Literature Review of the Solid Oxide Fuel Cell
Info: 5613 words (22 pages) Example Literature Review
Published: 20th Oct 2021
Tagged: Engineering
Literature review outline
- Solid Oxide Fuel Cell: basic principles; most used materials for anode, cathode and electrolyte
- SOFC systems including a Thermal Energy Storage
- PCM overview and PCMs for high temperature applications
- Thermal Energy Storage based on PCMs
1. Solid Oxide Fuel Cell
1.1 Overview
Solid oxide fuel cells (SOFCs) are the most efficient devices yet invented for conversion of chemical fuels directly into electrical power [1]. They consist of a solid dense ceramic electrolyte placed between two porous electrodes. The fuel is supplied to the anode side, air or oxygen to the cathode. The absence of a liquid electrolyte allows to eliminate one of the source of electrode corrosion and in general all the issues linked to the management of a liquid electrolyte (evaporation, for instance). Furthermore, simpler fabrication techniques can be adopted if the cell is a completely solid-state device. As concerns cell geometry, there are two main designs (Figure 1 and Figure 2) and each has its advantages.
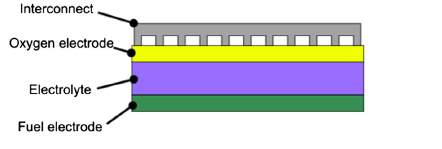
Figure 1. Planar configuration
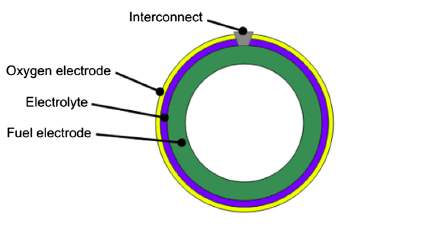
Figure 2. Tubular configuration
The planar cell is more widespread thanks to the higher volumetric power density, shorter electrons paths and cheaper cost [2]. The tubular design, instead, presents a higher thermomechanical stability. Planar design, in turn, can be of two types: electrolyte and electrode supported. In the electrolyte supported cell, the mechanical strength is ensured by the electrolyte. For this reason it is thicker (100-200 μm) than the other cell components. The working temperatures can reach 1000 °C in order to further increase the electrolyte ionic conductivity and so counterbalance the higher ohmic losses. As regards the electrode supported cell, cathode or anode are the thickest component of the cell (200-1000 μm). Therefore, due to a thinner electrolyte, the operating temperatures can be lower (700-800 °C) and the durability of the cell improves.
Each component of the cell must exhibit certain characteristics for the system to function effectively [3]. In the following lines, the requirements of each component are presented:
- electrolyte: it has to be characterised by high ionic conductivity and, at the same time, low electronic conduction at operating temperatures in order to reach good current efficiencies [4]; it must be sufficiently dense to be impermeable to gases, so avoiding H2 and O2 recombination; it should be chemically and mechanically stable at operating conditions; its thermal expansion coefficient has to be comparable to those of electrodes.
- electrodes: high electronic conductivity at operating temperatures; considerable porosity and adequate pores size to ensure an enough number of reaction sites (TPB) and simultaneously to promote products removal; optimised chemical composition and morphology to allow efficient transport of electrons and ions. In more detail: cathode composition must be such as to catalyse oxygen reduction and guarantee chemical stability in reducing condition; anode microstructure instead has to promote fuel oxidation and warrant chemical stability in oxidizing environment.
When an oxygen molecule comes into contact with the cathode/electrolyte interface, it reacts with the free electrons at the cathode, forming O2- ions. These oxide ions, which represent the charge carriers, are then transferred from the cathode through the electrolyte to the anode [5]. Here, they interact with hydrogen to produce water, releasing electrons to an external circuit. The half-cell reactions occurring at anode and cathode, and the total reaction in an SOFC operated with hydrogen, are:
Anode
H2+O2–→H2O+2e–
(1)
Cathode
12O2+2e–→O2–
(2)
Overall reaction
H2+12O2→H2O
(3)
The electrochemical reaction takes place at the triple phase boundary, that is the point of contact of electronic conductor, oxygen ion conductor and gas [6]. For the above-mentioned redox couple, the cell potential is 1.23 V under standard conditions and considering a completely reversible reaction. However, under the actual operating conditions, cell potential decreases up to 0.7 – 0.8 V due to the irreversibilities [7]. So, in order to reach more practical voltage levels, many cells are assembled in series (or in parallel) to form a stack. The connection element is named interconnector. It collects the current and guides the gas flow.
The use of a solid ceramic electrolyte (such as YSZ) results in very high operating temperatures, usually ranging from 800 °C and 1000 °C. At lower temperatures, in fact, the oxygen ion conductivity of the electrolyte and its surface exchange kinetics would not be sufficiently good to make the fuel cell practical. Besides being fundamental for an efficient operation of the cell, high working temperatures are also responsible for the main benefits that make SOFC technology so attractive. Below, the key advantages are listed:
- Hydrogen is not the only suitable fuel. In fact, thanks to the high temperature, hydrocarbon fluids can be reacted directly on the anode, where the so-called internal reforming process occurs. It consists in the conversion of conventional hydrocarbon fuels to syngas containing hydrogen.
- The possibility to use less precious catalysts. Kinetics is already promoted by the high operating temperatures.
- High exergy value of the exhaust heat, which can be used for CHP applications. The heat generated by the SOFC is due to the exothermicity of the overall reaction, ohmic losses, electrode overpotentials and in general all the irreversibilities that occur within the cell.
- High energy conversion efficiencies. The net electric efficiency is around 50% for a stand-alone SOFC, thanks to the absence of Carnot limitations. If the produced heat is not vented but represents a desirable product, then system efficiencies above 80% can be reached [6].
- Design flexibility thanks to the utilisation of a solid electrolyte
- Variety of applications, from small scale (W) to Megawatts
However, the high temperatures are also cause of many drawbacks. Below, the main ones are summarised.
- Long start-up time
- Difficulties in obtaining a gas-tight device
- Durability issues (high degradation rates), mainly caused by thermomechanical incompatibilities between cell components and chemical instability of the involved materials
- High cost of the materials
In the light of the above, a considerable scientific effort is currently in place to try to reduce the working temperatures without, however, affecting performance in a significant way.
Therefore, it can be said that the share of SOFC energy production in the world energy mix of the next future will strongly depend on the progress in materials science. In fact, the development of cheaper and more performing materials, especially at lower temperatures, will allow SOFCs to compete successfully with combustion devices, also without subsidies and incentives.
Considering, as just highlighted, the key role of materials in SOFC technology and its future development, it is needful to devote the following lines to give an overview on the most common and promising SOFC materials.
1.2 Electrolyte materials
Yttria Stabilised Zirconia (YSZ) iscurrently the most used electrolyte material consists of ZrO2 doped with Y2O3 [8]. This type of ionic (oxygen) conductor belongs to the so-called fluorite structure oxides. It is characterised by sufficiently high ionic conductivity and notable chemical stability in the range of temperatures between 800 and 1000 °C. In most cases zirconia is doped with 8 mol% Y2O3 (8YSZ) because this percentage allows obtaining a good compromise between ionic conductivity and robustness. However, it is quite expensive and presents significant grain size after calcination [9]. In addition, its ionic conductivity at lower temperatures is very poor [10] and then it would not be a good candidate in view of reducing the operating temperatures.
Scandia Stabilized Zirconia (ScSZ), as YSZ,is a fluorite structure oxide. At 850 °C, the ionic conductivity of 11 mol.% Sc2O3 doped ZrO2 is roughly 1.5 times higher than that of 8YSZ [11]. This discrepancy grows as temperature reduces, so operating temperature can be lowered. Unfortunately, its application is slowed by the high cost of the raw material and by the remarkable incidence of the aging process on its ionic conductivity [12].
Cerium oxide (CeO2) is another important electrolyte material that is characterised by a fluorite-type structure. Its microstructure and chemical properties are very similar to those of zirconia. Doping process with suitable divalent or trivalent cations (Y3+, Ca2+, Sm3+, Gd3+) allows increasing ceria ionic conductivity, which becomes higher than that of YSZ. For instance, at 800 °C the ionic conductivity values of Gd2O3-doped CeO2 (GDC) and Sm2O3-doped CeO2 (SDC) are five times higher than that of YSZ [13]. This difference is more pronounced at lower temperatures and so ceria-based materials are potential candidates for IT-SOFC electrolytes. However, the main issue that limits the use of ceria is the reduction of Ce4+ to Ce3+ at low O2 partial pressures and temperatures above 600 °C [14]. Reduction phenomenon determines electrons transfer through the electrolyte, with resulting decrease of current efficiency. A possible solution is represented by ceria-based composites (CBCs): they are obtained by adding a very thin layer (an YSZ one, for example) to the ceria structure in order to prevent electronic transfer. Therefore, this layer acts as an actual barrier for the electrons. Better performance has been achieved using GDC/YSZ and SDC/YSZ instead of only YSZ, GDC or SDC [15].
The high temperature cubic-form of bismuth oxide (δ-Bi2O3)is one of the two stable crystallographic polymorphs of bismuth oxide and it is known as the best oxide ion conductor among the fluorite structure based oxides [16]. However, δ-Bi2O3 is stable only between 730 and 804 °C. In fact, out of this range, the cubic form becomes unstable and phase transition occurs, leading to a huge drop in ionic conductivity [17]. In order to avoid phase transformation, δ-Bi2O3 can be stabilised to lower temperatures by adding proper dopant cations. Unfortunately, the stabilisation process is not sufficient to significantly enlarge the practical applicability of bismuth oxide as electrolyte material. Stabilised δ-Bi2O3 tends indeed to decompose into Bi metal under reducing conditions due to its low decomposition potential. Bilayered electrolytes have been used with the aim of solving this issue: the layer facing the anode is made of a more stable electrolyte material (doped zirconia for instance), while doped Bi2O3 is used to realize the layer facing the cathode.
Lanthanum Strontium Gallium Magnesium oxide (LSGM)is a perovskite-type oxide (chemical formula ABO3), obtained by adding Sr2+ and Mg2+ as acceptor dopant cations to LaGaO3. The doping process consists in the partial substitution of La3+ and Ga3+ ions with the low-valent alkaline ions Sr2+ and Mg2+. The effect is then the increase of the amount of oxygen vacancies in the structure, which results in an improvement of the ionic conductivity. In the last years, LSGM has attracted much attention because of its high ionic conductivity in an intermediate-temperature range [18], establishing itself as one of the most promising electrolyte materials for IT-SOFCs. Experimental studies confirm that systems using LSGM as electrolyte present lower Area Specific Resistance than YSZ at intermediate temperatures [2]. The extensive use of LSGM is however delayed by the following problems: Ga+3 can reduce to Ga+2 in reducing environments, which results in the occurrence of the short-circuiting phenomenon; incompatibility issues between LaGaO3 and metallic nickel contained in the cermet anode [16]; tendency of gallium oxide to volatilise; high cost of gallium compounds.
In the field of SOFCs operating at intermediate temperatures, proton conducting materials as electrolytes constitute a very interesting alternative to oxygen ion conductors due to their low activation energy and good ionic conductivity [19]. In proton conducting SOFCs, the electrochemical reactions at the electrodes are different from (1) and (2). In particular, they are:
Anode
H2→2H++2e–
(3)
Cathode
2H++O2+2e–→H2O
(4)
These materials, having a perovskite crystal structure, develop proton conductivity when they interact with water vapour or hydrogen. In other words, proton conductivity is the result of the combination between water molecules (or hydrogen), oxygen vacancy and lattice oxygen [20]. In order to increase proton conductivity, the number of proton defects is enhanced by doping the B site with proper trivalent cations (Gb, Yb, Sm or Y) [2]. One of the main advantages of proton-conducting SOFCs can be inferred by analysing reactions (3) and (4): since water is produced at the cathode side, there is no need for a fuel recirculation system [21]. BaCeO3 and BaZrO3 based materials are currently intensively studied as they are very promising as proton conductor electrolytes. In a more detail, BaCeO3 based materials are characterised by an excellent proton conductivity but low chemical stability in presence of CO2 and H2O [22]. An improvement of their chemical stability can be achieved by partially replacing CeO2 with appropriate oxides [23]. However, the side effect is the reduction of proton conductivity [20]. BaZrO3, for its part, presents the opposite behaviour: high chemical stability also if the material is exposed to CO2 or H2O but low proton conductivity [24]. Once again, the doping process can come to help. For example, Yushi et al [25] managed to increase BaZrO3 conductivity by means of Y-doping.
1.3 Anode materials
Ni-based cermets are the most common choice for SOFC anodes [26]. Nickel is able to catalyse hydrogen fuel oxidation but has almost no ionic conductivity. As a consequence, reaction zone is limited only to the anode-electrolyte interface. Therefore, to maximise TPB length and extend the active zone in the anode, Ni has to be mixed with proper ion conductor materials. Moreover, such materials provide structural support for the anode and thermal expansion match with the electrolyte [27]. The most used Ni-based cermet is Ni/YSZ thanks to its high conductivity and good compatibility with the other components of the cell [28]. Despite these advantages, the extent of Ni/YSZ weaknesses is such that this cermet is not the adequate choice to make SOFC anode sufficiently reliable, especially for direct oxidation of hydrocarbon fuels. In fact, Ni is a good catalyst not only for hydrogen fuel oxidation but also for coke formation. In addition, it is also affected by sulphur poisoning [29]. Both the above-mentioned phenomena cause the deactivation of the nickel. In the light of the above, researches have modified the chemical composition of Ni-YSZ cermet with the aim of solving, or at least mitigating, coke formation and/or sulphur poisoning issues. This goal is achieved by doping process, which consists in adding other materials to Ni/YSZ. A very promising result has been obtained by Wu and his co-workers [30]. They doped a Ni/YSZ anode with Ag, which is introduced in the anode structure by means of electroless silver plating method. Exposed to a hydrocarbon fuel, the modified Nickel zirconia anode experienced an improvement of its electrochemical performance, thanks also to a significant mitigation of the carbon deposition phenomenon. As regards instead sulphur contamination problem, Choi et al. [31] demonstrated that the addition of niobium oxide makes the Ni/YSZ anode stable even if the fuel contained traces of H2S (50 ppm). Another Ni-based cermet that has been intensively studied as SOFC anode is Ni-SDC. The reasons behind the interest for this material are mainly three: its high power density, the ability of ceria to absorb H2S at high temperature [32] and to be an excellent electro-catalyst for methane oxidation [33]. As already seen for Ni/YSZ cermet, also Ni-SDC composition has been modified in order to try to improve its electrochemical performance and soften coke formation phenomenon. Qu et al. [34] analysed the effect of the addition of some oxides (such as SrO, MgO, BaO, CaO) into Ni-SDC anode. The best result has been obtained doping the anode with CaO oxide. The modified Ni-SDC anode showed reduced carbon deposition rate and a peak of power density at 700 °C. This latter achievement makes Ni-SDC-Ca an interesting candidate for IT-SOFC anodes.
Another option is represented by perovskite materials. Since they are mixed ionic and electronic conductors, electrochemical reactions can occur not only at TPB sites but at any position of interfaces between the anode and gas phase [13]. Moreover, they proved to be less prone to sulphur poisoning than Ni-based anodes [35], compatible with the most used electrolytes and relatively cheap [26]. Among perovskites materials, doped strontium titanate composites (doped SrTiO3) seem very promising as SOFC anode materials. For instance, in the study conducted by Miller and Irvine [36], these materials were chemically stable under fuels containing carbon and sulphur. Ruiz-Morales et al. showed instead that they are eligible for the direct oxidation of methane at high temperatures [37]. A good tolerance to sulphur poisoning and coke formation is also showed by lanthanum chromium ferrite perovskites [38]. Despite the good sulphur resistance of the above-mentioned perovskites, they are also characterised by lower electrical conductivities than those of Ni-based cermets. As a consequence, some researches have decided to focus their attention to the double perovskites materials. Among them, one of the most appealing is Sr2MgMoO6-δ, which has been studied by [39]. Besides a notable tolerance to carbon and sulphur poisoning, it also showed a power density more in line with that of Ni/YSZ anodes.
1.4 Cathode materials
LSM-YSZ is the most common type of cathode for high temperature SOFCs [13]. LSM, which stands for Lanthanum Strontium Manganite, is synthesise by doping A-sites of LaMnO3 oxide with Sr. The choice of mixing LSM powders with those of yttria-stabilized zirconia derives from the need to extend the active area to the whole cathode. In fact, without the addition of an ionic conductor, the oxygen reduction on LSM would be limited to TPB sites. LSM-YSZ composite electrodes show high long-term stability, a thermal expansion coefficient comparable to YSZ one and good electrochemical properties [40]. A more performing alternative consists in infiltration of GDC particles in pure LSM. The infiltrated particles cause the following positive effects: decrease of electrode polarization resistance, more stability and lower tendency to delamination [41]. However, LSM has two main drawbacks: at temperatures higher than 1100 °C it can reacts with the YSZ of the electrolyte; it cannot be used as cathode in IT-SOFCs because of its low catalytic activity and high polarisation resistance when temperatures are below 800 °C [42]. Therefore, great efforts are being made in order to produce new cathode materials with better performance at lower temperatures. For example, the addition of Sc ions into B-site of LSM perovskite allows obtaining a material (LSMSc) that performs better than LSM, especially at lower temperatures [43].
Another promising LaSr perovskites is LSCF [44]. It is obtained from lanthanum cobaltite oxide (LaCoO3) by the doping of A-site with Sr and the doping of B-site with Fe. It is then impregnated onto GDC, which acts as sintering inhibitor, and LSCF-GDC is produced. Finally, Fe-doped LaNiO3 is added to LSCF-GDC to avoid the coarsening of LSCF particles.
Double perovskites with LnBaCo2O5+δ composition (where Ln can be La, Pr, Nd,…) have received particular attention recently. As many perovskites that contain Co in B-site, also this class of materials shows high electrochemical performance [45]. In particular they are characterised by high catalytic activity for oxygen reduction and notable electronic and ionic conductivities. Conversely, their thermal expansion coefficient is too high compared with those of the most common electrolytes and so structural stability issues may arise. However, proper dopants can decrease their thermal expansion coefficient, as well as further improve their performance. Moreover, BaCo2O5+δ double perovskites have shown a good compatibility with doped ceria-based electrolytes, as the studies of Fu et al. [46] and Xia et al. [47] prove. In more details, Fu and his colleagues analysed a LnBaCo2O5+δ double perovskite with Ln = Pr, doped on the A-site with Ca ions. Xia and co-workers studied instead a LnBaCo2O5+δ double perovskite with Ln = Sm and doped on the Co-site with Ni ions. Thanks to their superior electrochemical performance, LnBaCo2O5+δ double perovskites have been also considered for IT-SOFC cathodes. Choi et al. [48] developed a novel compound, adding GDC in different wt% ratio to LaBa0.5Sr0.5Co2O5+δ (LSBCO). LSBCO is a LnBaCo2O5+δ double perovskite with Ln = La in which the 50% of the Ba ions have been replaced by Sr ions. Choi et al. found out that LSBCO with 40 wt% of GDC reached the maximum power density at 650 °C, proposing itself as an appealing material for IT-SOFC.
Finally, another class of materials that could be applied in the context of IT-SOFC cathodes is represented by the so-called lanthanum nickelates La2-xNiO4+δ, where x varies between 0.01 and 0.05.
References
[1] K. Kendall, Introduction to SOFCs, 2nd ed., no. 1. Elsevier Ltd., 2016.
[2] S. Y. Gómez and D. Hotza, “Current developments in reversible solid oxide fuel cells,” Renew. Sustain. Energy Rev., vol. 61, pp. 155–174, 2016.
[3] S. J. Cooper and N. P. Brandon, An Introduction to Solid Oxide Fuel Cell Materials , Technology and Applications. Elsevier Ltd, 2017.
[4] M. Ni, M. K. . Leung, and D. Y. C. Leung, “Technological development of hydrogen production by solid oxide electrolyzer cell ( SOEC ),” Int. J. Hydrogen Energy, vol. 33, pp. 2337–2354, 2008.
[5] Y. N. Kim, J. Kim, and A. Manthiram, “Characterization of ( Y 1-x Ca x ) BaCo 4-y Zn y O 7 as cathodes for intermediate temperature solid oxide fuel cells,” Int. J. Hydrogen Energy, vol. 6, pp. 2–10, 2011.
[6] M. Irshad, K. Siraj, R. Raza, A. Ali, P. Tiwari, B. Zhu, A. Rafique, A. Ali, M. K. Ullah, and A. Usman, “A Brief Description of High Temperature Solid Oxide Fuel Cell ’ s Operation , Materials , Design , Fabrication Technologies and Performance,” Appl. Sci., 2016.
[7] Paul Breeze, “The Solid Oxide Fuel Cell,” in Fuel Cells, 1st ed., Paul Breeze, Ed. Academic Press, 2017, pp. 63–73.
[8] N. Q. Minh, J. Mizusaki, and S. C. Singhal, “Advances in Solid Oxide Fuel Cells: Review of Progress through Three Decades of the International Symposia on Solid Oxide Fuel Cells N. Q. Minh,” ECS Trans., vol. 78, no. 1, pp. 63–73, 2017.
[9] M. De Souza and F. Sartori, “Novel materials for solid oxide fuel cell technologies : A literature review,” Int. J. Hydrogen Energy, vol. 2, 2017.
[10] J. Larminie and A. Dicks, Fuel Cell Systems Explained, 2nd editio. John Wiley & Sons, 2003.
[11] S. P. S. Badwal and F. T. Ciacchi, “Oxygen-Ion Conducting Electrolyte Materials for Solid Oxide Fuel Cells,” Ionics (Kiel)., vol. 6, 2000.
[12] D. Lee, W. S. Kim, S. H. Choi, J. Kim, H. Lee, and J. Lee, “Characterization of ZrO 2 co-doped with Sc 2 O 3 and CeO 2 electrolyte for the application of intermediate temperature SOFCs,” Solid State Ionics, vol. 176, pp. 33–39, 2005.
[13] P. K. Shen, C.-Y. Wang, S. P. Jiang, X. Sun, and J. Zhang, “Advanced Materials for High-Temperature Solid Oxide Fuel Cells (SOFCs),” in Electrochemical Energy: Advanced Materials and Technologies, 1st ed., CRC Press, 2016, pp. 269–297.
[14] Z. Shao and M. O. Tadé, “Electrolyte Materials for IT-SOFCs,” in Intermediate-Temperature Solid Oxide Fuel Cells, Green Chemistry and Sustainable Technology, 2016, pp. 15–57.
[15] P. Kim-Lohsoontorn, N. Laosiripojana, and J. Bae, “Performance of solid oxide electrolysis cell having bi-layered electrolyte during steam electrolysis and carbon dioxide electrolysis,” Curr. Appl. Phys., vol. 11, no. 1 SUPPL., pp. S223–S228, 2011.
[16] N. Mahato, A. Banerjee, A. Gupta, S. Omar, and K. Balani, “Progress in material selection for solid oxide fuel cell technology : A review,” Prog. Mater. Sci., vol. 72, pp. 141–337, 2015.
[17] S. Boyapati, E. D. Wachsman, and N. Jiang, “Effect of oxygen sublattice ordering on interstitial transport mechanism and conductivity activation energies in phase-stabilized cubic bismuth oxides,” Solid State Ionics, vol. 140, no. 1–2, pp. 149–160, 2001.
[18] E. D. Wachsman and K. T. Lee, “Lowering the Temperature of Solid Oxide Fuel Cells,” Science (80-. )., vol. 334, no. 6058, pp. 935–939, 2011.
[19] L. Bi, E. H. Da’as, and S. P. Shafi, “Proton-conducting solid oxide fuel cell (SOFC) with Y-doped BaZrO3 electrolyte,” Electrochem. commun., vol. 80, pp. 20–23, 2017.
[20] F. Wang, Y. Lyu, D. Chu, Z. Jin, and G. Zhang, “The electrolyte materials for SOFCs of low- intermediate temperature : review,” Mater. Sci. Technol., vol. 836, 2019.
[21] N. Lina, R. Mohd, A. Abdul, and A. Azim, “Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell,” Ceram. Int., vol. 45, no. 6, pp. 6605–6615, 2019.
[22] L. Bi, S. Boulfrad, and E. Traversa, “Reversible solid oxide fuel cells (R-SOFCs) with chemically stable proton-conducting oxides,” Solid State Ionics, vol. 275, pp. 101–105, 2015.
[23] D. Medveded, A. Murashkina, P. Tsiakaras, E. Y. Pikalova, A. Demin, and A. Podias, “BaCeO3: Materials Development, Properties and Application,” Prog. Mater. Sci., pp. 72–129, 2014.
[24] S. Hossain, A. M. Abdalla, J. H. Jamain, Siti Noorazean Binti Zaini, and A. K. Azad, “A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells,” Renew. Sustain. Energy Rev., vol. 79, pp. 750–764, 2017.
[25] D. Yushi, L. I. Ying, D. Wenzhuo, H. Wenlong, and W. Changzhen, “Variation of optimum yttrium doping concentrations of perovskite type proton conductors BaZr1exYxO3ea(0_x_0.3) with temperature,” J. Rare Earths, vol. 31, no. 10, pp. 1017–1022, 2013.
[26] W. Wang, J. Qu, S. Barros, and Z. Shao, “Recent Advances in the Development of Anode Materials for Solid Oxide Fuel Cells Utilizing Liquid Oxygenated Hydrocarbon Fuels : A Mini Review,” Energy Technol., pp. 33–44, 2019.
[27] S. C. Singhal and K. Kendall, “Anodes,” in High-temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications, 1st ed., S.C. Singhal and K. Kendall, Eds. 2003.
[28] X. Song, X. Dong, M. Li, and H. Wang, “Effects of adding alumina to the nickel-zirconia anode materials for solid oxide fuel cells and a two-step sintering method for half-cells,” J. Power Sources, vol. 308, pp. 58–64, 2016.
[29] W. Wang, C. Su, Y. Wu, R. Ran, and Z. Shao, “Progress in Solid Oxide Fuel Cells with Nickel-Based Anodes Operating on Methane and Related Fuels,” Chem. Rev., vol. 113, no. 10, pp. 8104–8151, 2013.
[30] X. Wu, Y. Tian, J. Zhang, W. Zuo, X. Kong, J. Wang, K. Sun, and X. Zhou, “Enhanced electrochemical performance and carbon anti-coking ability of solid oxide fuel cells with silver modified nickel-yttrium stabilized zirconia anode by electroless plating,” J. Power Sources, vol. 301, pp. 143–150, 2016.
[31] S. Choi, J. Wang, Z. Cheng, and M. Liu, “Surface Modification of Ni-YSZ Using Niobium Oxide for Sulfur-Tolerant Anodes in Solid Oxide Fuel Cells,” J. Electrochem. Soc., vol. 155, pp. B449–B454, 2008.
[32] H. Chen, F. Wang, W. Wang, D. Chen, S.-D. Li, and Z. Shao, “H2S poisoning effect and ways to improve sulfur tolerance of nickel cermet anodes operating on carbonaceous fuels,” Appl. Energy, vol. 179, pp. 765–777, 2016.
[33] G. Kaur, “SOFC Technology: Its Working and Components,” in Solid Oxide Fuel Cell Components, Springer, 2016, pp. 79–122.
[34] J. Qu, W. Wang, Y. Chen, X. Deng, and Z. Shao, “Stable direct-methane solid oxide fuel cells with calcium-oxide-modified nickel-based anodes operating at reduced temperatures,” Appl. Energy, vol. 164, pp. 563–571, 2016.
[35] R. Mukundan, E. L. Brosha, and F. H. Garzon, “Sulfur Tolerant Anodes for SOFCs,” Electrochem. Solid-State Lett., vol. 7, no. 1, pp. A5–A7, 2004.
[36] D. N. Miller and J. T. S. Irvine, “B-site doping of lanthanum strontium titanate for solid oxide fuel cell anodes,” J. Power Sources, vol. 196, no. 17, pp. 7323–7327, 2011.
[37] J. C. Ruiz-Morales, J. Canales-Vázquez, C. Savaniu, D. Marrero-López, W. Zhou, and J. T. S. Irvine, “Disruption of extended defects in solid oxide fuel cell anodes for methane oxidation,” Nature, vol. 439, pp. 568–571, 2006.
[38] J. M. Haag, D. M. Bierschenk, S. A. Barnett, and K. R. Poeppelmeier, “Structural, chemical, and electrochemical characteristics of LaSr2Fe2CrO9-δ-based solid oxide fuel cell anodes,” Solid State Ionics, vol. 212, pp. 1–5, 2012.
[39] D. Marrero-López, J. Peña-Martínez, J. C. Ruiz-Morales, M. Gabás, P. Núnez, M. A. . Aranda, and J. R. Ramos-Barrado, “Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6 − δ as SOFC anode,” Solid State Ionics, vol. 180, no. 40, pp. 1672–1682, 2010.
[40] A. Nechache, M. Cassir, and A. Ringuedè, “Solid oxide electrolysis cell analysis by means of electrochemical impedance spectroscopy: A review,” J. Power Sources, vol. 258, pp. 164–181, 2014.
[41] K. Chen and S. P. Jiang, “Performance and structural stability of Gd0.2Ce0.8O1.9 infiltrated La0.8Sr0.2MnO3 nano-structured oxygen electrodes of solid oxide electrolysis cells,” Int. J. Hydrogen Energy, vol. 39, no. 20, pp. 10349–10358, 2014.
[42] N. A. Baharuddin, A. Muchtar, and M. R. Somalu, “Short review on cobalt-free cathodes for solid oxide fuel cells,” Int. J. Hydrogen Energy, vol. 42, no. 14, pp. 9149–9155, 2017.
[43] X. Yue, A. Yan, M. Zhang, L. Liu, Y. Dong, and M. Cheng, “Investigation on scandium-doped manganate La0.8Sr0.2Mn1−xScxO3−δ cathode for intermediate temperaturesolid oxide fuel cells,” J. Power Sources, vol. 185, no. 2, pp. 691–697, 2008.
[44] Y. Liu, F. Wang, B. Chi, J. Pu, L. Jian, and S. P. Jiang, “A stability study of impregnated LSCF–GDC composite cathodes of solid oxide fuel cells,” J. Alloys Compd., vol. 578, pp. 37–43, 2013.
[45] W. Zhou, Z. Shao, C. Kwak, and H. J. Park, “Advanced Cathodes for Solid Oxide Fuel Cells,” in Materials for High-Temperature Fuel Cells, 1st ed., S. P. Jiang and Y. Yan, Eds. Wiley-VCH, 2013, pp. 49–95.
[46] D. Fu, F. Jin, and T. He, “A-site calcium-doped Pr1−xCaxBaCo2O5+δ double perovskites as cathodes for intermediate-temperature solid oxide fuel cells,” J. Power Sources, vol. 313, no. 134–141, 2016.
[47] L.-N. Xia, Z.-P. He, X. W. Huang, and Y. Yu, “Synthesis and properties of SmBaCo2−xNixO5+δ perovskite oxide for IT-SOFC cathodes,” Ceram. Int., vol. 42, no. 1, pp. 1272–1280, 2016.
[48] S. Choi, S. Park, J. Kim, T.-H. Lim, J. Shin, and G. Kim, “Electrochemical properties of an ordered perovskite LaBaCo2O5 + δ–Ce0.9Gd0.1O2 − δ composite cathode with strontium doping for intermediate-temperature solid oxide fuel cells,” Electrochem. commun., vol. 34, pp. 5–8, 2013.
This literature review has been written by a student and is published as an example. See our guide on How to Write a Dissertation Literature Review for guidance on writing your own.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Engineering"
Engineering is the application of scientific principles and mathematics to designing and building of structures, such as bridges or buildings, roads, machines etc. and includes a range of specialised fields.
Related Articles
DMCA / Removal Request
If you are the original writer of this literature review and no longer wish to have your work published on the UKDiss.com website then please:




