Development of Bone Regeneration Technology
Info: 8680 words (35 pages) Example Research Project
Published: 3rd Jun 2021
Tagged: Medical
1.2 Isolation of Extracellular Vesicles
1.2.1 Techniques of EVs isolation
1.3.1 Techniques of EV characterization
1.4.1 Candidate biomaterials to incorporate with biomolecules during scaffold fabrication
1.5 Therapeutic delivery system
2.1 Cell cultures and sample preparation
2.1.1 Osteogenic/Non-osteogenic differentiation assays
2.3.1 Dynamic light scattering
2.3.2 Nanoparticle tracking analysis (NTA)
2.3.3 Transmission electron microscopy (TEM)
3.1 Dynamic light microscopy (DLS)
3.2 Transmission electron microscopy (TEM)
3.3 Nanoparticle tracking analysis (NTA)
Aim and objectives
The aim of this project is to develop a bone-regenerating technology (scaffolds and injectable hydrogels), which is based on delivery of osteogenic extracellular vesicles (exosomes) in order to promote fracture healing and treat critically sized bone defects. Exosomes are a well-studied class of extracellular vesicles known to deliver proteins, DNAs and RNAs between cells, regulating differentiation and proliferation. Extracellular vesicles (EVs) appear to have powerful bone regeneration properties. These particular properties make them a potential effective treatment for patients who suffer from non-healing bone fractures and defects.
To achieve this, osteoblastic cells were cultured in osteogenic media, for approximately 2 weeks, to induce osteogenic differentiation. Following, the supernatants were collected and differential ultracentrifugation was used to isolate vesicles. Vesicle diameter and size distribution were analysed using dynamic light scattering (DLS), nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). In later stages of this project, different stimuli to enhance the osteogenic capacity of extracellular vesicles will be explored as well as biomaterial delivery systems, including hydrogels and scaffolds, for localisation application and regeneration.
Chapter 1
Literature Review
The clinical requirement for effective bone regeneration therapies is in huge demand. Several studies reported that 4.8 – 11% of bone fractures, which occur from trauma, age and chronic diseases (osteoporosis, rheumatoid arthritis), may heal insufficiently due to limited bone regeneration. This particular bone performance is usually associated with loss of bone function and pain, influencing an enormous number of individuals around the world, causing chronic disabilities and in some cases shorter life expectancy. Every year more than 3.5 million individuals worldwide undergo graft surgeries to replace or repair bone lost to disease, genetic abnormalities or trauma. This has an associated annual cost of more than 1.9 billion, and is expected to increase a compound annual rate of 7-8% (Dinopoulos et al., 2012).
Fracture healing has been promoted through the use of various treatments methods, including internal and external fixation, cementless joints replacements and autologous/allogeneic bone grafting (Qin et al., 2016; Goodman et al., 2013; Bennett et al., 2005). The aim of these treatments is to provide stability and initiate osteogenesis. However, all these techniques have limitations. Cementless joint replacements are not always able to osseointegrate with the surrounding tissue or bone, and thus there is a possibility of loosening and implant failure (Goodman et al., 2013). Autologous/allogenic bone grafting is a current treatment which promotes bone regeneration. Nevertheless, roughly 25–35% of patients who undergo autologous/allogenic bone grafts suffer from morbidity and complications such as, infection and fracture, increasing significantly the treatment cost and operative time. Furthermore, there is a limit to the amount of autologous/allogenic bone that can be harvested and therefore large defects may not be treatable. In summary, this method is not optimal because is expensive, has limited availability and high risks of complication (Qin et al., 2016; Matassi et al., 2011).
In response to these limitations, researchers focus on regenerative medicine and tissue engineering as alternative strategies to regenerate and repair damaged bone/tissues using biomaterials and autologous cells, alone or in combination. Ceramics and Polymers have been widely investigated as candidate biomaterials for the development of natural or synthetic scaffolds and implants with embedded therapeutics to promote bone regeneration (Deng et al., 2011)
1.1 Regenerative medicine
Regenerative medicine (RegMed) is a field of translational research in tissue engineering (TE) focusing on the regeneration, replacement and repair of damaged-missing tissues or organs. The above processes can be achieved, stimulating irreparable organs to heal themselves or even incorporate/combine cells with biodegradable and biocompatible materials to participate in tissue formation (Polykandriotis et al., 2010; Lamichhane et al., 2014; De Jong et al., 2014).
Regenerative medicine consists of two critical approaches. The first approach is based on cell therapies during which the cells are regulated to restore a tissue through paracrine functions. The second approach aims at the integration and incorporation of cells with bio-compatible scaffolds to form a tissue (De Jong et al., 2014).
Recently, the identification of extracellular vesicles as carriers of cellular information has attracted attention in regenerative medicine. EVs and collagen sulfate offer a biomimetic coating on the surface of an implant or scaffold, enabling osteointergration once implanted in the body. Biomimetic implants/scaffolds, which are made of extracellular vesicles, have the ability to decrease inflammation and stem cell differentiation leading to bone/tissue formation from local osteoblastic cells (Goodman et al., 2013).
1.1.1 Extracellular Vesicles
Extracellular vesicles (EV) were first described in 1981, as exfoliated vesicles with ectoenzymatic activities (Tram et al., 1981). EVs are nano-sized and heterogeneous membrane-bound phospholipids produced by osteoblasts. EVs are released upon fusion with the plasma cell membrane into the cellular environment.
Osteoblastic cells constitute the 4–6% of the total bone resident cells. The main role of these cells is to promote/enhance/stimulate bone formation (Colub, 2011; Qin et al., 2016). During bone formation, calcium and phosphate minerals are produced by osteoblasts to form mineralized bone. However, extracellular vesicles (EVs) can also stimulate bone formation by controlling osteoblastic cells activity and proliferation (Qin et al., 2016).
