)
Arguably one of the most useful measurements determined by DSC, the Tm of a protein represents the point at which 50% of the protein is unfolded. In general, proteins with a higher Tm are more stable. The enthalpy of protein unfolding (
∆H), described by equation 1, is calculated from the area of the thermal transition or specifically, by the integral of excess heat capacity [Spink 2008] (Figure 4).
T1and
T2are the start and end temperatures of the thermal reaction, set by the user.
∆H=∫T1T2CpdT
(1)
The entropy change for unfolding is given by equation 2.
∆S=∫T1T2(Cp/T)dT
(2)
From here it is possible to calculate the Gibbs free energy (∆G) for protein unfolding using the modified Gibbs-Helmholtz equation (equation 3).
∆GuT=∆HuTm1-T/Tm+∆CpT-Tm-∆Cp(Tmln[T/Tm]
) (3)
The change in heat capacity
(∆Cp)is measurable from the baseline shift from before to after protein folding (Figure 4). Due to the opportunity for random variations to arise within the post-transitional baseline section, it can be more accurate to calculate
∆Cpusing Kirchhoffs law (equation 4) [Senske et al. 2014].
∆Cp=∆H∆Tm
(4)
The accuracy of these calculations relies on protein unfolding as a simple reversible, two-state transition [Bruylants et al. 2005]. Whilst this is reasonable for many small proteins, assumptions must be validated, which can be achieved in a number of ways. Usually, two DSC scans are performed, and the second endotherm checked against the first. Likewise, a downward scan can be performed following an upward scan and the inverted thermogram compared to that of the upward scan.
DSC is now extensively used by the biopharmaceutical industry for the assessment of a range biological molecules, particularly proteins [Chen & Oakley 1995]. The simplicity of its operation and interpretation of thermodynamic data contribute to the popularity of this technique and with technical improvements being reported each year, its use will only become more widespread.
5 Catechin
Catechins are small polyphenolic compounds belonging to the major subclass of plant phytochemicals called flavan-3-ols [Neilson & Ferruzzi 2011]. They exist naturally in cocoa, green tea and a variety of other plants as secondary metabolites [Harbowy et al. 1997; Andres-Lacueva et al. 2008]. Owing to their antioxidant and antimicrobial properties, they have served an important focus for dietary supplements, as well as anti-inflammatory, cancer and cardiovascular therapies [Freitas & Mateus 2012]. Any potential excipient must conform to stringent safety requirements and given its extensive consumption by man, catechin could serve as a promising molecule in biotherapeutic formulations, provided the required physiochemical functionalities are also met. Flavan-3-ols, in particular (+)-catechin and (-)-epicatechin serve an important role as the building blocks of long chain polymers or ‘tannins’ in grapes [Pinelo et al. 2006]. These diastereoisomers consist of two benzene rings and a dihydropyran ring with hydroxyl (-OH) groups at positions 3, 5, 7, 3’ and 4’ (Figure 5). The spatial orientation of the -OH at position 3 differs between the two molecules, resulting in distinct properties accentuated in large polymers [Scollary et al. 2010]. The potential for hydrogen bonds between flavan-3-ols and proteins are ample. The hydroxyl groups have proton donor capacity, whilst the carbonyl groups of the peptide backbone possess the ability to act as proton acceptors. Catechins and proteins interact strongly through both hydrophobic interactions and hydrogen bonding [Cui et al. 2015]. Owing to the multitude of possible interactions catechin can engage in with proteins, it is difficult to predict the effect on lysozyme stability. If the mechanisms follow those of the positively charged amino acids observed by Platts et al. [2016], at lower concentrations the stabilisation effects will be directed by interactions with the protein backbone and sidechains. At higher concentrations, the observed effects may occur as a result of competition for water between the cosolute and the unfolding protein, although the mechanisms of stabilisation by these amino acids are somewhat distinct amongst 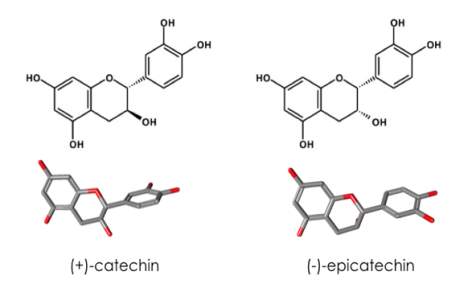
cosolutes.
Figure. 5 The structures of (+)-catechin and (-)-epicatechin. Two benzene rings are linked by a dihydropyran ring. The locations of the hydroxyl groups are depicted. Two different spatial configurations about the -OH in position 3 result in the 2,3-trans isomer (+)-catechin and the 2,3-cis isomer (-)-epicatechin
6 Interactions between proteins and small polyphenolics
The interactions between polyphenolics and proteins has wide implications in the commercial world and has occupied a considerable research effort over the last few decades. Proanthocyanidins, or condensed tannins, are polymeric flavonoid species generally comprised of subunits of flavan-3-ols, including catechin [Sarni-Manchado et al. 1999]. The significance of these molecules corresponds with their impact on organoleptic and technological properties of food and beverages, especially astringency in wine [Poncet-Legrand et al. 2003]. Astringency perception arises from tannin-induced interactions and precipitation of polyproline rich proteins (PRPs) in the oral cavity [Dinnella et al. 2009]. Both hydrophobic and hydrogen bonds have been shown to act as the driving force behind these interactions, with the structure of proanthocyanidins determining their relative contributions [Luck et al. 1994; Charlton et al. 2002; Simon et al. 2003; Kilmister et al. 2016; Nguela et al. 2016]. Several techniques have been used to investigate the interactions between polyphenolic compounds and proteins, including mass spectrometry, NMR and circular dichroism [Pascal et al. 2007]. Isothermal titration calorimetry (ITC) has also proved an invaluable tool for studying molecular associations and has found further application in analyses of tannins binding to proteins [Pascal et al. 2007; Poncet-Legrand et al. 2007; Frazier et al. 2010; McRae et al. 2010; Kilmister et al. 2016; Sun et al. 2017]. ITC, like DSC, provides a direct measure of ∆H associated with the making and breaking of non-covalent bonds within a molecular complex. A single experiment can also determine values for heat capacity
(∆Cp), free energy (∆G), entropy (∆S) and stoichiometry of binding. During ITC, a titration is performed by the addition of a titrant into a solution of a titrand, or its binding partner, within a sample cell maintained at a constant temperature [Jelesarov & Bosshard 1999]. The change in heat of the sample cell is measured in comparison to a reference cell of buffer. However, it should be taken into account that ITC measurements include heat associated with conformational changes, solvent rearrangement and protonation, alongside that from binding events [Ladbury 2010]. Consequently, thermodynamic interpretations of data are often more general.
A study by Poncet-Legrand et al. [2007] provides evidence to the dominating interactions between proteins and polyphenolics. The binding of various flavan-3-ols (catechin, epicatechin, epicatechingallate and epigallcatechingallate) to poly(L-proline) (PLP) was examined using ITC, although the heat changes upon titration with catechin and epicatechin were too small for investigation. The change in Gibbs free energy was characterised by the sum of both entropic and enthalpic terms. The negative ∆H, or enthalpic contribution, was interpreted as exothermic hydrogen bonding, thought to predominantly arise between H-acceptor sites within the peptide bond and the acidic polyphenol hydroxyl groups. The values for ∆S, or the entropic contributions, were considered to reflect hydrophobic interactions and the displacement of water molecules as a result of these. Further work that employed ITC for analysis of the interaction between grape seed and skin tannins with PLP provided observations in line with the mixed hydrophobic and hydrogen bonding that manifested as a biphasic titration curve [McRae et al. 2010]. Additional support of simultaneous hydrophobic interactions and hydrogen bonding was observed in tannin binding to bovine serum albumin (BSA), interpreted from the temperature dependence of the ITC titration curve [Kilminster et al. 2016], as well as in binding between proanthocyanidins and wheat gluten proteins [Girard et al. 2018].
- What happens to the structures? How does this relate to stabilizing proteins in formulations?
- ‘On the other hand, some polyphenols have important antioxi- dant properties that help to protect food from oxidation [12]’ then lead on to there are antioxidant polyphenolics? How do they work tho?
7 Concluding Remarks
8 References
Andres-Lacueva, C., Monagas, M., Khan, N., Izquierdo-Pulido, M., Upri-Sarda, M., Permanyer, J. & Lamuela-Raventos, R.M., (2008). Flavanol and Flavanol Contents of Cocoa Powder Products: Infleunce of the Manufacturing Process. Journal of Agricultural and Food Chemistry, 56, 3111-3117.
Arakawa, T. & Tsumoto, K., (2003). The effects of arginine on refolding of aggregated proteins: Not facilitate refolding, but suppress aggregation. Biochemical and Biophysical Research Communications, 304, 148–152.
Arakawa, T., Tsumoto, K., Kita, Y., Chang, B, & Ejima, D., (2007a). Biotechnology applications of amino acids in protein purification and formulations. Amino Acids, 33, 587-605
Arakawa, T., Ejima, D., et al., (2007b). Suppression of protein interactions by arginine: A proposed mechanism of the arginine effects. Biophysical Chemistry, 127, 1–8.
Batchelor, J. D., Olteanu, A., Tripathy, A. & Pielak, G.J., (2004). Impact of Protein Denaturants and Stabilizers on Water structure. Journal of the American Chemical Society, 126, 1958-1961.
Bee, J.S., Stevenson, J.L., Mehta, B., Svitel, J., Pollastrini, J., Platz, R., Freund, E., Carpenter, J.F. & Randolph, T.W., (2009). Response of a Concentrated Monoclonal Antibody Formulation to High Shear. Biotechnological Bioengineering, 103(5), 936-943.
Bruylants, G., Wouters, J. & Michaux, C., (2005). Differential scanning calorimetry in life science: Thermodynamics, stability, molecular recognition and application in drug design. Current Medicinal Chemistry, 12, 2011–2020.
Bye, J. W. & Falconer, R.J., (2013). Thermal stability of lysozyme as a function of ion concentration: A reappraisal of the relationship between the Hofmeister series and protein stability. Protein Science, 22, 1563-1570.
Bye, J. W. &Falconer, R. J. (2014). Three Stages of Lysozyme Thermal Stabilization by High and Medium Charge Density Anions. The Journal of Physical Chemistry B, 118, 4282-4286.
Bye, J. W., Platts, L. & Falconer, R. J. (2014). Biopharmaceutical Liquid Formulation: A Review of the Science of Protein Stability and Solubility in Aqueous Environments. Biotechnology Letters, 36(5), 869–875.
Cecile, S., Barathieu, K., Laguerre, M., Schmitter, J., Fouquet, E., Pianet, I. & Dufourc, E.J., (2003). Three-dimensional structure and dynamics of wine tannin-saliva protein complexes. A multitechnique approach. Biochemistry, 42, 10385-10395.
Charlton, A.J., Baxter, N.J., Khan, L., M., Moir, A.J.G., Haslam, E., Davies, A.P. & Williamson, M.P. (2002). Polyphenol/peptide binding and precipitation. Journal of Agricultural and Food Chemistry, 50, 1593-1601.
Chen, T. & Oakley, D.M., (1995). Thermal analysis of proteins of pharmaceutical interest. Thermochimica Acta, 248, 229-244.
Cheynie, V.& Vernhet, A., (2007). Interactions between a Non Glycosylated Human Proline-Rich Protein and Flavan-3-ols Are Affected by Protein Concentration and Polyphenol/Protein Ratio. Journal of Agricultural and Food Chemistry, 55, 4895-4901.
Collins, K.D., (2012). Why continuum electrostatics theories cannot explain biological structure, polyelectrolytes or ionic strength effects in ion–protein interactions. Biophysical Chemistry, 167, 43-59.
Collins, K.D. & Wasabaugh, M.W., (1985). The Hofmeister Effect and the Behaviour of Water at Interfaces. Quaterly Review of Biophysics, 18, 323-422.
Cui, F., Yang, K. & Li, Y., (2015). Investigate the Binding of Catechin to Trypsin Using Docking and Molecular Dynamics Simulation. PLoS One [online].10(5), 1-17. [Viewed 3 April 2018]. Available from: DOI:10.1371/journal.pone.0125848
Dinnella, C., Recchia, A., Fia, G., Bertuccioli, M., Monteleone, E., (2009). Saliva characteristics and individual sensitivity to phenolic astringent stimuli. Chem. Senses, 34, 295-304.
Frazier, R.A., Deaville, E.R., Green, R.J., Stringano, E., Willoughby, I., Plant, J. & Mueller-Harvey, I., (2010). Interactions of tea tannins and condensed tannins with proteins. Journal of Pharmaceutical and Biomedical Analysis, 51, 490-495.
Freitas, V. & Mateus, N., (2012). Protein/polyphenol interaction: Past and present contributions. Mechanisms of astringency perception. Current. Organic Chemistry, 16, 724-746.
Girard, A.L., Bean, S.R., Tilley, M., Adrianos, S.L. & Awika, J.M., (2018). Interaction mechanisms of condensed tannins (proanthocyanidins) with wheat gluten proteins. Food Chemistry, 245, 1154-1162 .
Goto, Y. & Fink, A.L., (1989). Conformational States of B-Lactamase: Molten-Globule States at Acidic and Alkaline pH with High Salt. Biochemistry, 28, 945-952.
Hagerman, A.E., Rice, M.E. & Ritchard, N.T., (1998). Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16 (4f8) Catechin (Procyanidin). Journal of Agricultural and Food Chemistry,46, 2590-2595.
Harbowy, M.E., Balentine, D.A., Davies, A.P & Cai, Y., (2010). Tea Chemistry. Critical Reviews in Plant Sciences, 16(5), 415-480.
Ishibashi, M. et al., (2005). Is arginine a protein-denaturant? Protein Expression & Purification, 42, 1–6.
Iyer, P.V. & Ananthanarayan, L., (2008). Enzyme stability and stabilization- Aqueous and non-aqueous environment. Process Biochemistry, 43, 1019-1032.
Jaenicke, R., (1991). Proteins stability and molecular adaptation to extreme conditions. European Journal of Biochemistry, 202, 715-728.
Jakobek, L., (2015). Interactions of polyphenols with carboyhdrayes, lipids and proteins. Food Chemistry, 175, 556-567.
Jelesarov, I. & Bosshard, H.R., (1999) Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. Journal of Molecular Recognition, 12, 3-18.
Jorgensen, L., Hostrup, S., Moeller, E. H. & Grohganz, H., (2009). Recent Trends in Stabilising Peptides and Proteins in Pharmaceutical Formulation – Considerations in the Choice of Excipients. Expert Opinion on Drug Delivery, 6(11), 1219–1230.
Jungwirth, P. & Cremer, P. S. (2014). Beyond Hofmeister. Nature Chemistry, 6, 261-263.
Kilmister, R.L., Faulkner, P., Downey, M.O., Darby, S.J. & Falconer, R.J., (2016) The complexity of condensed tannin binding to bovine serum albumin – An isothermal titration calorimetry study. Food Chemistry,190, 173-178.
Knowles, D.B., LaCroix, A.S., Deines, N.F., Shkel, I. & Record, M.T., (2011). Separation of preferential interaction and excluded volume effects on DNA duplex and hairpin stability. PNAS, 108(31), 12699-12704.
Ladbury, J.E., (2010) Calorimetry as a tool for understanding biomolecular interactions and an aid to drug design. Biochemical society transaction, 38(4), 888-893.
Lange, C. & Rudolph, R., (2009). Suppression of protein aggregation by L-arginine. Current Pharmaceutical Biotechnology, 10, 408–414.
Luck, G., Liao, H., Murray, N.J., Grimmer, H.R., Warminski, E.E., Williamson, M.P., Lilley, T.H. & Haslam, E. (1994). Polyphenols, astringency and proline-rich proteins. Phytochemistry, 37(2), 357-371.
Marcus, Y., (2009). Effect of Ions on the Structure of Water: Structure Making and Breaking. Chemical Reviews, 109, 1346-1370.
McRae, J. M., Falconer, R. J., & Kennedy, J. A., (2010). Thermodynamics of grape and wine tannin interaction with polyproline: Implications for red wine astringency. Journal of Agricultural and Food Chemistry, 58(23), 12510–12518
Mittheilung, Z. & Hofmeister, F., (1888). Zur Lehre von der Wirkung der Salze. Disease Archive, 17, 1-30.
Moorkens, E., Meuwissen, N., Huys, I., Declerck, P., Vulto, A. G. & Simoens, S., (2017). The Market of Biopharmaceutical Medicines: A Snapshot of a Diverse Industrial Landscape. Frontiers in Pharmacology, 8(314), 1-12.
Neilson, A.P. & Ferruzzi, M.G., (2010). Influence of Formulation and Processing on Absorption and Metabolism of Flavan-3-ols from Tea and Cocoa. Annual Reviews of Food Science and Technology, 2, 125-151.
Nguela, J.M., Poncent-Legrand, C., Sieczkowski, N. & Vernhet, A., (2016). Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and B-glucan. Food Chemistry,210, 671-682.
Omta, A. W., Kropman, M. F., Woutersen, S. & Bakker, H. J., (2003a). Influence of ions on the hydrogen-bond structure in liquid water. The Journal of Chemical Physics, 119, 12457.
Omta, A. W., Kropman, M. F., Woutersen, S. and Bakker, H. J. (2003b). Negligible effect of ions on the hydrogen-bond structure in liquid water. Science, 301, 347-349.
Pace, C.N., (1990). Conformational stability of globular proteins. Trends in Biochemical Sciences, 15, 14–17.
Pace, C.N. et al., (1996). Forces contributing to the conformational stability of proteins. Journal of the Federation of American Societies for Experimental Biology, 10, 75–83.
Pascal, C., Poncent-Legrand, C., Imberty, A., Gautier, C., Sarni-Manchado, P.,
Pinelo, M., Arnous, A. & Meyer, A.S. (2006). Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends in Food Science & Technology, 17, 579-590.
Platts, L. & Falconer, R.J., (2015). Controlling protein stability: Mechanisms revealed using formulations of arginine, glycine and guanidinium HCl with three globular proteins. International Journal of Pharmaceutics, 486, 131–135.
Platts, L., Darby, S.J. & Falconer, R.J., (2016). Control of Globular Protein Thermal Stability in Aqueous Formulations by the Positively Charged Amino Acid Excipients. Journal of Pharmaceutical Sciences, 105, 3532-3536.
Poncent-Legrand, C., Cartalade, D., Putaux, J.L., Cheynier, V. & Vernhet, A., (2003). Flavan-3-ol Aggregation in Model Ethanolic Solutions: Incidence of Polyphenol Structure, Concentration, Ethanol Content, and Ionic Strength. Langmuir, 19, 10563-10572.
Poncet-Legrand, C., Gautier, C., Cheynier, V., & Imberty, A., (2007). Interactions between flavan-3-ols and poly(L-proline) studied by isothermal titration calorimetry: Effect of the tannin structure. Journal of Agricultural and Food Chemistry, 55(22), 9235–9240.
Privalov, P.L. & Makhatadze, G.I., (1992). Contribution of hydration and non-covalent interactions to the heat capacity effect on protein unfolding. Journal of Molecular Biology, 224, 715–723.
Oh, H.I., Hoff, J.E., Armstrong, G.S. & Haff, L.A., (1980). Hydrophobic Interactions in Tannin-Protein Complexes. Journal of Agricultural and Food Chemistry, 28, 394-398.
Okur, H.I., Hladilkova, J., Rembert, K.B., Cho, Y., Heyda, J., Dzubiella, J., Cremer, P.S. & Jungwirth, P., (2017). Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. The Journal of Physical Chemistry B, 121, 1997-2014.
Razinkov, V. I., Treuheit, M. & Becker, G., (2015). Accelerated Formulation Development of Monoclonal Antibodies (mAbs) and mAb-Based Modalities: Review of Methods and Tools. Journal of Biomolecular Screening,20(4), 468-483.
Reddy, R.C. et al., (2005). L-Arginine increases the solubility of unfolded species of hen egg white lysozyme. Protein Science, 14, 929–935.
Sarni-Manchado, P., Deleris, A., Avallone, S., Cheynier, V. & Moutounet, M., (1999). Interactions of grape seed tannins with salivary proteins. Journal of Agricultural and Food Chemistry, 47, 42-47.
Scharnagl, C., Reif, M. & Friedrich, J., (2005). Stability of proteins: temperature, pressure and the role of the solvent. Biochimica et Biophysica Acta, 1749(2), 187-213.
Schneider, C.P., Shukla, D. & Trout, B.L., (2011). Arginine and the hofmeister series: The role of ion-ion interactions in protein aggregation suppression. Journal of Physical Chemistry B, 115, 7447–7458.
Senske, M., Törk, L., Born, B., Havenith, M., Herrmann, C. & Ebbinghaus, S. (2014). Protein Stabilization by Macromolecular Crowding through Enthalpy Rather Than Entropy. Journal of the American Chemical Society, 136, 9036-9041.
Shortle, D., (1996). The denatured state (The other half of the folding equation) and its role in protein stability. Journal of the Federation of American Societies for Experimental Biology, 10, 27–34.
Shukla, D. & Trout, B.L., (2010). Interaction of arginine with proteins and the mechanism by which it inhibits aggregation. Journal of Physical Chemistry B, 114, 13426–13438.
Spink, C.H., (2008). Differential Scanning Calorimetry. Methods Cell Biology, 84, 115-141.
Sun, L., Gidley, M.J. & Warren, F.J. (2017). The mechanism of interactions between tea polyphenols and porcine pancreatic alpha-amylase: Analysis by inhibition kinetics, fluorescence quenching, differential scanning calorimetry and isothermal titration calorimetry. Molecular Nutrition & Food Research, 61(10), 1-13.
Timasheff, S.N. (1993). The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annual Review of Biophysics and Biomolecular Structure, 22, 67-97.
Timasheff, S.N., (2002). Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry, 41, 13473-13482.
Vagenende, V. et al., (2013). Protein-associated cation clusters in aqueous arginine solutions and their effects on protein stability and size. ACS Chemical Biology, 8, 416–422.
von Hippel, P.H. & Schleich, T., (1969). Ion effects on the solution structure of biological macromolecules. Accounts of Chemical Research, 2, 257–265.
Wang, W., (1999). Instability, stabilization, and formulation of liquid protein pharmaceuticals. International Journal of Pharmaceutics, 185, 129–188.
Wang, W., (2015). Advanced Protein Formulations. Protein Science, 24(7), 1031-1039.
Williamson, M.P., (2012). How proteins work. 1st ed. New York: Garland Science.
Yancey, P.H., (2005). Organic osmolytes as compatible, metabolic and counteracting cryprotectants in high osmolarity and other stresses.Journal of Experimental Biology, 208, 2819-2830.
Xie, Q. et al., (2004). The guanidine like effects of arginine on aminoacylase and salt-induced molten globule state. International Journal of Biochemistry & Cell Biology, 36, 296–306.
Zhang, Y., Furyk, S., Bergbreiter, D.E. & Cremer, P.S., (2005). Specific Ion Effects on the Water Solubility of Macromolecules: PNIPAM and the Hofmeister Series. American Chemical Society, 127, 14505- 14510.
Zhang, Y. & Cremer, P., (2006). Interactions between macromolecules and ions: the Hofmeister series. Current opinions in Chemical Biology, 10, 658-663.
Zhang, Y. & Cremer, P. S. (2009). The inverse and direct Hofmeister series for lysozyme. Proceedings of the National Academy of Sciences, 106, 15249-15253.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




