Analysis of Brg1 in Keratinocytes During Wound Healing
Info: 8696 words (35 pages) Dissertation
Published: 21st Feb 2022
Tagged: BiologyPharmacology
ABSTRACT
Wound healing is a highly complex mechanism involving overlapping stages which starts with a phase of inflammation followed by proliferation and reepithelization. The overall effect is to remodel the injured skin back to normal through remodelling.
The SWI/SNF complex is involved in remodelling histone tails allowing nucleosome remodelling .This complex is composed Brm or Brg1 along with BAF complex that aid in expression of genes.
Our Research was conducted on the analysis of Brg1 in keratinocytes during wound healing.
This was conducted using an Immunohistochemistry approach using 4 skin tissues from mice including an 8 week Telogen tissue used for a point of comparison. Further three tissue showed progressive wound healing from day 3, 5 and 7.
Analysation of Fluorescent intensity was conducted quantitatively. Results showed a progressive increase expression of Brg1 from the distal to distal hyperproliferative regions from day 3 to day 7.
The change Brg1 expression suggests that Brg1 plays a role in the expression of genes involved in proliferation and differentiation of keratinocytes,
High intensity of Brg1 In the epithelial tongue was detected suggesting Brg1 plays a role in expressing the integrin genes allowing migration over the denuded area of the wound.
Qualitative visual analysis showed higher fluorescent intensity at hair follicle from the distal to hyper proliferative of wounds suggesting that Brg1 plays a role in maintaining the stem cell pool of hair follicle
1. INTRODUCTION
1.1 Functions of the skin
The mammalian skin provides a permeable barrier against the loss of water from nucleated epidermis layers whilst maintaining a ph of 5.5 supressing microbial growth. The skin also protects against UV and external factors such as chemicals and pathogens. Receptors on the surface of the skin allow thermoregulation as well as sensing pressure touch and pain (Boer et al,2016).
1.2 Structure of the Skin
The epidermis is a stratified epithelium formed up of organised layers. The keratinocytes are the most abundant cells which switch from a proliferative state in the basal layer to a differentiated state as they migrate through the suprabasal layer to form the stratum corneum. At the cornified layer the keratinocytes dehydrate and flatten forming corneocytes. This layer is resistant to abrasion forming a barrier against external factors whilst preventing water loss (Simpson et al 2011).
The intermediate filament Keratin form the infrastructure of keratinocytes as the cells move through the spinous and granular layer of the suprabasal layer undergoing differentiation there is a switch of keratins from K5 and K14 to K1 and k10.( Pastar et al 2014,moll et al 2008). The keratinocytes at the basal cells are attached to basement membrane through hemidesmosomes whilst at the suprabasal cells attach to neighbouring cells via desmosomes (Simpson et al 2011). Migration of keratinocytes during wound healing and maintenance of lost cells requires disconnection of attachments.
The process of differentiation requires MAP kinase pathways activated by various stimuli including a calcium influx and epidermal growth factors (Jost et al 2001 , Pastar et al 2014).
Further cells present in the epidermis include Melanocytes, Langerhans cells, Lymphocytes and Markel cells. These cells are involved in producing pigment and immunological defence respectively.
The extracellular matrix of the dermis composed of collagen; fibril and elastic fibres help provide structural support to the dermis. Fibroblasts cells are prominent in the dermis where they secrete collagen which forms a meshwork providing strength and elasticity. The network of capillaries and sweat glands are responsible for regulation of temperature through homeostasis (Tracy et al 2016).
The hypodermis is the last layer formed up of subcutaneous fat with a role in protection and insulation.
1.3 Wound healing
Injury to the skin involves cutaneous wound healing through overlapping processes. Immediately after a wound haemostasis begin with platelet aggregation leading to fibrin clot activation. Pro inflammatory cytokines are released from clot and surrounding tissue including Fibroblast, epidermal- Transforming and platelet derived growth factors. The role of neutrophils includes clearance of microbes and debris using lysosomal enzymes. The macrophages release cytokines which activate leukocytes and remove apoptotic cells allowing tissue regeneration. T cell lymphocytes maintain wound healing through tissue integrity maintenance by regulating inflammation (Guo et al, 2010).
During the inflammatory phase the proliferative phase begins characterized by the entry of fibroblasts and angiogenesis. The fibroblast produce components of the extracellular matrix (ECM) which aid migration composed of collagen, fibronectin and glycol proteins. The blood vessels, cells and the ECM contribute to the granulation tissue at the wound bed which allows reepithelization of the wound to occur (Guo et al, 2010).
To close the defect in epidermis the keratinocytes at the wound edge loosen their adhesion to each other and to basal lamina via disassembly of desmosomes and hemidesmosomes. Keratinocytes behind the migrating tongue proliferative whilst at the wound edge they migrate over the denuded area. This occurs via activation of PKCa which lead to conversion of calcium independent to dependent desmosomes. Transcription factor Slug allows for release of keratinocytes. Viscoelasticity is increased via the change of keratins as the keratinocyte migrate up the layer regulated by growth factors present within wound environment. (Pastar et al 2014)
This is followed by the remodelling phase where inflammatory cells go through apoptosis and collagen is replaced returning to normal skin.
1.4 Brg1 in embryonic development
Nanog is the transcription factor involved in self-renewal of undifferentiated embryonic stem cells (ESC) for pluripotency. Initially expressed widely during the morula-to-blastocyst transition, then expressed only in inner cell mass and silenced as the cells differentiate in the extraembryonic lineages (Moery et al 2015). Brg1 acts as a repressor and activator of transcription of genes through interaction with additional proteins.
The initial regulation of Nanog occurs in the embryonic development where it is vital to maintain the pluripotent characteristics of these stem cells hence this involves an increase in Noggin. Brg1 or its corepressor HDAC1 are disrupted leading to an increase in acyletayed H3K9/14 at the Nogginproximal enhancer allowing an open chromatin for expression.
Further development during the trophoblast lineage involves silencing of Noggin. Brg1 and HDAC1 are maintained the effect is acH3K9/14 are downregulated at Noggin proximal enhancer preventing an open chromatin structure.(Carey et al 2015)
Chromatin remodelling factors also interact with the tumour suppressor protein p53 to maintain the balance between proliferation and apoptosis. Chromatin transactivation protein BRG1 interact with p53 controlling the levels. Deletion of the brg1 in embryonic development results in peri-implantation lethality whilst an increase in Brg1 leads to low levels of p21 and p53 leading to rapid embryonic growth ( Marquez-Vilendrer et al 2016) (Singh et al 2016).
1.5 Brg1 and Hair follicles
Hair follicles contribute to epidermal healing through emigration of stem cells from follicle aiding in reepithelization. Absence of hair follicles results in a delay in wound closure and the epidermis hyper proliferate further away from site of wound. The hair follicle is divided into three phases consisting of: Anagen (Growth phase), Catagen (regression phase) and Telogen (resting phase) Replenishment of cells involves the epidermal stem cells (ESC) niches. These include the bulge of the hair follicle, the base of sebaceous glands and the basal layer of the interfollicular epidermis (Ito et al,2008).
The stem cells in the basal layer are unipotent In comparison to the multipotent stem cells found in the bulge cells of the hair follicles which are vital in maintenance of the IFE stem cells. During the Telogen the HFSC are kept inactive by BMP and FgF18. Activation of the hair follicle bulge cell progeny move into the lower outer root sheath towards the matrix. Entry into anagen involves BMP inhibitors from dermal papilla of hair follicle (Blanpain et al 2006). Brg1 binds to the promoter of Sonic hedgehog genes upon stimulation SHH signal causes bulge HFSC to divide symmetrically causing the outer root sheath to grow which moves signalling center away at dermal papilla returning the bulge to quiescence. Brg1 is activated by skin injury to facilitate early epidermal repair. Studies demonstrate a molecular circuit that integrates chromatin remodelling (Brg1), transcription regulation (NF-KB, Gli) and intercellular signalling Shh to control bulge stem cells during tissue regeneration (Xiong et al, 2013).
1.6 Brg1 in epidermal development
Epidermal development during embryogenesis occurs at E9.5 followed by stratification at E14.5. At E15.5 the spinous layer is formed. Terminal differentiation of the epidermis occurs at E18.5 (Koster et al 2007).The epidermis of the embryo lacking Brg1 still enters stratification but the terminal differentiation of keratinocytes is affected. Brg1-null mice showed disorganisation at stratum corneum due to alteration of lipid composition and number of corneodesmosomes that hold the stratum corneum together. (Indra et al 2005)
The transcription factor p63 regulates epidermal development by regulating a large number of genes. The epidermal differentiation complexes (EDC) are a cluster of genes involved in terminal differentiation and cornification of Keratinocytes found on the spanning 1.6 Mb on human 1q21 and 3q chromosome in mice.
P63 regulates the expression of the A-T rich binding protein SATB1. This protein is involved in the higher order chromatin folding of keratinocyte. P63 deficient mice showed a downregulation of chromatin regulating factors including Brg1 (Fessing et al 2011).
Regulation of Brg1 was found to be vital during epidermal development via p63. Brg1 was found to relocate the EDC towards the nuclear interior enclosed in SC35-positive speckles enriched in RNA metabolic factor which facilitated efficient transcription of genes (Mardarev et al 2014).
1.7 Aims of the project
The aim of the project is to analyse the expression of Brg1 in keratinocytes during cutaneous wound healing. Mice skin of progressive wound would be provided and using immunohistochemistry techniques to detect expression of the protein.
The project will allow a wider understanding of Brg1 in different stages of wound healing by looking at different areas of the wound including the distal, distal hyperproliferative and the epithelial tongue.
2. MATERIALS AND METHODS
2.1 Immunofluorescent staining
Mouse skin cyrosections were used for the detection of Brg1/Baf155. Tissue sections of variable times point from day 3, 5 and 7 showing progressive wound healing were used for analysis. A wild type 8 week Telogen tissue was used as a control for comparison.
The slides were removed from storage at -80°C and air dried for 10 minutes before being placed in 4% Formaldehyde for 10mins. This was followed by three consecutive washes with 1 x PBS for 5 mins (refer to table 1). The tissue sections were encircled with the PAP pen and dried for 2-3mins.
100µl of the blocking buffer (1% BSA/0.1%Triton x-100 / 0.05% Saponin in PBS) was added to each section. Incubation occurred at room temperature for 1 hour in a humidified chamber.
Optimum dilutions were investigated for Brg1 primary antibodies using the blocking buffer and at 8 week Telogen tissue sample. Brg1 santacruz was tested using 1:50, 1:100 and 1:200 dilutions. Brg1millipore and Santa cruz were tested using the same dilutions of 1:100, 1:200 and 1:400.
Optimum dilution for Baf155 was tested at 1:200 and 1:400. The preferred concentration of 1:400 was used using the blocking buffer produced. 100µl of these dilutions were placed on each tissue section. Overnight incubation in a moisturizing chamber occurred at 4°C.
Slides removed from incubation were washed with 1xPBS 3 times for 5 mins. Secondary antibody Donkey anti rabbit biotechnology was used with the recommended 1:200 dilution for 1 hour in a humidifying chamber in an incubator at 37°C by placing 100µl on each tissue section.
Goat anti Mouse IgG H&L (alex fluroa488) was used for Brg1 mouse primary antibody. The secondary antibody was diluted with1% BSA in 1x PBS.
Excess antibody was removed with 3 x washes with 1 x PBS for 5 mins. This was followed by fixing using vector labs vectashield medium with DAPI (abcam ab104139) and a coverslip (24-60mm) was placed on each slides. Nail varnish coat was applied on the edges of the coverslip.
2.2 Florescent and imaging slides
In order to visualise the expression of the proteins the slides were viewed and images captured using an epiflorescent microscope (Nikon ecilipse 80) and Image pro insight 64 Image j was used to analyse the images. A 10x magnification was used to capture images of the full wounds.
Analyses required 20x magnifications using an exposure of 10 milliseconds for the detection of DAPI of each sample whilst an exposure of 300 milliseconds was used to view the secondary antibody. Three images were taken of the tissue section to allow valid comparison these included distal ends to show normal epithelium and distal hyper proliferative and epithelial tongue images to show progressive wound healing. Again images were merged using image j.
2.3 Semi quantitative analysis
Images were analysed using Microsoft excel along with image J. A comparison of protein expression was calculated using same area boxes by calculating integrated density of the tissue with florescence and a region of blank space of background. These steps were repeated for other areas in field.
Once completed results were copied into programmed excel sheet which provided results for comparison .The average of the background was subtracted from the mean antibody expression. The results were then used to form a bar chart to show comparison between the distal and distal hyperproliferative regions and the epithelial tongue. This method was modified from a previous research project (McCloy et al 2014)
2.4 Statistical analysis
The mean and the standard deviation of the blank space background and the expression of protein was calculated using the programmed excel sheet.
Table - 1
| Reagent | Compounds | Supplier | Catalogue number |
| 10x PBS (phosphate buffer saline) |
|
BDH GPR
BDH GPR Fisher Scientific UK Fisher Scientific UK |
7447-40-7
7778-77-0 s/31600/60 7558-79-4 |
| Blocking buffer |
|
Sigma Aldrich
Sigma Aldrich Sigma Aldrich |
9048-46-8 8047-15-2
9002-93-1 |
Table – 2
| Primary Antibody | Secondary antibody |
| Antisnf2b/BRG1 Millipore Temecular California Rabbit
Brg1 h-88 B1411 Rabbit Santa cruz Biotechnology Baf155 SC10756 Rabbit Polyclonal IgG Biotechnology |
Anti Rabbit – Alexa 555 |
| Brg1 mouse antibody | Anti mouse-Alexa 488 |
3. Results
The purpose for this project was to analyse the expression of the ATP – dependent chromatin remodeller Brg1 during cutaneous wound healing. Examining the specificity of the antibody involved a range of dilution of primary antibody for optimisation at the right dilution with the least background staining and maximum specific nuclei staining. The use of visual analysis along with quantitative data of florescent intensity of resulting images was used. Mouse skin stained with an antibody was conjugated with the relevant florescent secondary antibody and mounted with DAPI. The four stages included, Telogen (Fig.4), day 3 (Fig.6abcd), day 5 (Fig 7 abcd) and day 7 wound (Fig.8abcd). Stained sections were visualised under an epifluorescence microscope using the same gain and exposure.
3.1 Optimisation of Antibodies
Optimisation was carried out using 8 week mouse skin. The expression of Brg-1 Antibody (Santacruz) was optimised at the recommended dilution of 1:200. Figure.1 shows high background and nonspecific staining. This means protein expression cannot be seen hence a change of antibody was needed. Optimization of Brg1 Santacruz and millipore Fig2a/b again shows non-specific staining with high background.
It is impossible to detect the Brg1 expression level using these antibodies hence Brg1 expression analysed using Baf155 antibody instead. Figure 3 and 4 show dilution 1:200 and 1:400. Both concentrations show specific staining distinguishing the epidermis from the dermis. But 1:400 is favoured due to lower background staining.
3.2 Visual expression of Brg-1 in wounds
BAF155 expression analysed in Telogen (Figure 5) shows weak expression in the epidermis layer and remains constant throughout. The hair follicles are present and show faint expression of BAF155.
Figure 6A shows wound day 3. The expression can be seen throughout the epidermis whilst there is minimal expression in the hypodermis. There is forming eschar around the wound whilst migrating epithelial tongue is visible.
Figure 7A leads on to day 5 showing Baf155 expression is still present in the epidermis where there is greater expression closer to the wound where keratinocytes are proliferating. There is forming granulation tissue in the hypodermis. The epithelial tongue is more defined as it migrates over the wound.
Figure 8a shows the wound at day 7. Expression of the protein can be seen at the distal hyper proliferative. Rather than migrating over the wound the epithelial tounge it folds over itself f rather than meeting in the middle hence why the expression is greater.
3.3 Quantitative analysis of the Expression of BAF-155 in Telogen and distal ends of wounds
The Telogen skin section (fig 5) showed similar expression patterns of BAF-155 compared to the distal wounded skin day 3 .5 and 7 (figures 6b, 7b and 8b). Expression is present but differentiation between the basal and suprabasal layer of the epidermis cannot be defined. The size of the hair follicle remains constant through the distal and Telogen tissues showing weak expression. Graph 1 also shows similar expression from Telogen to day 7 wound ranging from 24.62 to 20.52 integrated densities. Baf-155 expression is spread evenly throughout the epidermis of Telogen and the distal ends of the wounded epidermis supported by similar Standard Deviations (S.D) ranging from +/-2.20 to +/-2.52.
Expression of Baf-155 in Hyper proliferative distal ends of wounds
In comparison to distal ends of the wounds it is apparent that the BAF155 expression had increased at hyper proliferative distal ends shown by graph 1. The proliferation of the keratinocytes is shown by thickening of the epidermis where the basal and suprabasal layer can be differentiated. The hair follicles are larger showing greater expression of BAF155 in the dermal papilla.
Looking at graph 1 it shows an overall increase in comparison to the distal regions At day3 (figure 6c) distal hyper proliferative the expression of Baf155 increased 2 fold in comparison to Telogen. At day 5 (Figure 5c) the level of baf155 expression remain constant. The standard deviation shows that there’s variation of baf155 through the wounds as the s.d rises from +/-3.31 at day 3 to +/-5.17 at day 5.
Day 7 (figure 8c) showed the highest level of expression as the wound reaches the end of reepithelization.
The actual tissue split meaning keratinocytes proliferated at one area which affected the spread of expression as day 7 had the highest S.D of 8.095459.
3.4 Epithelial tongue of wounded skin
The epithelial tongue shows variation visually there is a highBaf155 expression in day3 which supports the density which reached a peak looking at graph 2. At day 5 it was visible that the length of the epithelial tongue increased and the density dropped. The low S,D at day5 suggest the antibody density remains constant along the tongue. At day 7 the epithelial tongue folds which causes a high density of fluorescence. This is shown in graph 2 as the density increases with a high S.D suggesting there is variation of expression of BAF155. There was limited background staining.
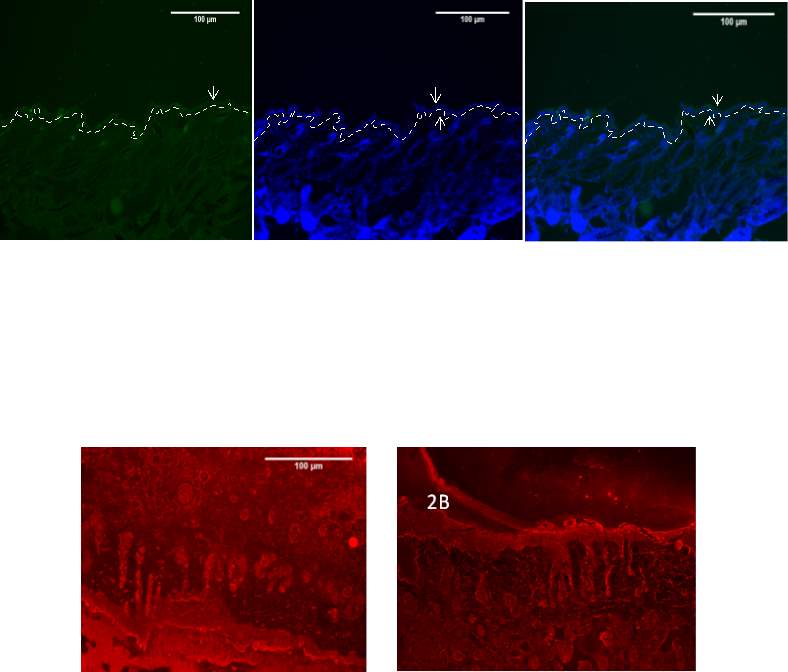
Figure 1- Optimization of the immunofluorescent staining of the 8 week old mouse skin with Santa Cruz anti-Brg1 antibody at 20x. The 8 week old mouse skin was stained with anti-Brg1 antibody at 1:200 dilutions (a) and nuclear DNA was stained with DAPI (b). The merged antibody and DAPI staining is also shown (c). The position of the basement membrane is indicated by the dotted line. The arrow indicates the Epidermis. Note high background staining of the antibody (BM-Basement Membrane EPI – Epidermis)
2A
Figure 2A and 2B- Optimization of the immunofluorescent staining of the 8 week old mouse skin with Millipore temecular california anti-Brg1 antibody) (A) and Santa cruz anti-Brg1 antibody (B) at 20x. The 8 week old mouse skin was stained with anti-Brg1 antibody at 1:200 dilutions. The merged antibody and DAPI staining is shown (a and b). Note high background staining of the antibody with no specific staining at all.
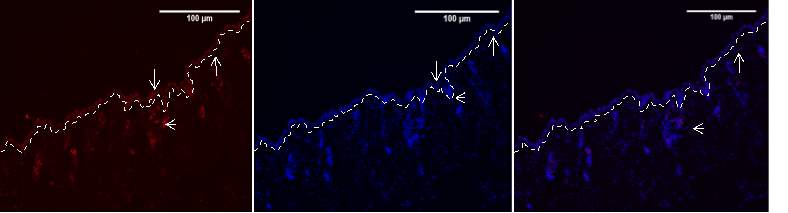

Figure 3,4- Optimization of the immunofluorescent staining of the 8 week old mouse skin with Baf155 Biotechnology antibody at 20x. The 8 weak old mouse skin was stained with anti-Brg1 antibody at 1:200 dilution (Figure 3) and 1:400 dilution (Figure 4) Nuclear DNA was stained with DAPI (3b and 4b). The merged antibody and DAPI staining is also shown (3c and 4c). The position of the basement membrane is indicated by the dotted line. The arrow indicates the Epidermis. Note high background staining of the antibody at 1:200 compared to 1:400 (BM-Basement Membrane EPI – Epidermis HF – hair follicle)
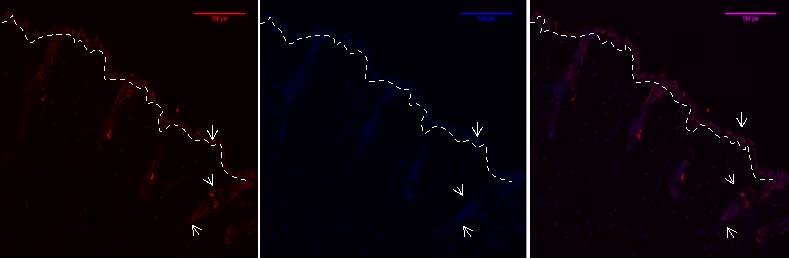
Figure 5A, B, C- Baf155 expression at Telogen 20x of 8 week old mouse skin at 1:400 dilution.
Expression of Baf155 a) and nuclear DNA was stained with DAPI (b). The merged antibody and DAPI staining is also shown (c). The position of the basement membrane is indicated by the dotted line. The arrow indicates the Epidermis, hair follicle and the dermal papilla
(EPI- Epidermis HF- Hair follicle DP – Dermal Papillae)
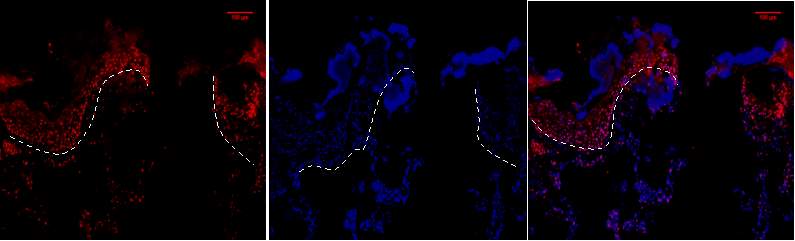
Figure 6A.1, 2,3- Baf-155 expression of day3 post wound at 10x wound .The dashed line showing the epithelial tongue, the wound bed shows forming granulation tissue
Baf155 antibody protein expression at 1:400(a.1) and nuclear DNA was stained with DAPI (A.2). The merged antibody and DAPI staining is also shown (A.3).
(ET- Epithelial Tongue WB – Wound bed
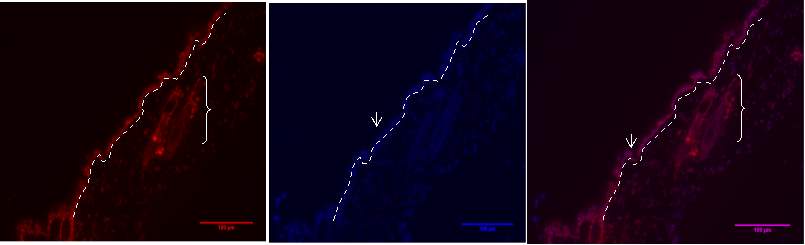
Figure 6B.1, 2, 3- Baf-155 expression of post wound day3 showing the distal ends of the wound at 20x
Baf155 antibody protein expression at 1:400(1) and nuclear DNA was stained with DAPI (2). The merged antibody and DAPI staining is also shown (3). The position of the basement membrane is indicated by the dotted line. Hair follicle is indicated)(EPI- epidermis HF-hair follicle)
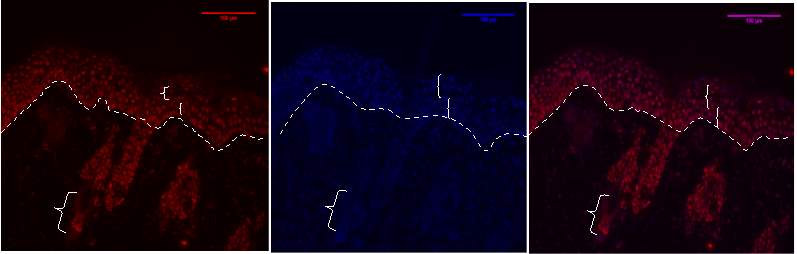
Figure 6C.1,2, 3- Baf-155 expression showing the distal hyper proliferative ends of post wound of day3 at 20x
Baf155 antibody protein expression at 1:400(1) and nuclear DNA was stained with DAPI (2). The merged antibody and DAPI staining is also shown (3). The position of the basement membrane is indicated by the dotted line. Suprabasal layer and basal layer can be distinguished at the epidermis
(EPI – epidermis SBL-suprabasal layer BL – Basal layer DP – dermal papillae)
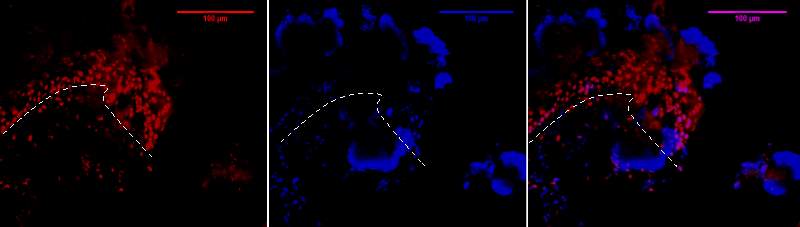
Figure 6D.1, 2, 3- Baf-155 expression at migrating epithelial tongue ends of wound day 3 at 20x
Baf155 antibody protein expression at 1:400(D.1) and nuclear DNA was stained with DAPI (D.2). The merged antibody and DAPI staining is also shown (D.3). The position of the basement membrane is indicated by the dotted line. (ET- Epithelial Tongue)
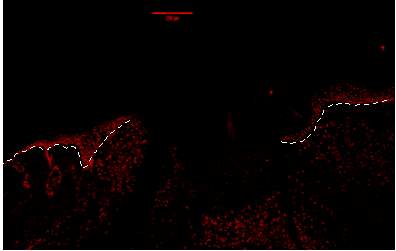
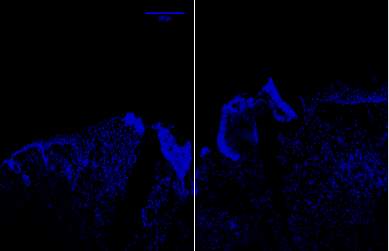
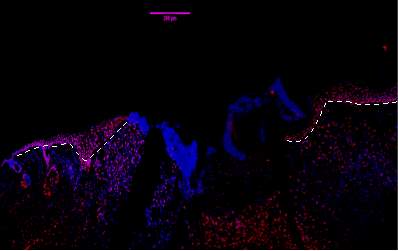
Figure 7A.1, 2, 3- Baf-155 expression of wound day 5 at 10x wound showing the epithelial tongue, the wound bed forming granulation tissue
Baf155 antibody protein expression at 1:400(a.1) and nuclear DNA was stained with DAPI (A.2). The merged antibody and DAPI staining is also shown (A.3). The position of the basement membrane is indicated by the dotted line. Granulation tissue can be seen at the dermis.
(ET- Epithelial Tongue) (WB – Wound bed)
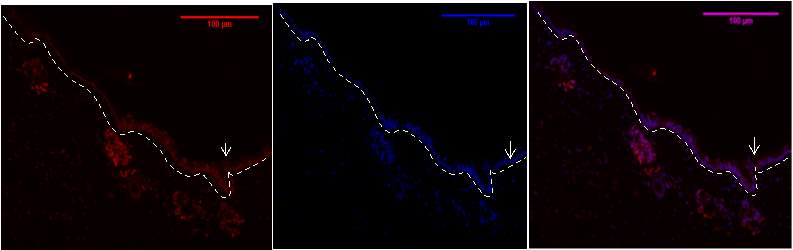
Figure 7b .1,2,3- Baf-155 expression of day 5 at 20x wound showing the Distal ends, the Hair Follicles can be seen
Baf155 antibody protein expression at 1:400(B.1) and nuclear DNA was stained with DAPI (B.2). The merged antibody and DAPI staining is also shown (B.3). The position of the basement membrane is indicated by the dotted line. is (EPI -Epidermis) (HF – Hair follicle)
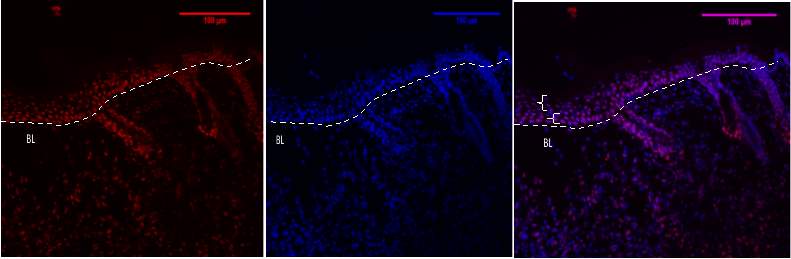
Figure 7C 1.2.3- Baf-155 expression of day 5 at 20x wound showing the Distal hyperproliferative ends, the Hair Follicles can be seen and the layers of the epidermis.
Baf155 antibody protein expression at 1:400(C.1) and nuclear DNA was stained with DAPI (C.2). The merged antibody and DAPI staining is also shown (C.3). The position of the basement membrane is indicated by the dotted line. is (EPI -Epidermis) (HF – Hair follicle) (SBL – Suprabasal layer) (DP – Dermal Papilla)
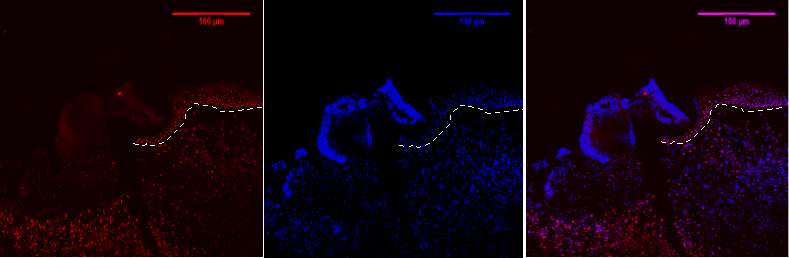
Figure 7D 1.2.3- Baf-155 expression of day 5 at 20x wound showing the migrating epithelial tongue, Baf155 antibody protein expression at 1:400(D.1) and nuclear DNA was stained with DAPI (D.2). The merged antibody and DAPI staining is also shown (D.3). The position of the basement membrane is indicated by the dotted line.ET- epithelial tongue
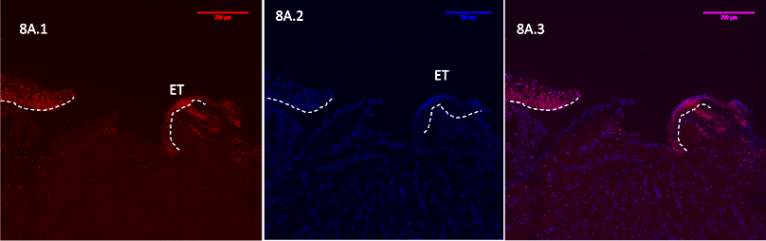
Figure 8A 1.2.3- Baf-155 expression of day 7 at 10x wound showing the wound, epithelial tongue , the formed dermis can be seen. Baf155 antibody protein expression at 1:400(a.1) and nuclear DNA was stained with DAPI (A.2). The merged antibody and DAPI staining is also shown (A.3). The position of the basement membrane is indicated by the dotted line. (ET- Epithelial Tongue) (WB – Wound bed)
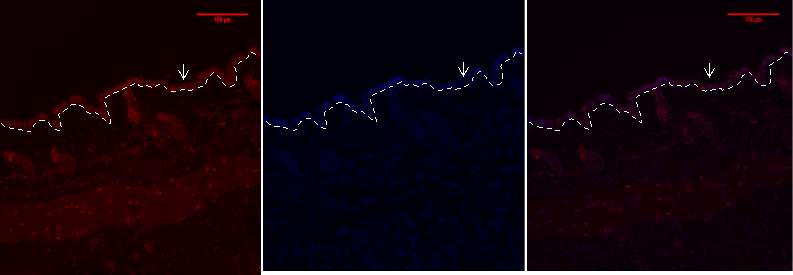
Figure 8b 1.2.3- Baf-155 expression of day 7 at 20x wound showing the Distal ends, the Hair Follicles can be seen
Baf155 antibody protein expression at 1:400(B.1) and nuclear DNA was stained with DAPI (B.2). The merged antibody and DAPI staining is also shown (B.3). The position of the basement membrane is indicated by the dotted line. (EPI -Epidermis) (HF – Hair follicle)
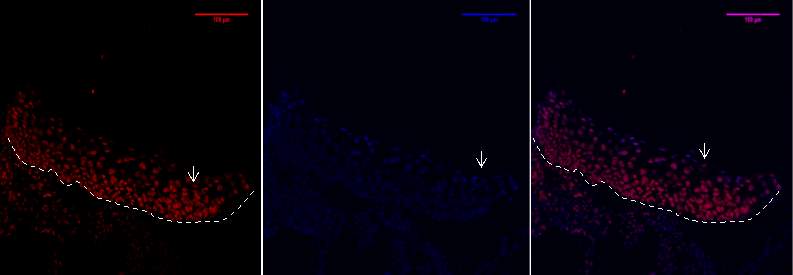
Figure 8C 1.2.3- Baf-155 expression of day 7 at 20x wound showing the distal hype rproliferative ends.
Baf155 antibody protein expression at 1:400(C.1) and nuclear DNA was stained with DAPI (C.2). The merged antibody and DAPI staining is also shown (C.3).
EPI- epidermis
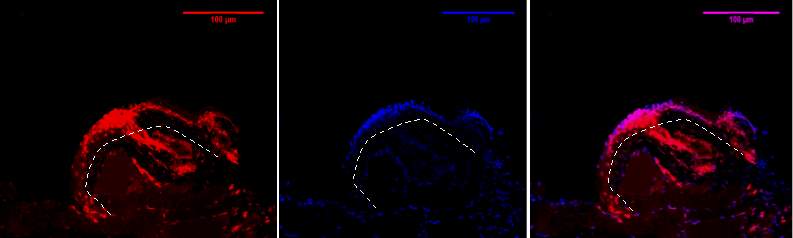
Figure 8D, 1.2.3- Baf-155 expression of day 7 at 20x wound at the migrating epithelial tongue. Shows folding over of the tongue. EPI- epidermis
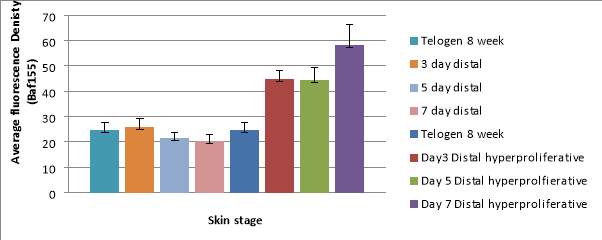
Graph 1 – A bar chart to show the difference in the average Baf155 Antibody fluorescence in Telogen and wounded skin between the distal region and the distal hyper proliferative regions.
The graph also shows the standard deviation of the fluorescent density.
The distal skin show similar fluorescence density ranging from 20.52 at day 7 to 25.95 at day 3. The spread of the density is consistent shown by low and similar standard deviation ranging from +/-3.26 to 2.19.
There is an increase of density at the hyper proliferative regions where it reaches a peak of 58.12 at day 7 whilst at day 3 and 5 it remains constant at around 44.
The standard deviation increases from day 3 to day 7 rising from +/-3.32 to +/-8.09 at the distal hyper proliferative region.
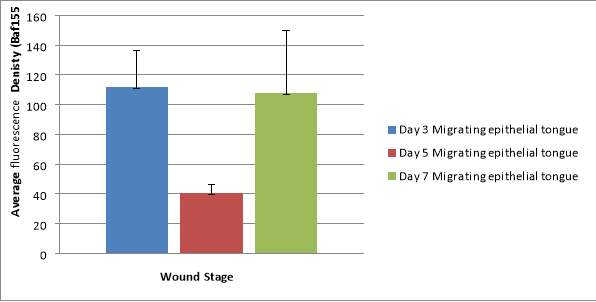
Graph 2 – A bar chart to show the average fluorescence density in the migrating epithelial tongue.
The density varies rising at day 3 reaching 111.57 before dropping to 40.75 at day 5 and rising again at day 7 to 108.
The average fluoresce density varies around the migrating tongue shown by the high standard deviations.
Discussion
The aim of the experiment was to analyse the Brg1 protein expression in different cellular compartments during the skin wound healing process. Upon optimising the Brg1 antibody it was found to have excessive non – specific background staining. Further tests with replacement Brg1 antibodies found similar results. This may have been due to the aging of the antibody which relies on proper storage conditions or possible contamination.
The BRG1 is a core ATPase of the SWI/SNF complex which contain additional subunits all cooperating to remodel the nucleosome. BAF155/BAF47 is a protein scaffolding proteins involved in assembling the SWI/SNF complex. Deletion of BAF155 led to embryonic lethality whilst Brg1 and Baf155 components showed an increase efficiency of somatic cell reprogramming. This shows that BRG1 works in cohesion with BAF155 hence detecting Baf155 expression will represent the presence of BRG1 in keratinocytes (Ho et al, 2009).
Expression of Baf155 observed in the hair follicles at different stages of the skin showed dynamic expression. At 8 week Telogen the hair follicles are in a state of quiescence caused by BMP pathway which inhibits WNT intracellular signalling.
The expression of the protein is similar to the distal ends of the wound where there is also weak expression in the hair follicles.
At the distal hyper proliferative regions of day 3, 5 and 7 the hair follicles elongate and the expression of Baf155 is greater shown by expression at the dermal papilla and hair matrix Wounding induces hair follicles into an anagen phase. This suggests Brg1 serves as a stress activated mechanism reprogramming bulge cells for wound healing.
The switch from a quiescent to a proliferative state involves Brg1 inhibiting the tumour suppressor gene p27Kip. Brg1 null bulge cells show defects in cell proliferation hence the stem cell pool is not replenished due to cell cycle arrest at G1 caused by p27. With activation of Brg1 the bulge cells escape arrest and proliferate maintaining the stem cell pool (Xiong et al,2013).
The epidermis during telogen showed moderate expression of BAF155 which was similar to the expression at the distal regions of post wound day 3, 5 and 7 (Graph 1 antibody density ranges from 20-25). Furthermore the overall layer of the epidermis is thin which suggest low proliferation and differentiation of keratinocytes at the epidermis. During the Telogen phase the hair follicle are resting yet the interfollicular (IF) epidermal stem cells in the epidermal basal layer are present which must mean Brg1 is still involved in proliferation of keratinocytes replacing lost cells at the stratum corneum and maintaining homeostasis of the skin.
Keratinocytes at the leading edge migrate shown by the epithelial tongue, whilst the progenitors behind the wound edge proliferate to restore the barrier function of the epithelium. The distal hyperproliferative show that the Baf155 expression is elevated and maintained at day3 and 5 whilst it rises at day 7.This must mean that proliferation of epidermal cells occurs throughout wound healing. The proliferation also allows the differentiation to be made between the basal and suprabasal layer.
In the basal layer keratinocytes are proliferative but when cells move towards the suprabasal layer they undergo differentiation. It was shown that deletion of Brg1 does not affect proliferation in early development (Indra et al 2005) yet in the figures during hyper proliferation of post wound day 3, 5, and 7 there is high expression of Baf155 in the basal layers. Studies show removal of Brg1 from the epidermis during development resulted in differentiation defects suggesting that the Brg1 has a prime role in keratinocyte differentiation (Indra et al , 2005).
The proliferative pathway of the epidermis is maintained by the actin like protein ACTL6A which is an epigenetic repressor of differentiation. It is also known as BAF53a forming part of the BAF complex which effectively increases the ATPase’s activity of the BRG1.
It was found that the relative ACTL6A mRNA levels were high in the basal layer which supports the high Baf155 expression found in the distal hyperproliferative regions.
ACTL6A regulates SWI/SNF to maintain the progenitor gene regulation. This protein was vital to impair BRG1 binding to the promotors of the differentiation genes such as KLF4 and maintaining the progenitor phenotype of keratinocytes (Bao et al, 2013).
In contrast differentiation of keratinocytes requires ACTL65 to be repressed this is shown by lower mRNA level of the protein in the suprabasal layers. Depletion of ACTL64 allows BRG1 to bind to gene promoters of KLF4 by relocating the SWI/SNF complexes to the chromatin (Bao et al, 2013).
Tissue injury leads to damage of blood vessel causing an acute tissue hypoxia. The inflammatory phase initiates a vascular response and inflammatory cell recruited lower oxygen levels leading to chronic hypoxia. Hypoxia induces the hypoxia induced factor a (HIF-a) forming the Hif complex activating angiogenesis during the proliferative phase of wound healing.
Research showed by inhibiting the HIFa regulator HIF prolyl hydroxylases the keratinocytes during reepithelization increased migratory rate. This was shown by greater aB3 integrin at migrating keratinocytes induced by HIF1α during the reepithelization stage (Kalucka et al, 2013).
Studies proposed that Brg1 promotes the expression of hypoxia inducible transcription factor HIFA and HIF2a gene. Suggesting that HIF recruits BRG1 to HIF target genes promotes increasing nucleosome remodelling and transcription via ATP (Sena et al, 2013).
Whilst proliferation occurs the keratinocytes migrate during reepithelization to restore the epidermal layer over the denuded area forming a migrating tongue. The expression of Baf155 can be seen at all time points of wound healing at the migrating tongue which suggests that Brg1 has role in the nucleosome remodelling of migrating genes.
TGFβ are a family of cytokines which play a vital role in cutaneous wound healing. TGFβ binds to the TGFβ transmembrane receptor. This activation phosphorylates Smad 2 and 3 which translocate into the nucleus, and act as transcription factors.
It was found that the TGFβ ligands and receptors were elevated in the leading edge of migrating epithelial tongue. Involvement of the TGFβ pathway in keratinocyte migration was further supported by the increase of phosphorylated Smad 2 found in the nuclei of the cells in the epithelial tongue.
At the migrating epithelial tongue the migration increased by the elevated expression integrin’s especially a3b1 prompted by the TGFβ pathway (Ramirez et al ,2014)
Suggesting that Brg1 is required for TGFβ gene response in wound healing. Using CTCF gene a TGFβ target involved in wound healing was highly dependent on BRG1. It was shown Smad2 and 3 in the nucleus bind to target promoters of genes which direct BRG1 SWI/SNF complex to remodel nucleosome structures (xi et al 2008)
The expression Baf155 was variable at day 3 , 5 and 7 post wound epithelial tongue. It is expected that migration of keratinocyte decreases as greater area of the wound is epithelized yet the Baf155 expression reached a peak at day 7 with variation of expression throughout the migrating tongue. This discrepancy may be caused by the folding over of the epithelial tongue forming a keloid wound at day 7 post wound which leads to a delay in reepithelization supporting the increase in Baf155 expression.
A novel therapeutic strategy for the treatment of wound healing was devised using ATP containing vesicles to increase tissue regeneration in chronic and acute wound healing. The use of ATP containing vesicle increased the expression of BRG1 mRNA levels. Using Western blotting indicated an increase BRG1 level with use of the ATP vesicle. This suggest that by increasing BRG1 it may be possible to treat chronic wounds and decrease time of wound healing (Sarajoni et al , 2017).
Cre lox p recombination system can be used to silence Brg1 gene as a future investigation on the effect on wound healing. This process involves a Cre recombinase and a lox p recognition site derived form a p1 bacteriophage. Lox p can be inserted at either side of Brg1 gene sites using crisper/cas 9 this is known as flanking. Cre is next inserted into the mouse using adenoviruses. Single loxp sites at ends of gene are bound by Cre protein. Loxp sites align as a circular piece of DNA which prevents maintenance leading to dysregulation. Insertion of loxp chosen sites over crisper/cas9 is preferred as it allows for specific manipulation (Bouabe et al,2015)
In summary it can be said that there is an epigenetic role of the BRG1 in cutaneous wound healing. The research showed that at Telogen and distal regions of post wound the expression is of Baf155 is low which increases in hyper proliferative and migrating epithelial tongue. This suggests that BRG1 plays a role in proliferation, differentiation and migration of keratinocytes during wound healing. The expression of Baf155 in the hair follicles suggests a role of BRG1 in maintaining the stem cell pool.
Nevertheless there are limitations to this study. Firstly the wound at day 7 did not heal forming a keloid wound producing a high standard deviation showing spread of density. Could overcome this by using a different mouse with ideal wounds and to improve the reliability it would be advised to use different animal models.
References
Arup Kumar Indra, Valérie Dupé, Jean-Marc Bornert, Nadia Messaddeq, Moshe Yaniv, Manuel Mark,Pierre Chambon, Daniel Metzger 2005 Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes Development 132: 4533-4544;
Bao, X., Tang, J., Lopez-Pajares, V., Tao, S., Qu, K., Crabtree, G. R., & Khavari, P. A. (2013). ACTL6a Enforces the Epidermal Progenitor State by Suppressing SWI/SNF-Dependent Induction of KLF4. Cell Stem Cell, 12(2), 193–203.
Blanpain, C., & Fuchs, E. (2006). Epidermal Stem Cells of the Skin. Annual Review of Cell and Developmental Biology, 22, 339–373.
Boer, M., Duchnik, E., Maleszka, R., & Marchlewicz, M. (2016). Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Advances in Dermatology and Allergology/Postȩpy Dermatologii I Alergologii, 33(1), 1–5.
Bouabe, H., & Okkenhaug, K. (2013). Gene Targeting in Mice: a Review. Methods in Molecular Biology (Clifton, N.J.), 1064, 315–336.
Carey TS1, Cao Z2, Choi I2, Ganguly A3, Wilson CA2, Paul S3, Knott JG4 BRG1 Governs Nanog Transcription in Early Mouse Embryos and Embryonic Stem Cells via Antagonism of Histone H3 Lysine 9/14 Acetylation.. Mol Cell Biol. 2015 Dec;35(24):4158-69.
Fessing, M. Y., Mardaryev, A. N., Gdula, M. R., Sharov, A. A., Sharova, T. Y., Rapisarda, V., … Botchkarev, V. A. (2011). p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. The Journal of Cell Biology, 194(6), 825–839.
Ho, L., Ronan, J. L., Wu, J., Staahl, B. T., Chen, L., Kuo, A., … Crabtree, G. R. (2009). An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proceedings of the National Academy of Sciences of the United States of America, 106(13), 5181–5186.
Ito, M., & Cotsarelis, G. (2008). Is the Hair Follicle Necessary for Normal Wound Healing? The Journal of Investigative Dermatology, 128(5), 1059–1061.
Johnny A. Senaa, Liyi Wangb and Cheng-Jun Hua,b (2013) “BRG1 and BRM Chromatin-Remodeling Complexes Regulate the Hypoxia Response by Acting as Coactivators for a Subset of Hypoxia-Inducible Transcription Factor Target Genes” Mol Cell Biol. 2013 Oct;33(19):3849-63.
Jost, M., Huggett, T. M., Kari, C., & Rodeck, U. (2001). Matrix-independent Survival of Human Keratinocytes through an EGF Receptor/MAPK-Kinase-dependent Pathway. Molecular Biology of the Cell, 12(5), 1519–1527.
Kalucka J1, Ettinger A, Franke K, Mamlouk S, Singh RP, Farhat K, Muschter A, Olbrich S, Breier G, Katschinski DM, Huttner W, Weidemann A, Wielockx B (2013) Loss of epithelial hypoxia-inducible factor prolyl hydroxylase 2 accelerates skin wound healing in mice. Mol Cell Biol. 2013 Sep;33(17):3426-38
Koster MI, Roop DR (2007) Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol 9:93–113
Mardaryev, A. N., Gdula, M. R., Yarker, J. L., Emelianov, V. N., Poterlowicz, K., Sharov, A. A., … Fessing, M. Y. (2014). p63 and Brg1 control developmentally regulated higher-order chromatin remodelling at the epidermal differentiation complex locus in epidermal progenitor cells. Development (Cambridge, England), 141(1), 101–111.
Marquez-Vilendrer, S. B., Rai, S. K., Gramling, S. J., Lu, L., & Reisman, D. N. (2016). BRG1 and BRM loss selectively impacts RB and P53, respectively: BRG1 and BRM have differential functions in vivo.
McCloy, R. A., Rogers, S., Caldon, C. E., Lorca, T., Castro, A., & Burgess, A. (2014). Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle, 13(9), 1400–1412. http://doi.org/10.4161/cc.28401
Moll, R., Divo, M., & Langbein, L. (2008). The human keratins: biology and pathology. Histochemistry and Cell Biology, 129(6), 705–733. -6
Morey, L., Santanach, A., & Di Croce, L. (2015). Pluripotency and Epigenetic Factors in Mouse Embryonic Stem Cell Fate Regulation. Molecular and Cellular Biology, 35(16), 2716–2728.
Pastar, I., Stojadinovic, O., Yin, N. C., Ramirez, H., Nusbaum, A. G., Sawaya, A., … Tomic-Canic, M. (2014). Epithelialization in Wound Healing: A Comprehensive Review. Advances in Wound Care, 3(7), 445–464.
Ramirez, H., Patel, S. B., & Pastar, I. (2014). The Role of TGFβ Signaling in Wound Epithelialization. Advances in Wound Care, 3(7), 482–491.
Sarojini, H., Billeter, A. T., Eichenberger, S., Druen, D., Barnett, R., Gardner, S. A., … Chien, S. (2017). Rapid tissue regeneration induced by intracellular ATP delivery—A preliminary mechanistic study. PLoS ONE, 12(4), e0174899
Simpson, C. L., Patel, D. M., & Green, K. J. (2011). Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nature Reviews. Molecular Cell Biology, 12(9), 565–580.
Singh AP, Foley JF, Rubino M, Boyle MC, Tandon A, Shah R, Archer TK. 2016. Brg1 enables rapid growth of the early embryo by suppressing genes that regulate apoptosis and cell growth arrest. Mol Cell Biol 36:1990–2010.
Tracy, L. E., Minasian, R. A., & Caterson, E. J. (2016). Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Advances in Wound Care,5(3), 119–136.
Watt FM, Lo Celso C, and Silva-Vargas V: Epidermal stem cells: an update. Curr Opin Genet Dev 2006; 16:518
Xi, Q., He, W., Zhang, X. H.-F., Le, H.-V., & Massagué, J. (2008). Genome-wide Impact of the BRG1 SWI/SNF Chromatin Remodeler on the Transforming Growth Factor β Transcriptional Program. The Journal of Biological Chemistry, 283(2), 1146–1155.
Xiong Y1, Li W, Shang C, Chen RM, Han P, Yang J, Stankunas K, Wu B, Pan M, Zhou B, Longaker MT, Chang CP.Brg1 governs a positive feedback circuit in the hair follicle for tissue regeneration and repair 2013 Apr 29;25(2):169-81.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Pharmacology"
Pharmacology involves the study of drugs and how they affect the body. A pharmacologist contributes to drug development by researching and testing how the body reacts to medication, and whether the medication can have a positive impact on the body in terms of fighting illness and disease.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




