Restoring Dynamic Accommodation via Accommodative Intraocular Lenses
Info: 11003 words (44 pages) Dissertation
Published: 13th Dec 2019
Tagged: MedicineSurgical Studies
Abstract
Research and current clinical studies are working on the correction of presbyopia and the restoration of dynamic accommodation, providing the eye with the ability to focus at distance, intermediate and near without the use of spectacles. Existing accommodating intraocular lenses (A-IOLs) can still be enhanced. They currently change their position or their shape in response to ciliary muscle contractions to provide an increase in dioptric power. Three main design principles exist. First, the single optic accommodative intraocular lenses rely on axial movement of the anterior eye. Second, dual optic accommodative intraocular lenses depend on the increased separation between the two optics to increase power. Third, curvature change accommodative intraocular lenses increase their optical power by modifying their surface curvature, in response to the ciliary muscle contractions. There are further A-IOL design concepts undergoing development, which deviate from the main design principles. Single optic A-IOLs were the first to be commercialized and have been used extensively. They move on average one millimeter anteriorly and generate a low dioptric change. A secondary action seems to be occurring, the central zone appears to be flexing, increasing ocular aberrations and enhancing near vision. Dual optic and curvature change A-IOLs are designed to provide greater amplitudes of accommodation than single optic devices; most of these implants are still in development and require additional work before commercialization. Dynamic accommodation can be restored with A-IOLs but a number of challenges must be addressed for long-term performance to be achieved.
1. Introduction
The definition of accommodation is the mechanism by which the eye changes refractive power by altering the shape of the crystalline in order to focus objects at variable distances 1. Far point is the term used to describe the image of an object that falls clearly on the retina with no accommodation 2. Near point is the term used when the clearest image is seen with maximum accommodation. Range of accommodation is anything seen between the far point and near point 2.
Helmholtz’s theory of accommodation, also known as the capsular theory, considers that the crystalline is elastic. In its non-accommodative state, whilst tension is caused by the zonules, it is stretched and flattened 3,4. During accommodation, the contraction of the ciliary muscle shortens the ciliary ring thus relaxing the zonules and the suspensory ligaments; as a result relieving strain. The crystalline then assumes a more spherical form, increasing the thickness and decreasing in diameter 3.
As the crystalline ages, it loses its ability to focus the image of a near object due to its progressive thickening and to the loss of its elasticity consequently resulting in presbyopia. The gradual loss of clear near vision starts approximately at forty years of age and reaches a maximum around sixty years of age 5.
Accommodation is a reflex action of the eye in response to focusing on a near object7. The reflex, controlled by the parasympathetic nervous system, involves three responses: the convergence of the eyes, pupil constriction and lens accommodation7. The eyes converge in order to prevent double vision. The pupils constrict to increase the depth of field. Finally, the crystalline accommodates by modifying its shape, increasing the total refractive power, resulting in a focused image at near distance3, 8. These responses are known as the near vision triad.
Dynamic accommodation is an active dioptric change in the eye’s refractive power helping to achieve clear near vision; this is a result of accommodative effort. Accommodative effort is the product of the change in power of the natural crystalline lens or of the effective power of an accommodative intraocular lens (pseudophakic accommodation) 3,8. Pseudoaccommodation is apparent accommodation, which achieves focused near vision without any true change in ocular power5, 7. The following methods are examples of pseudoaccommodative corrections: reading spectacles, bifocals, progressive, multifocal or monovision contact lenses, multifocal IOLs, refractive surgery, an increased depth of field caused by pupillary constriction and ocular aberrations 5,7. To prove the existence of pseudoaphakic accommodation, the following three factors are of importance: Objective measurements of biometric changes, objective measurements of dioptric changes in refractive power and subjective testing of distance corrected near vision acuity5, 7. The ideal method would provide the flexibility needed to focus in order to see clearly at distance, intermediate and near without glasses. This is how the natural lens works until, the lens stiffens with age, near vision is lost. A variety of different kinds of surgical methods are available or being developed to restore dynamic accommodation: surgical expansion of the sclera, using femtosecond lasers to treat the lens restoring its flexibility or accommodative intraocular lenses (A-IOLs) 6.
Accommodative intraocular lenses are designed to create a myopic refractive change in the eye in reaction to ciliary muscle contraction either through the movement of an optic or through a change in surface curvature 7. There are three main design principles: the single optic A-IOLs that rely on axial movement, the dual optic A-IOLs that rely on the increased separation between the two optics and the curvature change A-IOLs that modify their surface curvature in response to the ciliary muscle contractions in order to increase their optical power 6. The latest clinical studies in the presbyopic field are looking into crystalline regeneration using stem cells. Furthermore drug therapy can be used to avoid thickening of the lens as a preventive measure of presbyopia 8.
This paper will only focus on the different types of accommodative intraocular lenses as a method to restore dynamic accommodation.
2. Accommodating Intraocular Lenses
One of the methods used to restore accommodation to the presbyopic eye is by replacing the crystalline lens with an intraocular lens. A small incision is performed on the anterior surface of the capsule to remove the lens, leaving the capsule empty and ready to receive an IOL 11. Accommodative Intraocular lenses are created to use the contraction of the ciliary muscle, capsular bag elasticity and vitreous cavity pressure, to allow an optical power change 10,11. Accommodation can be produced in a number of different ways with A-IOLs. Single Optic A-IOLs move forward to simulate accommodation, while the increased separation of Dual Optic IOLs provide the necessary power change for accommodation. Moreover, Curvature Change A-IOLs increase accommodative power by modifying their surface curvature, similarly to a real crystalline lens. Finally, new types of A-IOLs, currently in development, use electro-active mechanical action and magnet-driven active shift to replicate accommodation.
2.1 Challenges encountered by A-IOLs
Posterior capsular opacification (PCO) and capsular fibrosis are challenges A-IOLs encounter; they are the most frequent complications of cataract surgery. Patients with PCO suffer from decreased visual acuity, impaired contrast sensitivity and glare disability 13. PCO is reported to occur twenty to forty percent of patients two to five years after surgery; it is age dependent with low incidence in older patients but higher rates in young patients especially children and infants 14,15. Clinically there are two types of PCO regenerating and fibrotic. It appears cataract surgery induces a wound-healing response, activating the leftover lens epithelia cells (LEC) into migrating and regenerating lens fibers 15. Regenerating PCO is much more common it is caused by LECs left behind in the capsular bag after the removal of the crystalline lens. LECs from the lens equator region migrate and proliferate into the space between posterior capsule and IOL. Fibrotic PCO is caused by LECs from the anterior capsule causing whitening and wrinkling of the posterior capsule 16. PCO can be treated with YAG laser capsulotomy however this may lead to complications including short term increased intraocular pressure, ocular inflammation, cystoid macular edema and retinal detachment 17. Efforts to prevent the development of PCO are taken. Drugs and gene therapy eliminated LECs but also damaged and affected neighboring cells and structures 74. The removal of LECs mechanically with a curette or by aspiration only seems to slow the growth and development of PCOs. Modifying lens designs to square-edges reduces the proliferations of PCO22, biocompatible lens materials are also chosen. Hydrophilic acrylic has a higher rate of PCO formation than silicone, acrylic or PMMA lenses.
All implants inserted in the capsular bag are much smaller in size compared to the crystalline lens. The wound healing process causes the lens capsule to shrink and wrap around the new implant, causing it to become rigid and reduce its flexibility. To offset this condition some designs are implanted in the sulcus and avoid the capsular bag while other designs use the shrinking capsule as a way to secure, stabilize the implant in place14.
