Solvent Extraction of Astaxanthin from Haematococcus Cyst Cells
Info: 6138 words (25 pages) Dissertation
Published: 17th Dec 2019
Tagged: CancerBiomedical Science
Solvent Extraction of Astaxanthin from Haematococcus Cyst Cells
Table of Contents
3.0 H. pluvialis as a Source of Astaxanthin
4.0 Solvent Extraction of Astaxanthin from H. pluvialis
1.0 Introduction
Haematococcus pluvialis is one of the potent organisms capable of synthesizing astaxanthin, which is a high value keto-carotenoid. Astaxanthin is believed to accumulate in thick walled cyst cells of Haematococcus, provided that the thick cell wall consists of sporopollenin-like material (algaenan) that can potentially hinders solvent extraction of astaxanthin. Therefore, disruption of cells, either via mechanical or non-mechanical methods, has to be accessed. Subsequently, the extraction of astaxanthin is performed by treating the cells with various solvents, for instance acetone, n-hexane, methanol, dimethyl sulfoxide (DMSO) etc. The effectiveness of these solvent extraction techniques can then be evaluated via two ways: spectrophotometry along with the use of Lichtenthaler equations, and high performance liquid chromatography (HPLC). The overall process flow describing the extraction of astaxanthin from Haematococcus pluvialis is summarized and illustrated as shown in Figure 1 below.
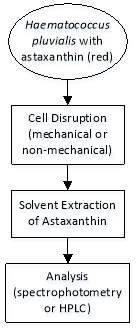
Figure 1: Solvent extraction of astaxanthin.
2.0 General Background
2.1 Astaxanthin
Astaxanthin (3,3’-dihydroxy--carotene-4,4’-dione) is a keto-carotenoid or tetraterpenoid, which belongs to a larger class of chemical compounds known as terpenes. Generally, terpenes are made up of five carbon precursors: isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). As a member of carotenoid compounds, astaxanthin attains oxygen-containing moieties, i.e. hydroxyl (-OH) or ketone (C=O) functional groups as shown in Figure 2 below.
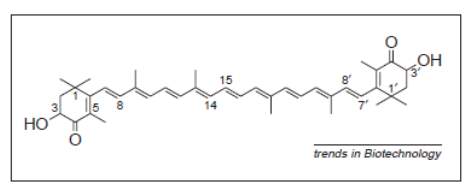
Figure 2: Structure of astaxanthin (Lorenz & Cysewski, 2000).
As can be seen in Figure 2, astaxanthin consists of two asymmetric carbons located at the 3 and 3’ positions of the benzenoid rings on either end of the molecule. Two types of configurations are concerned: R and S configurations which are largely dependent on the exact way of how -OH groups are attached to the carbon atoms at these centers of asymmetry, and hence results in different enantiomers of the astaxanthin molecule. R configuration particularly defines the situation where -OH group is attached and projected above the plane of the molecule, meanwhile the reverse statement implies that astaxanthin attains the S configuration. Thus, three possible enantiomers exist and are designated as 3R,3’R, 3S,3’S and 3R,3’S (Lorenz & Cysewski, 2000). Astaxanthin can be further classified into three: polar free astaxanthin (3 and 3’ -OH groups are not bonded), non-polar astaxanthin monoester (a fatty acid bonded to a 3 position) and diester (a fatty acid bonded to each of the 3 positions) (Todd, 2001).
Similar to most of the carotenoids, astaxanthin represents a colorful, lipid-soluble pigment. These characteristics are due to the long chain conjugated double bonds present at the centre of the compound (see Figure 2). In addition to astaxanthin’s role in the coloration of aquatic animals (usually red color), it possesses several essential bioactivities including anti-oxidation, enhancement of immune response and anticancer activities. Numerous studies have confirmed that astaxanthin possesses health-promoting effects in the prevention and treatment of various diseases, for example cancers, chronic inflammatory diseases, metabolic syndrome, diabetes, diabetic nephropathy, cardiovascular diseases, gastrointestinal diseases, liver diseases, neurodegenerative diseases, eye diseases, skin diseases, exercise induced fatigue, male infertility and HgCl2-induced acute renal failure (Herrero, Orosa García, Abalde, Rioboo Blanco, & Cid, 2012).
Astaxanthin is more commonly detected in some microalgae and phytoplankton, but it can also be found in some fungi, bacteria, yeast, salmon, trout, krill, shrimp, crayfish and crustaceans (Biswal, 2014) as evident in Table 1 below. As in the case of commercial production of astaxanthin, the primary natural sources comprise of:
- Euphausia pacifica (Pacific krill)
- Euphausia superba (Antarctic krill)
- Haematococcus pluvialis (Microalgae)
- Pandalus borealis (Arctic shrimp)
- Xanthophyllomyces dendrorhous, formerly Phaffia rhodozyma (yeast)
The approximate concentrations of astaxanthin in natural sources (Table 1) effectively demonstrate that Haematococcus pluvialis contains the highest level of astaxanthin in nature.
Table 1: Astaxanthin from various natural sources (Biswal, 2014).
| Source | Astaxanthin Concentration (ppm) |
| Salmonids | 5 |
| Plankton | 60 |
| Krill | 120 |
| Arctic shrimp (P. borealis) | 1200 |
| Phaffia yeast | 10000 |
| Haematococcus pluvialis | 40000 |
2.2 Haematococcus pluvialis
Haematococcus pluvialis (H. pluvialis) is an ubiquitous single cell biflagellate microalgae (Herrero et al., 2012) freshwater species of Chlorophyta from the family of Haematococcaceae. It is often found in temperate regions around the world, particularly in puddles, eaves and birth bathes. An eye-catching red to deep purple color of H. pluvialis as shown in Figure 3 indicates a massive accumulation of the secondary carotenoid astaxanthin, as well as its fatty acid mono or diesters, when the environmental conditions are unfavorable for normal cell growth.

Figure 3: Haematococcus pluvialis cysts (akinete forms in resting stage) full of astaxanthin (Fox, 2011).
3.0 H. pluvialis as a Source of Astaxanthin
Present technologies have widely exploit the microalgae Haematococcus pluvialis as the primary industrial source for natural astaxanthin synthesis. As proven in Table 1, H. pluvialis seems to accumulate the highest contents of astaxanthin in nature. According to Mendes-Pinto et al. (2001), Haematococcus can produce up to 8% dry weight of astaxanthin in the form of 3S, 3’S enantiomer, provided that it may consist about 70% monoesters, 10% diesters, 5% free astaxanthin, with the remainder comprising of - carotene, canthaxanthin, and lutein (Todd, 2001).
H. pluvialis has two types of cell morphology depending on the environmental conditions: green motile and non-motile forms. The cells tend to be green under optimal growth conditions, being capable of actively swimming with two flagella and increasing in cell number. However, adverse conditions cause unusual behaviour of green vegetative cells such that they cease to be motile, expand drastically, lose their flagella, form a hard, thick cyst-like wall and eventually enter a resting stage. Within this resting stage, astaxanthin is synthesized in the cyst cells as visualized by a red color due to astaxanthin accumulation (Herrero et al., 2012).
3.1 Synthesis Pathway
By referring to Herrero et al. (2012), higher plants and green algae share the same carotenoid biosynthetic pathway to -carotene. Enzymes of -carotene such as ketolase and hydroxylase catalyze further steps leading to astaxanthin in H. pluvialis.
Astaxanthin can be synthesized from β-carotene by two different pathways:
(a) With the use of diphenylamine (astaxanthin synthesis inhibitor), a synthesis pathway for Haematococcus has been proposed:
β-carotene→echinenone→cantaxanthin→adonirubin→astaxanthin
(b) Alternatively, astaxanthin biosynthesis occurs by:
β-carotene→β-cryptoxanthin→zeaxanthin→adonixanthin→astaxanthin
3.2 Astaxanthin Production
In general, the production of astaxanthin-rich Haematococcus is achieved through a two-step process: vegetative (green) and aplanospore (red) stages (Herrero et al., 2012). Firstly, the mass production of green vegetative cells is achieved under an approximate optimal growth conditions associated with careful control of pH, temperature and nutrient levels. Once a satisfactory viable cell density is accomplished, the broth culture is subjected to stresses to induce the formation of red cyst cells (Lorenz & Cysewski, 2000).
Several induction methods have been developed for the transformation of green cells to red cysts carrying high astaxanthin contents (see Figure 4). They can be divided into two classes according to the role of astaxanthin:
(a) First class utilizes various environmental stresses (including nutrients), except for strong light. This technique is aimed to cause retardation in cell multiplication, preferably via nitrogen starvation or the presence of excessive acetate, salt or other specific inhibitors targeting impaired cell division. Due to the scarcity of some factors necessary for normal cell growth, the cells store astaxanthin possessing an antioxidant function (Herrero et al., 2012).
(b) Second class refers to light induction method which exploits the photo-protective role of astaxanthin under high light intensity. Exposure towards high-intensity light leads to an accelerated astaxanthin accumulation that effectively protects the cells against photo and oxidative damages. Coupled action such as excess acetate addition or a supply of favorable CO2 concentration can be implemented along with the light induction method to enhance astaxanthin manufacture and accumulation (Herrero et al., 2012).
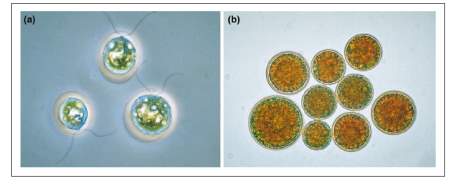
Figure 4: (a) Green Haematococcus vegetative cells and (b) Red haematocysts as a result of nutrient starvation and sunlight. 400x magnification (Lorenz & Cysewski, 2000).
Typical commercial systems introduce nitrate and phosphate deprivation, high temperature and light intensity, or addition of salt (NaCl) to the culture medium so as to facilitate the manufacture of astaxanthin. Usually within 2-3 days after stressing, red cysts are formed, and they are ready for harvest within 3-5 days following the haematocysts formation (contain about 1.5-3.0% astaxanthin) (Lorenz & Cysewski, 2000).
4.0 Solvent Extraction of Astaxanthin from H. pluvialis
Although Haematococcus pluvialis is one of the most important natural sources of the carotenoid astaxanthin, the thick sporopollenin cell wall in the cyst tends to hinder astaxanthin extraction and its subsequent bio-availability. Therefore, pretreatment of cyst cells (cell disruption) has to be accessed prior to solvent extraction of astaxanthin from H. pluvialis. The efficiency of various solvent extraction methods will then be evaluated through appropriate assays to be mentioned in the following subsections.
4.1 Cell Disruption
Theoretically, cell disruption involves the application of various effective techniques to open the cell wall in order to obtain the intracellular fluid without disrupting or damaging any of its essential components. Cell type and its cell wall composition remarkably determine the methods that are suitable for the disruption process (Naeem, 2016).
There are effectively two types of cell disruption methods: mechanical methods which utilize forces to separate out intracellular protein, and non-mechanical methods comprising physical, chemical and enzymatic methods (Naeem, 2016) stated in Table 2.
Table 2: Cell disruption methods (Naeem, 2016).
| Methods | Examples | Descriptions |
| Mechanical | Mortar & pestle/grinding | Most common method applicable to plant tissues frozen in liquid nitrogen. |
| Blender | High speed rotating blades can potentially disperse cells and tissues. This technique is similar to centrifugation. | |
| Bead beating | Glass or ceramic beads are used to crack open the cells (low mechanical shear keeps the organelles intact). | |
| Ultra sonication | Combined actions of vibration (high frequency sound waves via probe) and cavitation produce a local shockwave that disrupt the cell walls by pressure change. | |
| Homogenization (liquid-based) | Cell or tissue suspension are sheared by forcing them though a narrow space. |
| Non-mechanical | Physical | Freeze thaw | Cell disruption is achieved via a series of freezing and thawing cycles. Freezing forms ice crystals which expand upon thawing, thus ultimately rupture the cell wall. |
| Microwave | In addition to heat, the proteins within the cell wall denatures (disruption of bonds). This can be risky as excess heat can damage the cell. | ||
| Osmotic shock | The cells (without cell walls) is immersed in a hypotonic solution in which the water enters the cells until they burst. | ||
| Electrical discharges | Applicable to cells without cell walls (e.g. mammalian cells), thus allowing the researches to examine secretion by exocytosis. | ||
| Chemical | Permeation of cell walls can be achieved by applying organic or inorganic solvents. | ||
| Enzymatic | Digestive enzymes decompose the cell wall. Type of enzyme suitable for the operation depends on the cell type and strain (and hence the cell wall). | ||
According to the study performed by Mendes-Pinto et al. (2001), a range of physical and chemical processes were tested to promote the disruption of the encysted cells of Haematococcus pluvialis. The astaxanthin-rich biomass were subjected to the following processes:
(i) Autoclave at 121C, 1 atm for 30 minutes
(ii) 0.1 M HCl for 15 minutes
(iii) 0.1 M HCl for 30 minutes
(iv) 0.1 M NaOH for 15 minutes
(v) 0.1 M NaOH for 30 minutes
(vi) Enzymatic treatment for 1 hour with a mixture of 0.1% protease K and 0.5% driselase in a phosphate buffer, maintained at pH 5.8 and 30C
(vii) Spray drying with inlet and outlet temperatures of 180C and 115C respectively
(viii) Cell homogenization
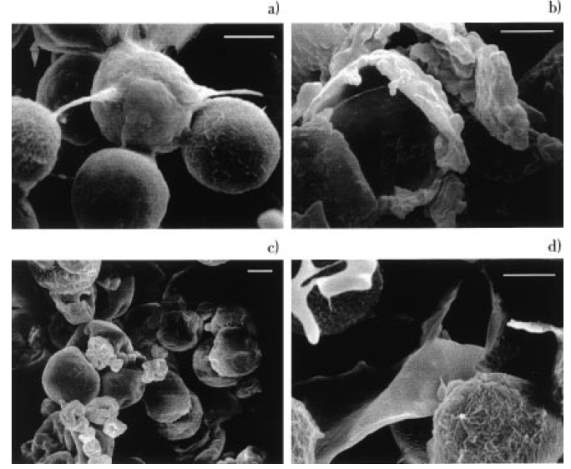
Figure 5: SEM of Haematococcus pluvialis (x 2000), bar size 10 μm: (a) control, (b) cell homogenization, (c) spray dry, and (d) autoclave (Mendes-Pinto, Raposo, Bowen, Young, & Morais, 2001).
Based on Figure 5, as compared to the intact cells (Figure 5a), obvious cell disruption was observed when H. pluvialis was treated by homogenization (Figure 5b) and autoclave (Figure 5d). An interesting phenomenon was noticed in the spray dried cells (Figure 5c) such that they tended to clump together even though the cells were not damaged by this process. On the other hand, Mendes-Pinto et al. (2001) mentioned that chemical treatment (NaOH or HCl) of H. pluvialis only resulted in slight distortion of some cells unless prolonged exposure was implemented. However, enzymatic treatment showed no visible effect on cell morphology (SEM not shown) (Mendes-Pinto et al., 2001).
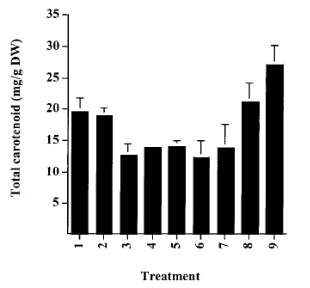
Figure 6: Total carotenoid content of Haematococcus cysts: 1. Control; 2. Autoclave; 3. HCl 15 min; 4. HCl 30 min; 5. NaOH 15 min; 6. NaOH 30 min; 7. Enzyme; 8. Cell homogenization; 9. Spray drying (Mendes-Pinto et al., 2001).
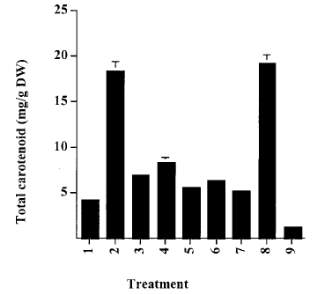
Figure 7: Extraction of total carotenoids into acetone from processed Haematococcus biomass. 1. Control; 2. Autoclave; 3. HCl 15 min; 4. HCl 30 min; 5. NaOH 15min; 6. NaOH 30 min; 7. Enzyme; 8. Cell homogenization; 9. Spray drying (Mendes-Pinto et al., 2001).
Distinct cell processing techniques affect the extractable total carotenoids from H. pluvialis as shown in Figures 6 and 7. By referring to Figure 6, the cells that had experienced autoclave, spray dry and homogenization attained the highest recovery of astaxanthin. However, the treatment of Haematococcus cells by acid, alkali or enzyme led to a significant loss of carotenoid in comparison to the unprocessed intact cells (Mendes-Pinto et al., 2001). Upon extraction (Figure 7), high total carotenoid contents were noticed for cells that had been autoclaved or homogenized. This discovery suggests that these two cell disruption methods may be the most suitable and effective ways of rupturing the cell walls and enhancing the extraction of astaxanthin from the lysed cells (Mendes-Pinto et al., 2001).
4.2 Solvent Extraction
Partition of components or compounds in a mixture can be easily accomplished by applying solvent extraction or liquid-liquid extraction. This process works based on the solubility of the desired component(s) in two different immiscible liquids: aqueous and organic phases. Several studies or research papers are focused in relation to the astaxanthin extraction methods from green alga Haematococcus pluvialis:
(a) An Efficient Method for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis (Sarada, Vidhyavathi, Usha, & Ravishankar, 2006).
This study had introduced three methodologies in the extraction of astaxanthin from H. pluvialis:
(i) Mortar, pestle and neutral glass powder were used to homogenize H. pluvialis, subsequently extracted with acetone.
(ii) Separate cell samples were treated or extracted with acetone (1 h and 24 h), methanol (24 h) and dimethylsulfoxide (DMSO) with 5 drops of glacial acetic acid.
(iii) Biomass were separately treated with HCl (1-10 N), and 2 N formic acid, citric acid, acetic acid and tartaric acid, followed by extraction in acetone for 1 h.
The results obtained from this study are shown in Table 3 and Figure 8, such that in this case the selected Haematococcus cells contained 2% total carotenoid (w/w) with astaxanthin constituted 88% of total carotenoids and 1.76% w/w of dry biomass (Sarada et al., 2006).
Table 3: Astaxanthin extractability in terms of different solvents with and without acid treatment (Sarada et al., 2006).
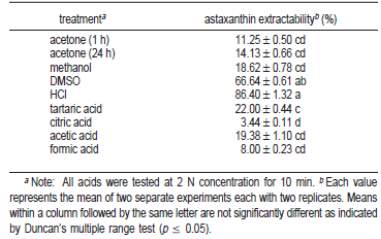
Treating H. pluvialis with solvents alone (methodology (ii)) yielded low astaxanthin extractability, except for DMSO in which the enhanced extractability was aided by the presence of glacial acetic acid. This observation is also proved by the fact that Aquasearch in-house research had confirmed and selected DMSO as the best choice among extractants for green algae or more specifically Haematococcus algae meal and cysts (Aquasearch, 1999a). On the other hand, among the acids tested (methodology (iii)), HCl treatment followed by acetone extraction gave the highest astaxanthin extractability (Table 3).
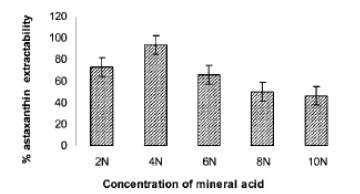
Figure 8: Astaxanthin extractability from Haematococcus encysted cells after treatment with HCl at 70 °C for 2 min (Sarada et al., 2006).
By referring to Figure 8, the astaxanthin extractability was also proved to increase with increasing HCl concentration. Yet, the extractability was observed to decline significantly at higher concentrations (> 4 N HCl) due to cell floating (probably dead). In addition, the astaxanthin extracted via methodology (i) and methodology (iii) were analyzed by both TLC and HPLC (data not shown), eventually demonstrating that both samples were identical, i.e. indicating that efficient extraction of astaxanthin can be achieved without homogenization by mechanical means (Sarada et al., 2006).
(b) Four Different Methods Comparison for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis (Dong, Huang, Zhang, Wang, & Liu, 2014).
In this research article, four different extraction methods were implemented and intensively evaluated for the extraction of astaxanthin from H. pluvialis:
(i) HCl pretreatment followed by acetone extraction (HCl-ACE Extraction)
(ii) Hexane/isopropanol (6:4, v/v) mixture solvent extraction (HEX-IPA Binary Solvents Extraction)
(iii) Methanol extraction followed by acetone extraction (MET-ACE 2-Step Extraction)
(iv) Soy-oil extraction (or Oil-Soy Extraction)
The outcomes of the study are tabulated in Table 4, showing the effect of different extraction methods on oil yield and total astaxanthin content (TAC).
Table 4: Oil yield and TAC of H. Pluvialis cell extracts (Dong et al., 2014).

It was observed that HCl-ACE extraction method presented the highest extraction oil yield and TAC. Although HEX-IPA and MET-ACE methods showed the similar extraction oil yield, the latter possessed a higher TAC extraction. In view of oil-soy method, the resultant TAC was significantly lower than those of other three extraction methods even though the extraction oil yield was relatively satisfactory (Dong et al., 2014).
The total oil yield and TCA extraction from H. pluvialis by four different techniques presented a strongly significantdifference, which are technically viable depending on theastaxanthin content of extracts. The best extraction method,in terms of oil yield, TAC and antioxidant of extract, wasHCl-ACE procedure. SEM (Scanning Electron Microscopy), NMR (Nuclear Magnetic Resonance), and DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical Scavenging Activity assays andreducing power experiments could be carried out to further confirm that HCl-ACE procedure was suitable for extractionof astaxanthin from H. pluvialis biomass (Dong et al., 2014).
(c) Extraction of Astaxanthin via Supercritical CO2 (Majewski, Perrut, & Valderrama, 2000)
Instead of extraction using organic solvents, supercritical CO2 can be an alternative for alga treatment due to its some unique characteristics and being an environmental friendly solvent. Majewski et al. (2000) had performed three runs involving the processing of samples by:
(1) Milling followed by supercritical CO2 extraction.
(2) Milling, then grinding with dry ice (solid CO2), followed by supercritical CO2 extraction.
(3) Similar to (2) but extracted using supercritical CO2 and 9.4% ethanol (co-solvent).
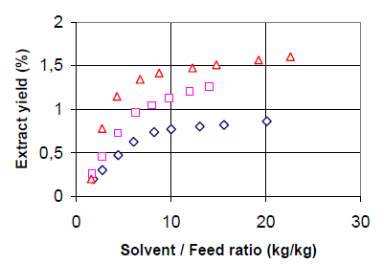
Figure 9: Extract yield of astaxanthin by supercritical CO2 (60C/300 bar). Run 1 (); Run 2 (); Run 3 () (Majewski et al., 2000).
Sample preparation and extraction conditions determine the astaxanthin contents in the extract. Figure 9 shows that the yield increased significantly when the samples were twice grinded (run (1) vs. run (2)). This fact reveals the importance of cellular wall breaking (subsection 4.1) on extraction efficiency. More efficient milling procedure yielded more extract, which in turns resulted in higher concentration of astaxanthin in samples. The addition of co-solvent (9.4% ethanol) into supercritical CO2 only increases the extraction yield to a small extent, but does improved the extraction and recovery of astaxanthin (Majewski et al., 2000).
4.3 Analyses
According to Cysewski (2006), analysis of astaxanthin content of a product can be conducted in two ways: spectrophotometric analysis and high performance liquid chromatography (HPLC) analysis.
4.3.1 Spectrophotometric Analysis
Via the usage of spectrophotometer (e.g. UV-VIS spectrophotometer), the maximum absorbance of astaxanthin (indicated by the wavelength in between 470-480 nm) is accessed accordingly. However, spectrophotometric assay method contributes to a problem such that astaxanthin cannot be distinguished from other carotenoids which also absorb light at the mentioned wavelength, hence leading to a false or rather an inaccurate results (Cysewski, 2006).
Instead, the efficiency of solvent extraction is approximately quantified by determining the carotenoid and chlorophyll contents through the usage of Lichtenthaler equations as mentioned in the following research papers.
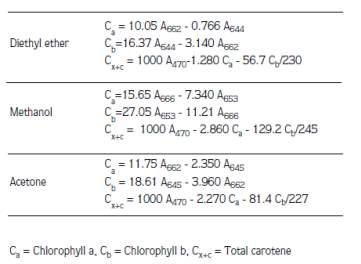
(a) The study conducted by Şükran et al. (1998) involved the determination of pigment levels of four algae species by using various extraction solvents (methanol, acetone and diethyl ether). Absorbances were read and the amount of chlorophyll a, chlorophyll b and carotenoids were calculated according to the formulas of Lichtenthaler and Wellburn (1985) as shown in Table 5 (Şükran, GÜNEŞ, & Sivaci, 1998).
Table 5: Lichtenthaler equations for different solvents (Şükran et al., 1998).
(b) Based on the paper written by Sumanta et al. (2014), the solvents defined in Table 6 were used and compared to investigate their extraction capabilities of pigments from fern leaves. Noticed that there is a trade-off between choosing the best solvent for efficient quantitative extraction of chlorophylls and use of a solvent best suited for spectrophotometric assay (Sumanta, Haque, Nishika, & Suprakash, 2014).
Table 6: Solvent properties (Sumanta et al., 2014).
| Solvents | Properties |
| Acetone | Acetone is volatile, highly flammable, narcotic and skin irritating.
It may not be suitable as an extractant but is ideal for spectrophotometric assay. Plastic cuvettes cannot not be used as acetone attacks polystyrene and polymethylacrylates (PMMA). |
| Methanol | Methanol is less volatile and flammable than acetone, but is relatively toxic and poisonous.
It can be a good extraction solvent for chlorophylls. False readings may result from the fogging of plastic cuvette by methanol. |
| Ethanol | Ethanol is much safer than acetone or methanol but not often used for spectrophotometric assay.
Flammable and less toxic. Plastic cuvettes are applicable. |
| Diethyl ether | DEE is the most prevalent solvent for chlorophylls.
Extremely volatile, flammable, explosive and narcotic. It attacks plastic cuvettes and most plastic laboratory ware. |
| Dimethyl sulfoxide (DMSO) | DMSO is ideal for both extraction and assay, probably more efficient when the pigments contents are low. |
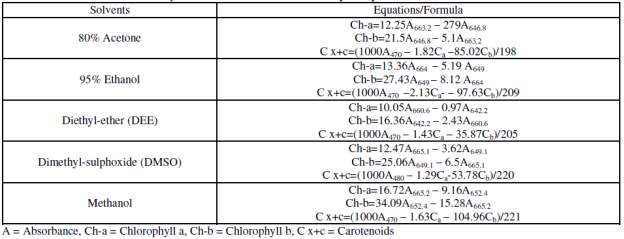
In relation to the type of extraction solvent, different equations were used for the quantification of chlorophyll a, chlorophyll b, and carotenoids as given in Table 7.
Table 7: Lichtenthaler formulas based on different extractant solvents (Sumanta et al., 2014).
4.3.2 HPLC Analysis
Astaxanthin content of a product can be analyzed via HPLC assay method, which is believed to be the most technically sound and accurate method in contrary to spectrophotometric analysis. This application has been accepted by the U.S. Food and Drug Administration (21 CFR 73.185) and the Canadian Food Inspection Agency (Registration No. 990535), and it is currently used by Japan’s official analysis agency, the Japan Food Research Laboratory. Basically, the underlying principles related to column chromatography are engaged to separate the pigments from one another in order to allow precise quantification of only astaxanthin. Nevertheless, the form of astaxanthin (free, mono or diesters) arises a complication in HPLC analysis. Many peaks will be observed, and calibration becomes relatively difficult as pure mono and diesters are not available. This issue can be overcome by applying cholesterol esterase to the cell extract containing astaxanthin. It uniquely induces saponification of the esters, thus leading to the transformation of esters to free astaxanthin. Through this modification in astaxanthin structure, HPLC analysis yields only free astaxanthin peaks, which are easily quantified using pure astaxanthin standards (Cysewski, 2006).
Aquasearch had produced a technical report stating the comparison of HPLC and spectrophotometric analyses of astaxanthin content in Haematococcus pluvialis algal meal (Aquasearch, 1999b). Spectrophotometric assay, which can be considered as a fairly accurate, reproducible and technically simple practice, is routinely utilized in their research laboratory for the determination of total astaxanthin contents in H. pluvialis. Detrimentally, the results obtained tend to be an overestimation of the total astaxanthin due to the problem mentioned previously in 4.3.1 (Aquasearch, 1999b).
They had therefore established another method with considerably high efficacy, i.e. reversed-phase HPLC for investigating both total astaxanthin and its distribution between the free and esterified forms (Aquasearch, 1999b).
Conclusively, astaxanthin and other carotenoids that absorb light in the same region of wavelength are not differentiated through spectrophotometry, and hence HPLC is more preferable in measuring astaxanthin concentration in Haematococcus pluvialis as separation among the pigments are initiated prior to quantification.
5.0 References
Aquasearch. (1999a). Analysis of Total Astaxanthin in Algae Meal Prepared from Haematococcus pluvialis. Retrieved from https://www.fda.gov/ohrms/dockets/dailys/00/jun00/061900/rpt0065_tab8.pdf
Aquasearch. (1999b). Comparison of HPLC and Spectrophotometric Analyses of Astaxanthin Content in Haematococcus pluvialis Algal Meal. Retrieved from https://www.fda.gov/ohrms/dockets/dailys/00/jun00/061900/rpt0065_tab8.pdf
Biswal, S. (2014). Oxidative Stress and Astaxanthin: The Novel Supernutrient Carotenoid. International Journal of Health & Allied Sciences, 3(3). doi:10.4103/2278-344x.138587
Cysewski, G. R. (2006, April 4). Analytical Methods for Measuring Astaxanthin.
Dong, S., Huang, Y., Zhang, R., Wang, S., & Liu, Y. (2014). Four Different Methods Comparison for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis. ScientificWorldJournal, 2014, 694305. doi:10.1155/2014/694305
Fox, F. (2011). Haematococcus pluvialis, Chlorophyta. Microscopic Recording with Differential Interference Contrast. In M. d.-B. 3.jpg (Ed.).
Herrero, C., Orosa García, M., Abalde, J., Rioboo Blanco, C., & Cid, Á. (2012). Astaxanthin Production in Cysts and Vegetative Cells of the Microalga Haematococcus pluvialis Flotow.
Lorenz, R. T., & Cysewski, G. R. (2000). Commercial potential for Haematococcus Microalgae as a Natural Source of Astaxanthin. Trends in Biotechnology, 18(4), 160-167. doi:http://dx.doi.org/10.1016/S0167-7799(00)01433-5
Majewski, W., Perrut, M., & Valderrama, J. O. (2000). Extraction of Astaxanthin from Haematococcus pluvialis by Supercritical Carbon Dioxide.
Mendes-Pinto, M. M., Raposo, M. F. J., Bowen, J., Young, A. J., & Morais, R. (2001). Evaluation of Different Cell Disruption Processes on Encysted Cells of Haematococcus pluvialis: Effects on Astaxanthin Recovery and Implications for Bio-availability. Journal of Applied Phycology, 13(1), 19-24. doi:10.1023/a:1008183429747
Naeem, S. (2016). Cell Disruption. Retrieved from https://www.slideshare.net/sabanaeem1/cell-disruption
Sarada, R., Vidhyavathi, R., Usha, D., & Ravishankar, G. A. (2006). An Efficient Method for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis. Journal of Agricultural and Food Chemistry, 54(20), 7585-7588. doi:10.1021/jf060737t
Şükran, D., GÜNEŞ, T., & Sivaci, R. (1998). Spectrophotometric Determination of Chlorophyll-A, B and Total Carotenoid Contents of Some Algae Species Using Different Solvents. Turkish Journal of Botany, 22(1), 13-18.
Sumanta, N., Haque, C. I., Nishika, J., & Suprakash, R. (2014). Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Research Journal of Chemical Sciences ISSN, 2231, 606X.
Todd, L. R. (2001). HPLC and Spectrophotometric Analysis of Carotenoids from Haematococcus Algae Powder.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biomedical Science"
Biomedical Science focuses on how cells, organs and systems function in the human body and underpins much of modern medicine. Biomedical Science applies parts of natural and/or formal sciences to help develop advances in healthcare.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




