Analysis of Denitrification Methods in Various Environments and Its Effectiveness
Info: 9481 words (38 pages) Dissertation
Published: 21st Feb 2022
Tagged: Chemistry
Abstract
Reactive nitrogen (Nr) in the environment has rapidly grown over the recent century, influenced by anthropogenic activities such as increased farming. Though it can be considered as an unavoidable product of agriculture, reactive nitrogen is a pollutant which imparts health risks, and environmental concerns. Reactive nitrogen enters and effects various ecosystems and environments with distinct qualities, which ultimately affects the efficiency of treatment and control of it. Environments such as rivers, groundwater sources, and wastewater plants. Nr can be effectively treated biologically by microorganisms, through the simultaneous work of two groups of microorganisms, nitrifiers, and denitrifiers. However, due to the various characteristics of each environment in which the process needs to be undertaken in, the growth and efficiency of the microorganisms may be severely impaired. In the wastewater treatment sector, anaerobic treatment has been increasing in interest due to its ability for energy recovery, and reduced costs through a reduction in aeration.
This paper aims to identify and propose various denitrifying methods that can be employed, with a focus on the biological methods, and the environments that they can be applied to, to improve the efficiency or Nr removal. It was determined that autotropic methods such as ANAMMOX, DAMO, and sulfur hold the most potential for wastewater treatment, whilst utilizing hydrogen gas and bio-electrochemical methods were useful for groundwater treatment.
Introduction
Nitrogen is found naturally in the environment and is an important plant nutrient (World Health Organisation, 2008). It is one of the major components for the growth of life, however, as atmospheric nitrogen (N2) is its most profuse form it presents itself as a limiting nutrient for most microorganisms (Vitousek & Howarth, 1990). Therefore, to support biomass growth a conversion from N2 to reactive nitrogen (Nr) is required,a process that either requires large amounts of energy through high temperature processes or through a small group of specialized N-fixing microbes (Galloway et al., 2017). The types of Nr fall into three broad categories, as seen in the figure below
Prior to the intensification of anthropogenic activities, there was little to no accumulation of Nr in the environment, as the microbial N-fixation and denitrification processes were in a state of equilibrium (Ayres, Schlesinger, & Socolow, 1994). However, in our recent history, there have been noticeable increases in Nr especially in areas of intensive agricultural practices from nitrogen fertilizer application and manure, and wastewater disposal by oxidation of nitrogenous waste products in human and animal excreta, including septic tanks (World Health Organisation, 2008). This, becomes an issue as reactive nitrogen in excess concentrations becomes a pollutant and an environmental concern.
Excess nitrogen levels in rivers, lakes, and groundwater can be toxic to humans and can cause problems with water quality in natural water systems (Hatfield, Keeney, & Hatfield, 2008). Eutrophication of water is a hazard to the stability and quality of aquatic ecosystems. It occurs when the water is enriched with excess amount of nutrients (including nitrate and nitrite). Eutrophication is characterized by the growth of plants and algae. The increased biomass load leads to oxygen depletion, decrease of quality and water clarity due to increased precipitation rate and is a threat to the biotic components of the ecosystem (Dorgham, 2011).
Other effects of nitrogen in water are those caused by the toxicity of nitrite to aquatic animals, particularly fish and crayfish. Hypoxia in fish and crayfish occurs when oxygen-carrying pigments are converted to forms that are incapable of carrying oxygen (Ansari, Gill, & Khan, 2011) Other toxic effects of nitrite on fish and crayfish include severe electrolyte imbalance, cancer, damage to mitochondria in liver cells and the repression of immune system decreasing the tolerance to bacterial and parasitic diseases (Ansari et al., 2011). While nitrate is considered to be relatively nontoxic to adults, high concentrations of NO3 -N can be fatal to infants under the age of six months. Hemoglobin in the blood combines with the reduced form of nitrate, nitrite, to form methemoglobin, a condition commonly known as “blue baby syndrome” (Kapoor & Viraraghavan, 1997; Kim, Nakano, Lee, Kanchanatawee, & Matsumura, 2002).
Nitrate contamination of ground waters has been a subject of concern since an association was made between the observation of elevated nitrate levels in well water, and the documentation of methemoglobinemia in 1945 (Comly, n.d.). Increased agricultural production and the use of fertilizers have severely affected groundwater pollution, furthermore nitrate can remain in groundwater for decades, accumulating to higher level as farming continues (Ju, Kou, Zhang, & Christie, 2017) The nitrate accumulation within groundwater sources occur due to the conditions with which the water remains, inhibiting the ability of N-fixing microbes to denitrify the pollutant into less harmful products. At this level, human interaction is required in order to bring nitrate levels back to acceptable levels, through engineered systems.
This study will look at separating the nitrogen removal process into a two-step system (nitritation and denitrification) due to the biological ease it provides, with an emphasis on developing a mainstream denitrification process to keep up with increasing nitrogen pollution in the environment, as in groundwater sources in particular, nitrogen pollution remains in the form of nitrate. The study will provide an in depth look at the potential methods that are currently employed and could be employed in terms of their efficiency, and sustainability.
Hence all the available denitrification methods will be considered, including their potential benefits or vices, to determine a suitable process which could be developed further for application purposes. In terms of denitrification, the two major areas which this could be utilized or enhanced in, will be in the wastewater treatment sector, and the groundwater treatment sector. Hence the review will be based on determining the most suitable denitrification method for these two sectors.
Methods currently employed
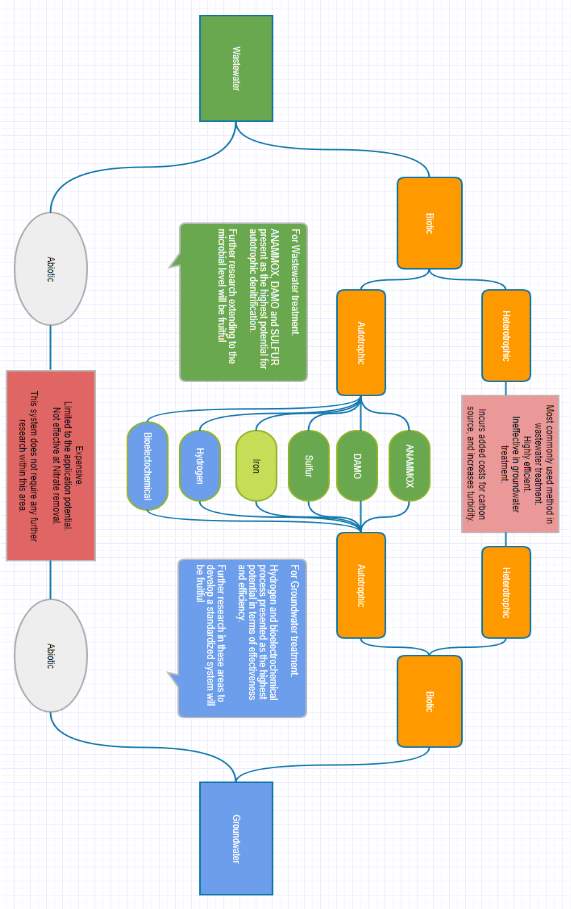
Treatment of reactive nitrogen in wastewater treatment plants can occur through two different mechanisms, abiotic or biotic. In most instances the treatment of nitrogen occurs through abiotic methods
Abiotic Nitrogen Removal
Within the abiotic nitrogen removal methods, the most common processes are reverse osmosis (RO), ion exchange (IE), electro-dialysis (ED) and chemical denitrification (Ghafari, Hasan, & Aroua, 2008). However, all abiotic methods are expensive and are limited in their application potential (Heu, Lahnsteiner, Frischherz, & Baumgartner, 1998), furthermore they show poor selectivity for nitrate removal and produce nitrate waste brine among other products (Choi, Maruthamuthu, Lee, Ha, & Bae, 2009; Rocca, Belgiorno, & Meriç, 2007).
| Abiotic Nitrogen Removal | ||||
| Ion Exchange | The most commonly used nitrate treatment method. Nitrate ions are removed from the treatment stream by displacing chloride on an anion exchange resin1. The resin is made to minimize adsorption of other anions/cations or interference from other anions/ cations so nitrate can be removed1. Subsequently, regeneration of the resin is necessary to remove the nitrate from the resin. | |||
| + | High availability of nitrate selective resins
Effective for removal in low to moderate nitrate concentration Can remove multiple contaminants (including arsenic, perchlorate, and chromium) Improved efficiency of low brine IX in recent years |
– | Production of a concentrated waste brine (rich in nitrate, chloride and sulphate),2 however, typically in a lower volume than from reverse osmosis
>The wastewater from the process must be treated before it is discharged. It must be disposed of (difficult and expensive) or treated further >Resin pre-treatment and frequency of replacement >May not be feasible for extremely high nitrate levels1 |
|
| Reverse Osmosis | Second most common nitrate treatment alternative. A semi-permeable membrane separates contaminants
(predominantly those with higher valences) when water is forced through1 The process will not change the compounds’ molecular structures3,4 |
|||
| + | Feasible for municipal and Point-of-Use
Applications. Can be used simultaneously for multiple contaminant removal and desalination1 |
– | >Produces concentrated waste brine that poses a disposal problem for
further treatment3,4 >NO3-is a monovalent ion, so RO is not as effective2. >Typically higher costs relative to IX (due to pre-treatment requirements and high power consumption) |
|
| Chemical Denitrification | Chemical denitrification uses metals to transform nitrate to other nitrogen species1 | |||
| + | >No waste brine produced, so no need to dispose
>Potential for multiple contaminant removal >Recent progress has been made in Improving efficiency |
– | >The nitrate reduction reactions are
inconsistent so there is the potential for incomplete denitrification. Therefore, there is a risk of nitrite formation1 >Dependence on temperature and pH |
|
| Electrodialysis | The process works involving ion flow across anion-exchange and cation-exchange membranes in a constant electric field5.
The membranes trap nitrate and other ions in a concentrated waste stream. Ion Exchange resin is used in a sheet form6. Build up on the membrane is minimized by reversing the polarity several times per hour to change the ions’ direction of movement1. |
|||
| + | >Multiple contaminant removal and
desalination >Less waste produced than RO >Fewer pre-treatment requirements than RO >Possible to selectively remove nitrate ions >Unaffected by silica |
– | >Pre-treatment requirements
>High energy demands >Operational complexity >Waste disposal >High operating costs (similar to RO) |
|
[1] (Groundwater, n.d.)
[2] (Song, Zhou, Li, & Mueller, 2012)
[3] (Ergas & Rheinheimer, 2004)
[4] (Shrimali & Singh, 2001)
[5] (Rozanska & Wisniewski, 2007)
[6] (Prato & Parent, 2017)
Biotic Nitrogen removal
Biological nitrogen removal presents itself as a more suitable approach than the abiotic methods due to its efficient performance (almost 100% effective), as Nr is almost completely decomposed into harmless nitrogen gas and released into the atmosphere (Hatfield et al., 2008; Prosnansky, Sakakibara, & Kuroda, 2002)The biological nitrogen removal process in its simplest form follows a cycle as seen in figure
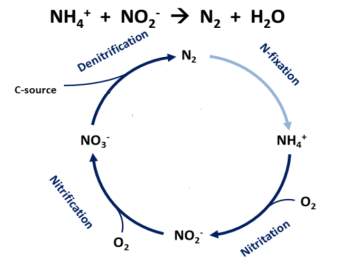
Traditionally the cycle relies on the coexistence of two phylogenetically distinct types of microorganisms, Ammonia Oxidizing bacteria (AOB) and Nitrite-oxidizing bacteria (NOB), finally to complete the cycle denitrification occurs in anaerobic conditions. This separation in the groups of microbes refers to the two step nitritition and nitritation process. In groundwater N2 exists in the form of NO3– , therefore strictly a group of denitrifying bacteria is required for treatment in such environments.
As can also be seen in figure below, denitrification occurs through a series of steps as follows:
NO3 -> NO2 -> NO -> N20 -> N2
Microorganisms reduce nitrate (NO3 ) to nitrite (NO2 ), nitric (NO), nitrous oxide (N2O), and finally to nitrogen gas (N2). Most of the time when denitrification occurs, through partial reduction nitric (NO), nitrous oxide (N2O) may be released into the atmosphere, which is a conern for the process. In a suitable environment, microorganisms reduce these nitrates by adjusting their metabolism to catalyze the above stages (Karanasios, Vasiliadou, Pavlou, & Vayenas, 2010). The denitrifiers use nitrate as their terminal acceptor in their respiratory process (Prosnansky et al., 2002), however different types of dentrifiers have adapted to specialize in utilizing different compounds as their electron donors. These microbes fall into two broad categories, heterotrophic denitrifiers and autotrophic denitrifiers.
It has been found that denitrifying populations maintain their denitrifying enzymes even when conditions are unsuitable for nitrate reduction (Smith & Tiedje, 1979). In these cases, denitrification will continue at least initially under changes in environment. Where this isn’t the case (Casey & Klaine, 2001), the population may synthesis the enzymes if there is a lack of nitrate, leading to their preservation and therefore durability for survival.
Heterotrophic denitrification
Heterotrophic bacteria use different carbon compounds as their electron donors required for cell growth, common carbon sources can be methanol, ethanol, methane, carbon monoxide, and acetic acid, of which, methanol has been most commonly used and documented (Swinarski, M. et al., 2009). A few of the most common and well known identified groups of heterotrophic nitrifying bacteria are displayed in the table below.
| Group | Source |
| Bacillus sp. | Yang et al. 2011 |
| Acinetobacter sp. | Yao et al. 2013 |
| Paracoccus denitrificans | Robertson and Kuenen 1983 |
| Pseudomonas stutzeri | Zhang et al. 2011 |
*Ammonium removal from high-salinity oilfield-produced water: assessing the microbial community dynamic at increasing salt concentrations
Heterotrophic denitrification has the advantages of high efficiency of reduction, however the efficiency is affected by the type of carbon source as well as the amount (Zhao et al., 2011). In certain wastewater treatment streams organic carbon is limited, and therefore must be provided at an added cost to maintain efficacy. Through previous studies it seemed that nitrification was also effected by type of carbon source, studies conducted by (Gómez & González-lópez, 2000; Pinar & L. Ramos, 1999) observed nitrite accumulation where sucrose was used, however when replaced with methanol or ethanol there was no accumulation. It is also important to consider the amount of carbon required, as excessive organic carbon can cause bacterial growth and increase turbidity (Zhao et al., 2011).
In instances like groundwater sources utilizing heterotrophic bacteria for nitrate treatment becomes a difficult process due to the lack of a carbon source, therefore an external carbon source has to be supplied for bacterial growth (B. Zhang et al., 2014). In the spirit of sustainability research has been conducted in order to analyze the possibility of woodchips, newspaper and sawdust as organic carbon alternatives for heterotrophic denitrification (Robertson, Vogan, & Lombardo, 2008; Saliling, Westerman, & Losordo, 2007; Volokita, Belkin, Abeliovich, & Soares, 1996). The challenges experienced by these methods for in situ treatment, involved containing the substance in a permeable reactive barrier and finding a matrix of solid carbon source which can promote biofilm growth in a flowing environment. Furthermore supplying organic compounds imparts an added cost to the process (Swinarski et al., 2009), which could otherwise be obsolete through autotropic denitrification. Hence for the purposes of nitrate treatment autotrophic denitrification bears added interest, which is just as well known to efficiently remove nitrate with less residual organics (Till, 1998; Zhou et al., 2011).
Autotrophic Denitrification
The reduction of nitrate through autotrophic denitrification is driven by inorganic compounds, generally by using sulfur, iron, methane, or hydrogen gas as their electron donors (Chorover, Dail, & Davidson, 2003), of which a summary of the benefits and limitations along with their catabolic stoichiometric equations for each process can be seen in the table below. The half equation for nitrate reduction illustrates the role of electrons (e–) in the process (Tesoriero, Liebscher, & Cox, 2000).
2NO3– + 12H+ + 10e– -> N2 + 6H2O
| Iron Reducing Denitrifiers | Few studies have been conducted in this area.
Studies conducted by (Li et al., 2015), highlighted the relevance of anaerobic ammonium oxidation coupled with ferric iron reduction for ammonium removal. (Haaijer, Crienen, Jetten, & Op den Camp, 2012) indicated the importance of iron-oxidizing nitrate reducers to the redox cycling of iron in groundwater |
| Sulfate Reducing Denitrifiers | Cheap and Easy to use.
Creates a high content of sulfur in the treated water, requires use of limestone for pH adjustment 3HS– + 5H+ + 8NO2– ↔ 3SO42- + 4N2 + 4H2O |
| Hydrogenotrophic Denitrifiers | Bacteria grows fastest (life cycle of 24 x to sulfur bacteria) (Cord-ruwisch, Seitz, & Conrad, 1988)
Hazardous nature of hydrogen, Low solubility of hydrogen in water. 2NO3- + 5H2 → N2 + 4H2O + 2OH− (Sharma & Sobti, 2012) |
| Methane | Circumvent the requirement at WWTPs for expensive electron donors, such as methanol or acetate, to lower the operational costs and carbon footprint of the denitrification process (Raghoebarsing et al., 2006; Thalasso & Vallecillo, 1997) reported NO3– removal rates indicate the process may not be economically viable (He et al., 2015)
5CH4 + 8NO3– + 8H+ → 4N2 + 5CO2 + 14H2O |
Sulfate reducing denitrifiers
Research based on sulfate is currently the most attractive method of autotrophic denitrification. Compared to heterotrophic denitrification it was revealed that denitrification with sulfide was a much faster process (Bill, Bott, & Murthy, 2009). Sulfide, also has presented as an inhibitor to NOB and AOM at different concentrations, and was hypothesized that through a controlled sulfide inhibition process a strategy that stimulates nitritation over nitration could be developed (Erguder, Boon, Vlaeminck, & Verstraete, 2008).
(further literature should be done for this section)
Anaerobic Ammonia Oxidation
Another form and widely studied method of autotrophic denitrification is through Anaerobic Ammonia Oxidation (ANAMMOX). This anammox process was first observed in the early 1990s by Mulder, et al., (1995), where they concluded that ammonium can be oxidized with nitrate serving as an electron acceptor under anaerobic conditions.
It was widely assumed that anammox bacteria played a minor role in the nitrogen cycle, however since 2001 research conducted by (Hamersley et al., 2007; M M M Kuypers et al., 2003; Marcel M.M. Kuypers et al., 2005) and (Thamdrup & Dalsgaard, 2002) revealed that around 20-50% of all nitrogen loss from marine environments can be attributed to anammox processes. Highlighting how prominent and useful the ANAMMOX prcoess can be. The equations for the ammonia reduction through ANAMMOX can be seen below.
NH4+ + NO2– -> N2 + 2H2O (I)
NH4+ + 1.5O2 -> NO2– + 2H+ + H2O (II)
2NH4+ + 1.5O2 -> N2 + 2H+ + 3H2O (I and II)
In order to make nitrogen removal from Ammonia an energy neutral or energy-positive process, supressing the growth of nitrite-oxidizing bacteria (NOB) and promoting anaerobic ammonium oxidation (anammox), is essential to the process. (Ma et al., 2015) managed to supress NOB growth, by using intermittent aeration with low dissolved oxygen, through flourescent in-situ Hybridization they revealed that the relative abundance of NOB was maintained between 2.0 -2.6% (Dokianakis, Kornaros, & Lyberatos, 2006). However it was observed that even with low DO (0.5 mg/l) after a long enrichment period, NOB may proliferate and rapidly diminish the nitrite accumulation (Lin Ye & Zhang, 2011). The issue is further complicated due to the varying affinity of DO from different groups of NOB (Nowka & Daims, 2015; Regmi et al., 2014). Other promising research determined that through maintaining salinity levels of 5 g/l NOB bacteria can be inhibited whilst still preserving the activity of AOB (Liu Ye, Tang, Zhao, Pijuan, & Peng, 2009)
Co-Coupling of DAMO
Due to the ubiquitous availability of methane in WWTP, studies have been conducted to study the coupling of denitrifying anaerobic methane oxidation (DAMO) with other autotrophic bacteria. (López et al., 2017) studied the simultaneous interdependent removal of CH4, H2S and NO3-, with respect to the influence on the nitrogen concentrations. They successfully managed to enrich a co-culture compromising of 60% DAMO, with promising NO3– removal rates, and a decrease in N2O production, however they also observed some SO42- accumulation which compromised the denitrification performance.
The coupling of DAMO with ANAMMOX bacteria was also studied by (Shi et al., 2013) of which after 24 months of operation they successfully managed to enrich a consortium of bacteria composing of 20-30% each of DAMO archaea, and anammox bacteria. It was also observed that with higher ammonium concentrations competition for nitrite was superseded by anammox bacteria ahead of damo (Winkler et al., 2015). However, nitrate depended damo co-exist well with anammox, as competition does not exist between the two, furthermore residual nitrate produced by anammox can be utilized in an efficient manner (Shi et al., 2013). Developing a potential method for wastewater plants to have an anaerobic nitrogen removal process, presenting a case for total nitrogen removal of 250 mgN L-1 D-1, this however is not to the level of conventional denitrification rates, and could be improved with a better understanding of the specific microorganisms involved in the process along with their enzymatic pathways.
Denitrification in the built environment
Wastewater treatment plants
Nutrient removal in wastewater treatment plants is a vital factor to consider, among those fixed nitrogen such as ammonium (NH4+) and nitrate (NO3–) must be removed to avoid toxic algal blooms in the environment (B. Kartal, Kuenen, & van Loosdrecht, 2010). Influent wastewater from domestic production generally has between 10-40 mg/l of Nitrogen (Tchobanoglous, & Angelakis, 1996) with industrial wastewater contributing to high nitrogen levels, among others such as sulfate and salinity (Davis, 2011). Common treatment of ammonium and nitrate in treatment plants utilize autotrophic nitrifiers followed by heterotrophic dentrifiers in anoxic conditions. As aforementioned to support and enhance the growth of denitrifiers, an external carbon source is commonly added to the system, as the natural wastewater characteristics may not have the required carbon levels to achieve nitrate removal (Henze, van Loosdrecht, Ekama, & Brdjanovic, 2008). Generally methanol is widely used as a carbon additive, however the related microbes (methylotrophs) are known to have a limited diversity of denitrifying bacteria, resulting in long start up times for the reactor (2-3 months), (Nyberg, Aspegren, Andersson, & Villadsen, 1992).
An autotrophic alternative may prove beneficial for wastewater treatment plants, this will have to be implemented via a separate side-stream process. Offering the comfort for a higher control of the parameters involved for the efficiency of the process. In particular having the added benefit of utilizing DAMO, by methane gas taken from anaerobic digestors. A denitrification ANAMMOX process could also be a more beneficial option in wastewater treatment plants, as ANAMMOX can utilize nitrite and convert ammonia into harmless gas with low energy requirements, furthermore denitrifiers will be able to utilize nitrates produced by ANAMMOX with minimal competition and interference between the two.
For a large period of time, the design and development of wastewater treatment plants were based on empirical data obtained from input vs output tests, leaving a gap in knowledge for the biochemical processes that control the system (Olsson & Newell, n.d.). This knowledge gap promotes performance issues in the system, in terms of efficiency, stability and cost effectiveness (Barnard & Steichen, 2006). However, over the past decades microbial techniques have been applied to obtain a deeper understanding of the denitrifying communities existing in the complex ecosystem, in terms of diversity and abundance of denitrification genes (Geets et al., 2007; Lu & Chandran, 2010). A study of the enzymatic pathways involved in this denitrifiers could look to bridge the gap in knowledge and help develop more efficient mainstream treatment processes which could be replicated around the world in terms of a specific regions requirements and parameters.
(hydrogen sulfide can also be removed by serving as an electron donor for autotrophic denitrification-autotrophic denitrification for combined hydrogen sulfide removal from biogas and post denitrification)
Groundwater
Groundwater is an important source of drinking water for many communities, unfortunately however, many groundwater sources are becoming increasingly polluted especially near agricultural areas (Bo, Tao, Xiang, & Mei-rong, 2008). This is due to the excessive use of nitrogen fertilizers in these areas, leading to leaching into groundwater sources, causing NO3– accumulation and pollution of these sources.
Abiotic methods such as ion exchange (IE) or reverse osmosis (RO) can be employed to remove nitrate from groundwater, however, these processes will produce concentrated nitrate brine which will require further treatment anyway (Prüsse, Hähnlein, Daum, & Vorlop, 2000). Furthermore, abiotic methods would be a form of ex situ treatment, which requires extra energy for pumping and further treating. Therefore, biotic methods for groundwater treatment is always the preferred option. Heterotrophic denitrification will eventuate to a costly process due to the lack of biodegradable organic materials in groundwater, because of this autotrophic denitrification is the subject of interest, where extensive research has already been conducted (Wan et al., 2009). In particular the use of hydrogen gas as an electron donor for the process (Rezania, Oleszkiewicz, & Cicek, 2007), this is due to hydrogen gas not leaving any residual harm to drinking water, however it is highly unstable and dangerous to control, as well as having a low solubility in water, impeding its performance. Studies conducted by (Wan et al., 2009) looked to minimize the disadvantages of hydrogen gas, by using a combined bioelectrical system with sulfur driven denitrification. This research was promising as H+ ions produced by sulfate denitrifiers can be consumed by the hydrogen denitrifiers, creating a mutually beneficial environment, the research was successful as they managed to have a nitrate removal efficiency of 95%-100% from nitrate loading rates of 0.12 – 24.0 kg N/m3 d, and hydraulic retention time of 2.1 – 4.2 hours.
The use of bioelectrochemical systems (BES) for nitrate reduction particularly for groundwater has been growing in interest. The system works by using autotrophic denitrifying bacteria capable of utilizing electrons at the cathode of an electrical circuit, as a food source. However, there is neither sufficient documentation nor wide implementation of bio-electrochemical removal of nitrate from water in full scale, further studies are needed (Ghafari et al., 2008). (Huang, Feng, Ding, & Zheng, 2013), demonstrated the effect of various carbon sources and the effect of current change on nitrate removal, whilst (Y. Zhang & Angelidaki, 2013) looked at proving the potential of a novel submerged denitrification cell, in order to treat groundwater in situ. The system utilized a cell, where bacteria produced current at the anode, in order to convert NO3– to N2 at the cathode. The results were highly promising, within the system wastewater was used as fuel, to give 90.5% reduction of nitrate with a 12 h wastewater hydraulic retention time. However, the reactor used was complex, making the construction of such a system an expensive one. (Tong & He, 2013) aimed to improve on that procedure by attracting the nitrate out of the groundwater by electricity generation, to reduce the nitrate at the cathode by heterotrophic denitrification. However, this still maintains the issue of requiring an added carbon source, nevertheless, due to the potential of this research it would be fruitful to further investigate the microbes involved in the process, with the aim of making the BES a completely autotrophic process. Through genomic techniques it could be possible to identify a highly efficient strain of bacteria to aid the autotrophic denitrification at the cathode. In the hopes of simplifying the above reactor systems, to a level where it could then be replicated and used for production.
Limitations
Temperature is a vital parameter that affects the rate of denitrification. It is common knowledge that for biological activity the optimum temperature should be around 25 to 35 degrees Celsius, however, for denitrification, the process can still be performed between 2 to 50 degrees Celcius, albeit not efficiently. (Volokita et al., 1996) and (Long-term performance of in situ reactive barriers for nitrate remediation) both experienced a reduction in the denitrification rates by half and a third respectively, when the temperature decreased from around 30 degrees to 14 degrees. This leaves opportunity for analysis of the microbes able to thrive at a wider range of temperatures, to identify and study strains of microorganism capable of thriving in more extreme conditions which can then be replicated and implemented to specific environments for nitrate reduction with improved efficiency.
Dissolved Oxygen is another contributing factor for denitrification rates, which becomes a greater issue for groundwater sources, where with nitrate pollution it is also known to contain oxygen (remediation of nitrate-nitrogen contaminated groundwater by a heterotrophic-autotrophic denitrification (HAD) approach in an aerobic environment). The presence of oxygen in environments creates a competitive effect, whereby microorganisms would choose oxygen as an electron acceptor instead of nitrates due to the energy yield, furthermore the presence of oxygen has been known to cause inhibition of the denitrification enzymes (effect of dissolved oxygen concentration on nitrate removal from groundwater using a denitrifying submerged filter 2002). Therefore, when developing in situ treatment of nitrate, the presence of DO needs to be considered and mitigated for effective treatment.
Apart from limitation with regards to efficiency, it should be noted that denitrification also has undesirable byproducts that could be produced. Greenhouse gas emissions in wastewater treatment has been identified as a concern for the denitrification process (Snip, Vanrolleghem, & Flores-alsina, 2011). During denitrification, not all of the NO3– is fully reduced to N2, and could be escaped in the form of N2O. “N2O is one of the most potent of the three biogenic greenhouse gases with a radiative forcing ~300x that of CO2” (Burgin, & Groffman, 2012, p.1). Studies regarding the production of Nitrous oxide emissions through various denitrification methods are minimal. In particular for damo, where it is yet to be reported (Hu, Zeng, Keller, Lant, & Yuan, 2011)
Studies conducted by (Takaya et al., 2003) identified strains of aerobic denitrifying bacteria with low N2O production. However, only a few other studies have been conducted to understand a microbial community’s influence on N2O emissions in wastewater treatment plants, with only a basic understanding of the parameters affecting N2O generation. The parameters that promote N2O generation include low pH, limited organic carbon, and higher dissolved oxygen content (Chung, 2000; Kuhni, 1995)
In the anammox process nitrous oxide emissions should be non-existent, as N2O is not part of the metabolism (Boran Kartal et al., 2007), which was also observed by (Terada, Smets, Love, Garland, & Gilmore, 2013) where N2O levels fell below the detection limit as they shifted their denitrification process to an ANAMMOX system.
For bioelectrochemical denitrification research conducted by (Doan, Kwon, Kumar, Tiedje, & Park, 2013) found that with increased current density, which is optimal for increased denitrification rates (Huang et al., 2013) corresponded to an increase in N2O accumulation, where the N2O generated accounted for around 70% of the removed nitrate, which is a concerning factor for greenhouse gas emissions. It was observed that at all current densities the N2O reduction gene was inhibited (nosZ) (Doan et al., 2013), this was a desired outcome of the research however, due to its ability for recovery and use as combustion propellant. Alternatively, through similar gene expression techniques microorganisms with nosZ within the bioelectrochemical denitrifiers could be identified and cultured in order to optimize the process where low levels of N2O are produced.
Conclusions and recommendations for future research
There are many different denitrification methods that could be utilized for a specific purpose. It is evident that the biotic method hold substantial benefits over abiotic methods, and therefore is the preferred method. Through a basic understanding of the various denitrifiers an experimental model could be developed to further understand the roles of the microorganisms involved in the process. In particular their responses to changes in the environment, whilst utilizing molecular techniques to understand and develop efficient engineered system that optimize the microbes performance in a given condition.
It was determined that for Groundwater treatment, bioelectrochemical and hydrogenotrophic processes would be the most plausible method of in-situ treatment, however due to its current inefficiencies and lack of knowledge, to develop a product, further research within this area will need to be done. For wastewater treatment the study of ANAMMOX and DAMO, to implement a separate side-stream process in the treatment plant could be improved on, in order to make the process the preffered method, due to the numerous benefits they provide, in terms of costs, and reduction in N2O emissions.
Biological process models can be developed with a better understanding of the microbial metabolic pathways of autotrophic dentitrifiers. The potential of utilizing electrochemical fuel cells to culture a brand of denitrifiers will create an opportunity for efficient and effective treatment of polluted groundwater sources. Particular care must be taken for the greenhouse gas emissions, when developing a process as this could deter the process from being implemented, despite its efficiency of treatment and cost effectiveness.
References
Ansari, A. A., Gill, S. S., & Khan, F. A. (2011). Eutrophication: Threat to Aquatic Ecosystems. In A. A. Ansari, S. Singh Gill, G. R. Lanza, & W. Rast (Eds.), Eutrophication: causes, consequences and control (pp. 143–170). Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-90-481-9625-8_7
Barnard, J. L., & Steichen, M. T. (2006). Where is biological nutrient removal going now ? https://doi.org/10.2166/wst.2006.088
Bill, K. A., Bott, C. B., & Murthy, S. N. (2009). Evaluation of alternative electron donors for denitrifying moving bed biofilm reactors ( MBBRs ), 2647–2657. https://doi.org/10.2166/wst.2009.622
Bo, Z. H. U., Tao, W., Xiang, Y. O. U., & Mei-rong, G. A. O. (2008). Nutrient Release from Weathering of Purplish Rocks in the Sichuan Basin , China ∗ 1, 18(2003), 257–264.
Choi, J., Maruthamuthu, S., Lee, H., Ha, T., & Bae, J. (2009). Nitrate removal by electro-bioremediation technology in Korean soil, 168(3), 1208–1216. https://doi.org/10.1016/j.jhazmat.2009.02.162
Chung, M. (n.d.). BNP test to evaluate the influence of C / N ratio on N 2 O production in biological denitrification, 23–27.
Comly, H. H. (n.d.). Cyanosis in Infants Caused by Nitrates in Well Water.
Cord-ruwisch, R., Seitz, H., & Conrad, R. (1988). Micro biolouy The capacity of hydrogenotrophic anaerobic bacteria on the redox potential of the terminal electron acceptor *, 1, 350–357.
Davis, B. M. L., Consid-, T. D., Growth, A., & Processes, B. (2011). Book reviews, 30(3), 266–267. https://doi.org/10.1002/ep.10602
Doan, T. Van, Kwon, T., Kumar, S., Tiedje, J. M., & Park, J. (2013). ScienceDirect Increased nitrous oxide accumulation by bioelectrochemical denitrification under autotrophic conditions : Kinetics and expression of denitrification pathway genes. Water Research, 47(19), 7087–7097. https://doi.org/10.1016/j.watres.2013.08.041
Dokianakis, S. N., Kornaros, M., & Lyberatos, G. (2006). Impact of Five Selected Xenobiotics on Isolated Ammonium Oxidizers and on Nitrifying Activated Sludge, 310–316. https://doi.org/10.1002/tox
Dorgham, M. M. (2011). Eutrophication Problem in Egypt. In A. A. Ansari, S. Singh Gill, G. R. Lanza, & W. Rast (Eds.), Eutrophication: causes, consequences and control (pp. 171–194). Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-90-481-9625-8_8
Ergas, S. J., & Rheinheimer, D. E. (2004). Drinking water denitrification using a membrane bioreactor, 38, 3225–3232. https://doi.org/10.1016/j.watres.2004.04.019
Erguder, T. H., Boon, N., Vlaeminck, S. E., & Verstraete, W. (2008). Partial Nitrification Achieved by Pulse Sulfide Doses in a Sequential Batch Reactor, 32(0), 8715–8720.
Galloway, J. N., Aber, J. D., Erisman, J. A. N. W., Sybil, P., Howarth, R. W., Cowling, E. B., … Sybil, P. (2017). The Nitrogen Cascade, 53(4), 341–356.
Geets, J., Cooman, M. De, Wittebolle, L., Heylen, K., Vanparys, B., Vos, P. De, … Boon, N. (2007). Real-time PCR assay for the simultaneous quantification of nitrifying and denitrifying bacteria in activated sludge, 211–221. https://doi.org/10.1007/s00253-006-0805-8
Ghafari, S., Hasan, M., & Aroua, M. K. (2008). Bio-electrochemical removal of nitrate from water and wastewater — A review, 99, 3965–3974. https://doi.org/10.1016/j.biortech.2007.05.026
Gómez, M. A., & González-lópez, J. (2000). Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter, 80, 69–80.
Groundwater, S. V. (n.d.). Addressing Nitrate in California ’ s Drinking Water.
Guidelines, W. H. O., & Quality, D. (n.d.). Nitrate and nitrite in drinking-water.
Haaijer, S. C. M., Crienen, G., Jetten, M. S. M., & Op den Camp, H. J. M. (2012). Anoxic iron cycling bacteria from an iron sulfide-and nitrate-rich freshwater environment. Frontiers in Microbiology, 3(FEB), 1–8. https://doi.org/10.3389/fmicb.2012.00026
Hamersley, M. R., Lavik, G., Woebken, D., Rattray, J. E., Lam, P., Hopmans, E. C., … Kuypers, M. M. M. (2007). Anaerobic ammonium oxidation in the Peruvian oxygen minimum zone. Limnol. Oceanogr., 52(3), 923–933. https://doi.org/10.4319/lo.2007.52.3.0923
Hatfield, J. L., Keeney, D. R., & Hatfield, J. L. (2008). Chapter 1 . The Nitrogen Cycle , Historical Perspective , and, 1–18.
He, Z., Geng, S., Cai, C., Liu, S., Liu, Y., Pan, Y., … Hu, B. (2015). Anaerobic oxidation of methane coupled to nitrite reduction by halophilic marine NC10 bacteria. Applied and Environmental Microbiology, 81(16), 5538–5545. https://doi.org/10.1128/AEM.00984-15
Henze, M., van Loosdrecht, M., Ekama, G., & Brdjanovic, D. (2008). Biological Wastewater Treatment: Principles, Modelling and Design.
Heu, F., Lahnsteiner, J., Frischherz, H., & Baumgartner, G. (1998). Experience with full-scale electrodialysis for nitrate and hardness removal, 7, 173–180.
Hu, S., Zeng, R. J., Keller, J., Lant, P. A., & Yuan, Z. (2011). Effect of nitrate and nitrite on the selection of microorganisms in the denitrifying anaerobic, 3, 315–319. https://doi.org/10.1111/j.1758-2229.2010.00227.x
Huang, B., Feng, H., Ding, Y., & Zheng, X. (2013). Electrochimica Acta Microbial metabolism and activity in terms of nitrate removal in bioelectrochemical systems, 113, 29–36. https://doi.org/10.1016/j.electacta.2013.08.172
Kapoor, B. A., & Viraraghavan, T. (1997). NITRATE REMOVAL FROM DRINKING WATER-REvIEW, 123(APRil), 371–380.
Karanasios, K. A., Vasiliadou, I. A., Pavlou, S., & Vayenas, D. V. (2010). Hydrogenotrophic denitrification of potable water : A review. Journal of Hazardous Materials, 180(1–3), 20–37. https://doi.org/10.1016/j.jhazmat.2010.04.090
Kartal, B., Kuenen, J. G., & van Loosdrecht, M. C. M. (2010). Sewage Treatment with Anammox. Science, 328(5979), 702–703. https://doi.org/10.1126/science.1185941
Kartal, B., Rattray, J., Niftrik, L. A. Van, Vossenberg, J. Van De, Schmid, M. C., Webb, R. I., … Strous, M. (2007). Candidatus “‘ Anammoxoglobus propionicus ’” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria, 30, 39–49. https://doi.org/10.1016/j.syapm.2006.03.004
Kim, Y., Nakano, K., Lee, T., Kanchanatawee, S., & Matsumura, M. (2002). On-Site Nitrate Removal of Groundwater by an Immobilized Psychrophilic Denitrifier Using Soluble Starch as a Carbon Source, 93(3), 303–308.
Kuypers, M. M. M., Lavik, G., Woebken, D., Schmid, M. C., Fuchs, B. M., Amann, R., … Jetten, M. S. M. (2005). Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci U S A, 102(18), 6478–6483. https://doi.org/10.1073/pnas.0502088102
Kuypers, M. M. M., Sliekers, A. O., Lavik, G., Schmid, M., Jorgensen, B. B., Kuenen, J. G., … Jetten, M. S. M. (2003). Anaerobic Ammonium Oxidation by Anammox Bacteria in the Black Sea. Nature, 422(6932), 608–611. https://doi.org/10.1038/nature01526.1.
Li, X., Hou, L., Liu, M., Zheng, Y., Yin, G., Lin, X., … Hu, X. (2015). Evidence of Nitrogen Loss from Anaerobic Ammonium Oxidation Coupled with Ferric Iron Reduction in an Intertidal Wetland. https://doi.org/10.1021/acs.est.5b03419
López, J. C., Porca, E., Collins, G., Pérez, R., Rodríguez-Alija, A., Muñoz, R., & Quijano, G. (2017). Biogas-based denitrification in a biotrickling filter: Influence of nitrate concentration and hydrogen sulfide. Biotechnology and Bioengineering, 114(3), 665–673. https://doi.org/10.1002/bit.26092
Lotti, T., Kleerebezem, R., Lubello, C., & Loosdrecht, M. C. M. Van. (2014). ScienceDirect Physiological and kinetic characterization of a suspended cell anammox culture. Water Research, 60, 1–14. https://doi.org/10.1016/j.watres.2014.04.017
Lu, H., & Chandran, K. (2010). Diagnosis and Quantification of Glycerol Assimilating Denitrifying Bacteria in an Integrated Fixed-Film Activated Sludge Reactor via 13 C DNA Stable-Isotope Probing, 44(23), 8943–8949.
Ma, B., Bao, P., Wei, Y., Zhu, G., Yuan, Z., & Peng, Y. (2015). Suppressing Nitrite-oxidizing Bacteria Growth to Achieve Nitrogen Removal from Domestic Wastewater via Anammox Using Intermittent Aeration with Low Dissolved Oxygen. Scientific Reports, 5(1), 13048. https://doi.org/10.1038/srep13048
Nitrogen, P., Ju, X. T., Kou, C. L., Zhang, F. S., & Christie, P. (2017). Nitrogen balance and groundwater nitrate contamination : Comparison among three intensive cropping systems on the North China Plain Nitrogen balance and groundwater nitrate contamination : Comparison among three intensive cropping systems on the North China Plain, 143(2006), 117–125. https://doi.org/10.1016/j.envpol.2005.11.005
Nowka, B., & Daims, H. (2015). Comparison of Oxidation Kinetics of Nitrite-Oxidizing Bacteria : Nitrite Availability as a Key Factor in Niche Differentiation, 81(2), 745–753. https://doi.org/10.1128/AEM.02734-14
Nyberg, U., Aspegren, H., Andersson, B., & Villadsen, I. S. (1992). Full-scale application of nitrogen remov al with methanol as carbon source, 26(5), 1077–1086.
Olsson, G., & Newell, B. (n.d.). Modelling , Diagnosis and Control.
Pinar, G., & L. Ramos, J. (1999). Recombinant Klebsiella oxytoca Strains with Improved Efficiency in Removal of High Nitrate Loads. Applied and Environmental Microbiology, 64, 5016–5019.
Prato, A. T., & Parent, R. G. (2017). nitrate and nitrite removal from municipal drinking water supplies with electrodialysis reversal, (May 1990).
Prosnansky, M., Sakakibara, Y., & Kuroda, M. (2002). High-rate denitrification and SS rejection by biofilm-electrode reactor ( BER ) combined with microfiltration, 36, 4801–4810.
Prüsse, U., Hähnlein, M., Daum, J., & Vorlop, K. (2000). Improving the catalytic nitrate reduction, 55(2), 79–90.
Raghoebarsing, A. A., Pol, A., Pas-schoonen, K. T. Van De, Smolders, A. J. P., Ettwig, K. F., Rijpstra, W. I. C., … Strous, M. (2006). LETTERS A microbial consortium couples anaerobic methane oxidation to denitrification, 440(April). https://doi.org/10.1038/nature04617
Regmi, P., Miller, M. W., Holgate, B., Bunce, R., Park, H., Chandran, K., … Bott, C. B. (2014). ScienceDirect Control of aeration , aerobic SRT and COD input for mainstream nitritation / denitritation. Water Research, 57, 162–171. https://doi.org/10.1016/j.watres.2014.03.035
Rezania, B., Oleszkiewicz, J. A., & Cicek, N. (2007). Hydrogen-dependent denitrification of water in an anaerobic submerged membrane bioreactor coupled with a novel hydrogen delivery system, 41, 1074–1080. https://doi.org/10.1016/j.watres.2006.11.016
Robertson, W., Vogan, J. L., & Lombardo, P. (2008). Nitrate Removal Rates in a 15‐Year‐Old Permeable Reactive Barrier Treating Septic System Nitrate. Ground Water Monitoring & Remediation, 28, 65–72.
Rocca, C. Della, Belgiorno, V., & Meriç, S. (2007). Overview of in-situ applicable nitrate removal processes, 204(May 2006), 46–62. https://doi.org/10.1016/j.desal.2006.04.023
Rozanska, A., & Wisniewski, J. (n.d.). No Title.
Saliling, W. J. B., Westerman, P. W., & Losordo, T. M. (2007). Wood chips and wheat straw as alternative biofilter media for denitrification reactors treating aquaculture and other wastewaters with high nitrate concentrations. Aquacultural Engineering, 37(3), 222–233. https://doi.org/https://doi.org/10.1016/j.aquaeng.2007.06.003
Science, E., Britain, G., Kuhni, M., & Science, E. (1995). RELEASE OF NITRIC AND NITROUS OXIDES FROM DENITRIFYING ACTIVATED SLUDGE NO3-, 29(1), 215–226.
Sharma, S. K., & Sobti, R. C. (2012). Nitrate Removal from Ground Water : A Review, 9(3), 1667–1675.
Shi, Y., Hu, S., Lou, J., Lu, P., Keller, J., & Yuan, Z. (2013). Nitrogen removal from wastewater by coupling anammox and methane-dependent denitrification in a membrane biofilm reactor. Environmental Science and Technology, 47(20), 11577–11583. https://doi.org/10.1021/es402775z
Shrimali, M., & Singh, K. P. (2001). New methods of nitrate removal from water, 112, 351–359.
Snip, L., Vanrolleghem, P. A., & Flores-alsina, X. (2011). Including greenhouse gas emissions during benchmarking of wastewater treatment plant control strategies, 5. https://doi.org/10.1016/j.watres.2011.04.040
Song, H., Zhou, Y., Li, A., & Mueller, S. (2012). Selective removal of nitrate from water by a macroporous strong basic anion exchange resin. DES, 296, 53–60. https://doi.org/10.1016/j.desal.2012.04.003
Takaya, N., Catalan-sakairi, M. A. B., Sakaguchi, Y., Kato, I., Zhou, Z., & Shoun, H. (2003). Aerobic Denitrifying Bacteria That Produce Low Levels of Nitrous Oxide, 69(6), 3152–3157. https://doi.org/10.1128/AEM.69.6.3152
Terada, A., Smets, B. F., Love, N. G., Garland, J. L., & Gilmore, K. R. (2013). Autotrophic Nitrogen Removal in a Membrane-Aerated Biofilm Reactor Under Continuous Aeration: A Demonstration 1, , { *, 30(1). https://doi.org/10.1089/ees.2012.0222
Tesoriero, A. J., Liebscher, H., & Cox, S. E. (2000). Mechanism and rate of denitrification in an agricultural Electron and mass balance along groundwater flow paths, 36(6), 1545–1559.
Thalasso, F., & Vallecillo, A. (1997). THE USE OF METHANE AS A SOLE CARBON SOURCE n o, 31(1), 1–6.
Thamdrup, B., & Dalsgaard, T. (2002). Production of N 2 through Anaerobic Ammonium Oxidation Coupled to Nitrate Reduction in Marine Sediments Production of N 2 through Anaerobic Ammonium Oxidation Coupled to Nitrate Reduction in Marine Sediments. Applied and Environmental Microbiology, 68(3), 1312–1318. https://doi.org/10.1128/AEM.68.3.1312
Till, B. A. (1998). Fe ( 0 ) -Supported Autotrophic Denitrification, 32(5), 634–639. https://doi.org/10.1021/es9707769
Tong, Y., & He, Z. (2013). Nitrate removal from groundwater driven by electricity generation and heterotrophic denitrification in a bioelectrochemical system. Journal of Hazardous Materials, 262, 614–619. https://doi.org/10.1016/j.jhazmat.2013.09.008
Vitousek, P. M., & Howarth, R. W. (1990). Nitrogen limitation on land and in the sea : How can it occur ?, 87–115.
Volokita, M., Belkin, S., Abeliovich, A., & Soares, M. I. M. (1996). Biological denitrification of drinking water using newspaper. Water Research, 30(4), 965–971. https://doi.org/https://doi.org/10.1016/0043-1354(95)00242-1
Wan, D., Liu, H., Qu, J., Lei, P., Xiao, S., & Hou, Y. (2009). Bioresource Technology Using the combined bioelectrochemical and sulfur autotrophic denitrification system for groundwater denitrification, 100, 142–148. https://doi.org/10.1016/j.biortech.2008.05.042
wastewater engineering_ treatment and reuse.pdf. (n.d.).
Water, J. (2009). Comparison of the Effects of Conventional and Alternative External …
Winkler, M. H., Ettwig, K. F., Vannecke, T. P. W., Stultiens, K., Bogdan, A., Kartal, B., & Volcke, E. I. P. (2015). ScienceDirect Modelling simultaneous anaerobic methane and ammonium removal in a granular sludge reactor. Water Research, 73, 323–331. https://doi.org/10.1016/j.watres.2015.01.039
Woods, T., & Hole, W. (2003). A mechanism of abiotic immobilization of nitrate in forest ecosystems : the ferrous wheel hypothesis, 228–236.
Ye, L., Tang, B., Zhao, K., Pijuan, M., & Peng, Y. (2009). Nitrogen removal via nitrite in domestic wastewater treatment using combined salt inhibition and on-line process control, (2007), 1633–1639. https://doi.org/10.2166/wst.2009.521
Ye, L., & Zhang, T. (2011). Ammonia-oxidizing bacteria dominates over ammonia-oxidizing archaea in a saline nitrification reactor under low DO and high nitrogen loading. Biotechnology and Bioengineering, 108(11), 2544–2552. https://doi.org/10.1002/bit.23211
Zhang, B., Liu, Y., Tong, S., Zheng, M., Zhao, Y., Tian, C., … Feng, C. (2014). Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. Journal of Power Sources, 268, 423–429. https://doi.org/10.1016/j.jpowsour.2014.06.076
Zhang, Y., & Angelidaki, I. (2013). A new method for in situ nitrate removal from groundwater using submerged microbial desalination-denitrification cell (SMDDC). Water Research, 47(5), 1827–1836. https://doi.org/10.1016/j.watres.2013.01.005
Zhao, Y., Feng, C., Wang, Q., Yang, Y., Zhang, Z., & Sugiura, N. (2011). Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm – electrode reactor. Journal of Hazardous Materials, 192(3), 1033–1039. https://doi.org/10.1016/j.jhazmat.2011.06.008
Zhou, W., Sun, Y., Wu, B., Zhang, Y., Huang, M., Miyanaga, T., & Zhang, Z. (2011). Autotrophic denitrification for nitrate and nitrite removal using sulfur-limestone. Journal of Environmental Sciences, 23(11), 1761–1769. https://doi.org/10.1016/S1001-0742(10)60635-3
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Chemistry"
Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