3. Single Optic Accommodative Intraocular Lenses
Single Optic A-IOLs depend on the movement of the ciliary muscle. These lenses are designed to translate forward and backward from the cornea, with the contraction and relaxation of the ciliary muscle and the anterior movement of the lens. This increases the refractive power when the intraocular lens is moved forward, for near vision, although the action might slightly vary among the different designs. The Crystalens and Turlign Toric from (Bausch & Lomb, Rochester, New York, USA) is the only design so far to have received FDA (Food and Drug Administration) approval in the United States. The most commonly used A-IOLs are the Crystalens, the Tetraflex (Lenstec, St. Petersburg, Florida, USA) and the 1CU (Human-Optics, Erlangen, Germany). They all have CE status (Conformité Européene) and are presently being prescribed in Europe. The Tek-Clear A-IOL (Tekia inc. Irvine, California, USA) recently received the CE certification in May 2015. The Tetraflex IOL and Tek-Clear A-IOL (Tekia inc. Irvine, California, USA) are currently undergoing pending FDA approval 12. Theoretically, the change in ocular power produced from the forward movement of an optic, can be easily calculated. Applying Bennett and Rabbetts schematic eye 18, a one millimeter forward shift to an IOL with a one millimeter thickness would result in 0.8 diopters of accommodation in an eye with 26.04mm of axial length, 1.3 diopters in an average sized eye of 24.09 mm in length or 1.85 diopters of accommodation in a short eye with 22.04 mm of axial length 18. Therefore, a single optic intraocular lens, which only relies on moving forward to provoke a change in power, would only create, on average, one diopter of accommodation. A secondary action seems to be occurring with Single Optic A-IOLs. Their central zone appears to be flexing and steepening; it is suspected to be caused by the vitreous or haptics during accommodation 10. These occurring alterations could result in change of power caused by a change in aberrations. The increased aberrations have a beneficial effect of amplifying the depth of field. This results in an increase to the pseudo accommodative effect, supplementing the power changes produced from the axial translation 19. Patients will perceive an elevated amplitude of accommodation when measured subjectively compared to measurements collected objectively. Single optic intraocular lenses move on average one millimeter anteriorly on accommodative effort 19. Although they are simple in design, single optic A-IOLs are unlikely to be successful over a wide range of dioptric powers. In addition, capsular bag flexibility remains an important factor for the success of their performance. Posterior cataract opacification not only affects the quality of vision but also makes the capsule rigid and hinders its flexibility.
 3.1) 1CU
3.1) 1CU
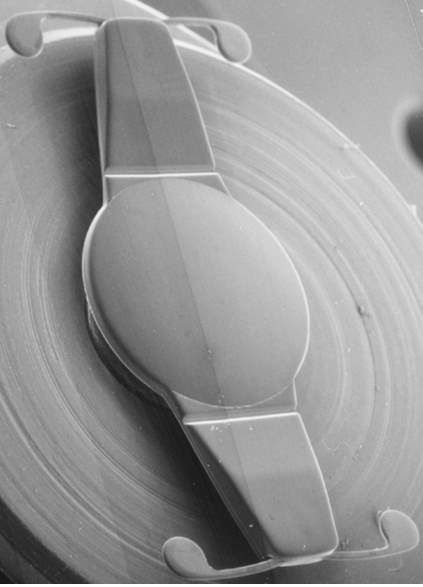
Figure 1. 1CU single optic A-IOL 35
The 1CU accommodative IOL is one of the older models approved by the Conformité Européene (CE). The 1CU is a hydrophilic acrylic IOL with a 5.5mm biconvex round optic (Fig.1), with a total diameter of 9.8mm and four precisely designed haptics that use the principles elaborated by K.D. Hanna 36.
The 1CU is meant to allow accommodation by anterior movement of the lens optic due to the contraction of the ciliary muscle 36. Following the contraction of the ciliary muscle, the capsular bag relaxes, causing the compression of the haptics thus moving the 1CU forward, resulting in increased near vision power 36,37. Patients with the 1CU showed a higher accommodative range and a better distance corrected near visual acuity than those in a control group with conventional IOLs 36,37. A two-year follow up study 32 on the long-term results of the 1 CU accommodative intraocular lens measured the amplitude of accommodation after 6 months, 1 year and 2 years. The amplitude of accommodation after 6 months was measured to be 1.9 ± 0.8 diopters, while after a year the amplitude of accommodation went down to 0.3 ± 0.2 diopters (ρ =0.004) and two years post operation the amplitude of accommodation were found to be 0.3 ± 0.2 diopters ρ (p=0.564) 38. The drop in performance is the result of post-capsular opacification, which is due to the rounded edges of the optic. Since solely the haptic edges are sharp-shaped, the migration of lens epithelial cells occurred, thus generating post capsular opacification 22. The choice of IOL material also influences the incidence of PCO 38. Some studies have demonstrated that hydrophilic acrylic IOLs are related to a high incidence of PCO, these IOLs have a weak adhesion to the capsule allowing the proliferation of lens epithelial cells 38,39. In conclusion, because of the high incidence of capsule opacification, due to the material and round shape and the low range of dioptric powers it offered the 1CU was not believed to be most successful design38.
 3.2) Crystalens
3.2) Crystalens
Figure 2. Crystalens single optic A-IOL23
The Crystalens (with 5.0mm diameter optic) and the Turlign Toric (with a 5.0mm diameter toric optic) are the only accommodative IOLs approved by the FDA and clinically prescribed in the USA for treating presbyopic patients with cataract 20,21. The Crystalens (Fig.2) is composed of a biconvex lens with two hinged haptic plates to retain a fixed position within the capsule. The lens is composed of a biocompatible silicone and is square-edged in shape to lower the formation of post capsular opacification 22.
The IOL is inserted in the capsular bag in a posteriorly vaulted position, with the optic positioned against the posterior capsule and anteriorly to the vitreous 24. Post surgery patients are typically placed on 1% cyclopentolate instilled three times a day for a week24. This ensures that the ciliary muscle is at rest as the Crystalens orients itself in the correct posteriorly vaulted position 24.The cycloplegic drops also allow the anterior and posterior surface of the capsule to fibrose around the haptics and seal the IOL within it 25. Inadequate cycloplegia can allow the lens to shift anteriorly in this critical time period 24. The manufacturer suggested that during accommodation, the contracting ciliary muscle bulk up within the vitreous cavity and increase the vitreous pressure against the sealed IOL. This causes a forward movement, a steepening of the central zone and an increase in depth of field consequently resulting in a rise in dioptric power 26,27,28. Marchini and associates29 measured the performance of the T-45 where the near point was used to induce accommodation. The results found were a mean of anterior shift of 0.33+/- 0.25mm, which alone is insufficient to provide full or functional dioptric change to improve near vision. Additionally, accommodation measured objectively with retinoscopy was found to be on average 2.42 +/- 0.39 diopters. This was a smaller value than the amplitude of accommodation perceived by the patients, which was found to be on average 5.79 diopters by near-point determination 29. These findings indicate that there is more than just axial translation occurring during accommodation, depth of field caused by aberrations seem to increase the amplitude of accommodation subjectively. A unique and very rare postoperative complication of the Crystalens could occur called “Z syndrome” 30,31. Z syndrome occurs when one haptic is pulled anteriorly whilst the other remains in the normal posterior position due to irregular capsular contraction. The IOL resembles the letter Z with tilted optic in the middle; this creates an asymmetric tilt known as Z syndrome 20,30,31.
Mild Z syndrome can be resolved with YAG capsulotomy but if unsuccessful the IOL would need to be surgically repositioned 31. The Trulign Toric Accommodating Intraocular lens is a modified plate haptic aspheric silicone A-IOL, same as the Crystalens 32. The axis markers align with the flat meridian of the lens. The lens comes in three powers: 1.25, 2.00 and 2.75 diopters 32. It is available in spherical powers ranging from ten diopters to thirty-three diopters in 0.50D steps 32. The Trulign Toric IOL can reduce astigmatism and offer presbyopic correction as well 32. The Trulign Toric IOL uses the same accommodative principles as the Crystalens 12.
 3.3) Tetraflex
3.3) Tetraflex
Figure 3. Tetraflex single optic A-IOL23
Tetraflex is a single optic A-IOL, made from HEMA material, has a 5.75 mm spherical optic (Fig. 3). It prevents glare effects and is square edged in shape to lower the formation of post-capsular opacification 22,33. Lenstec, the Tetraflex manufacturer, implies that Tetraflex is unique in design and is different from the other accommodative implants 34. Other A-IOLs have hinges or springs to provide mechanical advancement of the optic for near and intermediate vision whilst Tetraflex’s haptics are designed in a ribbon type flex 34. The highly flexible HEMA material provides a natural flex forward and backward, without the need for mechanical movement within the capsule, stimulated by the increased pressure of the vitreous chamber during accommodation 34. Tetraflex is designed with a five degrees forward angulation; this represents a five-degree start to near vision adaptation, which allow the patient to focus for near, intermediate and distance vision 34. Tetraflex is still undergoing pending FDA approval, therefore it isn’t available commercially in the United States but can be found in Europe, China, Australia, Taiwan, Canada and the Middle East 12. A FDA clinical trial was conducted in 2009, comparing the results of Tetraflex and monofocal IOLs control patients 33. To date 239 Tetraflex and 96 monofocal control patients were examined twelve months postoperatively. The results show that the Tetraflex patients read better than the control patients at print sizes of 6/24 (ρ=0.04), 6/18.9 (ρ=0.01), 6/15 (ρ<0.001), 6/12 (ρ=0.001), 6/9.6 (ρ <0.001), and 6/7.5 (ρ=0.001) 33. The proportion of patients reading at a speed of eighty words per minute or more was significantly higher with the Tetraflex IOL (ρ= 0.003) 33. Seventy-five percent of Tetraflex patients reported to never wear near spectacles or only occasionally for very small print compared with 46% of control patients (ρ<0.001) 33. Additional near power required for fully corrected near visual acuity was less in the Tetraflex group (ρ<0.001) than the control group. In conclusion the results support the effectiveness of the Tetraflex IOL to provide improved near reading vision and spectacle independence compared to a monofocal IOL control 33.
3.4) Tek-Clear
The Tek-Clear is a single optic accommodative IOL is currently undergoing clinical trials for FDA approval and was recently awarded CE certification in May 2015 12,40. It is a new product hence the only available information is provided by the manufacturer. Tek-Clear has an optic diameter of 5.5mm, with a 360-degree full haptic bag, which comes in customized haptic sizes (Figure 5). The lens has a dual square edge, designed to minimize PCO 41. Tek-Clear contains a bending beam mechanism to optimize optic axial movement. The full 360-degree haptic bag is designed to maximize axial vaulting from the ciliary body contractions 41. The axial movement is achieved without optic tilt or de-centration. Sagittal view of the axial movement, in vitro testing, has demonstrated 2.0 mm of axial movement.41 Tek-Clear A-IOL is placed into the posterior capsular bag, with the bending beam placed in the twelve and six o’clock orientation 41. Tek-Clear is made of a hydrophilic acrylic material and has a UV blocker; the lens is available from 17.0 diopters to 30.0 diopters in 0.50 diopter steps 41. The lens has a refractive index of 1.47 at thirty-five degrees Celsius and its light transmission is over 90% 41. Tek-Clear offers customized haptic sizes from 10.0mm to 11.0mm by 0.20 mm steps 41.
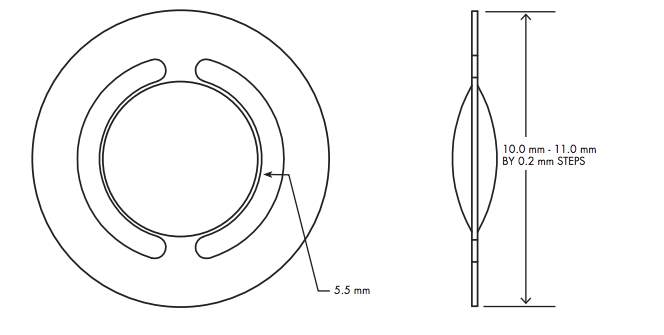 Figure 5. Tek-Clear single optic A-IOL 41
Figure 5. Tek-Clear single optic A-IOL 41
4. Dual Optic Accommodative Intraocular lenses
The single optic accommodative IOLs weak movements are considered pseudo-accommodative because of their limited accommodative ability 42. Their anterior movement is insufficient to provide a complete range of amplitude of accommodation 42. Therefore, dual optic accommodative intraocular lenses were developed. By increasing the distance between the two optics, an optical power change occurs 43,44. The dual optic design combines a more powerful positive lens with a weaker negative lens, similarly to the arrangement of a Galilean telescope. Different designs use springs or magnets to modify the distance between both lenses 44. The Synchrony IOL (AMO, Irvine, California, USA), the Sarfarazi IOL (Shenasa Medical, Carlsbad, California, USA) and the Lumina (AkkoLens Clinical b.v, Breda, The Netherlands) are different dual optic A-IOLs.
4.1) Synchrony
Figure 6. Synchrony dual optic A-IOL23
 The Visogen Company developed Synchrony. It is a mechanical accommodating intraocular lens with two lenses that move closer or farther apart to change focus. When Abbott Medical Optics (AMO) acquired Visogen for four hundred million dollars USD in 2009, Synchrony had just received CE certification in Europe. However, the lens got stalled in the FDA approval process for three years and eventually AMO discontinued it 12.
The Visogen Company developed Synchrony. It is a mechanical accommodating intraocular lens with two lenses that move closer or farther apart to change focus. When Abbott Medical Optics (AMO) acquired Visogen for four hundred million dollars USD in 2009, Synchrony had just received CE certification in Europe. However, the lens got stalled in the FDA approval process for three years and eventually AMO discontinued it 12.
The Synchrony is a single piece dual optic lens, made from silicone material, measuring 9.8 mm horizontally and 9.5 mm vertically. It contains a high plus anterior optic of +32.0 diopters measuring 5.5 mm in diameter, coupled to a smaller negatively powered posterior optic lens measuring 6.0 mm in diameter (Fig. 6). The negative lens’ power is varied in order to produce the desired overall net IOL power 34,37. The two lenses are separated by a spring-activated mechanism. During relaxation of the capsule, under accommodative effort, the haptics separate the lenses at a certain distance causing an increase in near vision power 43,45. Standard cataract surgery is employed during the insertion of the Synchrony, however special attention is paid to the anterior capsulotomy. Since the lens is designed to occupy the whole capsular bag, with the aqueous humor filing the space between the optics, capsule tension is critical for the well functioning of the lens 43,45. The capsulotomy must be well-centered, small and intact 43,45. Ophthalmologist Dr. Packer said that initially, post operation most patients will have a slight myopia 46. The reason is that when Synchrony is newly placed in the capsular bag, the capsule is still very elastic and open, hence the optics are further apart 46. The capsule will progressively shrink with time and by three months; the final distance refraction will be established. Patients that were myopic ended up plano at the three month follow up 46.
A clinical study, was performed on 74 patients undergoing cataract surgery, they were fitted with Synchrony dual optic A-IOL 47. The data collected at six months revealed that the mean binocular uncorrected and distance corrected visual acuity was 6/6 at distance, 6/6 at intermediate and 6/7.5 at near 47. Mesopic contrast sensitivity was within normal limits and seventy-eight percent of the patients had no spectacles, only one eye had IOL repositioning within one month of surgery47. Another clinical study was performed in 2010 to evaluate the long-term visual outcome with the Synchrony dual optic accommodating IOL48. In this study, the near visual performance was evaluated after four years. Fourteen patients (28 eyes) were fitted bilaterally with Synchrony A-IOL. Results were found to be equal to or better than 6/6 for uncorrected binocular visual acuities at all distances 48. Distance corrected near visual acuity in the Synchrony group was statistically better than the control group (monofocals IOLs) by 3-line in near VA 48. Four years after cataract surgery Synchrony A-IOL offers a full range of clear vision and enhanced near vision over standard monofocals IOLs 48.
4.2) Sarfarazi
 Sarfarazi dual optic elliptical accommodative intraocular lens was created by F. Mona Sarfarazi M.D. of Shenasa Medical and was licensed to Bausch and Lomb, in 2003 for further development and marketing 49. F. Mona Sarafazi sued Bausch & Lomb in 2013 for breach of contract, based on Bausch & Lomb’s alleged failure to use commercially reasonable efforts to develop an intraocular lens with the use of Sarfarazi’s intellectual property 49. This has affected the progress of the A-IOL and it is, to this day, still unavailable on the market. Sarfarazi elliptical A-IOL is made of an anterior biconvex positive lens and a posterior concave convex negative lens; both optics are connected by three haptics 50. The EA-IOL is 9.0 to 9.5 mm in length including the haptics and the lenses are 5.0 mm in diameter.
Sarfarazi dual optic elliptical accommodative intraocular lens was created by F. Mona Sarfarazi M.D. of Shenasa Medical and was licensed to Bausch and Lomb, in 2003 for further development and marketing 49. F. Mona Sarafazi sued Bausch & Lomb in 2013 for breach of contract, based on Bausch & Lomb’s alleged failure to use commercially reasonable efforts to develop an intraocular lens with the use of Sarfarazi’s intellectual property 49. This has affected the progress of the A-IOL and it is, to this day, still unavailable on the market. Sarfarazi elliptical A-IOL is made of an anterior biconvex positive lens and a posterior concave convex negative lens; both optics are connected by three haptics 50. The EA-IOL is 9.0 to 9.5 mm in length including the haptics and the lenses are 5.0 mm in diameter.
Figure 7. Sarfarazi dual optic elliptical A-IOL51
The elliptically shaped haptics (Figure 7) correspond to the natural shape of the capsule, making it easier to correctly position and center the optics 50.
The haptics provide the necessary resistance through the relaxation of the capsule caused by the ciliary muscle, which separates the optics and thus creates an increased power for near vision 50. Clinical studies performed on monkeys show that the Sarfarazi lens can perform seven to eight diopters of accommodative power 50. In the optic design recommended for humans, the movement generated between the optics will be of 1.9 mm; this will provide four diopters of accommodation 50.
4.3) Lumina
Lumen is a dual optic mechanical accommodating intraocular lens designed to be placed in the sulcus. The lens is undergoing pending FDA approval, clinical trials have been completed in Europe and Akkolens is anticipating Conformité Européene (CE) recognition 12. The lens is designed for placement in the sulcus (in front of the capsular bag), so it may be less affected by fibrosis and shrinkage of the capsular bag. Lumina is made of a hydrophilic acrylic material and is based on the principle of the Alvarez lens 52. The Alvarez principle stipulates that a change in optical power is produced by laterally translating two refractive plates (Fig. 8).
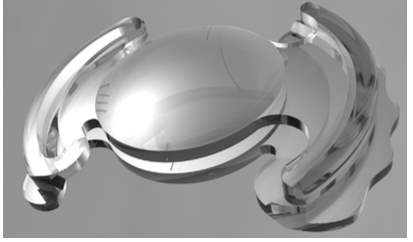
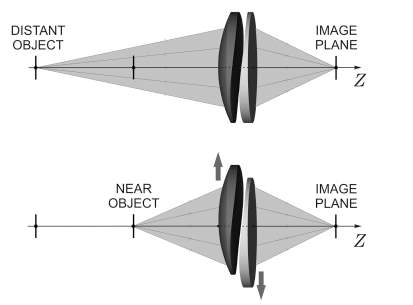
Figure 8. Lumina dual optic mechanical A-IOL52. Figure 9. The lateral translation of the two optics 52.
During accommodation, the ciliary body generates enough energy to trigger the sliding of the two sinusoidal optical surfaces across one another along the horizontal axis (Fig. 9). The anterior optic is a spherical lens. The optical power of the lens increases and changes in a continuous way allowing the patient to focus at all distances; it can yield from two to five diopters in add power 52. The Lumina is designed to be positioned at the sulcus plane, but the haptics on the flanges are meant to rest on top of the ciliary mass and do not considerably spread into the sulcus 52. A clinical study enrolled forty-three patients with cataract, sixty-one eyes were fitted with Lumina A-IOL while the twenty-five eyes were used as control and were fitted with a standard monofocal IOL from Alcon. Results were taken after a one-year follow up. Uncorrected and corrected distance visual acuities improved significantly in both groups (ρ 0.03) and were similar between them one year postoperatively. Uncorrected near visual acuity was 0.07 logRAD in the Lumina group and 0.37 logRAD in the control group at one year follow up; the difference was statistically important (ρ < 0.01). Corrected distance near visual acuity was 0.11 logRAD in the Lumina group and 0.41 logRAD in the control group after one year (ρ< 0.01). Depth of focus was considerably higher in the Lumina group at visual acuities of 0.10, 0.20 and 0.40 logMAR (ρ < 0.01). Contrast sensitivity was similar in both groups 53. The Lumina accommodative IOL successfully reinstated the visual function, accommodation and depth of focus52,53.
5. Curvature Change A-IOLs
The increase of three hundred micrometers in crystalline thickness and the decrease of three hundred micrometers in crystalline diameter size provoke the necessary accommodative power change of five diopters 54,55,56. These small physical modifications of the lens surface curvature elicit a strong change in optical power. A design that mimics the crystalline by generating a variation in optical power, caused by change in surface curvature, is most efficient. Two different designs that rely on change in surface curvature are presently undergoing pending FDA approval, the FluidVision (PowerVision Inc, Belmont, California, USA) and the NuLens (Herzliya Pituach, Israel) 12.
5.1) FluidVision
The FluidVision is a curvature change accommodating IOL; it relies on liquid to make accommodative changes. FluidVision consists of fluid filled haptics connected to a hollow optic. On ciliary contraction fluid is pushed into the optic increasing its curvature and dioptric power 57. The overall diameter of the IOL is 10 mm the optic diameter is 6.0 mm; the optic is suspended between the haptics, which measure 3.0 mm in height (Fig. 11).
Figure 10. FluidVision in accommodated state 57. Figure 11. FluidVision in dissaccommodated state 57.
FluidVision is made of hydrophobic acrylic material; the lens and hollow haptics are filled with silicone fluid of 1.48 refractive index 58. Movement of fluid from the haptics to the optic (Fig. 10) produces large increases in optical power59. A pilot study was conducted in South Africa; twenty patients were implanted with the FluidVision. The patients were followed up at one, three, six, twelve and eighteen months postoperatively 58. Their accommodation, amplitude of accommodation and visual acuities were evaluated. All patients had excellent distance best corrected visual acuity of on average 6/5.7, good intermediate visual acuity of 6/7.8 and near vision of 6/9.9 58. Patients also showed an average of 2.5 to 3 dioptres of accommodation when instilled with pilocarpine. The measurements remained stable throughout the follow up period58. There was little capsular fibrosis one-year post operation and there degradation of the lens was not apparent 57. The haptics fill the capsular bag and maintain an open capsule. The posterior capsule opacification as well as cell growth are constrained by the pressure contact of the IOL and the posterior surfaces of the capsule 57.
5.2) NuLens
NuLens is a curvature change accommodating IOL (Fig. 12), it is a single optic accommodating IOL and is designed to provide up to 10 D of accommodation 12. After the removal of the cataract from the capsule, NuLens is placed in the sulcus and rests behind the iris against the anterior surface of the collapsed capsular bag 60. When the patient accommodates, the collapsed bag moves forward and pushes the base unit, where a piston (Fig. 13), through an opening, pushes the silicone fluid forward and changes the radius of curvature, which leads to an increase in power 60.

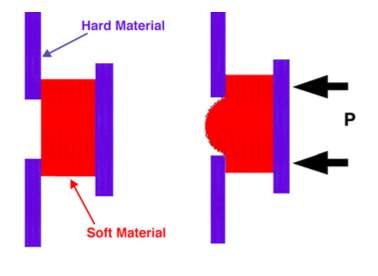
Figure 12. Nulens curvature change A-IOL 60 Figure 13. Nulens, piston applying pressure for curvature change 60
NuLens is not affected by capsule fibrosis but is subject to PCO that can be easily removed with YAG laser 61. A unique feature of the NuLens IOL is its basis of function; it works opposite to the normal accommodative process. Some neuroadaptation is required because accommodation occurs as the ciliary muscle relaxes not as it contracts. NuLens focuses for near when the ciliary muscle is relaxed and focuses for distance when the ciliary muscle is contracted. The relaxed state of the ciliary muscle creates tension in the zonules, which hold the capsular diaphragm in a rigid state 60,61. This applies a force against the posterior surface of the piston, forcing the gel through the opening into a convex shape. Therefore, the lens provides a higher power and the eye can be focused for near vision. When the ciliary muscle contracts, zonular tension and the tension on the capsular diaphragm is released60, 61. This alleviates the pressure on the posterior piston and allows the gel to withdraw from the opening, thus flattening the gel surface curvature. In this state, the eye would be focused for distance 60,61. In a clinical pilot study, a series of patients were inserted with the NuLens curvature change A-IOL and the single optic A-IOL Tek-Clear. Follow-up was three to six months post operation 62. The main results showed that NuLens achieved a mean clinical accommodation of 8.0 to 10.0 diopters while Tek-Clear revealed a mean near vision improvement of 1.25 diopters. In both instances, active ciliary body action was associated with the near vision improvement detected in patients 62. Another study conducted in Spain confirms these findings. After a year follow-up post operation NuLens was found to produce an ocular variation of ten diopters. Near visual acuity improved without compromising distance visual acuity 61. Sixty percent of the study patients were treated with YAG laser for PCO removal, however it did not affect the functioning of the IOL 61.
6. Additional A-IOLs designs
Further accommodative intraocular lenses concepts are undergoing development and deviate from the main design principles. These models are out of the box concepts compared to traditional A-IOLs. Refilling the empty capsular bag with a gel to mimic the crystalline lens, the use of magnets to actively shift an implant and the use electro-active mechanical action to replicate accommodation.
6.1) Lens Refilling
Lens refilling is a technique to restore accommodation by replacing the crystalline lens with an injectable flexible material. After cataract surgery, the removal of the opaque lens through a small capsular opening, the capsular bag is then filled with a transparent, nontoxic, elastic material 70. Ciliary muscle contractions were shown to function even in elderly patients. This suggests that the ciliary muscle may not be responsible for presbyopia. The idea behind this procedure proposes that the new material would mimic the behavior of the natural lens and restore vision; operating similarly to the crystalline lens before it became rigid. The Smart IOL (Medennium Inc., Irvine, California, USA) is a concept for a bag filling IOL. It has been implanted into cadaver eyes but needs to undergo further testing before clinical trials can begin 71. Several techniques of lens filling were used in animal experiments. One tactic is injecting the polymer in a liquid form, through a very small capsular opening, that would then transform into a gel inside the capsule (Fig.16 A) 72. However, this technique proved to be unsuccessful to completely fill the bag. An endocapsular balloon was used in another technique (Fig.16 B) 72. This prevented the silicone from leaking whilst reasonably preserving accommodation functions. However with this technique, the balloon and the capsule shape were occasionally inconsistent resulting in poor amplitude of accommodation. To improve on the balloon technique a plug to seal the small opening (CCC continuous curvilinear capsulorhexis) was added (Fig. 16 C) 72. Another technique uses a very thin intraocular lens as a plug for sealing the capsule opening as seen in (Fig. 16 E, F). In a study with primates, Haeflinger et al. 73 demonstrated that accommodative changes with silicone filling were similar to the changes carried out by the crystalline. A major obstacle to this method of restoring accommodation is “after cataract”, all lens refilling procedures showed high rate of posterior capsular opacification 73. Not only is the vision affected but the presence of after cataract causes the capsule to stiffen, rendering it inflexible and affecting the accommodation. Unlike other intraocular lenses, the YAG laser cannot be used to remove PCOs that would lead to leakage in the posterior segment and severely compromise accommodative function due to a decrease in capsular elasticity74. Although lens refilling seems like a promising and most natural way to restore accommodation, further research needs to be conducted in order to eliminate the obstacles it is facing.
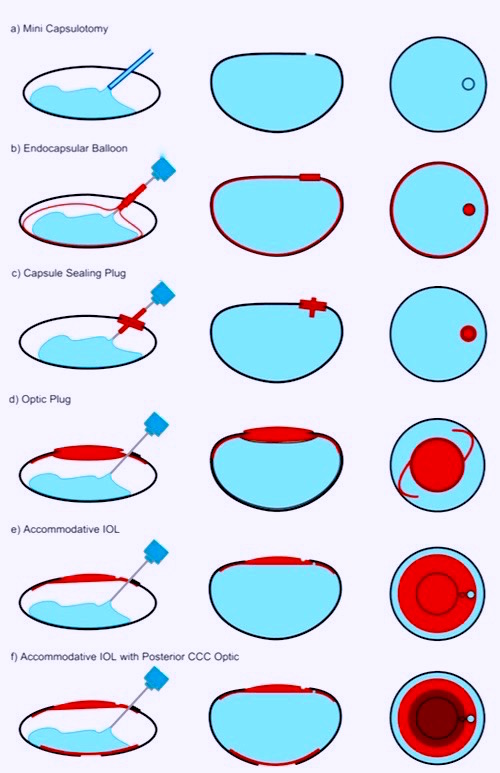
Fig.16 Several lens refilling techniques 72
6.2) Magnet Driven Accommodation Intraocular Lens
After cataract surgery, the capsular bag develops fibrosis, contracts and posterior capsular opacification frequently appears. Methods, which rely on the movement inside the capsular bag to restore accommodation, may not be stable or functional long-term 67,68. To bypass this issue, an accommodating intraocular lens based on magnetically induced movement, is under development at the University Eye Hospital in Mainz, Germany in collaboration with AcriTec (Henningsdorf, Germany). Preussner et al.69 initially suggested the employment of repulsive paired magnets implanted in the three and nine o’clock position. Instead of movement inside the capsular bag, this system moves the entire bag. The design consists of a single optic IOL that is implanted in the capsular bag, combined with pairs of repulsing magnets to drive the lens system forward during accommodation 67,68. The magnetic field created by the magnets converts the contraction of the ciliary muscle into an axial movement of the capsular bag. The amplitude of accommodation is 1.35 diopters for every one-millimeter shift of axial movement 68. Two inner magnets are inserted into the capsule with the IOL while two outer magnets are inserted beneath the superior and inferior rectus muscles; the inner and outer magnets repel each other. Magnetic repulsion will shift the capsule-IOL system anteriorly during ciliary muscle contraction 68.
A small clinical study showed that the welds used to fix the inner magnets to the capsule resisted the contraction forces of the capsular bag, in most cases. Contraction of the capsular bag may exercise important pull on the zonules and cause the loss of axial motion of the capsule-IOL structure 67. This may be due to inappropriate positioning of the welds or contraction forces from the fibrotic capsule. Further studies need to be performed before the magnet driven accommodative IOL is perfected 67.
6.3) Sapphire Autofocal
Sapphire Autofocal (ELENZA Inc., Roanoke, Virginia, USA) is presented as the first electro active accommodating IOL. After two years of development in 2010, ELENZA approached Alcon for financing. Over the next eleven months, Alcon reviewed and made a first round investment of several million dollars. In April 2011, Novartis acquired Alcon and it declined to invest in an additional round. ELENZA has claimed Novartis is attempting to exploit its technologies; the matter is in litigation. Presently, at the FDA, Sapphire Autofocal is under stalled status 12. Sapphire Autofocal contains an aspheric monofocal IOL to correct distance vision and utilizes liquid crystals to change power in response to an electronic signal 63. Liquid crystals have particular electro-optic properties. When an electric field is applied to the molecules of liquid crystals, it alters their orientation and their molecular configuration, which leads to a change in refractive index (Figure 14) 64. The lens has a photovoltaic cell (production of electric current when exposed to light) with photo-sensors that supervise the patient’s pupillary movements associated with accommodation 65. During accommodation, the eyes converge and the pupils contract (Figure 15) 66. Constant illumination and constriction of the pupils is a specific characteristic of accommodation, this triggers and activates Sapphire to provide three diopters for near vision add 65. The system also has a manual override, with a specific blink response that can commence accommodation and an emergency control to turn off the accommodation 63,65. Sapphire Autofocal doesn’t rely on the ciliary muscle contractions and the hardening of the capsular bag does not affect it 63. The IOL contains a microscopic rechargeable lithium-ion battery; it is currently the smallest know to man. Sapphire’s battery is encased in 24-carat gold, the sensors and liquid crystals are hermetically sealed in a glass wafer and the entirety is encapsulated in an acrylic IOL. The battery is estimated to have a life span of fifty years and it requires recharging every three to four days 63. The recharging process can be performed over distances as great as twenty centimeters, with a specialty sleep mask, pillow or neck pillow 65. As a safety feature, if the electronics fail for any reason or the battery is depleted, the lens defaults to a monofocal IOL 65. Clinical studies are presently suspended until the lawsuits get resolved.
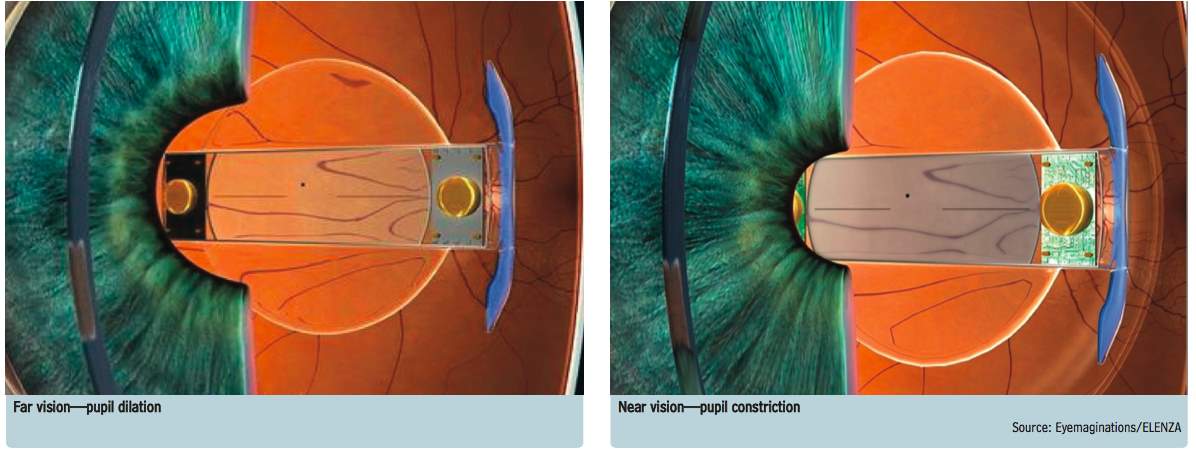
Figure 14. Sapphire Autofocal electro-active A-IOL 63.
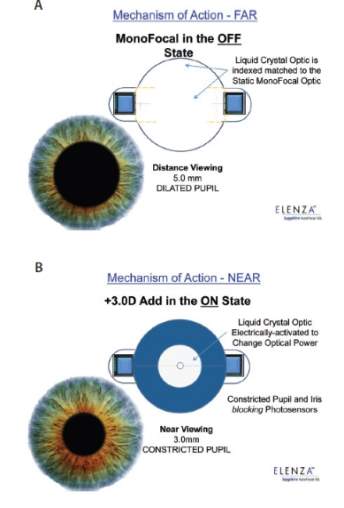
Figure 15. Mechanism of action of the Sapphire IOL: for distance vision (A), the lens works as a monofocal IOL; for near vision (B), the liquid crystals are converted to a near 3.00D add 65.
7. Conclusion
Dynamic accommodation can be restored with accommodative intraocular lenses. The possibility of eliminating the effects of presbyopia entirely could soon become reality. Presently, older generation A-IOLs such as single optic intraocular lenses are the most used but they offer a limited amount of dynamic accommodation. More recent and complex designs, still under development, such as dual optic A-IOLs and curvature change A-IOLs offer higher levels of accommodation. These newer technologies provide more genuine dynamic accommodation than their older counterparts. However, there are still challenges to be overcome regarding long-term stability and efficiency. PCO formation and capsular bag elasticity are the main issues concerning the long lasting function of these A-IOLs. Current techniques dealing with these problems lack in efficacy and may cause added complications. The use of YAG laser may lead to complications, it is costly and not readily available in developing countries. Moreover, pharmaceutical and gene therapy create complications regarding toxicity and cell damage of neighboring structures. Further research in this field is required in order to bypass these issues. Some designs try to avoid the capsular bag rigidity dilemma by implanting the A-IOL in the sulcus. Ultimately, new and exciting concepts such as liquid crystal and photo sensor technologies are being developed and combined with A-IOLs. These would completely overcome challenges faced by older generation A-IOLs. Innovation in this field is evolving rapidly and multiple A-IOLs are becoming widely available on the market. The role of optometrists and ophthalmologists is to carefully counsel patients and discuss the many options available to them so they may make the right choice and ensuring realistic expectations of visual performance.
References
1-Pallikaris, I.G., Plainis, S. and Charman, N.W. (2012) Presbyopia: Origins, effects, and treatment. United States: SLACK.
2-Rosenfield, M., Logan, N., Edwards, K., MCOptom, N.L. and Mark Rosenfield MCOptom PhD FAAO (2009) Optometry: Science, techniques and clinical management. 2nd edn. Edinburgh: Butterworth Heinemann Elsevier.
3-Von Helmholtz H. and Southall JPC. (2005) Treatise on physiological optics, volume I (Dover phoenix editions series). New York, NY, United States: Dover Publications.
4-Hub, S.L. Theories of eye accommodation. 2012. Available online at: http://sciencelearn.org.nz/Contexts/Light-and-Sight/Sci-Media/Images/Theories-of-eye-accommodation.
5-Schachar R. Cause and treatment of presbyopia with a method for increasing the amplitude of accommodation’, Ann Ophthalmol. 1992; 12:445-7, 452.
6-Naroo SA. Refractive surgery: A guide to assessment and management. 2003. Edinburgh: Butterworth-Heinemann.
7-Strenk SA, Strenk, LM. and Koretz JF. The mechanism of presbyopia, Progress in Retinal and Eye Research. 2005; 24:3; 379–393.
8-Lin H, Ouyang H, Zhu J, Huang S, Liu Z et al. Lens regeneration used using endogenous stem cells with gain of visual function. Nature Int. weekly journ. of science. 2016; 531; 323-328
9-Glasser A. and Kaufman PL.The mechanism of accommodation in primates. Ophthalmology. 1999; 106:5; 863–872.
10-Dick HB, Dell S. Single optic accommodative intraocular lenses. Ophthalmol Clin North Am. 2006; 19: 107-124, vi
11-Marchini G, Pedrotti E, Sartori P and Tosi R. Ultrasound biomicroscopic changes during accommodation in eyes with accommodating intraocular lenses. J Cataract Refract Surg 2004; 30: 2476-2482.
12-Smolinsky M. A look at Premium IOLs. OIS News Keeping an “eye on innovation” through news & analysis. Published 05 September 2015. Available online at: http://ois.net/a-look-at-premium-iols/
13- McDonnell, PJZarbin, MAGreen. WR Posterior capsule opacification in pseudophakic eyes. Ophthalmology 1983; 90 (12) 1548- 1553
14- Cobo, LMOhsawa, EChandler, DArguello, RGeorge. Pathogenesis of capsular opacification after extracapsular cataract extraction: an animal model. Ophthalmology. 1984; 91 (7) 857- 863
15- Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002; 74 (3) 337- 347
16- Apple, Solomon DJ, Tetz KD, et al. Posterior capsule opacification. Surv Ophthalmol. 1992; 37 (2) 73- 116
17- Hiles DA, Wallar PH. Phacoemulsification versus aspiration in infantile cataract surgery. Ophthalmic Surg. 1974; 5 (2) 13- 16
18-Bennett RB. The schematic eye. In: Rabbetts RB, ed. Bennett & Rabbett’s Clinical Visual Optics. Oxford, Boston: Butterworth-Heinemann, 1998.
19- Langenbucher A, Seitz B, Huber S, Nguyen NX, and Küchle M. Theoretical and measured pseudophakic accommodation after implantation of a new accommodative posterior chamber intraocular lens. Arch Ophthalmol. 2004; 121: 1722– 1727.
20- Calogero D. Food and Drug Administration (US). Intraocular Lens Regulation Division of Ophthalmic and Ear, Nose and Throat Devices, FDA/CDRH/ODE. Published May 2012. Available online at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/OphthalmicDevicesPanel/UCM347589.pdf
21-Cumming JS, Colvard DM, Dell SJ, Doane J, Fine IH, Hoffman RS, Packer M et al. Clinical evaluation of the Crystalens AT-45 accommodating intraocular lens: results of the US Food and Drug Administration clinical trial. J Cataract Refract Surg. 2006; 32: 812–825.
22- Hazra S, Palui H and Vemuganti GK. Comparison of design of intraocular lens versus the material for PCO prevention. International Journal of ophthalmology. 2012; 5(1): 59-63.
23-Burkhard Dick H. Accommodative intraocular lenses: current status. Curr Opin Ophthalmol. 2005, 16:8-26.
24-Fine H, Packer M, Hoffman R.S. Refractive Lens Surgery. Volume 1. Springer-Verlag Berlin Heidelberg (Germany): Science & Business Media. 2005; 7 Jul 2005: 87-97.
25-Adrian G. Restoration of accommodation: surgical options for correction of presbyopia. Clin Exp Optom. 2008; 91:3: 279-295.
26- Langenbucher A, Huber S, Nguyen NX, Seitz B and Küchle M. Cardinal points and image-object magnification with an accommodative lens implant (1CU). Ophthalmic Physiol Opt. 2003; 23: 61–70.
27- Rana A, Miller D, and Magnante P. Under- standing the accommodating intraocular lens. J Cataract Refract Surg. 2003; 29: 2284– 2287.
28- Prepose JS. Design strategies for new accommodating IOLs. Catarct Refract Surg Today. 2009; 9:39-45.
29-Marcai MS, Padnick-Silver L and Fontes BM. Visual outcomes after accommodating intraocular lens implantation. J Cataract Refract Surg. 2006; 32: 628-633.
30-Jardim D, Soloway B and Starr C. Asymmetric vault of an accommodating intraocular lens. J Cataract Refract Surg. 2006 Feb; 32(2): 347-350.
31- Yuen L, Trattler W and Boxer Wachler BS. Two cases of Z syndrome with the Crystalens aster uneventful cataract surgery. J cataract Refract Surg. 2008; 34:1986-1989.
32-Bausch and Lomb. Trulign Toric Posterior Chamber Intraocular Lens, product information brochure. Available online at: http://www.bausch.com/Portals/69/-/m/BL/United%20States/USFiles/Downloads/Consumer/Surgical/consumer-brochure-trulign.pdf?ver=2017-01-09-082946-043
33-Sanders DR, and Sanders ML. US FDA Clinical Trial of the Tetraflex Potentially Accommodating IOL: Comparison to Concurrent Age-matched Monofocal. Tetraflex Presbyopic IOL Study Group. Journal of Refractive Surgery 2010; 26(10): 723-730. Available online at: http://www.lenstec.com/uploads/2/6/7/1/26715076/jrs-2010-us-fda-clinical-trial-of-the-tetraflex-potentially-accommodating-iol.pdf
34-Lenstec. How the Tetraflex is designed to use the natural accommodation process. 2009. Available online at: http://www.lenstec.com/uploads/2/6/7/1/26715076/jrs-2010-mechanism-of-action-tetraflex.pdf
35-Bethke W. Accommodative IOL Come into Focus. Rev of Ophthalmol. 2011; published 15 February 2011. Available online at: https://www.reviewofophthalmology.com/article/accommodative-iols-come-into-focus
36-Fine H, Packer M and Hoffman RS. Refractive Lens Surgery. Volume 1. Springer-Verlag Berlin Heidelberg (Germany): Science & Business Media. 7 Jul 2005; 99-112.
37- Kuchle M, Seitz B, Langenbucher A, Martus P,and Nguyen NX. Stability of refraction, accommodation and lens position after implantation of the 1CU accommodating posterior chamber intraocular lens. J Cataract Refract Surg. 2003; 29: 2324–2329.
38-Mastropasqua L, Toto L, Falconio G, Nubile M, Carpineto P, Ciancaglini M, Di Nicola M, and Ballone E. Longterm results of 1CU accommodative intraocular lens implantation: 2-year follow-up study. Acta Ophthalmol. Scand. 2007; 85: 409-414.
39-Hayashi K & Hayashi. Posterior capsule opacification after implantation of a hydrogel intraocular lens. Br J Ophthalmol. 2014; 88: 182–185.
40-CE certification of the Tek-Clear Accommodative Intraocular Lens. Available online at: http://www.tekia.com/resources/certificate_cemark/Certificate_CE_II3-9611_Rev7_Accommodative_IOL.pdf
41- Vargas J.M. CEOVAL, Centro Oftalmologico de Valencia, Venezuela. 2015. Available online at: http://www.videris.cz/FTP/tek-clear_surgeon_Rev_D_v1.pdf
42- Gil-Cazorla R, Shah S, Naroo SA. A review of the surgical options for the correction of presbyopia. Br J Ophthalmol. 2016; 100: 62-70 originally published online April 23, 2015.
43- Ossma IL, Galvis A, Vargas LG, et al. Synchrony dual-optic accommodating intraocular lens—Part 2: pilot clinical evaluation. J Cataract Refract Surg. 2007; 33:47–52.
44- McLeod SD. Optical principles, biomechanics, and initial clinical performance of a dual-optic accommodating intraocular lens (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006; 104: 437–452.
45-McLeod SD, Vargas LG, Portney V and Ting A. Syn- chrony dual-optic accommodating intraocular lens. Part 1: Optical and biomechanical principles and design considerations. J Cataract Refract Surg. 2007; 33: 37–46.
46-Dalton M. New IOLs on the Horizon. Eye World. Published online December 2009. Available online at: www.eyeworld.org/article-new-iol
47- Marques EF, Castanheira-Dinis A. Clinical Performance of a new aspheric dual-optic accommodating intraocular lens. Clin Ophthalmol 2014; 8: 2289-2295.
48- Lau G, Kasthurirangan S and Vargas L. Long term visual outcome with the Synchrony dual-optic accommodating IOL. American Academy of Optometry. 2010. Available online at: http://www.aaopt.org/long-term-visual-outcome-synchrony-dual-optic-accommodating-iol
49-Telesca M.A. Bauch & Lomb Incorporated v. Sarfarazi, No. 6:2009cv06041-Document 82 (W.D.N.Y. 2013). July 2013. Available online at: http://law.justia.com/cases/federal/district-courts/new-york/nywdce/6:2009cv06041/72361/82/
50-Sarfarazi FM. Sarfarazi dual optic accommodative intraocular lens. Ophthalmol Clin North Am. 2006; 19: 125–128.
51-Sheppard AL, Bashir A, Wolffson JS and Davies L. Accommodating intraocular lenses: a review of design concepts, usage and assessment methods. Clin Exp Optom. 2010; 93: 6: 441-452.
52-Alio JL, Simonov A, Plaza-Puche AB, Angelov A, Angelov Y, Van Lawick W and Rombach M. Visual Outcomes and Accommodative Response of the Lumina Accommodative Intraocular Lens. AM j Ophthalmol. 2016 Apr; 164:37-48.
53- Alió JL, et al. Lumina accommodating IOL improves near visual acuity, contrast sensitivity. AM J Ophthalmol. 2016.
54- Vilupuru AS and Glasser A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005; 80: 349–360.
55-Ostrin L, Kasthurirangan S, Win-Hall D and Glasser A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci. 2006; 83: 657–665.
56- Glasser A, Wendt M and Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest Ophthalmol Vis Sci. 2006; 47: 278–286.
57-Groves, N. Fluid-Based injectable IOL maintains stability in capsular bag. Ophthalmology Times: North Olmsted. 2014; 39:16-20.
58-Charters L. Fluid-filled accommodative IOL shows stable visual function over 18 months. Ophthalmology Times; North Olmsted. 2015; 40: 9-28.
59-Charters L, and Nichamin L. Fluid-based IOL promising Foldable version of accommodating lens produces little capsular fibrosis, research shows. Ophthalmology Times; North Olmsted. 2015; 38:18-28.
60-Ben-Nun J and Alió JL. Overview of the NuLens accommodating IOL. Cataract & Refractive Surgery Today Europe. 2008; 3(1): 20-21.
61-Alió JL, Ben-Nun J, Rodríguez-Prats JL and Plaza AB. Visual and accommodative outcomes 1 year after implantation of an accommodating intraocular lens based on a new concept. J Cataract Refract Surg. 2009; 35:1671-1678.
62-Sanz JA and Ben-Nun J. Innovative IOL accommodative technologies: NuLens and TekClear. Acta Ophthalmologica. 2008; 86.
63-Hayden FA. Electronic IOLs: The future of cataract surgery. EW Feature. February 2012. Available online at: http://www.elenza.com/docs/Electronic_IOLS_EW_February_2012.pdf
64-Saleh BEA and Teich MC. Fundamentals of photonics. Hoboken, NJ: Wiley-Interscience; 2009, Chapter 19.
65-Donnenfeld E.D. An Autofocal Accommodating IOL, The Sapphire implant is the first to allow visual accommodation without movement of the lens. Cover Focus. January 2015. Available online at: http://crstodayeurope.com/articles/2015-jan/an-autofocal-accommodating-iol/
66-Donnenfeld E, Zikos G, Robilotto R, Gupta A and Morris M. The effect of cataract surgery on the pupil light response. Poster presented at: the XXXI Congress of the ESCRS; October 5-9, 2013; Amsterdam, Netherlands.
67-Menapace R and Preussner PR. Magnet-Driven Active-Shift IOL to Restore Accommodation, Magnets may improve the functionality of accommodating IOLs. UP FRONT. Jan 2009. Available online at: http://crstodayeurope.com/articles/2009-jan/0109_15-php/
68-Menapace R. Pre-loaded shift IOL systems: Concepts and first clinical experiences [German]. Proceedings of the 19th annual meeting of the DGII. 2005; 18–19. Magdeburg, Germany.
69-Preussner PR, Wahl J, Gerl R, et al. Akkommodatives Linsenimplantat. Ophthalmologe. 2001; 98:97-102.
70-Ford J, Werner L and Mamalis N. Adjustable intraocular lens power technology. J Cataract Sur. 2014; 40:1205-1223.
71-Stephenson M. What’s Next in Accommodative IOLs. Rev Ophthalmol, 2006; published 20 February 2006. Available online at: https://www.reviewofophthalmology.com/article/whats-next-in-accommodative-iols66-
72- Nishi Y, Mireskandari K, Khaw P and Findl O. Lens refilling to restore accommodation. J Cataract Refract Surg 2009; 35:374-382
73-Haefliger E. Parel JM, Fantes F, Norton EWD, Anderson DR, Forster RK, Hernandez E and Feuer WJ. Accommodation of an endocapsular silicone lens (phacoersatz) in the nonhuman primate. Ophthalmology. 1987; 94:471–477.
74- Hettlich HJ, Lucke K, Asiyo-Vogel MN, et al. Lens refilling and endocapsular polymerization of an injectable intraocular lens: in vitro and in vivo study of potential risks and benefits. J Cataract Refract Surg.1994; 20:115–123.
75-Nawa Y, Ueda T, Nakatsuka M, Tsuji H, Marutani H, Hara Y and Uozata H. Accommodation obtained per 1.0 mm forward movement of a posterior chamber intraocular lens. J Cataract Refract Surg. 2003; 29: 2069–2072.
76-Brown D, Dougherty P, Gills JP, Hunkeler J, Sanders DR and Sanders ML. Functional reading acuity and performance: comparison of 2 accommodating intraocular lenses. J Cataract Refract Surg. 2009; 35: 1711–1714.
77-McLeod SD, Portney V and Ting A. A dual optic accommodating foldable intraocular lens. Br J Ophthalmol. 2003; 87: 1083–1085.
78-ELENZA, Inc. – Electronic intraocular Lens. ELENZA, Inc. Electronic Intraocular Lens rechargeable and fully programmable Autofocal. Available online at: http://www.elenza.com/
Log of Meetings with Supervisor
| Date | Discussion |
| 28th of April 2016 | Initial meeting to go over topic at the end of second year. |
| 25th of January 2017
via e-mail |
Email written about dissertation progress. |
| 3rd of April 2017 | Office meeting, a quick look at dissertation, discussed the points to be refined. |
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Surgical Studies"
Surgery is the branch of medicine that involves the treatment of injuries, diseases and other conditions by operative methods, i.e. by cutting open the body and removing or repairing a damaged part. Technological advances mean that many surgeries can now be performed without large incisions using what is known as keyhole surgery.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: