What Were the Most Important Advances in Treating Wound Infection in Warfare?
Info: 10267 words (41 pages) Dissertation
Published: 9th Dec 2019
What were the most important advances in treating wound infection in warfare?
Introduction
The battle to heal traumatic wounds has been raging for our entire existence. From early chimpanzee’s efforts to lick and dab wounds through to nanoparticle infused bandages, humankind has been attempting to heal injuries and avoid infection. Advances in weapons and warfare have put modern day battlefields worlds away from the animal attacks that plagued early man but physicians have always been fighting to save life and limb and, despite advances in the methods of injuring and killing, doctors and surgeons are more successful than ever. This is due to thousands of years of experience and an increasingly scientific study of the process of healing wounds.
This paper aims to identify the most significant milestones discovered in the treatment of wound infection – specifically in the setting of trauma on the battlefield. Where possible scientific method has been applied and relevant studies have been used to demonstrate the significance of techniques throughout history. The first evidence of wound healing being hampered by infection is evident in prehistoric surgery, specifically trepanation. This early surgery involved drilling or scraping a hole in the skull and was done to treat any number of aliments from depression through to head trauma. Interestingly, upon examining prehistoric skulls, in most cases the surface of the skull around the operated site is smooth, indicating that bacteria caused no major complication. In only a few cases did osteomyelitis leave its marks. How it is that prehistoric man was performing an operation with a survival rate approaching 100% when the same procedure in the early 1800s, due mainly to infection, carried a death sentence? This can be explained by what we now know about the role of microorganisms in the infective process. In hospitals full of sick patients doctors were carrying infective organisms from patient to patient creating a breeding ground for virulent strains of bacteria (1).This 19th century problem still plagues us today, for example the emergence of MRSA (multi-drug resistant staphylococcus aureus) and other drug resistant strains of bacteria. As antibiotic resistance rises – finding new ways of treating infection is essential. This paper explores the treatments from the pre-antibiotic world with the hope of finding a new way of tackling the problem. Furthermore, this paper considers the potential for a cost-effective solution to treating wound infection based upon ancient techniques. With less economically developed countries having little access to expensive drugs and equipment, historical treatments may provide a cheap and highly effective alternative.
One of the most significant aspects of this timeline is the way that progress in traumatic wound treatment has fluctuated. Important discoveries by Ancient Egyptians were lost and rediscovered even as late as the 1800s and, alongside now proven treatments, there were hundreds of ‘cures’ which have subsequently proved to be more potent poisons than medicines. Therefore within this paper, the most important advances have been selected and the impact their discovery had at the time and on the field of medicine as a whole has been assessed. In addition, where available modern papers trialling historical treatments under controlled conditions have been included to get a more informed understanding of their efficacy.
Treatment: Defined as any action either to prevent or reverse the infection of combat wounds, in addition specifically looking at fighting infection as opposed to promoting quicker wound healing.
Wound: Any combat wound which has the potential to become infected.
Methodology
Searches were first conducted on PubMed, a source for published medical papers, for articles focusing on the work of Joseph Lister. During the research it was evident that, although his invention of antiseptic technique revolutionised patient outcomes, these did not necessarily change outcomes in the various major global conflicts that were to come (particularly World War One). Therefore, the search was expanded to include other methods applied to prevent wound infection. Upon widening the search, it was discovered that Lister was not the first to use antiseptic substances, in fact antisepsis was mentioned 10 times in the Lancet in the 10 years before Lister and even as far back as Ancient Egypt, there is mention of using effective antiseptics as wound treatment. Therefore, the search was expanded to cover all of history.
The initial aim was to identify the fundamental principles upon which the prevention of wound infection was built upon. To do this firstly treatment methods in use in military conflict today were identified using as the basis, the U.S. military guidelines for the prevention of infection associated with combat related injuries (2011)(2).
| Level of Care | Care Category | Recommendations |
| Role1/Level 1
(Prehospital |
Initial care in the field | -Bandage wounds with sterile dressings (avoid pressure over eye wounds)
Stabilize fractures Transfer to surgical support as soon as feasible |
| Post injury antimicrobials | Provide single-dose point-of-injury antimicrobials (Table 3) if evacuation is delayed or expected to be delayed (I C) | |
| Role 1/Level II Role 2/Level II without surgical support | Postinjury antimicrobials | Provide IV antimicrobials (Table 3) as soon as possible (within 3 h)
Provide tetanus toxoid and immune globulin as appropriate without surgical support ( Enhance gram-negative coverage with aminoglycoside or fluoroquinolone not recommended Addition of penicillin to prevent clostridial gangrene or streptococcal infection is not recommended Redose antimicrobials if large volume blood produce resuscitation (IC) Use only topical antimicrobials for burns (18) |
| Debridement and irrigation | Irrigate wounds to remove gross contamination with nonnal saline, sterile, or potable water, under low Postinjury antimicrobials pressure (bulb syringe or equivalent) without additives (18)
Do not attempt to remove retained deep soft tissue fragments if criteria met (18) Provide cefazolin 2 g IV X 1 dose |
|
| Role 2/Level II with surgical support (lib )I Role 3/ Level III | Postinjury antimicrobials | Provide IV antimicrobials (Table 3) as soon as possible (within 3 h) (18)
Provide tetanus toxoid and immune globulin as appropriate Enhance gram-negative coverage with aminoglycoside or fluoroquinolone not recommended (!B) Addition of penicillin to prevent clostridial gangrene or streptococcal infection is not recommended (IC) Redose antimicrobials if large volume blood produce resuscitation (I C) Use only topical antimicrobials for burns (18) Antimicrobial beads or pouches may be used (IB) Provide postspleneclomy immunizations if indicated {18 |
| Debridement and irrigation | Irrigate wounds to remove contamination with nonnal saline or sterile water, under low presswe (5-10 PSI, e.g., bulb syringe or gravity flow) without additives (usc 3 L for each Type I, 6 L for each Type II, and 9 L tor each Type III extremity fractures) (18) Do not attempt to remove retained deep soft tissue fragments if criteria met (18).
Provide cefazolin 2 g IV X 1 dose Do not obtain cultures unless infection is suspected (18) |
|
| Surgical wound management | Surgical evaluation as soon as possible (18)
Only dural and facial wounds should undergo primary closure (18) NPWT can be used (18) External fixation (temporary spanning) of temur/tibia fractures (18) External fixation (temporary spanning) or splint immobilization of open humerus/forearm fractures (18) |
|
| Role 4/Level IV | Postinjury antimicrobials | Complete course of postinjury antimicrobials (Table 3)
Antimicrobial beads or pouches may be used (18) Provide postsplenectomy immunizations if indicated (18) |
| Debridement and irrigation | Irrigate wounds to remove contamination with nonnal saline or sterile water, under low pressure (5-10 PSI, e.g., bulb syringe or gravity flow) without additives (use 3 L for each Type I, 6 L for each Type II, and 9 L for each Type III extremity fractures) (18) Do not attempt to remove retained deep soft tissue fragments if criteria met (18).t Provide cefazolin 2glVXldose
Do not obtain cultures unless infection is suspected (18) |
|
| Surgical wound management | Wounds should not be closed until 3-5 d postinjury (18)
Only dural and facial wounds should undergo primary closure (18) NPWT can be used (18) External fixation (temporary spanning) of femur/tibia fractures (18) External fixation (temporary spanning) or splint immobilization of open humerus/forearm fractures (18) |
Table 1. Recommendation to Prevent Infection Associated with Combat Related Injuries Based on Level of Care (2)
Secondly, historical documents and historian’s perspectives were examined to identify the way wounds have been treated historically and the impact advances in treatment methods had at the time of introduction. Using these in combination the most important advances were identified and grouped into two; Surgical interventions and Antimicrobial interventions.
Surgical Interventions
- Wound Closure
- Irrigation
- Debridement
- Negative pressure wound therapy (NPWT)
Antimicrobial interventions
- Systemic antibiotics
- Topical antiseptics:
- Modern antiseptics – carbolic acid
- Alcohol
- Honey
- Heavy metal ions
After these had be identified, they were individually examined, using data from historical trials, warfare statistics and patient records to assess the way these treatments individually changed patient outcomes at the time of their introduction. In cases where historical evidence is lacking modern papers performing research on ancient remedies under laboratory or clinical conditions were evaluated. Using this research the potential for future alternative treatment methods based upon historical remedies has also been analysed.
In order to gather accurate information on these time periods the University of Manchester Library was searched for sources translating and interpreting Egyptian papyri and Greek scrolls. In addition, PubMed and Google Scholar were utilised to collate modern sources on the area.
Analysis
Surgical interventions
Wound closure
Delayed primary wound closure is the practice of leaving heavily contaminated wounds open for an extended period (3-5 days) following debridement. After this time, the wound is re-assessed then sutured closed. This has been found to be especially beneficial in heavily contaminated wounds or when there is a high risk of infection. This can include wounds containing foreign bodies, severe wounds with extensive damage to tissues or when there is a delay in the patient reaching medical care.
Wound closure has been the subject of debate for centuries. Ancient Greeks discussed whether a wound should be immediately closed or allowed to suppurate. Indeed, with pus being seen as a beneficial and essential part of wound healing right the way through to the early 1800s, the problem of when to close wounds continued until the 14th century. It was only at this point that the removal of foreign objects, re-joining severed tissue and maintaining tissue continuity became a focus for battlefield surgery. Alongside this John Jones (1729–1791), a veteran surgeon of the French and Indian Wars, started advocating the avoidance of primary closure. He advised that, if primary closure was required, an onion was inserted to keep it open. Then, within 24-48hrs, the wound was reopened and re-assessed.(3) However, advice on avoidance of primary wound closure never gained traction and was still the subject of debate up until the First World War.
 During the first years of the First World Was military surgeons still used old fashioned methods which expressed the importance of primary closure. However, it soon became evident that this was not effective. Gas gangrene was more common than any war previously documented. On the allied side in the first years of the war 70% of amputations were to arrest infection rather than to remove a acutely damaged limb(4). This was due in part to the appalling conditions the soldiers were fighting in. Heavily fertilised soil and debris teaming with bacteria contaminated almost every wound. However, the primary closure technique was locking the bacteria inside the wound allowing them to develop into an infestation. In the harsh conditions of the battlefront, delayed primary closure was called upon and before long first-hand experience was repeatedly demonstrating its significant benefits. Soon clinical trials were performed which provided conclusive proof that delayed primary wound closure was saving lives.
During the first years of the First World Was military surgeons still used old fashioned methods which expressed the importance of primary closure. However, it soon became evident that this was not effective. Gas gangrene was more common than any war previously documented. On the allied side in the first years of the war 70% of amputations were to arrest infection rather than to remove a acutely damaged limb(4). This was due in part to the appalling conditions the soldiers were fighting in. Heavily fertilised soil and debris teaming with bacteria contaminated almost every wound. However, the primary closure technique was locking the bacteria inside the wound allowing them to develop into an infestation. In the harsh conditions of the battlefront, delayed primary closure was called upon and before long first-hand experience was repeatedly demonstrating its significant benefits. Soon clinical trials were performed which provided conclusive proof that delayed primary wound closure was saving lives.
Fig. 1 A study in 1917 showing improved wound adhesion using the delayed primary closure technique in a casualty clearing station on the western front (5)
The results showed that in battlefield environments, although primary wound closure was still the operation of choice for head wounds, chest and those involving joints, in general, delayed suture was safer and more certain in its results(5). Delayed primary closure allows for initial management of the wound with a few simple sutures, which can suffice for a few days until the patient can be treated at a casualty clearing station. At which time the wound can be thoroughly debrided and reassessed to decide if permanent closure is then appropriate. The open wound allows the surgeon to ascertain if there is virulent infection present before the wound is closed. If the infection has not been overcome the wound can be left open until the surgeon decides it is clean. As well as helping the body to fight the infection this method aided medical services during periods of high casualties as wounds could be initially dealt with by a simple suture, leaving more time to attend other injuries.
The physiology behind the technique has been further investigated in recent times. It has been demonstrated that keeping the wound open causes an increased blood flow to the wound edges. This improved blood flow leads to recruitment of functional phagocytes to the wound, and an increased resistance to infection. For example, a study demonstrated that, after a wound is created, with each day that passes the amount of bacteria required to cause infection increases 10 fold (up to 6 days) (6).
Modern day medicine still relies heavily on the technique of delayed primary wound closure to help treat severe combat casualties. On the battlefield, this approach has been proven to reduce wound infection rates, save tissue and reduce the risk of amputation. However, it has taken longer to impact civilian practice. In 1963 the first randomised control trial dedicated to delayed primary wound closure in the operative setting showed a decrease from 42% to 8% incidence of infection with the use of delayed primary wound closure. Other literature claimed 0 infections from a series of 300 highly contaminated wounds (7). More recently, out of a total of 158 patients who received treatment for perforated appendix in the Ayub Teaching Hospital, 6.3% of patients treated with Delayed Primary Closure developed infection. In comparison 39.2% developed infection following treatment with primary closure. This has led surgeons to conclude that even for modern surgery when there is significant contamination, delayed primary closure is the optimal management strategy(8). A 2013 meta-analysis from JAMA Surgery suggested that in contaminated or dirty abdominal wounds such as perforated appendix, DPC reduced the chances of surgical site infection. However, it was noted that this significance was dependant on the statistical handling of the data(9).
Whilst delayed primary closure is a technique that was developed to treat highly contaminated and severe laceration injuries during the war, the evidence researched as part of this report supports the use of delayed primary closure not only in combat wounds but also in civilian medicine such as contaminated surgical wounds.
Irrigation
Irrigation in its modern form is described as the steady flow of a solution across an open wound surface to achieve wound hydration, to remove deeper debris and to assist with the visual examination. It is seen as a more consistent method of wound cleansing, compared to bathing or swabbing.
Although modern irrigation techniques have their roots in the First World War, the principles of bathing and washing wounds pre-date the Ancient Egyptian civilisation. The Sumerian civilisation (3100- 500 B.C.) advocated the use of beer and hot water to wash wounds before applying a form of topical treatment. The Ancient Egyptians also saw the value in wound cleansing with the first mention of wine as an irrigating solution. This practice was to become a favourite of the Greeks and Romans with Hippocrates and later Galen believing that wounds should be irrigated with clean water and wine(1). The use of wine to bathe wounds was common place right through the classical period and into medieval medicine. However, it was not until World War One that irrigation evolved into a system of continuous fluid being applied to the wound surface. This development was spurred on by the failure of modern antiseptic solutions to control infection on the Western Front. Almost every wound was contaminated with soil rich in bacteria at the point of injury and the current method of using carbolic acid (and iodine) to cleanse the wound coagulated the superficial parts rendering the bacteria deeper down even harder to reach. At this point Henry Drysdale Dakin, an English chemist, and Alexis Carrel, a French surgeon, created a new method relying on irrigation to help cleanse wounds.
Their technique required wide excision of all devitalized tissue and the insertion of small rubber tubes containing side holes deep into the wound. These were then covered with clean dressings. Dakin created a solution (sodium hypochlorite) which was introduced through these tubes every 4 hours allowing a continuous flow of solution deep into the tissues. This solution was allowed to rest in the wound for maximal contact with the tissue. These flushes were continued until all microbes had been eradicated, which was determined by microscopic analysis. Only after the wounds were determined to be aseptic were they closed.
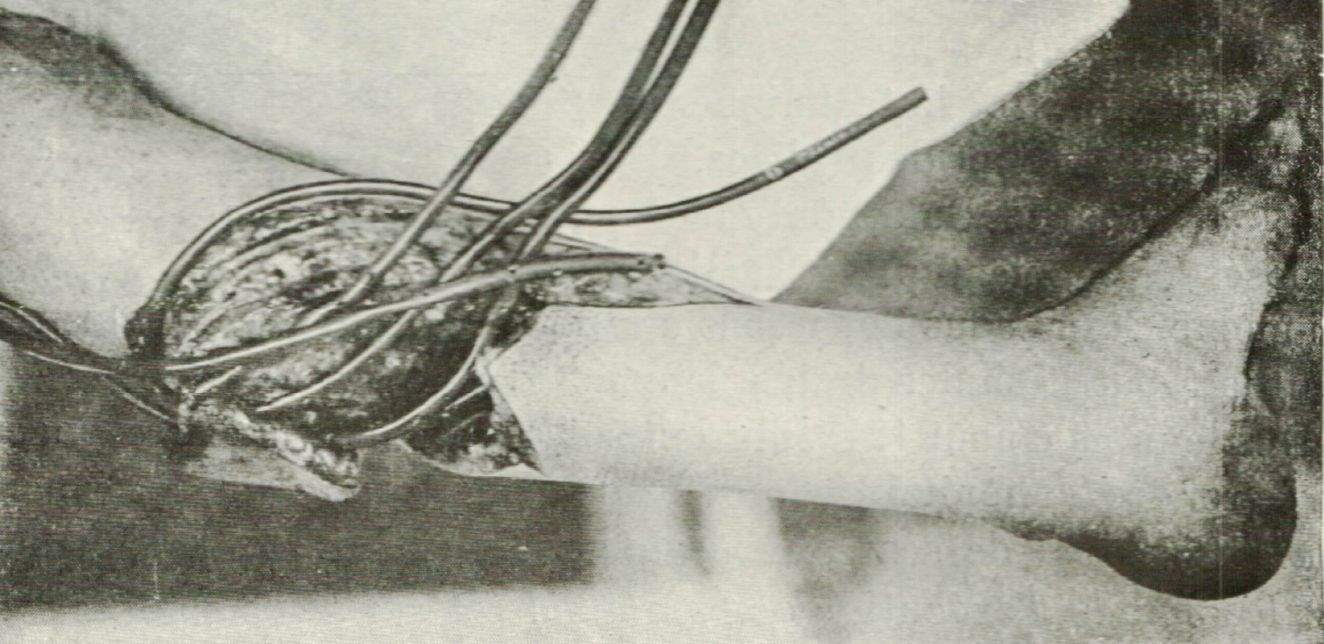
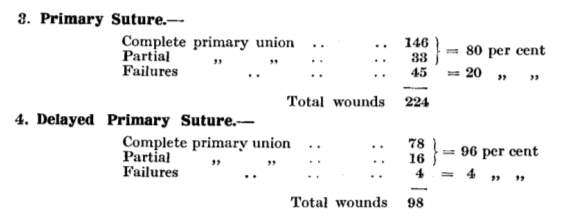
Fig. 2 Showing the arrangement of irrigation tubes used in the Carrel-Dakin method (10)
The so-named ‘Carrel-Dakin’ Method was given extensive clinical trial during World War I. Initially Carrel himself reported on treatment of 303 cases coming from a casualty clearing station and treated in hospital with his irrigation technique. After treatment and a hospital stay of 24hrs only 2 died of sepsis. He went on to comment that use of his method would “most certainly yield an immense improvement in results”(11). However, other clinical work at the time had more mixed reviews, showing that whilst use of this method prevented the local necrosis of wound suppuration, it failed to protect against invasive infection or to eradicate haemolytic streptococci in the wound. Alongside this, the method was extremely labour intensive with meticulous dressing technique required; these factors combined to severely hamper general adoption on the western front(12).
At the end of the war Alexander Fleming showed that Dakin’s solution was only effective at killing microbes in concentrations which were cytotoxic. He went on to call for the treatment with antiseptic solutions to be abandoned and surgeons rely on their skill alone(13). This explains some of the problems with the method, such as not eradicating haemolytic streptococci, and underlines the importance of wound flushing in the irrigation technique. It seems now that much of the good results of Carrel-Dakin’s method during the war were due to the fact that careful drainage of the wound was performed rather than any effect of the irrigating solution itself.
Still today the use of irrigation is strongly advocated. Various solutions are recommended but principally following debridement, wounds should be irrigated with warm, sterile fluids, through low-pressure flow sterile tubing, pulse irrigation systems or arthroscopy pumps. While interventions such as soaps and antibiotic solutions may lower initial bacterial counts, normal saline under low-pressure flow or bulb suction irrigation shows reduced rebound bacterial counts with less damage to host cells and is currently the recommended method of irrigation(14). This treatment has been backed up by clinical trials. Robert et al investigated the effect of irrigation on wound infection rates in penetrating combat wounds. Out of 44 patients receiving wound irrigation (83%) as a part of initial wound care infections developed within 48 hours in two (4.5%) patients. Compared to five (55%) without irrigation(15). Moscati et al demonstrated that irrigation with sterile aqueous solution effectively decreased the rate of wound infection in the emergency department(16).
Interestingly, future directions for irrigation may yet include Dakin’s solution. Using diluted Dakin’s solution one tenth the original “quarter strength” solution, Erdle et al suggests that cytotoxicity to native cells may be avoided whilst preserving antifungal and antibacterial properties. They go on to describe this being more effective than other antifungal agents such as mafenide acetate and amphotericin B(14).
Debridement
Debridement is defined as; the removal of all dead or devitalised tissue at the wound site. This process involves increasing the size of the wound, ensuring all foreign bodies have been removed and the cutting out of any non-viable tissue. In the case of highly contaminated wounds, viable tissue may also need to be removed. It is essential to remove the dead tissue as it harbours bacterial infections as well as interfering with the process of wound healing. This method has been shown to decrease the bacterial load and improve the inflammatory response encouraging healthy granulation tissue formation across the wound(17).
Debridement is not a new idea; in fact, it was described by Hippocrates in Ancient Greece (469-377 B.C.). He described the use of a heat cauterise the wound, cutting away and destroying the dead tissue to control infection(1). This method was in use for centuries and demonstrated good results in the 1800s to control “limp gangrene” as described by French surgeons Ponteau and Delpech. The Famous French surgeon Ambrose Paré (1510–1590) added further advice. He advocated thorough flushing of the wound, removing all foreign bodies, bruised or lacerated muscle and blood clots(18). Although he advised thorough cleansing the enlargement of wounds was still seen as counter-intuitive. During the French and Indian Wars (1754–1763) bullets were removed only if within easy reach of the surgeon and in many cases, even up until World War One foreign objects out of reach would be left inside the wound.
The term debridement came about in the 1800s during which time there is the first clinical description of wound enlargement to remove devitalised tissues by Desault. Also in the 1800s Russian military surgeon Karl Reyher demonstrated that the use of debridement combined with antiseptics in the treatment of gunshot wounds, dramatically reduced patient mortality(18).
It was during World War One that surgeons learnt through first-hand experience the importance of early debridement of tissues. Even in describing the effects of his irrigating solution Carrel expressed how much more delayed treatment was if necrosed tissue had remained for a long period inside the wound. Wangensteen noted in 1979, reflecting on the Great War. “The role of the surgeon prior to sulphanilamide was essentially that of pus evacuator. Debridement removes potential infection in its former stages, but when infection is well established, debridement is also useless”(19). This was documented for the first time in 1898 by Friedrich. He had shown that debridement is at its most effective within the first 6-hour window following contamination.(19) This has since been widely accepted to minimise the risk of deep infection and is commonplace is modern medicine.
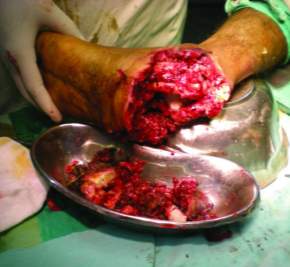
 Fig. 3 Blast injury to the foot after a mine explosion in Iraq, showing extensive debridement of devitalised tissues and removal of foreign bodies (20)
Fig. 3 Blast injury to the foot after a mine explosion in Iraq, showing extensive debridement of devitalised tissues and removal of foreign bodies (20)
In modern medicine debridement is still an essential technique in Emergency Departments around the world. Although, outside the military, due to simple wounds with low contamination surgeons tend to avoid radical debridement. It is advised for inexperienced surgeons (as there is less chance of wound dehiscence), if there is a delay in obtaining medical attention of greater than 12hrs or if there are multiple wounds. In combat military surgeons rely on aggressive debridement as their wounds almost always have significant contamination, are more complex and routinely involve peripheral neurovascular damage.
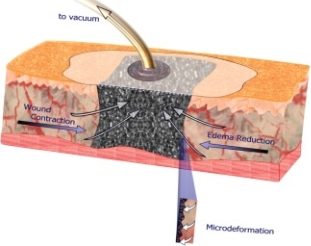 Negative-pressure wound therapy (NPWT)
Negative-pressure wound therapy (NPWT)
Negative pressure wound therapy is a modern technique of using suction to create a negative pressure in the wound. It consists of some form of filler material placed over the wound and covered with an air tight dressing. A tube is then used to apply 125mm of pressure to the wound (Fig. 4)(21).
Although the NPWT that we use today is only a recent advancement, the idea has been around for a millennium with the earliest vacuums being achieved by direct mouth suction on the wound. During the Roman era healers thought to possess special powers were responsible for sucking out deep and poisonous wounds. The idea consequently evolved to use heated glass cups to induce suction, similar to modern day cupping therapy. As the glass cooled the air inside shrank creating a negative pressure providing suction deep into the wound. This type of treatment was seemingly invaluable to the armies of the Roman Empire with Cato ensuring he had groups of “sucking healers” present during his African Campaign(22).
Fig. 4 Method used to apply NPWT
This form of therapy was still present even to as late as the 1800s with “wound suckers” still attached to French squadrons. Dominique Anel, a French Surgeon, noted that these healers would successfully remove clots and foreign bodies from injured soldier’s wounds(22).
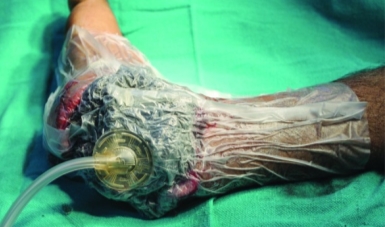 It was not until 1997 when Morykwas et al. performed studies on a pig model that the modern technique was established. Results of this experiment showed a fourfold increase in blood flow, significant granulation tissue formation and decrease in tissue bacterial counts(23). This work has been verified by many authors since. Lenininger et al reporting on contaminated soft tissue injuries in Iraq described an infection rate of 0% and a wound complication rate of 0%(24). Further studies by Peck et al into treatment of extremity vascular injury in Iraq showed infection rates of 3.7% and acute anastomotic disruption of 3%, with the use of NPWT(25). Alongside the physiological reduction in bacteria, NPWT also reduces the need for dressing changes. Pen-Barwell et al showed that 20 cases in which topical negative pressure was changed less than once per 4.9 days had significantly less rate of antibiotic prescription compared to 17 cases which had dressings changed more frequently. This allows more time for doctors and nurses to attend patients during periods of intense fighting, resulting in higher levels of care and reducing the levels of infection. Due to these factors negative pressure therapy is being widely used by modern military and civilian surgeons. (20)
It was not until 1997 when Morykwas et al. performed studies on a pig model that the modern technique was established. Results of this experiment showed a fourfold increase in blood flow, significant granulation tissue formation and decrease in tissue bacterial counts(23). This work has been verified by many authors since. Lenininger et al reporting on contaminated soft tissue injuries in Iraq described an infection rate of 0% and a wound complication rate of 0%(24). Further studies by Peck et al into treatment of extremity vascular injury in Iraq showed infection rates of 3.7% and acute anastomotic disruption of 3%, with the use of NPWT(25). Alongside the physiological reduction in bacteria, NPWT also reduces the need for dressing changes. Pen-Barwell et al showed that 20 cases in which topical negative pressure was changed less than once per 4.9 days had significantly less rate of antibiotic prescription compared to 17 cases which had dressings changed more frequently. This allows more time for doctors and nurses to attend patients during periods of intense fighting, resulting in higher levels of care and reducing the levels of infection. Due to these factors negative pressure therapy is being widely used by modern military and civilian surgeons. (20)
Fig. 5 Showing consequent NPWT being applied to the same blast wound of the foot (20)
There has been much research into the mechanism of action of this method since its rediscovery in 1997. It is evident that it is a complex and multifactorial process involving both primary and secondary mechanisms.
Initially there is wound contraction (Macrodeformation) which in turn induces microdeformation. This is the mechanical force generated by the negative pressure on individual cells and transmitted through the extracellular matrix. This force stimulates cell proliferation by a process of mechanotransduction(20).
Later in the wound healing process there are further increases in cellular proliferation. Scherer et al. noted an increase in biological marker of cell proliferation Ki-67 and other studies have shown an increased proliferation of fibroblasts, endothelial growth factor and epithelial cell migration into the wound(26, 27). The increase in pressure also causes relative hypoxia around the wound which stimulates angiogenesis by an increased expression of vascular endothelial growth factor. This ultimately results in an increase in granulation tissue formation, blood supply and wound healing(20).
Although the above mechanisms result in faster wound healing, NPWT also physiologically reduces infection in the wound. The mechanism by which it does this is fluid and bioburden reduction. Due to the negative pressure excess fluid is removed from the deep within the wound. This reduces local oedema and hydrostatic pressure while also removing any foreign debris, devitalised tissue and infective material. This both reduces the microbial load and enhances blood flow to the wound, promoting healing and preventing infection(20).
Fig. 6 Mechanisms of action of NPWT (20)
Antimicrobial interventions
Antibiotics
The discovery of antibiotics was a medical revolution. Science had finally created a ‘true magic bullet’, something that is harmful to infective organisms whilst sparing host cells. This is evident by not only the impact the new wonder drug had on the battlefield at the time but also our modern-day reliance on antibiotics for treating anything from pneumonia and tuberculosis to osteomyelitis.
Although Alexander Fleming first observed the bactericidal action of Penicillium notatum mold in 1928, therapeutic results were not realised until 1940, when Howard Florey and Ernst Chain prepared an extract and successfully treated mice injected with a lethal dose of Staphylococcus aureus. Consequent clinical trials conducted by the military during World War Two were a resounding success and penicillin development and production became a priority for the Allied Army(28). By the time the Allies arrived in Sicily large quantities of penicillin were available and were used via topical appliance to wounds and intramuscular injections. The results confirmed all the previous trials and it was soon demonstrated that penicillin was highly effective against the clostridia group of bacteria which were responsible for both gas gangrene and tetanus. By the time of the D-Day landings, penicillin was available for every casualty(4). Scott reflecting on the Second World War in 1954 described how, since the discovery of penicillin in 1928, there had been a marked reduction in post-operative septic complications. He goes on to state that the new drug was of even more importance during war situations with prolonged extraction times, and delayed surgical intervention (29)
Although antibiotic treatment is an essential part of modern military wound care, some studies have shown that surgical interventions may be more important in preventing and controlling infections. Robert et al comparing the use of systemic antibiotic prophylaxis and irrigation showed the following.
| Systemic antibiotic prophylaxis | No systemic antibiotic prophylaxis | |
| Wound irrigation | 2.6% (one of 38) | 17% (one of six) |
| No wound irrigation | 40% (two of five) | 75% (three of four) |
Fig. 7 Systemic antibiotic prophylaxis vs wound irrigation(15)
Regardless which is the most vital it is evident that in combination irrigation and SAP produce massive reductions in infection rates in combat wounds (15).
However, continued use of antibiotics in the future remains challenging, with antibiotic resistance an ever-growing problem. Antibiotics were introduced almost 100 years ago with little thought as to their appropriate use. Consequently, they were used in vast quantities without considering their effectiveness against various types of bacteria. As a result, resistance quickly developed and has been a problem ever since. This has never been a more important issue than today, as MRSA is threatening to become untreatable by antibiotics; scientists are looking at alternative methods. By exploring the history of the treatment of wound infection, the following ancient ideas are inspiring some exciting new treatment options.
Topical antiseptics
Throughout history there have been many attempts to create a topical treatment for wounds to help prevent infection.Although modern evidence doesn’t advocate extensive use of topical sterilising agents in wound treatment, they have been a central part of wound care ever since the first attempts at wound healing. Consequently, topical antiseptics have been looked at in more detail to assess why they fell out of favour and to see if they could ever be applied to today’s medicine. To do so they have been broken down into the following groups:
- Modern antiseptics – carbolic acid
- Honey
- Metal ions
- Alcohol
Modern antiseptics and carbolic acid
It is often thought that the dawn of infection control didn’t really begin until the work of Joseph Lister in 1867. Indeed, patient outcomes in the early 19th century were abysmal. As many as 80% of all operations were followed by hospital gangrene (presumably streptococcal infection and mixed synergistic infection), and almost one half of all patients died after a major operation, usually due to infection(30). This all changed when Lister published his antiseptic technique in 1867. This technique involved carbolic sterilising of the operating theatre, surgeon’s hands and tools. He also created the first antiseptic bandages (silk soaked in carbolic acid) which were put to use both in civilian and military treatment. His methods revolutionised surgical treatment. Mortality from amputation fell from 45% to 15%(31).
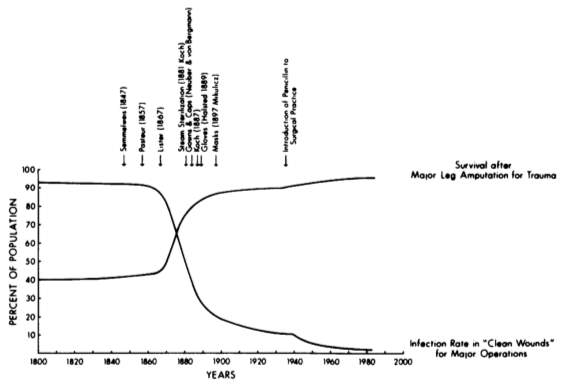
Fig. 8 showing impacts of antisepsis on infection and survival rates after major leg amputations. (Values are best estimates from a compilation of sources) (28)
On the battlefield, his new technique was immediately put into practice by the Germans in the Franko-Prussian War. Comparing the Germans and the French there is an early victory for antisepsis on the battlefield. The French using the traditional practice of packing the wound with unsterile lint fibres were plagued by sepsis and hospital gangrene with 25% of the wounded and 40-50% of amputees dying. Whilst, in comparison, the Germans using Lister’s eight-layered silk and carbolic acid bandage had remarkably low infection rates (Although, this result may be due to the French never using carbolic acid to sterilise operating theatres and instruments rather than the antiseptic effects of the bandage). The initial positive results were echoed by firstly the Spanish-American war – with mortality rates (compared with the American Civil War) almost halved in the 1320 patients treated for gun-shot wounds, and next the English Boer War with sepsis and gangrene markedly reduced and the majority of the 22,000 treated for wounds surviving.
However the results were not entirely positive. Casualty reports from the Franco-Prussian war as a whole were markedly worse than the earlier American Civil War, which had been fought without Listerian techniques. Mortality for thigh fractures stood at 65.8% in one series and ranged from 54.2% to 91.7% in others although, this result is probably compounded by the introduction of mass armies and new age weaponry.
In addition, when looking at these figures, it is hard to assess how much the impact on casualty figures is due to the adoption of antiseptic bandages and the consequent prevention of infection, or whether it is the introduction of Lister’s antiseptic surgery. Indeed, it is evident that antiseptic bandages were not as effective as previously believed from the casualty reports of the start of World War One. Surgeons faced with the onset of World War One had seen the fantastic results of antiseptic use in the Boer War and were ready to treat casualties using similar techniques. However, it was immediately evident that they were thoroughly unprepared and unable to deal with the horrific injuries on the Western Front; high explosive, high velocity missiles – machine gun -bullets, shell fragments, shrapnel – at close range on human tissues. In combination, compared to the arid desert of the Boer war, World War One was fought in fertile fields teaming with anaerobic clostridial organisms of gas gangrene and tetanus. The majority of wounds arrived heavily contaminated with the fertilised soil of Flanders and almost inevitably were infected(4). It was quickly discovered that whilst Lister’s methods proved effective at preventing infection, they struggled to treat infection once established. Wright, a British professor of pathology argued against the use of antiseptics, which he believed only served to retard the physiologic healing process. Alexander Fleming in 1919 showed that none of the commonly used antiseptics demonstrated appreciable bactericidal action in infected wounds and were in fact responsible for the destruction of leucocytes and a subsequent inhibition of the body’s own immune system. Consequently, he called for the abandonment of antiseptics in wound care(13).
Subsequently, gas gangrene was more common than in any war previously documented and tetanus complicated 8.8 per 1000 wounds. Pyaemia (sepsis) and erysipelas were common and secondary haemorrhage was a feared complication as ligatures slipped off blood vessels in infected wounds. As a result, in the early years of the war, compound fracture of the femur carried an 80% mortality and it was only with the introduction of modern surgical techniques such as debridement/delayed primary closure/Irrigation that patient outcomes started to improve(4).
It is indisputable that Lister reformed modern medicine and the aseptic surgery performed today would not be possible without him. His ideas of sterilised dressings, tools and equipment are commonplace in military and civilian medicine. However, in terms of treatment and prevention of infection on the battlefield use of antiseptic solutions and dressings in combat surgery has been phased out. Indeed, the U.S. military now only indicates topical antimicrobials for use in the case of severe burns (2).
Alcohol
The use of alcohol in healing wounds is one of the oldest medical treatments known to man. It was first mentioned in the form of washing wounds in beer by the Sumerians but the idea quickly evolved and before long the Ancient Egyptians were using wine in the dressing of wounds. This became the treatment of choice for the Greeks and Romans who would use it to treat almost any type of wound. Stronger alcoholic preparations were used after distillation was introduced, Nealaton (1863), effectively used alcohol on wounds resulting in less than 10% hospital mortality from elective operations(32). In the modern world alcohol is still being used extensively as a surface disinfectant.
Although alcohol has been used in many forms throughout history, it has most consistently been applied in the form of wine. This was applied to wounds by double padded dressings soaked in wine, bathing wounds in wine and even injected into wounds.
The historical basis for this treatment is extensive. Galen and Hippocrates advocated the use of wine in treatment of wounds and application of wine to wounds was also made in biblical times by the Good Samaritan (Luke 10:33-34). Despite this historical evidence, to assess its true impact in preventing wound infection, the scientific basis for its effectiveness as an antiseptic has been evaluated.
The very pure ethyl alcohol in use today obviously has scientific evidence to show its effectiveness in killing bacteria. In fact, it has been shown that the optimum strength by weight is 70% (alcohol-water mixtures against E.coli and staphylococci), However, many recent experiments show antiseptic properties in wine of strength 10-15%. Evidently, there are antiseptic properties to wine other than alcohol content Fig. 9 (1).
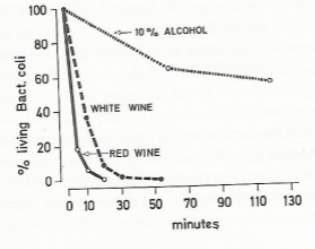 The mechanism has been shown to be due to anthocyanins, a subgroup within the larger group of polyphenols that exist in wine. The most prominent of these compounds, in terms of antibacterial effects, are malvoside and oenoside, which also happen to be responsible for the colour of red wine (there are colourless equivalents in white wines). Although this pigment is already present in grapes it is combined with a carbohydrate and hence not antiseptic. During alcoholic fermentation and under the highly acidic conditions in wine it undergoes hydrolytic cleavage and consequently becomes activated. In addition, red wines have an average ph. of 3.6, this degree of acidity allows for optimal solubility of malvoside and oenoside. Consequently, wines get stronger and darker over time and, in accordance with the action of the pigment, the bactericidal power of wine also increases (1).
The mechanism has been shown to be due to anthocyanins, a subgroup within the larger group of polyphenols that exist in wine. The most prominent of these compounds, in terms of antibacterial effects, are malvoside and oenoside, which also happen to be responsible for the colour of red wine (there are colourless equivalents in white wines). Although this pigment is already present in grapes it is combined with a carbohydrate and hence not antiseptic. During alcoholic fermentation and under the highly acidic conditions in wine it undergoes hydrolytic cleavage and consequently becomes activated. In addition, red wines have an average ph. of 3.6, this degree of acidity allows for optimal solubility of malvoside and oenoside. Consequently, wines get stronger and darker over time and, in accordance with the action of the pigment, the bactericidal power of wine also increases (1).
Fig. 9 Comparison of bactericidal powers of two wines (strength 9.8%) and ethyl alcohol (strength 10%), when tested on E.coli.
In the use of wine to treat wounds Greeks were utilising the antiseptic properties of polyphenol, a more complex version of Lister’s phenol (Carbolic acid) and, after investigation, it was discovered that the polyphenol in wine (malvoside) was discovered to be 33 times more potent than phenol when tested weight for weight on E.coli (1)
However, the drawback to wine is that its bactericidal power are short lived. The active components are quickly bound by proteins when applied to the wound and deactivated. Therefore, to be therapeutic, it must be used generously and frequently. This is why wine fell out of favour in the treatment of wounds(1).
In modern medicine although stronger preparations of alcohol are commonplace to disinfect surfaces and unbroken skin, the high alcohol content makes them cytotoxic to cells and therefore inappropriate for use as wound cleanser. Wine, on the other hand, seems to not be in use anywhere around the world. This could be an exciting area of research; if the active substance in wine could be isolated there could be potential for its use in modern medicine. Also, the efficacy of using wine in less economically developed countries where more expensive treatment options are unavailable could be explored.
Honey
Historically it has not just been alcohol that has been used to sterilise wounds. Honey is another ancient treatment which has been used for millennia. As far back as 1500BC, Ebers papyrus and Edwin Smith papyrus mention honey for burn and wound treatment. This was an essential treatment of the Ancient Egyptians and its use continued into the Greek and later Roman eras. It’s used was advocated by Hippocrates as well as Li Shizhen and Ancient Chinese and Indian medicine. In warfare honey as wound treatment was used by the Roman Army and in a remarkably similar way by the Russian and Chinese soldiers of WW1(33). However, specifically in modern medicine use of honey is seeing a resurgence following numerous papers showing potential antimicrobial properties. In addition, it is biologically safe being non-toxic, non-irritating and non-allergic. With the ever-growing threat of bacterial resistance honey could be an exciting alternative form of wound therapy.
Antimicrobial effects
The antimicrobial effects of honey have been attributed to a multitude of factors. First high sugar content, which produces an osmotic effect leading to bacterial dehydration and eventually cell disruption and lysis. Secondly, low pH (3.2-4.5) causing denaturing of proteins damaging the cell membrane, and finally changes to the size and shape of bacterial cells, compromising their structural integrity. These factors combine to create a hostile environment in which microorganisms struggle to survive. This outcome only increases with honey concentration, with lower concentrations producing a bacteriostatic effect and higher concentrations being an effective bactericidal agent(33).
However, even honey diluted with water demonstrated an antimicrobial effect. This has been explained firstly by the production of Hydrogen peroxide and secondly with ‘non-peroxide activity’ in form of methylglyoxal (MGO). Hydrogen peroxide is a form of broad-spectrum antimicrobial which is created by bees due to the action of the glucose oxidase enzyme(34). Although the concentration of this present in honey is not high enough to become toxic to human cells, it is at sufficient levels to have a proven antimicrobial effect.
Secondly, honey contains methylglyoxal (MGO). This exhibits an antimicrobial effect seemingly due to its ability to interfere with biofilm formation which persist in wounds and impair healing. As the work investigating honey continues more mechanisms are being discovered. Defensin 1 is a honey antimicrobial protein effective against Gram-positive bacteria. Leptosin, correlates with honey potency and may contribute to its antimicrobial activity(33).
Regardless of the mechanism, the effects of honey are beginning to be backed up by clinical trials. Manuka honey was used to treat Pseudomonas aeruginosa resulting in downregulation of flagella-associated proteins, destabilisation of the cell wall and reduced siderophore production (which act to scavenge iron from host cells – increasing bacterial virulence). Honey has even been useful against Methicillin-resistant staphylococcus aureus (MRSA). It has been shown to downregulate mecR1, a regulator of a penicillin-binding protein called mecA, in MRSA treated with Manuka honey which resulted in a reversal of oxacillin resistance(33).
Ant-inflammatory effects
The immunomodulatory effects of honey are again numerous and complex, so much so that further studies investigating them at depth are warranted. However, the current theories are as follows.
Studies have shown that honey can modulate cytokine release, including tumour necrosis factor alpha (TNF-alpha) and interleukin-beta, in such a way that they are stimulated during low inflammation and supressed in the setting of infection. Gelam honey has been shown to inhibit (COX-2) and inducible nitric oxide (NO) synthase expression in rats. Honey has also been shown to have an anti-oxidant effect and effect the production of immune cells. Arabinogalactan proteins of New Zealand Kanuka honey were shown by Gannabathula et al. to modify the immune system by activating monocytes. Further studies supported these results showing that honey elicits chemotaxis of neutrophils and inhibits leukocyte infiltration(33).
There are many clinical trials that support the scientific evidence that honey could be a very useful in the treatment of wounds. Novel uses are also being explored such as the use of honey as a wound treatment option in less economically developed countries(35). {nigera study}
Heavy metal ions
In modern day medicine, dressings impregnated with silver are beginning to see widespread use to fight infection and improve wound healing. However, once again, these are not novel treatments; in fact, the use of heavy metal ions has its roots in Ancient Egyptian medicine and went on to play an important role throughout the Greek and Roman civilisations. Heavy metals are distinct as they all contain partially filled d-orbitals which allow them to accept electrons and form complexes, either by redox reactions or by other biochemical reactions within the cell. Consequently, all the heavy metals exhibit degrees of toxicity towards human cells and bacterial cells with the most powerful antimicrobial following through to the weakest as such; Ag > Hg > Cu > Cd > Cr > Pb > Co > Au > Zn > Fe > Mn > Mo > Sn. The mechanisms by which these heavy metals exert their antimicrobial effect are variable due to the difference in biochemical properties however, it is generally theorised that metal cations interact with ionisable intracellular groups such as carboxylates and phosphates in the lipopolysaccharide layer of gram-negative bacterial cells and peptidoglycan and teichoic acids of gram-positive bacterial cells. Metal cations may get incorporated into the cell membrane, causing loss of fluidity, followed by further transport into the cell cytoplasm to inhibit vital biochemical processes, such as the production of enzymes. The use of heavy metals against microbes must be weighed against their toxicity to human cells and practicality. Taking this factor into account, the most feasible today and throughout history are silver, zinc and copper(36).
Silver (Ag+)
The antimicrobial properties of silver have been well documented. Starting in 1000 B.C. silver was used by Greeks and Romans as a method of storing perishable food and drinks. However, it wasn’t until the 19th century that Ag+ began to be used for medical purposes. But it quickly became an important treatment for a variety of ailments such as venereal diseases, bone and perianal abscesses and the removal of granulation tissue prior to epithelialisation. Although Ag+ fell out of fashion with the introduction of systemic antibiotics, investigation into its use is seeing a resurgence due to antibiotic resistance(36).
Silver displays the most potent antimicrobial action of all the heavy metals with broad spectrum antibacterial, antiviral, antiprotozoal and antifungal activity being achieved at very low concentrations. In combination, Ag+ exerts very little toxicity on human cells, making it a superb candidate for antibacterial dressings(36).
Ag+ expresses its antimicrobial activity in a multitude of ways. Initially it binds to receptors on the microbial cell membrane (especially protein residues including sulphydryl, amino, imidazole, phosphate and carboxyl groups), this results in membrane damage, impairment of solute and electron transport systems and reduced production of vital cell components. Ag+ then moves through the membrane and binds to other essential enzymes in the cytoplasm thus decreasing their activity and restricting the production of metabolites. In addition, in the presence of oxygen, Ag+ produces reactive oxygen species causing oxidation of the cell. The final phase involves binding to microbial DNA interfering with cell replication. Consequently, with a damaged cell wall and arrested biological processes the cell cannot continue and death occurs(36).
Silver salts, in combination with sulphonamide antibacterials, have been used to dress burn wounds since World War II. However, their use has not come without certain side effects and incidents. Although Ag is relatively non-toxic topical exposure of long periods of time can lead to its build-up in the dermis causing an irreversible blue-grey discolouration (argyria). There have also been reports of skin irritation in the form of rashes, stinging and burning. Finally, the most serious problem resulting from topical over-exposure can be electrolyte imbalances such as hyponatraemia or hypochloraemia(36).
Silver dressings are being used in warfare today, and are having a proven impact. In a large-scale literature review of the military uses of silver-nylon dressings David J. Barillo et al advocates their use not only just for burns but also as a universal military wound dressing and goes on to suggest stockpiling of silver dressings in the field of combat(37).
With silver already making an impact on the battlefield and recent advances in controlled delivery systems promising to overcome potential toxicity problems, silver dressings may play a key role in dealing with antibiotic resistant organisms, not only in a military setting but also in civilian medicine(38).
Copper
The Ancient Egyptians without any understanding of infection started the beginnings of antibacterial treatment with copper salts. The green pigment so popular in Ancient Egypt as eye shadow could only have been a few compounds, green stone malachite (copper carbonate), chrysocolla (copper silicate) and the scrapings of marine hardware – verdigris (copper acetate). All these compounds contain copper and consequently it has been shown that they all have varying degrees of bactericidal power. Therefore, worn as eye shadow this would have protected against eye infections by psudomonas aerueus and staphylococcus aureus and even today copper preparations are being used as treatment against trachoma, the blinding infection very common in Egypt(1). In addition, copper was also being applied to wounds and continued to be for thousands of years. Around 1700 Monsieur Jacques Dalibour, surgeon general to the army of louis XIV devised a treatment for “all Manners of Wounds, Cuts, Slashes by Word or Sabre, and Injuries by all Cutting and Bruising Devices”, the principle components of which are Copper and Zinc (1).
Although copper is an essential trace element in many proteins required for life, in high concentrations it can produce cellular damage and an antibacterial effect. Copper contains a partially filled d-orbital which allow it to accept electrons and form complexes, either by redox reactions or by other biochemical reactions within the cell. Because of this reactive nature, when exposed to oxygen copper quickly oxidises to form cuprous oxide (Cu20). This is unstable enough to disrupt organisms and bacteria. Copper, through cuprous oxide, kills germs through multiple different mechanisms when it comes into contact with them. Any of these methods can destroy bacteria, but overlapping mechanisms also helps to prevent resistance.
Firstly in the process of creating copper oxide molecules, copper pulls electrons from the membrane of the bacteria’s cell wall lipids, oxygen of proteins which weakens the bacterial cell wall. Once a gap opens in the cell wall, copper ions flood into the cell where they are highly toxic. Here they bind in excess to enzymes and prevent them from functioning. This disrupts DNA-writing, the production of ATP and causes displacement flooding.
Lastly, oxidizing copper releases free radicals in the forms of hydroxyl radicals. These atoms are extremely reactive due to their unpaired electrons making them highly unstable. These hydroxyl radicals then participate in various reactions harmful to cellular molecules, such as the oxidation of proteins and lipids; this can cause chain reactions in the bacterial cell’s membrane ultimately rupturing it(39).
This antibacterial property is seeing new applications in the field of infection and wound treatment. Although there have been problems associated with the use of silver dressings (irritation and discolouration of the skin) and zinc dressings (low binding affinity leading to an unstable matrix). Copper ions have reported high binding affinity combined with powerful antibacterial properties; therefore copper ions are being trailed in modern dressings(40). Cu2+ alignate hydrogels were successfully prepared using a two-step cross-linking procedure. These caused bacterial disinfection when using just 1.0% w/v of Cu2+ sulphate and the study concluded that they may be a promising candidate as an antibacterial wound dressing(40). Similarly Li J et al, confirmed nanocomposites combined Cu-containing GB nanocoatings with egg shell membrane are a promising biomaterial for wound healing application(41).
There has also been suggestion of using copper as a ’contact killer’ material. Even in solid form as a metal it displays impressive antibacterial powers, so much so that the U.S Enviromental Protection Agency recently registered it as the first solid antimicrobial material. Therefore, it has been proposed that copper be used for highly frequented sites like door handles and bathroom fittings within hospitals(42).
Zinc
Ancient Egyptians were shown to have used zinc in the form of calamine to treat wounds in 1000 B.C. Zinc has been a popular form of treatment ever since, being used in the form of sulphate, oxides and stearates(1). These were applied as powders, emollients and salves, but today zinc is most commonly seen in the form of impregnated bandages. However, like other Heavy Metals, the antibacterial activity of zinc has recently seen an influx of research focusing on novel treatment methods utilising new technologies such as hydrogels and nanoparticles(43).
Zinc nanoparticles specifically are an exciting area of research; multiple trials are showing ZnO nanoparticles to be effective bactericidal agents. Although the mechanism of action of ZnO nanoparticles is not entirely understood, there are theories explaining their action. Nanoparticles in particular are effective against bacteria because they have a larger surface area and can produce higher concentrations. This results in increased ZnO exposure to bacterial cells. Their small size also means ZnO can easily penetrate bacterial membranes and exert its toxic effects on the inside of the cell. Once inside the cell ZnO has been shown to induce measurable membrane leakage, significant morphological changes and substantial increases in expression of oxidative stress associated genes. Therefore, similarly to other heavy metals, the mechanism of action is theorised to include alteration of the permeability of cell membranes and the formation of reactive oxygen species (including hydrogen peroxide and hydroxyl radicals) resulting in an increased oxidation stress on bacteria and ultimately cell death (44, 45).
One current application of the ZnO antibacterial bioactivity is in the food industry where ZnO-NPs are added to packaged foods. Here they interact with pathogens causing bacterial cell death or inhibition. In wound care studies are showing ZnO nanoparticles can create effective antibacterial bandages. Alginate hydrogel/zinc oxide nanoparticles (nZnO) composite bandages were investigated and exhibited controlled degradation profile and faster blood clotting ability when compared to a control. They also showed excellent antimicrobial activity against Escherichia coli, Staphylococcus aureus, Candida albicans, and methicillin resistant S. aureus (MRSA)(46).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




