Socio-Economic Impacts of Arsenic Exposure
Info: 9404 words (38 pages) Dissertation
Published: 30th Sep 2021
Tagged: EconomicsSociologyPublic Health
Summary
Arsenic contamination of drinking water, which can occur naturally or because of human activities such as mining, is the single most important public health issue in Bangladesh. Fifty out of the 64 districts in the country have arsenic concentration of groundwater exceeding 50 µg L-1, the Bangladeshi threshold, affecting 35 – 77 million people or 21 – 48% of the total population. Chronic arsenic exposure through drinking water is also an important public health issue worldwide affecting hundreds of millions of people. Consequently, arsenic poisoning has attracted the attention of researchers and has been profiled extensively in the literature. Most of the literature has focused on characterising arsenic poisoning and factors associated with it. However, studies examining the socio-economic aspects of chronic exposure of arsenic through either drinking water or foods remain underexplored. The objectives of this paper are (i) to review arsenic exposure pathways to humans; (ii) to summarise public health impacts of chronic arsenic exposure; and (iii) to examine socio-economic implications and consequences of arsenicosis with a focus on Bangladesh. This scoping review evaluates the contributions of different exposure pathways by analysing arsenic concentrations in dietary and non-dietary sources. The socio-economic consequences of arsenicosis disease in Bangladesh are discussed in this review by considering food habits, nutritional status, socio-economic conditions, and socio-cultural behaviours of the people of the country. It has been found that the pathways of arsenic exposure in Bangladesh include drinking water, various plant foods and non-dietary sources such as soil. It has also been found that arsenic affected people are often abandoned by the society, lose their jobs and get divorced and are forced to live a sub-standard life. The fragile public health system in Bangladesh has been burdened by the management of thousands of arsenicosis victims in Bangladesh.
Keywords: Arsenic exposure, Socio-economic, socio-cultural, Public health, Bangladesh
Contents
- Introduction
- Methodology
- Result and Discussion
- Arsenic exposure to Human
- Dietary sources
- Non-dietary sources
- Public health impacts of arsenic exposure
- Non-carcinogenic effects
- Carcinogenic effects
- Other effects
- Socio-economic aspects of arsenicosis
- Ostracism
- Socio-economic hazards of arsenicosis
- Arsenic exposure to Human
- Conclusion
Acknowledgements
References
Introduction
Arsenic is ubiquitous in the earth’s crust, although generally constitutes less than 1% of most rocks, coals, and soils (Alam et al., 2002). Although the occurrence of arsenic in the environment is mainly from minerals and geogenic sources, human activities resulted in extensive soil and water contaminations in many parts of the world (Smith et al., 1998).
Groundwater contamination with high level of arsenic is an important environmental and public health issue in South and South-East Asian countries (Chakraborti et al., 2015). It is the single most important public health problem in Bangladesh, with between 35 and 77 million of its population being at risk of arsenic poisoning from arsenic-contaminated drinking water (Edmunds et al., 2015). In 2000, the World Health Organization (WHO) stated that Bangladesh has been experiencing “the largest mass poisoning of arsenic of population in history” (Smith et al., 2000).
The rural people of Bangladesh depend mainly on surface water until the mid-20th century to meet drinking water needs that was often contaminated by pathogen. From the 1960’s hand-pumped tube wells were widely introduced in the rural areas of Bangladesh especially by government and non-government agencies to provide pathogen-free drinking water. This practice accelerated significantly from the 1980s onwards as the installation of tube wells became relatively cheap and the technology became easily available (Edmunds et al., 2015). This led to a significant increase in the access of underground drinking water from the shallow alluvial aquifers (Smedley and Kinniburgh, 2002).
Chronic arsenic poisoning was first identified in West Bengal of India in the 1980s; however, the first diagnosis of arsenic poisoning in Bangladesh was made in 1993. It was reported that more than a quarter of shallow (
Although groundwater is the main source of drinking water, it is also an important source of irrigation water in south and south-east Asian countries (Meharg and Rahman, 2003). Underground water has been used extensively, particularly during the dry season, for rice cultivation in Bangladesh (Meharg and Rahman, 2003). The background levels of arsenic in paddy-growing soils in Bangladesh range from 4 to 8 mg kg-1; however, up to 83 mg kg-1 of arsenic was found in paddy soil that was irrigated with arsenic-contaminated groundwater water (Williams et al., 2006). High level of arsenic in paddy soils from contaminated irrigation water has resulted in arsenic uptake in rice grain (Rahman and Hasegawa, 2011; Williams et al., 2006) and vegetables (Alam et al., 2003; Rahman et al., 2013) that raised concern of potential human health risk in the country. The possibility of arsenic exposure to the people of arsenic-non-contaminated countries is an important concern with the expansion of global food trade (Rahman et al., 2014).
Widespread human exposure of arsenic from drinking water and food and associated carcinogenic and non-carcinogenic effects have been matters of growing concerns during the past three decades (Chatterjee et al., 2010; Rahman et al., 2009). Besides the public health impacts of arsenicosis, arsenic contamination may create widespread socio-economic consequences for the victims and their families in Bangladesh; however, this issue has received little attention from the point of view of social risk and hazards. Therefore, the purpose of this review is to fill in this knowledge gap with special reference to Bangladesh. The specific objectives of this paper are to:
- Discuss dietary and non-dietary pathways of arsenic exposure.
- Identify the socio-economic consequences of chronic arsenic exposure in Bangladesh considering food habits, nutritional status, socio-economic conditions, and socio-cultural behaviours of the population of the country.
- Identify the social risk and social hazards of arsenicosis.
Methodology
A scoping review is conducted following the framework outlined by Arksey and O’Malley (2005) and Levac et al. (2010). The framework outlined five steps that we implemented in this study: Identifying the research question; identifying relevant studies; study selection; charting the data; and collating, summarizing and reporting the results.
In step 1, we defined our research question as follows: What are the public health impacts of chronic arsenic exposure and the socio-economic consequences of arsenicosis in Bangladesh? In step 2, studies were identified through a selection of electronic databases, focusing on peer-reviewed publications, supported by citation tracking and hand searching. The target electronic databases were: Goggle Scholar, SCOPUS, Web of Science, and PubMed. Using a combination of keywords agreed upon by the research team and confirmed by MeSH terms and sub-headings associated with them, the search strategy was implemented. Data were extracted according to whether or not the study addressed our research question.
Over 683 research papers/documents were retrieved from the database of which, 60 papers were used in this review based on their relevance to the study research questions (Fig. 2). Year of publication, impact of the journals, study locations (mainly Bangladesh), and reliability of reported data in the documents were also considered in selecting the papers. Daily dietary intake (DI) of arsenic from different foods was calculated based on the following formula:
DI=DFC×MC…………………………………….. (1)
where, DFC is the daily food consumption rate (g/day), and MC is the mean arsenic concentration (µg/g fresh/dry weights) in individual food stuff. Dietary intake was calculated for individual adult male and female.
Qualitative data from multiple sources, as described by Hassan et al. (2005), were used to determine impacts of arsenic exposure on social lives of affected Bangladeshi population and their survival strategies.
Results and Discussion
Human Exposure to Arsenic
Arsenic-contaminated tube-well water is the main source of arsenic exposure to Bangladeshi adults; however, other important exposure pathways for arsenic include plant media (rice, vegetables, animal source foods, and water) and non-dietary sources such as air and soil.
Dietary sources
Plant media as an exposure pathway for arsenic occur in various forms including food preparation and consumption. Water used for the preparation of foods (e.g. rice and vegetables) and beverages (e.g. beverages prepared using arsenic-contaminated water ) represents an indirect route of arsenic exposure (Joseph et al., 2015a). In addition, the animal source foods such as milk, eggs and meat are potential exposure pathways for arsenic (Ahmed et al., 2016).
In Bangladesh, rice is the major staple food accounting for up to 80% of the daily caloric intake (Huq et al., 2006) with an average daily rice consumption of 400 to 650 g (Rahman et al., 2006), which is among the highest per capita rice consumption figures in the world (Abdullah et al., 2006). On average, arsenic concentration in Bangladeshi rice ranges from 0.1 to 0.95 µg g-1 (Williams et al., 2005). With a total arsenic content of 0.1 µg g-1, consumption of 650 g rice per day would translate into an ingestion of 65 µg or 0.065 mg arsenic, which is the highest among all dietary sources. It is worth mentioning that rice absorbs approximately twice its weight in water during cooking process, thus, arsenic levels in cooked rice might be higher if the cooking water is contaminated (Rahman et al., 2006; Sengupta et al., 2006). Based on past studies, the estimated retention of total arsenic in cooked rice can be between 45% – 107% (Joseph et al., 2015a) depending on the rice types, arsenic concentration in cooking water, and the methods of cooking. Commonly used traditional rice cooking method in Bangladesh include parboiling, and washing of raw rice and then cooking it with excess water followed by gruel decant. this cooking method are likely to reduce arsenic exposure from rice diet (Rahman et al., 2006).
Other important issues related to arsenic exposure from rice diet are concentration and bioavailability of arsenic species in rice (Laparra et al., 2005). On average, 50% of the total arsenic in rice is inorganic, the most toxic form of arsenic, which can vary from 10 to 90% (Zhu et al., 2008). The absolute bioavailability is the highest (103.9%) for arsenite followed by arsenate (92.5%), dimethylarsenate (DMA) (33.3%), and monomethylarsenate (MMA) (16.7%) (Juhasz et al., 2006). Therefore, human health risk of inorganic arsenic species is greater than organoarsenic species.
In case of vegetables, arsenic exposure to human is directly proportional to the vegetable consumption rate and arsenic concentrations in vegetables. Typically, root vegetables accumulate and store arsenic in their root tuber, while arsenic translocation to the above ground parts is generally limited (Rahman et al., 2013). Although arsenic concentrations in most of the vegetables did not exceed 4 mg kg-1 dry weight (dw), some vegetables contained up to 8 mg kg-1 dw (e.g. gourd leaf) and 158 mg kg-1 dw (e.g. peeled arum root) arsenic.
A recent study showed that arsenic concentrations in leafy and non-leafy vegetables in Bangladesh ranged between 0.1-2.0 mg kg-1 wet weight (ww) and 0.1-0.8 mg kg-1 ww, respectively (Tani et al., 2012). Another study reported arsenic concentrations in leafy and non-leafy Bangladeshi vegetables between 0.04-0.46 mg kg-1 ww (median is 0.11 mg kg-1 ww) and 0.011-0.15 mg kg-1 ww (median 0.03 mg kg-1 ww), respectively (Rahman et al., 2013). The per capita vegetables consumption in Bangladesh is reported to be 238 g/person/day (Joseph et al., 2015a) indicating that the consumers could be exposed to 26.18 mg and 7.14 mg of arsenic per day from their leafy and non-leafy vegetable diets, respectively. The above data suggest that in addition to water and rice, vegetables could be an important dietary source of arsenic exposure for Bangladeshi individuals.
Animal source foods such as meat, fish, and milk are the major dietary sources of arsenic in Bangladesh. Recent studies reported arsenic concentrations in animal-origin Bangladeshi foods as 0.20-0.27 mg kg-1 dw (mean is 0.20 mg kg-1) in chicken meat (Ghosh et al., 2012), 0.61-0.88 mg kg-1 dw in mutton meat, 0.41-0.67 mg kg-1 dw in beef meat (Tarafdar et al., 1991), 0.097-1.40 mg kg-1 dw (mean 0.40 mg kg-1) in fish (Islam et al., 2014), and 0.004-0.16 mg L-1 (mean 0.04 mg L-1) in cow milk (Ghosh et al., 2013). Dietary intake of arsenic from animal-origin foods depends on the daily consumptions of these foods. As reported by Ahmed et al. (2016), the consumption rates of beef, mutton, chicken and duck meats in Bangladesh are 4.77-12.63 g/day, 0.5-0.89 g/day, 9.01-17.42 g/day, and 5.31-13.42 g/day, respectively. Based on this data, the estimated daily dietary intake of arsenic from beef, mutton, chicken, and duck meats could be 0.038-0.833 mg, 0.305-0.783 mg, 1.80-3.83 mg, and 0.243-0.684 mg, respectively.
Fish is one of the regularly consumed foods in Bangladesh with a consumption rate of 15-96 g/person/day (Islam et al., 2015). Therefore, the estimated arsenic exposure to a Bangladeshi individual from their fish diet could be between 1.36-50.88 mg per day (based on the lowest and highest data reported here), which is substantially higher than that of meats.
Arsenic exposure from drinking water is directly proportional to the water intake and arsenic concentration in the water. The average direct and indirect water consumption by a Bangladeshi individual between the ages of 16 – 60 years is 6.1 L per day for males and 4.84 L per day for females (Hossain et al., 2013). Considering arsenic concentrations in drinking water of Bangladesh between 10 – 50 µg L-1 (as reported by Chakraborti et al. (2015), the estimated daily exposure of arsenic from drinking water varies between 61 – 305 µg and 48.4 – 242 µg for a Bangladeshi male and female, respectively.
Non-dietary sources
Non-dietary sources of arsenic are diverse. One possible pathway is the direct ingestion of contaminated soil. Surface soil is the main sink of arsenic from different environmental sources. Worldwide, the background concentration of arsenic in uncontaminated soils typically ranges between 4-10 mg kg-1 (Smedley and Kinniburgh, 2002), while in arsenic contaminated areas (e.g. Bangladesh) it can be as high as 81 mg kg-1 (Huq et al., 2003). During field works, arsenic contaminated soil particles may stick to hands and get ingested during hand-to-mouth activities, accidental soil ingestion, or via intake of foods (e.g. vegetables) to which contaminated soil is attached (Alam et al., 2003; Joseph et al., 2015a).
Soil ingestion by humans may be resulted from their occupation. A study by Doyle et al. (2012) reported a mean soil ingestion rate of 75 mg/day for a wilderness community (maily involved in outdoor activities) in Canada with the highest of 200 mg/day. Individuals involving in activities with extensive soil contact such as agriculture are likely to be exposed to much higher soil ingestion. With an estimated 35 million workers involved in agricultural sector in Bangladesh (Joseph et al., 2015a), it is likely that soil ingestion can be an important arsenic exposure pathway for the population of the country.
Another non-dietary source of arsenic is the exposure through inhalation. Inhalation of air containing arsenic particles or inhalation of gaseous forms of arsenic can also be a route of arsenic exposure to humans. In the rural areas of Bangladesh, biomass fuels provide 90% of the energy needs with agricultural residues – 45%, woods – 35%, and animal dung – 20% (Nath et al., 2013). Burning these biomass fuels for cooking and inhalation of smoke by the rural population of Bangladesh can be a potential route of arsenic exposure. Tobacco smoking can be another arsenic exposure pathway for Bangladeshi population. Bangladesh is one of the top tobacco consuming countries in the world (Barkat et al., 2012). Tobacco leaves in branded cigarette has been reported to contain arsenic concentrations ranging between 0.65-0.72 µg g-1, while a higher concentrations (2.12-2.64 µg g-1) of arsenic was found in non-branded cigarette and raw tobacco leaves (Arain et al., 2009). Another study reported 0.13-0.27 µg g-1 of arsenic in bidis (a locally produced low-price tobacco products commonly consumed by low-income people of Bangladesh) and cigarette in Bangladesh (Lindberg et al., 2010).
Public health impacts of arsenic exposure
According to the World Health Organization (WHO) estimation, over 200 million people worldwide are exposed to arsenic above the WHO safety level of 10 µg L-1 in drinking water (Table 1) (WHO, 2008). In Bangladesh, between 35 and 77 million people (Table 1) has been reported to be at risk of arsenic poisoning (Chakraborti et al., 2015).
The International Agency for Research on Cancer (IARC) classifies arsenic as a “Group I” carcinogen. The US Environmental Protection Agency (US EPA) lists inorganic arsenic as a “Group A” or known carcinogen (Joseph et al., 2015b). Arsenic ranks the No. 1 on the US Agency for Toxic Substances and Disease Registry (ATSDR) 2013 substance priority list (ATSDR, 2013). This ranking does not include full consideration of total exposure of a substance from all dietary and non-dietary sources. Therefore, human health risk of arsenic is even greater than its top ATSDR ranking.
Non-carcinogenic effects
Although skin cancer has also been identified, skin disorders and keratosis are the most common external indicators of arsenic poisoning.. Chronic arsenic poisoning can cause skin lesions, restrictive pulmonary disease, peripheral vascular disease, gangrene, hypertension, non-cirrhotic portal fibrosis, ischemic heart disease, and diabetes mellitus (Edmunds et al., 2015; Joseph et al., 2015b). Raindrop pattern of pigmentation and depigmentation of skin are particularly distinct on the edges and the chest. Hyperkeratosis (hardened skin) also seen predominantly on the feet (Hong et al., 2014).
The human health effects of arsenicosis is characterised by slow appearance of the external and internal symptoms. The main symptom of the disease is skin lesion (keratosis) that becomes visible usually around 10 years after first exposure (Edmunds et al., 2015). Black lesion (discoloured skin) on the feet and hand, a peripheral vascular disorder similar to gangrene, is another common symptom of arsenicosis. The skin discolouration caused by arsenicosis has been commonly known as “black-foot disease” that was first documented in Taiwan (Chen et al., 1985).
Cutaneous lesion is one of the best-known clinical indices of chronic arsenic exposure. This can occur within months or after several years of exposure (Das and Sengupta, 2008). Melanosis (hyperpigmentation) and keratosis are two frequently occurring dermatological non-cancer effects of arsenicosis. Melanosis is considered as an early symptom, while keratosis is a sensitive sign of more advanced stages of arsenicosis. Leucomelanosis (hypopigmentation) occurs less frequently than melanosis or keratosis (Naujokas et al., 2013). Numerous epidemiological research on skin lesions reveal that most of the individuals drinking water having arsenic concentrations of > 100 µg L-1 has skin lesions; however, occurrences of skin lesions have also been found at arsenic concentrations of
Carcinogenic effects
Arsenic is a known carcinogen. It can cause cancer in skin, lung, bladder, liver, and kidney. Some of the most common types of skin cancers related to chronic arsenic exposure are squamous cell carcinoma (Bowen’s disease), invasive squamous cell carcinoma, and basal cell carcinoma (Naujokas et al., 2013). Regardless of exposure pathways, chronic arsenic exposure is associated with lung cancer risk Numerous evidences suggest that lung cancer is the most common reason for arsenic-related mortality (IARC, 2012; Smith et al., 2009). . It is well established that individuals drinking water containing > 100 µg L-1 arsenic are more likely to be at risk of lung cancer (Putila and Guo, 2011).
Arsenic exposure can cause cancers in other organs of human body as well. Studies showed that arsenic exposure from drinking water for longer period (> 40 years) at high concentration (> 600 µg L-1) is associated with risk of bladder cancer (Gibb et al., 2011). Risk of kidney cancer from arsenic exposure corresponds to a dose-dependent manner for drinking water (Naujokas et al., 2013). Several studies have substantiated association between arsenic exposure and liver cancer(such as liver angiosarcoma) (Liaw et al., 2008).
Other effects
In addition to carcinogenic and non-carcinogenic health risks of chronic arsenic exposure, arsenic-associated health problems can affect many of the major organs and organ systems in human body (Table 2). For example, neurological impairments such as impaired cognitive abilities and motor functions have been reported in children and adults after arsenic exposure (Parvez et al., 2010). Wasserman et al. (2007) reported cognitive impairments in children at 6 and 10 years of age due to arsenic exposure. Hamadani et al. (2011) reported losses of verbal and full-scale IQ in girls due to arsenic exposure. Gong et al. (2011) found significant association between arsenic exposure from drinking water and impaired cognitive ability as well as education . Abhyankar et al. (2012) observed significant link between hypertension and arsenic exposure.
Arsenic exposure may also affect human immune system including altered immune-related gene expression and cytokine production in lymphocytes (Morzadec et al., 2012). Human and animal studies showed that arsenic exposure may affect multiple endocrine such as hormone regulation via the retinoic acid, thyroid hormone, and estrogen receptors (Barr et al., 2009). Increased occurrence of diabetes is also likely to be linked with arsenic exposure, particularly at high concentration and long exposure period (> 10 years) (Del Razo et al., 2011).
Socio-economic aspects of arsenicosis
Human health is defined not only by physical state of individuals rather it is a state of complete physical, psychological, and social wellbeing (Brinkel et al., 2009). From a literature search, only few scientific and technical reports have been found that addressed socio-economic implications and consequences of arsenic exposure to Bangladeshi populations. Evidence from literature review suggest that instead of being supported and consoled by their families and communities, sufferers from arsenicosis are avoided and neglected to the point of becoming social misfits or outcasts (Hassan et al., 2005). Although arsenicosis patients may not feel ill or look ill during the early stage of arsenicosis, they are treated as ‘dangerous people’ and stripped of their social status. The arsenicosis patients may face many economic problems including financial losses, decreased work efficiency, and inability to get suitable jobs due to discrimination (Ahmad et al., 2007).
Arsenicosis patients face ostracism. A study by Ahmed el al. (2007) found that a significant number of arsenicosis patients get ostracised. Because of lack of information and proper knowledge, many confuse the skin lesions with leprosy, which is considered a contagious killer by the illiterate village people. Patients having early symptoms of arsenicosis do not disclose their condition to avoid certain ostracism. Family members tend to avoid direct contact with the affected person. Sufferers in rural areas are discouraged to appear in public. School-age children having visible symptoms of arsenicosis are discouraged to attend schools and are avoided by their friends and classmates. In many cases, arsenic victim adults are barred from attending cultural/religious functions (Alam et al., 2002). Arsenic victims are abandoned not only by society but also by their family members. In many cases, husbands left wives or re-married and wifes left their husbands temporarily due to fear of being affected by arsenicosis (Chowdhury et al., 2006). Arsenic victim families may not be allowed to take water from neighbour’s tube wells and ponds, and even people refrain from making new relationship with arsenic victim families (Chowdhury et al., 2006).
In Bangladesh, arsenicosis female patients are the worst victims of ostracism, because they are the most vulnerable group in the society. Hassan et al. (2005) reported that an women of 31 years, who developed blisters and black spots on her body, was neglected by her husband. He stopped talking to her. There are some instances where wives were divorced or separated or sent back to their parents’ home because of their arsenicosis disease (Argos et al., 2007). In a study by Ahmed el al. (2007) it was found that out of 25 individuals having arsenicosis, 8% who were female said that their husband had abandoned them because of their arsenicosis disease. In the same study, 19 arsenicosis patients (76%) said they were hated by others, and 6 patients (24%) said that they were avoided by their friends and colleagues. Hassan et al. (2005) also reported that three wives (out of 37 affected women) were forced to return back to their parents and two had been divorced because of their arsenicosis illness.
Arsenicosis patients face problems in relationship within the family and in the community. Available evidence suggests that in Bangladesh arsenicosis patients become the burden to the family and the society (Ahmad et al., 2007; Argos et al., 2007; Bearak, 1998; Chowdhury et al., 2006; Hadi and Parveen, 2004; Hanchett, 2006; Mahmood and Halder, 2011). The problem can be classified into three groups: difficulty in getting married, conflicts within the family, and difficulties in socialisation.
Difficulties in getting married are manifested in various forms. For example, arsenicosis male patients mainly face economic problems, while the female patients face social problems mostly related to pre- as well as post-marital relationships. People become reluctant to establish marital relationship with arsenic victim families. Arsenic victim young men and women are advised by the their family to remain unmarried, which results in significant social instability of rural areas of the country (Chowdhury et al., 2006).
In arsenic affected rural areas of Bangladesh, it is the adult females who are generally the most undernourished and most vulnerable to disease due to their poor health condition. When the husband discovers symptoms of arsenicosis on his wife’s body, he often refuses to keep her under the same roof. If the woman is fortunate, the husband simply sends her back to her parents for treatment. In most cases, however, the husband finds it too risky to maintain the marital relationship and seeks divorce (Alam et al., 2002; Chowdhury et al., 2006).
In a rural setup in Bangladesh, divorcee is often socially maltreated even under normal circumstances. A divorcee with a fatal disease like arsenicosis is considered a social burden. Thus, the divorced women find no place in the society and, with their children, become destitute. It becomes a big problem for the arsenic victim’s parents to get their affected daughters married that make their life miserable (Chowdhury et al., 2006).
Problems within family relationships are characterised by the tendency to avoid arsenic victims. Parents feel hesitant about being close to their children and husbands keep a safe distance from their wives having arsenicosis disease. Hassan et al. (2005) reported that a father suffering from arsenicosis for 4 years were avoided by their sons tactfully – they did not like to come close to their father. It is an appalling situation in a family atmosphere in Bangladesh. This study also reported in-family situation of a 17-year girl who developed symptoms of arsenicosis (palms and skin lesions) in her whole body. The girl said her parents are rude to her, and she feel that she was a burden to her parents. This study also described in-family situation of a 26-year man having arsenicosis and claimed to be indirectly neglected and isolated by his family members.
Women are the worst victims of arsenicosis. Like many other household responsibilities such as cooking, feeding children and cleaning, women play a key role in securing arsenic-free drinking water for the family members. Sometimes, the women have to walk up to few kilometres to get arsenic-free water, which become a burden with additional responsibilities and they have to compromise with cultural and religious ideology (Chowdhury et al., 2006). The children of the poor have been drinking contaminated water since their birth. They may eventually suffer arsenicosis in their childhood or adult stage. The visible arsenicosis symptoms such as spotted melanosis, keratosis and ulcer keratosis make the adult unmarried women victims physically weaker and less appealing to the men. It will be very difficult, perhaps impossible, for the parents of an affected young woman to find a groom for her without offering a huge dowry. The provision of dowry is already a difficult social problem in Bangladesh, but will be further compounded by the arsenic problem.