Extracellular vesicles consist of proteins, nucleic acids and lipids; they allow intercellular communication between cells and transport DNA, mRNA, microRNA and proteins, enabling cells proliferation and differentiation (Zaborowski et al., 2015; Raposo and Stoorvogel, 2013; Lamichhane et al., 2014; Bruno et al., 2016; Qin et al., 2016; Mendes et al., 2016).
Different types of extracellular vesicles that vary in size, content and function are produced by cells. The main three classes of extracellular vesicles (EVs) based on the biogenesis mode are: apoptotic bodies, microvesicles and exosomes (Zaborowski et al., 2015; Lamichhane et al., 2014; Momen-Heravi et al., 2013; Mendes et al.,2016).
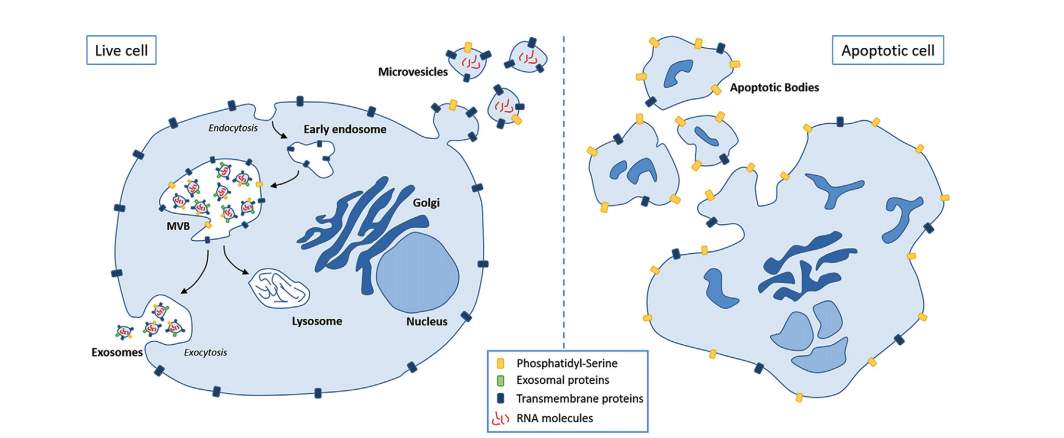
Figure 1. Schematic presentation of intracellular release of EVs (Bruno et al., 2016).
Apoptotic bodies are large size particles (1000-5000nm), which occurs during apoptosis and are released upon the fragmentation of plasma membrane (Bruno et al., 2016). In contrast, microvesicles are shed directly from the cell membrane. This type of vesicle is reported to be a lot larger than exosomes and smaller than apoptotic bodies with heterogeneous morphology. Their sizes range from 50 to 1000 nm (Zaborowski et al., 2015; De Jong et al., 2014; Bruno et al., 2016; Qin et al., 2016; Van Der Pol et al., 2010). Exosomes are released by the fusion between multivesicular bodies (MVB) and the cell membrane, having endosomal origin (Zaborowski et al., 2015; De Jong et al., 2014; Bruno et al., 2016). According to the literature, exosomes vary in size from 20 to 150nm with a density of 1.13-1.19 g/mL and their morphology is cup-shaped (Zaborowski et al., 2015; De Jong et al., 2014; Bruno et al., 2016; Qin et al., 2016; Raposo and Stoorvogel,2013; Kooijmans et al., 2012).
The natural capability of extracellular vesicles, both microvesicles and exosomes, to deliver biological cargo has attracted attention since they could be used as a delivery vehicle for osteoinductive proteins and nucleic acids that are highly effective for promoting bone regeneration. Particularly, exosomes may be most suitable for such delivery methods, because they are small (20–150 nm) and relatively homogenous in size. Their advantageous size (150 nm) makes them suitable for their use as therapeutic delivery system (Momen-Heravi et al., 2013). Nevertheless, the identification of these specific sub-populations is problematic due to the variability that exists in vesicles as well as to the continuing development vesicles biogenesis (Lamichhane et al., 2014).
1.2 Isolation of Extracellular Vesicles
EVs can be isolated from cells and biological fluids via several methods including, differential ultracentrifugation, immunoaffinity separation, chromatography, polymer-based precipitation and filtration (De Jong et al., 2014; Zaborowski et al., 2015; Yakimchuk, 2015; Bruno et al., 2016; Witwer at al., 2013; Raposo and Stoorvogel, 2013). Differential ultracentrifugation is the most widely used method for vesicles isolation and purification (Zaborowski et al., 2015). However, it should be noted that differential ultracentrifugation, like other existing methods, may not efficiently separate completely microvesicles from exosomes or other extracellular vesicles, such as apoptotic bodies and protein aggregates. The separation and identification of different types of vesicles is a difficult task because different sizes may mix together (Lamichhane et al., 2014; Zaborowski et al., 2015; De Jong et al., 2014). In addition, another factor which renders EVs extraction difficult is the variety of isolation protocols. Recently, several methods were utilised for extraction of extracellular vesicles, including microfluidic devices, antibody magnetic beads and filtration technologies. However, there is an urgent need for more efficient, reliable and reproducible EV extraction methods so that all downstream studies can be more standardized and efficient (Momen-Heravi et al., 2013).
1.2.1 Techniques of EVs isolation
1.2.1.1 Differential centrifugation and Ultracentrifugation
The differential centrifugation method consists of sequential centrifugation steps at different centrifugal forces (g/rpm), and aims to remove unwanted dendrites, cells and large vesicles (Yakimchuk, 2015; Szatanek et al., 2013).
Several protocols involve a few sequential centrifugation speed spins. The initial speed spin was designed to remove intact or dead cells and apoptotic debris with centrifugation forces ranging from 300 to 2,000 g for 10 to 30 minutes. Followed by a second spin to separate small and large extracellular vesicle populations with centrifugation forces from 10,000 to 20,000 g. Finally, the supernatant from the second spin is subjected to a final centrifugation to pellet exosomes. The speeds used for this final stage range from 100,000 to 200,000 g for 70 minutes to 2 hours. An additional vesicles purification can be achieved by multiple washings with PBS and centrifugations at 100,000 g for 70 minutes (Zaborowski et al., 2015; Yakimchuk, 2015; Szatanek et al., 2013; Momen-Heravi et al., 2013). The EVs isolation regardless of their cell of origin and subtypes, constitutes an important disadvantage of this method (Zaborowski et al., 2015).
1.2.1.2 Size exclusion chromatography
Size exclusion chromatography (SEC) is used to separate large (cells, debris) and small vesicles (exosomes) on the base of their size with low centrifugation speeds following by a filtration process to pre-concentrate the extracellular vesicles. This particular method appears to have advantages compared to centrifugation methods. Since the vesicles which are isolated by chromatography are not affected by shearing force. The shearing force can potentially change the vesicles structures (Yakimchuk, 2015; Szatanek et al., 2013). However, this method is time consuming and requires multiple biological samples (Yakimchuk, 2015).
1.2.1.3 Filtration
Ultrafiltration membranes (0.8-0.45μm) can be used to collect microvesicles larger than 400 nm to 800nm. Filtration allows microvesicles separation/isolation of soluble molecules and big particles (Yakimchuk, 2015; Witwer at al., 2013). However, this method has a significantly disadvantage; the additional forces which are applied to pass the liquid of particles through the cillicated membrane, can potentially cause damage on the vesicles surface (Yakimchuk, 2015).
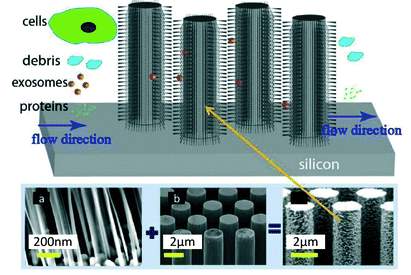
Figure 2. Filtration of EVs using ciliated porous structures (Yakimchuk, 2015).
1.2.1.4 Polymer-Based Prescription
Polymer-based prescription is used to isolate vesicles through the mixing process of a polymer containing solution with the biological fluid, followed by a low speed centrifugation. The polymeric material which is frequently used in this technique is polyethylene glycol (PEG). The polymer prescription is a quick process, enabling mild effects on vesicles. Nevertheless, the presence of the polymeric material may not be compatible with down/stream analysis (Yakimchuk, 2015; Szatanek et al., 2013).
1.2.1.5 Immunoaffinity separation
This method is based on the vesicles surface receptors, which are used to separate/isolate extracellular vesicles depending on their origin. Briefly, magnetic microbeads coated with an antibody can recognize certain intercellular markers, including tetraspanins (DC81, CD9, CD63, Alix) and cytosolic proteins (HSPs, Tsg101, ESCRT-3), which are presented on the vesicle surface. This process enables the selection of a specific vesicle population based on the marker expression regardless of its size. However, methods based on immunoaffinity may cause loss of the functional activity of the isolated vesicles (Szatanek et al., 2013; Bruno et al., 2016; Yakimchuk, 2015; Raposo and Stoorvogel, 2013).
1.3 EVs characterization
Various optical and non-optical methods have been developed to evaluate and characterise vesicles based on phenotypical features including size, concentration, morphology, charge and mobility (Kooijmans et al., 2012; Momen-Heravi et al., 2012).
1.3.1 Techniques of EV characterization
1.3.1.1 Optical methods – size, concentration and mobility
Optical methods have the potential to accurately obtain all clinically relevant properties of single microvesicles at a high speed.
1.3.1.1.1 Dynamic light scattering
Dynamic light scattering (DLS) is the most common optical method to determine the relative size distribution of nanometer-scale particles in solution. Particles sizes can be determined by measuring the random changes and temporal fluctuations in the intensity of light scattered. However, the results can be significantly affected by the presence of strongly particles, which may obscure the small particles (Van De Pol., 2010; Rupert at al., 2016).
Optical methods have the potential to accurately obtain all clinically relevant properties of single microvesicles at a high spee
1.3.1.1.2 Nanoparticle tracking analysis
Nanoparticle tracking analysis (NTA) is an alternative technique to DLS for obtaining the absolute size distribution and concentration of individual scattering particles with size ranging from 40 to 1000nm. Scattering particles are directly visualised and illuminated by a laser light scattering beam. The hydrodynamic radius of a particle is detected by the principle of Brownian motion. A highly sensitive camera records the particle sizes and locomotion. (Van De Pol., 2010; Momen-Heravi et al., 2012; Rupert at al., 2016; Witwer et al., 2013; Rupert at al., 2016).
1.3.1.1.3 Flow-cytometry
Flow cytometry is a powerful technology for scanning and counting single cells in fluids at a rate of thousands of particles or cells per second. This technique is widely used to record the scattering and the fluorescence signal which is generated by single particles. However, flow cytometers is difficult to detect vesicles with a few hundred nanometers sizes due to lower detection limit (Rupert at al., 2016; Van De Pol., 2010; Momen-Heravi et al., 2012; Witwer et al.,2013).
1.3.1.1.4 Western blot
Western blotting is used to demonstrate the presence of identification markers, including tetraspanins (CD81, CD9, CD82, CD63), stress proteins (Alix, Tsg101) and MHC molecules, which exist in vesicle populations (Witwer et al., 2013).
1.3.1.2 Non-optical methods – Structure and morphology
1.3.1.2.1 Electron microscopy
Transmission electron microscope (TEM) is the most common surface based imaging technique, which allows the detection of particles illustrating their structures and morphologies. TEM transmits a beam of electrons through an ultra thin specimen to create an image. TEM is performed in vacuum mode which implies that biological samples (extracellular vesicles) need to be fixated and dehydrated prior to imaging. Staining is also often applied to increase the imaging contrast. However, this technique may significantly damage fragile vesicles, their sizes and morphologies if no extra care is taken (Rupert at al., 2016; Momen-Heravi et al., 2012; Witwer et al., 2013; Rupert at al., 2016). On the other hand, scanning electron microscopy (SEM), scans a fine beam of electrons onto a thin specimen/over the surface and collects the electrons scattered on the surface, yielding a topographical image of the surface. (Momen-Heravi et al., 2012; Rupert at al., 2016).
1.3.1.2.2 Cryo- Electron Microscopy
Cryo-electron microscopy (cryofixation) enables particles analysis at low temperatures (below -100°C) avoiding the effects of chemical fixation and dehydration. Imaging is performed under vacuum conditions maintain the particles in frozen state (Momen-Heravi et al., 2012; Rupert et al., 2016; Witwer et al., 2013).
1.3.1.2.3 Atomic force microscopy (AFM)
AFM is a surface based imaging method, which provides a nanometers/sub-nanometers lateral and vertical resolution of topography. AFM employs an incident laser beam which is focused on the back of a cantilever with a very sharp tip that moves up and down (tapping mode) on the surface of a specimen and the deflections of the beam are captured by a diode. Atomic force microscopy can be used to detect size distribution of vesicles producing high resolution images. However, this technique is not suitable for vesicles characterization due to the sharp tip which may significantly damage the fragile vesicles (Van De Pol., 2010; Rupert at al., 2016; Witwer et al., 2013).
1.4 Biomaterials
Repair of bone fractures and tissue requires the presence of cells capable of restoring the damaged structure, and at the same time a micro-environment capable of promoting appropriate regeneration. Artificial porous scaffolds or implants provides the appropriate micro-environment for cell retention and consequently eventual tissue regeneration. Scaffolds and implants can either be of natural origin, such as natural hydrogels and collagen gels, or of synthetic origin including synthetic hydrogels and porous polymeric scaffolds (De Jong et al., 2014).
During the last few years, scientists have focused on the construction of biotics and bio-inspired materials, which can be easily incorporate with the body functions, integrate with extracellular matrix components and regenerate bone/tissues. Bio-inspired material also referred to as biomimetic, is any synthetic of natural elements or combination of elements whose structure and functions mimic those of natural materials. Bio-inspired materials are used to treat or to replace any organ, tissue or body function. The main purpose of these materials is to collaborate with a biological system. Since the environment of the human body is highly chemically and mechanically demanding, the requirements of the candidate materials’ properties are quite strict (high biocompatibility, high biodegradability, non-toxic) (Meyers et al., 2008; Dujardin and Mann, 2002; Molly M. Stevens, 2008; Cooper, 2015; Navarro et al, 2008; Reyes et al.,2007; Williams 2008).
1.4.1 Candidate biomaterials to incorporate with biomolecules during scaffold fabrication
1.4.1.1 Natural Hydrogels
Natural hydrogels including Collagen (Type-I), Chitosan, Alginate and Gellan, are widely used for the construction of natural biocompatible implants and scaffolds for wound healing, bone regeneration and drug delivery systems.
1.4.1.1.1 Hydrogels
Hydrogels (Hydrophilic gels) are crosslinked, hydrophilic, three dimensional polymeric networks. Their high water content makes them resemble the living tissue. Hydrogels (Hydrophilic gels) can be produced by different techniques including polymerisation, cross-linking of multifunctional monomers and multiple step processes. Hydrogel properties can be engineered for chemical and mechanical stability, biocompatibility and several other requirements, such as uniform cell distribution and immobilization. Hydrogels can be natural or synthetic origin. However, the last two decades, scientists have focused on natural origin hydrogels due to their biodegradable nature, biocompatibility and hydrophilic properties (Goodman et al., 2013; Tozzi et al., 2016; Jen et al., 1996; Ahmed, 2015).
1.4.1.1.2 Collagen (Type-I)
Human bone consists of two main components; hydroxyapatite (HA) and collagen. Hence, composites are produced using collagens and calcium phosphates, which are considered for regenerative applications. Collagen and hydroxyapatite coatings of implants have been shown to enhance and facilitate cell adhesion and proliferation, increasing osteointegration and osteoconduction (Sheikh et al., 2015).
1.4.1.1.3 Chitosan
Chitosan is a natural polymer derived from chitin. It is a polysaccharide, composed of N-acetyl and glucosamine. Chitosan has a hydrophilic surface and cationic behavior promoting cell attachment/adhesion and consequently cell differentiation (Sheikh et al., 2015).
1.4.1.1.4 Alginate
Alginate is a natural anionic polymer produced by brown seaweeds. Alginate usually used for scaffold construction due to low toxicity, high biocompatibility, high degradability and low cost of production (Lee and Mooney, 2012; Muzzarelli, 2011).
1.4.1.2 Synthetic bio-ceramics, bio-polymers
On the other hand, synthetic materials, including bio-ceramics and bio-polymers, are currently used for bone fracture repair, stabilisation, detect filling and replacement of diseased bone tissues. Bio-ceramic materials (Hydroxyapatite) are biocompatible and bioactive, having high corrosion resistance, demonstrating tremendous bioactivity.
1.5 Therapeutic delivery system
The microenvironment which is “created” from appropriate biomaterials is suitable for the cells maintenance, retention and immobilization to be used further as a therapeutic delivery vehicle.
Figure 3. Micrograph of cell-material microenvironment
The critical role of extracellular vesicles (EVs) to mediate therapeutic effects reveals potential applications for the vesicles as natural delivery vehicles of proteins, mRNA and DNA (biologicals) to promote bone and tissue regeneration (Kooijmans et al., 2012; Batrakova and Kim, 2015; Lamichhane et al., 2014).
The figure 4 below presents the consideration of extracellular vesicles in the design of therapeutic approaches including firstly cell culture conditions, secondly the interactions between cells and biomaterials, finally possible methods of therapeutic intervention (implanted or injectable scaffolds) (Kooijmans et al., 2012; Batrakova and Kim, 2015; Lamichhane et al., 2014).

Figure 4. Extracellular vesicles-therapeutic design parameters (Lamichhane et al., 2014).
Chapter 2
Materials and Methods
This section of the report covers details of all the materials and methods used including details of the specific techniques and equipment. As mentioned earlier, aim of this study is to examine the extracellular vesicles in the context of regenerative field as an alternative therapeutic approach. One of the critical points in this project is the isolation and characterisation of extracellular vesicles.
2.1 Cell cultures and sample preparation
Murine osteoblastic cells were obtained from MC3T3 cell line. MC3T3 cells from passage 17 were cultured in growth medium (α-minimal essential medium) supplemented with 10% foetal bovine serum (FBS), 2.4% L-glutamine and 1% penicillin. The media was changed three times per week to feed the cells with fresh media (α-MEM). Furthermore, the cells were sub-cultured every three to four days according to the standard techniques.
2.1.1 Osteogenic/Non-osteogenic differentiation assays
To induce osteogenic differentiation, MC3T3 cells were seeded at a concentration of 5,715 cells/cm2 in α- minimal essential growth media. During this process, the cells express markers (Collagen type I) known to be expressed by mineralizing osteoblasts. These cells are responsible for laying down the matrix and mineral during new bone formation (Birmingham et al., 2012). After five days, cultures maintained in osteogenic media which consists of 50 ml α- ΜΕΜ with 0.216 g sodium β-glycerophosphate and 500 μL L- ascorbic acid for a period of 14 days. The osteogenic growth supplement was changed every three days. The osteogenic supernatant placed into 50 ml tubes and stored into the fringe for 30 days.
To induce the non-osteogenic behaviour of MC3T3s, were cultured in non-osteogenic medium. MC3T3s were seeded at 5,715 cells/cm2 in α- minimal essential growth media. After five days, cultures maintained in non-osteogenic media which consists of 2.4% L-glutamine, 1% penicillin and 10% FBS.
2.3 Characterisation
Osteogenic and non-osteogenic pellets of extracellular vesicles were quantitated and visualised by DLS, NTA and TEM.
2.3.1 Dynamic light scattering
The Malvern DLS instrument used to analyze the particles diameters (z-average) and the size distributions. 20 μL of EV pellet was diluted in 700 μL of PBS into a cuvette. Then, the samples placed into a sonic bath in order to avoid vesicles aggregations. Light scattering analysis was conducted at 25°C using the Malvern software.
2.3.2 Nanoparticle tracking analysis (NTA)
Nanoparticle tracking analysis is the most common method for particle size distributions and concentrations. A NanoSight LM 10 microscope equipped with a 1000 nm laser used to determine size distributions and vesicle concentrations. Samples were diluted (1:100) in PBS and were injected to the laser beam manually. EV acquisition and videos of 30s were captured at 22°C and analysed using NTA 3.2 software.
2.3.3 Transmission electron microscopy (TEM)
Supernatants of extracellular vesicles were visualised using transmission electron microscopy. Briefly, 20μL of EV suspension was placed onto a carbon coated film grid (Agar Scientific, UK) and allowed to dry for 1 minute at room temperature. The excess suspension was removed by touching the grid to a lens soft tissue. Subsequently, negative staining process was conducted to contrast the sample. The carbon coated film grid was transferred to a 20μL drop of 2 % (w/v) uranyl acetate solution (UA) for another 1 minute. Subsequently, the excess suspension was gently removed using soft tissue. The carbon coated grids were observed with JEM-3200FS transmission electron microscope.
Chapter 3
Results and discussion
This section contains the analysis of data (osteogenic/non-osteogenic extracellular vesicles) which have been collected by using DLS, TEM and NTA microscopes. Firstly, I will discuss the results from the DLS microscope, following the results from TEM microscope and finally the results from NTA microscope.
3.1 Dynamic light microscopy (DLS)
The following graphs present the sizes of the osteogenic vesicles (nm) in relation to their intensity values (%). This sizes have accrued after the implementation of 5 different speeds, specifically of speed 1 at 10,000 rpm, speed 2 at 20,000 rpm, speed 3 at 50,000 rpm, speed 4 at 75,000 rpm and speed 5 at 120,000 rpm.
Graph 1 (Speed 1) displays that 30% of the sample has a relatively large size, that is 220 nm; also it shows that the 28% of the sample is 190 nm. Thus, the 58% of the sample (large peak) has a mean size value of 207.5 nm. The second peak in the graph (21.3% of the sample) has a mean size value of 11.2 nm.
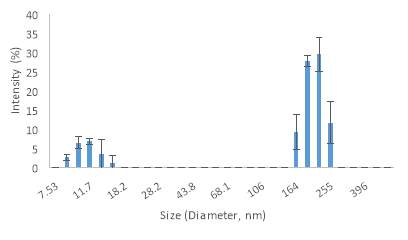
Graph 1. Vesicle sizes by DLS during the first speed at 10,000 rpm.
Graph 2 (Speed 2) shows that the percentage of the sample ranging between 190 nm to 220 nm has been reduced from 58% to 30%. Also, the percentage of the sample ranging between 142 nm to 164 nm has been increased significantly to 26.4% from 9.3% in speed 1. The graph below (graph 2) consists of one peak with mean size value of 242.3 nm.
Graph 2. Vesicle sizes by DLS during the second speed at 20,000 rpm.
Graph 3 (Speed 3) illustrates that 31.9% of the sample is 220 nm. Also, it shows that the 23% of the sample is 190 nm. Thus, the 54.9% of the sample ranges between 190 nm to 220 nm. The graph below (graph 3) consists of one peak with mean size value of 244.3 nm. This mean value is similar to speed 2. At this point it has to be noted that there is no much difference among the first three speeds in terms of vesicles sizes. However, at this point the standard deviations (error bars) are not close to the mean average (High StDev); this means that the vesicle sizes are spread out, in contrast to the first two speeds where the standard deviations are close to the mean average (Low StDev) which means that the most of the vesicle sizes are very close to the average.
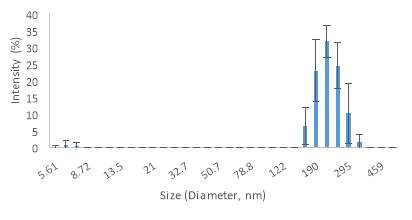
Graph 3. Vesicle sizes by DLS during the third speed at 50,000 rpm.
The graph below, Graph 4 (Speed 4), shows that 55% of the sample ranges between 122 nm to 164 nm. The mean value of the sizes is 147.9 nm. It is clear that when the speed increases by 75,000 rpm the percentage of large size vesicles is getting smaller and more and more small size emerge.
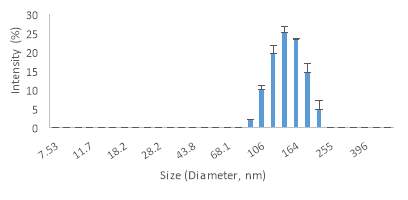
Graph 4. Vesicle sizes by DLS during the fourth speed at 75,000 rpm.
The same applies during the implementation of speed 5 (graph 5) where the speed has been increased by 70,000rpm and 45,000rpm compared to speed 3 and speed 4 respectively. Specifically, Graph 5 shows that 52.6% of the sample range between 78.8 nm to 142 nm. Also, it has to be noted that during speed 5, the 41.4% of the sample has very small size ranges between 4.85 nm to 68.1 nm. The mean value in the graph below (Graph 5) is 97.6 nm. Thus, during the implementation of speed 5 the vesicle sizes have been decreased significantly.
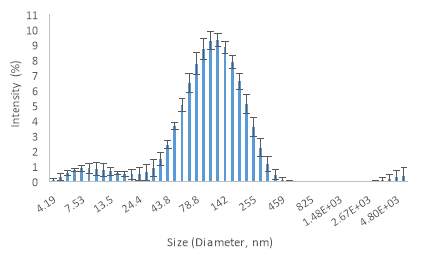
Graph 5. Vesicle sizes by DLS during the fifth speed at 120,000 rpm.
3.2 Transmission electron microscopy (TEM)
TEM was used to evaluate the morphology of EVs after the implementation of first speed at 10,000 rpm. According to recent bibliography, EVs present a circular – spherical shape (Zabeo et al., 2016). Figure 5A, illustrates that micro-vesicles which were isolated from osteogenic media, appear to have a spherical morphology with a diameter of 20 nm to 200 nm.
Afterwards, the non-osteogenic micro-vehicles were examined and the surface topography is presented in Figure 5B. It is clear that, there are not significant differences between the two samples (Osteogenic, Non-Osteogenic).



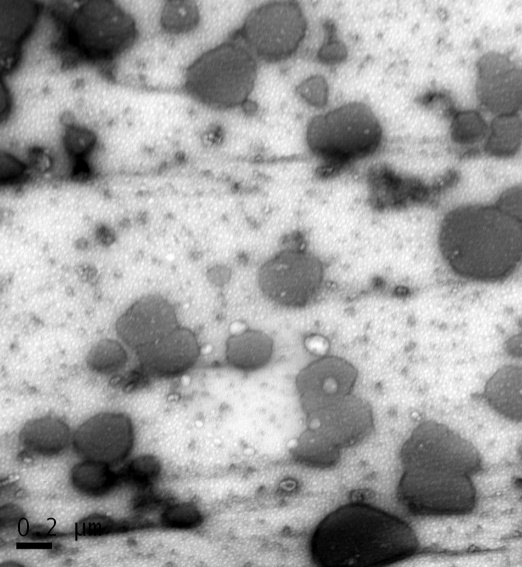

B
A
E V
200nm
200nm
Figure5. Transmission electron micrographs of osteogenic [A] and non-osteogenic [B] EVs.
3.3 Nanoparticle tracking analysis (NTA)
During the implementation of first speed (10,000 rpm), NTA was used to determine the absolute size distribution of osteogenic and non-osteogenic micro-vesicles. NTA provides a high precision for vesicles larger than 100 nm. However, NTA is not enough sensitive to detect vesicles smaller than 50 nm.
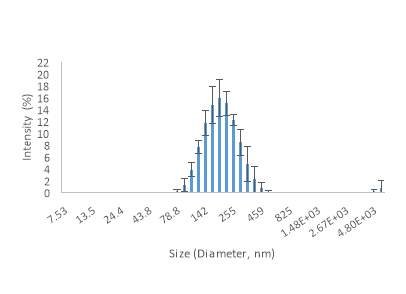
Graph 6, shows the absolute size distribution of osteogenic vesicles in relation to their concentration. The osteogenic vesicles sizes range between 100 nm to 506 nm. The mean size value of this sample is 195.6 nm.
Mean: 195.6 nm

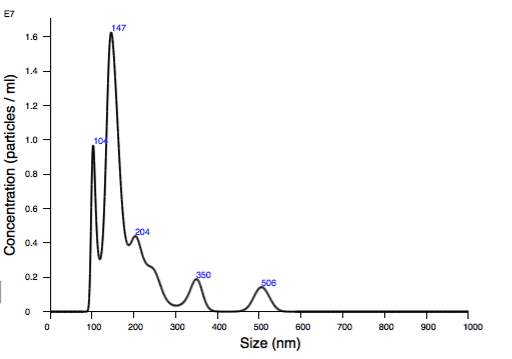
Graph 6. Size distribution from NTA of osteogenic micro-vesicles
The graph below (Graph 7) presents the absolute size distribution of non-osteogenic vesicles in relation to their concentration. The non-osteogenic vesicles sizes range between 99 nm to 293 nm. The mean size value for this particular sample is 145.7 nm. At this point, it has to be noted that the non-osteogenic vesicles are a bit smaller (145.7 nm) in size than the osteogenic vesicles (195.6 nm).
Mean: 145.7 nm

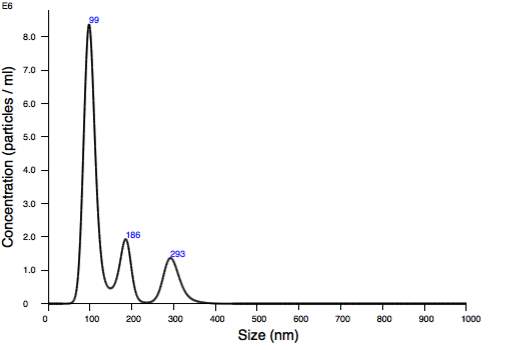
Graph 7. Size distribution from NTA of non-osteogenic micro-vesicles
Future Plan
The aim of the project is to examine the extracellular vesicles in the context of regenerative field, as an alternative therapeutic approach and more specifically as an alternative method for tissue and bone regeneration.
This project consists of 3 particular stages. The first stage involves the isolation and characterisation of extracellular vesicles; which will enable the evaluation of their sizes and morphological characteristics. The second stage concerns the examination of interactions between cells and candidate biomaterials. Finally, in stage I will examine possible methods of therapeutic intervention including implants or injectable scaffolds.
In this report I have analysed the vesicle sizes and morphologies, which were obtained from DLS, NTA and TEM microscopes. The results gathered so far are not very clear due to limited use of DLS, TEM and NTA microscopes. In order to gain reliable and publishable data regarding the vesicles sizes and morphologies, the above methods should be further explored and validated by comparing results from different microscopes including AFM, Western Blotting, Flow-cytometry and cryo-microscope.
Furthermore, the genetic and immunological profile of the vesicles need to be interpreted; this can be achieved with the use of Elisa Assay, Alizarin red and PCR. Following, osteogenic and non-osteogenic extracellular vesicles will be fed to MC3T3 cells in order to evaluate their effects.
Finally, several natural hydrogels including Alginate and Chitosan, will be investigated in order to provide a microenvironment on vesicles allowing cell retention and eventually proliferation.
References
- Ahmed, E.M., 2015. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research, 6(2), pp.105-121.
- Batrakova, E.V. and Kim, M.S., 2015. Using exosomes, naturally-equipped nanocarriers, for drug delivery. Journal of Controlled Release, 219, pp.396-405.
- Bennett, M.H., Stanford, R.E. and Turner, R., 2005. Hyperbaric oxygen therapy for promoting fracture healing and treating fracture non‐union. The Cochrane Library.
- Birmingham, E., Niebur, G.L. and McHugh, P.E., 2012. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche.
- Bruno, S., Porta, S. and Bussolati, B., 2016. Extracellular vesicles in renal tissue damage and regeneration. European Journal of Pharmacology, 790, pp.83-91.
- Cooper, I.R. (2015), Introduction to biomaterials and medical device- associated infections In: Barnes, L. and Cooper, I.R. Biomaterials and medical device- associated infections. pp. 3-17.
- De Jong, O.G., Van Balkom, B.W., Schiffelers, R.M., Bouten, C.V. and Verhaar, M.C., 2014. Extracellular vesicles: potential roles in regenerative medicine. Frontiers in immunology, 5, p.608.
- Deng, M., G Kumbar, S., W-H Lo, K., D Ulery, B. and T Laurencin, C., 2011. Novel polymer-ceramics for bone repair and regeneration. Recent Patents on Biomedical Engineering, 4(3), pp.168-184.
- Dujardin, E. and Mann, S., 2002. Bio-inspired materials chemistry. Advanced Materials, 14(11), p.775.
- Fleury, A., Martinez, M.C. and Le Lay, S., 2014. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Frontiers in immunology, 5, p.370.
- Golub, E.E., 2011, September. Biomineralization and matrix vesicles in biology and pathology. In Seminars in immunopathology (Vol. 33, No. 5, pp. 409-417). Springer-Verlag.
- Goodman, S.B., Yao, Z., Keeney, M. and Yang, F., 2013. The future of biologic coatings for orthopaedic implants. Biomaterials, 34(13), pp.3174-3183.
- Jagur‐Grodzinski, J., 2006. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polymers for advanced technologies, 17(6), pp.395-418.
- Jen, A.C., Wake, M.C. and Mikos, A.G., 1996. Review: Hydrogels for cell immobilization. Biotechnology and bioengineering, 50(4), pp.357-364.
- Johnstone, R.M., Mathew, A., Mason, A.B. and Teng, K., 1991. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. Journal of cellular physiology, 147(1), pp.27-36.
- Kooijmans, S.A., Vader, P., van Dommelen, S.M., van Solinge, W.W. and Schiffelers, R.M., 2012. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine, 7(1525), p.e41.
- Lamichhane, T.N., Sokic, S., Schardt, J.S., Raiker, R.S., Lin, J.W. and Jay, S.M., 2014. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Engineering Part B: Reviews, 21(1), pp.45-54.
- Lee, K.Y. and Mooney, D.J., 2012. Alginate: properties and biomedical applications. Progress in polymer science, 37(1), pp.106-126.
- Matassi, F., Nistri, L., Paez, D.C. and Innocenti, M., 2011. New biomaterials for bone regeneration. Clinical cases in mineral and bone metabolism, 8(1), pp.21-24.
- Mendes, P.M., Fernandez-Trillo, F., Grover, L., Stephenson-Brown, A. and Harrison, P., 2016. Vesicles and their multiple facets: underpinning biological and synthetic progress. Angewandte Chemie International Edition.
- Meyers, M.A., Chen, P.Y., Lin, A.Y.M. and Seki, Y., 2008. Biological materials: structure and mechanical properties. Progress in Materials Science, 53(1), pp.1-206.
- Momen-Heravi, F., Balaj, L., Alian, S., Mantel, P.Y., Halleck, A.E., Trachtenberg, A.J., Soria, C.E., Oquin, S., Bonebreak, C.M., Saracoglu, E. and Skog, J., 2013. Current methods for the isolation of extracellular vesicles. Biological chemistry, 394(10), pp.1253-1262.
- Momen-Heravi, F., Balaj, L., Alian, S., Tigges, J., Toxavidis, V., Ericsson, M., Distel, R.J., Ivanov, A.R., Skog, J. and Kuo, W.P., 2012. Alternative methods for characterization of extracellular vesicles. Frontiers in physiology, 3, p.354.
- Muzzarelli, R.A., 2011. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydrate Polymers, 83(4), pp.1433-1445.
- Navarro, M., Michiardi, A., Castano, O. and Planell, J.A., 2008. Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), pp.1137-1158.
- Polykandriotis, E., Popescu, L.M. and Horch, R.E., 2010. Regenerative medicine: then and now–an update of recent history into future possibilities. Journal of cellular and molecular medicine, 14(10), pp.2350-2358.
- Qin, Y., Sun, R., Wu, C., Wang, L. and Zhang, C., 2016. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. International journal of molecular sciences, 17(5), p.712.
- Raposo, G. and Stoorvogel, W., 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol, 200(4), pp.373-383.
- Raposo, G., Nijman, H.W., Stoorvogel, W., Liejendekker, R., Harding, C.V., Melief, C.J. and Geuze, H.J., 1996. B lymphocytes secrete antigen-presenting vesicles. Journal of Experimental Medicine, 183(3), pp.1161-1172.
- Reyes, C.D., Petrie, T.A., Burns, K.L., Schwartz, Z. and García, A.J., 2007. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials, 28(21), pp.3228-3235.
- Ribeiro, M., Monteiro, F.J. and Ferraz, M.P., 2012. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter, 2(4), pp.176-194.
- Robert E. Armstrong, 2010. Bio-inspired Innovation and National Security.
- Rupert, D.L., Claudio, V., Lässer, C. and Bally, M., 2017. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochimica et Biophysica Acta (BBA)-General Subjects, 1861(1), pp.3164-3179.
- Saini, M., Singh, Y., Arora, P., Arora, V. and Jain, K., 2015. Implant biomaterials: A comprehensive review. World Journal of Clinical Cases: WJCC, 3(1), p.52.
- Sheikh, Z., Najeeb, S., Khurshid, Z., Verma, V., Rashid, H. and Glogauer, M., 2015. Biodegradable materials for bone repair and tissue engineering applications. Materials, 8(9), pp.5744-5794.
- Stem cells: http://dels.nas.edu/resources/static-assets/materials-based-on-reports/booklets/Understanding_Stem_Cells.pdf
- Stevens, M.M., 2008. Biomaterials for bone tissue engineering. Materials today, 11(5), pp.18-25.
- Szatanek, R., Baran, J., Siedlar, M. and Baj-Krzyworzeka, M., 2015. Isolation of extracellular vesicles: Determining the correct approach (Review). International journal of molecular medicine, 36(1), pp.11-17.
- Tozzi, G., De Mori, A., Oliveira, A. and Roldo, M., 2016. Composite hydrogels for bone regeneration. Materials, 9(4), p.267.
- Trams, E.G., Lauter, C.J., Salem, J.N. and Heine, U., 1981. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et Biophysica Acta (BBA)-Biomembranes, 645(1), pp.63-70.
- Van Der Pol, E., Hoekstra, A.G., Sturk, A., Otto, C.V., Van Leeuwen, T.G. and Nieuwland, R., 2010. Optical and non‐optical methods for detection and characterization of microparticles and exosomes. Journal of Thrombosis and Haemostasis, 8(12), pp.2596-2607.
- van Dommelen, S.M., Vader, P., Lakhal, S., Kooijmans, S.A.A., van Solinge, W.W., Wood, M.J. and Schiffelers, R.M., 2012. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. Journal of Controlled Release, 161(2), pp.635-644.
- Williams, D.F., (2008) On the mechanisms of biocompatibility. Biomaterials,29(20), pp.2941-2953.
- Witwer, K.W., Buzas, E.I., Bemis, L.T., Bora, A., Lässer, C., Lötvall, J., Nolte, E.N., Piper, M.G., Sivaraman, S., Skog, J. and Théry, C., 2013. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of extracellular vesicles, 2.
- Yakimchuck Konstantin, 2015. Exosomes: isolation ans characterization methods as specific markers in https://www.labome.com/method/Exosomes-isolation-and-characterization-methods-and-specific-markers.html
- Zabeo, D., Cvjetkovic, A., Lasser, C., Schorb, M., Lotvall, J. and Hoog, J.L., 2016. Exosomes purified from a single cell type have diverse morphology and composition. bioRxiv, p.094045.
- Zaborowski, M.P., Balaj, L., Breakefield, X.O. and Lai, C.P., 2015. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience, 65(8), pp.783-797.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical"
The word Medical refers to preventing or treating injuries or illnesses, relating to the study or practice of medicine. Medical care involves caring for a patient and helping them through their journey to recovery.
Related Articles
DMCA / Removal Request
If you are the original writer of this research project and no longer wish to have your work published on the UKDiss.com website then please:




