Basal Cell Carcinoma Epidemiology and Treatment
Info: 9732 words (39 pages) Dissertation
Published: 19th Nov 2021
Click to expand Table of Contents
Table of Contents
Introduction
The Skin
Basal Cell Carcinoma
Aetiology
Ultra Violet Radiation
Genodermatoses
Xeroderma pigmentosum
Nevoid Basal Cell Syndrome (Gorlin syndrome)
Personal/Family History of BCC
Immune-Supressing Drugs
Exposure to Arsenic
Pathophysiology
Molecular
Hedgehog-Signalling pathway
TP53 Defects
Epidemiology
Clinical Features
Diagnostic Tests and Staging
Histological Analysis
Healthy Skin
Basal Cell Carcinoma
Stages and Grades
Stages
Grades
Treatment
Moh’s Micrographic Surgery (MMS)
Curettage and Electrocautery
Surgical Excision
Photodynamic Therapy (PDT)
Case Study
Future and Summary
Future
SMO & Gli inhibitors
Naproxen
Training Cell-Level Classifiers
Summary
References
Introduction
The Skin
The skin is considered one of, if not, the largest organ in the human body. The skin has numerous roles to ensure our bodies run efficiently and effectively. Primarily the skin acts as a physical barrier, protecting internal organs from any external noxious substances and radiation. The skin is important in homeostatic functions, including temperature and fluid regulation. Most notably, the skin is essential in the sensation “touch” acting as first contact with the environment. The skin is also involved in some metabolic processes, such as the synthesis of vitamin D and triglyceride (Ryan, 2015; MacKie, 1981).
The skin consists of three separate layers with their own structure and function. The most superficial layer is known as the epidermis and is primarily made up of four to five layers of squamous epithelium, along with keratinocytes, melanocyte, Langerhans cells and Merkel Cells. The deepest layer of the epidermis is known as the basement membrane and resides in a palisade configuration. It is this layer of cells, which connects the epidermis to the dermis (Losquadro, 2017).
The dermis consists of two distinct layers, the superficial papillary section and the deeper reticular section. Sebaceous and sweat glands are also found within the dermis along with hair follicles, blood vessels, and nerves. Consisting of mostly collagen, elastin, and reticulin, the dermis is also home to fibroblasts, macrophages, masts cells, and lymphocytes (Losquadro, 2017).
Below the dermis lies the subcutaneous fatty tissue layer which is used as a support for the overlying skin as well as a link between the superficial layers of skin and deeper tissues, such as bone and muscle (MacKie, 1981)
Basal Cell Carcinoma
Basal Cell Carcinoma (BCC) is one of the most prevalent forms of skin malignancy, especially in individuals of European ancestry (Epstein, 2008). BCC are usually found on areas of the skin over exposed to UV light. These areas include, but are not restricted to, the scalp, face, neck, and hands (Damjanov, 2005). This variation of skin cancer gets its name from the visual histological similarity to the cells found in the epidermis, running along the basal layer (Epstein, 2008). BCC is nonmelanocytic, meaning it is in fact an epithelium tumour, however some BCC’s can present with pigmentation. BCC have been known as rodent ulcers due to their rolled edges and characteristic central depression or plateau (BAD, 2015).
There are three main subtypes of basal cell carcinomas: nodular BCC that is the most commonly occurring variant, followed by superficial BCC’s, which are the second most common variant, and morpheaform BCC, which is an uncommon variant seen in around 5-10% of BCC cases (Marzuka and Book, 2015).
Aetiology
Ultra Violet Radiation
The skins physiology is greatly affected by UV radiation, and the damaged caused can manifest fairly quickly, as is the case in the acute inflammatory responses where UVB initiates the release of cytokines, vasoactive and neuroactive molecules, which together lead to sunburn (Slominski and Wortsman, 2000; Clydesdale et al., 2001; D’Orazio et al., 2013). Keratinocytes, after being exposed to UV radiation above their threshold, activate their apoptotic pathway and die in the process, these cells are identifiable via the pyknotic nuclei (Bayerl et al., 1995). The skin has a defence against UV radiation in the form of the melanin production pathway, and links have been made between defects in this pathway and cancer susceptibility (D’Orazio et al., 2013).
UV is also capable of causing damage in a delayed manor, with manifestations not surfacing until after years or decades of exposure. UVA damage is indirect and is mediated by the formation of free radicals and corruption of the cellular membranes, playing an important role in the carcinogenesis of stem cells within the skin (Gordon, 2013). UVB on the other hand is able to damage the DNA directly through tumorigenesis and inflammatory responses (Gordon, 2013).
UV exposure is also known to cause damage to cellular signalling pathways and has a temporary effect on mRNA. Disturbances to cellular pathways, such as the Hedgehog signalling pathway, are caused by the absorption of long wavelength UV by the linear repeats or ring structures, found within organic molecules. Such molecules can be found within DNA bases, and therefore genes are readily damaged by UV radiation (de Gruijl et al., 2001).
Mechanisms behind UV’s induced damage developing into cancer are intricate and convoluted process involving mutations to the p53 gene responsible for tumour suppression (Benjamin and Ananthaswamy, 2007). These genes are involved in apoptosis of UV damaged DNA, and mutations result in p53 gene being unable to assist in DNA repair process. Dysregulation of apoptosis caused by the mutation leads to mitosis of keratinocytes going unchecked, initiating the growth of skin cancer (Benjamin and Ananthaswamy, 2007; Brenner and Hearing, 2007).
As UV damage does not account for the cases of BCC that develop in areas not ordinarily exposed, such as in the groin or axilla (Betti et al., 1997). UV damage is not the singular cause of BCC.
Genodermatoses
Xeroderma pigmentosum
Xeroderma pigmentosum is an autosomal recessive condition that causes premature aging to areas of the skin, which are exposed, to UV. This condition reduces the cells ability to repair DNA damage caused by UV radiation (Roewert-Huber et al., 2007). Affecting only 2.3 live births per million within Europe, this is not a common condition, be that as it may, individuals with this condition have an extreme sensitivity to sunlight, and therefore increases the likelihood of DNA mutations and skin cancer (Lehmann et al., 2011).
Nevoid Basal Cell Syndrome (Gorlin syndrome)
Gorlin syndrome is characterised by the development of multiple BCC lesions from a young age. In this condition the cause of the BCC lesions are not related to UV exposure, rather a defect in the Hedgehog signalling pathway, which results in constitutive pathway activity and tumour cell proliferation (Bresler et al., 2016; Yamamoto et al., 2011). It is estimated that 1 in 55,600 people in England suffer from this condition, and 2% of cases of BCC in individuals under the age of 45, are also diagnosed with Gorlin syndrome (Muzio, 2008).
Personal/Family History of BCC
Family history of a patient is attained not only to investigate any genetic susceptibility, but it can also provide information on shared lifestyle, which could point to environmental or behavioural markers.
A study by Berlin et al., (2015) found that 62.2% of individuals with a BCC had some family history of a non-melanoma skin cancer. It was also discovered that individuals with a family history of non-melanoma or melanoma skin cancer, were three times more likely to develop early on set BCC, than those with no family history. The age at which the family member developed a BCC was also found to have an impact on the likelihood of developing BCC. It was stated that if the relative was ≥50 years old then the individual would be twice as likely to develop a BCC, compared to the individuals who reported no family history. This statistic increased when the relative was ≤50 years old (Berlin et al., 2015).
Although genetics play a part in BCC susceptibility, they are not the only reason it is necessary to attain a complete family history. It is a likely assumption that family members share behavioural and environmental characteristics, which can increase their risk of developing BCC. This is especially the case when these activities or behaviours involve UV exposure. Families are known to participate in group activities together and children develop behaviours and habits from their parents. Family members are likely to participate in outdoor activities and/or vacation together, sharing similar UV-protective behaviours e.g. using sun cream, therefore it is not illogical to assume they receive similar UV radiation exposure (Berlin et al., 2015). Recent studies have hypothesised that intense, intermittent outdoor UV exposure, as experienced by endurance athletics, can increase the risk of developing early onset BCC’s (Lee et al., 2017).
Immune-Supressing Drugs
In countries with a predominantly white population, non-melanoma skin cancers (NMSK) are the most common malignancy developed, after an organ transplant (Birkeland et al., 2000; Moloney et al., 2006). It has been found that individuals who are on immune-supressing drugs are more likely to develop NMSC at an earlier age, develop multiple lesions, experience local reoccurrences, and are at a higher risk of developing regional and or distant metastasis (Gutierrez-Dalmau and Campistol, 2007). There are factors effecting the risk of developing a post-transplant malignancy including the length of drug therapy, the type of immunosuppressant drug used, the number of drugs taken simultaneously, and the strength or intensity of the therapy (Gutierrez-Dalmau and Campistol, 2007). A supressed immune system results in the inability to detect and eradicate cancerous cells and viruses known to cause cancer (Epstein-Barr virus, and Hepatitis B&C amongst others). The risk of developing a NMSK post transplant was found increase sixfold compared to the general public (Krynitz et al., 2016).
Exposure to Arsenic
After exposure, arsenic is known to build up within the ectodermal tissues. Dangerous levels of arsenic within the tissues result in BCC’s as well as other types of skin cancer. It is difficult to determine histologically the causation of a BCC, as morphologically and histologically arsenic induced BCC’s do not differ from others(Centeno et al., 2002; Sarkar et al., 2016). Arsenic exposure has been linked to changes in gene expression associated with the molecular signalling pathways involved in arsenic-induced skin carcinogenesis. These changes include, increased transcription of keratinocyte growth factors and oxidative stress(Bailey et al., 2010; Martinez et al., 2011). It is the location of a BCC that can give an insight into the causation, and diagnosis of arsenic exposure usually relies on finding the BCC on sun-protected areas along with other related symptoms of arsenic poisoning (Centeno et al., 2002; Sarkar et al., 2016).
Pathophysiology
BCC has seen many hypotheses into the pathophysiology of the condition over the past 80 years, yet it has been the genodermatoses studies, which have provided the greatest breakthrough into the molecular changes associated with BCC.
The histopathologic variability seen within BCC tumours does not correspond with being derived from a singular epithelial cell type (Bale and Yu, 2001; Sehgal et al., 2014). As a generalisation, it is thought and accepted that BCC tumours arise from pluripotential cells. As these cells are responsible for the formation of hair, sebaceous glands, and apocrine glands in the basal layer of the epidermis, this possibly explains the tumour cells capacity to differentiate into any of the structures found in the epithelium. It is this well-established relationship between BCC and pilosebaceous units, that can explain why BCC’s are often found on areas of the skin that have hair (Sehgal et al., 2014).
The hair follicles in the epidermis are the source of pluripotential and stem cells, which are usually the origin of BCC’s. These stem cells are found just below the sebaceous gland duct in the bulge of the follicle (Sehgal et al., 2014), see Figure 1. This potential origin is justified as BCC’c rarely arise from precursory lesions, rather they arise due to cellular damage from radiation or inherited mutations (Sehgal et al., 2014).
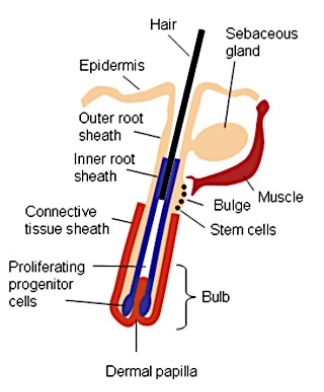
Figure 1. A diagram of a hair follicle, illustrating the location and positioning of the bulge and associated stem cells in the epidermis. Image retrieved from (Lyubovitsky et al., 2007).
The bulge section of the hair follicle is known to be a rich source of stem cells, which rapidly proliferate to heal damaged cells as well as replace dead cells. There is further evidence that suggest that these stem cells even have the ability to migrate out of the hair follicle to regenerate the epidermis after injury (Lyubovitsky et al., 2007). This rapid proliferation of stem cells, which also aid in molecular signalling between the mesenchymal dermal papillae and the developing hair follicle, have been found to be critical in understanding the histo-genesis of BCC (Sehgal et al., 2014).
Molecular
Hedgehog-Signalling pathway
The hedgehog-signalling pathway (Hh) controls the differentiation of tissues during embryonic development. After embryonic development the Hh pathway is responsible for the regulation of proliferation and differentiation of cells(Sehgal et al., 2014). Defects within this pathway are seen within 30% of sporadic BCC, and in almost all nevoid BCC cases (Sehgal et al., 2014; Van Domarus and Stevens, 1984; Hahn et al., 1996). In a fully functioning Hh pathway a Hh receptor, PTCH, inhibits the SMO signalling, see Figure 2a (Yang et al., 2010).
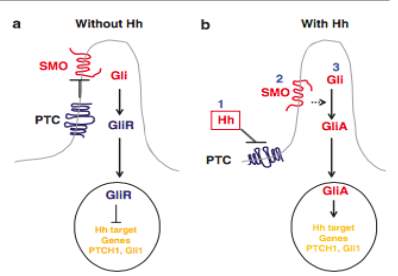
Figure 2. Simplified Hh signalling pathway model found in mammalian cells. (a) In the absence of Hh ligands, PTCH inhibits the SMO signalling. Gli molecules are changed into repressor forms, which switch off the Hh pathway (b) when Hh proteins are present PTCH is unable to bind to SMO. The SMO will then undergo conformational changes. Gli molecules are now changed to their active forms, activating the Hh targets genes. Taken from (Yang et al., 2010)
Over the past few years it has come to attention that the Hh signalling pathway is proprietor to tumour initiation and progression(Yang et al., 2010; Teglund and Toftgard, 2010). The deregulation of the Hh pathway is due to either a disruption of the PTCH1 signalling repressor, found in a majority of cases, or in some rare cases a mutated Smoothened signalling effector (SMO)(Wong and Dlugosz, 2014).
The PTCH receptor binds to hedgehog proteins (usually Sonic hedgehog, SHH, in mammals), which shuttles the PTCH receptor out of the cilium, hindering the PTCH’s ability to bind to SMO, See Figure 2b. This changes the SMO allowing them to couple with the G proteins forming SMO-G (Ogden et al., 2008; Yang et al., 2010). This unhindered SMO activity leads to the activation of the Hh pathways and high-level expressions of the Hh target genes, which ultimately plays a vital role in tumour pathogenesis(Wong and Dlugosz, 2014). These processes start a series of cell events leading to proliferation due to increased expression of transcription factor Gil 1 (Roewert-Huber et al., 2007) (Sehgal et al., 2014)
TP53 Defects
It was discovered by Brash et al., (1996) that 50% of individuals with BCC’s also process a defect in their TP53 gene, usually responsible for the synthesis of the p53 protein, and involved in genomic stability as it activates the repair of DNA and can promote apoptosis (Jayaraman et al., 2014). The TP53 mutation allows for the proliferation of abnormal cells created by UV exposure. The defect on the TP53 gene is not specific for BCC and has been found to play a role in other types of human cancer including squamous cell carcinoma (Brash et al., 1996; Roewert-Huber et al., 2007). Mutated TP53 genes have been identified in between 30 – 70% of basal cell tumours (Lacour, 2002; Reifenberger et al., 2005; Tang, 2011)
Epidemiology
BCC is one of the most common and easily preventable forms of skin cancer. The likelihood of a caucasian individual developing BCC in their lifetime is 30% (Glud et al., 2016). BCC’s are most commonly seen in the elderly population, yet it is possible to experience early on set BCC’s as a child or adolescent. It has been found that individuals with fair skin, red/blonde hair and light eyes are at a greater risk of developing BCC compared to individuals with dark skin, hair and eyes (Wu et al., 2013). Skin which has been subjected to severe or multiple bouts of sun damage are also at a higher risk of developing a BCC (Roewert-Huber et al., 2007).
An Australian study by (Staples et al., 1998) found that the incidences of males with BCC were double that of women. However within the
Currently in England the rate of BCC and other types of non-melanoma skin cancer is thought to be greater than reported by the government. This is due to the lack of efficient monitoring and reporting system for BCC, which is often omitted from, or represented as NMSK in the official cancer statistics, as little is known about the true scope of the condition (Levell et al., 2013). One reason for this being, many registries only include cancers confirmed by histological analysis, which does not take into consideration the cases of BCC which have been diagnosed by clinicians without histology (Reinau et al., 2014; Levell et al., 2013). English women are now overtaking men, as the incidences of NMSC, including BCC and squamous cell carcinoma, are increasing. Cancer research UK stated that during 2014 in England, there were 6490 NMSK cases in males and 6503 NMSK cases in females (Cancer Research UK, 2014).
Ethnicities which have a higher level of skin pigmentation are found to be 19 times less likely to develop a BCC compared to fairer skin caucasian (Roewert-Huber et al., 2007). This risk increases as the geographical location resided in, moves closer to the equator. Individuals most at risk of developing BCC’s are those of Caucasian origin, living close to the equator in countries with a higher UV radiance levels. (Roewert-Huber et al., 2007) stated that the Caucasian population of Australia were at the highest risk of developing BCC’s followed by the United States and Europe.
Clinical Features
BCC are one of the most common types of skin cancer and are known for their slow growing, yet locally destructive tendencies. BCC’s tend to be an indolent form of NMSK, rarely metastasising. Despite this, untreated BCC’s have the potential to extend through the dermis and subcutaneous fatty layer to the underlying muscles, bones and cartilage. Consequences of untreated BCC’s can be excessive and cause debilitating damage see figure 3.
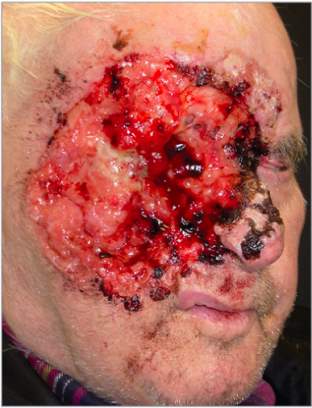
Figure 3. 76-year-old man presented with an osseous and muscular tumour spreading through both of the orbits and nasal bridge. Histology Confirmed ulcerated BCC, which had been growing for 20 years. Taken from (Maul et al., 2016).
BCC’s usually present with a set of features, similar to those observed in other forms of skin cancers and non-malignant neoplasms (Lamberg, 2002) Table 1 identifies the clinical features associated with the three main variants of BCC.
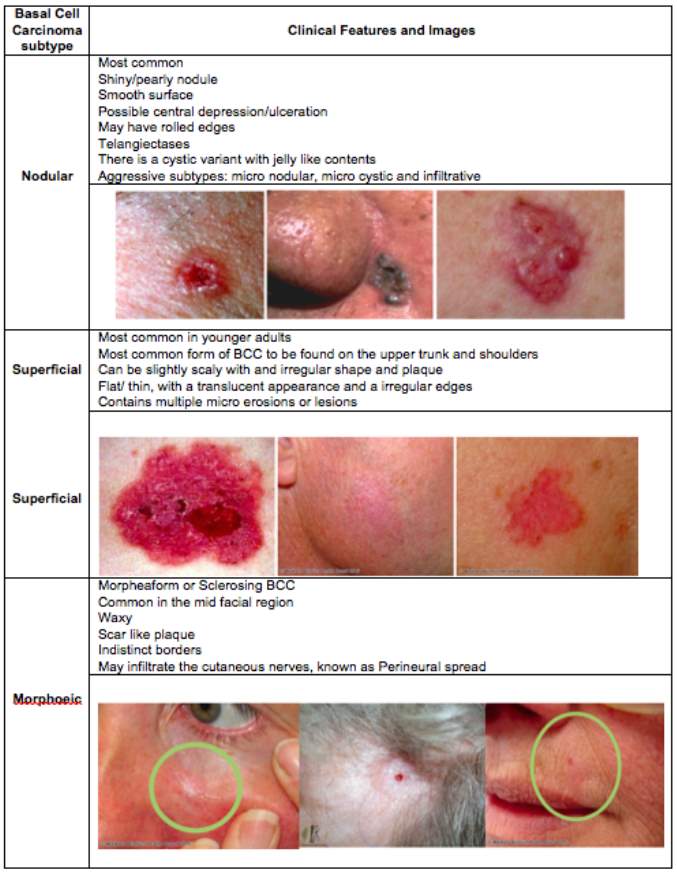
Table 1. Clinical features of the Basal cell carcinoma variants. Created using information and images from (Oakley, 2015).
After a dermatologist has visually inspected a suspicious lesion for signs of BCC, they may also choose to use a dermatoscope, which uses polarized light to examine the skin and lesions in more detail. The only way to definitively diagnose a BCC is through histological analysis of a biopsy, and no lesion suspected of being a BCC or other form of skin cancer should be treated without histological analysis. There are a few forms of biopsy, punch, shave, incisional, and excisional biopsies. The ideal biopsy method to use is the excisional biopsy of the whole lesion, as this can act as evidence for histological diagnosis and definitive therapy, however as BCC’s often occur in areas such as the face, clinicians must take into consideration the cosmetic outcome of such a procedure (Marzuka and Book, 2015).
Diagnostic Tests and Staging
Histological Analysis
Healthy Skin
The epidermis consists almost entirely of stratified, squamous epithelium. The four main cell types identifiable in the epidermis are described in Table 2. Alongside these; within the epidermis there are also paccinian corpuscles that are capsules of cells sensitive to pressure. The epidermis is devoid of any, and all blood vessels, and is reliant upon the dermis for all nutritional and waste disposal needs (Amirlak et al., 2015; University of Leeds, unknown).
Table 2. A description and illustration of the main four cell types found within the epidermis.
| Name of Cell | Description | Image |
|
Keratinocytes |
Also referred to as pickle cells due to their ‘fuzzy or prickly’ appearance, they make up 90% of the cells in the Epidermis. These cells are created in the basal layer and gradually move up through the epidermis undergoing keratinization, eventually loosing their nucleus and forming the superficial layer(Hirobe, 2014) . see Figure 4 | 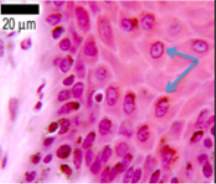 Figure 4. Section of the epidermis stained with haematoxylin and eosin. Keratinocytes are identified in front of the blue arrows. Adapted from (University of Leeds, unknown) |
|
Melanocytes |
Found in the Basal Layer these cells produce melanin pigments which are responsible for skin pigmentation (Hirobe, 2014). These cells have long dendrites or processes which run in between the keratinocytes(University of Leeds, unknown).
See Figure 5 |
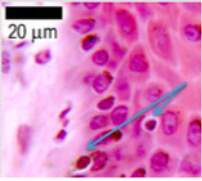 Figure 5. Section of the epidermis stained with haematoxylin and eosin. Melanocytes are identified in front of the blue arrows. Adapted from (University of Leeds, unknown) |
|
Langerhans Cells |
These cells facilitate in the skins response during an allergic reaction. They are irregularly shaped and are antigen-presenting cells. Langerhans cells are created in the bone marrow and found within the basal layer, granular layer and spinous layer of the epidermis(Amirlak et al., 2015). See Figure 6 | 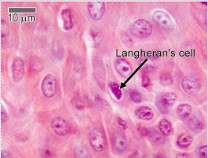 Figure 6. Section of the epidermis stained with haematoxylin and eosin. A Langerhans cell is identified in front of the black arrow. Adapted from (University of Leeds, unknown) |
|
Merkel Cells |
These cells are only found in areas of the skin which are sensitive to light touch, such as the pad of the fingers, genitalia, and within the nail beds (Amirlak et al., 2015). See Figure 7 |  Figure 7. Figure 7. 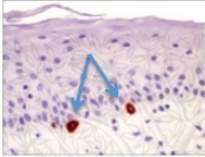 Section of epidermis contacting merkel cells, indicated by the blue arrows. Adapted from (Ovalle and Nahirney, 2007) Section of epidermis contacting merkel cells, indicated by the blue arrows. Adapted from (Ovalle and Nahirney, 2007) |
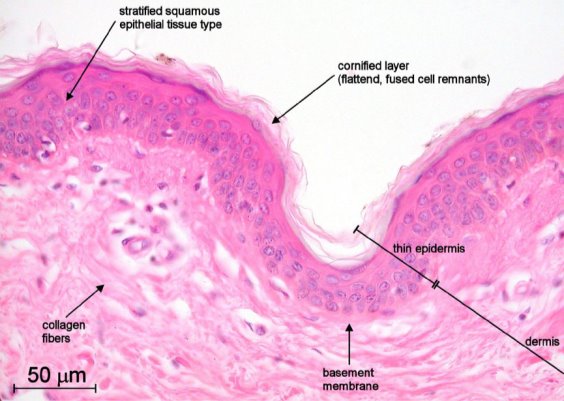
Figure 8. A section of thin human, non-pigmented skin seen under a light microscope at 40x objective and stained with haematoxylin and eosin (Overney, 2002).
Basal Cell Carcinoma
Indolent and Aggressive
Basal cell carcinomas are usually categorised into one of the two broad classifications; indolent and aggressive growth. Indolent-growth includes nodular and superficial BCC’s, where as aggressive-growth group includes the morpheaform and more severe forms of BCC such as infiltrative and basosquamous. The aggressive-growth lesions carry a higher risk of recurrence and cause more destructive local damage. The classification of BCC is complex, and may contain two or more histopathologic patterns, known as mixed histology tumours, which account for almost 40% of primary BCC cases (Marzuka and Book, 2015).
Nodular
It is the large nests of basliod cells (see Fig. 9A), which reside in either the papillary or reticular dermis, which characterise features of nodular BCC (Marzuka and Book, 2015).
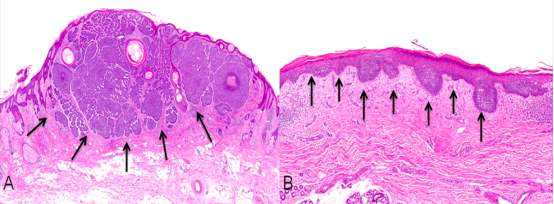
Figure 9. Sections of a nodular BCC viewed under the microscope, stained with Haematoxylin and Eosin. (A) Histology of nodular BCC with a circumscribed ball of cancer cells. (B) Histology of a superficial nodular BCC where the cancer cells spread in a budding pattern restricted to the superficial skin. Adapted from Figure 2 of (Baxter et al., 2012).
Superficial
Unlike in nodular BCC, the basliod cells in superficial BCC proliferate parallel to the surface of the epidermis (see Fig. 9B) and do not migrate deeper than the papillary dermis. In some cases there may be slit like withdrawals of the basal cells from the stroma surrounding the tumour (Marzuka and Book, 2015).
Morpheaform
Morpheaform BCC’s have a characteristic architecture consisting of strands of basal cells up to five cells deep. These strands extend down through the collagen fibres. These tumour types are very unorganised and it is not uncommon to see widespread invasion of reticular dermis cells, some may even infiltrate the subcutaneous fat. Also in this tumour type the palisading configuration of the basal cells is lost, see Figure 10 (Marzuka and Book, 2015).
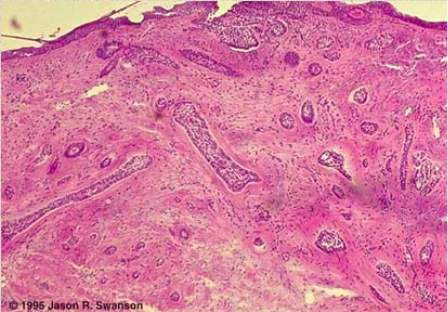
Figure 10. A section of a morpheaform BCC viewed under the microscope and stained with haematoxylin and eosin. The think purple strands of cells are the tumour cells and they extend deep into the dermis. The circumcision of the tumour is very poor. Adapted from (Swanson and Melton, 1996).
Stages and Grades
Stages
It is not very often that BCC’s will be assigned a stage as it is only in very rare cases that BCC’s actually metastasise. However, fairly large BCC’s will be assigned a stage, enabling clinicians to monitor the severity and possible progression of the tumour.
When a stage is assigned it adapts the same development as the staging system for squamous cell carcinomas (briefly outlined in Table 3).
Table 3. A brief overview of the staging system used to describe Basal cell carcinoma (Cancer Research UK, 2017).
| Stage | Description |
|
Stage 0 |
Bowen’s disease or Carcinoma in situ
Cancer cells in the place they started to develop Cells have become malignant but have not spread |
|
Stage 1 |
2cm or less across
OR No high risk features High risk features include: >2mm thick Grown down into the lower section of the dermis Perineural invasion Found to have developed on the lip or ear Microscopic examination reveals extreme abnormalities (poor differentiation) |
| Stage 2 | >2cm across
2 or more high risk factors |
|
Stage 3 |
Either:
Grown into the bones of the face Spread to a lymph node |
| Stage 4 | Either:
Grown into the spinal Colum, ribs or base of the skull |
Grades
Grades are assigned based on the similarity of cancerous cells to ‘normal’ or healthy cells. This grading system is designed to give clinicians an insight into the possible behaviour of the cancer and to help determine the best treatment therapy.
The grades run from 1 to 3:
- Low grade – look like normal cells
- Medium grade – look similar to normal cells with a few abnormalities
- High grade – look very abnormal with minimal similarity to normal cells
(Cancer Research UK, 2017)
Treatment
Moh’s Micrographic Surgery (MMS)
This pioneering excisional method was developed by Frederic Mohs (Mohs, 1941) and combines stages, resection, and extensive surgical margin examination to ensure the maximum removal of cancerous cells, with the minimal loss of skin. This method has demonstrated one of the highest success rates for curing even the most high risks BCC’s. Even with its high success rates, MMS is reserved for use on high-risk facial lesions, due to MMS’s ability to cure the cancer whilst preserving as much skin as possible, which is important when treating facial lesions. MMS is also time efficient, as most being carried out on an out-patient or day-case system, relieving the stress on post surgical care within hospitals (Telfer et al., 2008).
Fredric designed this method based on the principle that any trace of infiltrating BCC cells must be identified and removed, to ensure that the individual is completely cured. Studies from the Netherlands found that MMS is one of the most cost effective treatment options for BCC, despite its expensive running cost. MMS is more expensive than most surgery options for BCC treatment, however its high cure rate and low recurrence rate ensures that this method is cost effective (Telfer et al., 2008).
Curettage and Electrocautery
These two methods are very similar to surgical excision, except the surgeon uses a circular blade to scrape the cancerous cells away. The area is then burnt, destroying any remaining cancer cells. However, these methods are only suitable for small BCCs and this procedure may need to be repeated multiple times to ensure all of the cancer cells are completely removed, preventing recurrence. The success of these procedures relies on the skill and experience of the surgeon. These method can have a high success rate when preformed on low risk BCC, and are deemed to be poor treatment methods and leads to high recurrence rates (Telfer et al., 2008).
Surgical Excision
In this method a predetermined margin is used to excise, what is aimed to be the whole of the tumour. A wide margin is given to ensure any infiltrating BCC cells have been removed. After removal the peripheral and deep tissue margins of the excised tumour are analysed histologically, to ensure they are clean and there are no signs of BCC cells, which may indicate some cells have been missed, leading to BCC recurrence (Telfer et al., 2008). This method has been reported as extremely effective, with a five year recurrence rate of less than 2% (Walker and Hill, 2006; Griffiths et al., 2005). The cure rate of this method can be increased if combined with the curettage procedure, prior to the excision (Telfer et al., 2008).
Photodynamic Therapy (PDT)
Photodynamic therapy requires photosensitising agents, light, and an oxygen source, creating a photochemical reaction, which selectively destroys abnormal BCC cells. This method is usually used on superficial BCC’s. Photosensitising drugs may be administered orally, intravenously, or topically, and once in the body they migrate and congregate within the cancerous cells. Once these medications are exposed to light of a specific wavelength they are activated and this reaction destroys the BCC cells (Ngan, 2003; Matei et al., 2013).
PDT has achieved a great success and efficiency in treating BCC and is known to reduce the disfigurement associated with other BCC therapies. A study carried out by Benz et al.,(2008) found that PDT had an overall rate of complete remission which was 96.7%. Similar studies have boasted success rates of 92.7% (Kubler et al., 1999). Fantini et al. (2011) discovered that PDT had a higher complete remission rate in superficial BCC’s (82%) compared to nodular BCC’s (33%).
Case Study
Three females between the age of 29 and 34 years old, presented with suspicious lesions all present for three months or longer. Table 4 and Figure 11 illustrate the case information related to each patient and present the morphology and pathological diagnosis(Lee et al., 2017).
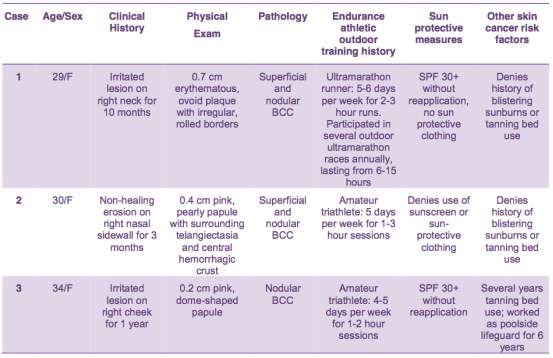
Table 4. Clinical data recovered from three patients diagnosed with early on set Basal Cell Carcinoma (Lee et al., 2017)
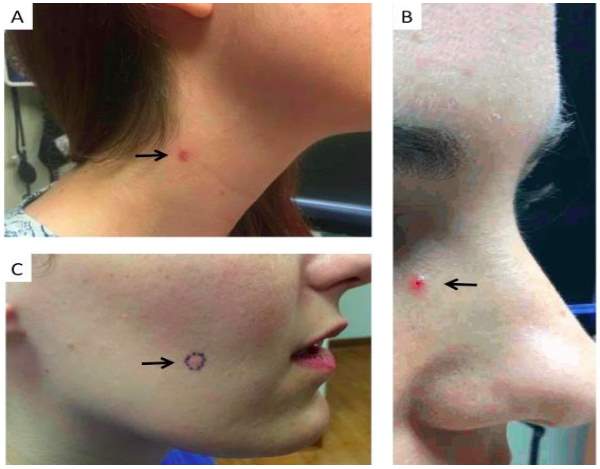
Figure 11. Clinical images of patients with earthy onset BCC’s. Treatment methods unknown. (A) 29 year old female presented with and oval lesion with rolled boarders, on the right side of her neck. Histological analysis determined that the lesion had a duel pathology and showed histologic features characteristic of both nodular BCC and superficial BCC. (B) 30 year old female presented with pearly papule with telangiectasia and a central “scab” located on her right nasal sidewall. Histological analysis determined that the lesion had a duel pathology and showed histologic features characteristic of both nodular BCC and superficial BCC. (C) 34 year old female presented with an irritated lesion on her right cheek, which has been present for a year. Histological analysis determined that the lesions histologic features were characteristic of a nodular BCC. Taken from (Lee et al., 2017)
Future and Summary
Future
SMO & Gli inhibitors
Over the past decade research has uncovered that targeting the Hh signalling pathway is an effective therapeutic treatment for BCC and other NMSK. One method of targeting the Hh pathway is to inhibit the ability of the SMO protein (Zhang et al., 2017). Currently there are two clinically approved SMO inhibitors; Vismodegib and Sonidegib. These two therapies are proving to be effective, however cancers in contact are starting to develop a resistance as they are acquiring different SMO inhibitors. To combat this many researchers have proposed targeting the downstream components directly below the SMO in the cascade. Beauchamp et al., (2011) and Kim et al., (2010) have experienced promising results when using arsenic trioxide molecules to target the Gli molecule. Hyman et al., (2009) also experienced positive outcomes to their trials using HPI-1, HPI-2 and HPI-3 to target the Gli molecule. Preventing the activation of Gli, could prove to be and effective alternative to SMO. Further research and studies are needed before this treatment is available for clinical use.
Naproxen
Naproxen has long been used by clinicians to treat inflammation, however its not widely known that Naproxen also has anti-proliferative and anti-apoptotic properties(Chaudhary et al., 2017). These properties are being utilised in mice as a therapeutic treatment. The drug is showing promising results against UVB induced BCC. The trials have shown that the number of tumours decreased throughout the trial. Data indicates that naproxen is a potent inhibitor of UVB induced carcinogenesis, inhibiting BCC’s by as much as 86%(Chaudhary et al., 2017).
Training Cell-Level Classifiers
Corredor et al., (2017) developed a computer programme which takes advantage of low-, med- and high-level imaging information in order to detect regions of interest on whole slide images. This new method is expected to increase the efficiency of histological analysis as it combines low level information attained from nuclear visual properties (distribution of cells, area of cells and colour of cells), with high level information attained from interactions with pathologists during diagnostic tasks (Corredor et al., 2017).
References
Amirlak, B., Caputy, G. and Shahabi, L. (2015) Skin Anatomy – Epidermis. Skin Anatomy. MedScape: MedScape. [Online] [Accessed on 24.10.2017]
BAD. (2015) Basal Cell Carcinoma. www.bad.org.uk.leaflets: British Association of Dermatologists.
Bailey, K., Hester, S., Knapp, G., Owen, R. and Thai, S. (2010) ‘Gene expression of normal human epidermal keratinocytes modulated by trivalent arsenicals.’ Molecular Carcinogenesis, 49(12) pp. 981 – 998.
Bale, A. and Yu, K. (2001) ‘The Hedgehog Pathway and Basal Cell Carcinomas.’ Human Molecular Genetics, 10 pp. 757 – 762.
Baxter, J. M., Patel, A. N. and Varma, S. (2012) ‘Facial Basal Cell Carcinoma.’ The British Medical Journal, 345 p. 9.
Bayerl, C., Taake, S., Moll, I. and Jung, E. (1995) ‘Characterisation of Sunburn Cells After Exposure to Ultraviolet Light.’ Photodermatology, Photoimmunology & Photomedicine, 11(4) pp. 149 – 154.
Beauchamp, E., Ringer, L., Bulut, G., Sajwan, K., Hall, M., Lee, Y., Peaceman, D., Ozdemirli, M., Rodriguez, O., Macdonald, T., Albanese, C., Toretsky, J. and Uren, A. (2011) ‘Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway.’ Journal of Clinical Investigation, 121(1) pp. 148 – 160.
Benjamin, C. and Ananthaswamy, H. (2007) ‘p53 and the Pathogenesis of Skin Cancer.’ Toxicology and Applied Pharmacology, 224 pp. 241 – 248.
Berlin, N. L., Cartmel, B., Leffell, D. J., Bale, A. E., Mayne, S. T. and Ferrucci, L. M. (2015) ‘Family History of Skin Cancer is Associated with Early-Onset Basal Cell Carcinoma Independent of MC1R Genotype.’ Cancer Epidemiology, 39(6) pp. 1078 – 1083.
Betti, R., Bruscagin, C., Inselvini, E. and Crosti, C. (1997) ‘Basal cell carcinomas of covered and unusual sites of the body.’ International Journal of Dermatology, 37(7)
Betz, C. S., Rauschning, W., Stranadko, E. P., Riabov, M. V., Albrecht, V., Nifantiev, N. E. and Hopper, C. (2008) ‘Optimization of treatment parameters for Foscan (R)-PDT of basal cell carcinomas.’ Lasers in Surgery and Medicine, 40(5), Jul, pp. 300-311.
Birkeland, S., Lokkegaard, H. and Storm, H. (2000) ‘Cancer Risk in Patients on Dialysis and After Renal Transplant.’ Lancet, 355(9218) pp. 1886 – 1887.
Brash, D., Ziegler, A., Johnson, A., Simon, J., Kunala, S. and Leffell, D. (1996) ‘Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion.’ Journal of Investigative Dermatology Symposium Proceedings, 1(2) pp. 136 – 142.
Brenner, M. and Hearing, V. (2007) ‘The Protective Role of Melanin Against UV Damage in Human Skin.’ Photochemistry and Photobiology, 84 pp. 539 – 549.
Bresler, S. C., Padwa, B. L. and Granter, S. R. (2016) ‘Nevoid Basal Cell Carcinoma Syndrome (Gorlin Syndrome).’ Head and Neck Pathology, 10(2)
Cancer Research UK. (2014) Non-Melanoma Skin Cancer Incidence by Sex and UK Country. CancerResearchUK.org: [Online] [Accessed on 24.10.2017] http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/skin-cancer/incidence – heading-Ten
Cancer Research UK. (2017) Skin Cancer: Stages and Grades. [Online] [Accessed on 25.10.2017] http://www.cancerresearchuk.org/about-cancer/skin-cancer/stages-grades
Centeno, J. A., Mullick, F. G., Martinez, L., Page, N. P., Gibb, H., Longfellow, D., Thompson, C. and Ladich, E. R. (2002) ‘Pathology Related to Chronic Arsenic Exposure.’ Environmental Perspectives, 110(5)
Chaudhary, S., Waseem, M., Rana, M., Xu, H., Kopelovich, L., Elmets, C. and Athar, M. (2017) ‘Naproxen Inhibits UVB-induced Basal Cell and Squamous Cell Carcinoma Development in Ptch1 +/- /SKH-1 hairless mice.’ Photochemistry and Photobiology, 93(4) pp. 1016 – 1024.
Clydesdale, G., Dandie, G. and Muller, H. (2001) ‘Ultraviolet Light Induced Injury: Immunological and Inflammatory Effects.’ Immunology Cell Biology, 79 pp. 579 – 568.
Corredor, G., Whitney, J., Arias, V., Madabhushi, A. and Romero, E. (2017) ‘Training a cell-level classifier for detecting basal-cell carcinoma by combining human visual attention maps with low-level handcrafted features.’ Journal of Medical Imaging, 4(2)
D’Orazio, J., Jarrett, S., Amaro-Oritz, A. and Scott, T. (2013) ‘UV Radiation and the Skin.’ International Journal of Molecular Science, 14(6) pp. 12222 – 12248.
Damjanov, I. (2005) ‘The Skin.’ In Pathology Secrets. second ed., United States of America: Elsevier Mosby,
de Gruijl, F. R., van Kranen, H. J. and Mullenders, L. H. F. (2001) ‘UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer.’ Journal of Photochemistry and Photobiology B: Biology, 63(1 – 3) pp. 19 – 27.
Epstein, E. (2008) ‘Basal cell carcinomas: attack of the hedgehog ‘ Nature Reviews Cancer, 8(10)
Fantini, F., Greco, A., Del Giovane, C., Cesinaro, A. M., Venturini, M., Zane, C., Surrenti, T., Peris, K. and Calzavara-Pinton, P. G. (2011) ‘Photodynamic therapy for basal cell carcinoma: clinical and pathological determinants of response.’ Journal of the European Academy of Dermatology and Venereology, 25(8), Aug, pp. 896-901.
Glud, M., Omland, S. and Gniadecki, R. (2016) ‘Basal Cell Carcinoma Surgery.’ Ugeskrift for Laeger, 178(20)
Gordon, R. (2013) ‘Skin Cancer: An Overview of Epidemiology and Risk Factors.’ Seminars in Oncology Nursing, 29(3) pp. 160 – 169.
Griffiths, R., Suvarna, S. and Stone, J. (2005) ‘Do basal cell carcinomas recur after complete conventional surgical excision?’ British Journal of Plastic Surgery, 58 pp. 795 – 805.
Gutierrez-Dalmau, A. and Campistol, J. M. (2007) ‘Immunosuppressive Therapy and Malignancy in Organ Transplant Recipients: A Systematic Review.’ Drugs, 67(8) pp. 1167 – 1198.
Hahn, H., Wicking, C., Zaphiropoulos, P. G., Gailani, M. R., Shanley, S., Chidambaram, A., Vorechovsky, I., Holmberg, E., Unden, A. B., Gillies, S., Negus, K., Smyth, I., Pressman, C., Leffell, D. J., Gerrard, B., Goldstein, A. M., Dean, M., Toftgard, R., ChenevixTrench, G., Wainwright, B. and Bale, A. E. (1996) ‘Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome.’ Cell, 85(6), Jun, pp. 841-851.
Hirobe, T. (2014) ‘Keratinocytes Regulate the Function of Melanocytes.’ Dermatologica Sinica, 32(4) pp. 200 – 204.
Hyman, J., Firestone, A., Heine, V., Zhao, Y., Ocasio, C., Han, K., Sun, M., Rack, P., Sinha, S., Wu, J., Solow-Cordero, D., Jiang, J., Rowitch, D. and Chen, J. (2009) ‘Small-molecule inhibitors reveal multi ple strategies for Hedgehog pathway blockade. .’ Proceedings of the National Academy of Sciences, 106(33) pp. 14132 – 14137.
Jayaraman, S., Rayhan, D., Hazany, S. and Kolodney, M. (2014) ‘Mutational Landscape of Basal Cell Carcinoma by Whole-Exome Sequencing.’ Journal of Investigative Dermatology, 134(1) pp. 213 – 220.
Karagas, M., Zens, S., Li, Z., Stukel, T., Perry, A., Gilbert-Diamond, D., Sayarath, V., Stephenson, R., Barton, D., Nelson, H. and Spencer, S. (2014) ‘Early-Onset Basal Cell Carcinoma and Indoor Tanning: A Population-Based Study.’ Pediatrics, 134(1)
Kim, J., Lee, J., Gardner, D. and Beachy, P. (2010) ‘Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector.’ Proceedings of the National Academy of Sciences, 107(30) pp. 13432 – 13437.
Krynitz, B., Olsson, H., Lundh Rozell, B., Lindelöf, B., Edgren, G. and Smedby, K. (2016) ‘Risk of Basal Cell Carcinoma in Swedish Organ Transplant Recipients: A Population Based Study.’ British Journal of Dermatology, 174(1) pp. 95 – 103.
Kubler, A. C., Haase, T., Staff, C., Kahle, B., Rheinwald, M. and Muhling, J. (1999) ‘Photodynamic therapy of primary nonmelanomatous skin tumours of the head and neck.’ Lasers in Surgery and Medicine, 25(1) pp. 60-68.
Lacour, J. (2002) ‘Carcinogenesis of basal cell carcinomas: genetics and molecular mechanisms.’ British Journal of Dermatology, 146 pp. 17 – 19.
Lamberg, S. (2002) ’11. Growths.’ In Blackwell’s Primary Care Essentials: Dermatology. Second ed., United States of America: Blackwell Science,
Lee, J., Dando, E., Bibee, K., Jedrych, J., Ho, J. and Patton, T. (2017) ‘Early-Onset Basal Cell Carcinoma in Young Female Endurance Athletes: A Report of Three Cases.’ Skin: The Journal of Cutaneous Medicine, 1(2) pp. 104 – 106.
Lehmann, A., McGibbon, D. and Stefanini, M. (2011) ‘Xeroderma pigmentosum.’ Orphanet Journal of Rare Diseases, 6(70)
Levell, N., Igali, L., Wright, K. and Greenberg, D. (2013) ‘Basal Cell Carcinoma Epidemiology in the UK: The Elephant in the Room.’ Clinical and Experimental Dermatology, 38(4) pp. 367 – 369.
Lim, J. and Stern, R. (2005) ‘High levels of ultraviolet B exposure increase the risk of non-melanoma skin cancer in psoralen and ultraviolet A-treated patients.’ Journal of Investigative Dermatology, 124 pp. 505 – 513.
Losquadro, W. D. (2017) ‘A natomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer.’ Facial Plastic Surgery clinics of North America, 25(3) pp. 283-289.
Lyubovitsky, J. G., Krasieva, T. B., Xu, X., Anderson, B. and Tromberg, B. J. (2007) ‘In situ multiphoton optical tomography of hair follicles
in mice ‘ Journal of Biomedical Optics, 12(4)
MacKie, R. M. (1981) ‘2. Basic Skin Biology.’ In Clinical Dermatology: An Illustrated Text Book. Hong Kong: Oxford University Press,
Martinez, V., Vucic, E., Becker-Santos, D., Gil, L. and Lam, W. (2011) ‘Arsenic Exposure and the Induction of Human Cancers.’ Journal of Toxicology, 2011
Marzuka, A. G. and Book, S. E. (2015) ‘Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management.’ Yale Journal of Biology and Medicine, 88(2) pp. 167 – 179.
Matei, C., Tampa, M., Poteca, T., Panea-Paunica, G., Georgescu, S., Ion, R., Popescu, S. and Giurcaneau, C. (2013) ‘Photodynamic Therapy in the Treatment of Basal Cell Carcinoma.’ Journal of Medicine and Life, 6(1) pp. 50 – 54.
Maul, L., Kähler, K. and Hauschild, A. (2016) ‘Effective and Tolerable Treatment of Advanced Basal Cell Carcinoma With Vismodegib Despite Renal Insufficiency.’ JAMA Dermatology, 152(12) pp. 1387 – 1388.
Mohs, F. (1941) ‘Microscopically Controlled Method of Cancer Excision.’ Archives of Surgery, 42(2) pp. 279 – 295.
Moloney, F., Comber, H. and O’Lorcain, P. (2006) ‘A population based
study of skin cancer incidence and prevalence in renal transplant recipients ‘ British Journal of Dermatology, 154 pp. 498 – 504.
Muzio, L. L. (2008) ‘Nevoid basal cell carcinoma syndrome (Gorlin syndrome).’ Orphanet Journal of Rare Diseases, 3(32)
Ngan, V. (2003) Photodynamic Therapy. dermnetnz.org: Dormant New Zealand. [Online] [Accessed on 25.10.17]
Oakley, A. (2015) Basal Cell Carcinoma. dermnetnz.org: Dormant New Zealand. [Online] [Accessed on 25.10.2017] https://www.dermnetnz.org/topics/basal-cell-carcinoma/
Ogden, S., Fei, D., Schilling, N., Ahmed, Y., HWA, J. and Robbins, D. (2008) ‘G Protein Galphai Functions Immediately Downstream of Smoothened in Hedgehog Signalling.’ Nature, 456 pp. 967 – 970.
Ovalle, W. K. and Nahirney, P. C. (2007) Netters Histology flash Cards. Elsevier.
Overney, G. T. (2002) Human Histology for Amateur Microscopists. In: Skin, N.-P. H. Micscape Magazine. pp. Section through non pigmented, thin human skin (objective 40x). Microscopy UK.
Reifenberger, J., Wolter, M. and Knobbe, C. (2005) ‘Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas.’ British Journal of Dermatology, 152 pp. 43 – 51.
Reinau, D., Surber, C., Jick, S. and Meier, C. (2014) ‘Epidemiology of Basal Cell Carcinoma in the United Kingdom: Incidence, Lifestyle Factors, and Comorbidities.’ British Journal of Cancer, 111 pp. 203 – 206.
Roewert-Huber, J., Lange-Asschenfeldt, B., Stockfleth, E. and Keri, H. (2007) ‘Epidemiology and aetiology of basal cell carcinoma.’ British Journal of Dermatology, 157 pp. 47 – 51.
Ryan, T. J. (2015) ‘Community Dermatology: A branch of Dermatology Embracing all Skin
Carers in The Restoration of Skin Function ‘ Health Care: Current Reviews, 3(2)
Sarkar, U., Chakrabarti, S. and Sarangi, S. (2016) ‘Simultaneous presentation of squamous cell carcinoma and basal cell carcinoma in a patient of chronic arsenic poisoning: a case report.’ International Journal of Medical Science and Public Health, 5(10)
Sehgal, V., Chatterjee, K., Pandhi, D. and Khurana, A. (2014) ‘Basal Cell Carcinoma : Pathophysiology.’ SkinMed: Dermatology for the Clinician, 12(3) pp. 176 – 181.
Slominski, A. and Wortsman, J. (2000) ‘Neuroendocrinology of the Skin.’ Endocrinology Review, 21 pp. 457 – 487.
Staples, M., Marks, R. and Giles, G. (1998) ‘Trends in the Incidence of non-melanocytic skin cancer (NMSC) treated in Australia.’ International Journal of Cancer, 78 pp. 144 – 148.
Swanson, J. R. and Melton, J. L. (1996) Histology Morpheaform Basal Cell Carcinoma. Loyola University Medical Education Network: Loyola University Chicago. [Online] [Accessed on 25.10.2017]
Tang, J. (2011) ‘Elucidating the role of molecular signaling pathways in the tumorigenesis of basal cell carcinoma.’ Seminars in cutaneous Medicine and Surgery, 30
Teglund, S. and Toftgard, R. (2010) ‘Hedgehog Beyond Medulloblastoma and Basal Cell Carcinoma.’ Biochimica et Biophysica Acta, 1805(2) pp. 181 – 208.
Telfer, N., Colver, G. and Morton, C. (2008) ‘Guidelines for the Management of Basal Cell Carcinoma.’ British Journal of Dermatology, 159 pp. 35 – 48.
University of Leeds. (unknown) Cells in the Dermis and Epidermis. University of Leeds. [Online] [Accessed on 24.10.2017] http://www.histology.leeds.ac.uk/skin/epidermis_cells.php
Van Domarus, H. and Stevens, P. (1984) ‘Metastatic basal call carcinoma: report of five cases and review of 170 cases in the literature ‘ Journal of the American Academy of Dermatology, 10 pp. 1043 – 1060.
Walker, P. and Hill, D. (2006) ‘Surgical Treatment of Basal Cell Carcinomas using Standard Postoperative Histological Assessment.’ Australias Journal of Dermatology, 47 pp. 1 – 12.
Wong, S. Y. and Dlugosz, A. A. (2014) ‘Basal Cell Carcinoma, Hedgehog Signalling, and Targeted Therapeutics: The Long and Winding Road.’ Journal of Investigative Dermatology, 134(3) pp. E18 – E22.
Wu, S., Han, J., Li, W.-Q., Li, T. and Abrar, A. (2013) ‘Basal-Cell Carcinoma Incidence and Associated Risk Factors in US Women and Men.’ American Journal of Epidemiology, 178(6) pp. 890 – 897.
Yamamoto, T., Ichioka, H., Yamamoto, K., Kanamura, N., Sumitomo, S., Shikimori, M. and Mori, M. (2011) ‘Nevoid basal cell carcinoma syndrome: Clinical features and implications of development of basal cell carcinoma in skin and keratocystic odontogenic tumor in jaw and their gene expressions.’ Asian journal of Oral and Maxillofacial Surgery, 23 pp. 105 – 112.
Yang, L., Xie, G., Fan, Q. and Xie, J. (2010) ‘Activation of the Hedgehog-Signalling Pathway in Human Cancer and the Clinical Implications.’ Oncogene, 29 pp. 469 – 481.
Zhang, X., Tian, Y., Yanf, Y. and Hao, J. (2017) ‘Development of Anticancer Agents Targeting the Hedgehog Signalling.’ Cellular and Molecular Life Sciences, 74(15) pp. 2773 – 2782.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Cancer"
Cancer is a disease in which cells grow or reproduce abnormally or uncontrollably. Cancerous cells have the potential to spread to other areas of the body in a process called metastasis.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




