Comparative Study Exploring Cognitive Patterns in Dementia and Functional Cognitive Impairment
Info: 12481 words (50 pages) Dissertation
Published: 1st Mar 2022
Tagged: Psychology
Abstract
Dementia syndrome is a common cause of disability and dependency among older people worldwide. It can be difficult to diagnose dementia, particularly during early stages due to an overlapping of symptoms. Functional cognitive impairment (FCI) is an increasingly common diagnosis in memory clinics as patients report significant memory failures in everyday life. It can be clinically challenging to differentiate these patients from those with memory difficulties due to early stages of a neurodegenerative condition. This study explores cognitive patterns in Alzheimer’s disease (AD), Lewy body dementia (LBD) and FCI. Patients with AD, LBD and FCI were compared on several neuropsychological tests assessing cognition. While subjective memory complaints are common in FCI, results showed that FCI patients performed significantly better than AD and LBD patients on tests of memory. Further analysis revealed that LBD patients performed significantly better than FCI patients on attention, while AD patients were significantly better than FCI patients on task switching tests. No significant differences were found between AD and LBD patients scores on cognitive tests. The current finding is consistent with previous findings suggesting that memory difficulties in FCI patients are different from those with memory deficits due to a neurodegenerative condition.
Introduction
Population aging is a worldwide phenomenon. With fertility rates steadily declining and a growing aging population in most places around the world, economic, social and health challenges are becoming far more prevalent (Ogura & Jakovljevic, 2018). In England, between 2005 and 2014 the number of people aged 65 or over increased by more than 1.5 million. Moreover, the office for national statistics predicts that in forty-seven years roughly 26% of the total UK population will be over the age of 65 (Office for national statistics, 2019). A growing older population means a greater demand for health and care services.
It is well-established that the risk of developing a disability or chronic health conditions increases substantially with age. While it is expected that men and women over 65 years old will live several years in good health throughout their remaining lives, life expectancy is rising more rapidly than healthy-life expectancy. This means that more people are living into older age with higher levels of dependencies, comorbidity and social care needs (Kingston, Comas-Herrera, & Jagger, 2018). Consequently, government funding has not been able to keep up with the rapidly growing demands. Reports on the health and care of older people in England indicate that investments in health care is failing to keep up with these demographic changes. In addition, NHS social care spending has dropped drastically over the last decade. It is estimated that NHS spending must increase between 3% and 6% a year in order to match the cost of increasing demands. However, since 2010 and 2011 NHS funding has only risen by an average of 0.8% a year and it is predicted that in 2021 the NHS funding gap will reach up to thirty-billion pounds a year (Mortimer & Green, 2015). This is a pressing issue for current and future generations, there clearly needs to be a significant change to the funding of our health care system if we want people to experience a good quality of care henceforth.
Age is a major risk factor for dementia syndrome and is a common cause of disability and dependency among older people worldwide. Currently, 1 in 14 people over the age of 65 and 1 in 6 people over the age of 80 will have a diagnosis of dementia (Alzheimer’s Research UK, 2019; Alzheimer’s Society, 2019). Dementia is a progressive syndrome that effects multiple higher cortical functions of the brain. It can be caused by various underlying diseases and is usually characterised by behavioural changes and prominent memory impairments along with additional deficits in abstract reasoning, attention, executive functions, semantic knowledge, visuospatial abilities and language which can significantly affect social functioning (Salmon & Bondi 1999). There is a growing interest in clinical research towards the classification and detection of cognitive impairments linked to age-related neurodegenerative diseases. This interest is driven by the need to accurately detect early cognitive changes that may indicate the start of a progressive dementia, as an early diagnosis could result in more effective treatment and slow down or in some cases stop progression of the disease (Charvin, Hannequin, Rebaî & Snoussi, 2011).
Alzheimer’s Disease (AD) has been a focus of neuropsychological research for decades as it is the most common cause of dementia and makes up 60 to 70 per cent of cases (“Dementia”, 2019). Alzheimer’s disease is a degenerative disorder that causes widespread neuropathological changes in the brain (Salmon & Bondi, 2009). Research suggests that these changes occur due to abnormal fluctuations of beta-amyloid and tau proteins in the brain (Ittner and Götz, 2010). Beta-amyloid proteins aggregate between neurons forming plaques which can disrupt cell function. Whereas elevated levels of tau protein in the brain can result in tangles inside neurons blocking the neuron’s transport system and damaging the synaptic communication between neurons (Tapiola et al., 2009). Additionally, these changes can cause the cerebral cortex to degenerate which in turn leads to cerebral atrophy (Tapiola et al., 2009). Due to the progressive nature of AD, it is important that an accurate and timely diagnosis is made. Still, this is often difficult to do, and diagnosis is usually delayed due to the overlap of symptoms with other types of dementia (Karantzoulis & Galvin, 2011).
Subtle changes in memory can occur as part of normal aging. However, memory loss is a strong indicator of early signs of dementia and is required for a diagnosis of Alzheimer’s disease, which is why an accurate and timely neuropsychological test of memory deficits is an important part of detecting early dementia (Mendez, Mastri, Sung & Frey, 1992). Memory can be described as the process of encoding, storing and retrieving information that has been learned or experienced over time (Sherwood, 2012). The different categories of memory are long-term and short-term (working) memory. Short-term memory is limited in capacity and only lasts for a number of seconds or occasionally minutes. In contrast, long-term memory encodes information and stores it over a long period of time. Semantic memory and episodic memory are both thought to be types of long-term memory. Semantic memory is defined as the ability to recall generic factual knowledge about the world, while episodic memory involves the conscious recollection of specific events and previous experience (Sherwood, 2012). In Alzheimer’s disease, short-term memory is typically affected in the earliest stages while long-term memory loss is affected gradually as the disease progresses. Braak and Braak (1991) suggests that the earliest changes can be seen in the medial temporal lobe structure, critical for episodic memory. This is consistent with other behavioural, imaging and longitudinal studies showing that episodic memory can be vulnerable to the effects of aging and extremely disrupted in Alzheimer’s disease, it is also thought to be the first memory system to deteriorate in normal and pathological aging. (Tromp et al., 2015; Ritchie, Artero & Touchon, 2001; Salmon, 2000).
Neuropsychological assessment has been essential over the last few decades in the characterisation of dementia associated with Alzheimer’s disease. Clinic-based studies have found that those showing severe impairment in episodic memory (word list learning, story recall) were most likely to progress to Alzheimer’s disease. Moreover, deficits in semantic memory (naming and category fluency) and mental speed (time taken to complete Trials B test) were also found to be consistent with AD progression (Flicker, Feris & Reisberg, 1991; Tierney et al., 1996; Albert, Moss, Tanzi & Jones, 2001). A cross-sectional analyses study involving non-demented participant and elderly volunteers (aged 60 to 96) who either had dementia or were suspected of having dementia with mild changes in a few cognitive domains, found significant slowing on part B of the trail making test, with older age groups showing greatest changes overall. There was no significant difference on part A of the Trail making test (Rasmusson, Zonderman, Kawas & Resnick, 1998).
Lewy body dementia (LBD) is another common type of dementia characterised by the formation of abnormal deposits of alpha-synuclein protein, called Lewy bodies, throughout the brain (Kim, Kågedal & Halliday, 2014). LBD symptoms include visual hallucination, motor deficits (parkinsonism) and fluctuating cognition, these symptoms can often overlap with AD and Parkinson’s disease (PD). Additionally, Lewy bodies can be found in LBD as well as in PD, making it difficult to distinguish LBD from other subtypes of dementia, especially during early stages (Ferman & Boeve, 2007). Studies investigating the early stages of Lewy body dementia suggest that there are pronounced visuospatial, attentional and frontal executive impairments, while memory functions are typically less impaired compared to AD patients. Moreover, patients with Lewy bodies tend to perform better on tests of verbal and episodic memory but consistently do worse on visual memory compared to Alzheimer patients (Salmon et al., 1996).
A follow-up community-based study investigating the cognitive differences in more than a thousand dementia patients found that those with Lewy body pathology appeared to have less severe impairments compared to patients with mild to moderate Alzheimer’s disease on verbal memory and confrontation naming. They also found that patients with Lewy body pathology performed worse on tests of attention (WAIS-R Digit Span) and executive function (Trails B), which is consistent with previous findings showing that Lewy Body patients are typically more impaired in tests of executive function and attention (Johns et al., 2009; Gomperts, 2016; Cooper, 2005). Furthermore, there were no significant differences among groups in part A of the Trail Making Test, which implies that patients with Lewy body pathology were more impaired in divided attention and did not perform the task more slowly due to motor impairments (Kraybill et al., 2005).
Although memory loss is more prominent in early Alzheimer’s compared to early Lewy body dementia, memory difficulties may also be present in advance stages of Lewy body dementia in addition to its more characteristic effects visual perception, judgment and planning (Alzheimer’s association, 2019). However, movement symptoms are far more likely to be an important cause of disability in early Lewy body dementia rather than in Alzheimer’s disease (Alzheimer’s association, 2019). Johnson, Morris and Galvin (2005) investigated verbal, visuospatial deficits and rates of progression in three groups participants from the longitudinal cohort of the Alzheimer disease research at Washington University with confirmed diagnosis of Lewy body, mixed (LBD/AD) and pure Alzheimer’s disease. They found that patients with pure AD performed worse on verbal memory tests, while patients with Lewy body pathology performed worse on tests of visuospatial abilities. Their results also showed that both LBD and AD declined at similar rates. Similarly, a comparative study looking at verbal and non-verbal learning and recall in patients with Alzheimer’s and Lewy body dementia using the Hopkins Verbal-Learning Test-Revised (HVLT-R) and the Brief Visuospatial Memory Test-Revised (BVMT-R) found that Alzheimer’s patients performed significantly worse on learning and delayed recall of the HVLT-R, as well as on the delayed free recall of the BVMT-R. These studies support previous evidence showing that individuals with LBD show impairments on visuospatial abilities that extends into the domain of memory components (McLaughlin, Chang & Malloy, 2012).
There is some evidence indicating that patients with LBD progresses to dementia much faster compared to AD (Olichney et al., 1998), while in more severe cases of LBD, there is higher mortality rates (Oesterhus et al., 2014) and higher rates of nursing home admissions compared to AD patients (Rongve et al., 2014). However, a systematic review investigating cognitive decline in LBD compared to AD found that AD patients decline more rapidly in delayed recall compared to LBD patients. Similarly, on the Hopkins verbal learning test- revised (HVLT-R) recognition was found to have a more rapid decline in AD compared to LBD, while verbal fluency was found to decline much faster in LBD patients (Breitve et al., 2014). Although one criticism is that most studies in this review used the Mini-Mental State Examination test (MMSE) eitheralone or combined with other cognitive tests. The MMSE may not be an ideal measure of cognition when comparing LBD and AD as it is heavily based on memory and language and is therefore more sensitive to changes in AD rather than LBD. Furthermore, the MMSE is extremely susceptible to ceiling and floor effects (Galasko et al., 2000).
Cognitive difficulties including memory impairments can also be seen in non-demented patients (Bharambe & Larner, 2018). Functional cognitive impairment (also known as functional cognitive disorder) is a common diagnosis in memory clinics. Pennington et al. (2015) describes functional cognitive disorder (FMD) as a persistent subjective impairment where patients present cognitive difficulties in memory, alongside concentration and attention without any evidence of an underlying cognitive disorder, head trauma or medication side effects to account for their symptoms. Patients with subjective memory impairment are typically younger (under 60 years-old) than those with a neurological disorder and are more likely to attend a memory clinic alone (Bharambe & Larner, 2018). The cognitive difficulties experienced by those with FCI can become a focus for health anxiety, especially if there is a family history of dementia or if the person is aware or concerned about dementia (Pennington et al., 2015).
There is evidence to suggest that cognitive symptoms of FCI are not likely to progress into an organic impairment. A retrospective clinic-based study has shown that more than fifty percent of FCI patients referred to the clinic do not go on to receive a diagnosis of dementia or mild cognitive impairment, and that this has been a consistent observation over many years in a number of memory clinics (Larner, 2018). It is, however, often difficult to differentiate the cognitive symptoms of FCI from those with early neurodegeneration. Therefore, a careful follow-up is needed to examine whether there is progressive cognitive decline (Pennington et al., 2015). A recent study by Wakefield et al. (2018) explored distinctive neuropsychological profiles of functional memory disorder (FMD) and amnestic mild cognitive impairment (a-MCI), They indicate that there are clear differences between a-MCI and FMD patients on tests of memory (semantic fluency, verbal and non-verbal memory). It was also found that FMD patients did not differ from healthy controls on the memory tests. Furthermore, objective impairment was not found on the FMD population even when using a cognitive battery sensitive to subtle deficits caused by early neurodegenerative diseases. FCD is a common diagnosis in memory clinics, but it continues to be extremely under-studied and research exploring effective treatment is still scarce (Pennington et al., 2015).
Memory perfectionism is a characteristic of FCI as patients expect a high level of memory performance and fixate on minor lapses that would otherwise be considered normal. This fixation and anxiety around how well their memory is performing can sometimes have a significant impact in their everyday life which can cause a considerable amount of anxiety and distress (Pennington et al., 2015). The anxiety and stress around memory in FCI patients can also be an issue during neuropsychological testing. Some studies have shown that patients with functional cognitive disorders often rate their own cognitive abilities to be much worse than what is shown in the objective memory tests (Pennington et al., 2015). The test of memory malingering (TOMM) is a frequently used test for examining underperformance on neuropsychological tests as it is highly sensitive to malingering or exaggerations of memory symptoms and has been shown to be unaffected by affective state such as depression and anxiety (Rees, Tombaugh & Boulay, 2001). Performance validity has been well studied in dementia, with previous evidence showing that dementia patients tend to score relatively well on tests of malingering (Tombaugh, 1997), however, the same is not true for patients with subjective memory complaints (Pennington et al., 2015).
