A Conceptual Model for Symptom Management
Info: 8702 words (35 pages) Dissertation
Published: 10th Dec 2019
Tagged: Medicine
CHAPTER 1: INTRODUCTION AND PROBLEM STATEMENT
Fibromyalgia: clinical and social construct
Peripheral and Central Nervous System Mechanisms of Pain
Peripheral and Central Nervous System Mechanisms of Pain in people diagnosed with Fibromyalgia
Polysomnographic registry of wakefulness and sleep
The construct of poor sleep quality in Fibromyalgia: the chicken and egg paradox
Sleep and Fibromyalgia: objective and subjective alterations
“It is all in their body” vs “It is all in their heads”: a biopsychosocial perspective of FM
A Conceptual Model for Symptom Management
The UCSF Symptom Management Model
A UCSF Symptom Management Model based intervention for women diagnosed with FM
CHAPTER 1: INTRODUCTION AND PROBLEM STATEMENT
Fibromyalgia (FM) is a chronic health condition that represents one of the most common causes of generalized musculoskeletal pain in the adult population (1) and it is currently conceived as a syndrome instead of a discrete disease as its etiology and cure treatment is still an issue of scientific discussion (2).
This syndrome affects up to 2.10% of people worldwide and 2.4% of the general population over 20 years of age in Spain, which in absolute numbers translates into about 1,900,000 people currently diagnosed with this syndrome. Regarding gender, the percentages are 4.2% for women and 0.2% for men, corresponding to a ratio of 21: 1 respectively (3,4).
Historically, the earliest references to pain of rheumatic origin date back more than 400 years (5) but it was not until 1906 when the British neurologist William Gowers described a new form of muscular rheumatism (6): “We are thus compelled to regard lumbago in particular, and muscular rheumatism in general, as a form of inflammation of the fibrous tissue of the muscles…we may conveniently follow the analogy of ‘cellulitis’ and term it ‘fibrositis”. This theory of inflammation was supported by the pathologist Ralph Stockman (7), in whose research he extracted and analyzed tissue samples from tender points of patients with muscular rheumatism, concluding that there was an evident inflammation and degeneration of the connective tissue. This research contributed to the acceptance and generalization of the term “fibrositis”, which was used during the following 72 years. However, this research generated a subsequent controversy since other authors could not observe the alleged lesions previously described (5). In fact, a Mayo Clinic researcher (8) re-examined the samples taken by Stockman, concluding that the tissue could be considered completely normal. Given the lack of scientific research demonstrating an actual inflammation at muscular level, many authors suggested that fibrositis was a misguided way to name this syndrome. Nevertheless, it was not until 1976 when Kahler Hench proposed the term fibromyalgia (FM) (fibro = fibrous tissue, myo = muscle and algia = pain) that the need to establishing a validated definition of this health condition emerged (9,10). Subsequently, the American College of Rheumatology (ACR) defined FM as a (11): “syndrome associated with generalized pain and multiple painful regions, particularly in the axial skeleton […] sleep disturbances, fatigue, and stiffness are the central symptoms of fibromyalgia […] other symptoms, such as anxiety, irritable bowel syndrome, modulating factors etc, are less common” and a standardized diagnostic criteria were developed.
Although in its own definition other symptoms besides pain were highlighted, the first diagnostic tool developed by the ACR was focused on the evaluation of this symptom and the palpation of certain sensitive points. Consequently, Crofford and Clauw (12) considered that the assessment of sensitive points supports excessively the inflammatory theory of FM. They also stated that, in the development of the 1990 diagnostic criteria, the evident alterations at the Central Nervous System in FM patients, which were directly linked to an inadequate processing of the nociceptive stimuli, were not taken into account. In addition, these criteria did not evaluate symptoms as prevalent in FM as sleep disturbances, fatigue or cognitive problems, among others. Despite the latter, the ACR did not develop new diagnostic criteria until 2010 (13).
People diagnosed with FM show an increase in the use of health services and, in terms of medical consultation, represents approximately the 5,7% of the total in the primary care setting and the 20% in the secondary or specialized care setting (3). Likewise, FM is one of the health conditions that causes greater total economic costs in Spain, being at the same level as chronic low back pain or Alzheimer’s disease. In relation to the group of rheumatic ailments, FM represents approximately the same economic burden as rheumatoid arthritis and much more than osteoarthritis, although these are much more prevalent pathologies (14). The Spanish Society of Pain (15) points out that, in Spain, each patient with FM generates a cost of approximately 10,000€ per year, assuming a total expenditure of 19,000 million euros per year.
This syndrome is one of the most common causes of generalized musculoskeletal pain in adults (1) and poor sleep quality is a prominent factor that is reported by up to 99% of these patients (16). Initially, it was thought that the sleep alterations that are present in FM patients were a consequence of pain but, since Moldofsky and Scarisbrick (17) showed that sleep deprivation induced FM-like symptoms in a sample of healthy participants, the potential role of sleep disorders as a cause chronic pain was recognized. Furthermore, in studies that included people diagnosed with FM, sleep disorders not only can predict exacerbation in pain (18) but also increase fatigue, negative mood and daytime sleepiness as well as affect executive and cognitive functions (19).
Bearing in mind the latter and that the pharmacological approaches have shown only modest symptomatic improvement (20), the development of new non-pharmacological strategies that integrate both pain and sleep, are key for the symptom management of people diagnosed with FM.
The biopsychosocial model points out the relevance of patient expectations and perceptions of symptoms and even the meaning that they attribute to them as an important contributor of a behavioral change which, in turn, could lead to a worsening of symptoms (21). So that, UCSF Revised Symptom Management Model provides direction for the development of a symptom management intervention for people diagnosed with FM in the present research.
Problem statement
…
CHAPTER 2: LITERATURE REVIEW
This chapter aims to provide an overview of FM not only from a clinical perspective but also including the social construct of this health condition. The current scientific evidence regarding the pathophysiology of FM, the alterations in the pain neuromatrix and the existent relationship between sleep alterations and FM symptoms were reviewed and summarized. Furthermore, as a result of the literature review, two systematic reviews were carried out and the outcomes are also presented and discussed in this section.
The chapter concludes with the description of both the Biopsychosocial Model, which is the conceptual framework of the present thesis and the UCSF Symptom Management Model that provides direction for the development of the interventional part of the research project.
Fibromyalgia: clinical and social construct
ICD-10 and ICF classification, diagnostic (how long is to the diagnostic process and its implications in terms of medical consultation and costs), overtreatment, sick leaves, economic compensations…
The social perspective of FM from the health professionals to general population…introduction of how the biomedical model has driven this social perspective and stigmatized this patients…
Etiology and Pathogenesis
….
Turk and Okifuji (22) suggested that FM symptoms could be described as a disturbance in the processing of information due to a dysregulation in the stress-response system. This hypothesis may provide an explanation for justifying disability, anxiety, fatigue, stiffness, depression and sleep disturbances in patients with FM. This model represents a hypothetical process by which a person may be at risk of developing FM. Thus, a stressor enables a physiological and psychological response, which in turn is mediated by a biological predisposition (e.g. genetic information) or based on experience or on environmental factors (e.g. previous learning). This process is usually self-corrected, however, in some people this process of self-correction may be inhibited because of predisposing (e.g. reactivity of central nervous system or dysfunctional thoughts) or environmental factors (e.g. strength and number of stressors at a given moment or level of social support). Time and the interaction of these two groups of factors, result in an altered regulation of the CNS (e.g. weakened reaction of hypothalamic-pituitary-adrenal axis), serotonin deficiency and cognitive and behavioral (e.g. cognitive errors) maladaptive responses. Therefore, a new and altered state of normalcy is created (predisposing factors that interact with the stressors are amended), further reinforcing the altered regulation of the system.
As a result of repeated activation of this altered regulatory system, these people could develop a set of responses (also called “set of answers of predisposition to FS”) that puts them at risk of having FM. Altered regulation of CNS reactions to stress and psychosocial adaptations results in increased attention to sensory information, negative mood, conception of being chronically ill (e.g. lack of control over the situation and catastrophic thoughts), reduction in activity and altered psychophysiological reaction. In addition, stressful situations, such as lights, noise and changing weather increased symptoms in people with FM (22).
Al final del apartado introducir el tema de las alteraciones en el “pain neuromatrix” y en el sueño, así como la interrelación entre ambos.
Peripheral and Central Nervous System Mechanisms of Pain
As reflected in the definition of pain by the International Association for the Study of Pain (23): “pain is an unpleasant sensory and emotional experience arising from actual or potential tissue damage, or described in terms of such damage”, psychological and social factors are involved in the pain experience, suggesting that pain is a sensation that goes beyond tissue damage.
Nociception is given by peripheral mechanisms activated by tissue damage, which may result in decreased nociceptor thresholds causing painful responses against low intensity stimuli (hyperalgesia), disappearing as the tissue damage is resolved. Therefore, these painful responses are an alarm and a transient process (24,25). Under these physiological conditions, nociception starts in nociceptors localized in visceral or somatic tissues. There are two main types of nociceptor terminals, low threshold nociceptors that conduct impulses through A-delta fibers (thinly myelinated) and high threshold nociceptors called C-fibers (unmyelinated). These A-delta and C fibers transform chemical, thermal or mechanical stimuli in electrical stimuli and make synapses, within the dorsal horn of the spinal cord, with second order neurons or interneurons. If the peripheral stimulus is sufficiently intense it causes an action potential, which through second order neurons, spreads to the Central Nervous System (CNS) areas such as the thalamus, Anterior Cingulate Cortex (ACC), Insular Cortex and Somatosensory Cortex (26,27).
From the CNS are launched descending pathways arriving to the dorsal horn of the spinal cord, releasing endogenous inhibitory substances, basically 5-HT (serotonin), opioids, norepinephrine and GABA. These inhibitory substances act by modulating the stimuli transmission, on the one hand by reducing the release of excitatory neurotransmitters and, on the other hand, hyperpolarizing the membrane of the postsynaptic neurons avoiding the binding of excitatory neurotransmitters with its respective receptors (28).
Finally, it is necessary to understand that not all nociceptive signals lead to pain and not all pain sensations are produced by nociception (27,29).
…
Peripheral and Central Nervous System Mechanisms of Pain in people diagnosed with Fibromyalgia
There is no scientific evidence demonstrating that the cause of pain in patients with FM is caused by peripheral nociception because of the absence of tissue damage or structural abnormalities. This means that, pain in this case does not have a protective function, suggesting that central sensitization could be involved in widespread pain and the exaggerated pain reaction seen in FM (30). Additionally, pain in FM does not have a specific body location hinting that the CNS is hyperexcitable (25,31). Central sensitization is defined as: ‘‘an augmentation of responsiveness of central pain-signalling neurons to input from low-threshold mechanoreceptors’’ (32). Because of this augmentation of responsiveness, glutamate binds not only to AMPA receptors, but also to NMDA receptors which are blocked by magnesium in physiological conditions. Thus, when CNS is sensitized, the removal of magnesium activates NMDA receptors allowing the influx of calcium, which activates NOS, PK-C and COX. This contributes not only to depolarize postsynaptic neurons, but also to generate a series of intracellular changes which amplify the nociceptive signal (33).
To evaluate if FM patients show alterations in spinal cord neurons, Banic and colleagues (34) applied, at random intervals of time, an electrical stimulation to assess the nociceptive withdrawal reflex given by the excitability state of spinal cord neurons. The authors measured the latency of EMG responses and the outcomes showed, clearly and objectively, the sensitization of spinal cord neurons in FM patients due to the exaggerated pain response to low intensity (hyperalgesia) or innocuous (allodynia) peripheral stimuli, partially providing a scientific explanation of pain without evident tissue damage.
Other authors (35,36)sustained the importance of psychological and behavioral aspects in central sensitization mechanisms providing an explanation of why this process can be developed by some persons but not by others. There is a large amount of scientific evidence showing, via neuroimaging techniques, significant differences in rCBF between fibromyalgic and healthy people in several brain structures involved in the processing and descending modulation of pain. In addition, the connection between areas of the midbrain related with mood, anxiety, fear and autonomic responses, and pathways that are activated by painful stimuli has been demonstrated. Low rCBF levels were observed in the caudate nucleus and in thalamic regions which are also related with affective cognition, cognitive functioning, personality and behaviors. High rCBF levels were noted on somatosensory cortex correlating these changes with the alteration and inhibition of pain modulation (37–39). There has also been observed that the ACC, which is a part of the limbic system, is extensively activated in people with chronic pain demonstrating an important implication of affective-motivational aspects in chronic painful experiences. An increased activity in PFC is noted in patients with chronic pain and this brain area is associated with the memory and cognitive-evaluative aspects of pain (36,39).
It remains unclear if behavioral and psychological aspects are involved in the development or maintenance of central sensitization but it is known that depression, dissatisfaction, catastrophic thoughts, anxiety and anger increased pain experience due to an abnormal activation of limbic structures (22). The fact that FM patients show alterations in these central nerve structures does not imply that these changes are caused by psychosocial aspects but it is noteworthy that these brain areas are related both with affective cognitions and pain processing, being possible that the dysregulation in one can affect the other and this, could produce alterations in pain sensations.
Phenomenology and Architecture of Sleep
Sleep is a complex behavior that, together with wakefulness, integrate the so-called wake-sleep cycle determined by its circadian rhythmicity and that results from the conjugated activity of encephalic regions located at brainstem, diencephalic and cortical levels (40).
Sleep is characterized by a decrease in the perception and response to the environment as well as in the motor activity. It is typically accompanied by a stereotyped posture and, unlike other altered states of consciousness, it is a self-regulated and rapidly reversible process (40–42). There are two well-defined types of sleep, slow-wave or non-rapid eye movement sleep (NREM) and rapid eye movement sleep (REM) or paradoxical sleep (40,43). These phases, as well as wakefulness and the transition from wakefulness to sleep, can be defined in terms of electrophysiological signs that are detected by polysomnography (PSG). PSG is considered the gold standard technique for the simultaneous recording of brain, muscular and ocular activity, measured with electroencephalogram (EEG), electromyogram (EMG) and electrooculogram (EOG), respectively (41,44).
Polysomnographic registry of wakefulness and sleep
In waking state with open eyes, brain activity is characterized by a desynchronized electroencephalographic record of high frequency waves, between 14-40 Hz, and low amplitude, called β and γ waves. It is considered that the low amplitude is due to a lack of synchrony that reflects the difference in the processing time of the motor, perceptual and cognitive functions (41). High muscle tone and rapid or saccadic eye movements, reading movements, eye reflexes, and blinking related to normal visual activity are also recorded (44,45). In a waking state with closed eyes, also called a quiet wake, an alpha wave is described, with a frequency of 8-13 Hz, a decrease in muscle tone, and slow and possibly asynchronous eye movements. It is considered that this phase precedes NREM sleep and therefore would be the transition phase from waking to sleep (41,42,44).
NREM sleep is characterized by a decrease in general physiological activity manifested by a reduction of various parameters such as blood pressure, heart and respiratory rate, body temperature and basal metabolic rate. It is also accompanied by a marked decrease in muscle tone and slow as well as poorly conjugated eye movements (43,44). NREM sleep is conventionally divided into four phases (41–44):
- In the first phase (N1), although the conscious perception of the environment begins to decrease, there is a great susceptibility to wake up to any stimulus, which is why this phase is described as “light sleep”. The α waves are replaced by slower oscillations that predominate in a range of 4-7 Hz characteristic of the θ rhythm and that reflect a greater synchrony at EEG level.
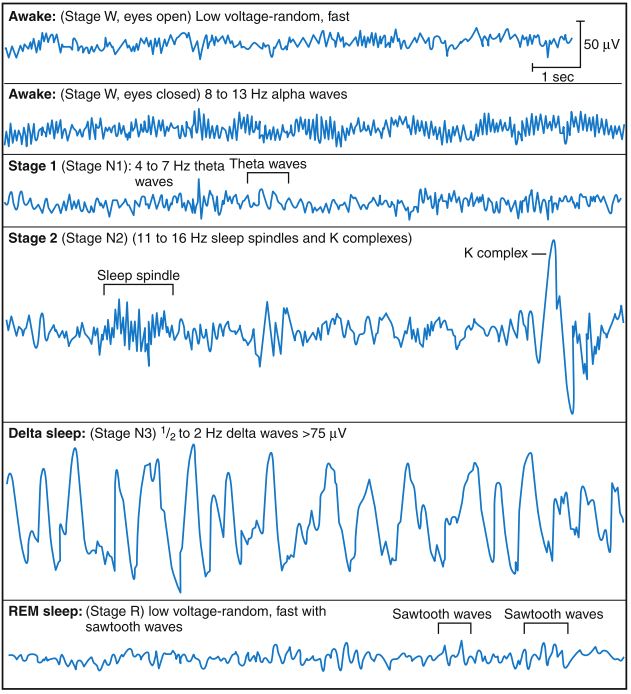 During phase 2 (N2) the perception of the environment completely disappears, increasing the threshold of activation and therefore a more intense stimulus is needed to wake up. As a result of the synchronization of neural groups at the thalamic level, bursts of fast waves known as sleep spindles occasionally appear with an activity of 11-16 Hz and a duration of approximately 1.5 seconds. K-complexes are also recorded in the EEG whose origin is still unknown. The eye movements are further slowed down and at the muscular level, periods of spontaneous activity are combined with periods of relaxation.
During phase 2 (N2) the perception of the environment completely disappears, increasing the threshold of activation and therefore a more intense stimulus is needed to wake up. As a result of the synchronization of neural groups at the thalamic level, bursts of fast waves known as sleep spindles occasionally appear with an activity of 11-16 Hz and a duration of approximately 1.5 seconds. K-complexes are also recorded in the EEG whose origin is still unknown. The eye movements are further slowed down and at the muscular level, periods of spontaneous activity are combined with periods of relaxation.
Figure X. Electroencephalographic characterization of the waking and sleeping states (41)
- Phases 3 and 4 (N3-4), together called slow wave sleep or delta sleep, are characterized by the presence of δ brain waves between 1 and 2 Hz. In these phases, considered the deepest of the NREM sleep, the physiological variables reach their lowest values, there are hardly any ocular movements and the muscle tone is minimal.
During REM sleep the brain waves are fast and desynchronized, similar to the α-rhythm registered in the wake with closed eyes (44). It is considered that this brain activity is associated with being dreaming because about 80% of the individuals who wake up in the REM phase report a vivid memory of the dream (42). Unlike NREM sleep, at this stage, the physiological variables become unstable, breathing becomes more rapid, irregular and superficial, and there is also an increase and variability of heart rate and blood pressure (43). Abundant rapid eye movements appear phasically, and the voluntary musculature is temporarily paralyzed but interrupted by small random fasciculations (42).
Sleep Architecture
During sleep, it is observed a pattern of alternation between NREM and REM sleep which is defined as “sleep architecture”. Normally sleep begins with N1 NREM and becomes deeper as N2 and N3-4 appear, followed by a brief first episode of REM sleep. Then, the NREM and REM phases will alternate in a predictable pattern of cycles that will last between 90 and 120 minutes each and will be repeated 3 to 7 times throughout the night (43,44).
NREM sleep usually involves between 75 and 80% of total sleep time. If we consider each phase of this sleep we find that N1 lasts only a few minutes and accounts for 2-5% of total sleep time, N2 lasts between 10 and 20 minutes and normally constitutes between 45 and 55% of the total time of sleep. N2 progresses to N3, which lasts for a few minutes, followed by N4 lasting 40 minutes, constituting 5-8% and 10-15% respectively. REM sleep accounts for 20-25% of total sleep time and its duration increases as alternating cycles occur (44).
The construct of poor sleep quality in Fibromyalgia: the chicken and egg paradox
Sleep and Fibromyalgia: objective and subjective alterations
Despite the wide range of research focused on describing sleep disturbances in patients with FM, there is no single consistent pattern that is pathognomonic of this syndrome. Moldofsky et al. (46) in the mid-70s were the first researchers to perform PSG records in patients with FM and described a characteristic pattern of α-wave intrusions during NREM sleep, which they called α-δ sleep. They speculated that some symptoms of FM were consequently produced by this pattern and proposed that FM should be considered as a “non-repairing sleep syndrome”. However, it is currently a controversial issue since there is research showing that α-δ rhythm is also recorded in subjects with other ailments and even in healthy individuals (25). In contrast, other authors conclude that the frequency of the α-δ rhythm is greater in subjects with FM and in people who present with sleep fragmentation than in healthy controls (26,27). Roizenblatt et al. (28) affirmed that this type of alteration is associated with an increase in the number of pain points, duration and intensity of pain. Despite this lack of agreement regarding α-δ alteration, there seems to be consensus regarding other important changes in continuity and sleep architecture in FM. There has been a significant increase in N1 NREM (29-32), reduction of N2 and N4 NREM and shorter REM sleep time (10,29,32-34), decrease in N2 sleep spindles (35), more latencies long onset of sleep and REM sleep and lower sleep efficiency (31,32). These alterations objectively show a fragmented sleep that is associated with a low quality of sleep. In addition, N4 deprivation is associated with increased sensitivity, worsening of musculoskeletal symptoms and mood disturbances (36). A greater number of awakenings (31) associated with greater awakening ability (37) and an increase in physical activity over the night recorded by actigraphy (AG) have also been reported (38). Rizzi et al. (39) described a high frequency of cyclic alternating pattern (PAC) with phases A2 and A3, which is a sign of instability of brain activity during sleep and poor sleep quality. In relation to subjective alterations, non-restorative sleep is, behind pain, the most reported symptom by people diagnosed with FM (12). They also describe a poor quality of sleep (37), greater difficulty falling asleep, frequent awakenings during the night and waking up very early with difficulty to reconcile sleep (40). In addition, they refer to excessive daytime drowsiness and fatigue (41).
“It is all in their body” vs “It is all in their heads”: a biopsychosocial perspective of FM
To introduce how the psychological and social factors could drive to a change in the patients’ behaviors that then are going to affect the symptoms experience… (this will allow to justify interventions directed to change the misconceptions and wrong behaviors)
Increasing evidence in support of the biopsychosocial model of pain suggests that cognitive and emotional processes are crucial contributors to inter-individual differences in the perception and impact of pain (47)
A Conceptual Model for Symptom Management
The UCSF Symptom Management Model
The USCF Symptom Management Model is a deductive, middle range model which was developed in the mid-90’s when the UCSF School of Nursing Symptom Management Faculty Group (48) identified that the models planned to date did not allow the nursing professionals to develop sufficiently comprehensive strategies for the symptomatic management of their patients. In addition, most of the research in the field of symptom management focused on strategies aimed at the improvement of a single feature of symptom management, did not provide enough guidance for health providers so as to implement integral symptom management strategies as well as did not allow the development of management strategies for chronic symptoms, emphasizing the need of more generic models, both at a clinical and a research level.
In response to this gap, Larson et al. (48) developed the Conceptual Model of Symptom Management (see Figure X) based on the assumption that, although it is important to cure the cause of a symptom, the development of symptom management strategies is essential as they are the most distressing factors and the main reason patients seek healthcare. In fact, the authors highlight that focusing treatment solely on the origin of the symptom may be ineffective, whereas the simultaneous treatment of the cause and the management of the symptom favors adherence to the treatment and, in turn, prevents patients not report or inappropriately hand severe symptoms requiring specialized care.
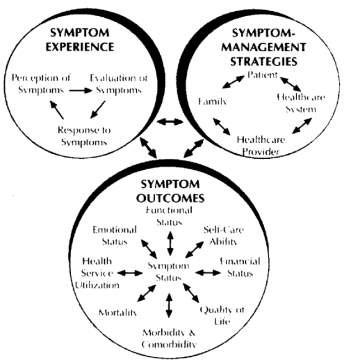
Figure X. Conceptual Model of Symptom Management (48)
After years of testing and collegial discussions, and on the basis of the following basic principles, a revised version of the Symptom Management Model (see Figure X) was finally published in 2001 (49):
- The exploration of symptoms should be based on the personal experience of each subject.
- The application of this model extends beyond the treatment of a symptom, i.e. it can be used as a prevention model if it is detected that the individual is at risk of developing a certain symptom.
- In subjects who do not have the ability to express themselves verbally, it will be imperative that a family member or a caregiver accurately detail the experience of the symptom so as to develop effective symptom management strategies.
- Symptom management strategies can be applied to the individual patient as well as to a group of people with similar characteristics, patients’ family or work environment.
- The process of symptomatic management should be understood as a dynamic process that can be modified depending on the results of each subject and the influence of the three domains of Person, Environment and Health & Illness.
The UCSF Symptom Management Model assumes that the symptom management is a multidimensional and changing process occurring at three main domains that are the Person, the Environment and the Health & Illness (49).
The person domain refers to how the demographic, biological, psychological and social variables, among others, understood as intrinsic variables of each subject, influence the symptomatic experience, not only in terms of perception but also in terms of evaluation and response to a concrete symptom. Likewise, when the model is used, the expansion of this domain depends on both the target population and the symptom/s.
The environment domain aims to contextualize the symptom experience around physical, social and cultural variables that may contribute to variance in the perception, evaluation and response to a symptom. The physical variable refers to the place where the symptom is occurring and it is considered important because the perception of a symptom would not be the same if it is experienced at home compared to in a working or hospital environment. Likewise, the symptom experience may be influenced by the social support network available to each individual as well as may be determined by cultural aspects such as religion, ethnicity or race, which build a certain system of beliefs and values.
Finally, the health or illness state is distinctive for each individual and is directly influenced by three main variables of the health & illness domain that are the risk factors, the general health status and the disease or injury characteristics.
In short, these three domains are key elements that alter the symptom experience and management strategies as well as the outcomes in a different manner in each subject and, therefore, they must be carefully analyzed so as to develop comprehensive and effective strategies directed to the symptom management.
The above described domains directly influence those that are considered the three dimensions of the original model, the symptom experience, the management strategies and, finally, the outcomes (48,49).
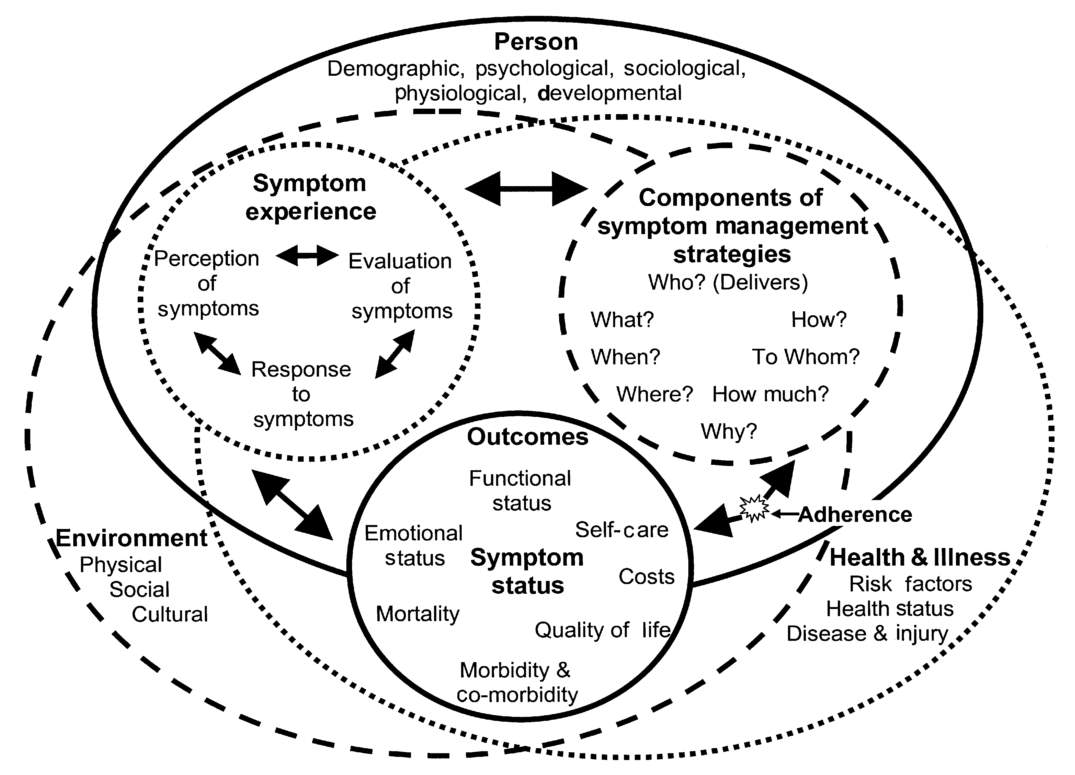
Figure X. Revised Symptom ManagementConceptual Model (49)
Symptom Experience
The general symptom experience of each individual is determined by three main factors, the perception, evaluation of the meaning and response that each subject provides to a symptom. These three factors show a bidirectional relationship between them, for example, the response that a patient gives to a symptom can alter the perception of the same and vice versa.
Symptom Management Strategies
Prior to the development of symptom management strategies, the experience of the symptoms should have been evaluated and analyzed if these strategies are to be truly effective. The strategies may be biomedical or psychosocial, but it must be ensured that they are specific for the perception, evaluation and / or response to a specific symptom. Both clinicians and researchers who base their intervention on the present model should clearly specify the nature of the intervention (WHAT) and the justification of its use (WHY) as well as to describe the WHEN (e.g. timing of day, frequency), WHERE (e.g. location of delivery), and HOW (e.g. in person, online, group). It is also considered essential to detail the dose of the intervention (HOW MUCH).
Outcomes
this dimension results from the interaction of the other two, that is, both the symptom experience and the strategies developed for its approach will influence the effectiveness of the intervention. The outcomes to be evaluated include functional and emotional status, self-management, quality of life, mortality, morbidity and comorbidity as well as social and economic costs, the latter being a factor incorporated in the revised version of the model.
Finally, it is mandatory to ensure and assess adherence to the symptom management intervention as it directly influences the obtained outcomes. Adherence depends on both the characteristics of patient and the health care provider and system.
A UCSF Symptom Management Model based intervention for women diagnosed with FM
(…)
As mentioned in previous sections, FM is a health condition whose pathophysiological basis is still a topic of scientific discussion, which prevent the development of curative treatments. Thus, the approaches developed so far focus on symptomatic improvement (50). (…)
In this study, the Symptom Management Model provides direction for the development of an educational intervention combined with neurofeedback for the integral and simultaneous approach to pain and poor sleep quality in women diagnosed with FM.
Symptom experience
Perception of symptoms
(…)
Evaluation of symptoms
As stated by Dodd et al. (49) “a person can perceive pain simply by recognizing the sensations, whereas evaluation involves a higher cognitive process of attaching meaning to the symptom”. Thus, the main difference between the perception of a symptom and its evaluation is that the latter implies giving meaning to the perception of a symptom.
To understand the symptomatic experience of people diagnosed with FM, it is essential to bear in mind that this syndrome is still currently interpreted under the perspective of a biomedical model, still prevailing both at the clinical and social levels, and that both the diagnosis and treatment process are well founded in said model. Therefore, patients are constantly confronted with the duality “it is all in your body” vs. “it is all in your head”, making them feel stigmatized and inevitably affecting how they interpret not only their health condition but also the symptoms derived from this (51).
As mentioned in previous sections, chronic widespread pain is the main symptom that characterizes FM and, therefore, it is not surprising that it is the most recurrent theme in qualitative studies that analyze the symptomatic experience of people affected and the main variable evaluated in quantitative research. Pain is a subjective symptom and its evaluation derives from both biological and psychosocial aspects, which contributes to making this evaluation a complex process for any patient who experiences it (52).
Sim & Madden (53) conducted a review of qualitative studies that analyzed the illness experience in people diagnosed with FM. Their results show that the discussion about pain for patients suffering from this health condition is conflicting given its non-specific and fluctuating nature. However, the factor that probably contributes most to the difficulty of communicating and making others understand the painful experience in people with FM, is that pain is “invisible” and verbal language is, to a large extent, a barrier for the expression of subjective experiences. Consequently, it is common for patients to use metaphors such as “stabbing”, “gnawing” or “boiling”, among others, to try to define their pain.
Marques et al. (52) found that the word most used by patients with FM (91%) and chronic low back pain (51%) to describe their pain was “sickening” and that there were some descriptors that were only used by people with FM as “wretched”, “blinding”, “exhausting”, “vicious”, “miserable” and “unbearable”, which were associated with a greater intensity of pain. Thus, these results suggest that people affected with FM experience their pain more intensely and with a greater psycho-emotional component compared to patients suffering from other health conditions.
Pain catastrophizing, understood as “a set of exaggerated and ruminating negative cognitions and emotions during actual or perceived painful stimulation” (54), together with depression, are the psychosocial factors with the greatest influence in the experience of pain (47). Although pain catastrophizing was initially conceived as another symptom of depression, the evidence shows that it is a phenomenon linked to pain independent of depression and even negative affect (35). In fact, in people with musculoskeletal conditions, pain catastrophizing is considered the most important risk factor and predictor of the prognosis of pain, both in the short and long term (55). There are studies that link pain catastrophizing with the intensity of pain (56–59), but the causal relationship between both phenomena is a little explored field of investigation. Some hypotheses consider the possibility that catastrophizing increases the fear of pain and contributes to a reduced capacity to move the focus away from it, which, in turn, can lead to an alteration in perceived intensity and in the evaluation of pain. (35). In this regard, Terry et al. (60) developed an experimental study to analyze the effects of reducing pain catastrophizing on pain intensity reported in a sample of healthy non-painful subjects. The authors also assessed whether pain catastrophizing had effects on the modulation of pain at the spinal level. The subjects were randomized to a pain catastrophizing reduction group or to a control group that received pain education. The results showed significant reductions in pain catastrophizing, as well as in the pain intensity and unpleasantness reported in the experimental group compared to the control group. However, although a decrease in the temporal summation of the nociceptive reflex at spinal level was found, it could not be demonstrated that pain catastrophizing mediated this decrease, which suggests that pain catastrophizing may modulate pain to a greater extent at supraspinal levels. Additionally, Kjøgx et al. (57) experimentally manipulated the levels of pain catastrophizing in a sample of patients diagnosed with chronic tension-type headache and healthy participants. All individuals were randomized either to a negative condition, based on hypnotic suggestions according to the parameters of the Pain Catatrophizing Scale (PCS); to a positive condition, also based on hypnotic suggestions, but with the content of the PCS reverted from negative to positive; or, finally, to a neutral condition in which a neutral hypnotic state was induced. The results of this study showed that pain catastrophizing can be manipulated both negatively and positively and that, in addition, this manipulation had a direct relationship with the reported pain intensity. Although more studies are needed to prove the causal relationship between pain catastrophizing and the experience of it, these findings may be key in developing future interventions in patients suffering from pain.
In patients with FM, a disruption of emotional pain modulation compared to people with rheumatoid arthritis and healthy controls has been shown (61) and pain catastrophism is directly related to increases in the intensity of pain, as well as in the number of painful points that they report (35)….
I HAVE TO DEVELOP MORE DEEPLY THIS PART OF PAIN CATASTROPHIZING IN PATIENTS WITH FM AND ALSO THE MISCONCEPTIONS ABOUT SLEEP
Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: cross-lagged panel analyses of dispositional and situational catastrophizing (58)
Catastrophizing was re- lated to a limited understanding of the symptoms of FM (62)
Response to symptoms
Central to successful coping was the ability of the self to understand FMS (53)
REFERENCES
1. Smith HS, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. 2011;14(2):E217–45.
2. Menzies V. Fibromyalgia Syndrome: Current Considerations in Symptom Management. Am J Nurs. 2016 Jan;116(1):24-32, 41.
3. Cabo-Meseguer A, Cerdá-Olmedo G, Trillo-Mata JL. Fibromialgia: prevalencia, perfiles epidemiológicos y costes económicos. Med Clínica. 2017;
4. Rivera J, Alegre C, Ballina FJ, Carbonell J, Carmona L, Castel B, et al. Documento de consenso de la Sociedad Española de Reumatología sobre la fibromialgia. Reum Clin. 2006;(1):55–66.
5. Inanici F, Yunus MB. History of fibromyalgia: past to present. Curr Pain Headache Rep. 2004;8(5):369–78.
6. Gowers WR. A Lecture on Lumbago: Its Lessons and Analogues: Delivered at the National Hospital for the Paralysed and Epileptic. Br Med J. 1904;1(2246):117–21.
7. Stockman R. The Causes, Pathology, and Treatment of Chronic Rheumatism. Edinb Med J. 1904;15:107–16.
8. Collins D. Fibrositis and infection. Ann Rheum Dis. 1940;2:114–25.
9. Bennett RM. Muscle physiology and cold reactivity in the fibromyalgia syndrome. Rheum Dis Clin North Am. 1989 Feb;15(1):135–47.
10. Clauw DJ, Wallace DJ. Fibromyalgia: The Essential Clinicians’s Guide. 10th Ed. Oxford: Oxford University Press; 2009.
11. Wolfe F, Smythe H, Yunus MB, Bennet R, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990;
12. Crofford LJ, Clauw DJ. Editorial: Fibromyalgia: Where are we a decade after the American College of Rheumatology classification criteria were developed? Arthritis Rheum. 2002;46(5):1136–8.
13. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010 May;62(5):600–10.
14. Rivera J, Rejas J, Esteve-Vives J, Vallejo M a. Resource utilisation and health care costs in patients diagnosed with fibromyalgia in Spain. Clin Exp Rheumatol. 2009;27(5 SUPPL. 56):S39–45.
15. Sociedad Española del Dolor. La fibromialgia provoca un gasto de 10.000 euros por paciente al año. Rev la Soc Española del Dolor. 2009 Oct;16(7):417–8.
16. Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62(2):145–51.
17. Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38(1):35–44.
18. Finan PH, Goodin BR, Smith MT. The Association of Sleep and Pain: An Update and a Path Forward. J Pain. 2013 Dec;14(12):1539–52.
19. Moldofsky H. The significance of dysfunctions of the sleeping/waking brain to the pathogenesis and treatment of fibromyalgia syndrome. Rheum Dis Clin North Am. 2009 May;35(2):275–83.
20. Fitzcharles M, Ste-marie P a, Goldenberg DL, John X, Abbey S, Choinière M, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome. Can Pain Soc. 2012;18(3):1–52.
21. Ferrari R. The biopsychosocial model—a tool for rheumatologists. Best Pract Res Clin Rheumatol. 2000 Dec;14(4):787–95.
22. Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002 Jun;70(3):678–90.
23. IASP. IASP Taxonomy [Internet]. 2012 [cited 2017 Jan 1]. Available from: https://www.iasp-pain.org/Taxonomy#Centralsensitization
24. Simms RW, Roy SH, Hrovat M, Anderson JJ, Skrinar G, Lepoole SR, et al. Lack of association between fibromyalgia syndrome and abnormalities in muscle energy metabolism. Arthritis Rheum. 1994 Jun 1;37(6):794–800.
25. Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007 Apr;26(4):465–73.
26. Reichling DB, Levine JD. The primary afferent nociceptor as pattern generator. Pain. 1999 Aug;Suppl 6:S103-9.
27. Merskey H, Bogduk N. Classification of Chronic Pain. IASP Pain Terminology. 1994. 240 p.
28. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–9.
29. Nijs J, Van Houdenhove B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: application of pain neurophysiology in manual therapy practice. Man Ther. 2009 Feb;14(1):3–12.
30. Clauw DJ, Chrousos GP. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. 4(3):134–53.
31. Staud R, Domingo MA. Evidence for abnormal pain processing in fibromyalgia syndrome. Vol. 2. 2001. p. 208–15.
32. Nijs J, Paul van Wilgen C, Van Oosterwijck J, van Ittersum M, Meeus M. How to explain central sensitization to patients with “unexplained” chronic musculoskeletal pain: practice guidelines. Man Ther. 2011 Oct;16(5):413–8.
33. Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002 Aug;4(4):299–305.
34. Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger P., Arendt-Nielsen L, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004 Jan;107(1–2):7–15.
35. Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(4):835–43.
36. van Wilgen CP, Keizer D. The Sensitization Model to Explain How Chronic Pain Exists Without Tissue Damage. Pain Manag Nurs. 2012;13(1):60–5.
37. Gracely RH, Ambrose KR. Neuroimaging of fibromyalgia. Best Pract Res Clin Rheumatol. 2011 Apr;25(2):271–84.
38. Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007 Feb 26;26(4):465–73.
39. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004 Dec;25(12):613–7.
40. Artiach Geiser G, del Cura González MI, Díaz del Campo Fontecha P, de la Puente MJ, Fernández Mendoza J, García Laborda A, et al. Guía de Práctica Clínica para el Manejo de Pacientes con Insomnio en Atención Primaria. Vol. 1, Ministerio de Sanidad y Política Social. 2009.
41. Arrigoni E, Fuller PM. An Overview of Sleep. In: Therapy in Sleep Medicine. Elsevier; 2012. p. 43–61.
42. Carskadon M a, Dement WC. Normal Human Sleep. In: Principles and Practice of Sleep Medicine. Elsevier; 2011. p. 16–26.
43. Kleinman L, Mannix S, Arnold LM, Burbridge C, Howard K, Mcquarrie K, et al. Assessment of sleep in patients with fibromyalgia: qualitative development of the fibromyalgia sleep diary.
44. Markov D, Goldman M, Doghramji K. Normal Sleep and Circadian Rhythms. Sleep Med Clin. 2012 Sep;7(3):417–26.
45. Marshall B, Robertson B, Carno M-A. Polysomnography for the sleep technologist: Instrumentation, Monitoring and Related Procedures. St. Louis: Elsevier Inc.; 2014. 134 p.
46. Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37(4):341–51.
47. Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–24.
48. Larson JP, Crrieri-Kohlman V, Dodd MJ, Douglas M, Faucett J, Froelicher E, et al. A Model for Symptom Management. Image J Nurs Scholarsh. 1994 Dec;26(4):272–6.
49. Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–76.
50. Fitzcharles MA, Ste-Marie PA, Pereira JX. Fibromyalgia: Evolving concepts over the past 2 decades. Cmaj. 2013;185(13):645–51.
51. Ashe SC, Furness PJ, Taylor SJ, Haywood-Small S, Lawson K. A qualitative exploration of the experiences of living with and being treated for fibromyalgia. Heal Psychol Open. 2017 Jul 7;4(2):205510291772433.
52. Marques AP, Rhoden L, Siqueira J de O, João SMA. Pain evaluation of patients with fibromyalgia, osteoarthritis, and low back pain. Rev Hosp Clin Fac Med Sao Paulo. 2001;56(1):5–10.
53. Sim J, Madden S. Illness experience in fibromyalgia syndrome: A metasynthesis of qualitative studies. Soc Sci Med. 2008 Jul 1;67(1):57–67.
54. Leung L. Pain catastrophizing: An updated review. Indian J Psychol Med. 2012;34(3):204.
55. Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006 Apr 15;55(2):325–32.
56. Linton SJ, Nicholas MK, MacDonald S, Boersma K, Bergbom S, Maher C, et al. The role of depression and catastrophizing in musculoskeletal pain. Eur J Pain. 2011;15(4):416–22.
57. Kjogx H, Kasch H, Zachariae R, Svensson P, Jensen TS, Vase L. Experimental manipulations of pain catastrophizing influence pain levels in patients with chronic pain and healthy volunteers. Pain. 2016;157(6):1287–96.
58. Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, et al. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther. 2012;14(5):R231.
59. Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical Perspectives on the Relation Between Catastrophizing and Pain. Clin J Pain. 2001;17(1):52–64.
60. Terry E, Thompson K, Rhudy J. Experimental reduction of pain catastrophizing modulates pain report but not spinal nociception as verified by mediation analyses. Pain. 2015;156(8):1477–88.
61. Rhudy JL, Delventura JL, Terry EL, Bartley EJ, Olech E, Palit S, et al. Emotional modulation of pain and spinal nociception in fibromyalgia. Pain. 2013;154(7):1045–56.
62. Van Wilgen CP, Van Ittersum MW, Kaptein AA, Van Wijhe M. Illness perceptions in patients with fibromyalgia and their relationship to quality of life and catastrophizing. Arthritis Rheum. 2008 Nov 1;58(11):3618–26.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




