REMOVAL OF METALS FROM INDUSTRIAL WASTEWATER
Info: 8826 words (35 pages) Dissertation
Published: 12th Dec 2019
Tagged: Environmental Science
Contents
2.0. Types of metals and their sources
3.0 Environmental and health risks
4.0 Wastewater Treatment Process
5.1.1Electrochemical treatments
5.1.2Physicochemical processes
5.2.1 Membrane filtration processes
5.2.4 Adsorption on new adsorbents
6.0 Liquid and biosolids discharge
7.0 Determining knowledge Gaps
List of tables
Table 1 Contaminants, their MCL and effects
Table 2 Removal of heavy metals by EC
Table 3 Removal of heavy metals by EF
Table 4 Removal of heavy metals using various adsorbents .
Table 5 Removal of heavy metals using NF .
Table 6 Removal of heavy metals by reverse osmosis process .
Table 7 Removal of heavy metals by UF .
Table 8 Adsorption capacity of natural materials for heavy metals.
Table 9 Adsorption capacity of agricultural waste .
List of figures
Figure 1 Flow diagram for wastewater treatment .
1.0 Introduction
The foremost thing required by all the living organisms is the access to clean water. Due to inflation of population, not only the pressure on the reserves increase but contaminations of these natural resources is increasing day by day. The pollutants discharging from various industries are making the scenario worse[1].Any water that has been affected by the use of humans is called wastewater. Therefore wastewater is the result of all the industrial, domestic, agricultural and commercial activities. The wastewater has different characteristics depending upon its source. Mainly wastewater can be divided into three types domestic wastewater(household), municipal wastewater(communities) and industrial wastewater(industrial activities)[1].
The term treatment of wastewater means upgrading or improving the quality of wastewater by using all the knowledge and technologies that we have[2]. There is a great need for treatment of wastewater so that human can efficiently use the water resources for various activities like swimming, fishing and for drinking water and animals can also have a healthy ecosystem for a living. Humans have devastating effects on the water resources with their various development activities. There is a massive increase in contaminants in waterways which leads to various problems like fish kills, low dissolved oxygen, and bacterial contamination. [4].
Typical contaminants in wastewater include BOD, TSS, Metals, Faecal Coliforms, Nutrients. The focus of this study is the treatment of heavy metals in industrial wastewater. The presence of heavy metals above the standard can cause serious health problems and environmental degradation. There are various efficient technologies to tackle this problem. Conventional techniques like Electrochemical treatments, chemical precipitation, and adsorption. More efficient and current techniques nanotechnology, membrane filtration process and adsorption on new adsorbents[6].
All the process are critically analyzed with respect to the technical, cost and environment perspective. Various technical and scientific challenges faced by these process. This study is not only reviewing these process but also pointing out the knowledge gaps or the areas of improvement for future studies.
2.0. Types of metals and their sources
Heavy metals are those metals which have very high density and are highly poisonous even at low concentrations. These heavy metals include mercury (Hg), cadmium (Cd), arsenic(As), chromium (Cr), thallium (Tl), zinc (Zn), nickel (Ni), copper (Cu) and lead (Pb). Municipal wastewater, industrial wastewaters, landfill leaches, mining wastes, and urban runoff are the main sources of contamination. [5[[6][7].Industries are the major source of heavy metals in wastewater. The electroplating industry’s wastewater contains a great number of metals like (cadmium zinc, lead, chromium. nickel, copper, platinum, silver, titanium). Wood processing industry is also a major source of metal in wastewater. Paint and enameling industries also release their effluents containing nickel into water bodies. PCB manufacturing industries are also a very significant source of producing metal waste[5][6][7].
3.0 Environmental and health risks
These metals are naturally found in the environment but the main concern is that their concentration in industrial wastewater is getting higher day by day. These metals are very soluble in an aquatic environment and can be transported with sediments. Metals are non-biodegradable in nature and can enter in our food chain. If these metals are presented more than permissible limits it a serious health-related issues, therefore, it becomes very important to treat the wastewater containing metals before discharging it into the environment.[6][7]
The effluent regulations are established under the Fisheries act by Canadian council of ministers of the environment Canada. It sets the mandatory minimum effluent quality that should be achieved before discharging the effluent into the environment. In order to reduce adverse effect on human health, fish habitat, and environment.
Table 1 Contaminants, their MCL and effects[5][6]
| Heavy Metals | Effects | MCL
(mg/l) |
||
|
|
0.050 | ||
| Cadmium |
|
0.01 | ||
| Chromium |
|
0.05 | ||
| Copper |
|
.25 | ||
| Mercury |
|
0.00003 | ||
| Zinc |
|
0.80 | ||
| Nickel |
|
0.20 | ||
| Lead |
|
0.006 |
4.0 Wastewater Treatment Process
The treatment of wastewater is broadly divided into four major parts[3].
1 Preliminary Treat 2 Primary Treatment 3 Secondary Treatment 4 Tertiary/Advanced Treatment
1. Preliminary Treatment This is the very first part of treatment it includes removal all those of solid objects or coarse material(rags, sticks etc) that can lead to maintenance and operational problems.
2. Primary treatment This includes partial removal of organic matter and suspended matter with help of filtration and some chemicals.
3.Secondary treatment This includes further removal of suspended solid along with biodegradable organic matter and some nutrients. Disinfectant is also added during this phase of treatment.
4.Tertiary treatment This is the last step of treatment it included removal of suspended solids and nutrients left after secondary treatment. It is included the removal of dissolved contaminants in wastewater.
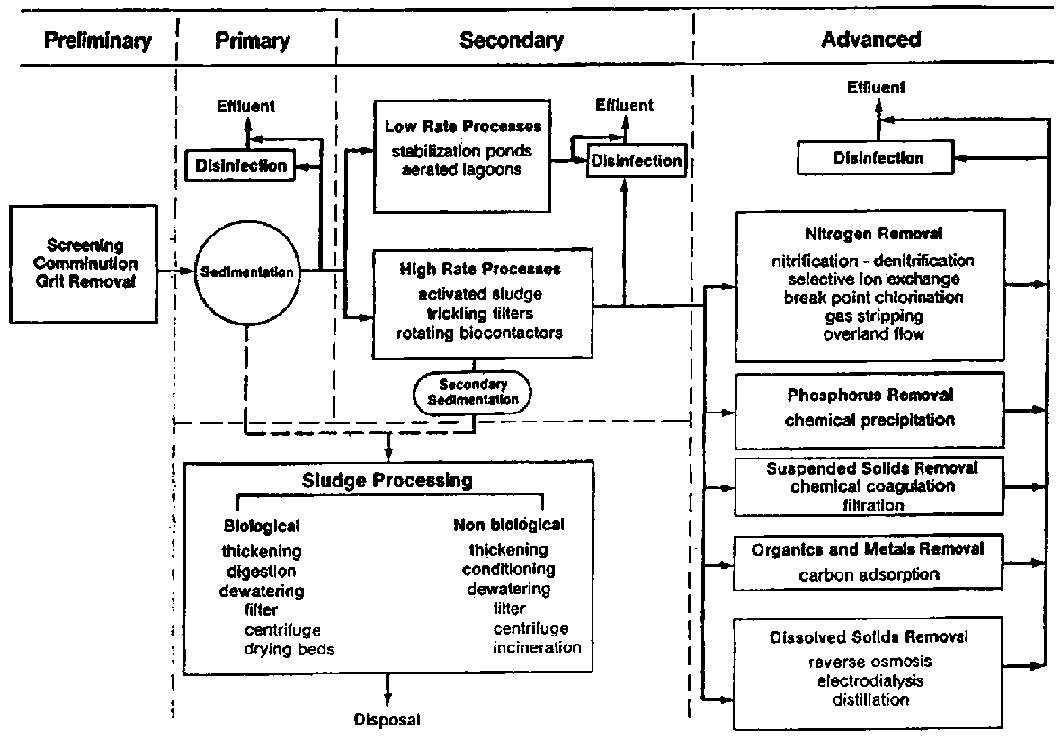
Figure 1 Flow diagram for wastewater treatment [2].
5.0 Remediation of wastewater
The treatment of wastewater containing metals can be broadly divided into two categories
- Conventional methods for wastewater treatment.
- Current methods for wastewater treatment
5.1 Conventional methods
Technologies for treatment of wastewater containing heavy metals were conventionally based on the principles of physiochemical electrochemical and advanced oxidation processes
5.1.1Electrochemical treatments This method include the use of electric current for heavy metal removal. It is divided into three parts
1.Electrocoagulation 2.Electroflotation 3.Electrodeposition
5.1.1.1 Electrocoagulation
It is very simple and useful method used for the treatment of wastewater[8]. The use of this method is limited by extremely poor design of the reactor and difficulties related to electrode reliability. A wide range of pollutants is removed by this method[9][6].
In simple words, the electrocoagulation reactor is an electrolytic cell. Which contains sacrificial electrodes that is one anode and one cathode. These electrodes are commonly made of aluminium or iron. These electrodes can be of same or different material[6]. In EC process small electric current is applied to wastewater solution. Firstly the pollutants like metals are retained in wastewater by the hydrogen bond or by their surface charge. When an electric field is introduced the electrostatic charges holding these pollutants are neutralized and then they will again form coagulated. Then the metals in wastewater bond together to form a floc[11][12]. The advantage of EC is that the flocs formed are of bigger size than in other techniques moreover these flocs are more stable and have less bound water. Later on processes like filtration remove these flocs easily [6][10].
Its procedure is summarised as below[10]
- Anode dissolution;
- OH− and H2 generation at the cathode;
- Electrolytic reactions at electrode surfaces;
- Coagulant adsorption on colloidal pollutants;
- Process of sedimentation or flotation removes collides
The main reactions for Al electrode are as follows
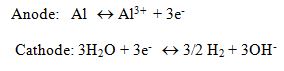
Al3+ and OH– ions formed at the electrode surfaces react in the wastewater:
One experiment also shows that metal ions can also be removed with electrocoagulation. This experiment uses hydroxide iron and steel flocs as adsorbents. Current density is directly proportional to the removal efficiency if current increases efficiency increases because of the rate of formation of hydroxide and steel flocs increases.EC is very environment-friendly because the reagents used in the process are electrons which are clean and eliminates the use of chemical additives which decreases the further pollutant generation up to great extent[10].
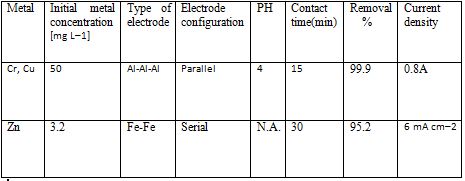
Table 2 Removal of heavy metals by EC [6]
5.1.1.2 Electroflotation
Electroflotation method is used to remove heavy metals from wastewater when the solution is very dilute. This process is simple in design and operation and is environmentally friendly. In electroflotation heavy metals are separated by floating metals to the surface of liquid phase this is done in three parts. First part involves attraction of pollutants to the reactor having two electrodes and a power supply[13]. The overall water electrolysis reaction occurring in a reactor is
2H2O2 O2 + 2H2
Heavy metal will take in molecules of hydrogen and oxygen then destabilization of emulsified particles will occur resulting in the formation of flocs.[6]. Then occurs the separation of foam generated and the settled flocs. Third and the last step is the removal of pollutant collected by methods like filtration[14][15].
The efficiency of EF is evaluated on the basis of pollutant removed to the power consumed. Factors affecting these two factors are material of the electrode, cell design, size of the bubble formed during electrolysis.
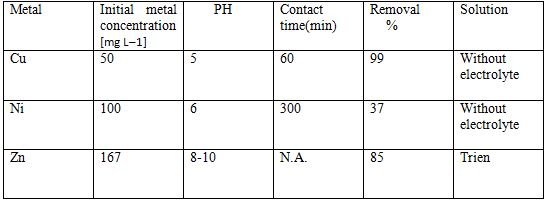
Table 3 Removal of heavy metals by EF [6]
To overcome the limitations of both the methods some researchers have combined the processes of electrocoagulation and electroflotation. This combined method is efficient in every way. The pollution removal efficiency of this combined method is more and safe in every way. The combined name of this method is Electro coagulation- floatation. Some tests were conducted to check its efficient it was able to remove 97% of heavy metals with very less power consumption[10].
5.1.1.3 Electrodeposition
This is a very advantageous process as there is no sludge formed in this process. This process has very low operating cost.[18][19]. This is the most efficient and environmentally friendly process among electrochemical processes for the removal of heavy metals from the wastewater[16][17].By deposition of ionic conductors, the dissolved metals ions are converted into solid particles to protect them from corrosion. This is a simple process containing a current source one anode and cathode in an electrolytic cell and simple oxidation and reduction of heavy metals take place in this cell[18].Finally, the reduced metals are electroplated on the cathode[20]. The reaction is as follows
Mn+ + ne– M
In order to prevent the anodes from obstructing the heavy metal recovery process, they are most likely made of insoluble or inert material[18]. A reaction at anode side is mentioned below
4OH─ O2+ 2H2O +4e–
There is a reduction of hydrogen ion H+ to hydrogen gas by one of the competing reactions occurring during the process. The reaction is mentioned below[18].
H+ + e– 0.5H2O
The physical parameters and initial concentration of the wastewater greatly affect the efficiency of the process[18]. This process can be applied to both the aqueous and non-aqueous solutions. There is a better removal of pollutants by using non-aqueous solution or solutions containing chelating agents[21].
There are problems related to both the process like in case of aqueous solutions liberation of the hydrogen molecule is one of the problems. On the other hand using non-aqueous solution face some technical issues like corrosion of cell components, low current efficiency etc[21].
5.1.2Physicochemical processes This process involves removal by a change in chemical and physical properties. It is mainly of three types
1. Chemical precipitation 2. Hydroxide precipitation 3. Sulfide precipitation
5.1.2.1 Chemical precipitation
This process is very commonly used for removing heavy metals from wastewater. This process is very simple easy and automated treatment process. In order to bring metal ions concentration within acceptable limits lot of chemicals are used in the process to treat wastewater containing heavy metals. All these chemicals are further a large source of pollution itself[22].
The PH is an important factor in this process. In basic conditions, PH should be maintained 11 for more efficient removal of heavy metals from wastewater. Insoluble solid particles are formed by reaction between chemical agents and heavy metal ions. These solid particles can be separated with the with help of processes like filtration and sedimentation[6].
There are various chemicals used for chemical precipitation but the most common ones are lime and calcium hydroxide. Lime is preferred over the other but the considerably high dose is required to reduce the heavy metal concentration to acceptable limits[6].
5.1.2.2 Hydroxide Precipitation
This process uses polymers and a variety of coagulants, for example, iron, salts, and alum for more efficient removal of heavy metal from wastewater. Processes like sedimentation and filtration lead to precipitation of soluble metals as hydroxide. Alkaline agents are used to increase the PH of the wastewater this, in turn, decreases the solubility of metal ions in wastewater and they precipitate out of wastewater[23]. Hydroxide precipitation reaction is as follows
M2+ 2OH– M(OH)2
M(OH)2 is insoluble. There is a formation of a soluble complex metal compound if PH is less than optimum.
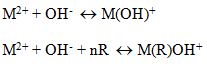
Hydroxide precipitation is effected by the presence of organic radicals. Moreover, alkaline compounds are just added to improve the hydroxide precipitation[23].
5.1.2.3 Sulfide Precipitation
The process of sulfide precipitation is quite similar to hydroxide precipitation. Metals ions are precipitated as metal sulfides using sulfide. Gravity settling or filtration method is used to remove the sludge produced. Both pre and post-treatment in this process is required due to the toxicity of the sulfide. Metal ions precipitate same as in hydroxide precipitation[23].
M2+ 2(OH)– M(OH)2
M2+ represents metal ion andOH– represents precipitant[23].
5.1.3 Adsorption It is another method used to remove heavy metals from wastewater. It works on the principle of mass transfer between the liquid phase and solid phase called adsorbent. The process of sorption of pollutants onto an adsorbent is divided into three parts.1 The penetration of pollutant onto an adsorbent from the bulk solution.2 Adsorbent of the pollution on the adsorbent surface.3 Penetration in the adsorbent structure[5].Most commonly used adsorbents are
1.Activated carbon 2.Carbon nanotubes 3.Wood sawdust
5.1.3.1 Activated carbon
Activated carbons are generally prepared from agricultural by-product when K2CO2 is used. Activated carbons are extensively used for the removal of heavy metals from wastewater. It has been studied that carbon prepared at 900is more effective[25].
The PH value also affects the adsorption value. A test was conducted and it showed 100% removal of nickel within the PH range 0f 2-5[25].
5.1.3.2 Carbon nanotubes
Carbon nanotubes are well known for their properties and applications. The CNTs have unique properties like thermal stability, high surface area, chemical stability. The removal of heavy metal by adsorption to carbon nanotubes is a very complex mechanism[24].
5.1.3.3 Wood sawdust
It is a low-cost adsorbent of heavy metals obtained as a waste product from the mechanical wood processing. Mainly comprises of materials that have a good capacity for binding metal cations. Cellulose(40%to 50%) and Lignin (20% to 30%)[6].
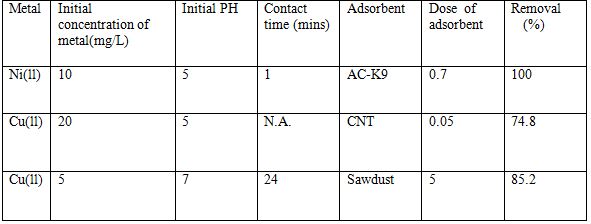
Table 4 Removal of heavy metals using various adsorbents [6].
5.1.4 Ion exchange method
In this process, a reversible exchange of ions between liquid and solid phase occurs. The first step is the initiation of ion exchange reactions and then physical absorption of heavy metal ions take place after that counterion and functional group form a complex. At last on the surface of the adsorbent hydration takes place. Various factors like temperature, time of contact, PH, the initial concentration of adsorbent affect the ion exchange method[6].
Ions from the electrolytic solution are removed with the help of resin and other ions of the similar charge in chemical equivalent amount is added to the electrolytic solution[6].
In some cases, physiochemical interactions take place during the capturing of heavy metal ions because the structure of some resins with acidic functional groups contains sulfonic acid[6].
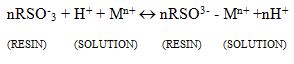
n is the constant of the reaction species, with respect to the metal ions oxidation state and the anionic group related to the ion exchange ion is represented by -RSO–[30].
This process shows the removal of the heavy metal ion from the wastewater with the help of insoluble resin and it releases other ions of similar charge and there is no change in the structure of the resin[32].
This ion exchange method is also very useful in recovering heavy metals from the inorganic effluents[30]. This method is usually preferred to the other methods because of various benefits like high efficiency, low cost, and less sludge volume. So this method is a good choice for removal of heavy metal ions from the waste water[6].
Some of the commonly used resins are synthetic polymer resins example styrene-divinylbenzene and the other are gel-like which are sometimes considered more efficient and costs less[31].
5.2 Current methods
5.2.1 Membrane filtration processes
These membrane processes are used due to their higher efficiency, no pollution and lower cost than the conventional treatment processes. All these advantages help this process in the treatment of heavy metals from wastewater. The basic procedure is shared by different membrane process with minor changes. In this technique, separation is achieved by higher trans-membrane pressure. There are various semi-permeable membranes with different pore sizes. When any membrane comes in contact with the suitable phase it lets the solution pass through it and catches the solute. The membrane used in these process have different aspects[6].
Membrane characteristics
It is a porous or a non-porous structure that removes the pollutant of various sizes and acts as a contact between two homogeneous phases.. The three types of membranes used in this process are hybrid pressure-driven and liquid membranes. Factors like pore size and composition can affect the efficiency and cost of the membrane processes[26]. Material plays an important role so material for membrane should have greater chemical resistance. In general, materials used for membrane are composite metallic and ceramic material[26][27].
Composite materials are generally are preferred for making membranes because of factors like cost and their porous structure. Moreover, the membranes of this material can be applied to various filtration process like microfiltration and reverse osmosis[26].
Membrane filtration process Five treatment processes of membrane filtration technology are[26].
1.Reverse osmosis 2.Ultrafiltration 3.Microfiltration
4.Nanofiltration 5.Electrodialysis
The above-mentioned techniques or processes have the same basic principle the only differences is in their pore structure, applied operating pressure and permeability of the membrane[26].
Microfiltration operates at low pressure (0.1 to 2 bar) and the membrane used is a membrane. porous[26]
Ultrafiltration operates at low pressure (1 to 5 bar) and membrane used is a porous membrane[26].
Reverse Osmosis operates at low pressure (10 to 20 bar) and the membrane used is non-porous [26].
Nanofiltration operates at low pressure( 10 to 20 bar) and the membrane used is porous[26].
5.2.1.1 Nanofiltration(NF)
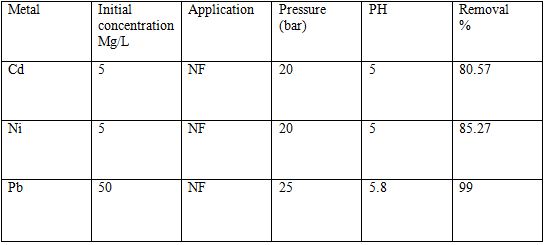
Nanofiltration is a new technology used for separation of heavy metals from wastewater this technique was developed to overcome the disadvantages of the conventional methods. This technique is used really small pores in order to remove larger molecules. The efficiency of the Nanofiltration process can be increased by introducing techniques like ultraviolet treatment and interfacial polymerization[6].
The whole process of nanofiltration can be divided into three steps. The first step is pre-filtration that requires pre-treatment of the influent before entering the system. Filtration, coagulation-adsorption process, ion exchange method, and flocculation are some of the adopted pre-treatment techniques [6]. The second step treatment of pollutant by nanofiltration membrane is achieved by the combination of three processes steric hindrance and dielectric effects. With the help of steric hindrance, neutral solutes are removed. Ion size and Donnan potential help in removal of charged components. The last step is the post-treatment of the effluent before discharging it into the environment[6].
Table 5 Removal of heavy metals using NF [6].
5.2.1.2 Reverse Osmosis(RO)
The process of reverse osmosis is widely used after 1950. This is a pressure driven process and now this technique is gaining lot of importance and is widely used in treatment of wastewater containing heavy metals. The process of reverse osmosis takes place under hydraulic operating pressure and the membrane used in semi-permeable. The flow of particles through the membranes occurs by diffusion[28][33].
There are three steps involved in the treatment of pollutants by reverse osmosis. Firstly the wastewater with heavy metal ion concentration is absorbed on the surface of the membrane. Presence of concentration gradient leads to the diffusion of ions through membrane.[28][33].
The molecules of water travel to the other side of the membrane by the diffusion process and the metal ions on the feed side when this process is over there is a nearly pure solution on the permeate side and heavy metal concentration on the feed side. These metal ions accumulated on the feed side needs proper treatment before they are released into the environment[28][33].
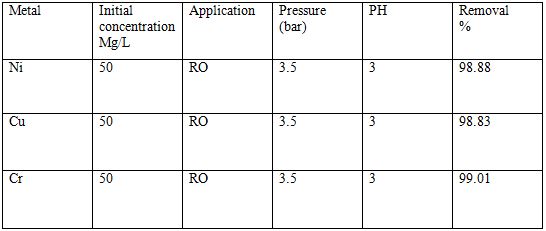
Table 6 Removal of heavy metals by reverse osmosis process [6].
5.2.1.3 Microfiltration and Ultrafiltration
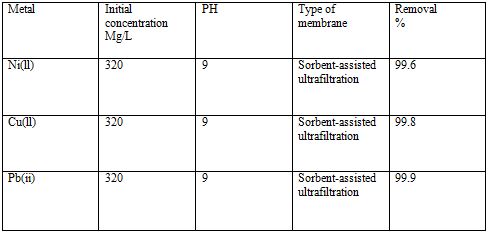
These processes are almost similar to each other both have usage in the same area, both use same separation procedure and both are pressure driven processes. The only difference is that the solutes removed by the ultrafiltration are smaller than removed by microfiltration, so the particle size is the deciding factor for these two processes[29]. Ultrafiltration rejects the pollutants that are in the form of dissolved macromolecules and colloidal materials. On the other hand, microfiltration rejects suspended particles. Both these processes are widely used in various industries but the most important is the treatment of wastewater with the help of these processes[29].
The membranes differ in their structure, material, and porosity. The efficiency of these processes greatly depend on the membranes o the membranes should be choosing appropriate membrane. Both of these processes use porous membranes[28]. On the basis of structure, there are to types of membranes symmetric membrane and asymmetric membrane. The symmetric membrane does not change its structural characteristics over the membrane cross-section. Symmetric membranes are usually used for microfiltration. Ultrafiltration uses asymmetrical membranes[28][29].
The material used for membranes in microfiltration and ultrafiltration process include hydrophilic and hydrophobic membranes and sometimes crystalline polymers are used. The use of hydrophilic membranes decreased due to their thermal resistance and other two are widely used[29].
There are two methods named as a dead-end method and cross-flow method used for filtration by the microfiltration and ultrafiltration processes[28][29].
In the dead-end method, the membrane is at 90 degrees to the feed flow and the feed flow passes tangentially through the membrane. The dead-end method have no reject stream[28]. The cross-flow method is the more complex method but the lifetime and flux rate is higher in this method[31].
The separation mechanism of microfiltration and ultrafiltration is based on the principle of molecular sieving with the help porous membranes. So the membrane rejects the particles larger than the pore size [28][29].
Table 7 Removal of heavy metals by UF [6].
5.2.1.4 Electrodialysis
This process is widely used for the treatment of wastewater. Electrodialysis is a novel liquid hybrid membrane process. The membrane used in electrodialysis is ion selective exchange membrane. These membranes do not accept anions and cations at the same time. These are further divided into two types. Anion exchange membrane and cation exchange membrane. Concentration gradient is the reason for the transportation of ions through the membrane [5].
The process that is done by reversing the polarity of electrodes of electrodialysis is called electrodialysis reversal . This process sometimes shows better results than the electrodialysis. The only drawback of this process is it requires more electrical control[5].
5.2.2 Photocatalysis process
This technique is developed lately and its usage can be found in various industries. This technique is very helpful in removing heavy metals from wastewater before releasing it into the environment[6]. This technique is preferred over the chemical process. In order to harness light of suitable wavelength semiconductors are used. These semiconductors are non-toxic. Other advantages that make this technique better than other methods include its higher removal efficiency, high stability, the design is simple and operational cost is low[6].
This is a multi step treatment technique. In the initial step, the pollutants in the aqueous phase rise to the surface and second step involve the absorption of these pollutants on the surface of semiconductors. Then the third and most important step of treatment in this step the photocatalytic reactions occur and at last pollutants are removed from the wastewater. The old process of thermal activation is removed because in this technique light is used as an activator[37].
There is excitation in photons of the semiconductor. The promotion of electrons to conduction band from valence band is done by illuminating them with a light whose energy is equal or greater than semiconductor’s energy(band gap energy). This light is usually visible light[6]. This leads to the formation of electron-hole pairs and a photoelectron(e–) is created in conduction band whereas a photo hole(h–) is created in the valence band. This reaction takes place[6]
SC + hv e– + h–
Alongside there is oxidation and reduction going on of the pollutants due to the formation of an electron-hole pair. The reactions are as follows[6]
A + e– A– Reduction reaction
D + h+ D+ Oxidation reaction
Alphabet A and Alphabet D here represents pollutants
The treatment of heavy metals is expressed by the following reaction
MN+ + e– M
The most suitable material for semiconductors till now is TiO2 because of various features like cheaper, chemically inert, stable against photo-corrosion and other environment-friendly features[6].
This techniques still have some drawbacks like unwanted by-products, faults related to absorption of visible light and loss of energy by recombination[38].
5.2.3 Nanotechnology
Some materials show great properties at nanoscale than in bulk. Properties like chemical reactivity and specific area are complete different at nanoscale. Due to their high specific area, they are perfect absorbents for treatment of wastewater containing heavy metals. Two techniques of nanotechnology are used for the treatment of wastewater named as in situ and ex-situ. In situ is the treatment of wastewater at the place of contamination. Ex situ is the treatment of wastewater by nanomaterials but before treatment wastewater contaminants are transferred to a more suitable place for treatment. In situ treatment is always preferred because it is cheaper and removal efficiency is more than ex situ treatment[35].
Adsorptive, reactive and hybrid magnetic are the types of nanoparticles. The mechanism of adsorption used by nanoparticles is explained in the section of nanomembranes. As the name suggests the hybrid magnetic nanopartieces consists of two or more nanoscale components and out of which one is magnetic[35]. They are widely used because of properties like a high surface area to volume to ratio, low toxicity, cheaper and magnetic properties. Commonly used hybrid magnetic nanoparticles are magnetite and maghemite. These particles can be regenerated through desorption process[35].
Overall the advantages there is a major drawback of these nanoparticles which include a high risk of nano pollutants in the environment. These nano pollutants are even more difficult to treat[34].
5.2.4 Adsorption on new adsorbents
Recently this process is recognized widely for removal of heavy metals from wastewater. Many cheap adsorbents had been developed lately. These adsorbents are widely use for treatment of wastewater containing heavy metals. These adsorbents are derived from the waste products generated from industrial activities, waste generated from agriculture and natural materials. Adsorption can be defined as a mass transfer process which transfers the substance from the liquid phase to the surface of a solid and becomes bound by physical and chemical interactions[5].
It’s a three-step treatment process 1 the pollutant is transferred to the sorbent surface from bulk solution 2 adsorption on particle surface 3 transportation within the sorbent particle. This technique is very cost effective[5].
1.Adsorption on modified natural materials
2.Adsorption on industrial by-products
3.Bio-sorption
5.2.4.1 Adsorption on modified natural materials
The properties like ion exchange capability make these zeolites very efficient in removal of heavy metals from wastewater. Some studies show that the techniques like conditioning and pre-treatment improve the removal efficiency. Ph also affects the removal efficiency of heavy metals[5].
Table 8 Adsorption capacity of natural materials for heavy metals[6].
| Adsorbent | Adsorption capacity(mg/g) |
| Pb2+ Zn2+ Cu2+ Ni2+ | |
| Zeolite | 1.6 0.5 1.64 0.4 |
| HCI-treated clay | 63.2 83.2 |
| Modified zeolite | 123 8 |
5.2.4.2 Adsorption on industrial by-products
The compound like fly ash, waste iron an iron slags can be chemically modified to improve the removal efficiency of heavy metals from wastewater. There are many studies showing the removal of heavy metals by these industrial by-products. Fly ash from sugar industry is used to remove Cd(ll) and Ni(ll) whereas fly ash from coal industry used to remove Cu(ll) and Pb(ll). Iron slag is also used to remove Cu(ll) and Pb(ll)[5].
5.2.4.3 Bio-sorption
Bioremediation of heavy metals with the help of agricultural by-products is known as bio-sorption. This technique of wastewater treatment is gaining a lot of interest lately. This technique uses inactive biomass and physicochemical process to bind and concentrate heavy metals in wastewater. New by-products used as an adsorbent for heavy metals include rice husk, hazelnut shell and jackfruit[5].
Table 9 Adsorption capacity of agricultural waste [6].
| Adsorbent | Adsorbent capacity(mg/g) |
| Pb2+ Zn2+ Cu2+ Ni2+ | |
| Husk | 456 495.9 |
| Orange peel | 158 |
| Pecan shells activated carbon | 13.9 31.7 |
6.0 Liquid and biosolids discharge
When we remove pollutants from wastewater there are always some left over known as biosolids. So in general bio-solids are the result of treatment of wastewater which removes sludge from wastewater. Each year more than 660,000 metric tons of dry stabilized bio-solids are produced by the Canadians. Out of the total cost of wastewater management around 50% is spent upon bio-solids management annually [36].
As it is believed that metal accumulation of metals in bio-solids can enter our food chain as we use these bio-solids in the various land application. So considering all these parameters certain laws and regulation are made that govern the use of bio-solids[36].
The utilization and disposal of residual substances are taken care under the laws of CWA, Bio-solid task group(BTG) these all come under the Canadian Council of Ministers of the Environment and OMAFRA (Ontario Ministry of Agriculture, Food and Rural Affairs)[40]. These Federal laws (Environmental Protection Act, Nutrition Management Act) reinforce the need to employ environmentally sound residuals management techniques and to beneficial use bio-solids[36].
Treatment of bio-solids can be divided into two parts
1.Land Application
2.Incineration
6.1 Land application
From many years bio-solids are used as fertilizers in farmlands. There are various regulations governing the land applications of bio-solids some of them are contaminant limits, field management practices and treatment requirements[4].
If the bio-solids are properly treated and applied on a farmland it can be beneficial in various ways. Bio-solid improves the soil structure and provides nitrogen, phosphorus and various nutrients required by plants. Bio-solids are very good for soil conditioner and fertilizer these are very useful in restoring the land for farming that has poor soil due to various activities like construction and mining[4].
These biosolids are transported to the treatment of farmland in semi-liquid or dewatered form. This semi-liquid or dewatered form is spread on land to be treated or on a farmland as a fertilizer. This technique helps in increasing fertility of a farmland and restoring a barren land to a green land. So this is a very good use of a waste material for the treatment of a land[40].
6.2 Incineration
This process burning of dried solids is done to reduce the dried solid to ash so that they can be disposed off or can be reused. These residual substances have a lot of fuel value so they must be properly dewatered in order to take full benefit of their fuel. To obtain a dry bio-solid dewatering equipments like pressure filtration are used[4].
The bio-solids after this treatment are sufficiently dry and they can burn without the continuous supply of additional fuel source for burning[4].
7.0 Determining knowledge Gaps
Current techniques used for the treatment of heavy metals in wastewater can remove up to 99% of metals from wastewater but still have some limiting factors like time duration, operational cost is more and generation of bio-solids in large amount. Below mentioned are the knowledge gaps or the problems faced by the current treatment processes.
Table 10 Problems faced by current treatment techniques[5].
| Treatment Technique | Disadvantages |
| Adsorption with the new adsorbent | Low selectivity
Production of waste products |
| Membrane filtration | High operational cost due to membrane fouling |
| Electrodialysis | Operational cost is high
Energy consumption |
| Photocatalysis | Long duration time
Limited application |
8.0 Conclusion
The presence of widely spread toxic substances like heavy metals in wastewater is creating huge problems for environmentalists. These contaminants pose a serious threat to human health and environment. In order to reduce their impact, the environmental laws and regulations are becoming more and more strict. Above mentioned are the most used methods for the treatment of the wastewater containing metals. These methods include various conventional methods like Electrochemical treatments, physicochemical process, ion exchange method and adsorption method. Various merits and demerits of these techniques, factors affecting the removal efficiency and the reasons why these techniques are not begin used now for treatment.
During recent years taking account of environmental degradation and effect to human health, there is a development of various new techniques for removal of metals from wastewater that are more effective than the previous techniques. These include techniques Membrane filtration process, Photocatalysis Process, Nanotechnology and Adsorption on new adsorbents all these methods are reviewed above and their advantage over the conventional methods. These methods have removal efficiency up to 99%. These methods beneficial in many ways.
But still, there are a lot of knowledge gaps that should be improved in order to maintain a more sustainable environment to live. There is a need to find a more eco-friendly process for treatment of wastewater and find ways to reduce the sludge production. These are the main knowledge gaps that are needed to fill in near future.
9.0 References
[1] Elizabeth Tilley, Lukas Ulrich, Christoph Luthi, Philippe Reymond and Christian Zurbrugg, ”Compendium of Sanitation Systems and Technology,” 2nd revised edition.
[2] Snheal Menon, ”Primary and secondary water treatment,” 2014.
[3] Metcalf and Eddy Inc., “Wastewater Engineering: Treatment, Disposal and Reuse,” 4th edition, (2003).
[4] ”Primer for Municipal Wastewater Treatment Systems,” pdf.’ 2004.
[5] M.A. Barakat, ” New trends in removing heavy metals from industrial wastewater- A review,” Arabian Journal of Chemistry, vol. 4, 2011, 361-377.
[6] Arezoo Azimi, Ahmad Azari, Mashallah Rezakazemi and Meisam Ansarp, ”Removal of Heavy Metals from Industrial Wastewaters- A review,” 2016.
[7] Ravindra K. Gautam, Sanjay K. Sharma, Suresh Mahiya and Mahesh C. Chattopadhyaya, ”Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation,” 2014.
[8] Emamjomeh MM. and Sivakumar M, “Pollutants removed by electrocoagulation and electrocoagulation/flotation processes- A review,” Journal of environmental management, 2009, 1663-1679.
[9] Holt PK., Barton GW. and Mitchell CA, ” The future for electrocoagulation as a localized water treatment technology,” Chemosphere, 2005, 355-367.
[10]V. Khandegar and A. K. Saroha, “Electrocoagulation for the treatment of textile industry effluent-A review,” Journal of Environmental Management, 2013, 949-963.
[11] J. A. Gomes, Daida P, Kesmez M, Weir M, Moreno H, Parga JR, Irwin G, Mc Whinney H, Grady T, Peterson E, and Cocke DL, “Arsenic removal by electrocoagulation using combined Al-Fe electrode system and characterization of products,” Journal of Hazardous Material, 2007, 220-231.
[12] M. Y. Mollah, Morkovsky P, Gomes JA, Kesmez M, Parga J and Cocke DL, ” Fundamentals, present and future perspectives of electrocoagulation,” Journal of Hazardous Material, 2004, 199-210.
[13] G. Chen, “Electrochemical technologies in wastewater treatment,” Separation and Purification Technology, vol. 38, 2004, 11-41.
[14] Fu, Fenglian, and Qi Wang. “Removal of heavy metal ions from wastewaters: a review.” Journal of environmental management 92, no. 3 (2011): 407-418.
[15] Zodi, Salim, Olivier Potier, François Lapicque, and Jean-Pierre Leclerc. “Treatment of the textile wastewaters by electrocoagulation: Effect of operating parameters on the sludge settling characteristics,” Separation and Purification Technology vol. 69, no. 1 (2009, 29-36.
[16] Y. Oztekin and Z. Yazicigil, “Recovery of metals from complexed solution by electrodeposition,” Desalination, vol.190, 2006, 79-88.
[17] K. Scott and E. M. Paton, “An analysis of metal recovery by electrodeposition from mixed ion solutions-part II. Electrodeposition of cadmium from process solutions,” Electrochemica. Acta., vol. 38, 1993, 2191-2197.
[18] J. P. Chen and L. L. Lim, “Key factors in chemical reduction by hydrazine for recovery of precious metals,” Chemosphere, 2002, 362-370.
[19] Agarwal, I. C., A. M. Rochon, H. D. Gesser, and A. B. Sparling. “Electrodeposition of six heavy metals on reticulated vitreous carbon electrode.” Water Research 18, no. 2 1984, 227-232.
[20] V.S. Saji and R.M. Cook, “Corrosion Protection and Control Using Nanomaterials,” 2012.
[21] W. Simka, D. Puszczyk and G. Nawrat, ” Electrodeposition of metals from non-aqueous solutions,” Electrochemica. Acta., vol. 54, 2009, 5307-5319.
[22] K. Juttner, U. Galla and H. Schmieder,” Electrochemical approaches to environmental problems in the process industry,” Electrochemica. Acta., vol. 45, 2000, 2575-2595.
[23]L. K. Wang Lawrence K., Hung, Yung-Tse, Shammas and Nazih K, ” Physicochemical Treatment Processes,” vol. 3, 2005.
[24] N. M. Mubarak, J. N. Sahu , E. C. Abdullah & N. S. Jayakumar, “Removal of Heavy Metals from Wastewater Using Carbon Nanotubes,” Separation and Purification Reviews, vol. 43, 2013.
[25] Erdoğan, S., Y. Önal, C. Akmil-Başar, S. Bilmez-Erdemoğlu, Ç. Sarıcı-Özdemir, E. Köseoğlu, and G. Icduygu. “Optimization of nickel adsorption from aqueous solution by using activated carbon prepared from waste apricot by chemical activation,” Applied Surface Science, vol. 252, no. 5 2005, 1324-1331.
[26] Hai, F. I. and Yamamoto, K, “Membrane Biological Reactors,” P. Wilderer (Eds.), Treatise on Water Science, 2011, 571-613.
[27] Angelo Basile, Alfredo Cassano and Navin Rastogi,” Advances in Membrane Technologies for Water Treatment,” 2015.
[28] R. Singh , “Membrane Technology and Engineering for Water Purification,” 2nd ed., Butterworth‐Heinemann, Oxford, 2015.
[29] A. Cassano , A. Basile , ” Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications,” (Eds: A. Basile, S. P. Nunes ), Cambridge,2011.
[30] A. Dabrowski, Hubicki Z. Podkoscielny P. and Robens E, “Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method,” Chemosphere, 2004, 91-106.
[31] F. Gode and E. Pehlivan, “Removal of chromium(III) from aqueous solutions using Lewatit S 100: the effect of pH, time, metal concentration and temperature,” Journal of Hazardous materials, 2006, 330-337.
[32] L. K. Wang Lawrence K., Hung, Yung-Tse, Shammas and Nazih K, “Advances in Physicochemical Treatment Processes,” 2006.
[33] Greenlee LF, Lawle DF, Freeman BD, Marrot B and Moulin P,” Reverse osmosis desalination: Water sources, technology and today’s challenges,” Water Research, Pages 217-2348, 2009, 217-
[34] R. K. Gautam, A. Jaiswal, M. C. Chattopadhyaya, “Advanced Materials for Agriculture, Food, and Environmental Safety,” Advanced Material Series, John Wiley & Sons, Hoboken 2014.
[35] S. Kar, P. K. Tewari, “Nanotechnology in Eco-Efficient Construction,” (Eds: F. Pacheco-Torgal et al.), 2013, 364-427.
[36] Canadian Council of Ministers of the Environment. https://www.ccme.ca/en/resources/waste/biosolids.html
[37] J. M. Herrmann, C. Guillard and P. Pichat, “Heterogeneous Photocatalysis: An Emerging Technology for Water Treatment,” Catalysis Today, vol. 17, Pages 7-20, 1993.
[38] Meng Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy,” Recent developments in photocatalytic water-splitting using TiO2 and hydrogen production-A review,” Renewable and sustainable energy Reviews, vol. 11, Pages 401-425, 2007.
[39] Ronald L. Droste, “Theory and Practice of waste and wastewater treatment,” John Wiley and Sons, 1997.
[40] OMAFRA Ontario Ministry of Agriculture, Food and Rural Affairs mailto:http://www.omafra.gov.on.ca/english/
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Environmental Science"
Environmental science is an interdisciplinary field focused on the study of the physical, chemical, and biological conditions of the environment and environmental effects on organisms, and solutions to environmental issues.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: