Chemical, Physical, and Mechanical Properties of the Human Skin
Info: 6948 words (28 pages) Dissertation
Published: 12th Jan 2022
Tagged: BiologyPhysiology
ABSTRACT
The chemical, physical, and mechanical properties of the skin are very extensive. The skin is made up of tissues that work together to perform unique functions. The skin makes up 16 percent of body weight. Understanding the chemistry and physical properties of the skin assists industry in manufacturing products, such as deodorant, lipstick, and moisturizers to name a few. The mechanical properties of the skin were not always reproducible. The results obtained had a wide range of references. Recently, a new method and technique were developed to help clinicians refer to a database with reproducible results and comparative analysis. New developments and understanding such as this will help with new discoveries and development of products.
1. Introduction
The human body contains numerous systems critical to maintaining life. One of the most vitally important systems of the human body is the integumentary system. Intugugment by definitionmeans a covering or outer protective layer. The integumentary system plays an essential role in human physiology. It is made up of the skin and the skin’s appendages such as hair, nails, sebaceous, ceruminous, and sweat glands [1]. Human skin makes up the largest organ of the human body and accounts for about 16% of total body weight [2]. Skin provides the body with a protective barrier from external physical, chemical, and biological injury. This includes but is not limited to physical injury, external fluids penetrating its relatively impermeable outer layer, stopping internal fluids from escaping, providing physical protection from the ultraviolet rays of sunlight, aiding in stability during temperature fluctuations, and protecting against harmful living agents [3].
The skin is also responsible for the synthesis of vitamin D3, balance of water and electrolytes, excretion of toxins, urea, ammonia, uric acid, etc., balance of the skin pH to ward off bacteria, and providing sensation through it abundant receptors. The dermis and hypodermis provide storage for fat and water, structure and rigidity, and vessels for the transport of blood and lymphatic cells. The epidermis aids in physical appearance which has important implications on social interactions as well providing information about the health, age, gender etc. [4]. The skin appearance can also help alert indication of infectious diseases, allergic reactions to foreign materials, or personal identification by way of fingerprints. Skin is a complex system containing interacting elements of lipids, cells, blood vessels, nerve endings, glands, and fiber networks that all contribute to its functionality and the vitality of human life.
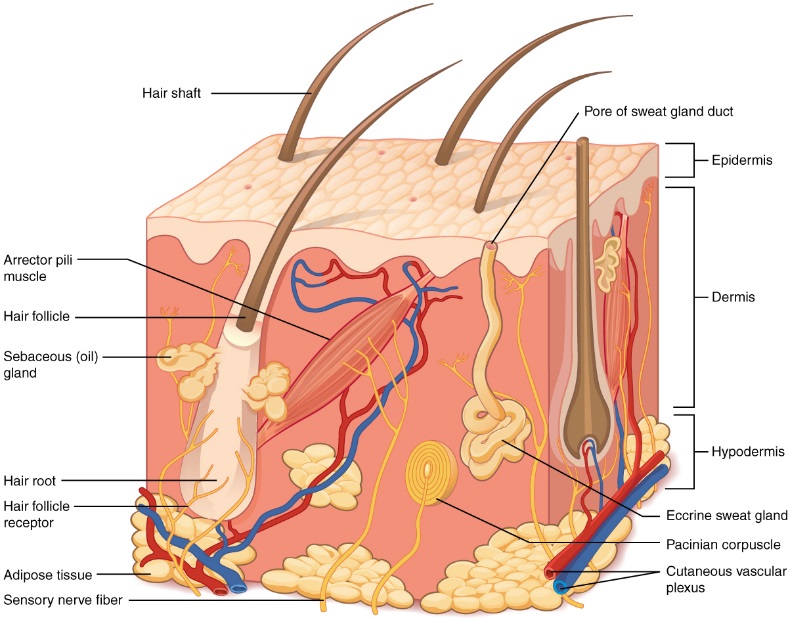
Fig. 1. The skin is composed of two main layers: the epidermis, made of closely packed epithelial cells, and the dermis, made of dense, irregular connective tissue that houses blood vessels, hair follicles, sweat glands, and other structures. Beneath the dermis lies the hypodermis, which is composed mainly of loose connective and fatty tissues.[5]
2. Chemical Properties
2.1 Chemical Composition of the Epidermis Layer
Skin has several chemical properties that aid in its barrier function. Human skin consists of two distinct regions: the epidermis and dermis [6]. The epidermis is stratified into 5 layers. The barrier function of skin resides in the outer layer of the epidermis, the stratum corneum. The stratum corneum chemical makeup is unique from other biological membranes [7]. The two major structural components of the stratum corneum are the corneocytes and lipids [6]. Corneocytes are non-living cells that are make up about 80% of the volume of the stratum corneum and are imbedded in a highly hydrophobic lipid lamellae matrix. The main lipids of this matrix are ceramides, free fatty acids, and cholesterol. Glucosylceramides and cholesterol sulfate are also present, but in much smaller quantities. The lipids are organized in lamellar phases and the phases are oriented approximately parallel to the surface of the corneocytes [8]. The lipids are organized in lamellar bilayer structures resulting lipid chains that are highly organized in gel or crystalline phases [9].
The interface between the epidermal layers and the outer environment, which covers the human skin, is a continuous hydrolipidic film. The skin surface lipids are a mixture of fatty (sebaceous) and epidermal lipids. The highest concentration of sebaceous glands is found in the areas of the forehead, upper chest, and dorsum. This reflects sebaceous secretion, flowing from those sites to areas with lower concentration, where the influence of cellular lipid components, which are rich in oleic and linoleic acid, becomes more relevant [10]. The major lipid components in human sebum (oily secretion of the sebaceous glands) include squalene, wax esters, and triglycerides (Fig. 2-4). Usually, sebum is rich in long chain fatty acids, linear or branched, and mainly saturated or monounsaturated.
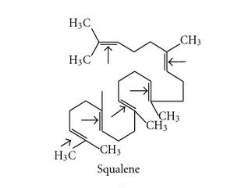
Figure 2. Chemical structure of squalene.[11]

Fig. 3. Chemical structure of human skin wax esters [12]
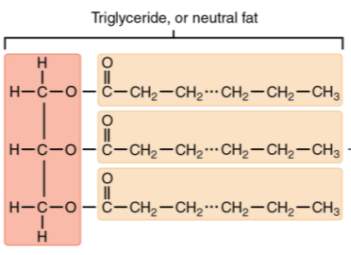
Fig. 4. Chemical structure of a triglyceride. [11]
In a study conducted at Boston University, the surface lipids from the foreheads of 51 subjects, ranging in ages of five days and fifteen years, were analyzed by thin-layer chromatography (TLC). The cholesterol levels were lowest at birth and highest at six years, and drops again at nine years. It was hypothesized that cholesterol on the surface of human skin is much higher in young children than in adults due to the fact that as a person ages, the sebaceous gland activity increases secretion of sebum. Sebum contains very little cholesterol. Therefore the cholesterol present early on in life is diluted as the sebaceous gland become more active in later developmental stages. Contrariwise, the average concentration of wax esters was high at birth, low between the ages of three and six, and high again by nine years. There was no significant change in combined triglyceride and free fatty acid concentration during development. However, it was found that at birth no hydrolysis of triglycerides to free fatty acids occurred. It is believed that as a person ages the appropriate bacteria begin to populate the skin and hydrolysis of triglycerides to free fatty acids occurs [10].
The ceramides, a family of waxy lipid molecules present in the stratum corneum layer of the epidermis, covalently bond to the protein envelope of the corneocytes and influence macroscopic lipid organization. In a study conducted by Moore and Rerek, stratum corneum models containing ceramide 2 and ceramide 5 (Fig. 5) were investigated using Fourier transform infrared spectroscopy. It was found that the ceramide 2 and ceramide 5 participate in strong hydrogen bonding patterns, thus providing cohesiveness and organization to the stratum corneum barrier [9]. Ceramides play a functional role in structuring the and maintaining the water permeability barrier function of skin [13].
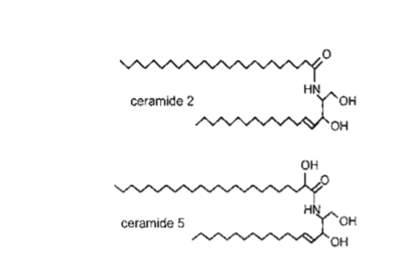
Fig. 5. The chemical structure of ceramides 2 and 5. [9]
Even though lipids do not make up the major portion of volume in the stratum corneum, the barrier function lipids provide is significant for preventing fluid permeability and fluid loss from the body [9].
2.2 Chemical Composition of the Dermis
2.2.1. Fibroblast
A fibroblast is a type of cell which synthesizes the extracellular matrix and collagen, and assist in wound healing. They are the most common cells of connective tissues in animal cells. They can be found in the dermis, specifically the reticular dermis layer, of the skin. Its branched cytoplasm surrounds an elliptical nucleus with two or more nucleoli (Fig. 6). The rough endoplasmic reticulum of the fibroblasts, help in recognizing them. They make collagens, glycosaminoglycans, reticular, elastic fibers, and glycoproteins which are found in the extracellular matrix and cytokine TSLP[14]. Tissue damage increases the activity of fibrocytes and gives rise to the mitosis of fibroblasts.
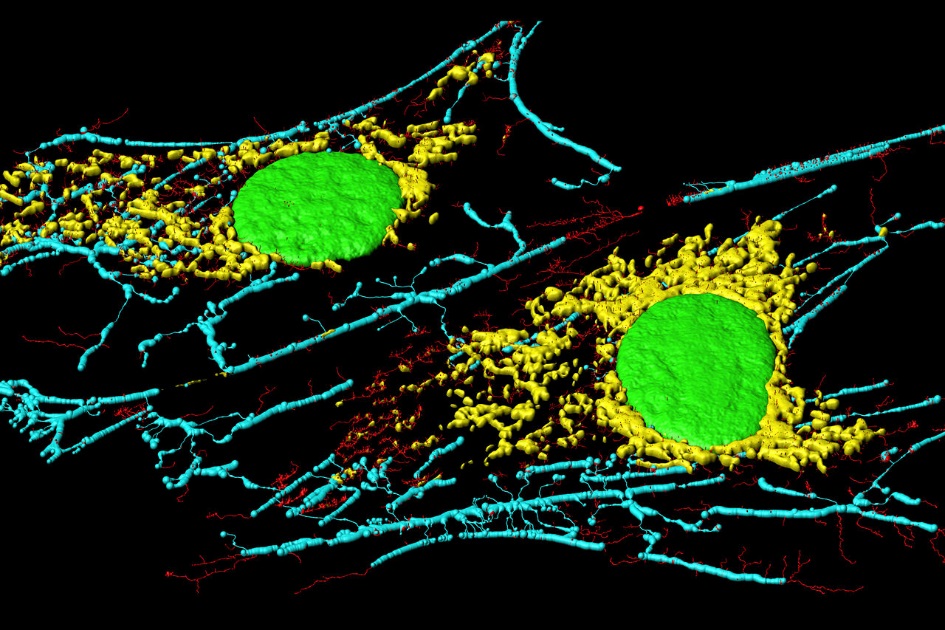
Fig. 6. Microfilaments (blue and red), mitochondria (yellow), and nuclei (green) in fibroblast cells. [15]
Studies from the Department of Medicine and the Department of Biological Chemistry at Washington University, St. Louis, showed that human skin fibroblast produce collagenase as a proenzyme and methods were developed which allowed the purification of the procollagenase to homogeneity [16]. The resulting proteins were shown to consist of a set of two zymogens, which could be converted proteolytically to a corresponding active form.
2.2.2. Lipids of the Dermis Layer
Essential oils are aromatic molecules, which occur naturally in plants. There are very limited studies on the biological effects of essential oils on human skin cells. However, studies have shown that essential oils in human dermal fibroblasts affect proteins, genes, and pathways related to inflammation and tissue remodeling process [17]. The experiment was conducted using a Biologically Multiplexed Activity Profiling (BioMAP) system HDF3CGF, designed to model the pathology of chronic inflammation in a vigorous and reproducible manner. The system is made up of a cell type, molecular stimuli to recreate the disease environment and a set of protein biomarkers readouts to examine treatment effects on the diseased environment.
There are studies, which have shown the anti-inflammatory and immunomodulatory activities of geranium essential oil, anti-fungal activity of patchouli essential oil (Fig. 7), antimicrobial, and antioxidant activities of petitgrain essential oil, and the anticancer and anti-proliferative activity of sandalwood essential oil. These studies are consistent with the existing studies on the anti-proliferative activity of the essential oils in human dermal fibroblasts.
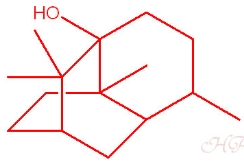
Fig. 7. Patchouli essential oil structure. [18]
Essential oils may apply beneficial effects on the human skin. Further studies on the mechanisms of actions, together with clinical efficacy and safety of these essential oils, can increase the understanding of their therapeutic benefits. A clinical trial showed that aroma inhalation of geranium essential oil effectively reduced anxiety during childbirth [19].
2.3. Hydration of skin
Water is a large component of the human body and represents about 60% of the body’s composition [20]. Skin not only acts as a protective water tight barrier against external substances, but also prevents excessive tranepidermal water loss and drying-out of the skin. It is important to maintain the intactness of the stratum corneum barrier to conserve this vital function. In a study conducted by Heinrich and associates, significant changes were observed in skin hydration after different cosmetic formulations were used by 60 females or a period of 4 weeks. It was found the more lipids a cream contained the higher its hydration effect. The high lipophilic content of the cream had an occlusion effect on the skin surface that resulted in a reduction of transepidermial water loss (Heinrich, Heinrich et al. 2014). In a study conducted by Palma and associates, it was found that higher inputs of dietary water positively impacted the normal skin physiology of epidermal superficial and deep hydration, particularly in those individuals with lower daily water consumption [20].
The hydration of skin can also be negatively be influenced by a number of other components in cosmetic products. In the same study by Heinrich and associates, it was found that skin barrier penetrators such as liposome, commonly used for penetration of the stratum corneum and transporting active agents to the deeper skin layers, broke down the intact skin surface of the stratum corneum. Openings in this surface caused transepidermal water loss and drying out of the skin [21]. The absorption behavior of skin is quite complex and results from not only the nature of the skin barrier but also the solubility, size, and hydrophobicity of the materials being applied to skin [22].
3. Physical Properties
3.1. Structure and Function of the Epidermis Layer of Skin
The structure of the skin plays a vital role in protection, stability, and vitality of the human body. Skin structure is comprised of three layers: epidermis, dermis, and hypodermis. The epidermis is the outer layer of the skin. The epidermis is also the thinnest layer between 20-150 µm [4]. The thickness of the epidermis is dependent on the region of the body. Typically palms and soles contain the thickest epidermis [23]. The epidermis is nonvascular and nourished by the tissue fluid from the inner region of the skin. The epidermis is stratified consisting of 5 layers [24]. The outermost strata to the innermost strata of the layers are respectively the stratus corneum, stratum lucidum, stratum granulosum, stratum spinosum, and the stratum basale. The stratum basle, also known as the stratum germinativum or malpighian layer is the bottom most layer of the epidermis. New live cells are produced in this layer and passed out ward. This layer is responsible for repair of minor injuries to the outer skin [1].
As the cells pass through the other layers of the epidermis, also known as the transitional region, flattening of the cells takes place. The cytoplasm of the cell slowly dies and is replaced with keratin or horn. In the stratum spinosum layer cells become slightly flattened and have a prickly surface. In the stratum granulosm layer, the cells contain kertohyalin granules. In the stratum lucidum layer the cells are more transparent and contain eleidin, an intermediate product of the formation of keratin [25]. These cells end in the outermost layer of the epidermis, the stratum corneum as non-living keratinized cells called corneocytes. This process is known as cornification [26]. The cells together make up the horny layer of the stratum corneum that is waterproof and germ resistant. The stratum corneum provides the epidermis its characteristic roughness and is the thickest of the 5 layers of the epidermis at about 14 µm [4]. The rate at which cells are shed from the stratum corneum is the same as which cells are formed in the stratum basle layer.
Sunlight, humidity, detergents, and various ingredients of cosmetics can influence the intactness of the stratum corneum skin barrier. Disturbance to the barrier can results in compromise the ability to prevent bacterial infections or water loss from the body through the epidermis [21].
3.2. Structure and Function of the Dermis Layer of Skin
The dermis is the inner vascular region of skin. It is made of 2 regions (Fig. 8). The outer thin papillary layer contains loose connective tissue and send projections to the epidermis forming dermoepidermial junctions [24]. The thicker inner layer is called the reticular layer and consists of a thick connective tissue as well as reticular, collagen, and elastin fibers. It is 2-3 times as thick as the epidermis and provides the structural strength of the skin because it contains collagen and keratin fibers. The dermis thickness varies between 1-4 mm [4]. The dermis connective tissue contains bundles of wavy unbranched collagen fibers, straight branching elastin, and various cell types including but not limited to fibroblasts, histiocytes, and mast cells. The fibroblast cells secrete collagen and elastic fibers as well as the matrix these fibers lie. Histiocytes aid in the destruction and removal of injured cells and foreign bacterial cells penetrating the skin. The mast cells secrete heparin and histamine. Heparin acts as an anticoagulant, while the histamine helps to activate the inflammatory system to fight pathogens in infected tissues.
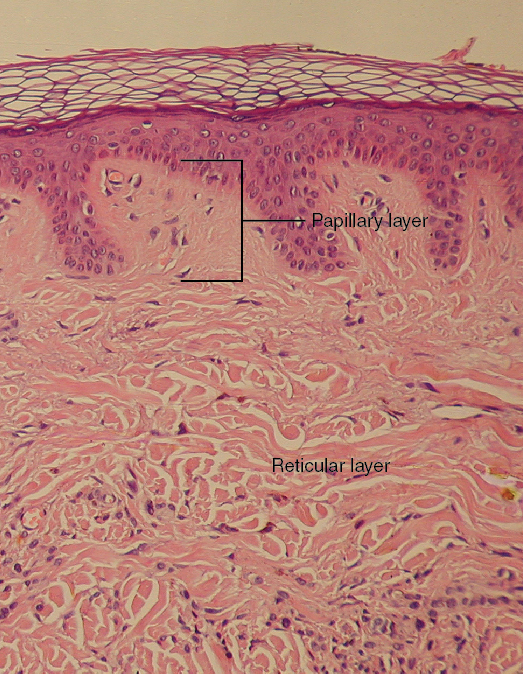
Fig. 8. Layers of the dermis: This stained slide shows the two components of the dermis—the papillary layer and the reticular layer. [25]
The collagen fibers attach the skin firmly to the underlying muscles. These fibers are strong and limit stretching of the skin giving rigidity to the skin. As the skin ages, the collagen fibers begin to be damaged causing wrinkles later in life. The elastic fibers help with recovery after the skin is stretched. The dermis also contains blood vessels, nerves fibers, and lymph vessels [6]. The blood vessels send capillaries to the epidermis. The nerve fibers make up a network of communication and feedback control mechanisms from sensory receptors in the dermis to the muscles fibers of the body. The receptors detect fluctuations in temperature, pain, and pressure. The receptors send nerve impulses to the rest of the body in order to maintain total body homeostasis. The dermis also contains arteriovenous anastomes, also known as shunts, which can pass blood directly from the arteries to veins. This is important for temperature and blood pressure regulation [27].
3.3. Structure and Function of the Hypodermis Layer of Skin
The hypodermis, also referred to as the subcutaneous layer, lies beneath the dermis. It contains subcutaneous tissue that loosely attaches the skin to body muscles. This allows for the skin to slide over the muscles. Body fat is deposited in many regions of the subcutaneous tissue and is typically 1 mm in thickness [4]. The function of the fat layer is to insulate the body against cold and heat and acts as a shock absorber. In the palms of hands and soles of feet the subcutaneous tissue is more tightly woven to the dermis than other regions of the body. This is why skin is more firmly attached in the palms and soles regions Kanitakis.
3.4. Surface pattern of skin
The outer surface of the skin is imprinted with patterned intersecting line unique to the region on the body and the individual. The study of these skin markings and patterns is also known as dermatoglyphics. As an individual ages lines, ridges, furrows, and folds develop. The skin’s first ridges are developed before birth. Ridges on the palmar and plantar tips of the fingers and toes start to develop during the third and fourth month of fetal growth and never change afterwards. Ridges are typically wider in men than in women. The skin’s ridges are very irregular and rarely pursue a straight course, they follow arched sweeps or form recognizable patterns. Only the palms and fingers the skin has numerous ridges that form highly characteristic patterns of arches, whorls, and loops. Fingerprints are commonly used to identify individuals based on the unique design of whorls, loops, and arches [6]. This practice is most commonly used in the crime and punishment field of work.
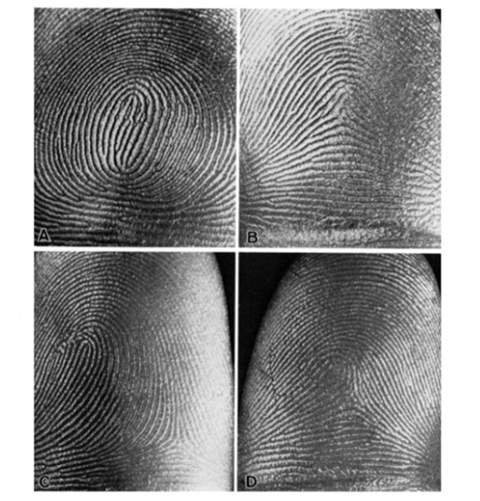
Figure 9: Major patterns of dermatoglyphics: (A) whorl, (B) arch, (C) loop, (D) combined form. [6]
3.5 Protection from ultraviolet rays
Skin provides some barrier to sunlight and the damaging effects of ultraviolet rays. The lower layer of the epidermis, the straum basale, contains melanocytes. Melanocytes are responsible for producing the melanin protein family of dark pigments as granules called melanosomes. Individuals with darker skin have a higher density of melanosomes. Melanosomes are capable of transferring melanin pigments to keratinocytes. Melanin pigment absorbs ultraviolet light which aids in protection of light with wavelengths 400 nm and less [28]. Another important element of protection is the thickness of the epidermis. Regions of skin with a thick epidermis is nearly void of melanin [24]. In order for radiation to damage viable tissues light must pass through the stratum corneum. When radiation passes through the stratum corneum, it is refracted due to the difference in refractive index of air and the refractive index of the stratum corneum [29]. Scattering by collagen fibers largely determines the depths to which the wavelengths can penetrate [28].
3.6. Temperature Regulation
Skin adapts with its ever changing environment through constant communication [6]. Receptor sites in the skin detect temperature changes in the external environment [1]. Human temperature regulation is an important body process to establish consistency of deep body temperature to maintain functioning enzymes. Normal body temperature is approximately 98.6 °F (37 °C) [1]. The skin has a remarkable ability to adapt to its surroundings particularly when exposed to temperature fluctuation by external influences. The initial feedback control mechanism is an attempt to reach homeostasis. Temperature control is a dynamic system of signals caused by temperature, heat flow, and neuronal commands that needs feedback controls to maintain a relatively constant body temperature [27].
Thermosensors are non-uniformly spread throughout the body. The three autonomic effector mechanisms of temperature regulation are metabolic heat production, sweat production, and blood dilation. The most essential property of temperature regulation is the existence of feedback control[30]. Body temperature is sensed by thermosensors which sends signals to the central controllers which in turn activate mechanisms acting on the heat transfer process to maintain a relatively constant body temperature. The increase or decrease of body temperature is evoked by atmospheric temperature and the feedback control-loop system. When atmospheric temperature is higher than steady state body temperature, the feedback thermosensors trigger vasodilatation and sweat production. Heat flows from the body core (organs, bones, muscles) to the skin via blood and tissue. Heat is released by way of blood flow from the overheated organs to the surface of the skin and is lost through convection, conduction, or radiation heat exchange. The heat released through sweat production is from sweat evaporation on the surface of the skin and heat exchange with the atmospheric air [27]. Heat loss occurs until homeostasis of temperature in the body is met.
When atmospheric temperature is less than that of the body temperature, at first the blood vessels in the dermis dilate to bring heat to the surface of the extremities. This is why light-skinned individuals will have rosy cheeks when first exposed to cold atmospheric temperatures [1]. However the body cannot maintain vasodilation as the internal vital organ must maintain temperature homeostasis. Therefore, the thermoregulatory control-loop triggers vasoconstriction to bring blood back to the vital organs. In addition the feedback control-loops trigger shivering in an attempt to produce heat [27]. If prolonged exposure to cold atmospheric temperature remains, the risk of frostbite may occur due to the lack of blood flow to the skin extremities in order to conserve heat for the vital organs.
3.7 Bacteria Barrier
Skin provides a barrier to bacteria and other microorganisms trying to penetrate the surface barrier. The ability of the skin to control microbial flora is due to factors including such as bacterial antagonism, skin lipids, skin hydration, and pH. The surface of human skin is slightly acidic with a pH below 5 [31]. This is why many soaps and shampoos will often promote the fact that their product is pH balanced. In a study conducted by Lambers and associates, it was found that when skin pH increased to alkaline levels of 8-9 the normal bacterial flora of the skin was reduced [32]. As normal bacterial of the skin decreases this increases the susceptibility of harmful bacteria to penetrate the skin and cause infections. When the acidity of the skin is off balance the skin is also no longer self-sterilizing. In addition to maintaining an acidic environment to prevent bacterial infection, the sebaceous gland secretes sebum. Sebum is a lipid excretion that also contains antifungal and antibacterial properties [1].
4. Mechanical Properties of the Skin
As stated previously, the skin is made up of three connected layers, which are distinct in their nature, structure, and properties. It is known that the epidermis is involved in protecting the skin from its environment, but the fibrous dermis also protects the inner layer of the skin. The fibrous dermis is viscoelastic envelope, and along with the hypodermis, it plays an important role in protecting the skin from mechanical stress. The fibrous dermis can withstand a certain degree of mechanical stress, imposed on it by environmental factors, and it is also required to accompany the different movements of the body. The mechanical function of the skin is the conveying of the biomechanical nature of its components and their structural organization. The modifications it undergoes caused by aging, disease, and the effects of environmental conditions can be reflected by the study of its biomechanical properties. Structural and molecular alterations gives rise to functional deficit in the skin produced by aging.
A study conducted at the laboratories in L’Oreal, France showed that skin keeps its thickness and extensibility until the age of seventy. The skin becomes more rigid at that age. The scientists hypothesized that the decrease in the skin’s thickness and extensibility, which occurs after 70 years of age, may be a consequence of the compacting of the skin, where many spaces in the fibrous dermis of the adult disappear [33]. The increased interaction between the fibrous network, the unraveling of the collagen network, and the intermolecular and intramolecular cross-linking of the collagen, found in the skin of older people, would make the skin stiffer. The recovering capacity of the skin decreases after deformation with age, which is related to the morphometric changes that occur in the elastic network of the dermis. They concluded their study by stating that the viscoelastic parameters vary with age in two different ways. Some of the parameters can change, continuously, from an early age (elasticity of the skin, relaxation time, following deformation). Other parameters may stay constant until about the age of 70 years, then change rapidly (skin thickness, extensibility)[34].
There have been many different non-invasive techniques used to evaluate the skin’s mechanical properties. The most commonly used methods are based on measurements of suction, torsion, and traction. Other methods measure the skin’s mechanical behavior like extensometry, or elastic wave propagation. The data acquired, however, with these methods are mainly descriptive and different, depending of the experimental conditions. These methods are disadvantageous because they modify the skin’s natural state of stress, as the experimental device has to be fixed to the skin throughout the test. The consequence of this is that the measured value of the mechanical properties may be affected, and it can be challenging to estimate the pre-stress value induced by the devices [35].
The suction test is normally used to study the mechanical properties of the human skin, in vivo. The values of the local stresses and deformation cannot be calculated directly from displacement and force applied by the test apparatus. This makes it complicated to acquire the intrinsic mechanical parameters of the skin in vivo. Generally, the negative pressure applied and the elevation of the dome of the skin drawn up is taken into account in order to deduce the properties of the skin. The major disadvantage of this method is that it is dependent on the experimental conditions, which are used, specifically the size of the suction cup and the negative pressure applied [36].
A torsion test is conducted to test the torsional properties of skin. These properties include modulus of elasticity, yield shear strength, ultimate shear strength, modulus of rupture in shear, and ductility. Similar to the suction test, it is difficult to attain the values of the stressors and deformation.
Skin traction uses stressors attached to the skin to indirectly apply pulling force. Researchers at Lyon University, France, proposed a new method to determine the human skin mechanical properties, in vivo, using indentation test. The indentation method is not commonly used to measure the skin mechanical properties, although it allows to achieve the skin mechanical properties, in normal direction, without pre-stressing the skin, prior to testing [37]. The aim of the study was to describe the skin mechanical properties using indentation test. In literature the Young’s modulus for skin, E, ranges from 0.42 MPa and 0.85 MPa for torsion tests [38], 4.6 MPa and 20 MPa for tensile tests [39], and between 0.05 MPa and 0.15 MPa for suction tests. Their results showed that it is necessary to develop a two-layer elastic model to describe the variation of the measured Young’s modulus versus penetration depth.
As already discussed, objective measurements are necessary for reproducible results and comparison between studies which is why specific devices are needed. In the cosmetics industry, this type or reproducibility and comparison are very important. Clinicians need to be able to compare the tissues of the skin before and after a product is applied in order to evaluate potential improvement of the properties of the skin. The knowledge of the properties of the skin has the potential of creating a database for the comparison of measurements with references. There are many techniques which have assessed the mechanical properties of the skin. A new technique has recently been developed, non-contact techniques based on air. This technique assesses the mechanical properties of the skin without disturbing and preconditioning of the stressed area [40]. The other techniques require double sided tape to bond the probe to the skin. This procedure modifies the properties of the skin which hinders other testing on that area. A new specific non-contact device, which is based on air flow and high speed triangulation laser measurement has been proposed.
The device developed for this new method is called Tonoderm (Fig.10). The air flow is provided by an air compressor, and is controlled by the Air Mass Flow Controller (AMFC). A first solenoid valve SVb (electromechanically operated valve) is placed at the output of the AMFC which allows the release of the air system. A second solenoid valve SVs is placed right before the output pipe and allows the immediate stop of the flow. The gap between the end of the output pipe and the skin is measured by using a Laser Displacement Sensor Head. The laser beam passes through a glass plate allowing coaxiality between measurement and stress axis. A specific software developed under LabView language (National Instruments) allows to control the whole system.
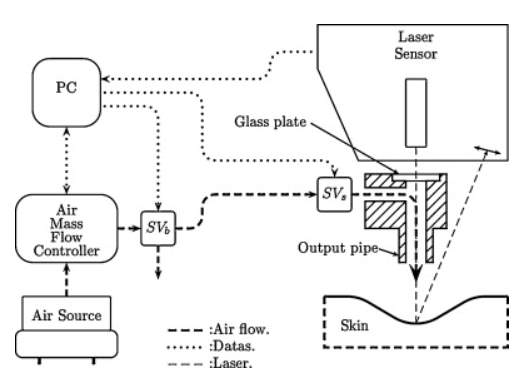
Fig 10. Schematic of the whole developed device [40]
5. Conclusion
Skin is a complex system of chemical, physical, and mechanical properties. Insight to these properties is important to support skin health. The skin’s three connected layers are distinct in nature, structure, and functionality. The chemical properties of lipids play a critical role in creating a gel or crystalline like structure on the surface of the skin. This impermeable barrier helps to maintain skin hydration, block UV rays, keep bacteria out, and support the abundant nerve receptors at the surface of the skin. The rigidity and elasticity of the skin comes from fibroblast synthesis of collagen and elastin fibers. Overtime stressors reduce production of collagen and elastic fibers which lead to the reduction of this fibrous network causing wrinkles, deformations, and sagging. As an individual ages feature line, ridges, furrows, and folds increase.
In the hypodermis, or subcutaneous layer, the subcutaneous tissue is allows the skin to slide over the muscles easier. This is also were body fat is often deposited. In the cosmetic industry, understanding the chemical properties of each layer is important for the formulation of skin care products. Formulating skin care product with lipids will support the barrier function of the skin. If water soluble active components need to be delivered deeper to the skin, specialized ingredients such as liposomes can be used to get past the hydrophobic surface layer of the skin. However, maintaining the skins natural physical properties is critical for retaining skins natural physical protection characteristics.
As a person ages, the skin endures many stress factors. The mechanical properties of the skin, such as elasticity, extensibility, deformation recovery, and rigidity changes. Measurements of skins mechanical properties in the cosmetic industry should be done using non-invasive techniques. Non-contact techniques based on air, such as the device Tonoderm, are viable options to avoid equipment influence on the skin’s natural state of stress. Such techniques are critical to the measurement of mechanical properties of skin before and after application of bioactive skin care products should be used to understand their effectiveness. Further research on the chemical, physical, and mechanical properties of skin is likely to improve the efficacy of the skin care treatments and products.
Citations
1. Rizzo, D.C., Fundamentals of anatomy & physiology. 2010, Delmar Cenagae Learning: Clifton Park, NY. p. 1 online resource (xxxv, 529 p.).
2. Kanitakis, J., Anatomy, histology and immunohistochemistry of normal human skin. European journal of dermatology: EJD, 2002. 12(4): p. 390-9; quiz 400-1.
3. Robledo, A.A. Skin associated lympphoid tisues (SALT). Its normal and pathological function. in Anales de la Real Academia Nacional de Medicina. 2006.
4. Dąbrowska, A., et al., Materials used to simulate physical properties of human skin. Skin Research and Technology, 2016. 22(1): p. 3-14.
5. Betts, J.G., et al. Anatomy & physiology.(2013). Open Stax College.
6. Montagna, W., The structure and function of skin. 2012: Elsevier.
7. Menon, G.K., New insights into skin structure: scratching the surface. Advanced drug delivery reviews, 2002. 54: p. S3-S17.
8. Bouwstra, J., et al., The lipid organisation in the skin barrier. Acta Dermato Venereologica-Supplement, 2000(208): p. 23-30.
9. Moore, D.J. and M.E. Rerek, Insights into the Molecular Organization of Lipids in the Skin Barrier from Infrared Spectroscopy Studies of Stratum Corneum Lipid Models. Acta Dermato-Venereologica, 2000. 80: p. 16-22.
10. Ramasastry, P., et al., Chemical composition of human skin surface lipids from birth to puberty. Journal of Investigative Dermatology, 1970. 54(2): p. 139-144.
11. De Luca, C. and G. Valacchi, Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators of inflammation, 2010. 2010.
12. Wellness, S.P. 2016 12.5.17]; Available from:http://www.stpwellness.com.
13. Coderch, L., et al., Ceramides and skin function. American journal of clinical dermatology, 2003. 4(2): p. 107-129.
14. Stricklin, G.P., et al., Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry, 1977. 16(8): p. 1607-1615.
15. Paves, H., Microfilaments, Mitochondria and Nuclei in Fibroblast Cells. 2012.
16. Stricklin, G.P., et al., Human skin fibroblast collagenase: chemical properties of precursor and active forms. Biochemistry, 1978. 17(12): p. 2331-2337.
17. Han, X., et al., Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochimie Open, 2017.
18. Habtemariam. Herbal Analysis Services. 2017 12.5.17]; Available from: http://www.herbalanalysis.co.uk/Patchouli-Essential-Oil-Analysis.html.
19. Fakari, F.R., et al., Effect of inhalation of aroma of geranium essence on anxiety and physiological parameters during first stage of labor in nulliparous women: a randomized clinical trial. Journal of caring sciences, 2015. 4(2): p. 135.
20. Palma, L., et al., Dietary water affects human skin hydration and biomechanics. Clinical, cosmetic and investigational dermatology, 2015. 8: p. 413.
21. Heinrich, K., U. Heinrich, and H. Tronnier, Influence of different cosmetic formulations on the human skin barrier. Skin pharmacology and physiology, 2014. 27(3): p. 141-147.
22. Bolzinger, M.A., S. Briançon, and Y. Chevalier, Nanoparticles through the skin: managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 2011. 3(5): p. 463-478.
23. Koehler, M.J., et al., In vivo measurement of the human epidermal thickness in different localizations by multiphoton laser tomography. Skin Research and Technology, 2010. 16(3): p. 259-264.
24. Shlivko, I.L., et al., Identification of layers in optical coherence tomography of skin: comparative analysis of experimental and Monte Carlo simulated images. Skin Research & Technology, 2015. 21(4): p. 419-425.
25. Saladin, K.S. and L. Miller, Anatomy & physiology. 1998: WCB/McGraw-Hill New York (NY).
26. Candi, E., R.A. Knight, and G. Melino, Cornification of the Skin: A Non‐apoptotic Cell Death Mechanism. eLS, 2009.
27. Werner, J., System properties, feedback control and effector coordination of human temperature regulation. European journal of applied physiology, 2010. 109(1): p. 13-25.
28. Anderson, B.E., The Netter Collection of Medical Illustrations-Integumentary System E-Book. 2012: Elsevier Health Sciences.
29. Anderson, R.R. and J.A. Parrish, The optics of human skin. Journal of investigative dermatology, 1981. 77(1): p. 13-19.
30. Birgersson, U., et al., A methodology for extracting the electrical properties of human skin. Physiological measurement, 2013. 34(6): p. 723.
31. Aly, R., et al., Effect of prolonged occlusion on the microbial flora, pH, carbon dioxide and transepidermal water loss on human skin. Journal of Investigative Dermatology, 1978. 71(6): p. 378-381.
32. Lambers, H., et al., Natural skin surface pH is on average below 5, which is beneficial for its resident flora. International journal of cosmetic science, 2006. 28(5): p. 359-370.
33. Lavker, R.M., P. Zheng, and G. Dong, Aged skin: a study by light, transmission electron, and scanning electron microscopy. Journal of investigative dermatology, 1987. 88.
34. Escoffier, C., et al., Age-related mechanical properties of human skin: an in vivo study. Journal of Investigative Dermatology, 1989. 93(3): p. 353-357.
35. Pailler-Mattei, C., S. Bec, and H. Zahouani, In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Medical engineering & physics, 2008. 30(5): p. 599-606.
36. Khatyr, F., et al., Model of the viscoelastic behaviour of skin in vivo and study of anisotropy. Skin research and technology, 2004. 10(2): p. 96-103.
37. Bader, D. and P. Bowker, Mechanical characteristics of skin and underlying tissues in vivo. Biomaterials, 1983. 4(4): p. 305-308.
38. Agache, P., et al., Mechanical properties and Young’s modulus of human skin in vivo. Archives of dermatological research, 1980. 269(3): p. 221-232.
39. Manschot, J. and A. Brakkee, The measurement and modelling of the mechanical properties of human skin in vivo—I. The measurement. Journal of Biomechanics, 1986. 19(7): p. 511-515.
40. Boyer, G., et al., Non contact method for in vivo assessment of skin mechanical properties for assessing effect of ageing. Medical engineering & physics, 2012. 34(2): p. 172-178.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Physiology"
Physiology is related to biology, and is the study of living organisms and how they function. Physiology covers all living organisms, exploring how the body performs basic functions in relation to physics and chemistry.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




