Could Epigenetic Therapy Replace Current Treatment Methods for Acute Myeloid Leukaemia?
Info: 8720 words (35 pages) Dissertation
Published: 14th Feb 2022
Tagged: MedicineCancerBiomedical Science
Abstract
Acute myeloid leukaemia is the most frequent type of acute leukaemia present in adults. The current treatments, which are quite effective, have rather severe side effects and the mortality rates are quite high. With the emergence of a new field, epigenetics offers hope of restoring the cell’s proper function instead of just killing cells which is what the current treatment for AMl. Epigenetics could prevent the suppression of tumour suppressor genes that cause AML to rise in the first place and reduce the need for a bone marrow transplant. Unfortunately, epigenetic drugs (azacitidine and decitabine) do not reduce mortality rates in comparison to cytarabine and daunorubicin, but Midostaurin, a targeted drug does reduce mortality rates for patients with the FLT3 mutation which suggests that targeted therapy may be more effective than general epigenetic drugs, thus suggesting that this may be a far superior treatment method than epigenetic drugs.
Table of Contents
Introduction
Literature Review
Glossary
Discussion
Conclusion
Evaluation
Bibliography
Introduction
With the completion of the human genome project in 2003 Bill Clinton triumphantly declared that ‘it is now conceivable that our children’s children will know the term cancer only as a constellation of stars’ (National Human Genome Research Institute, 2000). Unfortunately, fourteen years later this does not seem likely to happen. We have made great strides in the field of disease and cancer but there is still a great deal left to learn. When this project was completed many thought that this was the answer to all maladies. But this is certainly not this case with the emergence of the field of epigenetics.
There is just more to disease than simply faulty genes. The addition of extra information to the DNA code, without actually changing the underlying DNA code, is called the epigenome. It is derived from the Greek word ‘epi’ which means ‘on’. Your epigenome controls which genes are switched on and which genes are switched off in all of your cells. For example, the gene to make insulin is turned on in your b cells in your pancreas but this same gene is also turned off in your muscle cells. This then leads one to think how this could perhaps be affected in cancerous cells, where tumour suppressor genes are turned off which eventually leads to rapid proliferation of these cells.
In this study, I will be looking at two primary chemotherapeutic agents (cytarabine the main drug and midostaurin, which is primarily used if a patient has an flt3 mutation) and the use of allogenic haemopoietic stem cell transplant and I will look at the efficacy of three epigenetic therapeutic agents, azacitidine and saha or suberoylanilide hydroxamic acid and decitabine. I will look at how survival rates are affected when these new agents are introduced and how this may also affect morbidity. Morbidity rates are slightly different to mortality rates in the fact that morbidity assess the quality of life of the patient, if the drugs are toxic then what problems this causes for the patient and how this affects their quality of life. I will be looking at how an emerging field, epigenetics, could change the way we treat disease, specifically cancers of the blood. Epigenetic therapy holds great hope for patients because unlike gene therapy the effect of the epigenetic drugs is reversible, histones can be deacetylated and DNA could be unmethylated again. Furthermore, the germ line will not be affected as when a sperm cell fertilises an egg cell, agents in the cytoplasm of the egg cell remove all the epigenetic markers that allowed those cells to become so specialised in the first place and what is left if a totipotent zygote[1]. Thus, if epigenetic drugs do have unintended consequences that have not yet been discovered then it is very unlikely that they will be transferred to the next generation.
Looking at AML is particularly important because it is a ‘disease of the elderly’ and with an ageing population, there will most likely be higher incidences of the disease and we must come up with less toxic treatment methods. Moreover, about 15-20% of patients who had a solid tumour and were treated with chemotherapy usually develop AML. This is a terrible consequence of treatment and highlights the desperate need for better treatments of cancer not just for AML but for a wide variety of cancers. (Kumar, 2011)
I will not however be looking at the economical aspect of these drugs. I think that efficacy must certainly be established before we look at the economic viability of the drugs. Whilst this would certainly be an interesting topic, as the potential lifelong requirement of some epigenetic drugs such as azacitidine may cause a great deal of stress and anxiety for patients who live in countries where lifesaving drugs are not necessarily guaranteed, America for example. This could be further investigated, but this essay will focus on efficacy. In order to determine whether epigenetic therapy can replace current chemotherapy agents, a higher efficacy for epigenetic agents needs to be determined when compared to standard chemotherapy. Efficacy can be defined by how well a drug achieves the desired goal, in this case removing as many cancerous cells as possible and preventing them from returning for as longs as possible. Efficacy can be measured in mortality rates but this must be then compared with morbidities and side effects of the drugs.
Literature Review
What is Acute Myeloid Leukaemia?
The bone marrow contains lots of stem cells, which eventually become either white blood cells (also known as leukocytes), red blood cells (also known as erythrocytes) or platelets. These cells are different to other cells in the body because the red blood cells do not just divide by mitosis in order to create more red blood cells because they do not contain any DNA. Therefore, new red blood cells need to be made in the bone marrow in order to replace any erythrocytes that have died. In healthy bone marrow stem cells differentiate into different types of cells depending on their environment. The differentiation pathway of various cells is illustrated in the diagram below. When these stem cells differentiate the cells become more specialised which allows them to perform a certain function better. An example is that erythrocytes do not contain any DNA to allow the cells to contain more haemoglobin (a protein that carries oxygen) which in turn allows the cell to transport more oxygen.
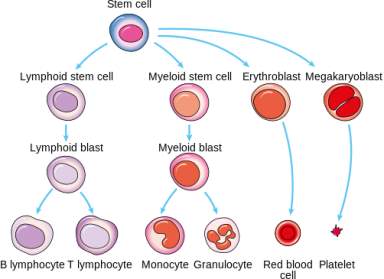
Figure 1. shows a schematic representation of the pathway that stem cells take in order to become different cells in the blood. (Martin, 2014)
However, in acute myeloid leukaemia, myeloid blasts, which are a type of immature white blood cell that have not fully differentiated, are abnormal. They do not follow the normal pathway to become healthy functioning monocytes and granulocytes. These abnormal cells are called ‘leukaemia cells’ and they build up in the bone marrow which means that there is less space for healthy white blood cells, red blood cells and platelets to develop. The cells also build up in the blood and causes severe problems. It means that there are not enough healthy blood cells and this is important as red blood cells carry oxygen to cells around the body and white blood cells are essential to the immune system as they help fight off invading pathogens. Platelets are also very important as they form blood clots at the site of a wound to prevent bacteria from getting in and infecting the wound and to also prevent more blood from escaping the body.
Because there are leukaemia cells present in the blood, this means that it is very easy for the cancer to metastasize (travel to different parts of the body and begin to rapidly divide at the new site, forming new tumours). The cancerous cells can spread to the brain, spinal cord, skin and even the gums. There are 12 different subtypes of AML and they are defined by how mature the cancer cells are when the patient is diagnosed and just how different the cells are to normal cells. (National Cancer Institute , 2017)
What Causes AML to Arise?
Cancerous cells usually arise from mutation in the DNA. A mutation is a change to the original DNA sequence. DNA consists of 4 bases (A, T, C, G). For example, is we had a fictional DNA sequence of ATTCGAGGCT, a mutation could mean substituting the first A for a C or removing the A altogether.
Mutations occur when DNA is not replicated faithfully. Many patients with AML have mutations in specific genes such as the FLT3, KIT or RAS gene. The FLT3 and KIT gene code for proteins that belong to the tyrosine kinase family. These proteins are situated on the cell membrane and they transmit signals into the cell. These genes control cell division and the KIT gene also codes for proteins that are important for the development of hematopoietic stem cells, which are cells in the bone marrow which eventually become blood cells, and immune cells called mast cells. (U.S. national Library of Medicine, 2017).
Mutations in the FLT3 occur in about 30% of patients with AML and is said to be ‘the most common molecular abnormality in acute myeloid leukaemia’. There are two prominent types of mutations that occur in this gene. One of them is an internal tandem duplication of DNA sequences found in exons 14 and 15. And the other type of mutation is a D835 mutation. Both of these mutations activate the FLT3 receptor tyrosine kinase protein in early haematopoietic cells (Mayo Clinic, n.d.). FLT3 mutations also become more common with age. (U.S. national Library of Medicine, 2017). A mutation in the FLT3 gene ‘leads to production of proteins that cause cell growth and inhibit cell death through apoptosis’. (Mayo Clinic, n.d.)
Larger changes that can occur to the DNA are changes to the chromosomes which occurs in most cases as 40-50% of patients with AML have the right number of chromosomes which are all the right shape and size. This is called a normal karyotype. (Kumar, 2011). There are four major changes that can occur to the chromosomes. One of them is translocation, which is the most common cause of leukaemia. It occurs when one part of a chromosome breaks off and attaches itself to a different chromosome. This can turn on oncogenes or turn off genes that would usually cause blood cells to mature such as RUNX1 and RARa. A deletion can also occur in a chromosome where a piece of a chromosome is lost. This results in a gene being lost and could potentially be a tumour suppressing gene. An inversion can also occur when a part of a chromosome is reversed which can also result in losing a gene because the genes on the chromosome can now no longer be read. Addition and duplication can also occur in chromosomes which leads to multiple copies of genes, which could potentially be devastating if a gene or several genes are oncogenes (American Cancer Society, 2016).
One of the characteristic traits of cancer cells is their uninhibited proliferation, where they divide many, many times (much more than the average cell) to produce a mass of cells, a tumour. This rapid division is associated with oncogenes and tumour suppressor genes. The former is associated with proteins that tell the cell to divide and the latter tells the cell when to stop dividing. It ensures that the cell does not begin to divide rapidly, which ensures that the cell does not become cancerous. But of course, this can go wrong. Patients with AML have a distinct lack of DNA methylation. This will be explored in the next section but DNA methylation is associated with genes being turned off so if a gene is hypomethylated (under methylated) then it does not have as many methyl groups as it should do, resulting in a gene being turned on. This of course is disastrous when this is a tumour suppressor gene. (Esteller, 2002)
What are the current treatments for leukaemia?
The treatment for AML is separated into different parts. When a patient is first diagnosed with AML they undergo induction therapy, which is the first part of their treatment and aims to eliminate as many leukaemia cells as possible. The intensity of the treatment is dependent on the person’s age and health. The most intensive chemotherapy is given to people under the age of 60. As the goal is to get rid of as many cancerous cells as possible usually very toxic drugs are prescribed. One chemotherapy drug is called cytarabine. This works by inhibiting DNA replication. It is converted to a certain form by the cell and is then incorporated into the DNA instead of cytidine (a DNA base). This then means that the DNA molecule cannot rotate so the DNA molecule cannot be replicated. Furthermore, it ‘also inhibits DNA polymerase, resulting in a decrease in DNA replication and repair’ (U.S. National Library of Medicine, 2005). Because it affects DNA replication this will most affect cells that rapidly divide, the leukaemia cells and stops them in their tracks. But this also means that the drug will attack hair growth as well and healthy tissue as well.
Another drug that is used is daunorubicin, which is an anthracycline. It is an antibiotic. The drug also affects the replication of DNA by interacting with it and it also affects the repair of DNA by repair mechanisms. And affects protein synthesis of the cell, causing it to die. (U.S. National Library of Medicine, 2005)
A variety of other drugs can also be given to patients, depending on the patients themselves and the stage of the cancer but this report will be looking at the two main drugs: Cytarabine and daunorubicin; It will also look at a drug given to patients who have the FLT3 mutation. This drug is called Midostaurin.
Cytarabine is known as a ‘cytotoxic antimetabolite’. Antimetabolites are very similar to normal substances within the cell, they attack cells at very specific phases in the cell cycle which in this case is the dividing phase as cancerous cells rapidly divide, therefore they are taken up by the cancerous cells. (Chemocare, n.d.) When they are inside the cells, they cause a deficiency of DNA and RNA in cancer cells (which are necessary to for cells to grow and multiply). This means that the cells grow in an unbalanced way and causes the cell to die. Unfortunately, as cytarabine affects rapidly dividing cells they can also affect normal healthy cells such as blood cells and hair cells, the reduction in the production of blood cells means that people are susceptible to infection. For this reason, doses of cytarabine are administered in various intervals to allow normal cells to recover from the detrimental effects of the drug. However, during this period, cancer cells will also recover and begin to replicate again. In most chemotherapy regimens, doses are administered in courses at various intervals to allow normal cells to recover from the adverse effects of the anticancer medicines between doses.
Radiation therapy may also be used as well if the leukaemia has spread to other parts of the body such as the brain or the spinal cord, or chemotherapy may also be given into the cerebrospinal fluid.
A stem cell or bone marrow transplant may be recommended a couple of weeks after the chemotherapy has been completed. A biopsy will be completed and it should show a few bone marrow cells and only a small portion of blasts. An autologous stem cell transplant means that stem cells from the patient are removed from their bone marrow or blood and is frozen before chemotherapy begins. After the treatment has finished, the stem cells are injected into the patient and after a couple of week the stem cells begin to differentiate into new white blood cells. For a bone marrow transplant a small portion of another person’s bone marrow, which is a match for the patients’, is taken and injected into the patient. This replaces the blood cells lost during chemotherapy. (American Cancer Society, 2017)
What is Epigenetics?
DNA codes for proteins, however, the specific proteins that each specialised cell produces is controlled by epigenetics. Epigenetics is additional to DNA, they are chemical ‘tags’, that changes the phenotype without changing the genotype. This means that the genotype, the complete set of alleles in a cell stays the same but the phenotype, the physical trait of the cell, what can be observed changes. This is way beta cells is the pancreas produce insulin but muscle cells do not. The complete set of chemical tags on a personas DNA makes up their epigenome. One chemical tag is a methyl group (one carbon atom and three hydrogen atoms, CH3). This can be attached to cytosine, base C, but only if the C is followed by a G (guanine). This C and G pair are known as CpG islands. If a methyl group is attached to a CpG island then other proteins are attracted to it and these proteins effectively turn the gene off, this is called DNA methylation. This means that the polypeptide that the gene codes for is no longer produced. However, if there are not many methyl groups attached to the CpG islands then the gene is turned on and the polypeptide will be produced (Carey, The Epigenetic Reolution, 2011). This is shown in the diagram below.
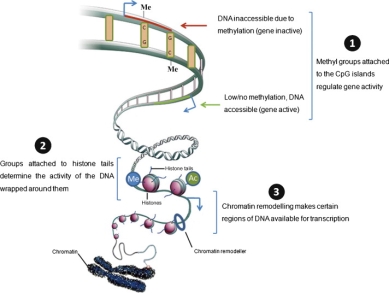
Figure 2: Shows A schematic representation of epigenetic markers on dna and how this affects gene expression
Epigenetic changes, the amount of methylation or other chemical tags, can be affected by the environment and any modifications to the epigenome can be passed on to daughter cells when the cell divides as the epigenetic tags are copied along with the DNA. There are many epigenetic processes such as methylation, acetylation, phosphorylation, ubiquitylation, and sumolyation, but in this report DNA methylation and histone acetylation will be the primary focus.
Epigenetic tags are added and removed by gene regulatory proteins, which use enzymes to add tags to the DNA or histones, which is the protein that DNA is wrapped around to prevent it from getting tangled up, or both the DNA and histones. (Carey, The Epigenetic Reolution, 2011)
The most widely studied process is DNA methylation. This is when a methyl group (CH3) is attached to a specific sequence of DNA. This makes the DNA wrap tighter around the histones. This makes the DNA harder to read by the enzyme RNA polymerase and therefore it is harder to be transcribed into RNA. Therefore, the protein that that specific sequence of DNA codes for is not produced by the ribosomes. (Weinhold, 2006)
What is Epigenetic Therapy?
Epigenetic therapy is when drugs are used to change the existing chemical tags on DNA. This could be adding or taking away a methyl group or histone modification. In the genome, there exist tumour suppressing genes which prevent a cell from dividing uncontrollably. So, if by demethylation that gene this could stop leukemic cells from endless proliferation. This would then be passed onto its daughter cells as epigenetic tags are copied, along with the DNA so this change would be apparent in all new cells, thus stopping more tumours from forming.
Furthermore, chromatin, a combination of DNA and histones, compresses the DNA so it can fit into cells. This also controls gene expression and any changes to the histones also change the structure of the chromatin. If the chromatin is very loose then that gene will be expressed but if it is tight then the gene will not be expressed. So, a drug could be used to change the structure of the chromatin which again will mean that an oncogene is not expressed.
For some tumour suppressor genes, the fact that it is compressed by the chromatin means that it behaves almost like an embryonic cell. However embryonic cells stop making copies of themselves when they receive a certain signal but cancerous cells never receive that signal because a growth limiting signal has been silenced by the addition of a methyl group.
In one study researchers removed a methyl group from a specific sequence of bases in the genome using a drug called 5-azacytidine and a HDAC inhibitor, which blocks histones. The chromatin coils loosed and some expression was restored. This could then be applied to restore the expression of growth limiting factor genes. (Johns Hopkins, n.d.)
Azacitidine and decitabine and SAHA are all drugs that have been approved for the use of treatment of AML and they are all epigenetic drugs. They change the chemical tags in a cell, causing that cell to behave differently. (Carey, The Epigenetic Revoltion, 2011)
Discussion
Mortality Rates
There are two factors to consider when attempting to introduce a new treatment with a higher efficacy: mortality rates and morbidity rates. This section will talk about mortality rates.
Chemotherapy drugs are usually used in combination and the combinations vary depending on the patient. But one of the most common drugs is cytarabine. 66% of patients who receive standard induction therapy (cytarabine and daunorubicin) achieve remission. Remission is when the bone marrow ‘contains fewer than 5% blast cells, the blood cell counts are within normal limits, and there are no signs or symptoms of the disease’ (American Cancer Society, 2014). But this varies with each patient as age or genetic changes affect a patient’s prognosis. This is the short-term survival rate but over 5 years the survival rate lies around 30%, which means that the mortality rate lies at 70%. This can be seen in Figure 3 where patients who received high doses and intermediate doses had similar survival rates after five years. This shows that the efficacy of cytarabine remains the same regardless of dose. But in other study, higher doses of cytarabine were observed to be more effective in increasing remission rates.
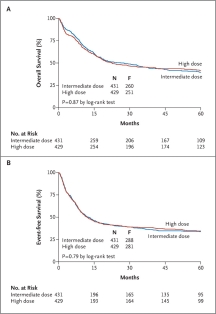
Figure 3 Shows survival rates for high dose and intermediate doses of cytarabine. Graph A shows overall survival rates and graph B shows event free survival rates (Löwenberg, 2011)
A possible reason that cytarabine is not as effective as it could be, is the fact that it can be deactivated by the liver. The liver contains enzymes which remove an amino acid from cytarabine. This is normally a very important process as it converts dangerous products into urea by deamination, but in this instance, it renders cytarabine useless. It also means that cytarabine cannot affect any leukaemia cells that may be present in the liver, as there is a low concentration of it in the liver. If the leukaemia cells are not affected they can build up their and produce tumours. This will drastically affect survival rates because the liver performs so many functions and if it is dysfunctional then a liver transplant may be required and that in itself comes with a host of risks.
But on the other hand, this may not be as devastating as if these leukemic cells accumulated in other organs because the liver does have the ability to regenerate, to an extent, so if a patient were to undergo surgery to remove a, relatively small to medium sized, mass from the liver then they would not require an organ transplant, which would unfortunately not be the case with other organs.
On the other hand, one of the reasons that cytarabine is so effective as a drug is that it blocks the leukemic cells from replicating. By preventing the cells from replicating it is stopping the cancer at the very first stage. This is far more effective at getting rid of lots of leukemic cells, which is very important as induction therapy attempts to get rid of as many leukemic cells as possible in the first stage in order to minimise the devastating effects of the aggressive cancer. Cytarabine, when compared to decitabine (a hypomethylating agent) has been shown to kill more leukemic cells in vitro (in a colony assay) than decitabine[2]. This shows the higher toxicity of cytarabine for cancerous cells which indicates higher efficacy than epigenetic drugs. But these cells were observed under laboratory conditions instead of inside the body.
Although cytarabine has been shown to kill more leukemic cells in vitro this does not translate into better survival rates. When we look at the mortality rate when decitabine was used the mortality rate after 1 year was 28%. This is much lower than when cytarabine was used. In Figure 1 after 1 year the survival rate is 60%. From this the mortality rate can be deduced as 40%. Therefore, decitabine decreases mortality rate. This is probably due to the lower toxicity of decitabine. This is particularly important in elderly patients as highly toxic drugs will increase mortality rates as elderly patients are less able to tolerate the toxic chemicals.
This supports the idea that decitabine could replace cytotoxic agents for elderly patients who are less able to deal with aggressive treatments (Ramos, 2015)
In addition, there is a 12-hour gap between doses of cytarabine which could allow the cancerous cells time to regroup and allows them to build up again. If the time between doses were reduced this may prevent the cells from rapidly reproducing and prevent the number of these cancerous cells from increasing. However, this in itself comes with adverse side effects as it does not give the body time to recover and may in fact have more adverse side effects as the treatment progresses with more rounds of chemotherapy.
However, some patients are resistant to cytarabine, there is interference with the normal apoptic pathway in AML cells and decitabine reactivates the regulation of this pathway so can be used to treat patients who are resistant to cytarabine. (Momparler, 2013)
Epigenetic changes do not occur in a vacuum and actually the reduced efficacy of cytarabine is through epigenetic silencing. There is a protein that tells a cell to die. When the gene that codes for that protein is silenced through DNA methylation the protein is not produced and the cell does not die. Decitabine reactivates this gene, ‘Foxo3’, which then increases the TRAIL pathway. This pathway tells the cell to die, if it is abnormal. In doing this, cancerous cells are prevented from migrating to other parts of the body, preventing metastasis.
In one study 114 patients (17% of the total group) were given either azacitidine or decitabine or both of the drugs. They were also given a histone deacetlyase inhibitor. These patients received these drugs as part of their induction therapy, the first part of the treatment.

Figure 4 shows the probability of survival 10 years after initial diagnosis: A Kaplan-Meier Curve (Quintás-Cardama, 2012)
Patients who received epigenetic therapy had lower rates of achieving complete remission, in one study 28% of patients receiving epigenetic therapy achieved complete remission but 42% of patients who received chemotherapy achieved complete remission. Furthermore, the reduction of tumours was also less for the people receiving epigenetic therapy than for people receiving chemotherapy. In this study, the reduction of tumours was defined as:
‘ORR = CR + CR with incomplete platelet recovery’
It was 29% for patients receiving epigenetic therapy but 47% in patients who received standard chemotherapy treatments. The epigenetic therapy used in this study was a hypomethylating agent. However, the mortality rate was lower for epigenetic therapy:
‘The early mortality rates (mortality within the first 8 weeks of therapy) were 18% with intensive chemotherapy and 11% with hypomethylating agents’
and ‘The 2-year relapse-free survival rates with intensive chemotherapy and epigenetic therapy were 30% and 40%, respectively (P = .843). The median survival times were 6.7 and 6.5 months (P = .413), respectively’.
Therefore, although a higher percentage of patients achieved complete remission, this, unfortunately, did not mean that mortality rates decreased. (Quintás-Cardama, 2012)
Whilst decitabine and azacitidine improved complete remission rates but did not significantly decrease mortality rates, targeted therapy seems to have a different outcome.
Midostaurin is used to target patients with the FLT3 mutation. Patients who received this drug had a decrease of risk of death by 22% compared to a placebo. And 51.4% of patients were alive 4 years after initial diagnosis. (National Cancer Institute, 2017)
An example of a histone deacetlyase inhibitor is saha. It has the same effects as DNA methylation, in that it prevents acetyl group from attaching on to the lysine on the histone tails of certain gens. This means that the gene is not expressed as much. In one study SAHA increased the regulation of the TRAIL pathway which is what regulates the death of cancerous cells. The effect of SAHA was improved by using it with homoharringtonine. The combination prevented the growth of a culture of leukaemia cells in vivo and increased cells death. Therefore, SAHA in combination with homoharringtonine, at low concentrations can re-regulate the TRAIL pathway and can be used in treatments. (Cao, 2013)
This shows us that whilst SAHA may not be particularly effective on its own, it may be effective in combination with other drugs to regulate proper apoptic pathways. This is a very complex pathway and moves away from the tumour suppression gene into actually killing cells. This could be very useful as if it merely reactivated the tumour suppressor gene, this may be permanent and may result in patients taking lifelong medication with the fear of the cancer returning at any time. This is particularly similar to the effects of cytarabine which also induces apoptosis in leukemic cells. Therefore, this may work extremely well in induction therapy, in which the aim is to get rid of as many cancerous cells as possible but it may not stop the production of more cancerous cells once therapy stops. (Glozak & Seto, 2007)
Another study looked at the effects of histone deacetylases instead of hypomethylating agents, histone deacetylases are quite effective as they allow tumour suppressor genes to be expressed again. (Quintas-Cardama, 2010)
In one study involving decitabine which compared it against cytarabine, ‘patients demonstrated significantly improved complete remission rates, but the survival difference did not reach significance’ (Malik, 2014). This is the opposite of azacitidine which we saw earlier. However, the insignificant difference in survival rates could be attributed to the fact that the patients who took part in this study were elderly and elderly patients, unfortunately do have a lower overall survival rate when compared with younger adult patients. This is due to the fact that it is difficult for them to take on intense therapies and leads to comorbidities
Morbidity Rates
Another way to look at the efficacy of treatments is to look at morbidity rates. This looks at the problems that come with the use of therapeutic agents. If two agents have similar mortality rates then a drug with the higher efficacy will be the drug with the lower morbidity, i.e. fewer side effects or fewer severe side effects.
Cytarabine, for it to be most effective, requires the use of high doses but this comes with the side effect of cardiotoxicity. This means that the drug could cause heart disease, such as myocarditis (inflammation of the heart tissue) or arrhythmias (irregular beating of the hear) or cardiogenic shock (Albini, 2010). Furthermore, this is also a problem with daunorubicin, that it causes high levels of cardiotoxicity. It is also an antibiotic which comes with its own problems.
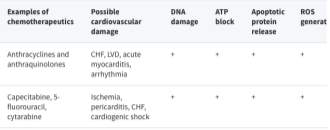
Figure 5 shows possible damage to the heart and the mechanisms through which they are likely, where + = likely. (Albini, 2010)
Anthracyclines and cytarabine interfere with DNA replication as they get in between DNA base pairs. This action produces free hydroxyl radicals [OH] instead of the usual ion [OH]–. These are extremely reactive molecules, as the oxygen has an unpaired electron. The cardiac muscle is particularly affected by these radicals and thus has the greatest damage done to it, even though this helps reduce tumours. (Hortobágyi, 2012)
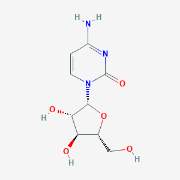
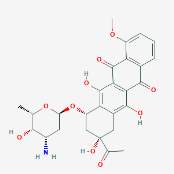
Figure 6 shows the skeletal formula of Daunorubicin and Cytarabine (National Center for Biotechnology Information., 2017)
On the other hand, epigenetic agents do not have this terrible side effect but SAHA does have other very similar side effects. These Include:
- Fatigue
- Diarrhea
- Nausea
- Taste changes
- Increased blood glucose level (hyperglycemia)
- Increased creatinine level (transient)
- Increased level of protein in the urine
- Low platelet count (thrombocytopenia) (ChemoCare, n.d.)
When compared to the side effects of cytarabine, these side effects appear quite similar and therefore it may not be a good replacement. On the other hand, if the side effects are very similar but does not have the side effect of cardiotoxicity and both drugs have similar mortality rates then SAHA could be a viable alternative because the morbidity of the drug will be lower than current chemotherapeutic agents. This could improve the quality of life of patients.
Again, the side effects of taking decitabine are very similar to the side effects associated with SAHA. They are:
- Low blood counts. White and red blood cells and platelets may temporarily decrease. This can put you at increased risk for infection, anaemia and bleeding, and may increase need for blood or platelet transfusions.
- Fatigue
- Fever
- Nausea
- Cough
- Petechiae (Tiny red dots on your skin,) and can occur with low platelet count
- Constipation
- Diarrhoea
- Hyperglycaemia – high blood glucose levels (Chemocare, n.d.)
But again, SAHA has the benefit of not increasing the chances of heart failure.
If remission is achieved, patients may then get more chemo (consolidation). Up to half of patients that get this go into long-term remission (and may be cured). But this number is also affected by prognostic factors, such as a person’s age and whether the leukaemia cells have certain gene or chromosome changes. Using an allogeneic stem cell transplant as consolidation has a higher success rate, but it also has a higher risk of death as a complication. (American Cancer Society, 2014)
Bone marrow transplants have been common treatment for AML for a while. In one cancer centre the survival rate one year after an allogenic bone marrow transplant was 75%, which surpassed their predictions of 62%. They used another type of therapy when transplanting the bone marrow cells, called t cell therapy, in which they removed the t cells from the donor’s bone marrow cells. This meant that the patient’s immune system did not recognise the donor’s bone marrow cells as foreign cells and started to attack these cells. This is a very common problem with transplants and means that patients have to be on immunosuppressant’s for a while and this means that they are at a greater risk of infection. This is known as graft – v – host disease, where the transplant is recognised as a foreign cell by the body’s immune system and it then produces a response in order to try and kill these invading cells. This reduces the efficacy of the treatment. (Memorial Sloan Kettering, 2012) The side effects of bone marrow transplants can be quite severe and some of the symptoms include an itchy rash, dry eyes, dry flaky skin, shortness of breath, joint pain or jaundice, where the skin and the whites of the eyes gain a yellowish hue. Due to the risk of the patient rejecting the transplant and the severity of these side effects it is normally recommended that patients are relatively young in order to reap the most benefit from the treatment. (NHS, 2015). This is a problem as most cases of AML occur in elderly patients, therefore, for them this is not the ideal treatment despite it being relatively effective. Furthermore, bone marrow transplants are often allogenic (the transplant cells come from a donor rather than the patient) rather than autologous (where the cells come from the patient) because some of the cells taken from the patient may be leukaemia cells, therefore this may re-introduce the cancer into the body during the transplant. Furthermore, if bone marrow transplants are to be allogenic then a donor must be found who is a match for the patient. This means that the proteins on the donor cells and the patient cells are the same. This therefore reduce the risk of rejection by the patient’s immune system. (Cancer Research UK, 2015)in one study that analysed the outcome of allogenic and autologous stem cell transplants, the patients who received allogenic transplants had a 100% success rate, all of the patients had a successful outcome whereas only 94.7% of patients who received an autologous transplant had a successful outcome, but bearing in mind that all patients involved in the study were in complete remission.
Conclusion
In conclusion, epigenetic therapy cannot replace current treatment methods. The main reason is that every patient is different and using a very specific targeted course of treatment, for example using Midostaurin if they have the FLT3 mutation will be far more effective than using epigenetic agents. As the majority of patients who get diagnosed with AML are elderly epigenetic drugs could prove to be a less aggressive approach and decrease mortality rates in that age bracket although this is quite hard to determine due to the presence of comorbidities.
Furthermore, the use of targeted therapy which is specifically designed to deal with genetic mutations in patients reduces mortality rates much more than epigenetic therapy does. And since the goal of treatment is to reduce mortality rates targeted therapy appears to be the way forward in terms of treatments. In addition, epigenetic therapy will only affect the patient’s epigenetics and not all the causes of AML are epigenetic, some are genetic and the use of hypomethylating drugs will not be particularly useful.
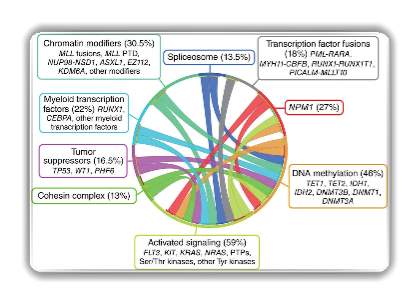
Figure 7 shows a circos plot in which the cause of AML are linked to one another and demonstrates that there is no one single cause. For EXAMPLE, patients who have mutations with activation signalling could also have problems with Myeloid transcription factors.
Therefore, the use of decitabine or azacitidine would not be effective if a patient has a problem with myeloid transcription factors than if a targeted therapy was used for it.
Evaluation
I did achieve my aims, I found out what epigenetic agents are currently being trialled, how they work and how they compare to standard chemotherapeutic agents. I faced quite a few challenges in doing the project. The first of which was actually understanding the journals. They were quite complex, using complicated terms and the statistical analysis included in the journal were often quite a challenge to comprehend. I also struggled with time management, stumbling through the first few weeks as I was not sure about my topic and once I did choose a topic I still found it quite hard to start my research as I felt like there was so much information to sort through. To overcome this, I conducted some very preliminary research and identified some key questions to start with, such as: what exactly is epigenetics? What exactly is acute myeloid leukaemia? How does it arise? This allowed me to gain a foothold in the vast amount of research available online and narrow down my focus. I also scheduled in specific times in the week to do my research and write up my project so that I would be consistent over the weeks. I scheduled my research for Wednesday evenings, Thursday mornings and Sunday evenings as these were convenient times for me and still allowed me to do other things.
Another problem I faced was that over the summer holidays I was on holiday and I did not have access to the internet. Anticipating this I brought two books with me, which whilst I had read previously I could not remember precise details, and I reread them and made detailed notes on them, particularly the mechanisms by which epigenetic agents work. Therefore, in the autumn term I had quite a bit of extra work but scheduled in some extra time in order to catch up.
During this project, I have learned that detailed planning is necessary to write a coherent argument that flows, I think this is particularly true for my project as there were so many technical terms that I had to keep track of. I have also learnt to narrow my research instead of just using google. At first, I used Google which provided me with not particularly relevant sources but then I came across the blood journal which was extremely useful as it focused on blood disorders and cancers of the blood. I learned that when putting together a project you should keep a detailed log of what you have done and make sure you write down everything you have learnt from your sources as at first, I did not do this and later on I forgot what I had learned from them.
The main limitation of my project is that I only used secondary data, which was the only available data for me. Furthermore, the studies I used in my discussion did not have the same number of patients and the doses of the drugs administered to each patient varied. This means that this is a naïve direct comparison, in which ‘comparison between two drugs refers to an assessment or analysis where clinical trial results for one drug are directly compared with clinical trial results for another drug. There is no attempt to adjust for any discordance in comparators between/among the trials’. (Kim, 2013)
Bibliography
Albini, A. e. (2010, January 6). Cardiotoxicity of Anticancer Drugs: The Need for Cardio-Oncology and Cardio-Oncological Prevention. journal of the National Cancer Institute.
American Cancer Society. (2014, December 9). Treatment Response Rates for Acute Myeloid Leukemia. Retrieved October 2017, from American Cancer Society: https://www.cancer.org/cancer/acute-myeloid-leukemia/treating/response-rates.html
American Cancer Society. (2016, Feburary 22). Do We Know What Causes Acute Myeloid Leukemia? Retrieved from American Cancer Society: https://www.cancer.org/cancer/acute-myeloid-leukemia/causes-risks-prevention/what-causes.html
American Cancer Society. (2017, November 21). Typical Treatment of Most Types of Acute Myeloid Leukemia (Except Acute Promyelocytic M3). Retrieved from American Cancer Society: https://www.cancer.org/cancer/acute-myeloid-leukemia/treating/typical-treatment-of-aml.html
Cancer Research UK. (2015, March 18). Who can donate bone marrow or stem cells. Retrieved October 2017, from Cancer Research UK: http://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/bone-marrow-stem-cell-transplants/who-can-donate-bone-marrow-or-stem-cells
Cao, H. e. (2013, June 7). Homoharringtonine and SAHA synergistically enhance apoptosis in human acute myeloid leukemia cells through upregulation of TRAIL and death receptors. . Molecular Medicine Reports, 1838-1844.
Carey, N. (2011). The Epigenetic Reolution. London: Icon Books.
Carey, N. (2011). The Epigenetic Revoltion. London: Icon Books.
Chemocare. (n.d.). Cytarabine. Retrieved from Chemocare: http://chemocare.com/chemotherapy/drug-info/cytarabine.aspx
Chemocare. (n.d.). Decitabine. Retrieved from Chemocare: http://chemocare.com/chemotherapy/drug-info/decitabine.aspx
ChemoCare. (n.d.). Vorinostat. Retrieved October 2017, from ChemoCare: http://chemocare.com/chemotherapy/drug-info/Vorinostat.aspx
Esteller, M. (2002, August 12). CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene, 5427-5440.
Glozak, M. A., & Seto, E. (2007, August 13). Histone deacetylases and cancer. Oncogene, 5420-5432.
Hortobágyi, G. (2012, October 23). Anthracyclines in the Treatment of Cancer. Drugs.
Jing, Y. e. (2012, September 24). Efficacy of allogeneic and autologous hematopoietic SCT in patients with AML after first complete remission. Retrieved October 2017, from Nature.com: https://www.nature.com/articles/bmt2012154
Johns Hopkins. (n.d.). What is Epigenetics? The Cancer Epigenome. Retrieved from Johns Hopkins Medicine: https://www.hopkinsmedicine.org/kimmel_cancer_center/research_clinical_trials/research/su2c/what_is_epigenetics.html
Kim, H. e. (2013, April 25). Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. British Journal of Clinical Pharmocology, 116-121.
Kumar, C. C. (2011, May 17). Genetic Abnormalities and Challenges in the Treatment of Acute Myeloid Leukemia. Genes and Cancer, 95-107.
Löwenberg, B. e. (2011, March 17). Cytarabine Dose for Acute Myeloid Leukemia. Retrieved October 2017, from The New England Journal of Medicine: http://www.nejm.org/doi/full/10.1056/NEJMoa1010222#t=article
Malik, P. e. (2014, Feburary 4). Decitabine in the treatment of acute myeloid leukemia in elderly patients. Cancer Management and Research, 53-61.
Martin, A. (2014, September 26). Classifying Leukaemia: One size doesn’t fit all. Retrieved September 2017, from Cancerresearch UK / Science Blog: http://scienceblog.cancerresearchuk.org/2014/09/26/classifying-leukaemia-one-size-doesnt-fit-all/
Mayo Clinic. (n.d.). FLT3 Mutation Analysis, Varies. Retrieved from Mayo Medical Laboratories: https://www.mayomedicallaboratories.com/test-catalog/Overview/19739
Mayo Clinic. (n.d.). Test ID FLT; FLT3 Mutation Analysis, Varies. Retrieved from Mayo Medical Laboratories: https://www.mayomedicallaboratories.com/test-catalog/Overview/19739
Memorial Sloan Kettering. (2012, March 26). MSK’s One-Year Survival Rate after Allogeneic Bone Marrow Transplant Exceeds Expectations. Retrieved October 2017, from Memorial Sloan Kettering Cancer Centre: https://www.mskcc.org/blog/msk-s-one-year-survival-rate-after-allogeneic-bone-marrow-transplant-exceeds-expectations
Momparler, R. L. (2013, August 6). Optimization of cytarabine (ARA-C) therapy for acute myeloid leukemia. Retrieved October 2017, from Biomedcentral: https://ehoonline.biomedcentral.com/articles/10.1186/2162-3619-2-20
National Cancer Institute . (2017, March 6). General Information About Adult Acute Myeloid Leukemia. Retrieved September 2017, from National Cancer Institute: https://www.cancer.gov/types/leukemia/patient/adult-aml-treatment-pdq
National Cancer Institute. (2017, June 1). Midostaurin Approved by FDA for Acute Myeloid Leukemia. Retrieved October 2017, from National Cancer Institute: https://www.cancer.gov/news-events/cancer-currents-blog/2017/fda-midostaurin-aml
National Center for Biotechnology Information. (2017, November 26). Daunorubicin. Retrieved November 2017, from PubChem Compound Database: https://pubchem.ncbi.nlm.nih.gov/compound/30323#section=Top
National Human Genome Research Institute. (2000, June 26). June 2000 White House Event. Retrieved November 2017, from National Human Genome Research Institute: https://www.genome.gov/10001356/june-2000-white-house-event/
NHS. (2015, October 8). Stem cell and bone marrow transplants . Retrieved October 2017, from NHS : https://www.nhs.uk/conditions/stem-cell-transplant/risks/
Quintás-Cardama, A. e. (2012, September 27). Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Retrieved October 2017, from The Blood Journal : http://www.bloodjournal.org/content/120/24/4840.full?sso-checked=true
Quintas-Cardama, A. e. (2010, November 30). Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukaemia, 226-235.
Ramos, N. R. (2015, April 10). Current Approaches in the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. Retrieved October 2017, from Journal of clinical medicine: http://www.mdpi.com/2077-0383/4/4/665
U.S. National Library of Medicine. (2005, June 24). Pubchem: Open Chemistry Database. Retrieved from Cytarabine: https://pubchem.ncbi.nlm.nih.gov/compound/cytarabine#section=Top
U.S. National Library of Medicine. (2005, June 24). Pubchem: Open Chemistry Database. Retrieved from Daunorubicin: https://pubchem.ncbi.nlm.nih.gov/compound/daunorubicin#section=Top
U.S. national Library of Medicine. (2017, November 21). KIT proto-oncogene receptor tyrosine kinase. Retrieved November 2017, from Genetic Home Reference: https://ghr.nlm.nih.gov/gene/KIT
Weinhold, B. (2006, March). Epigenetics: The Science of Change. Environ health Perspect, 160-167.
[1] In reality, not all epigenetic markers are removed. A very select few genes are ‘imprinted’ with these markers to allow the cell to recognise which gene came from which parent. For more information look into ‘The Epigenetic Revolution’ Chapter 8 ‘The Battle of the Sexes’
[2] Reference needed
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biomedical Science"
Biomedical Science focuses on how cells, organs and systems function in the human body and underpins much of modern medicine. Biomedical Science applies parts of natural and/or formal sciences to help develop advances in healthcare.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




