Temporomandibular Disorders and Single Motor Unit (SMU) EMG Activity
Info: 4576 words (18 pages) Dissertation
Published: 10th Dec 2019
Tagged: Physiology
1.1 Jaw Motor system
The jaw motor system is anatomically and physiologically a very complex structure consisting of the maxillae and mandible, teeth, periodontium, tongue, temporomandibular joints and orofacial muscles. Because of its complexity, the jaw system is able to generate large compressive forces during mastication and also to achieve very fine motor control with precise positioning of the upper and lower teeth whenever it is required (Peck et al., 2010). Given the frequent demands imposed on the jaw motor system from, for example, the need for clearly articulated speech and efficient masticatory activities, then it may be not surprising that this highly complex motor system can develop pain and/or dysfunction.
1.2 Pain and TMD
The development of a universally accepted definition of pain and related concepts was indicated by John J Bonica as one of the main goals of the then rising International Association for the Study of Pain (IASP) (Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy, 1986) (Raffaeli and Arnaudo, 2017).
Therefore, pain has been defined, according to IASP as ‘‘an unpleasant, sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’’ (Lipton, 1991). This definition includes the sensory aspect of pain as well as the emotional and interpretive or cognitive aspects.
In fact, the attempt to understand pain represents one of the oldest challenges in the history of medicine (Raffaeli and Arnaudo, 2017). It leads patients to seek medical relief, perhaps more than any other one symptom of disease (Wagner, 1906).
Orofacial pain refers to pain in the face and/or oral cavity. Studying one population dataset from the UK Biobank between 2006 and 2010, researchers reported an overall prevalence of self-reported facial pain as 1.9% (women 2.4%, men 1.2%) of which 48% was chronic (Macfarlane et al., 2014). Another study, analysing the prevalence of orofacial pain (OFP) among young adults (30-31 years old), reported the prevalence of OFP as high as 23% (Macfarlane et al., 2009).
Despite the fact that it is very prevalent in the population, it also includes different types, for example, associated with pulpal and periodontal processes, sinusitis, trigeminal neuralgia, and pain in any of the masticatory muscles or temporomandibular joints (TMJ) (Conti et al., 2012).
Temporomandibular disorders (TMD) are clinical conditions that often involve pain in the masticatory muscles, the temporomandibular jaw joint and/or associated structures. The most common symptom is pain, but often patients with TMD present other symptoms such as limited or asymmetric mandibular movements and/or joint sounds (Yap et al., 2002) and which vary in severity from case to case.
Temporomandibular disorders are a very common problem affecting up to 33% of individuals within their lifetime (Wright and North, 2009). However, this prevalence can vary from study to study as the number of patients suffering from TMD seems to be increasing worldwide. Temporomandibular disorders are in fact one of the most common chronic pain conditions, along with headaches and back pain (Ghurye and McMillan, 2015).
Epidemiological studies show that nearly 10% to 15% of the general population has some kind of TMD, and from those, around 5% require treatment (Tournavitis et al., 2017). They impose significant personal and economic burdens on ~5% of Australians (Blyth et al., 2001; Sessle, 2000) and also have working related impacts. Treatments often require a multi-disciplinary approach and they represent a significant health-care cost with non-surgical treatments often costing over $1,000 per episode.
In general, TMD is more prevalent in women during their reproductive years than men and post-menopausal women. Some authors have suggested that TMD can have estrogen as a risk factor as it could contribute to enhanced central sensitization processes (see section 1.8.3 below) and which might predispose to the development of painful TMD (Ribeiro-Dasilva et al., 2017). To support this idea, another study showed no difference in the prevalence of TMD between boys and girls during the pre-puberty phase of their lives (Ghurye and McMillan, 2015).
Nowadays, there are a large number of therapeutic options that can help with the elimination or reduction of the symptoms in muscles and jaw joints. For example, some therapeutic options include the occlusal oral plates (Restrepo et al., 2011), physical therapy (Morell, 2016; Tuncer et al., 2013), cognitive-behavioural therapy (CBT) (Dura-Ferrandis et al., 2017), acupuncture (Fernandes et al., 2017), stress reduction strategies (Reissmann et al., 2012), physical exercises (Haggman-Henrikson et al., 2017b), attention to the improvement of sleep quality (Babiec, 2017), pharmacotherapy (Haggman-Henrikson et al., 2017a), counselling and self-care management (Henien and Sproat, 2017; Nilsson and Willman, 2016), laser therapy (Demirkol et al., 2017) or even a combination of two or more of these options depending on the case and the recommendation of the dentist. However it has been reported that there is a lack of therapeutic confidence in the treatment of pain related to TMD among dentists (Kakudate et al., 2017).
The stabilization appliance using a flat occlusal splint made of hard acrylic or polycarbonate material is among the most popular current treatment worldwide (Altok et al., 2016; Shukla and Muthusekhar, 2016). Yet, insufficient evidence for its effects on musculature is found on the literature (Al-Ani et al., 2004; Dahlstrom et al., 1982) and further studies are recommended in regards to occlusal stabilization as a TMD treatment (Kuzmanovic Pficer et al., 2017).
Nonetheless, although it has been widely discussed by researchers and clinicians, the cause and pathophysiology of TMD remains unclear and many current treatments are based on little scientific evidence, and there is a significant placebo effect. Therefore, patients with TMD can go from one clinician to the next in a desperate attempt to obtain symptom relief. Besides, not much is known about the reason and mechanisms whereby acute TMD episodes became chronic TMD.
Chronic pain is usually defined as pain lasting more than 3 months and almost certainly has some element of central sensitization (Crofford, 2015; Gil-Martinez et al., 2016). It is a complex sensory and emotional experience that varies widely between people depending on the context and meaning of the pain and the psychological state of the person (Bushnell et al., 2013). It can be really disabling with a negative impact on people’s quality of life. The value of this definition is its ability to describe all the conditions that can be defined as chronic pain even if it does not refer to the impairment brought about by the pain, the presence of specific symptoms, and the supposed etiologic framework. This is also because chronic pain is a term employed to define several diverse conditions whose common feature is the presence of persistent pain (Raffaeli and Arnaudo, 2017).
In this context, TMD may also be associated with other problems of general health, depression and anxiety, or psychological disabilities that affect the patient’s quality of life or even elevated levels of suicidal ideation (Bertoli and de Leeuw, 2016; Stohler, 1999). In fact, it is frequently associated with physical symptoms of other chronic pain disorders and comorbidities, as generalised muscle and joint pain (Beiter et al., 2015; Moreno-Fernandez et al., 2017), or irritable bowel syndrome (IBS) (Gallotta et al., 2017), for example. TMD actually shares similarities with other chronic pain conditions such as chronic tension-type headache or migraine, low back pain, and fibromyalgia in physiopathologic mechanisms (Sessle, 2009). Also, one study found no differences between TMD patients and non-TMD patients with a chronic pain disorder in terms of the use of medicines; levels of depression, anxiety, somatization, hostility, or psychoticism; behavior in their social surroundings; or ratings of problems with work, family, self-esteem, or impulses for suicide (McKinney et al., 1990).
1.3 TMD and diagnoses
The diagnosis of TMD is complex. Most current research recognizes that TMD is not caused by a single factor but it is multifactorial. Temporomandibular Disorders are complex disorders that are best viewed from a biopsychosocial perspective, that is, they exhibit a range of different physical signs and symptoms, as well as changes in behaviours, and emotional and social interactions (Slade et al., 2016). This has led to acceptance of a multifactorial ethology of TMD pain.
There are several diagnostic systems proposed for TMD.
The Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) was published in 1992 and it was the first classification that incorporated the biopsychosocial pain model. It has been translated into 22 languages with a Dual-axis system: clinical conditions (Axis I) with the purpose of finding possible abnormalities of structures and functions of the masticatory muscles and temporomandibular joints, and pain-related disability and psychological status (Axis II) (Dworkin and LeResche, 1992).
The RDC/TMD has been the most extensively used diagnostic protocol for TMD research since its publication. The intent was to provide simultaneously a physical diagnosis and to identify other relevant aspects of the patient that could influence the expression of TMD and maybe help with the management of their pain (Schiffman et al., 2014).
A series of studies has been done since its publication to verify its diagnostic validity and to identify some points of conflict. In this context, the Diagnostic Criteria for TMD (DC/TMD) is the revised version of the RDC/TMD and evaluations indicate that it is reliable and valid. This diagnostic protocol cover the most frequent kinds of TMD, such as disorders related with pain (e.g., myalgia, TMD-related headache and arthralgia) and disorders linked to the TMJ (e.g., disc displacements and degenerative disease) (List and Jensen, 2017).
1.4 Single Motor Unit (SMU) EMG activity
Knowledge of the anatomy and physiology of the motor mechanism that is required to produce muscle contractility in human beings and other animals is essential in order to understand the variety of disturbances that can occur in human motion and locomotion. Therefore, electromyography is the study of the electrical activity of the muscle (Pruzansky, 1952) and is used for studying muscular functioning – See figure 1-1.
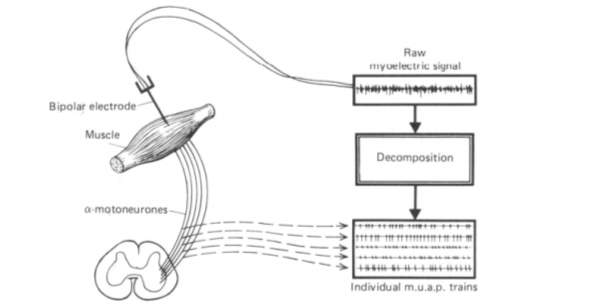
Figure 1‑1: Schematic illustration of the origination, myoelectric recording and subsequent decomposition of five superimposed motor-unit action potential. M.u.a.p.: motor unit action potentials From: (De Luca et al., 1982).
Consequently, EMG analysis might be helpful to elucidate the normal function of the masticatory muscles as well as how the muscles adapt in patients with TMD (Dagsdottir et al., 2015).
Movement is accomplished by the controlled activation of motor unit populations. The motor unit is mostly under the control of the central nervous system (CNS). The motor unit is the final common pathway whereby converging sensory and descending neural inputs are translated into forces to generate movement. It is therefore a neuromechanical transducer.
1.4.1 Definition of a motor unit
The motor unit (Figure 1-2) consists of two components:
- One α-motoneurone and
- The muscle fibres innervated by that motoneurone (Heckman and Enoka, 2012).
Each muscle fibre is innervated by only one motoneurone, and each motoneurone can innervate between ten and thousands of muscle fibres. The muscles that act on the largest body masses have motor units that contain more muscle fibres, whereas smaller muscles contain fewer muscle fibres in each motor unit (Buchthal and Schmalbruch, 1980). Jaw muscle motor units contain several hundred muscle fibres (Lenman and Ritchie, 1987).
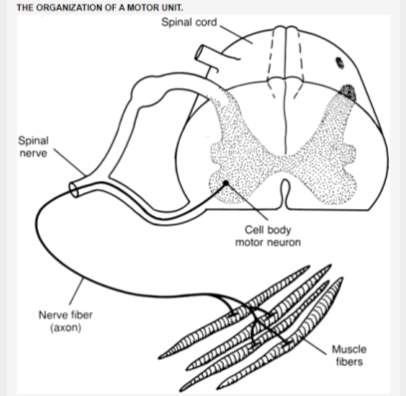
Figure 1‑2: The organization of a motor unit from the spinal motor system. The motor unit consists of a motoneurone (motor neuron in the figure) and all the muscle fibres it innervates. From (Hamilton et al., 2011).
1.4.2 Fibre types
Skeletal muscles are composed of numerous fibres that range from 10 to 80 micrometers in diameter. These fibres contain myofibrils, which can be defined as the real force generators. Each myofibril consists of sarcomeres, the final functional units of the muscle contraction. The sarcomeres, in turn, contain mainly thick (myosin) and thin (actin) filaments. Nonetheless, there are also other proteins called troponin and tropomyosin –See figure 1-3. The interaction between all these filaments is the cornerstone for muscle contraction (Bottinelli and Reggiani, 2000).
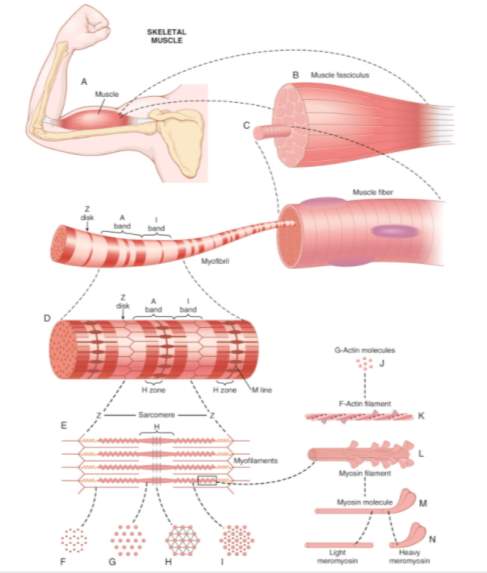
Figure 1‑3: Organization of skeletal muscle (A and B), from the gross to the molecular level. Each muscle fibre (C) can contain from several hundred up to several thousand myofibrils (D). The sarcomere (E) is the part of the myofibril between two successive Z discs. (F), (G), (H), and (I) are cross sections at the levels indicated. Each myofibril is composed of around 1500 adjacent myosin and 3000 actin filaments, which are large polymerized protein molecules responsible for the actual muscle contraction (J), (K), (L), (M), (N). From: (Hall, 2016).
Scientists have been aware that skeletal muscles can be distinguished on the basis of their colour as red or white and their contractile properties as fast and slow since the first half of the 19th century (Schiaffino and Reggiani, 2011).
Initially, three types of muscle fibres were histochemically distinguished exclusively on the basis of qualitative differences in their actomyosin ATPase which could distinguish them between type I (slow) and type II (fast) fibres (Guth and Samaha, 1970). Later on, type II fibres were subdivided into IIA and IIB and some new fibre types (IIX, IIC and IM) were also identified by this method (Staron and Pette, 1986).
Also, based on histochemical techniques using both mitochondrial enzyme and myosin adenosine triphosphatase (ATPase) activities to differentiate fibres, muscle fibres can be classified as slow oxidative (SO), fast oxidative glycolytic (FOG), and fast glycolytic (FG) (Delp and Duan, 1996; Pette and Staron, 1990).
In addition, the structural and functional properties of the fibres, which are generally referred to as fibre phenotype, can change in response to hormonal and neural influences, nerve-activity being a major determinant of the fibre type profile. This property is defined muscle plasticity or malleability (Schiaffino and Reggiani, 2011).
The association of different muscle fibre types with motor neurones of different sizes is the basis of an elegantly simple mechanism for grading the force of muscle contraction.
1.4.3 Small x Large motor units
Motor units may vary in size. The size of a motor unit in a muscle is defined by the innervation ratio, which is the ratio between the number of muscle fibres in a muscle and the number of supplying motoneurone axons. Small motor units have small-diameter axons typically innervating slow muscle fibres that are resistant to fatigue. Those motor units play one important role in activities that require sustained muscular contraction, as, for example, in the maintenance of an upright posture. Large motor units have, on the other hand, large-diameter axons that typically innervate faster muscle fibres that are more fatigable and play an important role in brief exertions that require large forces, as for example running or jumping. Lastly, there is also an intermediate-size motor unit called fast intermediate fatigable or fast fatigue-resistant –See figure 1-4 (Burke, 1967; Burke et al., 1970; Burke et al., 1973; Purves D, 2001).
The size of a motor-unit is especially important in determining the magnitude of the motor-unit force and the amplitude of the motor-unit action potential (Goldberg and Derfler, 1977).
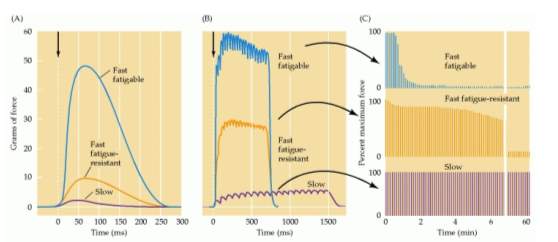
Figure 1‑4: Three different types of motor units and their force and fatigability. (A), (B) and (C) shows the change in muscle tension due to (A): A single motor neurone action potential. (B): Repetitive stimulation of the motor neurones. (C): Repeated stimulation at a level that evokes maximum tension. The ordinate represents the force generated by each stimulus with the different fatigue rates. From: (Purves D, 2001).
1.4.4 Motor unit variability
Most information on the physiological properties of single motor units comes from animal experiments involving stimulation of motoneurones directly by insertion of electrodes into the motor nucleus supplying the limb muscles (Freund, 1983).
SMUs can vary in morphology (innervation ratio, cross-sectional area, geometric distribution of muscle fibres) and in physiological and biochemical properties (force output, contraction velocity, resistance to fatigue, oxidative and glycolytic capacities, ATPase activities, and myosin heavy-chain (MyHC) isoform contents) (van Eijden and Turkawski, 2001). Masticatory muscles contain, therefore, a large variety of motor units with different physiological and morphological properties (Turkawski and van Eijden, 2000).
Many differences can be found between and within the jaw muscles. In fact, a comparison of jaw opening muscles with jaw closing muscles shows that jaw openers are simpler with respect to activation, architecture and fibre type composition than the jaw closing muscles (Tsuruyama et al., 2002). In a study analyzing fibre type compositions and fibre cross-sectional areas of masticatory muscles in eight cadavers (Korfage et al., 2000), it was demonstrated that the temporalis, masseter and pterygoid muscles could be characterized by a relatively large number of fibres containing more than one MyHC isoform (hybrid fibres) with a large number of fibres expressing MyHC-I, MyHC-fetal and MyHC-cardiac alpha. Besides, in these muscles type I fibres had larger cross-sectional areas than type II fibres. In the meantime, the mylohyoid, geniohyoid and digastric muscle were characterized by less fibres expressing MyHC-I, MyHC-fetal, and MyHC-cardiac alpha, and by more fibres expressing MyHC-IIA; the cross-sectional areas of type I and type II fibres in these muscles did not differ significantly. Furthermore, jaw closers muscles contain 40% of hybride muscle fibres as oppose to only 10% fibres of jaw openers muscles (Korfage et al., 2001).
1.4.5 Orderly recruitment of motor units
Adrian and Bronk (Adrian and Bronk, 1929) initially discussed that increases in force were achieved by the recruitment of additional motor units and an increase in discharge rate of motor units that had already been recruited. Derek Denny-Brown (1901–1981) and Joseph Pennybacker (1907–1982) reported later, in 1938, that “a particular voluntary movement appears to begin with discharge of the same motor unit. More intense contraction is secured by the addition of more and more units added in a particular sequence. This ‘recruitment’ of motor units into willed contraction is identical to that occurring in certain reflexes. The early motor units in normal graded voluntary contraction are always in our experience small ones. The larger and more powerful units, each controlling many more muscle fibres, enter contraction late” (Denny-Brown, 1938).
Elwood Henneman et al. (Henneman, 1957; Henneman et al., 1965) finally proposed that, with increasing levels of motor activation, motor units would be recruited in order, starting from the smallest to the largest and this is termed the Size Principle of Motor Unit activation. On the basis of this, the recruitment of the smallest, most fatigue-resistant motor units would be recruited in tasks requiring fine motor control over long periods of time, while the larger motor units would be required for brief bursts of high force production – See figure 1-5(Mendell, 2005).
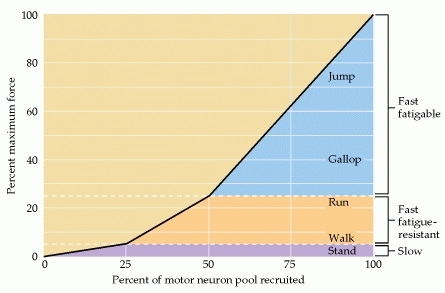
Figure 1‑5: The recruitment of motor neurones in the cat medial gastrocnemius muscle under different behavioural conditions. Slow motor units, for instance, provide the tension necessary for standing. Fast fatigue-resistant (FR) units, in turn, provide additional force required for activities such as walking and light running. Lastly, fast fatigable units are recruited for the most demanding activities. From: (Purves D, 2001). -After (Walmsley et al., 1978).
1.4.6 Motor unit territories
In humans the size of the individual motor unit is investigated by electrophysical delineation of the territory occupied by the fibres of a motor unit. Fibres are identified as belonging to the same motor unit when their potentials have identical time relationships irrespective of the frequency of discharge (Buchthal and Schmalbruch, 1980). In this technique, an electrode is moved through the territory of an active motor unit. The length of the path over which motor unit activity is registered is used as an estimation of motor unit width (van Eijden and Turkawski, 2001).
In animal muscles, motor unit territories can be mapped with the glycogen-depletion method that was originally described by Edstrom and Kugelberg (1968). In this method, a neurone is stimulated until the muscle fibres it innervates are depleted of glycogen. These depleted fibres can then be visualized in a muscle histological section, where they are the only ones that are not stained by the periodic acid Schiff (PAS) method (van Eijden and Turkawski, 2001).
Muscle fibres of motor units are intermixed with each other and are restricted to a particular region of the muscle, named the motor unit territory. Within the territory of a motor unit, motor units of human limb muscles have an average territory of 5-10 mm in diameter; this allows space for the fibres of 15-30 motor units (Buchthal and Schmalbruch, 1980). The distance between fibres of a particular motor unit varies from zero (fibres in contact) to hundreds of micrometres.
In two muscles with equal average territory of the motor units (e.g., the brachial biceps and the extensor digitorum communis), the maximum amplitude of the motor-unit potential is highest in the muscle with the greatest number of fibres per motor units (Buchthal and Schmalbruch, 1980).
Motor unit territories in masticatory muscles appear to be smaller than territories in limb muscles, and this would suggest a more localized organization of motor control in masticatory muscles. Motor unit cross-sectional areas show a wide range of values, which explains the large variability of motor unit force output. The proportion of motor unit muscle fibres containing more than one MHC isoform is considerably larger in masticatory muscles than in limb and trunk muscles. In fact, in trunk and limb muscles, this phenomenon is primarily observed in elderly subjects (Andersen et al., 1999). Hence, in masticatory muscles, a finer gradation of force and contraction speeds appears when compared to limb and trunk muscles (van Eijden and Turkawski, 2001).
The presence of localized motor unit territories and task-specific motor unit activity facilitates differential control of separate muscle portions. This gives the masticatory muscles the capacity of producing a large diversity of mechanical actions (van Eijden and Turkawski, 2001).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Physiology"
Physiology is related to biology, and is the study of living organisms and how they function. Physiology covers all living organisms, exploring how the body performs basic functions in relation to physics and chemistry.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