Due to the patriarchal nature of society and socio-cultural position of women in society in Bangladesh, unmarried and divorced women having arsenicosis live inhumanely. They are the most negligible among all victims of the disease. A study conducted in Samta village of Jessore, Rajarampur of Chapainawabgong, and Courtpara of Kushtia in Bangladesh, where arsenic contamination is very high, involving 55 female victims at the verse of social violence revealed that a significant portion of the women with arsenicosis disease were subject to domestic violence such as dowry (40%), divorce (17%), separation (17%), desertion (11%), physical torture (9%) and polygamy (7%) (Chowdhury et al., 2006).
Difficulties in socialisation arise due to ignorance and poor literacy level, especially in rural areas. Superstitions, prejudices and fairy tales are constructed surrounding the arsenic victims. People stay away from the arsenic victims, neglect or become scared of them due to such superstition (Chowdhury et al., 2006). Due to lack of proper knowledge about arsenicosis, some people think of it as an act of devil or evil spirits or impure air, and keep themselves and their family members away of the patients. Because of such superstitions and prejudices, most of the patients did not receive any treatment, and have been passing miserable lives (Chowdhury et al., 2006). Arsenic victim school children are also faced prejudice. Friends of arsenic victim school children keep themselves away of them, do not like to share books and pencils, and do not let them taking parts in sports. Even, teachers restrict their access to school (Hassan et al., 2005).
Arsenicosis also causes extreme instability in social life. Case study showed that arsenicosis patients received social injustice and the negligence of their local leaders. In some cases, the local leaders are not cooperative to the arsenic victims in getting financial support from the local government and treatment from appropriate medical practitioner (Hassan et al., 2005).
In a socio-economic rural setup in Bangladesh, the male is generally the sole earning person in a family. Socio-economic analysis of arsenic contamination in Bangladesh showed a strong link between poverty and arsenicosis (Argos et al., 2007; Brinkel et al., 2009; Hassan et al., 2005). Many of the rural poor people are engaged in either agriculture or daily labouring. If the employers identify the employees as arsenicosis patient, the employers do not hire them. Even after appointment, if the employers discover their sickness, the arsenic victim workers lose their jobs immediately. Once detected and dismissed from an employment because of arsenicosis, no other local employer will provide them with alternative employment (Chowdhury et al., 2006).
Hassan et al. (2005) studied the socio-economic consequences of arsenicosis in Bangladesh. In the study, many patients said that they faced difficulties in getting job due to arsenicosis. Ahmed et al. (2007) surveyed the socio-economic problems of arsenicosis in arsenic affected areas of Bangladesh. They found that 58.6% out of 140 respondents having arsenicosis patient in their family experienced economic problems because of arsenicosis. The problems they identified for the arsenic victims included decreased work efficiency, financial difficulties due to lose of their work, and inability to get a job. The early symptoms of arsenicosis become more pronounced day by day depending on patient’s arsenic exposure rate and the availability of treatment. Limbs can be affected by gangrene, and internal organs including liver, kidney, and lung can also be badly affected. In extreme cases, cancer can occur in skin and internal organs. Thus, the victims become crippled and incapable of doing laborious work.
Socio-economic hazards of arsenicosis
A social hazard of a particular event is characterised by the nature and magnitude of harm the event pose to people’s social norms and social structure. Several studies reveal that arsenicosis has created extensive social and economic problems for its victims and their families in affected areas including social degradation, social injustice, and social isolation. Therefore, arsenicosis has been considered not only a health problem, but also as a major socioeconomic hazard of Bangladesh as this health problem represents a challenge to the victims and their families’ social status, lifestyle, and financial condition.
Studies identified a strong relationship between poverty and arsenicosis disease. Compared to higher income group, arsenicosis patients of lower income group are at risks of social as well as economic risk in Bangladesh (Ahmad et al., 2007; Mahmood and Halder, 2011). This is likely due to the poor health condition of the patients of lower income group, and hardship in accessing safe drinking water, and difficulties in getting proper treatment of the disease as they cannot afford the costly medicine. Chowdhury et al. (2006) found that between 20-70% of the arsenicosis patients in Bangladesh did not receive any treatment due to financial problems. Because the untreated victims become physically weak day by day, they are incapable of doing hard work. The lack of treatment eventually deteriorates the health and economic conditions of the arsenicosis victims.
In some cases, arsenicosis patients were found to be leading miserable lives. It has been reported that one woman patient attempted to commit suicide by taking poison after failing to be cured by the treatment of the local doctors. In rural Bangladesh, some local religious cleric refuse to bury with Muslim rites when an arsenicosis patient die (Hassan et al., 2005).
Social conflicts over arsenic contamination in drinking water undermine the social harmony, network relationship, and isolation as arsenicosis victims are often wrongly identified as leprosy patients (Brinkel et al., 2009). Arsenicosis also hampers socialization by social stigmatization and discrimination. For example, arsenic victims are often ostracized and barred from social functions. Children of arsenic victims are not allowed to attend social and religious gatherings as well as deprived of taking arsenic-free water from a neighbour’s tube well (Keya, 2004). Arsenicosis victims are not allowed to take bath in the village ponds in a fear that they may contaminate the ponds’ water. The unaffected people are rude and unfriendly to the arsenic victims, and think that the patients should stay in their home or leave the village (Hassan et al., 2005).
Social instability has become a serious social problem in arsenic affected areas of Bangladesh. The arsenic victims are facing isolation within their families as well as in the society. Unemployment, domestic violence, discrimination in workplace, isolation, physical torture, divorce, and separation are common social problems that the arsenicosis victims are facing. It has been evidenced from several studies that social hazard faced by the arsenic-affected people, driven by loneliness, social injustice, frustration, damage to social bonds results in social instability in arsenic affected areas of Bangladesh (Argos et al., 2007; Hassan et al., 2005).
CONCLUSION
During the last two decades, national/international newspapers and research groups working on arsenic contamination in Bangladesh with a focus on social, environmental, and human health issues reveal that arsenicosis patients become the burden to the family and the society. Because hundreds of thousands of people have been affected by arsenic contamination, health complications related to arsenic poisoning will place a heavy burden on the already hard-pressed health services of Bangladesh. Evidences from literature review reveal that the poorest fraction of the society faces the worst arsenic contamination-related problems, particularly those who are already undernourished. Severe social and economic hazards have emerged in the arsenic affected areas of the country, and this problem has triggered several unexpected social problems that were still not fully recognized. In addition to health problems, arsenic poisoning has created many socio-economic problems. Perhaps the worst social disaster is yet to come.
REFERENCES
Abdullah AB, Ito S, Adhana K. Estimate of rice consumption in Asian countries and the world towards 2050. Proceedings for Workshop and Conference on Rice in the World at Stake. 2, 2006, pp. 28.
Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environmental Health Perspectives 2012; 120: 494-500.
Ahmad SA, Sayed MHS, Khan MH, Karim MN, Haque MA, Bhuiyan MSA, et al. Sociocultural aspects of arsenicosis in Bangladesh: Community perspective. Journal of Environmental Science and Health, Part A 2007; 42: 1945-1958.
Ahmed MK, Shaheen N, Islam MS, Habibullah-Al-Mamun M, Islam S, Islam MM, et al. A comprehensive assessment of arsenic in commonly consumed foodstuffs to evaluate the potential health risk in Bangladesh. Science of the Total Environment 2016; 544: 125-133.
Alam M, Allinson G, Stagnitti F, Tanaka A, Westbrooke M. Arsenic contamination in Bangladesh groundwater: a major environmental and social disaster. International Journal of Environmental Health Research 2002; 12: 235-253.
Alam MGM, Snow ET, Tanaka A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Science of the Total Environment 2003; 308: 83-96.
Arain MB, Kazi TG, Baig JA, Jamali MK, Afridi HI, Jalbani N, et al. Respiratory effects in people exposed to arsenic via the drinking water and tobacco smoking in southern part of Pakistan. Science of the Total Environment 2009; 407: 5524-5530.
Argos M, Kalra T, Pierce BL, Chen Y, Parvez F, Islam T, et al. A prospective study of arsenic exposure from drinking water and incidence of skin lesions in Bangladesh. American Journal of Epidemiology 2011; 174: 185-194.
Argos M, Parvez F, Chen Y, Hussain AZMI, Momotaj H, Howe GR, et al. Socioeconomic status and risk for arsenic-related skin lesions in Bangladesh. American Journal of Public Health 2007; 97: 825-831.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology 2005; 8: 19-32.
ATSDR. The ATSDR 2011 Substance Priority List. http://www.atsdr.cdc.gov/SPL/index.html. Agency for Toxic Substances and Disease Registry, Atlanta, 2013.
Barkat A, Chowdhury AU, Nargis N, Rahman M, Khan MS, Kumar A, et al. The economics of tobacco and tobacco taxation in Bangladesh. Available at:. http://global.tobaccofreekids.org/files/pdfs/en/Bangladesh_tobacco_taxes_report.pdf. International Union Against Tuberculosis and Lung Disease, Paris – FRANCE, 2012.
Barr FD, Krohmer LJ, Hamilton JW, Sheldon LA. Disruption of histone modification and CARM1 recruitment by arsenic represses transcription at glucocorticoid receptor-regulated promoters. PLoS One 2009; 4: e6766.
Bearak B. New Bangladesh Disaster: Wells That Pump Poison. New York TImes, NY, USA, 1998.
Brinkel J, Khan MH, Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. International journal of environmental research and public health 2009; 6: 1609-1619.
Chakraborti D, Rahman MM, Mukherjee A, Alauddin M, Hassan M, Dutta RN, et al. Groundwater arsenic contamination in Bangladesh – 21 Years of research. Journal of Trace Elements in Medicine and Biology 2015; 31: 237-248.
Chatterjee D, Halder D, Majumder S, Biswas A, Nath B, Bhattacharya P, et al. Assessment of arsenic exposure from groundwater and rice in Bengal Delta Region, West Bengal, India. Water Research 2010; 44: 5803-5812.
Chen C-J, Chuang Y-C, Lin T-M, Wu H-Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Research 1985; 45: 5895-5899.
Chen C-L, Chiou H-Y, Hsu L-I, Hsueh Y-M, Wu M-M, Wang Y-H, et al. Arsenic in drinking water and risk of urinary tract cancer: a follow-up study from northeastern Taiwan. Cancer Epidemiology Biomarkers & Prevention 2010; 19: 101-110.
Chowdhury MAI, Uddin MT, Ahmed MF, Ali MA, Rasul SMA, Hoque MA, et al. Collapse of socio-economic base of Bangladesh by arsenic contamination in groundwater. Pakistan Journal of Biological Science 2006; 9: 1617-1627.
Das NK, Sengupta SR. Arsenicosis: diagnosis and treatment. Indian Journal of Dermatology, Venereology, and Leprology 2008; 74: 571.
Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environmental Health 2011; 10: 73-84.
Doyle J, Blais J, Holmes R, White P. A soil ingestion pilot study of a population following a traditional lifestyle typical of rural or wilderness areas. Science of the Total Environment 2012; 424: 110-120.
Edmunds WM, Ahmed KM, Whitehead PG. A review of arsenic and its impacts in groundwater of the Ganges-Brahmaputra-Meghna delta, Bangladesh. Environmental Science: Processes & Impacts 2015; 17: 1032-1046.
Ghosh A, Awal MA, Majumder S, Sikder MH, Rao DR. Arsenic residues in broiler meat and excreta at arsenic prone areas of Bangladesh. Bangladesh Journal of Pharmacology 2012; 7: 178-185.
Ghosh A, Majumder S, Awal MA, Rao DR. Arsenic exposure to dairy cows in Bangladesh. Archives of Environmental Contamination and Toxicology 2013; 64: 151-159.
Gibb H, Haver C, Gaylor D, Ramasamy S, Lee JS, Lobdell D, et al. Utility of recent studies to assess the National Research Council 2001 estimates of cancer risk from ingested arsenic. Environmental Health Perspectives 2011; 119: 284-290.
Gong G, Hargrave KA, Hobson V, David Lefforge MS. Low-level groundwater arsenic exposure impacts cognition: a project FRONTIER study. Journal of Environmental Health 2011; 74: 16-22.
Hadi A, Parveen R. Arsenicosis in Bangladesh: prevalence and socio-economic correlates. Public Health 2004; 118: 559-564.
Hamadani J, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. International Journal of Epidemiology 2011; 40: 1593-1604.
Hanchett S. Social aspects of the arsenic contamination of drinking water: a review of knowledge and practice in Bangladesh and West Bengal. APSU Selected papers on the social aspects of arsenic and arsenic mitigation in Bangladesh. Arsenic Policy Support Unit, Dhaka, Bangladesh, 2006, pp. 1-51.
Hassan MM, Atkins PJ, Dunn CE. Social implications of arsenic poisoning in Bangladesh. Social Science & Medicine 2005; 61: 2201-2211.
Hong Y-S, Song K-H, Chung J-Y. Health effects of chronic arsenic exposure. Journal of preventive medicine and public health 2014; 47: 245-252.
Hossain MA, Rahman MM, Murrill M, Das B, Roy B, Dey S, et al. Water consumption patterns and factors contributing to water consumption in arsenic affected population of rural West Bengal, India. Science of the Total Environment 2013; 463: 1217-1224.
Huq SI, Joardar J, Parvin S, Correll R, Naidu R. Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation. Journal of Health, Population and Nutrition 2006: 305-316.
Huq SI, Rahman A, Sultana N, Naidu R. Extent and severity of arsenic contamination in soils of Bangladesh. Fate of arsenic in the environment. Dhaka: Bangladesh University of Engineering and Technology 2003: 69-84.
IARC. Arsenic, Metals, Fibres, and Dusts: A Review of Human Carcinogens. Available: http://monographs.iarc.fr/ENG/Monographs/vol100C/. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 100C. International Agency for Research on Cancer, Lyon, France, 2012, pp. 501.
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Islam KN, Ibrahim M, Masunaga S. Arsenic and lead in foods: a potential threat to human health in Bangladesh. Food Additives & Contaminants: Part A 2014; 31: 1982-1992.
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Masunaga S. Assessment of trace metals in fish species of urban rivers in Bangladesh and health implications. Environmental Toxicology and Pharmacology 2015; 39: 347-357.
Joseph T, Dubey B, McBean EA. A critical review of arsenic exposures for Bangladeshi adults. Science of the Total Environment 2015a; 527: 540-551.
Joseph T, Dubey B, McBean EA. Human health risk assessment from arsenic exposures in Bangladesh. Science of The Total Environment 2015b; 527: 552-560.
Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, et al. In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environmental Health Perspectives 2006; 114: 1826-1831.
Keya MK. Mental health of arsenic victims in Bangladesh. South African Anthropol 2004; 4: 215-23.
Laparra JM, Velez D, Barbera R, Farre R, Montoro R. Bioavailability of inorganic arsenic in cooked rice: Practical aspects for human health risk assessments. Journal of Agricultural and Food Chemistry 2005; 53: 8829-8833.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implementation Science 2010; 5: 1.
Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH. Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiology Biomarkers & Prevention 2008; 17: 1982-1987.
Lindberg A-L, Sohel N, Rahman M, Persson L, Vahter M. Impact of smoking and chewing tobacco on arsenic-induced skin lesions. Environmental Health Perspectives 2010; 118: 533.
Mahmood SAI, Halder AK. The socioeconomic impact of Arsenic poisoning in Bangladesh. Journal of Toxicology and Environmental Health Sciences 2011; 3: 65-73.
Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta 2002; 58: 201-235.
Meharg AA, Rahman M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environmental Science & Technology 2003; 37: 229-234.
Morzadec C, Bouezzedine F, Macoch M, Fardel O, Vernhet L. Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes. Toxicology 2012; 300: 46-56.
Nath TK, Baul TK, Rahman MM, Islam MT, Harun M. Traditional biomass fuel consumption by rural households in degraded sal (shorea robusta) forest areas of Bangladesh. International Journal of Emerging Technology and Advanced Engineering 2013; 3: 537-544.
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental Health Perspectives (Online) 2013; 121: 295.
Parvez F, Chen Y, Brandt-Rauf PW, Slavkovich V, Islam T, Ahmed A, et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax 2010; 65: 528-533.
Putila JJ, Guo NL. Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS One 2011; 6: e25886.
Rahman MA, Hasegawa H. High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Science of the Total Environment 2011; 409: 4645-4655.
Rahman MA, Hasegawa H, Rahman MA, Rahman MM, Miah MAM. Influence of cooking method on arsenic retention in cooked rice related to dietary exposure. Science of the Total Environment 2006; 370: 51-60.
Rahman MA, Hasegawa H, Rahman MM, Miah MM, Tasmin A. Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicology and environmental safety 2008; 69: 317-324.
Rahman MA, Rahman MM, Naidu R. Arsenic in rice: Sources and human health risk. Wheat and Rice in Disease Prevention and Health. 1. Elsevier, 2014, pp. 365-376.
Rahman MM, Asaduzzaman M, Naidu R. Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. Journal of hazardous materials 2013; 262: 1056-1063.
Rahman MM, Naidu R, Bhattacharya P. Arsenic contamination in groundwater in the Southeast Asia region. Environmental Geochemistry and Health 2009; 31: 9-21.
Sengupta MK, Hossain MA, Mukherjee A, Ahamed S, Das B, Nayak B, et al. Arsenic burden of cooked rice: Traditional and modern methods. Food and Chemical Toxicology 2006; 44: 1823-1829.
Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry 2002; 17: 517-568.
Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. Journal of Exposure Science and Environmental Epidemiology 2009; 19: 343-348.
Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bulletin of the World Health Organization 2000; 78: 1093-1103.
Smith E, Naidu R, Alston A. Arsenic in the soil environment. Advances in Agronomy 1998; 64: 149-195.
Tani M, Jahiruddin M, Egashira K, Kurosawa K, Moslehuddin A, Rahman M. Dietary intake of arsenic by households in Marua village in Jessore. Journal of Environmental Science and Natural Resources 2012; 5: 283-288.
Tarafdar S, Ali M, Islam A, Khan A. Level of some minor and trace elements in Bangladeshi meat products. Journal of Radioanalytical and Nuclear Chemistry 1991; 152: 3-9.
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environmental Health Perspectives 2007: 285-289.
WHO. Guidelines for drinking-water quality: incorporating first and second addenda to third edition, Vol. 1, Recommendations. World Health Organization, Geneva, 2008, pp. 515.
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, et al. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environmental Science & Technology 2006; 40: 4903-4908.
Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environmental Science & Technology 2005; 39: 5531-5540.
Zhu YG, Williams PN, Meharg AA. Exposure to inorganic arsenic from rice: A global health issue? Environmental Pollution 2008; 154: 169-171.
Table 1: Arsenic exposure concerns worldwide, adopted from (Naujokas et al., 2013) with permision.
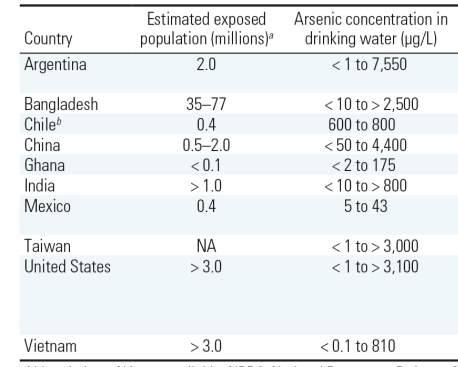
Table 2: Human health effects of arsenic exposure, adopted from (Naujokas et al., 2013) with permision.
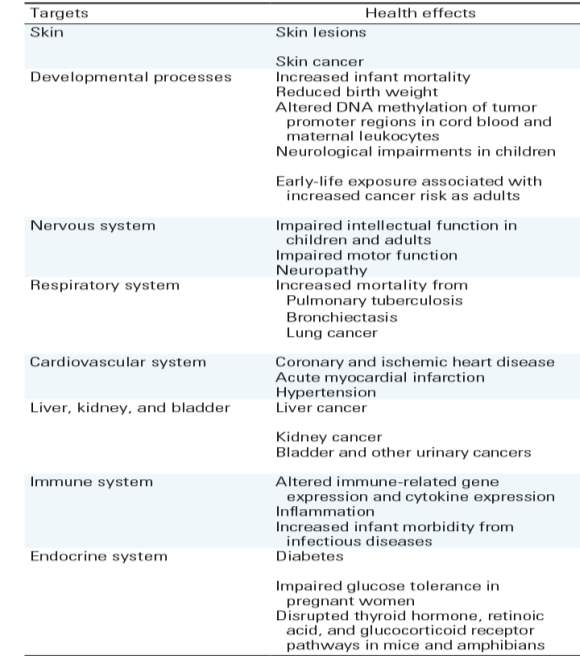
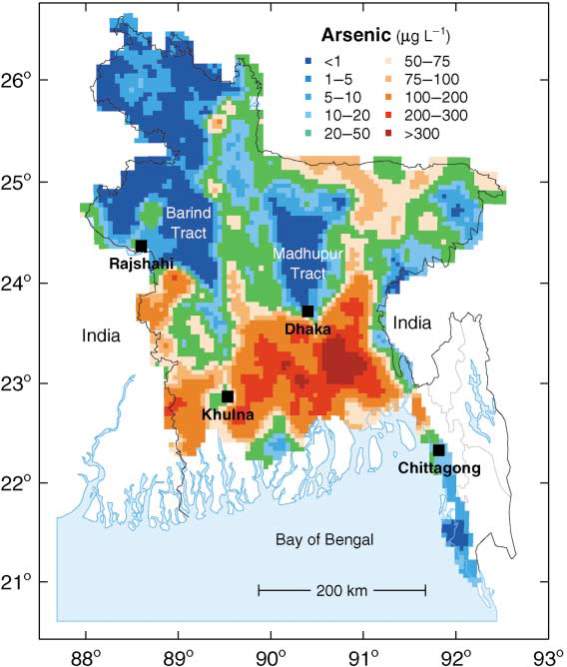
Fig. 1: Map showing the distribution of arsenic in groundwater of Bangladesh, adopted from Smedley & Kinniburgh (2002) with permission.
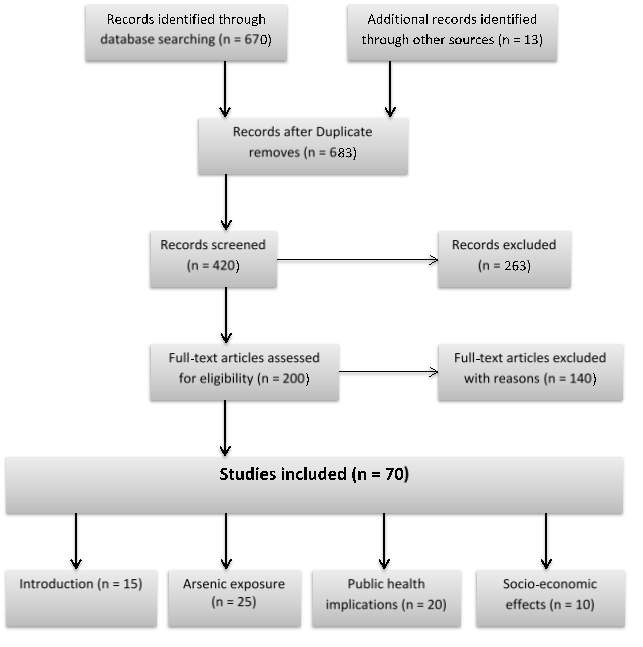
Fig. 2: PRISMA flow diagram for the scoping review process.
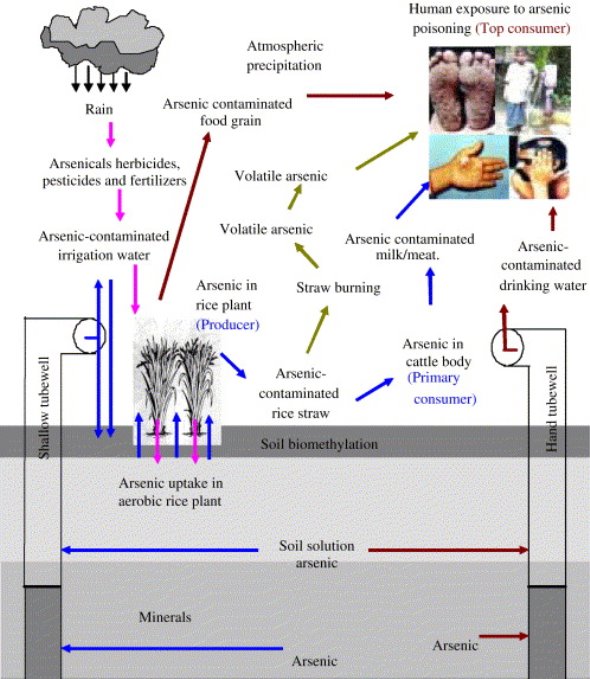
Fig. 3: Different exposure pathways of arsenic to human, adopted from Rahman et al. (2008) with permission.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Public Health"
Public health concerns the wellbeing and health of the general public as a whole. Through organised efforts, public health bodies look to inform the choices of society to help protect from threats to their health and prolong and improve the quality of life.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




