Understanding the P2X7 Receptors' Response to Pain
Info: 6102 words (24 pages) Example Research Project
Published: 2nd Nov 2021
The Neural P2X7 Receptor Is Insignificant in Brain inflammation In Mice Models, Regarding IL-1β And ATP Levels, When Mice Models Are Electrically Stimulated.
LAY SUMMARY
Protein P2X7 is found in the nervous systems. When the protein binds to the receptor (P2X7), it activates it and encourages ATP- a pivotal energy source molecule, to attach in large quantities. Recently emerging studies highlight how this receptor is a fascinating tool in responding to chronic pain caused by neuroinflammatory conditions - however, our research aims to be more specific in addressing this further. Our intentions are to see if we can adapt our understanding of the P2X7 receptors' response to pain for future models that will hopefully enable successful human therapy and treatment. The response-process originates from a series of specialised cells named satellite glial cells, that are found covering the surface lining of nervous bodies. These cells are what aid the P2X7 receptor to mediate neuropathic and inflammatory pain in humans.
P2X7 receptors also creates high concentrations of proteins named cytokines, which function by regulating the cells for signalling purposes. Il-1β is a cytokine that the P2X7 receptors generate, and it is important to study how they provide relief to chronic pain. When expressed by the P2X7 receptors during the response-process, IL-1β matures through cleavage from a primitive form of the molecule to affect cell function in a positive, helpful manner so that chronic pain is alleviated from a patient. Under ethical treatment, we aim to artificially trigger IL-1β secretion in mice model samples to establish enhanced understanding of neuropathic pain and its eventual dissolution in patients going forward.
TECHNICAL SUMMARY
Protein P2X7 is commonly found in the central and peripheral nervous systems, often paired with a complimentary receptor (Sluyter, 2017). Our research aim is to investigate the effect of activation of the P2X7 receptor on satellite glial cells in mice and determine their impact in controlling pain from inflammation in the brain, which is related to significant health related issues. (Sacks et al., 2015). The objective is to monitor levels of ATP and IL-1β as well as where it is concentrated in the dorsal root ganglia using mice as a formative model sample. We are researching this as a model of how it would affect humans as neurological disorders are a major concern within the world's population and are becoming more prevalent in society, however there is no cure as yet .
We will use two randomised groups of five mice, each mouse being male and 10- weeks old. One group will contain transgenic mice (the blind-trial group) and the other will contain non-transgenic mice which is known as the control. From this we will measure the different areas of the mice's brain which are being stimulated through electrical stimulation of the mice using electrodes. We will measure the levels of ATP and IL-1β released from cells. They will be measured twice a day for a duration of six months. The first group will be a control to validate any observed improvement in inflammation in the brain is due to elevated P2X7 receptor levels. The second group will be known as a blind- trial group. In this group, the severity of electrical stimulation they receive will be varied, which will in turn affect the amount of ATP and IL-1β produced by cells. We will measure this using various analysis methods; an MRI to show where the ATP is most concentrated as well as the use of an ELISA test and HPLC chromatography. We expect that the more the mice are stimulated, higher levels of P2X7 receptors are active in the mice and more ATP as well as cytokine (IL-1β) will be present in cells.
BACKGROUND, IMPORTANCE AND OBJECTIVES
Currently, there is not a sound understanding on P2X7 receptors and their function, we hope to broaden our understanding once we have analysed our results at the end of this experiment.
Reading into currently published work has provided us with sufficient knowledge to initiate our research project into P2X7 receptors.
Purinergic receptors, known as purinoceptors, are simply receptors that are mediated by purines such as ATP. Purinoceptors can be divided into five subclasses; P2X, P2Y, P2Z, P2U and P2T, which each have various functions (Pangalos and Davies, 2002) and are found in virtually all mammalian cells (Sluyter, 2017). There are seven subtypes of P2X receptors (P2X1-7).
The focus of our research will be on the P2X7 receptor which is an ionotropic ligand gated ion channel which interacts with cations such as Na+, Ca2+and K+ (Burnstock, 2006). Figure 1 shows the structure of the P2X7 receptor and how ATP will activate a change in its structure to allow cations to pass through the cell membrane. It is important to note that after synaptic signalling, purinergic signalling is the second most abundant signalling mechanism in both healthy and diseased cells in the central nervous system and is involved in the development and management of pain and inflammatory responses (Cotrina and Neergaard, 2009). P2X7 receptors in particular are associated with inflammation as a study by Chessell et al. revealed that neuropathic pain levels decreased when P2X7 receptors underwent conformational changes.

Patients that suffer from chronic pain have been found to have significantly more P2X7 receptors than healthy individuals. Also, conformational changes to the shape of P2X7 receptors reduces the production of various types of interleukin such as IL1β, IL-10, and IL-6, which are all involved in the production of erythrocytes, immunology regulation and inflammatory reactions (Solle et al., 2001, Sims et al., 1988). This provides sufficient evidence that P2X7 receptors are involved with immunology, particularly in regard to inflammation.
The topic of our research was further narrowed down when it became clear that these receptors are considerably involved in neuro-immune mechanisms, which is reinforced by the fact that P2X7 receptors are abundantly present in the spinal cord and the brain (Deuchars et al., 2001). Refer to Table 1 for a brief overview of where P2X7 receptors can be found in neuronal cells. In 1979, Crockcroft and Gomperts wrote in Nature that the presence of ATP over the duration of several seconds will produce a much larger pore in the P2X7 receptor. This will allow it to become permeable to significantly larger ions such as N-Methyl-d-aspartic acid (NMDG). These findings were then built upon by Suprenant et al. in 1996, when it was concluded that P2X7 receptors are essential for mechanisms within the immune system, more specifically for inflammatory responses.

Further research into the specific roles of the P2X7 receptor revealed that there is contribution to the progressive loss of structure of neurons and in some cases, apoptosis of neuronal cells. One way that this role is carried out is due to P2X7's activation of several different cysteine-aspartic proteases (CASPRs) which have a vital role in inflammation and apoptosis (Wilson et al., 1994). Other tasks that the P2X7 receptor carries out includes; the processing and release of interleukin-1β(IL-1β) and the release of glutamate; which is the most commonly used neurotransmitter in vertebrates (Meldrum, 2000).
Lastly, in order for us to produce an experiment, we were required to understand how P2X7 receptors influence recovery from inflammation in the brain and more specifically ATP levels and IL1β production. One study by Virgilio et al. found that P2X7 acts as a proinflammatory mediator, and that ATP activity modulates inflammatory responses. This information is the basis of where our investigation stems from, we will be investigating the binding of ATP to P2X7 receptors in the brain and spinal cord and how this impacts neuro-immunology based on the release of IL-1β from cells.
Although there have been great advances in scientific understanding in recent years, one field that has been difficult to make progress in, is the treatment of neuropathic pain. This study will bridge the gaps in scientific knowledge and help to broaden the understanding of P2X7 receptors and its role in neural inflammation. The results gained from this experiment will provide further data that can be used by academics for further investigation in order to expand our medical understanding to increase life expectancy and improve the quality of life. The objective of this experiment is to discover how the production of IL-1βeffects recovery from inflammation in the brain with respect to the activation of P2X7 receptors.
RESEARCH PLAN
Outline
Our experimental intentions are to induce the activation of P2X7 receptors based in the glial cells of our sample mice models. P2X7 receptor samples will be obtained ethically from healthy mice donor populations. We will determine the receptor's efficiency in apprehending neuropathic pain, by stimulating the mice through electrode-based electrical stimulation. The levels of stimulation will be at a moderate variation to ensure simultaneous ethical handling of sample donors and highly accurate results. Our variables of interest are interlinked; the levels of extracellular ATP and the subsequent release of IL-1β will be monitored and recorded over a clinical period of six months in laboratory conditions. ATP concentrations on the P2X7 samples will be quantitatively measured using the luciferase enzyme to enable spatial visualisation of the spread using an MRI scanner. In addition, we will be incorporating high-performance liquid chromatography (HPLC) to aid this further.
P2X7 Receptor
P2X receptors are readily identified by distinct ligand-gated ion channels- which display observable sensitivity to extracellular ATP. This triggers the channels to open, enabling cation influx (Torres et al., 1999). The P2X7 is the seventh protein subunit in the P2X purinoreceptor family and can generate homomeric receptors. It operates efficiently under high concentrations of extracellular ATP that surpass a minimum threshold, meaning the receptor can maintain high sensitivity for prolonged periods, rendering it ideal for clinical studies. Figure 2 shows how the components of the P2X7 receptor come together to link to its function (North, 2002). A vital aspect to consider going forward when studying the P2X7 receptor is that when successfully activated, it commands the mass secretion of bound ATP. This in turn, optimises signalling in the purinergic channels (Savio et al., 2018). The signalling via the ATP and P2X7 association also encourages the participation of specialised cytokine proteins, most notably IL-1β. This is a crucial component when addressing the P2X7 receptor's response to pain caused by neuroinflammatory conditions, which we will allude to when articulating below.

IL-1β
IL-1β is a cytokine protein that originates from the microglia regions of cell bodies in organisms. Activation of inflammasomes triggers the cytokine to be released in vast quantities from the dorsal horn region of the spinal cord, in conjunction with Caspase-1- a proteolytic enzyme that specialises in cleavage of the cytokine for activation. Subsequent coordination of downstream signalling facilitates a proinflammatory response (Clark et al., 2006). One aspect of the cytokine that ties into our investigation is increased expression in patients that have been diagnosed with conditions (such as COPD), which supplies evidence of it being a measurable factor associated with inflammation. IL1β mediates the overall response to inflammation, which according to a model proposed by MacKenzie et al. in 2001, postulates the IL-1β being packaged into micro vesicles for exocytosis and expression.
Studies by Clark et al. in 2006 and 2009 provide us with cross-sections samples of mice donor dorsal horn. In doing so we incur the risk of obtaining samples that show inconsistent IL-1β congregations. As we are prioritising ATP-induced IL-1β release, we will conduct multiple experiments focusing on experimental methods as detailed by Clark et al. in 2010. All the experiments will be conducted in vivo, and to reduce protein deformation, we will use Kreb's Solution mixed with 0.1% Bovine Serum Albumin (initialized as BSA). Our samples will be continuously exposed to the Kreb's Solution and BSA and would be fractionated into several 10cm slices to be preserved under cold temperatures to retain the levels of IL-1β already present. It is imperative to be consistent in experimental technique and handling of materials; this will eradicate unnecessary errors in our results likely to be caused by careless form. We will obtain and assess fractions before, during and post-electrode stimulation to track the progression of the IL-1β release, using ten-minute intervals across an hour. This will be performed on all 10 mice using random selection to eliminate bias. Additionally, we will introduce a specific antagonist- A839977 for the P2X7 receptor, a tetrazole-based molecule (as these types are strongly potent enough to produce pharmacologic responses that are desirable to study) (Honore et al., 2009). This provides a diverse insight as to how the P2X7 communicates in terms of providing a response to chronic pain.
To quantify and analyse the IL-1β levels released after our experiments, we then will utilize an ELISA test pack conduct a complimentary assessment. Before this, we will acquire 10 ml samples of our results for centrifugation in a Beckman Coulter Optima X Ultracentrifuge. The samples will be immersed in buffer solutions and undergo the assay after which they will be enclosed in specialized colorimetric plates at a 450nm wavelength. All data will be recorded in tabular and graphical format as percentages to two decimal places.
Figure 3: IL-1β release expressed as basal percentages via ATP/LPS-induced expression.
These results provide model expectations of what to observe through similar procedures detailed above, whilst also illustrating the various factors at work. (Clark et al, 2010).
Figure 3: illustrating model results from an experiment showing release of IL-1β and ATP
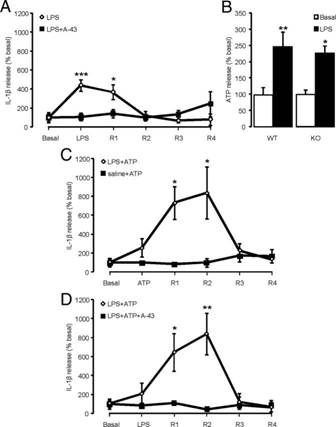
Response to Inflammation
P2X7 receptors have been established as crucial participants in responding to and mediating neuroinflammatory pain. This is an interesting component as ethanol-induced conditions can desensitise P2X7 receptors. (figure 3)
Mice and Associated Materials
10 eight-week old male mice models will be involved in the study, having ensured we received ethical consent from the donor organisations to test upon. We will allocate them into two groups of 5; a control group containing non-transgenic mice and a blind-trial group consisting of transgenic mice, so as to eliminate if not reduce bias. To assess nociceptive pain response, the electrode stimulation will be controlled, and all risks will be curbed by using pre-determined scans to see which areas of the brain receive stimulation the best. Testing will occur twice every day of the duration over six months to provide systematic data over prolonged periods of time. At two junctions per week, MRI software will be used to monitor all cellular development and corresponding imagery will be presented to show the densely congregated areas for ATP populations within the mice brain samples.
To conduct this experiment successfully, it is imperative to perform ordinate laboratory techniques, and cleave out improper testing practices. All concentrations and stock volumes, serial dilutions will be stated clearly and agreed upon, kept constant throughout. GraphPad Prism Version 7 will be used to perform statistical tests and logarithmic tests will be implemented to yield parameter comparisons. Flow cytometry using an Attune NxT Flow Cytometer (Thermo Fischer Scientific) can enable analysis of cellular expression in our results. An ELISA assay kit will be arranged for and delivered courtesy of Thermo Fischer Scientific (Loughborough, United Kingdom). Our continued association with Thermo Fischer will incorporate the use of another scientific apparatus- the Vanquish Horizon UHPLC System to ensure quantitative measurements of ATP concentrations on the P2X7 receptor. Data will be expressed as percentages to two decimal places in tabular form, and the mean of each series of results as well as the standard deviation will be calculated to provide relative variation.
This experiment comprises of two experimental groups. The first of these experimental groups are a control group, to clarify whether our results are due to the specific parameter we are investigating. The second group is a blind group, and this means the person carrying out the experiment is unaware. This reduces accidental bias and thus reduces variation. All 10 of these mice must be male and 8 weeks old, and certain conditions must be constant to ensure results are accurate and valid. These conditions include mouse size, weight, and breed although they can be of any breed provided the breed is constant across all the test subjects. We are inducing pain in these mice subjects by electrical stimulation of the brain using electrodes. Although this method is more invasive, it can offer us more accurate results and can determine which part of the brain is being activated when looking at neuropathic pain.
Bias Elimination
Throughout the experiment, numerous variables can affect the results we obtain and as such we employ several techniques to reduce variation as much as possible. One of the main techniques used is referred to as "randomisation". This technique involves assigning each subject mouse to its experimental group at random and avoiding self-selection. This method of research minimises bias and as such allows us to obtain more reliable results. Another technique we use to reduce variation is known as "blinding". This means the member of the group taking measurements is unaware which experimental group is which. This helps to prevent accidental bias, as the person taking measurements here cannot be influenced by their predicted outcome and misinterpret the data or reach a conclusion prematurely. Furthermore, we use controls to help determine if other factors are to play. We create identical conditions and administer treatment that appears identical to the experimental treatment, except for the parameter under investigation. This confirms to us that this specific parameter has the proposed impact on the subjects.
IMPACT SUMMARY
Research indicates that neurological disorders were responsible for the highest percentage of deaths throughout England and Wales (Jones and Goldeck, 2014). It is said to lead to significant tissue damage i.e. peripheral neuropathy (Freire et al., 2018) as well as being a leading cause of premature mortality (Stahre et al., 2014). As a result, it cannot be said that this treatment or therapy will reverse the organ damage, however it may slow down the progression of damage in the brain as the P2X7 receptor is abundant in the central nervous system.
The data from this project can be used by researchers to create better treatments with minimal side effects and improve the effectiveness of using the P2X7 receptor. It helps to show how the effectiveness can assist in slowing down brain degradation from neuropathic disorders in humans. The P2X7 receptor has been proven as useful in the pharmacological inhibition approach which is important for the generation of inflammatory responses during a number of pathologies as evidenced. (Freire et al., 2018).
The data from this study will be made available to a wider audience through many mediums. These mediums of media will provide the information to a wide audience and provide platforms for the general public, researchers, clinicians and primarily those affected by neurological disorders. For professionals who may understand basic concepts and have knowledge about this receptor, it will be published in science journals which can be accessed via a subscription for everybody except students who will be able to access if for free.
As this research also needs to benefit the wider population, selective parts of this will also be published in a simplified form on neurological health awareness websites online for example neurosymptoms or NHS and will be displayed in conferences to raise awareness such as the Europe and UK Neurology congress, Switzerland neurophysiology conference etc. People who may be affected by neurological disorders will also be delivered talks regarding this issue and this research may be used in a way in which they will comprehend.
This exchange of information provides knowledge to further the understanding of the brain and how the P2X7 receptor associates with ATP and IL-1β in response to neurological stimuli. This in turn may result in scientific collaborations to overcome the degradation to parts of the brain due to unknown effects of neuropathic pain.
We hope that this will help in reducing the cases of neurological disorders and may allow a cure to be found. As a result, this will improve people's quality of life and help the economy as well as public services by having to spend less on neurological and inflammatory related treatments as they cannot be treated simultaneously.
Appendix 2: Application for Ethical Approval (BS2000)
1. Applicant Details:
|
Names: |
Email: |
2. Project title:
The Neural P2X7 Receptor Is Insignificant In Brain inflammation In Mice Models, Regarding IL-1β And ATP Levels, When Mice Models Are Electrically Stimulated.
3. Project aims & research questions:
|
Our research project aim is to combat inflammation in the brain, whilst looking at the activation of the neural P2X7 receptor in mice models. This is to determine the P2X7 receptor impact in targeting neuropathic pain, and what effect ATP and IL-1β contributes towards this. 1- 10, eight-week-old male mouse models will be involved in this study. 2- The mice will be split into 2 groups of 5 (a control group, which consists of non-transgenic mice) and a blind-trial group (which consists of transgenic mice). 3- IL-1β levels will be measured via an enzyme linked immunosorbent assay. 4- Mice models are also being exposed to ATP. 5- We will measure pain by electrical stimulation with electrodes, this is an invasive method, yet it is accurate and can determine which part of the brain is being activated. 6- ATP sensitive dye will show where the ATP is mostly concentrated on an MRI scan. 7- ATP concentrations on the P2X7 receptor will be quantitively measured via high performance liquid chromatography and luciferase. |
4. Does your proposal involve (tick all that apply) and complete the relevant sections below:
Only complete the sections relevant to your research proposal. You will be marked on the quality of what you write. You will not lose marks if your proposal does not encompass all five of these areas.
|
a) Human subjects |
|
|
X |
b) Experimental animals (defined by the Animals (Scientific Procedures) Act 1986) |
|
c) Genetic modified organisms (GMOs) (defined by the UK Health & Safety Executive) |
|
|
d) Dangerous pathogens(defined by the UK Advisory Committee on Dangerous Pathogens (ACDP)) |
|
|
e) Environmental Risk (defined as the chance of harmful effects to human health or ecological systems resulting from exposure to physical, chemical or biological entities that can induce an adverse response. The probability/likelihood and magnitude of possible/potential harms to participants/subjects should be no greater than those encountered by them in any aspects of their everyday life which may be related to the research) |
4a) Human Subjects -describe number of participants, explain how they will be recruited and explain how your proposal will meet current UK legislation covering Human Subjects
N/A
4b) Experimental Animals -describe number of animals, explain how this number has been calculated and explain how your proposal will meet current UK legislation covering Experimental Animals:
Our research project will require 10 mice, as we will be doing 2 trials. Therefore, 5 mice will be used per trial. The mice will be randomised for fairness.
Alpha (significance) = 0.05; required power = 80%; expected effect size = 60%; Stdev = 0.33
n = 2(1.96 + 0.85)2 x (0.33 )2/ (0.62)= 15.8 x 0.11 / 0.36 = 4.8 (5 mice)
How we chose the expected effect size; the mean of treatment group, minus the mean of the control group.
We will strictly follow the Animal Scientific Procedures Act (ASPA) 1986 (amended 2013). In order to complete this study legally we must comply with current UK legislation by attaining licences; we will acquire licences from the home office for the use of mice. The Establishment Licence is held already by the University of Leicester. For each group member, a personal licence is required. For completion of the 5 categories, a project licence is required.
We will conform with the 3 R's; refine, reduce, replace. Refine: in order to reduce animal suffering and improve their welfare, the mice will reside in pathogen-free, temperature-controlled room with comfortable, large, cages with other mice so they can have social interaction (5 animals per cage). The mice will have access to water and a monitored diet. The mice will be handled compassionately and with care. The staff handling the mice will all have the right skills and training. (House of Commons, 1986, p.3)
Care procedures will include pain management via analgesia which will be pre-emptive, and postoperative. However, under section 10(2)(b) of ASPA 1986, if the mice are presenting signs of pain or suffering which cannot be relieved, we will guarantee the mouse is killed humanely without delay regardless of the research project. (House of commons, 1986, p.3)
Reduce (fewer number of mice as possible) and replace (if/where applicable).
The codes of practice we will follow for the humane death of the mice is Schedule 1, under section 21, ASPA 1986, we will use a technique which is appropriate for the size of the mouse (approximately 1.5kg) A veterinary surgeon will give the mice a humane death via euthanasia as early as scientifically justifiable. This will be done by, increasing carbon dioxide concentration in a chamber and exposing the mouse to it. This is quick and less distressing. (House of commons, 1986, p.6)
Furthermore, under section 3.2 of ASPA 1986, mice will not be killed in the presence of other mice, to prevent distress and antagonisation. (House of commons, 1986, p.3)
Moreover, under section 4 of ASPA 1986, we will ensure the mice are dead before the mouse is left unattended/or disposed of (rigor mortis will be the determinant). (House of commons, 1986, p.4)
4c) Genetically Modified Organisms- describe proposed experiments and explain how your proposal will meet current UK legislation covering Genetic Manipulation:
N/A
4d) Dangerous Pathogens - describe proposed experiments and explain how your proposal will meet current UK legislation Dangerous Pathogens:
N/A
4e) Environmental Risk -describe proposed experiments, explain how you will minimize environmental risk and explain how your proposal will meet current UK legislation covering Environmental Impact:
N/A
5. Are there any cultural, religious or other implications to conducting the study and if so, how will these issues be addressed?
Animal activists advocating rights for the mice, we will ensure the mice used in our research will be treated with compassion and dignity.
Also, in regard to religion, we are not using any animals that in religious traditions are considered holy or avoided.
6. Are there any other ethical issues that you think might be raised in the proposed research and if so how will they be addressed?
N/A
REFERENCES
Burnstock, G., 2006. Purinergic signalling. British journal of pharmacology, 147(S1), pp.S172-S181.
Chessell, I.P., Hatcher, J.P., Bountra, C., Michel, A.D., Hughes, J.P., Green, P., Egerton, J., Murfin, M., Richardson, J., Peck, W.L. and Grahames, C.B., 2005. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain.Pain, 114(3), pp.386-396.
Clark, A.K., D'Aquisto, F., Gentry, C., Marchand, F., McMahon, S.B. and Malcangio, M., 2006. Rapid corelease of interleukin 1β and caspase 1 in spinal cord inflammation. Journal of neurochemistry, 99(3), pp.573-582.
Clark, A.K., D'Aquisto, F., Gentry, C., Marchand, F., McMahon, S.B. and Malcangio, M., 2006. Rapid corelease of interleukin 1β and caspase 1 in spinal cord inflammation. Journal of neurochemistry, 99(3), pp.868-880.
Cockcroft, S. and Gomperts, B.D., 1979. ATP induces nucleotide permeability in rat mast cells. Nature, 279(5713), p.541.
Cotrina, M.L. and Nedergaard, M., 2009. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic signalling, 5(2), pp.223-232.
Davalos, D., Grutzendler, J., Yang, G., Kim, J.V., Zuo, Y., Jung, S., Littman, D.R., Dustin, M.L. and Gan, W.B., 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience, 8(6), p.752.
Deuchars, S.A., Atkinson, L., Brooke, R.E., Musa, H., Milligan, C.J., Batten, T.F., Buckley, N.J., Parson, S.H. and Deuchars, J., 2001. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. Journal of Neuroscience, 21(18), pp.7143-7152.
Di Virgilio, F., Dal Ben, D., Sarti, A.C., Giuliani, A.L. and Falzoni, S., 2017. The P2X7 receptor in infection and inflammation. Immunity, 47(1), pp.15-31.
Freire, D., Reyes, R., Baghram, A., Davies, D. and Asatryan, L. (2018). P2X7 Receptor Antagonist A804598 Inhibits Inflammation in Brain and Liver in C57BL/6J Mice Exposed to Chronic Ethanol and High Fat Diet. Journal of Neuroimmune Pharmacology, 14(2), pp.263-277.
Haran Jaykaran, et al. (2013). How to calculate sample size in animal studies? Journal of pharmacology and Pharmacotherapeutics. 4 (4), 303-306.
Honore, P., Donnelly-Roberts, D., Namovic, M., Zhong, C., Wade, C., Chandran, P., Zhu, C., Carroll, W., Perez-Medrano, A., Iwakura, Y. and Jarvis, M.F., 2009. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1αβ knockout mice. Behavioural brain research, 204(1), pp.77-81.
House of Commons (13/01/1997). The Humane Killing of Animals under Schedule 1 to the Animals (Scientific Procedures) Act 1986. London : Stationery office limited . p3-6
Jones, R. and Goldeck, D. (2014). Unexpected and unexplained increase in death due to neurological disorders in 2012 in England and Wales: Is cytomegalovirus implicated?. Medical Hypotheses, 83(1), pp.25-31.
Meldrum, B.S., 2000. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. The Journal of nutrition, 130(4), pp.1007S-1015S.
North, R.A., 2002. Molecular physiology of P2X receptors. Physiological reviews, 82(4), pp.1013-1067.
Pangalos, M.N. and Davies, C.H. eds., 2002. Understanding G protein-coupled receptors and their role in the CNS. Oxford University Press.
Sacks, J., Gonzales, K., Bouchery, E., Tomedi, L. and Brewer, R. (2015). 2010 National and State Costs of Excessive Alcohol Consumption. American Journal of Preventive Medicine, 49(5), pp.e73-e79.
Savio, L.E., de Andrade Mello, P., da Silva, C.G. and Coutinho-Silva, R., 2018. The P2X7 receptor in inflammatory diseases: angel or demon?. Frontiers in pharmacology, 9, p.52.
Shang, Y. et al (2018). Leucodin attenuates inflammatory response in macrophages and lipid accumulation in steatotic hepatocytes via P2x7 receptor pathway: A potential role in alcoholic liver disease. Biomedicine and Pharmacotherapy, 107, (pp.374-381).
Sims, J.E., March, C.J., Cosman, D., Widmer, M.B., MacDonald, H.R., McMahan, C.J., Grubin, C.E., Wignall, J.M., Jackson, J.L. and Call, S.M., 1988. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science, 241(4865), pp.585-589.
Sluyter, R., 2017. The P2X7 receptor. In Protein Reviews (pp. 17-53). Springer, Singapore.
Solle, M., Labasi, J., Perregaux, D.G., Stam, E., Petrushova, N., Koller, B.H., Griffiths, R.J. and Gabel, C.A., 2001. Altered cytokine production in mice lacking P2X7 Receptors. Journal of Biological Chemistry, 276(1), pp.125-132.
Stahre, M., Roeber, J., Kanny, D., Brewer, R. and Zhang, X. (2014). Contribution of Excessive Alcohol Consumption to Deaths and Years of Potential Life Lost in the United States. Preventing Chronic Disease, 11.
MacKenzie, A., Wilson, H.L., Kiss-Toth, E., Dower, S.K., North, R.A. and Surprenant, A., 2001. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity, 15(5), pp.825-835.
Torres, G.E., Egan, T.M. and Voigt, M.M., 1999. Hetero-oligomeric assembly of P2X receptor subunits specificities exist with regard to possible partners. Journal of Biological Chemistry, 274(10), pp.6653-6659.
Figure 1: Di Virgilio, F., Dal Ben, D., Sarti, A.C., Giuliani, A.L. and Falzoni, S., 2017. The P2X7 receptor in infection and inflammation. Immunity, 47(1), pp.15-31.
Figure 2: Mortaz et al, (2012). Role of P2X7 Receptors in Release of IL-1β: A Possible Mediator of Pulmonary Inflammation. Tanaffos. 2012; 11(2), (pp. 6–11).
Figure 3: Clark, K.A et al (2010). P2X7-Dependent Release of Interleukin-1β and Nociception in the Spinal Cord following Lipopolysaccharide. The Journal of Neuroscience, 30(2), (pp-573-582).
Table 1: Cotrina, M.L. and Nedergaard, M., 2009. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic signalling, 5(2), pp.223-232.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this research project and no longer wish to have your work published on the UKDiss.com website then please:



