Dissertation Proposal on Dye Removal from Wastewater
Info: 7539 words (30 pages) Dissertation
Published: 17th Nov 2021
Dye Removal from Wastewater Utilizing Stirred-Tank Impeller-Bed Adsorption System (STIBA).
ABSTRACT
Wastewater from textile industry, especially from batik industry is raising alarm on impact on environment. This issue should be dealt to ensure the preserve quality of the surroundings. Various dye contained in the batik wastewater is causes serious effect not only on environment but to human heatlh too. Current efforts to inhibit the issue are by implementing dye removal methods comprising chemical, biological and physical treatments. Among these methods, adsorption is the commonly used as it encompasses with some benefit. Good removal of wide variety of dyes, no sludge formation and cheap. To enhance the effect of adsorption, novel process, Stirred Tank Impeller Bed Adsorption (STIBA) System is used. Since there were only little data available on the STIBA system, this research aimed to study the ability of adsorbate to utilize STIBA to achieve higher adsorption.
TABLE OF CONTENTS
Chapter 1: Introduction
1.1. BACKGROUND OF STUDY
1.2. PROBLEM STATEMENT
1.3. OBJECTIVES
1.4. SCOPE OF STUDY
Chapter 2: Literature Review and Theory
2.1. BATIK WASTEWATER
2.2. DYE
Chapter 3: METHODOLOGY/ PROJECT WORK
3.1. STIRRED-TANK IMPELLER-BED ADSORPTION (STIBA) SYSTEM
3.2. PROJECT FLOWCHART
3.3. EXPERIMENTAL SETUP
3.4. STIBA SYSTEM SETUP
3.5. OPERATING PARAMETERS
3.6. EXPERIMENTAL PROCEDURE
REFERENCES
Chapter 1: Introduction
1.1. BACKGROUND OF STUDY
Batik industry is one of the most prominent textile industry in Malaysia. The batik was also recognised by UNESCO as Intangible Cultural Heritage. Biggest share of local South-East Asian families’ source of income is from batik industry. This industry reveals negative impact on the environment, especially the generation of waste and wastewater.
Dyes are released as pollutant to environment from this dyeing process in batik. Nowadays, the treatment of wastewater containing dyes is of greatest importance because it has low biodegradability and it pollutes environment. This industry has a high pollution tendencies because of its unregulated nature, lack of environmental control and nature of its discharges. Malaysian government has imposed stringent limits on the quality of discharged wastewater, as the adverse harmful impacts of wastewater on the environment and society.
Various techniques have been employed to remove dyes from wastewater, including biological treatment, adsorption, chemical oxidation, coagulation, ion exchange, reverse osmosis, membrane filtration and photochemical degradation or a combination of these wastewater treatments. Most of these methods are complex treatment, complicated, imposes high cost and inefficient in large volume of wastewater.
The adsorption is the method considered to viable treatment as it gives finest removal capacity as it can remove various coloring materials in wastewater. No fragments in the effluent is left as adsorption removes the dye molecule as whole. The efficiency of adsorption is significantly affected by the type of adsorbent. Activated carbon is the most commonly used as adsorbent in dye removing processes. As recent researches focused in using adsorbent from agricultural by-products, activated carbon becomes the choice.
1.2. PROBLEM STATEMENT
Waste effluent from batik industry can cause harm to environment especially water pollution as it is usually directed to nearby river or water source. The wastewater from batik industry contains elevated level of dyes, heavy metals, grease, wax and suspended solids. This can also cause harm for aquatic organisms as they can raise the COD and BOD levels of water. This should be addressed to inhibit the harm on environment. As this industry is mainly done by small and medium enterprises (SME), advanced technology for waste treatment not affordable. Therefore, cost efficiency and simplicity are among the factors to be considered.
1.3. OBJECTIVES
- To determine the effect of adsorbent loading towards the efficiency of dye removal from wastewater for STIBA system.
- To determine initial dye concentration to the adsorption of dye by STIBA System.
- To determine the effect of agitation mechanism (impeller speed) that would contribute to high efficiency of dye removal from wastewater.
1.4. SCOPE OF STUDY
In this research, it is focused on the adsorption of methylene blue from aqueous solution using Stirred-Tank Impeller-Bed Adsorption(STIBA) System. This research focuses utilizing agricultural by-product activated carbon on understanding STIBA system.
Chapter 2: Literature Review and Theory
2.1. BATIK WASTEWATER
Wastewater is stated as water that contains any material either solid, liquid, semisolid, contained gas or other forms resulting industrial, commercial, mining or agricultural operations, or from community and household activities that is devoid of usage and discarded. The quality of water content in the wastewater is adversely affected by anthropogenic influence. Wastewater can originate from a combination of domestic, industrial, commercial or agricultural activities, surface runoff or stormwater, and from sewer inflow or infiltration.
One of the main sources is the textile industry, which discharges substantial amounts of industrial waste water[1]. Some constituents of concern in wastewater treatment are suspended solids biodegradable organics, pathogens, nutrients; mainly nitrogen and phosphorus that leads to eutrophication, carcinogens, mutagens, pesticides, phenols, surfactants, oil, heavy metals, dissolved inorganics. Release of this industrial wastewater into the environment creates a significant footprint and may also create various other hazards. Some of the impact are interference with the sunlight penetration, retardation of the photosynthetic processes and therefore, the growth inhibition of aquatic biota[2].
Batik has been both an art and craft for centuries and is part of an ancient tradition. Over 1000 batik factories are scattered mainly throughout Kelantan and Terengganu on the east coast of Malaysia[3]. The word batik is derived from the Javanese word ‘Amba’, means ‘to write’, the suffix ‘titik’ means to make dots[4]. Batik is now well known as UNESCO has recognized batik as world cultural heritage[5]. This makes the batik industry to boom in recent years. According to Zainudin, et al. [6], discharged wastewaters from batik industry contain dyes, heavy metals, grease, wax and also suspended solids with high chemical oxygen demand (COD).
As the most basic, water resources are limited vital element, are utterly affected by industrial exploit. For a pollution-free world, proper treatment dye removal from wastewater in an environment friendly and economic way is crucial.
2.2. DYE
Dye is important as it is used to add aesthetic value in many manufacturing industries. Appeal in colored materials have been responsible for the growth of dye usage. Thousands of different dyestuffs and pigments are utilized in chemical industries, such as textile, plastic, paper, printing, pharmaceutical and food industries.[7]
Dye molecules consists of two key components: the chromophores, which are largely responsible for producing the color, and the auxochromes, which not only supplement the chromophore but also render the molecule soluble in water and enhance its affinity (to attach) toward the fibers [8].
There are numerous ways to classify dye such as in term of colour, chemical structure and application methods. Dyes can be classified in accordance to their solubility in water as cationic (basic dyes), anionic (acid, direct, and reactive dyes), and non-ionic (vat and disperse dyes)[8, 9]. Table 2.1 shows the details on types of dyes and the application on principal substrates. Based on research by Peláez-Cid, et al. [9], basic dyes are used to dye acrylic fibers; acid dyes are used on wool, silk, or nylon; disperse dyes are used on polyester or nylon. As for cotton, direct, reactive or vat dyes are used
Table 2.1: Classification of dye based on their chemical nature[8].
| Class | Principal Substrate | Method of Application | Chemical Types |
| Acid | Nylon, wool, silk, paper, inks, and leather | Usually from neutral to acidic dye baths | Azo (including premetalized), anthraquinone, triphenylmethane, azine, xanthene, nitro and nitroso |
| Azoic components and compositions | Cotton, rayon, cellulose acetate and polyester | Fiber impregnated with coupling component and treated with a solution of stabilized diazonium salt | Azo |
| Basic | Paper, polyacrylonitrile, modified nylon, polyester and inks | Applied from acidic dye baths. | Cyanine, hemicyanine, diazahemicyanine, diphenylmethane, triarylmethane, azo, azine, xanthene, acridine, oxazine and anthraquinone |
| Direct | Cotton, rayon, paper, leather and nylon | Applied from neutral or slightly alkaline baths containing additional electrolyte. | Azo, phthalocyanine, stilbene, and oxazine |
| Disperse | Polyester, polyamide, acetate, acrylic and plastic | Fine aqueous dispersion often applied by high temperature/ pressure or lower temperature carrier methods; dye may be padded on cloth and baked on or thermofixed | Azo, anthraquinone, styryl, nitro, and benzodifuranone |
| Fluorescent brighteners | Soaps and detergents, and all fibers, oils, paints and plastics | From solution, dispersion or suspension in a mass. | Stilbene, pyrazoles, coumarin, and Naphthalimides |
| Food, drug, and cosmetics | Foods, drugs, and cosmetics | – | Azo, anthraquinone, carotenoid and triarylmethane |
| Mordant | Wool, leather, and anodized aluminum | Applied in conjunction with Cr salts | Azo and anthraquinone |
| Oxidation bases | Hair, fur, and cotton | Aromatic amines and phenols oxidized on the substrate | Aniline black and indeterminate structures |
| Reactive | Cotton, wool, silk, and nylon | Reactive site on dye reacts with functional group on fiber to bind dye covalently under influence of heat and pH (alkaline) | Azo, anthraquinone, phthalocyanine, formazan, oxazine and basic |
| Solvent | Plastics, gasoline, varnishes, lacquers, stains, inks, fats, oils, waxes | Dissolution in the substrate | Azo, triphenylmethane, anthraquinone, and phthalocyanine |
| Sulfur | Cotton and rayon | Aromatic substrate vatted with sodium sulfide and reoxidized to insoluble sulfur-containing products on fiber | Indeterminate structures |
| Vat | Cotton, rayon and wool | Water-insoluble dyes solubilized by reducing with sodium hydrogensulphite, then exhausted on fiber and reoxidized | Anthraquinone (including polycyclic quinines) and indigoids |
2.2.1. METHYLENE BLUE (MB)
Methylene blue (MB), also known as tetramethylthioninechloride is a cationic dye. It is used to dye in textile industry for dying cotton, wool and silk[10, 11]. MB has been reported to cause increased heart rate, cyanosis, nausea, jaundice, quadriplegia, Heinz body formation and tissue necrosis in humans[11]. It also has effect on human health such as eye burns, breathing problem, gastritis, diarrhea and vomiting.[10, 12-14]. Thus, it imposes greater need for wastewater treatment.
2.2.2. DYE REMOVAL METHODS
Dye in wastewater is the discharge of dye from manufacturing, dyeing printing, and textile industries. Many techniques have been employed to remove dyes from wastewater. The technique is including biological treatment, adsorption, chemical oxidation, coagulation, membrane filtration and photochemical degradation or a combination of these wastewater treatments. [7, 14-16]. Table 2.2 illustrates the advantages and disadvantages of dye removal methods.
Table 2.2: Advantages and disadvantages of dyes removal methods.[8]
| Chemical Treatments | ||
| Methods | Advantages | Disadvantages |
| Oxidative process | Simplicity of application | (H2O2) agent needs to activate by some means |
| H2O2+Fe(II) salts (Fenton’s reagent) | Fenton’s reagent is a suitable chemical means | Sludge generation |
| Ozonation | Ozone can be applied in its gaseous state and does not increase the volume of wastewater and sludge | Short half-life (20 min) |
| Photochemical | No sludge is produced and foul odors are produced | Formation of by-products |
| Sodium hypochlorite (NaOCl) | Initiate and accelerates azo bond cleavage | Release of aromatic amines |
| Electrochemical destruction | No consumption of chemicals and no sludge build up | Relatively high flow rates cause a direct decrease in dye removal |
| Biological Treatments | ||
| Methods | Advantages | Disadvantages |
| Decolorization by white rot fungi | White-rot fungi can degrade dyes using enzymes | Enzyme production has also been shown to be unreliable |
| Other microbial cultures (mixed bacterial) | Decolorized in 24–30 h | Under aerobic conditions azo dyes are not readily metabolized |
| Adsorption by living/dead microbial biomass | Certain dyes have an affinity for binding with microbial species | Not effective for all dyes |
| Anaerobic textile – dye bioremediation systems | Allows azo and other water-soluble dyes to be decolorized | Anaerobic breakdown yields methane and hydrogen sulfide |
| Physical Treatments | ||
| Methods | Advantages | Disadvantages |
| Adsorption by activated carbon | Good removal of wide variety of dyes | Very expensive |
| Membrane filtration | Removes all dye types | Concentrated sludge production |
| Ion exchange | Regeneration: no adsorbent loss | Not effective for all dyes |
| Irradiation | Effective oxidation at laboratory scale | Requires a lot of dissolved O2 |
| Electro-kinetic coagulation | Economically feasible | High sludge production |
Among these methods, adsorption is often used due to its effectiveness, simplicity in the operational, and low energy requirements.[15]
2.2.3. ADSORPTION METHOD
Adsorption process has been found to be superior among the existing processes for water treatment due to high efficiency, flexibility, ease of operation, insensitivity towards toxic pollutants and economic feasibility[11, 13, 15]. Adsorption is a process which involves the accumulation of substance at the interface of the two phases, such as, liquid-liquid, gas-liquid, gas-solid, or liquid-solid interface. Adsorbate is the substance which is adsorbed on the surface. The substance on which surface the adsorbate is adsorbed is the adsorbent. In fact, adsorption onto activated carbon has been cited by US Environmental Protection Agency as the Best Available Technology to reduce the environmental pollution in water, soil or air by separation and purification processes[2].
2.2.4. ACTIVATED CARBON
Adsorption has the advantage on removal of dyes compared to other methods based on the Table 2.2. According to Peláez-Cid, et al. [9] and Amin and Nizam [16], activated carbon (AC) is one of the most widely used adsorbents with very varied applications It has shown considerable good dye removal efficiency. Commercial activated carbon (CAC) has been widely used as adsorbents in wastewater treatment processes of textile industry due to its advantages such as large surface area, large adsorption capacity and easy application[2, 15]. However, practical application of AC is limited because of its regeneration or disposal, sludge production and economic feasibility problems[11]. Recently, agricultural and industrial wastes have dictated the researcher’s interest owing to their wide availability, low cost, less commercial value and biodegradable properties. Table 2.3 shows the adsorption capacities of agricultural wastes for methylene blue dye removal.
Table 2.3: Adsorption capacities of agricultural wastes for methylene blue dye removal[8].
| Adsorbents | qe (mg/g) | Reference
|
| Coconut Shell (NaOH Activated) | 916.26 | [17] |
| Rice Husk | 690 | [8] |
| Posidonia Oceanica Fibres (H2O2 Activated) | 600 | [18] |
| Cassava Rind | 565 | [19] |
| Cashew Nut Shell (ZnCl2 Activated) | 476 | [20] |
| Coconut Husk (KOH Activated+ Microwave Heating) | 418.15 | [21] |
| Durian Shell (NaOH Activated + Microwave Heating) | 410.85 | [22] |
| Albizia Lebbeckseed Pods | 381.22 | [18] |
| Oil Palm Empty Fruit Bunch (KOH Activated + Microwave Heating) | 344.83 | [23] |
| Teak Tree Bark | 333.3 | [8] |
| Palm Oil Fiber (Microwave Heating) | 312.5 | [24] |
| Cotton Stalk (KOH Activated) | 294.12 | [18] |
| Durian Shell (Conventional Heating) | 289.26 | [24] |
| Cotton Stalk (K2CO3 Activated) | 285.71 | [18] |
| Z-Ac Composite from Oil Palm Ash | 285.71 | [25] |
| Durian Peel (CO2 Activated + Conventional Heating) | 284 | [22] |
| Palm Oil Fiber (Conventional Heating) | 277.78 | [24] |
| Factory Rejected Tea (NaOH Activated) | 242 | [26] |
| Karanj Fruit Hull | 239.4 | [18] |
| Maize Silk Powder | 234.1 | [27] |
| Palm Kernel Fiber | 223.41 | [28] |
| Rambutan Peel (KOH Activated) | 215.05 | [18] |
| Macadamia Nut Endocarp (ZnCl2 Activated) | 194.7 | [18] |
| Peach Stone (ZnCl2 Activated) | 191.3 | [18] |
| Coffee Grounds (H3PO4 Activated) | 181.8 | [18] |
| Corn Comb (CO2 Activated) | 165 | [29] |
| Magnetite-Loaded Palm Shell-Waste | 163.3 | [30] |
| Posidonia Oceanica Fibres (H3PO4 Activated) | 137.27 | [18] |
| Corn Comb (Steam Activated) | 135 | [29] |
| Posidonia Oceanica Fibres (KOH Activated) | 133.62 | [18] |
| Oil Palm Shell (KOH Activation + Microwave Heating) | 133.13 | [31] |
| Rice Straw ((NH4)4HPO4 + Conventional Heating) | 129.5 | [22] |
| Posidonia Oceanica Fibres (ZnCl2 Activated) | 114.89 | [18] |
| Oil Palm Leaves | 103.02 | [32] |
| Coffee Grounds (KOH Activated + Conventional Heating) | 99.43 | [23] |
| Hazelnut Shell | 76.9 | [8] |
| Coconut Bunch Waste | 70.92 | [32] |
| Walnut Saw Dust | 59.17 | [8] |
| Ficus Carica Bast | 55.56 | [33] |
| Sunflower Seed Husk | 45.25 | [8] |
| Oil Palm Shell (KOH Activation + Conventional Heating) | 40.86 | [31] |
| Cherry Saw Dust | 39 | [8] |
| Posidonia Oceanica Fibres (H3PO4+HNO3 Activated) | 36.68 | [18] |
| Rice Straw | 20.38 | [34] |
| Coconut Coir Pith | 5.8 | [35] |
AC may be presented in diverse forms depending on its application and the precursor used in its preparation. It can be found as granules (GAC), powder (PAC), fibers (ACF), cloths of activated carbon fibers (ACFC), carbon nanotubes (CNT), monoliths, composites, and extruded or pellet forms.[9]. Granular activated carbon and the powdered activate carbon are the most commonly used in adsorption process. Powdered activated carbon is usually used in batch process due to the much larger surface area. As for fixed bed adsorption column, granular type of activated carbon is used because it is easier pack into the column.
Although the adsorbents shown in above table is promising, the are some limitation such as waste production capacity by the country. Country such as Malaysia produces oil palm and coconut related waste more than the above said adsorbents. After oil palm and rubber, coconut is the third most important industrial crop in Malaysia in terms of cultivated area. Other than food values, coconut shell and husk are very promising sources of valuable activated carbon. About 151,000 ha of land were being used for coconut plantation in the year 2001 in Malaysia but this is gradually decreasing every year due to competition with oil palm for land. Recently, a coconut industry revitalizing plan (2008–2015) was initiated to replant and rehabilitate the coconut plantation area.[36] Therefore, dye removal via adsorption using coconut shell activated carbon as adsorbent is the best option compared to the other methods.
Chapter 3: METHODOLOGY/PROJECT WORK
3.1. STIRRED-TANK IMPELLER-BED ADSORPTION (STIBA) SYSTEM
Batch reactor is the common setup that is usually used for adsorption process. Even though the advantages of the batch reactor are abundant. However, the difficulty experienced to remove the adsorbent at the end of the process makes it less effective.
Therefore, STIBA system is introduced to improve the prevailing system. STIBA system contains hollow fin which is filled with adsorbent. This fin is connected to an impeller. This enables the STIBA system to be more effective removing dye. The saturated adsorbents can be removed at ease by withdrawing the impeller for replacement. STIBA improved design contributes time saving and reduces operating cost which allows study on dye adsorption effectively
3.2. PROJECT FLOWCHART
The project will be conducted based on the flowchart below.
Problem Statement & Objectives
Literature Review
Experimentation
Column Designation and Samples Preparation
Change parameters
Successful
No
 Yes
Yes
Data Gathering

Data Analysis
Report Writing
Figure 3.1: Project Flowchart
3.3. EXPERIMENTAL SETUP
This project will study the effect of amount of absorbent towards the efficiency of dye removal from wastewater in the STIBA system. Besides, this experiment will examine the effect of agitation mechanism on the adsorption rate for the STIBA system. These objectives can be achieved by following the below experimental design.
Design the impeller (STIBA)

Batik wastewater from collection
Study the effect of dye adsorption for STIBA system and batch system

Change Parameter
Study the effect of impeller speed on STIBA efficiency
Successful
No
 Yes
Yes
Collect the dye samples (before and after adsorption)

Data Analysis
Report Writing
Figure 3.2: Experimental setup
3.4. STIBA SYSTEM SETUP
As STIBA impeller have been fabricated by previous FYP student, Muhamad Haziq bin Halmi, the same STIBA impeller would be used to conduct this project. The following figures are extracted from Muhamad Haziq’s report.
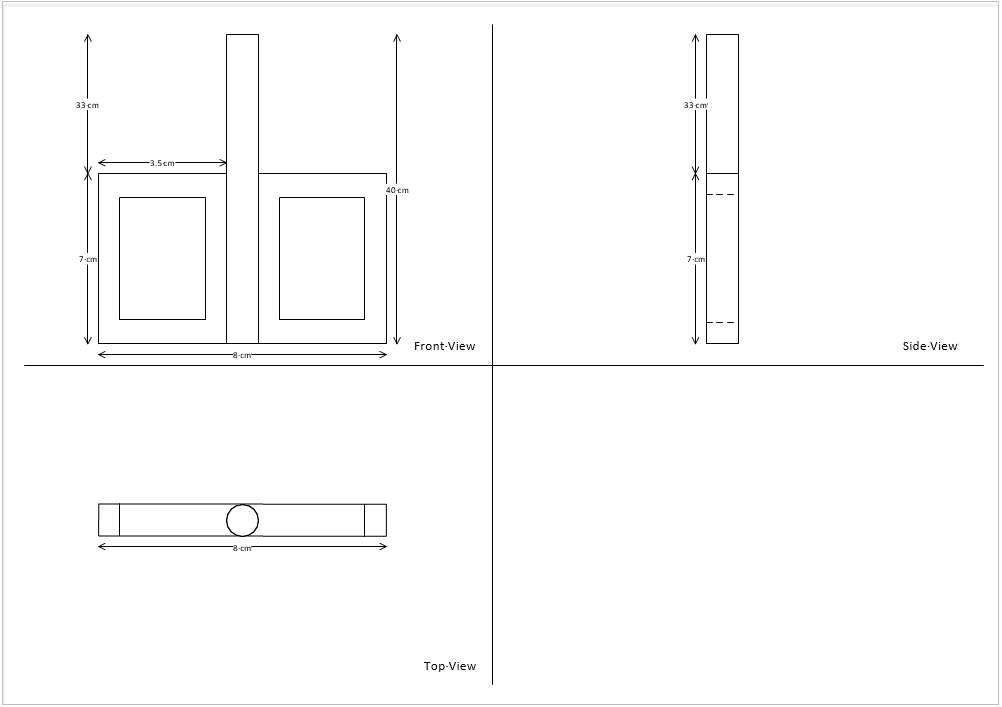
Figure 3.3: Orthographic drawing of STIBA impeller together with dimensions.

Figure 3.4: STIBA impeller attached to overhead stirrer
3.5. OPERATING PARAMETERS
Time, volume and initial concentration will be kept constant throughout the study whereas, the impeller speed and amount of adsorbent will be varied to study the adsorption mechanism for dye removal of wastewater. Table 3.1 and Table 3.2 shows the proposed operating parameters that will be used throughout this study.
Table 3.1: STIBA system operating parameters
| Parameters | Value |
| Type of adsorbent | Activated carbon (Coconut Shell) |
| Dye | Methylene blue |
| Volume (ml) | 1000 |
| Time (min) | 120 |
| Impeller speed (rpm) | 50, 100, 200 |
| Dye solution concentration (ppm) | 10, 20, 30 |
| Amount of adsorbent used (g) | 10 |
| Temperature (℃) | 25 |
Table 4: Batch system operating parameter
| Parameters | Value |
| Type of adsorbent | Activated carbon (Coconut Shell) |
| Dye | Methylene blue |
| Volume (ml) | 1000 |
| Time (min) | 120 |
| Dye solution concentration (ppm) | 10 |
| Amount of adsorbent used (g) | 10 |
3.6. EXPERIMENTAL PROCEDURE
3.6.1. RAW MATERIALS
Coconuts used for preparation of AC to be obtained from localmarket in Maringá, Paraná, Brazil. The fruits shall be washed with distilled water, and subsequently dried at 110 ◦C for 48 h. After that, the shells will be removed from the fruits, dried at 110 ◦C for 48 h, ground and granulometrically separated. The proximate analysis of the raw material used in this study by ASTM-D1762 Standards revealed moisture, ash, volatile matter, and fixed carbon content values of 3.52, 1.28, 74.60, and 20.60%, respectively.[17]
3.6.2. PREPARATION OF ACTIVATED CARBON
The raw material with particle size between 250 µm and 425 µm, to be placed in a horizontal stainless steel reactor and heated in a furnace at the rate of 20 ◦C min−1 from room temperature to 500 ◦C, and maintained at this temperature for 2 hour. The obtained char should be mixed with NaOH pellets and 10 mL of water, at the ratio of 3:1 (NaOH:char) in a vertical stainless steel reactor under magnetic stirring for 2 hour and then dried at 130 ◦C for 4 hour. The reactor containing the dry mixture should be set into a furnace under N2 flow of 100 cm3 min−1, and heated at the rate of 20 ◦C min−1 to the final temperature of 700 ◦C, which to be maintained for 1.5 hour. After cooling, the resulting mixture should be washed with a 0.1 M solution of HCl followed by hot distilled water until pH value approximately 6.5 to eliminate activating agent residues and other inorganic species formed during the process. In the washing step, the activated carbon will be separated using 0.45-µm membrane filters. The carbon obtained to be dried at 110 ◦C for 24 hour and kept in tightly closed bottles for further analysis. The prepared activated carbon at NaOH:char ratio of 3:1 to be labeled as AC-3. The activated carbon yield will be defined as the final weight of product after activation, washing, and drying. The percent yield will be determined from the relation:
Yield %= wcwox100
where wc and wo are the final activated carbon dry weight (g) and the precursor dry weight (g), respectively.
3.6.3. METHYLENE BLUE REMOVAL
Initially, the experiment to be conducted in a conventional batch system and basic stirred system as explained. These two results will then be compared to find out which system has better adsorption efficiency of dye removal. Then, system that gives the better adsorption rate will be studied to find the optimum condition that will give the maximum adsorption rate.
Batch Experiment
10mg of methylene blue will be added into 1 liter of water in a beaker to prepare 10 ppm of methylene blue solution. Then, 10g of activated carbon (adsorbent) will be added into the solution and stirred (200 rpm) for 2 hours to study the adsorption rate of the dye. Agilent Cary 60 UV-Vis spectrophotometer will be used to analyze the concentration of dye in the solution.
STIBA System Experiment
10mg of methylene blue will be added into 1 liter of water in a beaker to prepare 10 ppm of methylene blue solution. Then, 10g of activated carbon (adsorbent) will be fit into the fin of the impeller which will be then attached to the overhead stirrer as shown in Figure 3.4Figure 3.4. Then the solution will be stirred (200 rpm) for 2 hours. Agilent Cary 60 UV-Vis spectrophotometer will be used to analyze the concentration of dye in the solution.
STIBA System with 10ppm, 20ppm and 30ppm with Variable Speed
10mg of methylene blue will be added into 1 liter of water in a beaker to prepare 10 ppm of methylene blue solution. Then, 10g of activated carbon (adsorbent) will be fit into the fin of the impeller which will be then attached to the overhead stirrer as shown in Figure 3.4Figure 3.4Figure 3.4. Next, the solution will be stirred for 50 rpm, 100 rpm and 200 rpm for 2 hours. The manipulated variable for this experiment is the impeller speed. Agilent Cary 60 UV-Vis spectrophotometer will be used to analyze the concentration of dye in the solution. This steps will be repeated with 20mg of methylene blue to prepare 20 ppm of methylene blue solution and 30mg of methylene blue to prepare 30 ppm of methylene blue solution.
4 REFERENCES
[1] K. Shen and M. A. Gondal, “Removal of hazardous Rhodamine dye from water by adsorption onto exhausted coffee ground,” Journal of Saudi Chemical Society, vol. 21, Supplement 1, pp. S120-S127, 1// 2017.
[2] C. Palma, L. Lloret, A. Puen, M. Tobar, and E. Contreras, “Production of carbonaceous material from avocado peel for its application as alternative adsorbent for dyes removal,” Chinese Journal of Chemical Engineering, vol. 24, no. 4, pp. 521-528, 2016/04/01/ 2016.
[3] P. M. Birgani et al., “An efficient and economical treatment for batik textile wastewater containing high levels of silicate and organic pollutants using a sequential process of acidification, magnesium oxide, and palm shell-based activated carbon application,” Journal of Environmental Management, vol. 184, Part 2, pp. 229-239, 12/15/ 2016.
[4] A. Susanty, D. Puspitasari, D. I. Rinawati, and T. Monika, “Achieving cleaner production in SMEs batik toward innovation in production process,” in 2013 International Conference on Engineering, Technology and Innovation, ICE 2013 and IEEE International Technology Management Conference, ITMC 2013, 2015.
[5] Sutisna et al., “Batik Wastewater Treatment Using TiO2 Nanoparticles Coated on the Surface of Plastic Sheet,” Procedia Engineering, vol. 170, pp. 78-83, // 2017.
[6] N. S. Zainudin, M. H. Yaacob, and N. Z. M. Muslim, “Voltammetric determination of reactive black 5 (RB5) in waste water samples from the batik industry,” Malaysian Journal of Analytical Sciences, Article vol. 20, no. 6, pp. 1254-1268, 2016.
[7] H. Fida, G. Zhang, S. Guo, and A. Naeem, “Heterogeneous Fenton degradation of organic dyes in batch and fixed bed using La-Fe montmorillonite as catalyst,” Journal of Colloid and Interface Science, vol. 490, pp. 859-868, 3/15/ 2017.
[8] K. A. Adegoke and O. S. Bello, “Dye sequestration using agricultural wastes as adsorbents,” Water Resources and Industry, vol. 12, pp. 8-24, 2015/12/01/ 2015.
[9] A.-A. Peláez-Cid, A.-M. Herrera-González, M. Salazar-Villanueva, and A. Bautista-Hernández, “Elimination of textile dyes using activated carbons prepared from vegetable residues and their characterization,” Journal of Environmental Management, vol. 181, pp. 269-278, 2016/10/01/ 2016.
[10] G. Upendar, S. Dutta, J. Chakraborty, and P. Bhattacharyya, “Removal of methylene blue dye using immobilized bacillus subtilis in batch & column reactor,” Materials Today: Proceedings, vol. 3, no. 10, pp. 3467-3472, 2016/01/01/ 2016.
[11] H. Singh, G. Chauhan, A. K. Jain, and S. K. Sharma, “Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions,” Journal of Environmental Chemical Engineering, vol. 5, no. 1, pp. 122-135, 2// 2017.
[12] V. Russo, D. Masiello, M. Trifuoggi, M. Di Serio, and R. Tesser, “Design of an adsorption column for methylene blue abatement over silica: From batch to continuous modeling,” Chemical Engineering Journal, vol. 302, pp. 287-295, 10/15/ 2016.
[13] N. Mohammed, N. Grishkewich, H. A. Waeijen, R. M. Berry, and K. C. Tam, “Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns,” Carbohydrate Polymers, vol. 136, pp. 1194-1202, 2016/01/20/ 2016.
[14] M. I. Khan, T. K. Min, K. Azizli, S. Sufian, H. Ullah, and Z. Man, “Effective removal of methylene blue from water using phosphoric acid based geopolymers: Synthesis, characterizations and adsorption studies,” RSC Advances, Article vol. 5, no. 75, pp. 61410-61420, 2015.
[15] Kusmiyati, P. A. Listyanto, D. Vitasary, R. Indra, D. Islamica, and Hadiyanto, “Coal bottom ash and activated carbon for removal of vertigo blue dye in batik textile waste water: Adsorbent characteristic, isotherms, and kinetics studies,” Walailak Journal of Science and Technology, Article vol. 14, no. 5, pp. 427-439, 2017.
[16] I. N. H. M. Amin and M. H. M. Nizam, “Assessment of membrane fouling indices during removal of reactive dye from batik wastewater,” Journal of Water Reuse and Desalination, Article vol. 6, no. 4, pp. 505-514, 2016.
[17] A. L. Cazetta et al., “NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption,” Chemical Engineering Journal, vol. 174, no. 1, pp. 117-125, 2011/10/15/ 2011.
[18] M. A. Islam, S. Sabar, A. Benhouria, W. A. Khanday, M. Asif, and B. H. Hameed, “Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption,” Journal of the Taiwan Institute of Chemical Engineers, vol. 74, pp. 96-104, 2017/05/01/ 2017.
[19] B. H. Beakou, K. El Hassani, M. A. Houssaini, M. Belbahloul, E. Oukani, and A. Anouar, “Novel activated carbon from Manihot esculenta Crantz for removal of Methylene Blue,” Sustainable Environment Research, 2017/07/04/ 2017.
[20] A. A. Spagnoli, D. A. Giannakoudakis, and S. Bashkova, “Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters,” Journal of Molecular Liquids, vol. 229, pp. 465-471, 2017/03/01/ 2017.
[21] K. Y. Foo and B. H. Hameed, “Coconut husk derived activated carbon via microwave induced activation: Effects of activation agents, preparation parameters and adsorption performance,” Chemical Engineering Journal, vol. 184, pp. 57-65, 2012/03/01/ 2012.
[22] K. Y. Foo and B. H. Hameed, “Textural porosity, surface chemistry and adsorptive properties of durian shell derived activated carbon prepared by microwave assisted NaOH activation,” Chemical Engineering Journal, vol. 187, pp. 53-62, 2012/04/01/ 2012.
[23] K. Y. Foo and B. H. Hameed, “Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue,” Desalination, vol. 275, no. 1, pp. 302-305, 2011/07/15/ 2011.
[24] K. Y. Foo and B. H. Hameed, “Microwave-assisted preparation of oil palm fiber activated carbon for methylene blue adsorption,” Chemical Engineering Journal, vol. 166, no. 2, pp. 792-795, 2011/01/15/ 2011.
[25] W. A. Khanday, F. Marrakchi, M. Asif, and B. H. Hameed, “Mesoporous zeolite–activated carbon composite from oil palm ash as an effective adsorbent for methylene blue,” Journal of the Taiwan Institute of Chemical Engineers, vol. 70, pp. 32-41, 2017/01/01/ 2017.
[26] M. A. Islam, A. Benhouria, M. Asif, and B. H. Hameed, “Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes,” Journal of the Taiwan Institute of Chemical Engineers, vol. 52, pp. 57-64, 2015/07/01/ 2015.
[27] S. M. Miraboutalebi, S. K. Nikouzad, M. Peydayesh, N. Allahgholi, L. Vafajoo, and G. McKay, “Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis,” Process Safety and Environmental Protection, vol. 106, pp. 191-202, 2017/02/01/ 2017.
[28] A. E. Ofomaja, “Sorption dynamics and isotherm studies of methylene blue uptake on to palm kernel fibre,” Chemical Engineering Journal, vol. 126, no. 1, pp. 35-43, 2007/02/01/ 2007.
[29] P. M. K. Reddy, P. Verma, and C. Subrahmanyam, “Bio-waste derived adsorbent material for methylene blue adsorption,” Journal of the Taiwan Institute of Chemical Engineers, vol. 58, pp. 500-508, 2016/01/01/ 2016.
[30] K. T. Wong, N. C. Eu, S. Ibrahim, H. Kim, Y. Yoon, and M. Jang, “Recyclable magnetite-loaded palm shell-waste based activated carbon for the effective removal of methylene blue from aqueous solution,” Journal of Cleaner Production, vol. 115, pp. 337-342, 2016/03/01/ 2016.
[31] K. Y. Foo and B. H. Hameed, “Dynamic adsorption behavior of methylene blue onto oil palm shell granular activated carbon prepared by microwave heating,” Chemical Engineering Journal, vol. 203, pp. 81-87, 2012/09/01/ 2012.
[32] H. D. Setiabudi, R. Jusoh, S. F. R. M. Suhaimi, and S. F. Masrur, “Adsorption of methylene blue onto oil palm (Elaeis guineensis) leaves: Process optimization, isotherm, kinetics and thermodynamic studies,” Journal of the Taiwan Institute of Chemical Engineers, vol. 63, pp. 363-370, 2016/06/01/ 2016.
[33] D. Pathania, S. Sharma, and P. Singh, “Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast,” Arabian Journal of Chemistry, vol. 10, pp. S1445-S1451, 2017/02/01/ 2017.
[34] S. Zhang, Z. Wang, Y. Zhang, H. Pan, and L. Tao, “Adsorption of Methylene Blue on Organosolv Lignin from Rice Straw,” Procedia Environmental Sciences, vol. 31, pp. 3-11, 2016/01/01/ 2016.
[35] G. O. El-Sayed, “Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber,” Desalination, vol. 272, no. 1, pp. 225-232, 2011/05/03/ 2011.
[36] V. O. Njoku, M. A. Islam, M. Asif, and B. H. Hameed, “Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide,” Journal of Analytical and Applied Pyrolysis, vol. 110, pp. 172-180, 2014/11/01/ 2014.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Manufacturing"
Manufacturing describes the process of using raw materials to produce goods. Manufacturing involves workers making use of heavy machinery, tools, and other resources throughout the process.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