The aim of this study was to explore cognitive patterns in Alzheimer’s disease, Lewy body dementia and functional cognitive impairment by comparing memory performance on the Hopkins verbal learning test- revised (HVLT-R) and the Wechsler Memory Scale Logical Memory immediate and delayed recall, and performance on tests of attention and executive function using the Digit Span (forwards and backwards) and Trail Making Test (TMT).
It is hypothesised that AD patients will show significant lower scores on Logical Memory immediate recall (LMI) and delayed recall (LMII) compared to DLB and FCI patients. In addition, AD patients will also perform significantly worse than the LBD patients on the learning and delayed free recall scores of the HVLT-R. FCI patients will perform significantly better on all tests of memory compared to dementia patients, but will perform significantly worse on tests of attention compared AD and LBD patients. It is also hypothesised that LBD patients will show significantly lower scores on the Digit Span and trails B compared to AD and FCI.
Method
This was an archival research project sponsored by the University of Bristol in accordance with the North Bristol NHS Trust. Due to the nature of this study, consent and ethics application was not required. This study was based at the Brain Centre at Southmead Hospital where a retrospective search through patient files was made to select patients who had undergone a full neuropsychological battery. Final diagnosis was made by a consultant neurologist based on magnetic resonance imaging (MRI) scans, blood tests and neuropsychological reports. A search through the Brain centre’s database was made to draw neurological clinical data about each patient’s diagnosis. The relevant data was extracted and anonymised prior to statistical analysis.
Inclusion Criteria:
- Must have undergone a full neuropsychological battery.
- Must have a diagnosis of Alzheimer’s disease (AD), Lewy Body Dementia (LBD) or Functional Cognitive Impairment (FCI).
- Sufficient level of English language proficiency.
Exclusion Criteria:
- The Wechsler Adult Intelligence Scale-III (WAIS-III) and Wechsler Memory Scale (WMS-III).
- Brief individualised assessments e.g. Addenbrooke’s Cognitive Examination (ACE), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Mild Cognitive Impairment screen and Bespoke assessments.
- Unclear diagnosis.
- Visual and hearing impairments.
Patients
All selected patients had been administered a full neuropsychological test battery when attending the ReMemBr group cognitive clinic at North Bristol Trust between between 1st June 2011 – 1st June 2019. Medical notes of patients documented as having an unclear diagnosis were excluded. After exclusionary criterion was applied, 72 patients were suitable for final analysis. There was a total of 32 (44.4%) FCI patients, 27 (37.5%) AD patients, and 13 (18.1%) LBD patients. This reduction in sample size was primarily due to diagnosis type. Patients with a Parkinson’s disease dementia (PDD) diagnosis was included in the Lewy body dementia sample as there is clinical evidence demonstrating that they share similar pathologies, and that most PDD patients display core clinical diagnostic features of LBD (Jellinger, 2017; Friedman, 2018; McKeith and Mosimann, 2004).
Definition of functional cognitive impairment
Since an exact definition of functional cognitive impairment (FCI) is still up for debate, it is important to describe what this study means by the diagnostic term FCI. A definition by Pennington et al. (2015) was used in this case, where functional cognitive disorder (FCD) is defined as persistent cognitive complaints with no evidence of an underlying cognitive disorder, head trauma or medication side effects to account for their symptoms. FCI patients with psychiatric symptoms such as depression and anxiety were not excluded from final analysis, as those experiencing subjective memory difficulties due to a psychiatric disorder were under a different diagnosis on the medical database. The decision to include patients over the age of 70 was made based on the evidence that it is unlikely for patients with functional cognitive disorders to progress and later diagnosed with an organic condition such as Alzheimer’s disease (Larner, 2018; Carson, 2003).
Demographics
All patients were considered regardless of age and level of education, with age ranging from 29 to 89 (M = 63.39, SD =13.74), while years of education ranged from 5 to 23 (M =13.48, SD = 2.90). This sample comprised of 32 (44.4%) females and 40 (55.6%) males, of which 62 (86.1%) were right-handed, 8 (11.1%) were left-handed and 2 (2.8%) were ambidextrous.
Degree of Impairment
The Montreal Cognitive Assessment (MoCA) is one of the most frequently used scales to test cognitive impairment in Alzheimer’s and Lewy body disease. In total, 57 out of 72 patients were assessed using the MoCA. The total mean scores were calculated in order to compare the level of impairment between diagnosis. One point (+1) is usually added if patients have 12 years or less of formal education. In this sample, points for education were not added to the calculation. The table below demonstrates the demographics and mean scores on the MoCA for each diagnosis. There were no major differences between mean scores on the MoCA and type of diagnosis.
|
Diagnosis |
N |
Mean |
Std. Deviation |
Std. Error |
Minimum |
Maximum |
|
AD |
19 |
21.16 |
2.32 |
0.53 |
15 |
25 |
|
LBD |
9 |
21.44 |
2.81 |
1.27 |
17 |
28 |
|
FCI |
29 |
22.31 |
4.96 |
0.92 |
11 |
30 |
|
Total |
57 |
21.79 |
4.05 |
0.54 |
11 |
30 |
Table 1.
Descriptive of the total mean scores on the MoCA.
Performance Validity test
As previously mentioned, the test of memory malingering (TOMM) is commonly used in memory clinics to detect faking or exaggerations of symptoms (Tombaugh, 1996). The TOMM is a neuropsychological test designed to appear difficult but is in fact easy to complete, meaning that a fail would demonstrate poor effort. Falling the task is usually unrelated to demographic variables (age, education or gender) or the presence of a neurological conditions (Tombaugh, 1997). A recent study verified the use of the TOMM 1 with a cut off score of ≤ 43 as being the substandard effort (Rai & Erdodi, 2019). In this study, a total of 67 patients completed the TOMM 1. The mean score of the TOMM 1 was calculated to compare performance between each diagnosis. Although lower scores were more frequently seen in FCI patients, there were no significant differences reported between the mean scores.
Table 2.
Descriptive of the total mean scores on the TOMM 1 for each diagnosis.
|
TOMM 1 |
||||||
|
Diagnosis |
N |
Mean |
Std. Deviation |
Std. Error |
Minimum |
Maximum |
|
AD |
22 |
44.52 |
9.29 |
1.98 |
7 |
50 |
|
LBD |
13 |
47.62 |
4.01 |
1.11 |
36 |
50 |
|
FCI |
32 |
42.31 |
8.56 |
1.51 |
23 |
50 |
|
Total |
67 |
44.06 |
8.29 |
1.01 |
7 |
50 |
Assessments of Memory
The Hopkins Verbal Learning Test – Revised (HVLT-R)
The Hopkins Verbal Learning Test – Revised (Brandt & Benedict, 2001) is a well-established short test of verbal learning and memory with six alternative forms each containing 12 words belonging to three semantic categories (e.g. animals, precious gems, clothing), the semantic categories vary across the six forms. The test involves three learning trials (total recall), and a delayed recall trial administered approximately 20 minutes after the learning trials.
Logical Memory (WMS-IV; The Wechsler Memory Scale)
Logical memory I and II are subtests from the Wechsler Memory Scale IV where two short stories are presented verbally. It involves two conditions, the immediate condition (logical memory I) requires the examinee to retell each story from memory immediately after, whereas the delayed condition (logical memory II) measures long-term narrative memory with free recall and recognition tasks (Abikoff et al., 1987).
Assessments of attention and executive function
Trail Making Test A & B
The Trail making test (TMT) is a test of attention and set shifting consisting of two parts with 25 circles distributed on a sheet of paper. During part A of the test, circles are numbered 1 to 25 and examinees are asked to draw lines to connect the numbers in ascending order. In part B, the circles are labelled as numbers (1-13) and letters (A-L). Examinees are asked to draw lines to connect the circles in an ascending order, alternating between numbers to letters i.e. 1-A-2-B-3-C and so forth.
Digit Span Forwards and Backwards (WAIS-IV; The Wechsler Adult Intelligence Scale)
The Digit Span is a subtest from the Wechsler Adult Intelligence Scale – IV. It is a test of short-term memory; in that it measures an individual’s ability to store information for a short period of time. The Digit span Forwards involves rote learning and memory as well as attention, encoding and auditory processing. Digit Span backwards involves working memory, mental manipulation and visuospatial imaging.
Statistical Analysis
All statistical analyses were performed using the program packages SPSS 25.0 for windows. For statistical analysis, raw scores were used for HVLT-R, Digit Span and Trail Making Test, meaning that they were not corrected for age. Standard scores were used for logical memory 1 and 2 due to the inclusion of different versions of the test (young vs old), these scores were corrected for age. Sample demographics were established by analysing frequencies of types of diagnosis, handedness and gender. Analysis of age and years of education were made using descriptive statistics. Comparisons between types of diagnosis and memory tests (HVLT-R and logical memory) were made using a one-way ANOVA, with diagnosis as the factor with three levels (AD, LBD and FCI). The dependent variables were the cognitive measures HVLT-R, Logical memory 1 and 2, Digit Span and Trail Making Test (TMT) A and B. For descriptive statistics of cognitive tests, see appendix A.
Normality and homogeneity of variance were tested using levene’s test. A Tukey’s Post Hoc test was conducted in order to confirm where the differences occurred between groups. The Kruskal-Wallis test was used as a nonparametric alternative when assumptions on the one-way ANOVA were not met. A Pearson’s correlation was conducted to assess the relationship between age and memory test scores. Further analysis using Pearson’s correlation was conducted to assess the relationship between years of education and memory test scores. An alpha level of .05 was used for comparison analyses, while correlation was significant at the 0.01 level (2-tailed). Effects size estimates are reported using Partial Eta squared (ƞ2).
Results
Memory measures
Tests of homogeneity of variance found that for both HVLT-R and logical memory were found to be normally distributed and equal variances were assumed on tests of delayed recall based on results of Levene’s test (LM 2: F(2, 66) = 1.40, p = .253; HVLT-R Trial 4: F(2, 47) = .406, p = .070). Learning trials (1-3) on the HVLT-R were also tested and satisfied on Leverne’s F(2, 66) = 2.37, p = .101. The tests of homogeneity of variance was not significant on logical memory Immediate recall (LM 1) with Levene’s F(2, 47) = 4.38, p p = .097) were found among the three types of diagnosis. Therefore, a post hoc test was not necessary for logical memory 1.
A one-way ANOVA was conducted to examine differences between mean scores on the HVLT-R and logical memory 2. The results revealed a statistically significant difference between groups on learning trials F(2, 66) = 13.82, p
Post hoc analysis was conducted using Tukey HSD. The results showed that the mean scores for AD (M = 15.12, SD = 4.36) and LBD (M = 16.93, SD = 6.93) was significantly different from FCI (M = 22.77, SD = 5.95) on the HVLT-R learning trial. It was revealed that the mean score for AD (M = 2.98, SD = 3.01) and LBD (M = 5.00, SD = 4.10) was also significantly different from FCI (M = 7.83, SD = 2.89) on HVLT-R delayed recall and a statistically significant difference between AD (M = 5.11, SD =3.07) and FCI (M = 8.09, SD = 3.41) in logical memory delayed recall (LM 2). A significant difference was not found between AD and LBD on the HVLT-R and logical memory 2 (all p scores > .05).
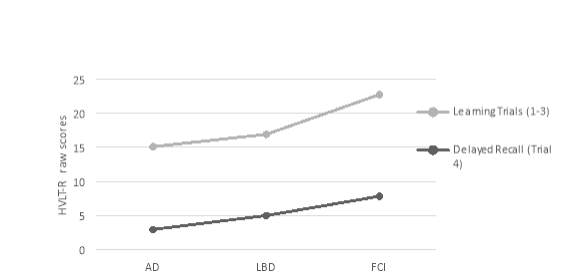
Figure 1. A graph illustrating the mean scores (raw scores) of each type of diagnosis on the HVLT-R learning trial and delayed recall. HVLT-R1-3raw = learning trials, HVLT-R4raw = delayed recall.
Diagnosis
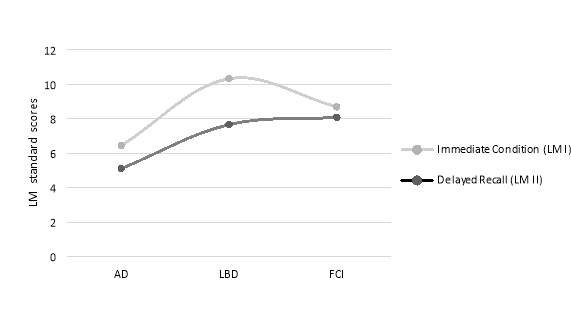
Figure 2. A graph depicting the types of diagnosis versus the mean scores on logical memory immidiate condition (LMI) and delayed recall (LMII). LMIss = immediate condition, LMIIss = delayed recall.
Diagnosis
Other cognitive measures
Tests of homogeneity of variance found that both Trails A and B were normally distributed, equal variances were assumed on Trails A based on results of Levene’s test F(2, 68) = 2.42, p = .097 and Trails B F(2, 68) = 1.79, p = .103. Digit span forwards and backwards was also found to be homogeneous based on Levene’s test F(2, 49) = .983, p = .38 and F(2, 49) = .177, p = .838.
A One-way ANOVA was used to analyse the relationship between diagnosis and cognitive test performance. A significant difference was found between groups on part A of the trail making test F( 2, 68) = 4.39, p = .016, ƞ2= .114 and part B F(2, 52) = 9.63, p = .000, ƞ2 = .270. There was no statistically significant difference between groups on the digit span forwards F(2, 49) = 1.43, p = .250, ƞ2 = .055 and backwards F(2, 49) = .043, p = .958, ƞ2 = .002.
Post hoc analysis using Tukey HSD procedure revealed significant differences between LBD (M = 84.40, SD = 54.81) and FCI (M = 43.51, SD = 32.15) on Trails A. There was also a statistically significant difference between AD (M =192.57, SD =89.47) and FCI (M = 104.59, SD = 55.28) on Trails B. There was no significant difference between AD and LBD in trails A and B (all p scores > .05).
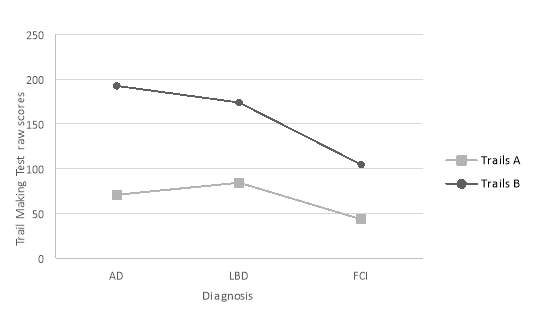
Figure 3. Graph illustrating the types of diagnosis (AD, LBD, FCI) and their mean scores (raw scores) on the Trail Making test A and B. TrailsAraw = Attention, TrailsBraw = Executive Function.
Correlation Analysis
A Pearson’s correlation analysis between age and cognitive test scores revealed that there was a moderate negative correlation between age and HVLT-R learning trials scores r = -.432, n = 69, p = .000 and HVLT-R delayed recall scores r = -.491, n = 69, p = .000. A moderate positive correlation was also found between age and Trails B r = 507, n = 55, p = .000. For scatter plots of correlations, see appendix B.
Further analysis of Pearson’s correlations between memory test scores and years of education were made. There was a weak positive correlation between years of education logical memory 2 (delayed recall) scores r = .392, n = 50, p = .005. A weak negative correlation was also observed in Trails B r = -.355, n = 54, p = .008. In conclusion, there were no statistically significant correlation between years of education and cognitive test performance.
Discussion
The purpose of this study was to explore cognitive patterns in Alzheimer’s disease, Lewy body dementia and functional cognitive impairment using neuropsychological tests including the Hopkins verbal learning test-revised (HVLT-R), Logical Memory 1 and 2, Digit Span (forwards and backwards) and the Trail Making test (A & B). As previously reported, the results from this study found that FCI patients scored significantly better than AD and LBD patients on the HVLT-R immediate and delayed recall test. There was also a significant difference in mean scores between FCI and AD in Logical Memory 2. Despite the subject memory complaints of FCI patients, there was no evidence of significant memory impairment in FCI compared to dementia patients. These findings support previous evidence indicating significant differences in neuropsychological test performance between dementia patients and FCI patients (Wakefield et al., 2018). Additionally, these findings also provide supporting evidence that memory difficulties experienced by FCI patients are not similar to memory deficits caused by an underlying neurodegenerative disorder, and that there may be other psychological factors that could possibly explain symptoms of FCI (Steinberg et al., 2015). Interestingly, a study found that therapy programs including stress management, cognitive behavioural therapy and mindfulness can help improve memory self-efficacy in FMD patients (Metternich, Schmidtke, Dykierek & Hüll, 2008).
Examinations of correlation between age and memory tests revealed that as age increased, the scores on memory tests decreased. A significant negative correlation was found between age and HVLT-R delayed recall scores. Overall, there was a moderate correlation between age and HVLT-R. These results are somewhat consistent with previous finding indicating a negatively correlation between age and scores on the HVLT-R in elderly patients (Duff, 2015). There was no significant correlation between years of education and HVLT-R scores, which is also consistent with other studies who found that years of education did not significantly affected HVLT-R scores in older adults (Vanderploeg et al., 2000). Furthermore, years of education did not seem to have an effect on cognitive performance overall. There was a correlation between scores on Trails B and age, it was found that as scores increased age also increased (see Appendix B for scatter plot). This is similar to previous findings indicating that the Trail Making Test is sensitive to age and years of education (Tombaugh, 2004). Despite there being a moderate correlation between variables, the results from this study suggests that there is a possibility that age could influence neuropsychological test performance.
On tests of attention, executive function and working memory, it was hypothesised that LBD patients will show significantly lower scores on the Digit Span and trails B compared to AD and FCI. However, no significant differences were found between groups on the Digit Span forwards and backwards. The results did show that FCI patients performed significantly worse on the attention test (Trails A) compared to LBD patients. It was also reported that FCI patients performed significantly worse compared to AD patients on the executive function test (Trails B). Studies comparing executive function and attention between FCI and dementia patients are particularly limited. Some studies have shown that higher levels of subjective memory complaints are associated with significantly poorer performance on tests of executive function and attentional set-shifting compared to those with lower levels of subjective complaints, they also noted that symptoms of depression, chronic stress and sleep problems are extremely likely to influence poor executive cognitive functioning in subjective memory disorders (Stenfors, Marklund, Magnusson Hanson, Theorell & Nilsson, 2013). Another study using healthy participants, reported differences in performance between FMD groups and healthy control groups in tests of attention and working memory. They noted that these differences could be caused by stressful environments, and that a calming testing environment may remove significant differences between FMD and healthy controls. They also argue that with cognitive speed and attention, everyday memory deficits experienced by FMD patients in real world environments could be caused by distractedness (Metternich et al., 2009).
Cognitive impairment in LBD patients has been reasonably well documented, especially when it is compared with AD and Parkinson’s disease patients (Noe et al., 2004; Metzler-Baddeley, 2007; Troster, 2008). The findings from this study showed no statistically significant differences between AD and LBD in cognitive tests. However, most studies investigating differences in neuropsychological test performance between AD and LBD seem to find an overall pattern indicating that LBD patients are more likely to develop earlier and more pronounce attentional, visuospatial and frontal-executive impairments, whereas AD patients are more likely to have significant memory impairments (McLaughlin, Chang & Malloy, 2012; Johnson, Morris and Galvin, 2005; Metzler-Baddeley, 2007; Salmon et al., 1996). A clinic-based study examining the neuropsychological test results of four-hundred and two AD and LBD patients found that AD patients performed significantly worse on memory tests, such as delayed recall and orientation on the MMSE and logical memory I and II. They also found that LBD patients performed significantly worse than AD patients on visual information processing on the Raven’s Coloured Progressive Matrices (RCPM), digit span backwards, pentagon copying on the MMSE and tests of working memory and attention (Kawai et al., 2013).
Neuroimaging studies have made important contributions towards the distinction of different types of dementia. Burton et al. (2008) reported a strong correlation between hippocampal atrophy and neurofibrillary tangles and amyloid-beta plaques found in Alzheimer’s. In addition, Chow et al. (2012) used magnetic reasoning imaging to demonstrate that although hippocampal atrophy is present in patients with LBD, it is relatively less severe compared to patients with AD, which is a strong indication that episodic memory abilities are generally more preserved in LBD compared to AD patients. Moreover, Mak, Su, Williams and O’Brien (2014) highlighted that one of the most characteristic positron emission tomography findings of LBD is the reduction of dopaminergic activity in the basal ganglia, which eventually leads to problems with movement and motor functions. They also observed that the medial temporal lobe (MTL) remains relatively preserved in LBD compared to AD, which may also explain preserved memory functions in LBD. It is important to note that a common limitation in neuroimaging studies is that they typically have a small sample size, and that larger studies may help confirm the utility of many imaging techniques. However, given the considerable neuropsychological and neuroimaging evidence demonstrating significant differences between AD and LBD patients, the results from this study should be treated with caution.
The present study had several limitations that are worth discussion. Firstly, the sample size was relatively small partly due to the exclusion criteria. There were also sample size differences between diagnosis with considerably less LBD patients than AD and FCI patients, which could have impacted the findings from this study. Secondly, missing data was also an issue as some patients who had undergone a full extensive neuropsychological battery missed or did not complete certain tests due to several different reasons. Thus, the total number of patients who had completed the tests chosen for this study varied throughout. The absence of data can reduce statistical power and representativeness of a sample, missing data can also cause bias in the estimation of parameters and obscure the analysis of the study (Kang, 2013).
Finally, this study is consistent with previous findings indicating that memory difficulties experienced by those with FCI might not be similar to the memory difficulties experience by those with a neurodegenerative disorder, and that poor performance on tests of attention and executive function may be caused by stressful testing environments, such as a memory clinic. The subjective memory complaints of FCI patients seems to be much worse than what it is usually found in objective measures (Pennington et al., 2015). These concerns may be due to various reasons depending on the individual. Fear of dementia can occur with increasing age and/or a family history of dementia, stressful life events and personality factors can also affect an individual’s attitude towards their memory function (Wakefield et al., 2017; Steinberg et al., 2015). It is important to note that due to the slow progression of many types of neurodegenerative diseases, follow-ups with FCI patients are essential in order to rule out completely the possibility of neurodegeneration (Pennington et al., 2015). The relationship between subjective cognitive impairment and psychiatric conditions, such as anxiety, depression and chronic stress should be an area of interest for future studies with FCI patients.
References
Alzheimer’s association (2019) Lewy Body Dementia. Retrieved from https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/lewy-body-dementia.
Abikoff, H., Alvir, J., Hong, G., Sukoff, R., Orazio, J., Solomon, S., & Saravay, S. (1987). Logical memory subtest of the wechsler memory scale: Age and education norms and alternate-form reliability of two scoring systems. Journal Of Clinical And Experimental Neuropsychology, 9(4), 435-448. doi: 10.1080/01688638708405063
Albert, M., Moss, M., Tanzi, R., & Jones, K. (2001). Preclinical prediction of AD using neuropsychological tests. Journal Of The International Neuropsychological Society, 7(5), 631-639. doi: 10.1017/s1355617701755105
Bharambe, V., & Larner, A. (2018). Functional cognitive disorders: memory clinic study. Progress In Neurology And Psychiatry, 22(3), 19-22. doi: 10.1002/pnp.509
Burton, E., Barber, R., Mukaetova-Ladinska, E., Robson, J., Perry, R., & Jaros, E. et al. (2008). Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain, 132(1), 195-203. doi: 10.1093/brain/awn298
Braak H, Braak E. (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82: 239–59.
Brandt, J., & Benedict, R. (2001). Hopkins verbal learning test-revised.
Breitve, M., Chwiszczuk, L., Hynninen, M., Rongve, A., Brønnick, K., Janvin, C., & Aarsland, D. (2014). A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer’s disease. Alzheimer’s Research & Therapy, 6(5-8). doi: 10.1186/s13195-014-0053-6
Carson, A. (2003). The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. Journal Of Neurology, Neurosurgery & Psychiatry, 74(7), 897-900. doi: 10.1136/jnnp.74.7.897
Charvin, H., Hannequin, D., Rebaî, M., & Snoussi, M. (2011). Early diagnosis of Alzheimer’s disease. Alzheimer’s & Dementia, 7(4), S266. doi: 10.1016/j.jalz.2011.05.761
Chow, N., Aarsland, D., Honarpisheh, H., Beyer, M., Somme, J., & Elashoff, D. et al. (2012). Comparing Hippocampal Atrophy in Alzheimer’s Dementia and Dementia with Lewy Bodies. Dementia And Geriatric Cognitive Disorders, 34(1), 44-50. doi: 10.1159/000339727
Cooper, S. (2005). The clinical assessment of the patient with early dementia. Journal Of Neurology, Neurosurgery & Psychiatry, 76(suppl_5), v15-v24. doi: 10.1136/jnnp.2005.081133
Dementia. (2019). Retrieved 3 September 2019, from https://www.who.int/news-room/fact-sheets/detail/dementia
Duff, K. (2015). Demographically corrected normative data for the Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised in an elderly sample. Applied Neuropsychology: Adult, 23(3), 179-185. doi: 10.1080/23279095.2015.1030019
Ferman, T. J., & Boeve, B. F. (2007). Dementia with Lewy bodies. Neurologic clinics, 25 (3), 741–vii. doi:10.1016/j.ncl.2007.03.001
Friedman, J. (2018). Dementia with Lewy Bodies and Parkinson Disease Dementia: It is the Same Disease. Parkinsonism & Related Disorders, 46, S6-S9. doi: 10.1016/j.parkreldis.2017.07.013
Galasko, D., Gould, R., Abramson, I. and Salmon, D. (2000). Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Statistics in Medicine, 19(1112), pp.1421-1432.
Gomperts, S. (2016). Lewy Body Dementias. CONTINUUM: Lifelong Learning In Neurology, 22(2, Dementia), 435-463. doi: 10.1212/con.0000000000000309
Ittner, L., & Götz, J. (2010). Amyloid-β and tau — a toxic pas de deux in Alzheimer’s disease. Nature Reviews Neuroscience, 12(2), 67-72. doi: 10.1038/nrn2967
Jellinger, K. (2017). Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. Journal Of Neural Transmission, 125(4), 615-650. doi: 10.1007/s00702-017-1821-9
Johns, E., Phillips, N., Belleville, S., Goupil, D., Babins, L., & Kelner, N. et al. (2009). Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology, 23(6), 765-777. doi: 10.1037/a0016792
Johnson, D., Morris, J., & Galvin, J. (2005). Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology, 65(8), 1232-1238. doi: 10.1212/01.wnl.0000180964.60708.c2
Kang, H. (2013). The prevention and handling of the missing data. Korean Journal Of Anesthesiology, 64(5), 402. doi: 10.4097/kjae.2013.64.5.402
Karantzoulis, S., & Galvin, J. (2011). Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Review Of Neurotherapeutics, 11(11), 1579-1591. doi: 10.1586/ern.11.155
Kawai, Y., Miura, R., Tsujimoto, M., Sakurai, T., Yamaoka, A., & Takeda, A. et al. (2013). Neuropsychological differentiation between Alzheimer’s disease and dementia with Lewy bodies in a memory clinic. Psychogeriatrics, 13(3), 157-163. doi: 10.1111/psyg.12019
Kim, W., Kågedal, K., & Halliday, G. (2014). Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Research & Therapy, 6(5-8). doi: 10.1186/s13195-014-0073-2
Kingston, A., Comas-Herrera, A., & Jagger, C. (2018). Forecasting the care needs of the older population in England over the next 20 years: estimates from the Population Ageing and Care Simulation (PACSim) modelling study. The Lancet Public Health, 3(9), e447-e455. doi: 10.1016/s2468-2667(18)30118-x
Kraybill, M. L., Larson, E. B., Tsuang, D. W., Teri, L., McCormick, W. C., Bowen, J. D., Cherrier, M. M. (2005). Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology, 64(12), 2069–2073. doi:10.1212/01.WNL.0000165987.89198.65
Larner AJ. (2018) Dementia in clinical practice: a neurological perspective. Pragmatic studies in the Cognitive Function Clinic (3rd edition). London: Springer.
Mak, E., Su, L., Williams, G., & O’Brien, J. (2014). Neuroimaging characteristics of dementia with Lewy bodies. Alzheimer’s Research & Therapy, 6(2), 18. doi: 10.1186/alzrt248
McLaughlin, N., Chang, A., & Malloy, P. (2012). Verbal and Nonverbal Learning and Recall in Dementia with Lewy Bodies and Alzheimer’s Disease. Applied Neuropsychology: Adult, 19(2), 86-89. doi: 10.1080/09084282.2011.643944
McKeith I. G., Mosimann U. P. (2004) Dementia with Lewy bodies and Parkinson’s disease. Parkinsonism and Related Disorders, 10, 15-18.
Mendez, M., Mastri, A., Sung, J., & Frey, W. (1992). Clinically Diagnosed Alzheimer Disease. Alzheimer Disease & Associated Disorders, 6(1), 35-43. doi: 10.1097/00002093-199205000-00004
Metternich, B., Schmidtke, K., Dykierek, P., & Hüll, M. (2008). A Pilot Group Therapy for Functional Memory Disorder. Psychotherapy And Psychosomatics, 77(4), 259-260. doi: 10.1159/000128166
Metternich, B., Schmidtke, K., & Hüll, M. (2009). How are memory complaints in functional memory disorder related to measures of affect, metamemory and cognition?. Journal Of Psychosomatic Research, 66(5), 435-444. doi: 10.1016/j.jpsychores.2008.07.005
Metzler-Baddeley C. (2007) A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex, 43(5), 583–600.
Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. (2004) Comparison of dementia with Lewy bodies to Alzheimer’s disease and Parkinson’s disease with dementia. Mov Disord, 19(1), 60–7.
Oesterhus R, Soennesyn H, Rongve A, Ballard C, Aarsland D, Vossius C. Long-term mortality in a cohort of home-dwelling elderly with mild Alzheimer’s disease and Lewy body dementia. Dement Geriatr Cogn Disord. 2014;8:161–169. doi: 10.1159/000358051.
Office for national statistics. (2019). Living longer: how our population is changing and why it matters.
Ogura, S., & Jakovljevic, M. (2018). Editorial: Global Population Aging – Health Care, Social and Economic Consequences. Frontiers In Public Health, 6. doi: 10.3389/fpubh.2018.00335
Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. (1998). Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology 51, 351–357. doi: 10.1212/WNL.51.2.351.
Rai, J., & Erdodi, L. (2019). Impact of criterion measures on the classification accuracy of TOMM-1. Applied Neuropsychology: Adult, 1-12. doi: 10.1080/23279095.2019.1613994
Rasmusson, X., Zonderman, A., Kawas, C., & Resnick, S. (1998). Effects of Age and Dementia on the Trail Making Test. The Clinical Neuropsychologist, 12(2), 169-178. doi: 10.1076/clin.12.2.169.2005
Rees, L., Tombaugh, T., & Boulay, L. (2001). Depression and the Test of Memory Malingering. Archives Of Clinical Neuropsychology, 16(5), 501-506. doi: 10.1093/arclin/16.5.501
Ritchie, K., Artero, S. & Touchon, J. (2001). Classification criteria for mild cognitive impairment—a population-based validation study. Neurology 56, 37–42.
Rongve A, Vossius C, Nore S, Testad I, Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer’s dementia. Int J Geriat Psychiatry. 2014;29:392–398. doi: 10.1002/gps.4015.
Salmon, D. P., & Bondi, M. W. (2009). Neuropsychological assessment of dementia. Annual review of psychology, 60, 257–282.
Salmon DP, Bondi MW (1999). Neuropsychology of Alzheimer’s disease. Alzheimer Disease. 2nd Philadelphia, PA: Lippincott: Williams & Wilkens; pp. 39–56.
Salmon, D., Galasko, D., Hansen, L., Masliah, E., Butters, N., Thal, L., & Katzman, R. (1996). Neuropsychological Deficits Associated with Diffuse Lewy Body Disease. Brain And Cognition, 31(2), 148-165. doi: 10.1006/brcg.1996.0039
Sherwood, L. (2012). Human physiology. CA: Wadsworth Publishing Co Inc. The cerebral cortex, 5(4), 70-73.
Steinberg, S., Negash, S., Sammel, M., Bogner, H., Harel, B., Livney, M., McCoubrey, H., Wolk, D., Kling, M. and Arnold, S. (2013). Subjective Memory Complaints, Cognitive Performance, and Psychological Factors in Healthy Older Adults. American Journal of Alzheimer’s Disease & Other Dementiasr, 28(8), pp.776-783.
Stenfors, C., Marklund, P., Magnusson Hanson, L., Theorell, T., & Nilsson, L. (2013). Subjective Cognitive Complaints and the Role of Executive Cognitive Functioning in the Working Population: A Case-Control Study. Plos ONE, 8(12), e83351. doi: 10.1371/journal.pone.0083351
Tapiola, T., Alafuzoff, I., Herukka, S., Parkkinen, L., Hartikainen, P., Soininen, H., & Pirttilä, T. (2009). Cerebrospinal Fluid β-Amyloid 42 and Tau Proteins as Biomarkers of Alzheimer-Type Pathologic Changes in the Brain. Archives Of Neurology, 66(3). doi: 10.1001/archneurol.2008.596
Tierney, M., Szalai, J., Snow, W., Fisher, R., Nores, A., & Nadon, G. et al. (1996). Prediction of probable Alzheimer’s disease in memory-impaired patients: A prospective longitudinal study. Neurology, 46(3), 661-665. doi: 10.1212/wnl.46.3.661
Tombaugh, T. (1997). The Test of Memory Malingering (TOMM): Normative data from cognitively intact and cognitively impaired individuals. Psychological Assessment, 9(3), 260-268. doi: 10.1037//1040-3590.9.3.260
Tombaugh, T. (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives Of Clinical Neuropsychology, 19(2), 203-214. doi: 10.1016/s0887-6177(03)00039-8
Troster A. I. (2008) Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev., 18(1), 103–19.
Tromp, D., Dufour, A., Lithfous, S., Pebayle, T., & Després, O. (2015). Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Research Reviews, 24, 232-262. doi: 10.1016/j.arr.2015.08.006
van der Flier WM., Scheltens P. (2005) Epidemiology and risk factors of dementia Journal of Neurology. Neurosurgery & Psychiatry, 76.
Vanderploeg, R., Schinka, J., Jones, T., Small, B., Borenstein Graves, A., & Mortimer, J. (2000). Elderly Norms for the Hopkins Verbal Learning Test-Revised. The Clinical Neuropsychologist, 14(3), 318-324. doi: 10.1076/1385-4046(200008)14:3;1-p;ft318
Wakefield, S., Blackburn, D., Harkness, K., Khan, A., Reuber, M., & Venneri, A. (2017). Distinctive neuropsychological profiles differentiate patients with functional memory disorder from patients with amnestic-mild cognitive impairment. Acta Neuropsychiatrica, 30(2), 90-96. doi: 10.1017/neu.2017.2
Appendix A
Tables displaying descriptive statistics of tests from the one-way ANOVA.
|
Table 3. Descriptive statistics of Hopkins Verbal Learning Test – Revised using raw scores. |
|||||||||
|
N |
Mean |
Std. Deviation |
Std. Error |
95% Confidence Interval for Mean |
Minimum |
Maximum |
|||
|
Lower Bound |
Upper Bound |
||||||||
|
HVLT-R trials 1-3 |
AD |
26 |
15.12 |
4.357 |
.855 |
13.36 |
16.88 |
7 |
24 |
|
LBD |
13 |
16.92 |
6.934 |
1.923 |
12.73 |
21.11 |
8 |
31 |
|
|
FCI |
30 |
22.77 |
5.952 |
1.087 |
20.54 |
24.99 |
13 |
33 |
|
|
Total |
69 |
18.78 |
6.586 |
.793 |
17.20 |
20.36 |
7 |
33 |
|
|
HVLT-R trial 4 |
AD |
26 |
2.96 |
3.013 |
.591 |
1.74 |
4.18 |
0 |
9 |
|
LBD |
13 |
5.00 |
4.103 |
1.138 |
2.52 |
7.48 |
0 |
12 |
|
|
FCI |
30 |
7.83 |
2.890 |
.528 |
6.75 |
8.91 |
0 |
12 |
|
|
Total |
69 |
5.46 |
3.845 |
.463 |
4.54 |
6.39 |
0 |
12 |
|
|
Table 4. Descriptive Statistics of Logical Memory I & II (WMS-IV) using standard scores. |
|||||||||
|
N |
Mean |
Std. Deviation |
Std. Error |
95% Confidence Interval for Mean |
Minimum |
Maximum |
|||
|
Lower Bound |
Upper Bound |
||||||||
|
LM 1 |
AD |
18 |
6.44 |
2.995 |
.706 |
4.96 |
7.93 |
1 |
11 |
|
LBD |
9 |
10.33 |
11.587 |
3.862 |
1.43 |
19.24 |
2 |
40 |
|
|
FCI |
23 |
8.70 |
3.336 |
.696 |
7.25 |
10.14 |
2 |
13 |
|
|
Total |
50 |
8.18 |
5.667 |
.801 |
6.57 |
9.79 |
1 |
40 |
|
|
LM 2 |
AD |
18 |
5.11 |
3.066 |
.723 |
3.59 |
6.64 |
1 |
11 |
|
LBD |
9 |
7.67 |
3.606 |
1.202 |
4.90 |
10.44 |
3 |
14 |
|
|
FCI |
23 |
8.09 |
3.410 |
.711 |
6.61 |
9.56 |
2 |
13 |
|
|
Total |
50 |
6.94 |
3.542 |
.501 |
5.93 |
7.95 |
1 |
14 |
|
|
Table 5. Descriptive statistics for Trail Making Tests (TMT) A & B using raw scores. |
|||||||||
|
N |
Mean |
Std. Deviation |
Std. Error |
95% Confidence Interval for Mean |
Minimum |
Maximum |
|||
|
Lower Bound |
Upper Bound |
||||||||
|
TMT part A |
AD |
27 |
70.86 |
56.15 |
10.81 |
48.64 |
93.07 |
25.00 |
333.00 |
|
LBD |
12 |
84.40 |
54.81 |
15.82 |
49.58 |
119.23 |
27.00 |
183.39 |
|
|
FCI |
32 |
43.51 |
32.15 |
5.68 |
31.91 |
55.09 |
10.00 |
153.00 |
|
|
Total |
71 |
60.82 |
48.71 |
5.78 |
49.29 |
72.35 |
10.00 |
333.00 |
|
|
TMT part B |
AD |
21 |
192.57 |
89.47 |
19.52 |
151.85 |
233.29 |
88 |
456 |
|
LBD |
5 |
174.00 |
73.75 |
32.98 |
82.43 |
265.57 |
121 |
300 |
|
|
FCI |
29 |
104.59 |
55.28 |
10.27 |
83.56 |
125.62 |
48 |
340 |
|
|
Total |
55 |
144.49 |
82.38 |
11.11 |
122.22 |
166.76 |
48 |
456 |
|
|
Table 6. Descriptive statistics for the Digit Span Forward and Backwards using raw scores. |
|||||||||||
|
N |
Mean |
Std. Deviation |
Std. Error |
95% Confidence Interval for Mean Lower Upper Bound Bound |
Minimum |
Maximum |
|||||
|
DS-F |
AD |
18 |
9.50 |
2.229 |
.525 |
8.39 |
10.61 |
6 |
14 |
||
|
LBD |
9 |
8.89 |
2.147 |
.716 |
7.24 |
10.54 |
5 |
12 |
|||
|
FCI |
25 |
8.16 |
2.925 |
.585 |
6.95 |
9.37 |
3 |
14 |
|||
|
Total |
52 |
8.75 |
2.604 |
.361 |
8.03 |
9.47 |
3 |
14 |
|||
|
DS-B |
AD |
18 |
6.39 |
2.227 |
.525 |
5.28 |
7.50 |
3 |
11 |
||
|
LBD |
9 |
6.67 |
2.449 |
.816 |
4.78 |
8.55 |
2 |
11 |
|||
|
FCI |
25 |
6.48 |
2.365 |
.473 |
5.50 |
7.46 |
3 |
12 |
|||
|
Total |
52 |
6.48 |
2.288 |
.317 |
5.84 |
7.12 |
2 |
12 |
|||
Appendix B
Simple scatter graphs with line of best fit showing correlations between age/years of education and cognitive tests.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Psychology"
Psychology is the study of human behaviour and the mind, taking into account external factors, experiences, social influences and other factors. Psychologists set out to understand the mind of humans, exploring how different factors can contribute to behaviour, thoughts, and feelings.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




