Dissertation: Identifying the most effective medications for the self-management of acute asthma
See for yourself why we're the world's leading academic writing company. One of our expert writers has created this bespoke sample medicine dissertation that shows the incredible quality that's guaranteed with every piece of work ordered. Secure your academic success and place an order today or view our dissertation writing service.
This sample is part of a set:
- Medicine Dissertation Topic with Titles
- Medicine Dissertation Proposal
- Full Education Dissertation
Table of Contents
- 1. Background
- 2. Methodology
- 3. Results
- 4. Discussion
- 5. Conclusion and Future Recommendations
- References
- Appendices
1. Background
1.1 Definition
Asthma is defined by the National Institute of Health as a chronic respiratory disease of the airways, that is characterised by airway inflammation and hyper-responsiveness, which causes variable airflow obstruction and upon provocation, intermittent acute exacerbations, with sudden and progressive increases in dyspnoea (Mims, 2015). Notably, asthma has been increasingly recognised as a heterogenous disorder with varying clinical and molecular phenotypes (Ray et al., 2015). The phenotypes are generally classified as being either atopic (allergic), or non-atopic, with the former being the most common and early-onset presentations, whilst the latter includes late-onset eosinophilic and obesity-related phenotypes, which are generally associated with more severe and treatment-resistant disease (Wenzel, 2012). Moreover, severe asthma accounts for 5-10% of all cases, and may comprise varying subtypes, such as ‘brittle asthma’, which refers to patients with unstable asthma despite high doses of corticosteroids (Papiris et al., 2002).
If you need assistance with writing your dissertation, our professional Dissertation Writing Service is here to help!
Find out more1.2 Epidemiology
Asthma is one of the most prevalent chronic diseases, estimated to affect more than 300 million persons, worldwide, and has thus, been described as a major public health problem by the World Health Organisation (2018). The highest prevalence rates of approximately 10% are observed among developed western countries, whilst the prevalence among countries with the lowest incomes is less than 1% (Holgate et al., 2015). However, the adoption of western lifestyles among low- and intermediate-income countries has seen rates of asthma increase substantially over the past few decades, see figure 1, appendices (Bousquet et al., 2005). Asthma rates have followed an increasing trend with other immune-mediated conditions, such as type 1 diabetes mellitus and inflammatory bowel disease, with research correlating such increases with maternal and foetal nutritional factors but this has remained inconclusive (Jackson et al., 2014). Notably, asthma is the commonest chronic disease in children, with 80% of those diagnosed by the age of 6 years being attributable to the atopic allergic phenotype, inferring a genetic predisposition for immunological hypersensitivity (Yunginger et al., 1992). Furthermore, the clinical course of asthma is variable with patients experiencing permanent remission, intermittent remission or late onset asthma, which have been suggested to be related to hormonal variances in puberty and young adulthood, as well as other environmental and occupational stimuli (Butland and Strachan, 2007; Dumas et al., 2014).
The Global Asthma Network (2018) have reported that asthma is associated with significant morbidity and mortality, being in the top 20 leading causes of years lived with disability and accounting for more than 1,000 deaths per day, worldwide. The age-adjusted mortality rate for asthma is estimated to be 150 per 100,000 persons, which is equivalent to a loss of almost 11 million life-years. In the UK asthma affects more than 4.3 million adults and 1.1 million children, of whom, a third observe functional impairment and reduced quality of life, which has resulted in millions of missed days from education and productivity at work (Lipstein et al., 2009; Mukherjee et al., 2016). It is reported that more than 180 persons with potentially life-threatening airflow obstruction are admitted to acute hospitals in the UK every day, and there has consistently been approximately 1,000 deaths per annum over the past few decades (Mukherjee et al., 2016). On a more international scale, asthma accounts for approximately 0.6% of all hospital admissions, but this can vary by as much as 10-fold between different nations (Global Asthma Network, 2018). Due to the high prevalence, asthma is responsible for high socioeconomic costs, with a high demand for primary and secondary care services, medicines, and losses in productivity, due to disease-related absence or disability (Nunes et al., 2017). The economic costs related to asthma have been estimated by Sadatsafavi et al. (2014), who found that pharmacotherapy accounted for the majority of costs, with the annual cost per person varying between $150 and 3000, which was equivalent to totals of $24 billion in Europe and £5 billion in the UK (Sadatsafavi et al., 2014).
1.3 Morbidity and Mortality
Whilst there have been advances in the understanding of asthma and increasing availability of pharmacological treatments, the trends in morbidity and mortality have shown little improvement (D'Amato et al., 2016). It is reported that the majority of all asthma-related hospitalisations and deaths could be prevented with improved adoption and optimisation of self-management strategies, including the use of personalised action plans and maintaining adherence to treatment (Torjesen, 2014). In a UK-based confidential enquiry into nearly 200 asthma deaths that occurred over the course of one year, approximately two thirds of deaths were attributable to suboptimal medication management and were thus, considered preventable. Suboptimal management in this report mostly referred to under-treatment of asthma, where poor prescribing practices resulted in patients receiving medication that was not congruent with asthma guidelines (Levy, 2015). Furthermore, and unexpectedly almost half of all asthma deaths occurred in patients with mild or moderate airflow restriction, rather than those with severe disease, which may infer that medical and patient level complacency in prescribing practices and adherence to medication was partly responsible for the poor outcomes (D'Amato et al., 2016; Levy et al., 2014).
Acute exacerbations of airway obstruction are the event the precedes and leads to morbidity and mortality in asthma (D'Amato et al., 2016). Exacerbations of asthma represent a medical emergency and necessitate immediate pharmacological intervention to reverse airflow limitation and hypoxaemia, such to avoid the need for hospital admission and its associated sequalae (Fuhlbrigge et al., 2012). The occurrence and frequency of exacerbations can be limited by long-term stringent adherence to asthma medication and indeed, poor adherence has been shown to result in higher exacerbation relapse rates and failure to achieve individual treatment targets (Global Initiative for Asthma, 2018).
1.4 Self-Management
The self-management of asthma has long been recommended by official guidelines for several decades, whereby the development of individualised action plans can assist service users in optimising chronic disease management, having an aid memoire and being more prepared for treating acute exacerbations (British Thoracic Society, 2016). Asthma UK (2016) has developed a universal personalised asthma action plan template for national use, which contains information regarding the use of preventer and reliever inhalers during exacerbations. However, whilst rescue oral steroids are mentioned this does not form a routine aspect of the urgent self-management of asthma exacerbations. An example of the form can be viewed in figure 2, appendices. Current treatment recommendations are guided by the severity of exacerbation, which is predominantly dictated by the degree of reduction in peak expiratory flow rate that can be easily self-monitored at home (Alangari, 2014). In patients with mild exacerbations (peak expiratory flow >70% of baseline), the use of inhaled beta-agonists, such as salbutamol, are normally sufficient to achieve resolution and such patients only rarely require systemic corticosteroids. In contrast, patients with more moderate exacerbations (peak expiratory flow 40-69% of baseline) often require the addition of nebulisation to assist the delivery of beta-agonists but should also receive systemic corticosteroids to achieve faster resolution of symptoms and reduce relapse rates (Camargo et al., 2003; Rowe et al., 2004). Patients with severe exacerbations (peak expiratory flow <40% of baseline) should receive aggressive therapy with high dose nebulised beta-agonists and other bronchodilators, such as ipratropium, as well as systemic corticosteroids and consideration of additional drugs, such as magnesium, montelukast and aminophylline (Alangari, 2014).
Given that patients with moderate and severe exacerbations and a small proportion of those with mild exacerbations will require systemic corticosteroids, the opportunity to self-administer the drug in the community setting may help to improve asthma outcomes (Ramsahai and Wark, 2018). Indeed, oral systemic corticosteroids could be more widely utilised among patients’ acute asthma action plans to achieve this goal, but the evidence supporting its efficacy in this regard will require future primary research. The importance of personalised action plans in asthma have been reflected in the UK independent review of asthma related deaths, where suboptimal and untimely responses to worsening asthma symptoms resulted in preventable mortality and notably, less than 25% of patients had been provided with self-management education (Levy et al., 2014). Whilst optimising self-management has been advocated as an essential component of improving outcomes associated with chronic disease, reports suggest that only a small proportion of asthma patients have been provided with a personalised action plan (Asthma UK, 2014; Coulter et al., 2013). Furthermore, whilst current action plans are largely restricted to recommending the use of inhaled asthma medication, the scope to routinely include oral corticosteroids may confer additional benefits to morbidity and mortality arising from acute exacerbations. Indeed, the self-administration of antibiotics and oral corticosteroids by patients with chronic obstructive pulmonary disease has led to improvements in morbidity and unclear but likely benefits to mortality, which presents the question whether similar outcomes could be attained for asthma (Nici et al., 2014).
1.5 Rationale
The rationale for this work has been formulated from the background section discussed above, and also from the researcher’s observation of a patient admitted to the Emergency Department with an acute asthma exacerbation, as noted in the proposal. In this case it was evident that the patient had not received an asthma review for the preceding six months, which meant that their current medication was inappropriate and not optimised for their current asthma status. In addition, there was evidence of intermittency in regard to the use of inhalers, suggesting poor adherence to treatment, which is known to lead to worse outcomes, than compared to patients with optimal adherence, as is reviewed above. Whilst the researcher notes that hospitals receive most patients presenting with acute asthma exacerbations and therefore have the opportunity to review patients medication regimes, there is an earlier window of opportunity to improving outcomes, which is in the community. Therefore, this dissertation will use this rationale to explore the following aims and objectives.
1.6 Aims and Objectives
Given the background literature discussed above, this research aims to explore and evaluate whether systemic corticosteroids offer any benefit to morbidity and mortality, than compared to inhaled steroids, of which could then be recommended for incorporation into asthma action plans for self-administration in the management of acute asthma exacerbations. In order to meet these aims, the following objectives have been identified:
- Identify and evaluate the impact of systemic corticosteroids upon physiological outcomes related to asthma exacerbations.
- Evaluate the impact of systemic corticosteroids in regard to outcomes related to morbidity and mortality.
- Evaluate whether oral corticosteroids would be feasible for use in personalised asthma action plans.
- Identify recommendations for future research and practice.
2. Methodology
2.1 Overview
Methodology refers to the logic and theoretical grounding of research and contains its methods or strategies, which are collectively, essential to the planning, conduct, analysis and interpretation of data that focuses upon meeting the aims and objectives (Long, 2014). Given the objectives and that this research is expected to largely comprise quantitative findings, a general epistemological and ontological approach will be utilised to generate knowledge and relationships from the findings (Goertz and Mahoney, 2012). Whilst primary research can add significant novel information to the evidence-base, the abundance of information already available on asthma medication and the time restrictions placed upon the researcher suggest that secondary methodology is more appropriate. This will facilitate synthesis of findings from a number of primary and heterogenous research articles that are relevant to the research domain of interest, such to generate summarised and meaningful results that can inform current practice and guide future research (Gough et al., 2012).
2.2 Research Question
The construction of a central research question is recognised as an essential component of literature reviews, as it ensures that the research process remains focused upon achieving the aims and objectives (Farrugia et al., 2010). The ‘PICO’ (population, intervention, comparator and outcome) framework was used to help derive the principle research question subsections and to also generate basic terms for the search strategy that will be detailed in the proceeding section (Scells et al., 2017). The ‘PICO’ terms generated (table 1, below) allowed derivation of the following research question:
Are systemic corticosteroids effective at reducing morbidity and mortality from acute asthma exacerbations and are they feasible for use in personalised asthma action plans?
Table 1. ‘PICO’ subsections for research question development.
|
‘PICO’ |
|
|
Population |
Patients with acute asthma exacerbations |
|
Intervention |
Systemic corticosteroids |
|
Comparator |
Inhaled corticosteroids |
|
Outcomes |
Respiratory Symptoms Morbidity Mortality |
2.3 Search Strategy
The sole use of online electronic databases was used to identify and retrieve literature relevant to the research question, as this is considered the ‘gold standard’ approach to constructing modern and credible literature reviews (Bowling, 2009). Moreover, the use of electronic databases allows for efficiency when filtering the search results and applying study-specific inclusion and exclusion criteria, which will be detailed in the next section (Grewal et al., 2016). The following databases were selected for the search: PubMed/MEDLINE, EMBASE, CINAHL and the Cochrane registry. This series of databases was chosen as they contain the vast majority of published, high quality, peer-reviewed and up-to-date healthcare literature, which adds initial credibility to this literature review (Bowling, 2009). In addition, evidence has shown that using a series of databases helps to avoid reporting bias, whereby, the reliance upon one database alone may lead to the incidental exclusion of relevant studies to the research question (Qiu and Wang, 2016).
In order to address and maintain congruence with the research question, the ‘PICO’ framework was also used to generate basic search terms to help inform and develop terms for incorporation into electronic database searching. The use of ‘PICO’ in this regard is similarly considered to be a credible method of ensuring that the search does not lead to incidental exclusion of important studies. However, the ‘comparator’ component was excluded, as this has been shown to lead to an overly-selective search that can result in exclusion of relevant studies (Bettany-Saltikov, 2012). After development of the basic search terms, these were converted into alternative words, phrases and acronyms, to form final terms, which were applied to electronic database searching (table 2, below). For each database, the relevant terms were matched to their subject headings, to generate greater precision and avoid retrieval of excess irrelevant research. The applied search terms were also truncated where appropriate and combined using Boolean logic.
Table 2. Search strategy.
|
|
Population |
Intervention |
Outcome |
|
Generic Terms |
Patients with acute asthma exacerbation |
Systemic Corticosteroids |
Resolution of acute asthma exacerbation Morbidity Mortality |
|
Applied search terms (columns combined with “AND”) (*truncation) |
1-Patient* 2-Asthma 3-Acute 4-Exacerbation |
5-Systemic 6-Corticosteroid* 7-Steroid* |
8-Outcome* 9-Symptom* 10-Morbidity 11-Mortality |
|
Boolean Combinations |
1 AND 2 AND 3 OR 4 |
5 AND 6 OR 7 |
8 OR 9 OR 10 OR 11 |
2.4 Inclusion and Exclusion Criteria
After retrieval of studies from electronic database searching it is important that inclusion and exclusion criteria are applied to the results, such that irrelevant studies are dismissed and only those pertaining to the research question are considered for full eligibility (Booth et al., 2012). The inclusion criteria defined articles published in the last two decades (1998-2018), contained within a peer-reviewed journal, primary rather than secondary research or reviews, available in English language and reporting of relevant data to the research question. This series of criteria ensures that the findings in this review are informed and comprehensive, such that any recommendations will be meaningful to current practice. In addition, peer-review helps to establish a level of validity among primary studies and only reviewing studies of English language negates the need for translation, which is time-consuming and may increase the risk of error (Cowell, 2014; Meline, 2006). The used of exclusion criteria is also important to help refine the filtering of retrieved studies, to identify those that are most relevant to the research domain; however, no selective exclusions were applied as this can result in the exclusion of important studies (Garg, 2016). Thus, the exclusion criteria directly contrasted the inclusion criteria; articles published prior to 1988, lacking peer-review, non-primary research, not available in English language and reporting of irrelevant data to the research question.
2.5 Ethical Considerations
The adherence to ethical and moral principles during the conduct of research is essential to ensuring sufficient protection of participants and in maintaining proper research practices, that help to generate high quality data that is error free and can thus, help to inform practice and improve patient care (Resnik, 2015). Such practices have been formally published by the UK Research Integrity Office in their Code of Practice for Research (2009), which was developed to encourage proper conduct and prevent misconduct, to ensure the provision of high quality research. In order to adhere to best research ethics and practices, the researcher screened all eligible studies used for synthesising results in this review, to ensure that the original researchers adequately protected participants, such as using proper consenting and confidentiality procedures. In addition, the researcher considered whether the review topic would raise any ethical issues, however, given the nature of the topic and after discussion with the local Research and Development department, no formal ethical approval was deemed to be required. Of note, any studies lacking proper ethical practices would constitute exclusion, which if evident, will be detailed in the proceeding study eligibility section.
3. Results
3.1 Study Eligibility
The PRISMA statement for reporting of systematic reviews and meta-analyses in health was used to guide the eligibility process, which can be viewed in figure 3, below. Indeed, it is reported that the use of PRISMA ensures that literature reviews comply with best research practices through adopting a thorough and transparent inclusion and exclusion assessment, such that the collective findings are not influenced by reporting bias (Liberati et al., 2009). After electronic database searching, a total of 2,695 articles were retrieved and after the removal of duplicates, 2,579 studies were left for title and abstract screening. The inclusion and exclusion criteria were applied to this process, which resulted in the large exclusion of 2,547 studies. Notably, such large exclusions can be expected in literature reviews, as the use of relatively broad search criteria ensures that the literature base is thoroughly screened for studies relevant to the research question, whilst overly-selective searches can lead to incidental exclusions of pertinent research (Tsafnat et al., 2018). A total of 32 studies remained for full text review, which resulted in the further exclusion of 25 studies, due to the reporting of intervention and/or outcome data that was irrelevant to the research domain of interest. Thus, seven studies were considered fully eligible to inform the findings in this review.
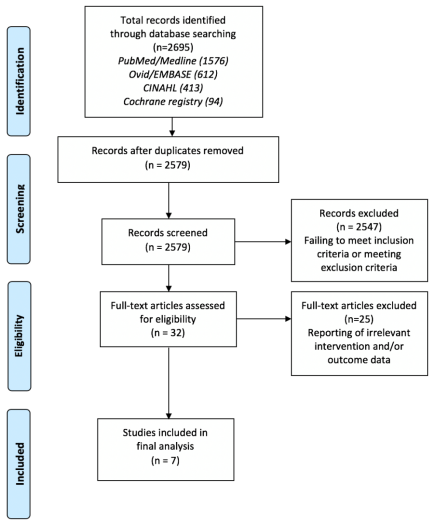
Figure 3. PRISMA flow diagram (2015) of filtering process for electronic database search results.
3.2 Synthesis of Results
To develop the latter aspect of this literature review, whilst all eligible studies report upon quantitative data, they will be analysed summarily and descriptively due to the marked heterogeneity across the studied populations, interventions and outcome measures. This will allow the complex range of information to be comprehended by the reader, and a thematic-style discussion, will facilitate the generation of more meaningful findings for current research and clinical practice. Prior to the reporting of the outcome measures, the included studies will be subject to critical appraisal, to ensure their methodological quality and the consequent validity and reliability is sufficient enough to support a credible literature review (Mhaskar et al., 2009). As all included studies were of randomised controlled trial (RCT) design, The Critical Appraisal Skills Program (2018) framework for RCTs was used to support and structure the appraisal process in this review.
3.3 Critical Appraisal
Section A: Are the results of the study valid?
Did the study address a clearly focused issue?
The aims of included studies are summarised in table 3, appendices, but generally, all studies evaluated the impact of oral corticosteroids upon acute asthma exacerbation related outcomes. The populations analysed varied among included studies with four studies (Becker et al., 1999; Devidayal et al., 1999; Schuh et al., 2006; Schuh et al., 2000) including paediatric patients aged <18 years and three studies (Lin et al., 1999; Lee-Wong et al., 2002; Rodrigo, 2005) including persons aged up to a maximum of 55 years. All RCTs included patients with acute asthma exacerbations but the characteristics of those included and excluded, as detailed in table 4, below, varied. Of note, there were no apparent inappropriate exclusions, which may otherwise increase the risk of selection bias (Ahmed et al., 2012). All studies pre-defined their outcome measures, which is essential to ensuring that the risk of false-positive and false-negative error upon statistical analysis is minimised (Andrade, 2015). The outcomes measures will be discussed in the relevant section of this review.
Table 4. Participant characteristics.
|
Study |
Number of subjects |
Age (years) |
Inclusion Criteria |
Exclusion Criteria |
|
Becker et al. (1999) |
66 |
2-18 |
Acute asthma exacerbation Treated in Emergency Department |
Significant chronic disease Oral steroids in past 5 days Recent varicella exposure Requiring intensive care support Continuous need for beta-agonists or intravenous formulations |
|
Devidayal et al. (1999) |
80 |
2-12 |
More than one acute asthma attack in last six months Acute exacerbation diagnosed clinically |
Significant acute or chronic diseases Oral or inhaled steroids in past 24 hours |
|
Lee-Wong et al. (2002) |
40 |
18-55 |
Acute asthma exacerbation Requiring hospital admission |
Pregnancy, lactation Substance abuse Severe mental illness Intubation required Current users of corticosteroids Severe co-morbidities Inability to attend follow-up |
|
Lin et al. (1999) |
56 |
>18 (43.1; mean) |
Acute asthma with peak flow <50% of predicted value after initial beta-agonist therapy |
Inability to perform peak flow Smoking history >20 pack years Pregnancy |
|
Rodrigo (2005) |
106 |
18-50 |
Acute asthma with peak flow <50% of predicted value |
Pyrexial Pregnancy Significant co-morbidities |
|
Schuh et al. (2000) |
100 |
5-17 |
Acute asthma with peak flow <60% of predicted value Able to use inhaler |
No prior history of wheezing Requiring immediate steroid therapy or intubation Received steroids in past 7 days or taking high dose inhaled steroids Significant co-morbidities Recent varicella exposure |
|
Schuh et al. (2006) |
69 |
5-17 |
Acute asthma with baseline peak flow 50-79% of predicted value |
Airway instability Persistent vomiting Received corticosteroids in past 7 days Significant co-morbidities Recent varicella exposure Previous intensive care admission for asthma Language barrier |
Was the assignment of patients to treatment randomised?
All studies randomised participants to intervention groups and their respective controls, using appropriate methods, such as computer-generated randomisation lists and block randomisation. The use of randomisation has led to RCTs being considered the gold standard of evidence for evaluating the effect of interventions, as the design reduces the adverse influence of sample size and participant covariable imbalances between groups, to reduce the impact of confounding and bias (Kang et al., 2008). Moreover, adequate randomisation ensures that the initial allocation of participants to groups is concealed and the validity of statistical tests is supported, which further minimises the risk of bias. Indeed, in situations where randomisation is inadequate or lacking, the size of reported effects may be largely over-estimated by as much as 40%, than compared to studies utilising proper randomisation techniques (Suresh, 2011).
Were all of the patients who entered the trial properly accounted for at its conclusion?
All studies were completed to study end with no reported premature terminations, such as due to adverse events or circumstances that would raise concerns regarding truncation bias (Briel et al., 2009). All studies did not observe significant drop-outs or losses of participants to follow-up (<5% of initial eligible subjects) as a result of the immediate/short duration to follow-up, which reduces the overall risk of attrition bias (Nunan et al., 2018). Furthermore, in the studies by Lin et al. (1999) and Rodrigo (2005) the authors used intention-to-treat analysis to minimise the impact of non-compliance and missing data, which generates unbiased effects in RCTs and provides high levels of generalisability, than compared to RCTs lacking intention-to-treat analyses (Gupta, 2011).
Were patients, health workers and study personnel ‘blind’ to treatment?
All included studies utilised double rather than single blinding, which refers to the concealment of participants and care providers knowledge as to the assignment intervention or control. The use of blinding is an essential component of RCT design, as it helps to prevent differential treatment of subjects between groups and differential reporting from participants, that could lead to biased estimates of the pre-defined outcomes. As such, blinding helps to sustain the allocation concealment initiated by randomisation at trial onset to study end, which reduces the risk of performance and ascertainment bias (Karanicolas et al., 2010). The controls in six of seven studies comprised placebo (Becker et al., 1999; Devidayal et al., 1999; Lin et al., 1999; Schuh et al., 2006; Schuh et al., 2000; Lee-Wong et al., 2002), whilst the remaining study (Rodrigo, 2005) defined the control as being standard treatment with intravenous hydrocortisone. Notably, the use of placebo as the control, rather than an alternative treatment as a comparator, increases the effect of blinding, meaning that majority of studies are at low risk of bias in this particular regard (Karanicolas et al., 2010).
Were the groups similar at the start of the trial?
In RCTs it is imperative that confounding variables are defined, controlled for during statistical testing, and balanced between groups, in order to reduce biased effects and to support the randomisation process (Roberts and Torgerson, 1999). The baseline covariables among participants were comparable for the studies by Rodrigo (2005), Schuh et al. (2000) and Lee-Wong et al. (2002), with p values exceeding the standard 0.05 cut-off value for significance. Whilst most variables for comparable for Lin et al. (1999) the intervention group had modestly shorter median durations of acute symptoms (36 hours v. 60 hours), which may comprise a confounding factor that could lead to over-estimates of the effect size. This was similarly true for Schuh et al. (2006) but there was a higher proportion of subjects in the prednisolone group using inhaled corticosteroids (55.9% v. 51.4%), which may have led to unreliable outcome estimates. In the study by Becker et al. (1999), whilst there are no significant differences in baseline covariables between the intervention groups, the authors have only included a small number of confounders, which increases concern for biased outcomes due to unmeasured confounders. This was similarly true for the study reported by Devidayal et al. (1999).
Aside from the experimental intervention, were the groups treated equally?
The reported interventions and controls or comparators among included studies are complex and have therefore been summarised below in table 5. Notably, aside from the defined interventions and controls, all participants in all studies were treated equally, which is important in ensuring that there are no additional factors that could have confounded the outcome effects reported (Kendall, 2003). Note, in the study by Lee-Wong et al. (2002) systemic corticosteroids were comprised as part of standard treatment, which means that only non-inferiority can be determined when evaluating the reported outcomes. Table 5. Interventions and controls of included studies.
|
Study |
Intervention |
Control/Comparator |
|
Becker et al. (1999) |
Prednisolone (oral) + Methylprednisolone (intravenous) |
Placebo (oral sucrose + intravenous saline) |
|
Devidayal et al. (1999) |
Prednisolone (oral) + Salbutamol (nebulised) |
Placebo (oral tablet) + Salbutamol (nebulised) Budesonide (nebulised) |
|
Lee-Wong et al. (2002) |
Flunisolide (inhaled) |
Placebo (inhaled) |
|
Lin et al. (1999) |
Methylprednisolone (intravenous) |
Placebo (intravenous saline) |
|
Rodrigo (2005) |
Fluticasone (inhaled) |
Hydrocortisone (intravenous) |
|
Schuh et al. (2000) |
Fluticasone (inhaled) |
Placebo (inhaled) + Prednisolone (oral) |
|
Schuh et al. (2006) |
Fluticasone (inhaled) + Placebo (oral) |
Placebo (inhaled) + Prednisolone (oral) |
Section B: What are the results?
How large was the treatment effect and how precise was the estimate of the treatment effect?
The reporting of outcome measures for all included studies and the size and precision of the effects are discussed in the latter section of this literature review.
Section C: Will the results help locally?
Can the results be applied to the local population, or in your context?
Given the collective characteristics of participants among the included studies, as noted in table 4, above, and that all had received a diagnosis of acute asthma exacerbation, the findings of this review can therefore, be applied to paediatric and young adult populations. In addition, most studies were derived from western populations in the United States (Lin et al., 1999; Becker et al., 1999; Lee-Wong et al., 2002) and Canada (Schuh et al., 2006; Schuh et al., 2000), which from an epidemiological perspective, can be inferred to be generalisable to UK-based populations, who are likely to observe similar disease patterns. However, two included studies evaluated populations from India (Devidayal et al., 1999) and Uruguay (Rodrigo, 2005), which may not be representative to western populations, and thus, the applicability is partly impaired by this dual inclusion.
Were all clinically important outcomes considered?
A range of outcomes were considered by studies in this review. This included physiological outcomes, such as oxygen saturations and peak expiratory flow rate, and those pertaining to morbidity, such as length of stay and readmission rates. For each individual study, the reported outcomes were all appropriate to their respective aims, however, for the objectives of this research, no studies reported upon mortality as an outcome measure.
Are the benefits worth the harms and costs?
This signalling question from the Critical Skills Appraisal Program framework for RCTs has not been considered by most of the included studies in this review. Only Becker et al. (1999) reported upon the estimated costs of prednisolone and methylprednisolone, where the latter was markedly more expensive. Despite this, the researcher cannot confidently comment on this aspect in further detail, given that corticosteroids are known to be associated with significant adverse effects, which would require specific consideration in future research, to make determinations regarding benefit versus harms and cost.
Other Quality Considerations Not Addressed by CASP
All studies in this review were subject to peer-review and maintained proper ethical standards by protecting patients and obtaining ethical approval, which is in keeping with best research practice (Grove et al., 2013). Some studies received funding from official institutions (Becker et al., 1999; Schuh et al., 2000), but funding sources among others were not well-defined or unclear (Rodrigo, 2005; Devidayal et al., 1999; Schuh et al., 2006; Lin et al., 1999). Notably, Lee-Wong et al. (2002) received a grant from a pharmaceutical laboratory, which increases the concern for conflicts of interests and funding bias in the publication and results of this study. All studies selected to use an appropriate range of statistical tests and software to analyse data, which minimises the risk of reporting bias related to outcome measures (Pirracchio et al., 2013). Finally, calculations of power to ensure that the statistical analysis of data is unlikely to produce false-positive or false-negative results is essential to the credibility of RCTs (Dumas-Mallet et al., 2017). Indeed, five of seven studies (Rodrigo, 2005; Schuh et al., 2006; Schuh et al., 2000; Lin et al., 1999; Becker et al., 1999) generated sufficient power (>80%) in their samples to ensure this risk was minimised, however, Devidayal et al. (1999) and Lee-Wong et al. (2002) did not report on the use of a power calculation meaning the reliability of the results could be inadequate.
Summary
Overall, the quality of included studies in this review has been evaluated using the above CASP structuring for RCTs. From these determinations, the collective risk of bias and concerns for generalisability have been summarised for the reader in table 6, below.
Table 6. Risk of bias and applicability.
|
Study |
Risk of Bias |
Applicability Concern |
|
Becker et al. (1999) |
Low |
Low |
|
Devidayal et al. (1999) |
High |
High |
|
Lee-Wong et al. (2002) |
High |
Low |
|
Lin et al. (1999) |
Moderate |
Low |
|
Rodrigo (2005) |
Low |
High |
|
Schuh et al. (2000) |
Low |
Low |
|
Schuh et al. (2006) |
Low |
Low |
3.4 Outcome Measures
3.4.1 Overview
Given the marked heterogeneity among the populations and outcomes measures of included studies in this review, the merging of data and collective statistical analysis was not feasible. Therefore, the outcomes are discussed descriptively under two main identified themes; physiological outcomes and morbidity. Although, a thematic analysis has not been conducted, a thematic-style allowed the findings to be appropriately structured and presented. Notably, the descriptive outcome data is summarily presented in table 7, below, which details the outcomes in regard to corticosteroid use.
3.4.2 Physiological Outcomes
All included studies reported upon a range of physiological outcome parameters. Becker et al. (1999) found that both the prednisolone and methylprednisolone groups observed significant improvements in peak expiratory flow rate (p<0.001), but there was no statistically significant difference between groups (p=0.2). Moreover, children among the prednisolone group required significantly shorter mean durations of oxygen administration (30 hours), than those in the methylprednisolone group (52 hours, p=0.04). However, the prednisolone group observed non-significant improvements in the weaning of beta-agonists to four times daily intervals, than compared to the methylprednisolone group (p=0.47). Similarly, Lee-Wong et al. (2002) found that improvements in peak expiratory flow rate were observed for both the intervention (prednisolone) and control groups (inhaled corticosteroid) but the between group difference was insignificant (p=0.95). This non-significant effect was also demonstrated for improvements in symptoms and forced expiratory volume in one second (p=0.39 and p=0.33, respectively).
Rodrigo (2005) also showed that peak expiratory flow significantly improved from baseline for the inhaled corticosteroid (fluticasone) and hydrocortisone groups (p<0.001), with the fluticasone group observing a significant 30.5% greater mean improvement (p=0.03) and higher peak expiratory flow rates at 120, 150 and 180 minutes (p<0.05). This trend was also demonstrated for forced expiratory volume in one second; from baseline (p<0.01) and between groups (p=0.04), and a 46.4% greater mean improvement was observed for the fluticasone group, than compared to the hydrocortisone group (p=0.001). In the earlier study by Schuh et al. (2000), the authors found that the prednisolone group observed significant improvements in forced expiratory volume in one second (18.9% v. 9.4%, p<0.001), forced vital capacity (18.6% v. 9.0%, p=0.008) and peak expiratory flow (20.0% v. 7.6%, p<0.001) at 240 minutes from baseline, than compared to the fluticasone group. However, no significant differences between groups were observed for respiratory rate and oxygen saturations (p=0.86 and p=0.14, respectively). In the later study, Schuh et al. (2006) the authors found that the prednisolone group experience significantly faster and overall improvements in forced expiratory volume at 240 minutes from baseline, than compared to the fluticasone group (p=0.001). However, the effect at 48 hours post-onset of asthma exacerbation did not remain significant between groups (p=0.47) and adjustment for covariables failed to alter this effect. Notably, a significantly higher proportion of subjects in the prednisolone group (76.7%) observed excellent treatment responses at 240 minutes, than compared to the fluticasone group (46.7%, p=0.017) and the number needed to treat with prednisolone to observe this effect was 3.3. Other reported outcomes including respiratory rate, oxygen saturations, additional corticosteroid requirements and rescue beta-agonist use were not significantly different between groups.
Lin et al. (1999) found that the methylprednisolone group observed significantly greater improvements in peak expiratory flow over time (baseline to 120 minutes), than compared to placebo (p=0.002). This effect remained after altering the dependent variable to percent predicted peak expiratory flow (p=0.005) and when patients who had received a beta-agonist (salmeterol) had been excluded in sub-analysis (p<0.005). Moreover, whilst there were no significant improvements in acute asthma symptoms between groups, when stratified as >48 hours, the authors found that the methylprednisolone group observed significant reductions in mean heart rate, as compared to placebo (p=0.029). In comparison, Devidayal et al. (1999) found that the inhaled corticosteroid (budesonide) and prednisolone groups observed significant improvements in respiratory rate, heart rate, pulsus parodoxus, pulmonary index, respiratory distress score and peak expiratory flow rate, however, the improvement was greater among those receiving budesonide (p<0.05). In contrast, significantly more subjects receiving prednisolone had oxygen saturations >95%, absence of accessory respiratory muscle use, wheeze, and respiratory distress (p<0.01). However, reduction in the severity of respiratory distress occurred significantly faster in those receiving budesonide, than compared to prednisolone (1.7 hours v. 2.5 hours, p<0.01). Moreover, significantly more patients in the prednisolone group required the additional administration of intravenous hydrocortisone, than compared to the budesonide group (p<0.001). Furthermore, among the budesonide group significantly more patients had shown clinical improvement and recovered from the acute exacerbation after three nebulisations, than compared to the prednisolone group (p<0.001). In support of this finding, Lin et al. (1999) showed that the methylprednisolone group required significantly less unblinded additional intravenous steroid, than compared to placebo (2 v. 9 cases, p=0.16).
3.4.3 Morbidity
Six of seven studies (Becker et al., 1999; Lin et al., 1999; Devidayal et al., 1999; Rodrigo, 2005; Schuh et al., 2000; Lee-Wong et al., 2002) reported upon factors related to morbidity, as considered in this review, such as length of stay and need for admission. Becker et al. (1999) found that children in the prednisolone group had a shorter mean length of hospital stay (70 hours), than compared to the methylprednisolone group (78 hours) but the difference was not statistically significant (p=0.52). Devidayal et al. (1999) also showed that budesonide patients observed significantly shorter mean durations of stay (2.9 hours), than compared to those receiving prednisolone (5.5 hours, p<0.001). A shorter length of stay for subjects receiving prednisolone was also found in the study by Lee-Wong et al. (2002) but this was insignificant when compared to the inhaled corticosteroid group (2.3 days v. 2.7 days, p=0.34).
Devidayal et al. (1999) found that five patients in the prednisolone group required hospital admission, as compared to only one patient in the budesonide group. In contrast, Lin et al. (1999) found that the need for hospital admission was comparable between methylprednisolone group and placebo (p=0.118). Comparable admission rates were also found in the study by Rodrigo (2005), where 11.1% and 7.7% of participants in the hydrocortisone and fluticasone groups required ongoing care (p=0.7). However, substantially more patients in the fluticasone group reached criteria for discharge at the 90, 120 and 150 minute thresholds, than compared to the hydrocortisone group (log rank test 0.0003). In multiple regression analysis, Rodrigo (2005) also found that in patients with more severe airway obstruction, those receiving fluticasone observed significantly reduced hospitalisation rates, than compared to the hydrocortisone group (p=0.05). In this analysis, the marked effect upon discharge criteria at the 90, 120 and 150 minute thresholds remained (log rank test 0.004). Schuh et al. (2000) found that the need for hospital admission was significantly higher in the fluticasone group (31%), than compared to the prednisolone group (10%, p=0.01), which remained significant after adjustment for gender and previous hospital admissions.
3.4.4 Summary
Table 7. Summary of outcomes in respect of oral/intravenous corticosteroid use.
|
Study |
Physiological Parameters |
Morbidity |
|
Becker et al. (1999) |
↑ Weaned to routine beta-agonist therapy ↑ Lower oxygen requirements |
⇔ Length of hospital stay ↓ Corticosteroid requirement ↓ Length of hospital stay |
|
Devidayal et al. (1999) |
↓ Respiratory rate ↓ Oxygen saturations ↓ Peak expiratory flow rate ↓ Respiratory distress severity |
↓ Discharge criteria met |
|
Lee-Wong et al. (2002) |
⇔ Peak expiratory flow rate ⇔ Symptoms |
⇔ Length of hospital stay |
|
Lin et al. (1999) |
↑ Peak expiratory flow rate ↑ Mean heart rate ↑ Accessory muscle use |
↑ Hospital admission rate ↑ Corticosteroid requirement |
|
Rodrigo (2005) |
↓ Peak expiratory flow rate ↓ Forced expiratory volume |
↓ Hospital admission rate |
|
Schuh et al. (2000) |
↑ Forced expiratory volume ↑ Peak expiratory flow rate ↑ Forced vital capacity |
↑ Hospital admission rate |
|
Schuh et al. (2006) |
↑ Forced expiratory volume |
- |
*↑; superior to comparator, ↓; inferior to comparator, ⇔; no difference.
4. Discussion
4.1 Overview
This literature review has aimed to determine whether the use of corticosteroids could be incorporated into personalised asthma action plans for the routine acute management of asthma exacerbations, which confer most of the morbidity and mortality attributed to the condition. To achieve this aim two main objectives were identified; 1) evaluate the impact of corticosteroids upon physiological outcomes in patients with acute asthma exacerbations and 2) evaluate addition outcomes related to morbidity and mortality. Notably the first objective has been achieved, however, no included studies in this review reported upon mortality and thus, objective two has only been partly achieved. The third and fourth objectives will be addressed within the discussion and concluding sections.
4.2 Thematic-Style Discussion
To retain logical structure and consistency with the results section, the main body of the discussion will be detailed under the respective subheadings; physiological outcomes and morbidity.
4.2.1 Physiological Outcomes
All included studies in this review reported upon the effect of inhaled, oral or intravenously administered corticosteroids on a range of physiological parameters related to asthma exacerbations. When considering the comparison between oral or intravenous corticosteroids and inhaled corticosteroids, the findings in this review have shown mixed results. The findings by Becker et al. (1999), Lin et al. (1999), Schuh et al. (2000) and Schuh et al. (2006) showed that oral or intravenous corticosteroids can improve measures of lung function during acute asthma exacerbations, including peak expiratory flow rate, forced expiratory volume in one second, and forced vital capacity, as well as reducing oxygen and beta-agonist requirements. Indeed, the use of corticosteroids in improving lung function to a degree that reduces airway obstruction has been previously reported by Fanta et al. (1983) who compared hydrocortisone to placebo. Indeed, such improvements in lung function indices represent markers of improvement in the degree of airway obstruction and airflow restriction, that are ultimately responsible for hypoxaemia, respiratory failure and other consequent sequalae among patients with asthma exacerbations (Maselli and Paciocco, 2000). However, whilst corticosteroids are reported to be effective at reducing inflammatory processes, resistance to steroids among a minority of asthma patients could confound the results of any trials assessing their efficacy (Ramsahai and Wark, 2018).
In contrast, studies by Devidayal et al. (1999) and Rodrigo (2005) showed that oral or intravenous corticosteroids were inferior to their comparators as inhaled corticosteroids in regard to measures of lung function, oxygen requirements and indicators of respiratory distress, whilst the respective findings by Lee-Wong et al. (2002) were non-inferior or superior for either the intervention or control groups. The comparable efficacy between oral and inhaled corticosteroids has been previously shown in randomised trials by Levy et al. (1996) and Scarfone et al. (1995). In trials demonstrating superiority or non-inferiority of inhaled corticosteroids, it is suggested that the effect may be due to rapid non-genomic effects, whereby the induction of vasoconstriction in the large airways can reverse vascular and inflammatory related hyperpermeability - which normally predisposes asthma patients to airway narrowing (Horvath and Wanner, 2006). Indeed, this biological effect may account for findings of the studies in this review (Devidayal et al., 1999; Rodrigo, 2005), that demonstrated the superiority of inhaled corticosteroids, as compared to enteral and parental steroid formulations.
4.2.2 Morbidity
The majority of studies in this review reported upon the impact of corticosteroids on acute asthma patients morbidity, in regard to the need for hospital admission and length of stay, and again, the results were mixed. In the studies by Lin et al. (1999) and Schuh et al. (2000), oral and intravenous corticosteroids were found to provide reductions in hospital admission rates, as compared to inhaled corticosteroids, which has been supported among previous literature (Littenberg and Gluck, 1986). Other literature has also shown that systemic corticosteroids can reduce the frequency of exacerbation relapses after discharge (Schneider et al., 1988; MRC Collaborators, 1956) and readmission rates (Scarfone et al., 1995), although this was not found by Rodrigo and Rodrigo (1994). Notably, in the most recent systematic review evaluating the effect of corticosteroid therapy for patients with asthma, Krishnan et al. (2009) reported that systemic formulations can accelerate the resolution of acute exacerbations and reduce the risk of relapse. Moreover, the oral, intravenous and intramuscular formulations were found to be of comparable efficacy, whilst combined inhaled and oral corticosteroids were also suggested to minimise the risk of repeat exacerbations.
In the Global Strategy for Asthma Prevention and Management (2018), the Global Initiative for Asthma report that inhaled corticosteroids are as effective as systemic formulations at preventing exacerbation relapses; however, the authors note that there is insufficient evidence to advocate patient-initiated oral steroids into routine practice. Given that the findings in this review have shown mixed effectiveness for systemic corticosteroids for managing acute asthma exacerbations and that there are no identified studies evaluating oral steroids among ambulatory populations, the Global Asthma Initiatives recommendation is iterated by the researcher. There have also been marked safety concerns regarding the adverse long-term effects of corticosteroids, which has largely restricted research to evaluating the effects of inhaled steroids in asthma patients (Poetker and Reh, 2010). As such, the potential benefits of intermittent oral steroid use for ambulatory patients experiencing acute exacerbations of asthma may remain under-evaluated given the likely ethical issues that prevent such trials from being undertaken. Whilst, the superiority of systemic corticosteroids for resolving the acute phase of asthma exacerbations remains largely unclear, their administration has shown delayed benefits. In a systematic review, Rowe et al. (2007) evaluated the benefit of systemic corticosteroids in asthmatic patients who were discharged after experiencing an acute exacerbation. The authors identified six trials of 374 patients and found that a significantly lower number of subjects in the corticosteroid group observed relapses within the first seven days post-exacerbation (relative risk (RR) 0.38; 95% CI 0.2, 0.74), which was an effect sustained by three weeks (RR 0.47; 95% CI 0.25, 0.89). In addition, patients receiving systemic steroids required less subsequent emergency hospital admissions (RR 0.35; 95% CI 0.13, 0.95), had reduced beta-agonist requirements (mean difference -3.3; 95% CI -5.6, -0.1). Moreover, there were no significant increases in side effects among systemic corticosteroid users (mean difference 0.03; 95% CI -0.38, 0.44), which overall, supports the use of corticosteroids for patients requiring assessment for acute asthma exacerbations. Notably, no such reviews or studies have been conducted in the community setting. Whilst acknowledging the lack of evidence, the European Asthma Exacerbation Advisory Panel suggest that select groups of patients may benefit from oral corticosteroids and these can be prescribed as part of their self-management plan to help treat exacerbations (Reddel and Barnes, 2006). However, the selection of patients who are most appropriate for oral corticosteroids is complicated by the emergence of specific asthma phenotypes, some of which, may comprise steroid resistance (Jayaram et al., 2006; Bonsignore et al., 2015).
4.3 Summary
Overall, the results of this review can only report that systemic corticosteroids are as effective as inhaled formulations in regard to asthma outcomes, which is supported by previous research as indicated above. Given that inhaled corticosteroids are already a component of personalised asthma plans and the lack of primary research studies evaluating the efficacy of systemic corticosteroids in ambulatory patients, it appears that it would not be feasible to incorporate them into individuals routine asthma management. The recommendations for future research are noted below in the conclusion section, but the findings in the systematic review by Pinnock et al. (2017) provide important justification. The authors evaluated the effectiveness of self-management plans upon asthma control and healthcare resource utilisation among 27 systematic reviews, including 244 RCTs, as well as 13 updated RCTs. The results showed that self-management plans can reduce hospital admission rates, reducing the need for unscheduled care, as well as improving asthma control and associated morbidity. Importantly, the adoption of self-management strategies was not associated with excess healthcare costs, which overall, supports their utility in the routine management of asthma from a clinical, organisation and policy level perspective.
4.3 Strengths and Limitations
In keeping with best research practice recommendations, the researcher has identified a variety of strengths and limitations of this literature review. Firstly, the structured and systematic approach to conducting this review, including the use of ‘PICO’ and a precise search strategy, ensured that all literature relevant to the research question was identified and used to inform the collective findings, such that the aims and objectives were achieved and the research domain addressed. Secondly, the objectivity demonstrated throughout the review process, including the use of a formal critical appraisal framework to evaluate the credibility of included studies, means that the review is not influenced by reporting bias. Thirdly, as all included studies were screened for proper ethical practices, this review can confidently report that the original participants were adequately protected. Fourthly, all included studies were of randomised, double-blind, controlled trial design, which is considered to be the ‘gold standard’ design to producing the highest quality of research evidence. The fifth and final strength of this review is the objectivity and proportionate statements produced in regard to the implications of this research and recommendations for future practice, as will be noted in the conclusion section.
The principle limitations of this review are mostly related to factors that were external to the researcher’s direct control. Firstly, the researcher is not a medically trained specialist in respiratory medicine or asthma, and nor do they have significant experience and expertise in the research field. This may have had implications for this research, such as limited scope of data analysis and discussion and inadequately informed recommendations for future practice and research. Secondly, the review is inherently limited by the studies included herein and as noted in the critical appraisal section, many studies were subject to bias and concerns for applicability, which limits the value and use of this review to the current evidence base. Thirdly, whilst efforts were made to ensure that all relevant studies were obtained for results synthesis, it is possible that some studies were incidentally excluded from the electronic databases and also, studies of non-English language may have overall, resulted in a degree of reporting bias.
5. Conclusion and Future Recommendations
Asthma is a highly prevalent respiratory condition that is considered to be a major global public health problem, contributing to substantial morbidity and to a lesser extent, mortality, worldwide. As many nations are experiencing huge pressures and demand upon their health systems, the drive for patients to adopt greater self-efficacy in managing their chronic conditions has been increasingly emphasised by official governing bodies. In the UK, the emergence of personalised asthma action plans arose from findings of substandard pharmacological management and adherence in an independent review of asthma-related deaths, which were in part, also designed to assist users in better managing their own disease. The currently used action plan places much focus upon using ‘reliever’ and ‘preventer’ inhalers during acute exacerbations, which relate to beta-agonists and corticosteroids, respectively. However, in some instances, this combination is insufficient to resolve exacerbations and prevent the need for hospitalisation and/or more serious sequalae.
Therefore, this review aimed to determine whether systemic corticosteroids are associated with better asthma related outcomes, than compared to their inhaled formulations and to determine whether it would be feasible to introduce oral steroids into personalised action plans. However, the findings showed that there were mixed results, although there was an apparent minor trend towards greater efficacy among systemic corticosteroid use and physiological and morbidity related outcomes. Given the inconclusive nature of the results, a variety of recommendations for future research and practice have been identified. Firstly, it is imperative that all care providers involved in the management of patients with asthma ensure that patients are equipped with individualised action plans, which may help to improve uptake rates and reduce avoidable hospital admissions and its associated costs, as well as morbidity and potentially mortality. Secondly, adherence to steroids should be widely promoted and the importance emphasised to patients, as the intermittent use can lead to poorer asthma control. Thirdly, whilst the findings in this review and of that recommended by the Global Asthma Initiative do not support the routine use of oral steroids for acute exacerbations in ambulatory populations, a minor subset of patients with severe asthma may benefit from this, despite the potential long-term adverse effects. This is a clinician decision that should be diligently considered by primary and secondary care physicians. Finally, it is apparent that there is a lack of literature available upon evaluating the use of oral corticosteroids during acute asthma upon outcomes, such as symptom resolution, hospital admission, length of stay and mortality. Therefore, future research should attempt to address this research domain using longitudinal observational or randomised controlled trial design, although this may prove ethically challenging.
References
Ahmed, I., Sutton, A. J. and Riley, R. D. (2012) Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ, 344 (1), 1-7. Available from: http://www.bmj.com/content/344/bmj.d7762.abstract [accessed 06/04/2018].
Alangari, A. A. (2014) Corticosteroids in the treatment of acute asthma. Ann Thorac Med, 9 (4), 187-192. Available from: https://doi.org/10.4103/1817-1737.140120 [accessed 05/10/2018].
Andrade, C. (2015) The primary outcome measure and its importance in clinical trials. J Clin Psychiatry, 76 (10), 1320-1323. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26528658 [accessed 08/06/2018].
Asthma UK. (2014). Time to take action on asthma [online]. Available from: https://www.asthma.org.uk/globalassets/campaigns/compare-your-care-2014.pdf [accessed 05/10/2018].
Asthma UK. (2016). Your asthma action plan [online]. Available from: https://www.asthma.org.uk/globalassets/health-advice/resources/adults/adult-asthma-action-plan.pdf [accessed 05/10/2018].
Becker, J. M., Arora, A., Scarfone, R. J., Spector, N. D., Fontana-Penn, M. E., Gracely, E., Joffe, M. D., Goldsmith, D. P. and Malatack, J. J. (1999) Oral versus intravenous corticosteroids in children hospitalized with asthma. J Allergy Clin Immunol, 103 (4), 586-590. Available from: http://doi.org/10.1016/S0091-6749(99)70228-9 [accessed 05/10/2018].
Bettany-Saltikov, J. (2012) How to do a systematic literature review in nursing: a step-by-step guide. 1st edition. New York, NY, Open University Press.
Bonsignore, M. R., Profita, M., Gagliardo, R., Riccobono, L., Chiappara, G., Pace, E. and Gjomarkaj, M. (2015) Advances in asthma pathophysiology: stepping forward from the Maurizio Vignola experience. European Respiratory Review, 24 (135), 30-39. Available from: http://err.ersjournals.com/content/errev/24/135/30.full.pdf [accessed 30/09/2018].
Booth, A., Papaioannou, D. and Sutton, A. (2012) Systematic Approaches to a Successful Literature Review. 1st edition. London, UK, SAGE.
Bousquet, J., Bousquet, P. J., Godard, P. and Daures, J. P. (2005) The public health implications of asthma. Bull World Health Organ, 83 (7), 548-554. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16175830 [accessed 30/09/2018].
Bowling, A. (2009) Research Methods In Health: Investigating Health and Health Services. 1st edition. New York, NY, Open University Press.
Briel, M., Lane, M., Montori, V. M., Bassler, D., Glasziou, P., Malaga, G., Akl, E. A., Ferreira-Gonzalez, I., Alonso-Coello, P., Urrutia, G., Kunz, R., Culebro, C. R., da Silva, S. A., Flynn, D. N., Elamin, M. B., Strahm, B., Murad, M. H., Djulbegovic, B., Adhikari, N. K., Mills, E. J., Gwadry-Sridhar, F., Kirpalani, H., Soares, H. P., Abu Elnour, N. O., You, J. J., Karanicolas, P. J., Bucher, H. C., Lampropulos, J. F., Nordmann, A. J., Burns, K. E., Mulla, S. M., Raatz, H., Sood, A., Kaur, J., Bankhead, C. R., Mullan, R. J., Nerenberg, K. A., Vandvik, P. O., Coto-Yglesias, F., Schunemann, H., Tuche, F., Chrispim, P. P., Cook, D. J., Lutz, K., Ribic, C. M., Vale, N., Erwin, P. J., Perera, R., Zhou, Q., Heels-Ansdell, D., Ramsay, T., Walter, S. D. and Guyatt, G. H. (2009) Stopping randomized trials early for benefit: a protocol of the Study Of Trial Policy Of Interim Truncation-2 (STOPIT-2). Trials, 10 49-56. Available from: https://doi.org/10.1186/1745-6215-10-49 [accessed 08/08/2018].
British Thoracic Society. (2016). British guideline on the management of asthma [online]. Available from: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-quick-reference-guide-2016/ [accessed 30/09/2018].
Butland, B. K. and Strachan, D. P. (2007) Asthma onset and relapse in adult life: the British 1958 birth cohort study. Ann Allergy Asthma Immunol, 98 (4), 337-343. Available from: https://doi.org/10.1016/S1081-1206(10)60879-4 [accessed 30/09/2018].
Camargo, C. A., Spooner, C. H. and Rowe, B. H. (2003) Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev, (4), 1-12. Available from: https://doi.org/10.1002/14651858.CD001115 [accessed 05/10/2018].
CASP. (2018). CASP Checklists [online]. Available from: https://casp-uk.net/casp-tools-checklists/ [accessed 04/04/2018].
Coulter, A., Roberts, S. and Dixon, A. (2013). Delivering better services for people with long-term conditions [online]. Available from: https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/delivering-better-services-for-people-with-long-term-conditions.pdf [accessed 05/10/2018].
Cowell, J. M. (2014) Importance of Peer Review. The Journal of School Nursing, 30 (6), 394-395. Available from: https://doi.org/10.1177/1059840514557235 [accessed 02/09/2018].
D'Amato, G., Vitale, C., Molino, A., Stanziola, A., Sanduzzi, A., Vatrella, A., Mormile, M., Lanza, M., Calabrese, G., Antonicelli, L. and D'Amato, M. (2016) Asthma-related deaths. Multidiscip Respir Med, 11 37-41. Available from: https://doi.org/10.1186/s40248-016-0073-0 [accessed 25/08/2018].
Devidayal, Singhi, S., Kumar, L. and Jayshree, M. (1999) Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatr, 88 (8), 835-840. Available from: https://doi.org/10.1111/j.1651-2227.1999.tb00057.x [accessed 05/10/2018].
Dumas, O., Laurent, E., Bousquet, J., Metspalu, A., Milani, L., Kauffmann, F. and Le Moual, N. (2014) Occupational irritants and asthma: an Estonian cross-sectional study of 34,000 adults. Eur Respir J, 44 (3), 647-656. Available from: https://doi.org/10.1183/09031936.00172213 [accessed 30/09/2018].
Dumas-Mallet, E., Button, K. S., Boraud, T., Gonon, F. and Munafo, M. R. (2017) Low statistical power in biomedical science: a review of three human research domains. R Soc Open Sci, 4 (2), 1-6. Available from: https://doi.org/10.1098/rsos.160254 [accessed 05/08/2018].
Fanta, C. H., Rossing, T. H. and McFadden, E. R., Jr. (1983) Glucocorticoids in acute asthma. A critical controlled trial. Am J Med, 74 (5), 845-851. Available from: https://www.ncbi.nlm.nih.gov/pubmed/6340496/ [accessed 03/10/2018].
Farrugia, P., Petrisor, B. A., Farrokhyar, F. and Bhandari, M. (2010) Research questions, hypotheses and objectives. Canadian Journal of Surgery, 53 (4), 278-281. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2912019/ [accessed 23/04/2018].
Fuhlbrigge, A., Peden, D., Apter, A. J., Boushey, H. A., Camargo, C. A., Jr., Gern, J., Heymann, P. W., Martinez, F. D., Mauger, D., Teague, W. G. and Blaisdell, C. (2012) Asthma outcomes: exacerbations. J Allergy Clin Immunol, 129 (3), 34-48. Available from: https://doi.org/10.1016/j.jaci.2011.12.983 [accessed 28/08/2018].
Garg, R. (2016) Methodology for research I. Indian J Anaesth, 60 (9), 640-645. Available from: https://doi.org/10.4103/0019-5049.190619 [accessed 27/07/2018].
Global Asthma Network. (2018). The Global Asthma Report 2018 [online]. Available from: http://globalasthmareport.org/Global%20Asthma%20Report%202018.pdf [accessed 28/08/2018].
Global Initiative for Asthma. (2018). Global Strategy For Asthma Management And Prevention [online]. Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/ [accessed 02/06/2018].
Goertz, G. and Mahoney, J. (2012) Concepts and measurement: Ontology and epistemology. Social Science Information, 51 (2), 205-216. Available from: https://doi.org/10.1177/0539018412437108 [accessed 05/10/2018].
Gough, D., Thomas, J. and Oliver, S. (2012) Clarifying differences between review designs and methods. Syst Rev, 1 1-28. Available from: https://doi.org/10.1186/2046-4053-1-28 [accessed 26/07/2018].
Grewal, A., Kataria, H. and Dhawan, I. (2016) Literature search for research planning and identification of research problem. Indian J Anaesth, 60 (9), 635-639. Available from: https://doi.org/10.4103/0019-5049.190618 [accessed 27/07/2018].
Grove, S. K., Burns, N. and Gray, J. R. (2013) The Practice of Nursing Research: Appraisal, Synthesis, and Generation of Evidence. 7th edition. St Louis, MI, Saunders Elsevier.
Gupta, S. K. (2011) Intention-to-treat concept: A review. Perspectives in Clinical Research, 2 (3), 109-112. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3159210/ [accessed 03/07/2018].
Holgate, S. T., Wenzel, S., Postma, D. S., Weiss, S. T., Renz, H. and Sly, P. D. (2015) Asthma. Nature Reviews Disease Primers, 1 1-22. Available from: http://dx.doi.org/10.1038/nrdp.2015.25 [accessed 02/05/2018].
Horvath, G. and Wanner, A. (2006) Inhaled corticosteroids: effects on the airway vasculature in bronchial asthma. Eur Respir J, 27 (1), 172-187. Available from: https://doi.org/10.1183/09031936.06.00048605 [accessed 05/10/2018].
Jackson, D. J., Hartert, T. V., Martinez, F. D., Weiss, S. T. and Fahy, J. V. (2014) Asthma: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc, 11 139-145. Available from: https://doi.org/10.1513/AnnalsATS.201312-448LD [accessed 30/09/2018].
Jayaram, L., Pizzichini, M. M., Cook, R. J., Boulet, L. P., Lemiere, C., Pizzichini, E., Cartier, A., Hussack, P., Goldsmith, C. H., Laviolette, M., Parameswaran, K. and Hargreave, F. E. (2006) Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J, 27 (3), 483-494. Available from: https://doi.org/10.1183/09031936.06.00137704 [accessed 05/10/2018].
Kang, M., Ragan, B. G. and Park, J.-H. (2008) Issues in Outcomes Research: An Overview of Randomization Techniques for Clinical Trials. Journal of Athletic Training, 43 (2), 215-221. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2267325/ [accessed 26/06/2018].
Karanicolas, P. J., Farrokhyar, F. and Bhandari, M. (2010) Blinding: Who, what, when, why, how? Canadian Journal of Surgery, 53 (5), 345-348. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2947122/ [accessed 26/06/2018].
Kendall, J. M. (2003) Designing a research project: randomised controlled trials and their principles. Emergency Medicine Journal, 20 (2), 164-168. Available from: https://emj.bmj.com/content/emermed/20/2/164.full.pdf [accessed 02/07/2018].
Krishnan, J. A., Davis, S. Q., Naureckas, E. T., Gibson, P. and Rowe, B. H. (2009) An umbrella review: corticosteroid therapy for adults with acute asthma. Am J Med, 122 (11), 977-991. Available from: https://doi.org/10.1016/j.amjmed.2009.02.013 [accessed 05/10/2018].
Lee-Wong, M., Dayrit, F. M., Kohli, A. R., Acquah, S. and Mayo, P. H. (2002) Comparison of high-dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest, 122 (4), 1208-1213. Available from: http://doi.org/10.1378/chest.122.4.1208 [accessed 05/10/2018].
Levy, M. L. (2015) The national review of asthma deaths: what did we learn and what needs to change? Breathe, 11 (1), 14-24. Available from: https://doi.org/10.1183/20734735.008914 [accessed 28/08/2018].
Levy, M. L., Andrews, R., Buckingham, R., Evans, H., Francis, C., Houston, R., Lowe, D., Nasser, S., Paton, J. Y., Puri, N., Strewart, K. and Thomas, M. (2014). Why asthma still kills: The National Review of Asthma Deaths (NRAD) [online]. Available from: https://www.rcplondon.ac.uk/projects/national-review-asthma-deaths [accessed 28/08/2018].
Levy, M. L., Stevenson, C. and Maslen, T. (1996) Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax, 51 (11), 1087-1092. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8958890/ [accessed 05/10/2018].
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., Clarke, M., Devereaux, P. J., Kleijnen, J. and Moher, D. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med, 151 (4), 65-94. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19622512/ [accessed 30/05/2018].
Lin, R. Y., Pesola, G. R., Bakalchuk, L., Heyl, G. T., Dow, A. M., Tenenbaum, C., Curry, A. and Westfal, R. E. (1999) Rapid improvement of peak flow in asthmatic patients treated with parenteral methylprednisolone in the emergency department: A randomized controlled study. Ann Emerg Med, 33 (5), 487-494. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10216323 [accessed 05/10/2018].
Lipstein, E. A., Perrin, J. M. and Kuhlthau, K. A. (2009) School absenteeism, health status, and health care utilization among children with asthma: associations with parental chronic disease. Pediatrics, 123 (1), 60-66. Available from: https://doi.org/10.1542/peds.2008-1890 [accessed 28/08/2018].
Littenberg, B. and Gluck, E. H. (1986) A controlled trial of methylprednisolone in the emergency treatment of acute asthma. N Engl J Med, 314 (3), 150-152. Available from: https://doi.org/10.1056/NEJM198601163140304 [accessed 05/10/2018].
Long, H. (2014) An Empirical Review of Research Methodologies and Methods in Creativity Studies (2003–2012). Creativity Research Journal, 26 (4), 427-438. Available from: https://doi.org/10.1080/10400419.2014.961781 [accessed 05/10/2018].
Maselli, R. and Paciocco, G. (2000) Asthma: pathophysiology of the bronchial obstruction. Allergy, 55 49-51. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10919507 [accessed 30/09/2018].
Meline, T. (2006). Selecting Studies for Systematic Review: Inclusion and Exclusion Criteria [online]. Available from: https://pdfs.semanticscholar.org/a6b4/d6d01bd19a67e794db4b70207a45d47d82f3.pdf [accessed 01/09/2018].
Mhaskar, R., Emmanuel, P., Mishra, S., Patel, S., Naik, E. and Kumar, A. (2009) Critical appraisal skills are essential to informed decision-making. Indian Journal of Sexually Transmitted Diseases, 30 (2), 112-119. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3168054/ [accessed 13/05/2018].
Mims, J. W. (2015) Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol, 5 2-6. Available from: https://doi.org/10.1002/alr.21609 [accessed 30/09/2018].
MRC Collaborators (1956) CONTROLLED trial of effects of cortisone acetate in status asthmaticus; report to the Medical Research Council by the subcommittee on clinical trials in asthma. Lancet, 271 (6947), 803-806. Available from: https://www.ncbi.nlm.nih.gov/pubmed/13368522/ [accessed 05/10/2018].
Mukherjee, M., Stoddart, A., Gupta, R. P., Nwaru, B. I., Farr, A., Heaven, M., Fitzsimmons, D., Bandyopadhyay, A., Aftab, C., Simpson, C. R., Lyons, R. A., Fischbacher, C., Dibben, C., Shields, M. D., Phillips, C. J., Strachan, D. P., Davies, G. A., McKinstry, B. and Sheikh, A. (2016) The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Medicine, 14 (1), 113-118. Available from: https://doi.org/10.1186/s12916-016-0657-8 [accessed 28/08/2018].
Nici, L., Bontly, T. D., Zuwallack, R. and Gross, N. (2014) Self-management in chronic obstructive pulmonary disease. Time for a paradigm shift? Ann Am Thorac Soc, 11 (1), 101-107. Available from: https://doi.org/10.1513/AnnalsATS.201306-150FR [accessed 05/10/2018].
Nunan, D., Aronson, J. and Bankhead, C. (2018) Catalogue of bias: attrition bias. BMJ Evidence-Based Medicine, 23 (1), 21-22. Available from: https://ebm.bmj.com/content/ebmed/23/1/21.full.pdf [accessed 27/07/2018].
Nunes, C., Pereira, A. M. and Morais-Almeida, M. (2017) Asthma costs and social impact. Asthma Res Pract, 3 1-11. Available from: https://doi.org/10.1186/s40733-016-0029-3 [accessed 30/08/2018].
Papiris, S., Kotanidou, A., Malagari, K. and Roussos, C. (2002) Clinical review: severe asthma. Crit Care, 6 (1), 30-44. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11940264 [accessed 30/09/2018].
Pinnock, H., Parke, H. L., Panagioti, M., Daines, L., Pearce, G., Epiphaniou, E., Bower, P., Sheikh, A., Griffiths, C. J. and Taylor, S. J. (2017) Systematic meta-review of supported self-management for asthma: a healthcare perspective. BMC Med, 15 (1), 64-69. Available from: https://doi.org/10.1186/s12916-017-0823-7 [accessed 05/10/2018].
Pirracchio, R., Resche-Rigon, M., Chevret, S. and Journois, D. (2013) Do simple screening statistical tools help to detect reporting bias? Annals of Intensive Care, 3 29-32. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3847052/ [accessed 02/07/2018].
Poetker, D. M. and Reh, D. D. (2010) A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am, 43 (4), 753-768. Available from: https://doi.org/10.1016/j.otc.2010.04.003 [accessed 05/10/2018].
PRISMA. (2015). Prisma flow diagram [online]. Available from: http://www.prisma-statement.org/documents/PRISMA%202009%20flow%20diagram.doc [accessed 12/08/2018].
Qiu, X. and Wang, C. (2016) Literature Searches in the Conduct of Systematic Reviews and Evaluations. Shanghai Arch Psychiatry, 28 (3), 154-159. Available from: https://doi.org/10.11919/j.issn.1002-0829.215008 [accessed 15/09/2018].
Ramsahai, J. M. and Wark, P. A. B. (2018) Appropriate use of oral corticosteroids for severe asthma. MJA, 209 (2), 18-21. Available from: http://doi.org/10.5694/mja18.00134 [accessed 05/10/2018].
Ray, A., Oriss, T. B. and Wenzel, S. E. (2015) Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol, 308 (2), 130-140. Available from: https://doi.org/10.1152/ajplung.00070.2014 [accessed 30/09/2018].
Reddel, H. K. and Barnes, D. J. (2006) Pharmacological strategies for self-management of asthma exacerbations. Eur Respir J, 28 (1), 182-199. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16816348 [accessed 02/05/2018].
Resnik, D. B. (2015). What is Ethics in Research & Why is it Important? [online]. Available from: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm [accessed 23/05/2018].
Roberts, C. and Torgerson, D. J. (1999) Baseline imbalance in randomised controlled trials. BMJ, 319 (7203), 185-185. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1116277/ [accessed 06/06/2018].
Rodrigo, C. and Rodrigo, G. (1994) Early administration of hydrocortisone in the emergency room treatment of acute asthma: a controlled clinical trial. Respir Med, 88 (10), 755-761. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7846337/ [accessed 05/10/2018].
Rodrigo, G. J. (2005) Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med, 171 (11), 1231-1236. Available from: https://doi.org/10.1164/rccm.200410-1415OC [accessed 05/10/2018].
Rowe, B. H., Edmonds, M. L., Spooner, C. H., Diner, B. and Camargo, C. A. (2004) Corticosteroid therapy for acute asthma. Respir Med, 98 (4), 275-284. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15072167/ [accessed 05/10/2018].
Rowe, B. H., Spooner, C. H., Ducharme, F. M., Bretzlaff, J. A. and Bota, G. W. (2007) Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev, (3), 1-12. Available from: https://doi.org/10.1002/14651858.CD000195.pub2 [accessed 05/10/2018].
Sadatsafavi, M., Rousseau, R., Chen, W., Zhang, W., Lynd, L. and FitzGerald, J. M. (2014) The preventable burden of productivity loss due to suboptimal asthma control: a population-based study. Chest, 145 (4), 787-793. Available from: https://doi.org/10.1378/chest.13-1619 [accessed 28/08/2018].
Scarfone, R. J., Loiselle, J. M., Wiley, J. F., Decker, J. M., Henretig, F. M. and Joffe, M. D. (1995) Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med, 26 (4), 480-486. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7574132/ [accessed 05/10/2018].
Scells, H., Zuccon, G., Koopman, B., Deacon, B., Azzopardi, L. and Geva, S. (2017). Integrating the Framing of Clinical Questions via PICO into the Retrieval of Medical Literature for Systematic Reviews [online]. Available from: https://dl.acm.org/citation.cfm?id=3133080 [accessed 27/07/2018].
Schneider, S. M., Pipher, A., Britton, H. L., Borok, Z. and Harcup, C. H. (1988) High-dose methylprednisolone as initial therapy in patients with acute bronchospasm. J Asthma, 25 (4), 189-193. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3053605/ [accessed 05/10/2018].
Schuh, S., Dick, P. T., Stephens, D., Hartley, M., Khaikin, S., Rodrigues, L. and Coates, A. L. (2006) High-dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics, 118 (2), 644-650. Available from: https://doi.org/10.1542/peds.2005-2842 [accessed 06/10/2018].
Schuh, S., Reisman, J., Alshehri, M., Dupuis, A., Corey, M., Arseneault, R., Alothman, G., Tennis, O. and Canny, G. (2000) A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. N Engl J Med, 343 (10), 689-694. Available from: https://doi.org/10.1056/NEJM200009073431003 [accessed 05/10/2018].
Suresh, K. (2011) An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci, 4 (1), 8-11. Available from: https://doi.org/10.4103/0974-1208.82352 [accessed 05/08/2018].
Torjesen, I. (2014) Two thirds of deaths from asthma are preventable, confidential inquiry finds. BMJ, 348 1-3. Available from: https://www.bmj.com/content/bmj/348/bmj.g3108.full.pdf [accessed 28/08/2018].
Tsafnat, G., Glasziou, P., Karystianis, G. and Coiera, E. (2018) Automated screening of research studies for systematic reviews using study characteristics. Syst Rev, 7 (1), 64-68. Available from: https://doi.org/10.1186/s13643-018-0724-7 [accessed 02/09/2018].
UK Research Integrity Office. (2009). Code Of Practice For Research: Promoting good practice and preventing misconduct [online]. Available from: http://ukrio.org/wp-content/uploads/UKRIO-Code-of-Practice-for-Research.pdf [accessed 01/09/2018].
Wenzel, S. (2012) Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy, 42 (5), 650-658. Available from: https://doi.org/10.1111/j.1365-2222.2011.03929.x [accessed 30/09/2018].
World Health Organisation. (2018). Chronic respiratory diseases: Asthma [online]. Available from: http://www.who.int/respiratory/asthma/en/ [accessed 28/08/2018].
Yunginger, J. W., Reed, C. E., O'Connell, E. J., Melton, L. J., O'Fallon, W. M. and Silverstein, M. D. (1992) A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis, 146 (4), 888-894. Available from: https://doi.org/10.1164/ajrccm/146.4.888 [accessed 30/09/2018].
Appendices
Table 3. Summary of included studies.
|
Article details |
Aim |
Study Design |
Details of Participants |
Results |
Limitations |
|
Becker et al. (1999) United States |
Determine the effectiveness of oral prednisolone versus intravenous methylprednisolone for acute asthma exacerbations |
Randomised, double-blind, placebo controlled trial |
Children aged 2-18 years N=66 |
Prednisolone group observed non-significant shorter mean length of stay (p=0.52), than the methylprednisolone group. Patients receiving prednisolone observed significant reductions in oxygen requirements (p=0.04), than the comparator group. |
Authors used a higher than recommended dose of oral prednisolone, which means the results may not be applicable to standard practice. Variability in Emergency Department management may increase the risk of confounding and bias. |
|
Devidayal et al. (1999) India |
Evaluate the efficacy of nebulised budesonide as compared to oral prednisolone in acute asthma |
Randomised, double-blind, placebo controlled trial |
Children aged 2-12 years N=80 |
The oxygen saturations, respiratory rate, pulmonary index and respiratory distress score were significantly improvement among patients in the budesonide group, as compared to prednisolone (p<0.01). Moreover, a greater proportion of patients receiving budesonide met the criteria for discharge. |
No control for the dose of budesonide administered, given the large variability in dose delivery to the target tissue when using nebulisation. This could have resulted in under- or over-estimated of the outcome effects. |
|
Lee-Wong et al. (2002) United States |
Investigate whether the use of inhaled Flunisolide is as effective as systemic corticosteroids in patients with severe asthma exacerbations |
Randomised, double-blind, placebo controlled trial |
Adults aged 18-55 years N=40 |
Both groups observed significant improvements in peak expiratory flow rate, but there were no significant between group differences observed (p=0.33). The same effect was demonstrated for forced expiratory volume in one second (p=0.39). |
The study was reported to be underpowered although no calculation was provided, which markedly increases the risk of reporting bias. Did not report upon patient symptoms, which may have been an additional useful outcome measure. |
|
Lin et al. (1999) United States |
Evaluate the impact of intravenous methylprednisolone upon airflow in patients with acute asthma |
Randomised, double-blind, placebo controlled trial |
Adults aged >18 years N=56 |
The methylprednisolone group observed significant increases in peak expiratory flow rate and its predicted value, than compared to control (p=0.002 and p=0.005, respectively). There were also significant improvements in mean heart rate among methylprednisolone users, than control (p=0.029). |
The study is inadequately powered as to detect clinically meaningful differences in the defined outcome measures, which increases the risk of reporting bias. Inclusion of patients who smoked cigarettes, although less than 20 pack years, may have confounded the outcomes. |
|
Rodrigo (2005) Uruguay |
Compare the effect of inhaled fluticasone with standard treatment of systemic corticosteroids in severe acute asthma |
Randomised, double-blind, placebo controlled trial |
Adults aged 18-50 years N=106 |
Subjects who received fluticasone observed significant improvements in peak expiratory flow and forced expiratory volume, than compared to hydrocortisone (p<0.05). Patients with more severe baseline asthma and treated with fluticasone showed significant need for hospital admission, than compared to hydrocortisone (p=0.05). |
The inclusion of patients from Uruguay may not be generalisable to more western populations, who may observe differing disease courses and risk factors. In addition, the inclusion of those with only severe asthma further limits the applicability to more general asthmatic populations. |
|
Schuh et al. (2000) Canada |
Compare the efficacy of fluticasone to prednisolone in patients with severe asthma. |
Randomised, double-blind trial |
Children aged 5-17 years N=100 |
Both groups observed significant improvements in forced expiratory volume after fours hours of therapy (p<0.001), but no between group differences were demonstrated. Significantly more patients in the fluticasone group required hospital admission, as compared to the prednisolone group (p=0.01). |
The limited use of pulmonary function tests as the main outcome measure, limits the meaning of the findings, as symptoms for example were not considered. The inclusion of patients with only severe asthma limits the applicability of the findings to more general asthma populations. |
|
Schuh et al. (2006) Canada |
Determine the effect of fluticasone and prednisolone upon forced expiratory volume in mild and moderate asthma |
Randomised, double-blind trial |
Children aged 5-17 years N=69 |
After 240 minutes, the forced expiratory volume significantly increased in both groups, but this became insignificant at 48 hours (p=0.14). Moreover, there were no significant between group differences. Relapse rates were higher among the fluticasone group, but this was not statistically significant. |
The sole use of forced expiratory volume as the outcome measure, limits the meaning of the findings, as symptoms and other measures of lung function were not considered. The inclusion of patients with mild to moderate asthma as defined in the study, limits applicability to this group and not those with very mild or severe asthma. |
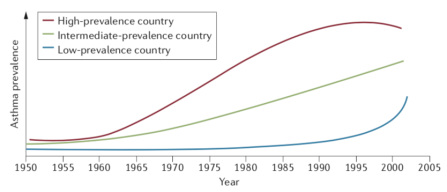
Figure 1. Prevalence of asthma according to gross domestic product (GDP; red (high GDP), green (intermediate) GDP and blue (low GDP) (Bousquet et al., 2005).

Figure 2. Asthma UK (2016) personalised action plan template.
Figure 4. Example screenshot of PubMed/MEDLINE search.
Cite This Work
To export a reference to this article please select a referencing stye below: