Development and Improvement on a Plant-derived Vaccine for Hepatitis B
Info: 9120 words (36 pages) Dissertation
Published: 16th Dec 2019
Tagged: Infectious DiseasesPharmacology
Contents
What is a plant-derived edible vaccine?
What edible vaccines are there at the moment?
Target Evaluation: The Solution
What the plant derived edible vaccine needs to be?
Lay Summary
A plant-derived edible vaccine is when a plant is bioengineered to produce the vaccination in its tissues. Once the plant have been consumed by an individual, the vaccine in the plant causes an immune response, which leads to the production of memory cells. This means if the individual is exposed to the disease again the memory cells produced from the vaccination will reproduce and fight the disease, protecting the individual. There a several different types of edible vaccinations being developed and trialled at this moment in time however this report will focus on the development of an edible vaccination for hepatitis B virus (HBV).
Hepatitis B is a virus that affects the liver causing scarring, liver failure and liver cancer. Those at risk of being infected with hepatitis B are those who inject drugs and those who have unprotected sex. (https://www.webmd.boots.com/digestive-disorders/digestive-diseases-hepatitis-b). The (HBV) is made up of four major proteins, and exposure to just one (specifically HBsAg), leads to immunity (Guan et al, 2010). Therefore the plant will express HBsAg in its tissues to ensure immunity of hepatitis B is achieved.
There are several advantages to using plant-derived edible vaccines, these include the low cost of production, storage and administration. These advantages mean the vaccine can be made available to third world countries, benefitting the poorest and most vulnerable people. The main challenge scientists face with edible vaccines is the low levels of the vaccine protein in the plant. Low levels of proteins in the plant-derived vaccine will result in an insufficient immune response, meaning that memory cells won’t be produced and stored. This means that the individual has not been immunized and re-infection could occur if exposure to the disease occurred again.
When designing a plant-edible vaccine, there are several ways to maximise the protein production. These include choosing a suitable tissue for the protein to be expressed and stored in and choosing a suitable plant which has high levels of protein expression. For the plant to produce the foreign protein, the gene encoding for that protein must be inserted into the plants own genetic material (genome) so it is expressed. This is done by inserting the gene of interest into a vector which will insert itself into the plants genome. The engineering of the vector is important and the methods of engineering the vector to produce the most protein possible is explored in the report.
Abstract
Hepatitis B is a virus that severely affects 350mn people, while there is a parental vaccine available, it is expensive and people in the poorest countries in the world do not have access, or can’t afford the vaccine. This report explores edible vaccines, their advantages and disadvantages and explores the development of a plant-derived edible vaccine that, once consumed, would immunize the individual from hepatitis B. The main problem identified with plant-derived edible vaccines is the low protein expression levels. The target evaluation of the report discusses how to increase the protein expression in the plant. This can be achieved by codon optimization, the correct and efficient use of promoters and the design of the expression cassette that will transform the plants genome.
To develop a plant-derived edible vaccine there are several approaches and factors that need to be considered. Firstly and most importantly what protein will be expressed in the plant, it has to be able to induce a sufficient immune response that will protect the individual if the virus re-infects the individual. Secondly the transformation and expression system the plant will use to produce the protein must be decided. Next the genome that will undergo the transformation must be selected, this can be the nuclear genome or plastid genomes. The target plant species and tissue must then be chosen. Then the storage of the protein at a cellular level and strategies to ensure the levels of protein expression are sufficient are discussed.
Introduction
What is a plant-derived edible vaccine?
A plant-derived edible vaccine is a plant made pharmaceutical. The transgenic plant must trigger an immune response in the patient that has ingested the transgenic material (Chaitanya, Kumar, 2006). The plant is transformed by adding specific genes into the genome to produce antigenic proteins (Jan et al, 2016). This report is going to focus on the plant side of edible vaccines, namely the development of one for HIV. Plant based edible vaccines can use cereals like wheat and barley, fruits like bananas and vegetables like lettuce and potatoes to produce the antigens. Edible vaccines not only trigger systemic immunity, but also mucosal immunity when the plant material hits the digestive tract after ingestion (Jan et al, 2016). Mucosal immunity is very important as it is the first line of defence against the infecting pathogens (Jan et al, 2016).
How do they work?
Edible vaccines work by triggering immune responses as they contain a dead or inactive form of the disease. Because plant cell have such a tough outer wall the cells aren’t hydrolysed by gastric enzymes (Jan et al, 2016). The transgenic plant cells act by bio-encapsulation, where the cells are hydrolysed in the intestine (Jan et al, 2016). The antigens in the cell are released and taken up by M cells (Jan et al 2016). M cells are specialised epithelial cells of the mucosa associated lymphoid tissues (Gebert et al, 1996). These M cells are found on Peyer’s patches, which are made up of GALTs (gut- associated lymphoid tissue). Peyer’s patches consist of aggregated lymphoid follicles and contains about 70% of the bodies immunocytes (white blood cells that can produce antibodies) (Jung et al, 2010). The M cell can transport antigens from the intestine lumen to cells of the immune system, including macrophages and local lymphocyte populations. This in turn initiates an immune response (Jung et al, 2010) creating memory cells so the patient’s immune system can fight the disease next time pathogens enter the body.
Advantages and disadvantages?
There are a number of advantages and challenges with edible vaccines. From an economical point pf view edible vaccines are efficient as they do not require trained medical staff to administer them as they are taken orally (Jan et al, 2016). They also do not require syringes which reduces the cost of the vaccine and removes the risk of blood borne diseases and contamination (Jan et al, 2016). Edible vaccines also do not need the ‘cold chain storage’, a system which transports and stores vaccines at the appropriate temperature (Jan et al, 2016). This system is not needed as edible vaccines do not need to be kept cool as they would be either seeds, or plant tissue, both of which are stored at room temperature. The vaccine is also easier to produce. Instead of being produced in the lab, edible vaccines can be grown in the soil which again is more economical. The production process can be made more economical yet by its ability to be scaled up very quickly through breeding more transgenic plants. Edible vaccines also have greater storage advantages as seeds of the transgenic plants can be easily dried (Jan et al, 2016).
From a biological point of view edible vaccines are also more efficient as they do not require subsidiary elements to stimulate an immune response (Jan et al, 2016). Also they not only induce the systemic immune response but also the mucosal immune response (Jan et al, 2016) which is the first line of defence the body has when a pathogen first invades the host. The vaccines are safer as they do not contain the heat killed pathogens, rather just proteins from the pathogen (Jan et al, 2016).
There are however limitations to edible vaccines, namely that dosage would be a problem. The amount of protein each plant would produce would differ and so getting the right dosage in say the fruit of a plant would be challenging as the fruit size, weight, and protein synthesis levels in individual plants would differ (Jan et al, 2016). This is a problem because if the dosage is too low then the individual doesn’t have a sufficient immune response which means the individual wouldn’t be protected if the pathogen invaded them. On the other hand if the dosage was too high then the individual could become tolerant to the vaccine protein antigen and so the vaccine wouldn’t be effective (Jan et al, 2016). Another issue is the stability of the plant and protein. If the protein is expressed in potato for example then the protein has to be able to withstand high temperatures as the potato is cooked without denaturing (Jan et al, 2016). Like all plants transgene plants are also susceptible to microbial infestation (Jan et al, 2016) and dependent on factors like water, carbon dioxide concentrations, light intensity etc which all costs money to regulate.
Another key issue is the separation of the transgene and normal plants to avoid vaccine tolerance (Jan et al, 2016). Keeping these separate is of extreme importance as failure to do so would involve many people eating more than one dose and therefore gaining immune tolerance to the vaccine and therefore the vaccine would no longer be effective. Also some individuals might not want to eat transgenic plants and therefore should be able to be correctly informed as to whether the food they are eating is transgenic and contains the vaccine.
Also a consideration is whether the protein function is hampered due to the differences in post translational modifications in plant and human cells, particularly glycosylation (Jan et al, 2016). More than 50% of proteins in eukaryotes and 1/3 of proteins in biopharmaceuticals are glycoproteins (proteins with carbohydrate groups bound to them) (Gomord et al, 2010). Plant cells aren’t able to glycosylate proteins in the same pattern as human cells (Gomord et al, 2010) which is an issue as incorrect glycosylation affects the proteins structure and therefore function. The antigens produced by the plants in edible vaccines are complex and require these post-translational modifications, including glycosylation (Gomord et al, 2010), therefore it is paramount that they undergo the correct glycosylation modifications. Having said this there have been advances recently in humanising the N-glycan structures in plants using different techniques which include targeting the expression of recombinant protein to the endoplasmic reticulum (ER) (Gomord et al, 2010). This is because the N-glycans in the ER that are glycosylated to the proteins are high-Man-type (high in mannose) N-glycans which are structurally similar in plant and human cells (Gomord et al, 2010). Another approach was to inactivate the plants glycosyltransferases and add in the expression of mammalian glycoslytransferases (Gomord et al, 2010).
There are also many challenges associated with edible vaccines, namely the lack of scientific research, funding and investments (Jan et al, 2016). This is because mainstream recombinant vaccines have become much cheaper to produce (Jan et al, 2016). However this is still expensive for third world countries with about 1/5th of infants not being vaccinated against the diseases that WHO aimed to have universal vaccinations by 2000 (diphtheria, whooping cough, tetanus, measles, poliomyelitis and tuberculosis) (Jan et al, 2016). Edible vaccines that can be grown and administered without trained medical staff and equipment would revolutionise vaccines in these parts of the world.
What edible vaccines are there at the moment?
At the moment there are very few edible vaccines that have passed through the trial phases and are being used on the general public. At this moment in time edible vaccines for cholera, measles, hepatitis B and HIV are being trialled (Lal et al, 2007). For cholera, a transgenic potato with the CT-B gene transformed into it has provided promising results in trials in mice (Lal et al, 2007). In this trial one potato a week for a month plus boosters provides the mice immunity (Lal et al, 2007). A measles vaccine is being trialled on mice in tobacco. The transgenic tobacco expressing MV- H could attain antibody levels five times over the level considered practical for humans (Lal et al, 2007), therefore is looking increasing promising with the trials moving from mice to other primates (Lal et al, 2007). The vaccine is also being developed in rice, lettuce and baby food (Lal et al, 2007).
The Problem
Around 2 billion people worldwide are infected by Hepatitis B, with 350 million being chronically infected (Streatfield, 2000), making it a global health problem. While there are vaccines for Hepatitis B available, they are restricted from being used worldwide because of the costs of buying the vaccine, and paying for the trained personnel to administer the vaccine (Streatfield, 2005). These costs mean that developing countries struggle to afford them leaving the poorest vulnerable to contracting Hepatitis B. Therefore oral delivery of the vaccine would be a great positive, as it would reduce the expense of the vaccine and therefore increase the likelihood of people from developing countries being protected from this disease.
Hepatitis B is one of the smallest eukaryotic DNA disease’s and therefore has a very advanced and complex genome (Guan et al, 2010). Because the genome is so small there are four open reading frames (ORFs) which each encode for one of the main proteins that make the hepatitis B virus (HBV), namely the core antigen (HBeAg/HBcAg), the HBV DNA polymerase (HBV DNA p), the surface antigen (HBsAg) and the X antigen (HBxAg) (Guan et al, 2010). Seeing as an immune response to the HBsAg protein is enough to protect an individual from hepatitis B (Guan et al, 2010), scientists are focusing on producing vaccines that contain it. The current recombinant HBV vaccination is produced using yeast cells which is expensive and difficult to scale up (Guan et al, 2010), hence the interest in edible vaccines using plants.
Multiple vaccine proteins that can be produced in plants, like tobacco, but cannot be administered orally as the patient obviously cannot eat tobacco. Therefore the proteins have to extracted, purified and administered parenterally. While the production of the protein in plants is cheaper than the mainstream approach of producing the protein through yeast cells, it still isn’t as efficient and cost effective as it could be. This is due to the extraction, purification, packaging and administering the vaccine. Diseases whose vaccine protein can be produced in plants include diseases like hepatitis b, which has been produced in tobacco (Mason et al, 1995).
Currently the Hepatitis B surface antigen (HBsAg) has been produced in transgenic tobacco where it accounts for 0.01% of the total soluble protein (Joung et al, 2016). The tobacco derived HBsAg have similar antigenicity and buoyant density to the yeast derived HBsAg (Mason et al, 1995). This means that the plant derived HBsAg will be able to instigate a similar immunological response to the vaccine available to the general public now. With this producing promising results (Streatfield, 2005) it has attracted a lot of attention and therefore investment in this area of research has increased, with the HBsAg being produced in a variety of different transgenic plant systems like potato, lettuce, maize and tomatoes (Joung et al, 2016). More work has been done into creating a Hepatitis B vaccine that can be eaten (Streatfield, 2005), as the protein expression levels in these cases are too low to be efficacious The main problem encountered so far is the low expression level, in tomatoes and lettuce leaves the HBsAg accounts for as little as 0.000001% of the fresh weight which is far too low to even attempt being able to increase (Streatfield, 2005). The HBsAg protein has been expressed in corn seed by College Station in Texas where approximately 0.2% of the total soluble protein in the first generation of the transgenic plant was HBsAg, which equates to around 0.003% of the total weight, which would be easier to increase to a practical level (Streatfield, 2005).
There are several different approaches that can be taken in order to improve the possibility of an orally administered Hepatitis B vaccine by increasing antigen expression. Firstly choosing the right promoter for the tissue, organelle and environment (Joung et al, 2016). Secondly, optimizing the use of codons. Another key strategy is increasing the efficiency of translation, this can be done using leader sequences (untranslated 5’ region which is directly upstream from the initiation codon) (Joung et al, 2016). The use of different vector systems would also vary the levels of antigens expression (Joung et al, 2016).
In terms of dosage, oral vaccinations are thought to be at least on par with parenteral doses if not more (Streatfield, 2005). The current vaccine dosage varies from 5µg to 40µg depending on the patient’s age, weight and vaccine history. Oral vaccines need to have a higher dosage because when the vaccine reaches the stomach the proteins are subject to digestive enzymes, therefore the higher dosage is needed to ensure that enough HBsAg enters the gut where it can reach the GALT (Gut-associated lymphoid tissue) and initiate mucosal and systemic immune responses (Mason et al, 1995). The problem with the Hepatitis B vaccines is that because of multiple companies producing the vaccine in multiple different ways, there is no international set antigen protein per dose (Joung et al, 2016), which makes determining a dosage for an edible vaccine even more challenging. A clinical trial with plant based Hepatitis B vaccines administered two to three doses of 100g of raw potato which contained ~1mg of HBsAg per dose (Streatfield, 2005). The results where positive, as more than half of the 33 volunteers showed increases antibody titres (Streatfield, 2005). While they had previously been vaccinated against Hepatitis B (Streatfield, 2005), these results show the ability of the vaccine to induce an immune response and therefore the result are promising and should prompt further development of this vaccine.
The problems that have to be overcome to produce a commercial plant-derived edible Hepatitis B vaccine are the low expression levels and also choosing the correct plant for the antigen proteins to be expressed in. The plant should be able to be stored for extended periods of time without going off, be able to be eaten raw, and be thermostable (Joung et al, 2016).
Target Evaluation: The Solution
What the plant derived edible vaccine needs to be?
In order for an edible vaccine to be successfully integrated into today’s vaccination market on a commercial level the vaccine should hit certain criteria. Namely the antigen dosage should be able to instigate a sufficient immune response that memory cells are formed, while not exceeding more than 100g for convenience of the consumer (Streatfield, 2005). Secondly the concentration of HBsAg should be consistent across plants (Streatfield, 2005) so that the dosage can be accurate without working out the amount of HBsAg in each plant. Thirdly, the vaccine should taste pleasant, to increase compliance and encourage the public to take it. Finally the vaccine should be able to be stored at room temperature for extended period of times so that storage costs implementing the cold chain aren’t encountered (Streatfield, 2005). On top of this it also obviously has to be effective and safe.
There are several different approaches that can be taken when trying to produce edible vaccines, the most important considerations include what plant expression system to use (stable or transient), what promoter use, where to have the antigen expressed and whether the plant should be crop or non-crop. This part of the report debates these considerations, and decide which the new Hepatitis B vaccine will have.
Plant expression system?
When considering what plant expression system to express the HBsAg in there are two main approaches, transient or stable expression. Stable expression introduces the foreign DNA into the host genome by Agrobacterium T-DNA vectors or biolistic particle delivery systems (Dirisala et al, 2016). Transient expression uses viral vectors or agrobacterium to insert foreign DNA into the hosts’ genome (Mason et al, 1995).
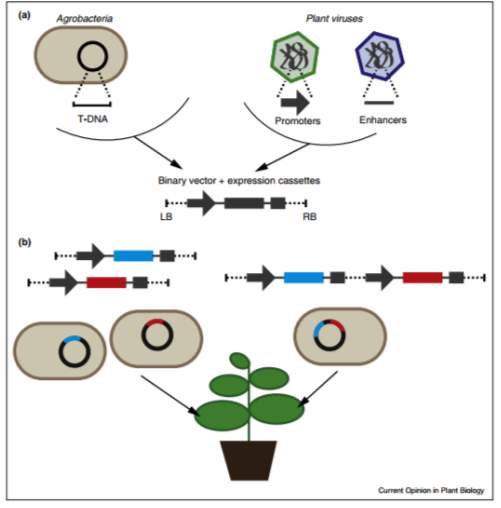
Figure 1. Different methods of transient expression in plant systems. (a) Using binary vectors from T-DNA vectors which contain high yielding expression cassettes from the plants viral regulatory sequences. (b) Here co-expression of multiple proteins means multiple proteins can be produced in one plant. This can be achieved either by co-infiltration of Agrobacteria cultures that contain different binary vectors, or by incorporating multiple expression cassettes into single binary vector. (Sainsbury et al, 2014).
Transient expression occurs when viral vectors containing the gene of interest are inserted into the plant cells via agroinfiltration (Sainsbury et al, 2014). Agroinfiltration is the process by which the expression cassette in the viral vectors in the A. tumefaciens are inserted into the plant tissue to be expressed (Chen et al, 2013). There are several different techniques for agroinfiltration, namely syringe agroinfiltration and vacuum infiltration (Chen et al, 2013). Syringe infiltration is the preferred method due to the lack of expensive and complex equipment required, it also requires a lower quantity of A. tumefaciens medium (Chen et al, 2013). Syringe infiltration occur through nicking the plant tissue and then applying the A. tumefaciens medium onto the wound via a needless syringe (Chen et al, 2013). Vacuum infiltration occurs by submerging the host plant in the A. tumefaciens medium and applying a negative pressure in the vacuum chamber. This forces the air in the plant out and the air is replaced by the A. tumefaciens medium (Chen et al, 2013). One advantage of syringe infiltration is that it can be localized on the plant so one plant can undergo agroinfiltration multiple times with multiple proteins in multiple locations (Leuzinger et l, 2013), whereas vacuum agroinfiltration does not have the same flexibility. Once the vectors have been delivered to the plant cells successfully the gene construct begins to transiently express the target protein (Sainsbury et al, 2014), in this case HBsAg. The gene of interest does not integrate into the nuclear genome, which means that the plant does not need to be grown with a genetic alteration. This means that the protein can be harvested around 1-2 weeks after agroinfiltration (Leuzinger et al, 2013).
Stable transformation differs from transient expression as the exogenous DNA is integrated into the genome of the nucleus or chloroplast. This is achieved through the use of A. tumefaciens, the vectors containing the expression cassettes allows for the insertion and integration of the gene of interest into the genome of the nucleus in the target host (Leuzinger et al, 2013). Hormones in the plants are then used to initiate growth and differentiation of roots and leave (genetically modified plant tissues) (Leuzinger et al, 2013). For chloroplast transformation the use of A. tumefaciens does not work, therefore DNA-coated gold or tungsten particles are shot into the plant cells (Leuzinger et al, 2013).
There are certain advantages and limitations to using both strategies. Transient expression produces higher levels of the antigen therefore overcoming the problem of low expression in the plants whereas stable transformation produces relatively low levels of the antigen (Joung et al, 2016). This drawback of stable transformation can be counter-acted by transforming plastids like chloroplasts instead of the nucleus as there is much higher expression due to the increased numbers in each cell (Joung et al, 2016). This however also has its drawbacks as chloroplasts are prokaryotic in nature and so cannot carry out all the post translational modifications the antigen might require including complex glycosylation and creating disulphide bridges (Gomez et al, 2009). While this is a major drawback of stable transformation it is worth considering that stable transformation has many other advantages including a reliable harvest of antigens over numerous generations, and once the transgenic plant has been developed, the time, resources and labour are minimal (Joung et al, 2016). While transient expression lasts for only one generation of plant as the plants genomes (neither the nucleus’s nor the chloroplasts) are transformed. Transient expression on the other hand has advantages like being environmentally friendly (Joung et al, 2016), being able to manipulate the hosts genome quickly and cheaply, and being able to produce lots of the HBsAg quickly due to the rapid manufacturing cycles (Joung et al, 2016). Normally with transient expression the time between transformation via agroinfiltration and harvesting the target protein is 1-2 weeks, with stable transformation it can be several months to a year before harvesting (Leuzinger et al, 2013). This is because the plant has to be grown with the transformation in its genome.
For the Hepatitis B vaccine stable transformation will be used although it takes longer to produces the plants, and costs more short term, once the transgenic plants have been produced future generations only have be to grown and require no further lab work. If HBsAg was expressed transiently, while it would produce more protein and cost less to do short term, every generation of the plant would need to undergo the process of agroinfiltration as the genome is not altered in transient expression, meaning that the exogenous DNA coding for HBsAg will not be passed on to future generations. This would mean that people in third world countries would have to buy the vaccine each time instead of just being able to buy seeds and grow the vaccine like they would be able to if the gene was transformed into the plants genome via stable transformation.
What genome to transform
Now we have established that the target plant will be stably transformed the next important consideration for producing an effective plant-derived vaccine would be which genome to transform, namely the nucleus or the chloroplast.
Transformation of the chloroplast has many key advantages. Firstly the chloroplast has a much higher level of transgene expression (Chebolu et al, 2009) due to the greater numbers of chloroplast in each cell compared to the singular nucleus. This means that they can produce more HBsAg which would initiate a sufficient immune response. Chloroplast transformed genomes also allow for multigene engineering in a single transformation event (Watson et al, 2003). This is when multiple genes can be transformed into the genome, thereby increasing the amount of proteins expressed. Because of the higher expression levels of the exogenous DNA, chloroplast transformation is suitable for hyper-expression of antibodies (Watson et al, 2003). On top of this because of maternal inheritance of the chloroplast, the transgenicity of the plant is contained. This makes it easy to keep the transgenic and regular plants separate (Watson et al, 2003) which is important for making sure dosage is correct and also controls the publics resistance to the vaccine. Another key advantage is that, unlike nuclear transformation, chloroplast transformation does not have gene silencing (Watson et al, 2003). Gene silencing occurs because of the random integration of the expression cassettes causing mutational inserts which disrupts genes (Kindle, 1998). The nature of chloroplasts gene expression would mean that the transformed chloroplast would express the proteins uniformly because of the site specific integration of the expression cassettes (Watson et al, 2003). The site specific integration is due to the specific flanking sequences in the transformation vector, and these undergo homologous recombination to allow the expression cassette to become integrated into the chloroplast genome.
However due to the chloroplasts prokaryotic nature, it cannot carry out the range post-translational modifications that the nucleus can, namely very little glycosylation can occur in the stroma. This being said the chloroplast can process some eukaryotic proteins (Daniell et al, 2001), they can form disulphide bridges and correctly fold proteins due to the presence of chaperonin proteins in the stroma (Daniell et al, 2001). HBsAg is a very complex protein that requires very specific post-translational modifications. As its being translated, HBsAg in mammalian cells is inserted into the Endoplasmic Reticulum (ER) where it undergoes dimerization (Sacti et al, 2009). It is then transported to the post-ER and pre-Golgi compartment (Sacti et al, 2009), here they are oligomerized and form VLPs before being secreted from the cell (Sacti et al, 2009). When the nucleus is transformed translation will happen in the cytosol. In the chloroplast however, translation occurs in the stroma and issues occur in transporting complex proteins across the chloroplasts membrane into the cytosol to be further modified post-translation. However as the translated protein is inserted into the ER straight after translation there shouldn’t be any issue with the transportation of the protein across the chloroplasts membrane. As most of the post-translational modifications of HBsAg occurs in the cytosol it should make no difference where the protein is translated (be it in the nucleus or the chloroplast) as it is transported to the ER as it is being translated (Sacti et al, 2009).
Nuclear transformation allows for more complex post-translational modifications including glycosylation which is key to producing a functional antibody. However nuclear transformation results in lower expression levels of protein (Watson et al, 2003) due to the fact that there is only one per cell, compared to the multiple numbers of different plasmids. Also in nuclear transformation the integration of the expression cassette is random which leads to an increased amount of mutants (Kindle et al, 1998). This leads to phenotypic mutations which need to be screened out, normally several transgenic lines are produced and the best are picked to go forwards (Sacti et al, 2009). The multiple locus inserts are also responsible for increased incidences of unstable gene expression and gene silencing (Sacti et al, 2009). Another key limitation is that nuclear transformation is very time consuming as the transgenic lines need to be screened to check that they have been transformed correctly (Sacti et al, 2009).
While both have their merits and limitations, when considering the high expression levels available using chloroplast transformation, the fact that HBsAg expressed in the chloroplast would be functional, and that there are no real advantages to transforming the nucleus over the chloroplast it makes sense to transform the chloroplast over nucleus.
Target plant species?
When deciding what the target plant species should be there are several factors that should be considered. The plant should be easily transformable and the plant should have tissue suitable for the protein to be expressed and accumulate. The tissue needs to be suitable for consumption in its raw form and have no toxic molecules in that tissue. Also the cells must be able to carry out the correct post-translational modifications and folding for the protein to be active.
In this vaccine there were a number of options as to what the target plant species should be including tobacco, potatoes, tomatoes and bananas (Guan et al, 2010). However tobacco cannot be eaten raw and would need the protein to be extracted and processed which isn’t the edible vaccine desired. At this moment in time while potatoes are expressing the most protein (11µg g-1 (Guan et al, 2010)), are easy to transform and easy to grow, the problem is the fact that is has to be cooked to be eaten which would destroy large amounts if not all of the proteins expressed in the tuber. Bananas are a relatively new fruit that are being transformed to produce HBsAg. They have advantages like being cheap, the ability to be eaten raw and the fact that they have a wide cultivated area (Guan et al, 2010). However because they haven’t been extensively researched yet they have a low transformation ratio, there is no found fruit tissue specific promoter and the transgenic analysis is very time consuming (Guan et al, 2010). Tomato plants have the advantages of being able to be eaten raw, relatively easy and successful transformation and expression ~8µg g-1 in the fruit tissues (Guan et al, 2010) which is relatively high. Therefore while potatoes produce more HBsAg, it is more sensible to choose tomato plants as the target plant species as the fruit can be eaten, it tasty which will encourage the oral administration. Tomato plants also similar levels of protein production to potatoes which are the maximal expression rates of proteins in plants so far (Guan et al, 2010).
Protein accumulation?
Once the HBsAg has been transcribed in the stroma, translated and transported to the ER and oligomerized and folded to become a fully formed protein the next issue is where to store the protein. Here the proteins can be localised by tissue, including the roots, leaves or fruit.
The main problem with edible vaccines and particularly with the fruit tissues of the plants, are the low levels of protein expression. There are several strategies that can increase these protein levels so they are able to instigate an immune response, these include a good target plant species to transform, the correct transformation method, codon optimization, design of the recombinant gene expression cassette and what plant tissue to harvest (Ko, 2014).
The transformation method for this vaccine is stable transformation which while it doesn’t produce as much protein as transient transformation, does ensure a steady harvest with every generation without interference from the labs.
Codon optimization is slightly altering the codons of the gene so that they are more common, as it is easier for the plant to translate proteins with commonly used codons (Ko, 2014). The use of codons can increase the efficiency of translation which results in more proteins being produced more quickly. Rate-limiting steps of translation include ribosomal initiation so making sure that the codons around this point are optimised which will reduce the risk of the site being blocked here allowing for efficient translation of HBsAg (M.-R. Ma et al, 2015).
The design of the recombinant gene expression cassette is important if the cassette is poorly designed then there will be a low transformation ratio and the expression rates of HBsAg will be low. The cassette is bound to gold or tungsten particles and fired into the chloroplast where the border sequences determine where integration into the genome occurred. The cassette also has to include sequences that promote and terminate the expression of the gene thereby controlling the levels of HBsAg produced. The HbsAg expression cassette is comprised of several different components. An essential component is of course the site where the gene of interest will be added (Shan et al). Also key are plasmid functions for replication and integration (Shan et al) to allow the T-DNA vector to integrate itself into the plants genome. There also must be a chimeric selectable marker like neomycin phosphotransferase which makes the plant resistant to kanamycin (Shan et al), this allows us to see which vectors have successfully integrated into the plants and therefore which plants will be expressing HBsAg. The border sequences of the T-DNA must also be included, they are used as recognition sites for recombination of the T-DNA vector (Shan et al). The inclusion of these components ensures high protein production levels.
The plant tissue that the HBsAg expression will be localised to will be the fruit tissue as it means the public can simply eat the fruit raw with no need to process or cook it. Protein expression is localised to tissues by the use of tissue-specific promoters (Rukavtsova et al, 2007). The cauliflower mosaic virus 35S promoter (35S CaMV) is used extensively in transgenic plants to express certain genes and proteins (Lim et al, 2012). This has resulted in 80% of transgene constructs using 35S CaMV (Hull et al, 2000), making it one of the most extensively researched promoters to date. It is constitutively active meaning that the genes are expressed ectopically (Leeuwen et al, 2001) resulting in high levels of protein production. Therefore the use of the 35S CaMV promoter will ensure increased levels of HBsAg are produced which should initiate an immune response. At a cellular level, HBsAg expressed in tomatoes previously accumulate in the Endoplasmic Reticulum (ER) vesicles (Guan et al, 2010). There is no need to change this as the protein is translated and transported to the ER simultaneously, to undergo post-translational modifications. The storage of these newly formed proteins near the ER makes sense as it means they don’t have to be transported far, and being stored in vesicles means that they have an element of protection in the cell.
Conclusions
To summarize, the main problems with the hepatitis B recombinant vaccine (HBV) at the moment is the fact that it is fairly expensive to produce, and can’t be used for mass scale production (Guan et al, 2010). An edible vaccine for HBV would therefore be beneficial as it would reduce costs or production, storage and administration. It would also initiate mucosal as well as systemic immune responses.
There are several approaches to designing an edible vaccine including the expression system, which genome to transform, where the protein will accumulate and choosing the target plant species. The problem with edible vaccines for HBV at this moment in time is that the protein expression levels are too low. The use of CaMV 35S promoter will increase the amount of HBsAg protein expressed in the plant. The vaccine will also have codon optimization, especially near the ribosome initiation site to allow for the most efficient rate of translation of HBsAg. The expression cassette will be engineered so that the border sequences of the T-DNA integrate specifically into the chloroplast genome so that the protein doesn’t suffer pleiotropic or position effects. The expression system selected for this vaccine is stable transformation as while it takes longer short term, the genome itself is transformed and this is inherited by future generations of the plants. Transient expression doesn’t however integrate into the genome so the foreign protein is only expressed in that generation, which is short-lived and defeats the point of people from developing countries being able to grow their own vaccine. The chloroplast genome was picked to transform as it produces more proteins more rapidly than nuclear genome transformation, therefore also contributing to the solution of increasing the protein production in the plant. Finally the choice of host plant ensures relatively high protein expression, compared to the other plants available for transformation. Tomato plants produced a similar level of protein in the fruit as potato tubers and can be consumed raw, unlike potatoes which, when cooked would destroy the majority of the HBsAg stored.
Looking towards the future, developments could be made in regards to the use of promoters to allow for even greater expression in the plant. Also the expansion of plants which can be grown to produce this vaccine so that the public can choose which plant to consume based on their personal preferences. Furthermore, greater research should go into increasing the complexity of the post-translational modifications that can occur in the chloroplast to allow for more complex proteins and antibodies to be produced. Following the successful development of an edible hepatitis B vaccine, the development of edible vaccines for other diseases like HIV/AIDs and malaria should be explored as they are diseases that plight people in developing countries and affordable vaccines should be made available to them.
References
Introduction references:
A. Gebert, H.J. Rotherkӧtter, R. Pabst. (1996). M cells in Peyer’s patches of the intestine. In Rev Cytol. 167(91) pp159.
C. Jung, J.P. Hugot, F. Barraeu. (2010) Peyer’s patches: The immune sensors of the intestine. International Journal of inflammation.
H.S. Mason, C.J. Arntzen. (1995) Transgenic plants as vaccine production systems. Trends in biotechnology journal. 13 pp388-392.
N. Jan, F. Shafi, O.B. Hameed, K. Muzuffar, S. Mohammad Dar, I. Majid, N. Ga. (2016). An overview on edible vaccines and immunisation. Austin journal of nutrition and food sciences. 4(2) pp1078.
P. Lal, V.G. Ramachandran, R. Goyal, R. Sharma. (2007). Edible Vaccines: current status and future. Indian Journal of Medical Microbiology. 25(2) pp93-102.
S. J. Streatfield. (2005). Oral Hepatitis B Vaccine candidates produced and derived in plant material. Immunology and Cell biology. 83 pp257-263.
V. Gomord, A.C. Fitchette, L. Menu-Bouaouiche, C. Saint-Jar-Dupa, C. Plasson, D. Michaud, L. Faye. (2010). Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant biotechnology Journal. 8(5) pp564-587.
V.K. Chaitanya, J.U. Kumar. (2006) Edible vaccines. Sri Ramachandra journal of medicine. 1(1).
Y.H. Joung, S.H.Park, K.B. Moon, J.H. Jeon, H.S. Cho, H.S. Kim. (2016). The last ten years of advancements in plant-derived recombinant vaccines against hepatitis B. International Journal of Molecular Sciences. 17(1715).
Target Evaluation references:
Chebolu, S., & Daniell, H. (2009). Chloroplast-Derived Vaccine Antigens and Biopharmaceuticals: Expression, Folding, Assembly and Functionality. Current Topics in Microbiology and Immunology, 332, 33–54. http://doi.org/10.1007/978-3-540-70868-1_3
Chen, Q., Lai, H., Hurtado, J., Stahnke, J., Leuzinger, K., & Dent, M. (2013). Agroinfiltration as an Effective and Scalable Strategy of Gene Delivery for Production of Pharmaceutical Proteins. Advanced Techniques in Biology & Medicine, 1(1), 103. http://doi.org/10.4172/atbm.1000103
D.M. Shan, N.E. Tumer, D.A. Fischhoff, R.B. Horsch, S.G. Rogers, R.T. Fraley, E.G. Jaworski. The introduction and expression of foreign genes in plants. http://www.nottingham.ac.uk/ncmh/documents/bger/volume-5/bger5-3.pdf (Accessed 19/12/17)
Daniell, H., Streatfield, S. J., & Wycoff, K. (2001). Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends in Plant Science, 6(5), 219–226.
E. Gomez, S.C. Zoth, A. Berinstein. (2000). Production of Hepatitis B surface antigen in transgenic plants for oral immunization. Nature Biotechnology. 18 pp1167-1171. https://doi.org/10.1038/81153
E. Gomez, S.C. Zoth, A. Berinstein. (2009). Plant based vaccines for potential human application. Human Vaccines. 5(11) pp738-744.
F. Sainsbury, G.P. Lomonossoff. (2014). Transient expressions of synthetic biology in plants. Current opinion in plant biology. 19 pp1-7. https://ac.els-cdn.com/S1369526614000193/1-s2.0-S1369526614000193-main.pdf?_tid=1be7886a-df28-11e7-b289-00000aacb35f&acdnat=1513075082_3000f6ce998f2455799f945e8d8f1677
Guan Z.J, Guo B, Huo Y.L, Guan Z.P, Wei Y.H. (2010) Overview of expression of hepatitis B surface antigen in transgenic plants. Vaccine. 28(46) pp7351-7362. https://doi.org/10.1016/j.vaccine.2010.08.100
H. Daniell, N.D. Singh, H. Mason, S.J. Streatfield. (2009) Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 14(12) pp669-679. https://www.ncbi.nlm.nih.gov/portal/utils/pageresolver.fcgi?log$=activity&recordid=5a04651ffdc41d0194924796&absref=https://www.ncbi.nlm.nih.gov
J Appl Phycol. 6: 231. https://doi.org/10.1007/BF02186076
Kindle K.L. (1998) Nuclear Transformation: Technology and Applications. In: Rochaix J.D., Goldschmidt-Clermont M., Merchant S. (eds) The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Advances in Photosynthesis and Respiration, vol 7. Springer, Dordrecht
Kindle, K.L. & Sodeinde, O.A. (1994) Nuclear and chloroplast transformation in Chlamydomonas reinhardtii: strategies for genetic manipulation and gene expression
Ko, K. (2014). Expression of Recombinant Vaccines and Antibodies in Plants. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 33(3), 192–198. http://doi.org/10.1089/mab.2014.0049
Kwon, K.-C., Chan, H.-T., León, I. R., Williams-Carrier, R., Barkan, A., & Daniell, H. (2016). Codon Optimization to Enhance Expression Yields Insights into Chloroplast Translation. Plant Physiology, 172(1), 62–77. http://doi.org/10.1104/pp.16.00981
Leeuwen W.V, Ruttink. T, Borst-Vrenssen A.W.M, van der Plas L.H.W, van der Krol A.R. (2001). Characterization of position induced spatial and temporal regulation of transgene promoter activity in plants. Journal of experimental Botony. 52(358) pp949-959.
Leuzinger, K., Dent, M., Hurtado, J., Stahnke, J., Lai, H., Zhou, X., & Chen, Q. (2013). Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. Journal of Visualized Experiments : JoVE, (77), 50521. Advance online publication. http://doi.org/10.3791/50521
Lim, C. J., Lee, H. Y., Kim, W. B., Lee, B.-S., Kim, J., Ahmad, R. Kwon, S.-Y. (2012). Screening of Tissue-Specific Genes and Promoters in Tomato by Comparing Genome Wide Expression Profiles of Arabidopsis Orthologues. Molecules and Cells, 34(1), 53–59. http://doi.org/10.1007/s10059-012-0068-4
Lindbo, J. A. (2007). High-efficiency protein expression in plants from agroinfection-compatible Tobacco mosaic virus expression vectors. BMC Biotechnology, 7, 52. http://doi.org/10.1186/1472-6750-7-52
M. Grabsztunowicz, M.M. Koskela, P. Mulo. (2017). Post-translational Modifications in Regulation of Chloroplast Function: Recent Advances. Front Plant Sci. 8(240). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5322211/?log$=activity
M.-R. Ma, L. Hui, M.-L. Wang, Y. Tang, Y.-W. Chang, Q.-H. Jia, X.-P. Yang, X.-H. Wang and X.-Q. Ha. (2015) Synonymous codon selection in the hepatitis B virus translation initiation region. Genetics and Molecular research. 14(3) pp 8955-8963 DOI http://dx.doi.org/10.4238/2015.August.7.4
R. Hull, S. N. Covey, P. Dale (2000) Genetically modified plants and the 35S promoter: assessing the risks and enhancing the debate, Microbial Ecology in Health and Disease, 12:1, 1- 5, DOI: 10.1080/089106000435527
Rukavtsova, E.B., Abramikhina, T.V., Shulga, N.Y. et al. (2007). Tissue specific expression of hepatitis B virus surface antigen in transgenic plant cells and tissue culture. Russ J Plant Physiol. 54: 770. https://doi.org/10.1134/S1021443707060088
Santi, L., Huang, Z., & Mason, H. (2006). Virus like particles production in green plants. Methods (San Diego, Calif.), 40(1), 66–76. http://doi.org/10.1016/j.ymeth.2006.05.020
Watson, J., Koya, V., Leppla, S. H., & Daniell, H. (2004). Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine, 22(31-32), 4374–4384. http://doi.org/10.1016/j.vaccine.2004.01.069
M.A. Barocchi, R. Rappuoli. (2015). Delivering vaccines to the people who need them most. Philos Trans R Soc lond B Biol Sci. 370(1671).
D.W. Pascual. (2007). Vaccine are for dinner. Proc Natl Acad Sci USA. 104(26) pp10757- 10758.
https://www.nature.com/icb/journal/v83/n3/full/icb200536a.html
M.M. Rigano, A.M. Walmsley. (2005). Expression systems and developments in plant-made vaccines. Immunology and Cell Biology. 83, pp271-277.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4667769/
N. Takeyama, H. Kiyono, Y. Yuki. (2015) Plant-based vaccines for animals and humans: Recent advances in tech and clinical trials. Therapeutic advances in vaccines. 3(5-6) pp139-154
https://link.springer.com/article/10.1007/s11738-016-2315-3
V.R. Dirisala, R.R. Nair, K. Srirama, P.N. Reddy, K.R.S.S. Rao, N.S.S. Kumar, G.Parvatam. (2017). Recombinant pharmaceutical protein production in plants: unravelling the therapeutic potential of molecular pharming. Acta Physiol Plant. 39(18)
https://doi.org/10.1111/pbi.12796
M. Yang, H. Sun, H. Lai, J. Hurtado, Q. Chen. (2017). Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnology Journal.
G.P. Lomonosoff, M.A. D’Aoust. (2016). Plant-produced biopharmaceuticals: A case of technical developments driving clinical deployment. Science. 353(6305) pp1237-1240.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5074611/
Hepatitis B:
https://www.nature.com/articles/icb200534
https://www.jstage.jst.go.jp/article/biochemistry1922/127/4/127_4_543/_pdf/-char/en
https://www.ncbi.nlm.nih.gov/pubmed/8659125
Target evaluation links:
http://www.sciencedirect.com/science/article/pii/S0958166900000707
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Pharmacology"
Pharmacology involves the study of drugs and how they affect the body. A pharmacologist contributes to drug development by researching and testing how the body reacts to medication, and whether the medication can have a positive impact on the body in terms of fighting illness and disease.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




