ImmunoPET Imaging of Human CA6
Info: 7191 words (29 pages) Dissertation
Published: 15th Feb 2022
Tagged: CancerBiomedical Science
First in human study of a companion diagnostic immunoPET tracer for measuring human CA6 expression in cancer for antibody drug conjugate (ADC) therapy
Key words: BFab, human CA6, immunohistochemistry, Positron Emission Tomography (PET), companion diagnostics
Conflict of interest
The authors declared no potential conflicts of interest with respect to the work presented in this manuscript.
Abstract
Purpose: Antigen binding fragment (BFab) derived from antibody (huDS6) targeting a tumor-associated mucin 1–sialoglycotope antigen (CA6) was developed. We synthesized a companion diagnostic PET tracer by radiolabeling B-Fab with 64Cu to measure CA6 expression on cancer tissues prior to anti-CA6 huDS6-DM4 drug conjugate (antibody drug conjugate: ADC) therapy for ovarian and breast cancer patients.
Methods: Two female patients (47 and 62 yrs.) were previously diagnosed with either breast or ovarian cancer. They had initial resections of the primary tumors and had undergone subsequent chemotherapy treatment. Immunohistochemistry (IHC) staining for the CA6 sialoglycotope was performed on the tumor tissue biopsies and post chemotherapy using the mouse anti-human CA6 of DS6 mAb at the time of their initial diagnosis. The levels of CA6 expression in was quantified as H-score that quantitates the intensity of membrane staining. Each patient was imaged by PET with 18F-2-fluoro-2-deoxyglucose ([18F]FDG) and 64Cu-DOTA-BFab (64Cu-BFab). They were each injected intravenously with 64Cu-BFab tracer (5.5 – 11.7 mCi) approximately 1- 4 weeks after their FDG PET scan. Whole body positron emission tomography (PET)/computed tomography (CT) (from head to toe) was performed at 1 hour and 24 hours after 64Cu-BFab tracer administration. Blood pressure, electrocardiogram (EKG), oxygen levels, heart rate, and complete blood count (CBC) were collected from each patient before and 30 min, 1h, 24 h after tracer administration. Staining of immunohistochemistry (IHC) and Hematoxylin eosin saffron (HES) of pre- and post-therapy of ovarian, and post-therapy breast patient lesions were analyzed for CA6 expression. All PET/CT scans were reviewed by two nuclear medicine physicians.
Results: The 64Cu-BFab tracer was well tolerated by both patients at the dose administered without safety concerns. No significant tracer uptake was observed in both patients using 64Cu-BFab scans, whereas [18F]FDG-PET of the breast cancer patient indicated FDG-avid tumor metastasis to the liver, bilateral hila and thoracic spine scanned prior to chemotherapy, and ovarian patient show no uptake. Ovarian lesion IHC data indicated CA6 expression levels were intermediate, prior to chemo therapy. After chemo therapy, the liver metastases in breast cancer patient displayed weak CA6 expression level with an H-score of 35. For lymph node metastases in the ovarian patient indicate H-scores of 80 and 35, for pre- and post- chemo therapy, respectively.
Conclusion: A novel 64Cu-BFab PET tracer was administered to image human CA6 receptor expression in ovarian and breast cancer patients. The study showed the 64Cu-BFab PET/CT imaging tracer was safe with no observed adverse effects. Further PET studies with 64Cu-BFab are needed in order to determine the potential utility of this new tracer as a diagnostic tool for selecting patients who may respond to CA6 directed huDS6-drug conjugate therapy based on CA6 expression.
Introduction
ADC refers to the delivery of cytotoxic drug compounds selectively using an antibody to tumor-associated antigens [1, 2]. In this approach the toxicity of chemotherapeutics is delivered specifically to the target while minimizing the normal cells [3]. This approach has been augmented by the recent Food and Drug Administration (FDA) approval of brentuximab vedotin (Adcentis, Seattle Genetics) targeting CD30 [4], and ado-trastuzumab emtansine (T-DM1, Roche) targeting human epidermal growth factor receptor 2 (HER2) metastatic breast cancer [5] in 2011 and 2013, respectively. In addition, over 60 other ADCs were being evaluated in clinical trials [6-9] at various stages. ADCs are designed to deliver toxic components to selective patients with cancers that are expressing high level of the specific antigens. Given the many obstacles including internalization of ADC selectively in tumor cells and release of payload in the cytoplasm are two key examples to hinder the development of ADC therapeutics [10, 11], the transition of ADCs into the clinic would benefit greatly from non-invasive companion diagnostics that could potentially allow for patient selection and early efficacy evaluation.
Measuring antigen levels expressed by tumors is the most important requirement prior to administering ADC for patient therapy. Traditionally, antigen levels were scored by an invasive tissue biopsy followed by immunohistochemistry. In this technique, tumor biopsy samples harvested are subject to a sampling error, in that they may not represent the entire antigen expression from a cancer. Further, it is difficult to find samples from multiple metastatic lesions which can occur anywhere in the body. To overcome this, non-invasive molecular imaging modalities have been developed. For example, positron emission tomography (PET) has the in vivo ability for visualization, characterization, and quantification of cancer depending on the properties of the radiotracer which is used.
These PET tracers can be used for the following applications: a) as a companion drug to select the patients for ADC therapy regimen, and b) the same tracer can be used for therapy monitoring, after ADC administration by measuring antigen levels in the whole body [12, 13]. These advantages make PET tracers a unique companion imaging agent when targeted to cancer biomarkers. We strive to develop for stratification and response-assessment to deliver an ADC (SAR566658; huDS6-DM4), a humanized monoclonal antibody (huDS6) against the tumor-associated MUC1-sialoglycotope, CA6 biomarker [14], conjugated to the cytotoxic maytansinoid derivative, DM4 [15] that targets the CA6 antigen. The CA6 epitope is found on a variety of solid tumors, including breast, ovarian, cervical, lung and pancreatic tumors. The CA6 antigen has a limited distribution in normal adult tissues and is most characteristically detected in fallopian tube epithelium, inner urothelium, and type 2 pneumocytes. CA6 has limited distribution in normal adult tissues and is often over-expressed in carcinomas of the pancreas, ovary, breast and bladder [16]. Upon antibody/antigen binding and internalization, the immunoconjugate releases DM4, which binds to microtubules and disrupts their assembly/disassembly dynamics, resulting in mitotic arrest of CA6-expressing tumor cells.
Many reports indicated that engineered antibody fragments have been showing short half-lives in circulation [17, 18], compared to the full antibody. We have developed one such antibody fragment named BFab (~50 kDa) based on the full length humanized CA6 antibody and labeled it with 64Cu (t1/2 = 12.7h). This tracer (64Cu-BFab) was developed and characterized for immunoreactivity, specificity, and serum stability. In vivo tracer optimization, and evaluation of targeting ability of CA6 were carried out in a tumor bearing mouse model and reported elsewhere [19]. Pre-clinical study results indicated that this tracer demonstrated improved tumor to background ratios at earlier time points (at 24h p.i. the uptake ratio was 1.6-fold higher for CA6 positive when compared to CA6 negative tumor), which encouraged us to translate it into human subjects.
In this study, we have performed the first-in-human study of 64Cu-BFab to measure the expression of the CA6 biomarker in ovarian and breast cancer patients using PET/CT. Further, prior to injection of 64Cu-BFab for immunoPET imaging, we also performed tissue biopsies of the patients pre- and post-surgery and/or chemotherapy to evaluate the CA6 antigen expression level by IHC.
Materials and Methods
BFab
The selection and production of BFab derived from huDS6, a humanized monoclonal antibody against the tumor-associated sialoglycotope human CA6 have been described previously [20]. BFab was engineered specifically to bind the cell surface antigen CA6, which is overexpressed in tumors and homogeneous in 96% of breast and ovarian cancers [14, 21]. Pharmaceutical grade anti-CA6 BFab was manufactured by Sanofi Aventis. The BFab was engineered from light variable domain (VL), and heavy variable domain (VH), sequences from huDS6 (Fig 1). The BFab construct was produced in bacterial cells with His (GGCGGHHHHHH) tag sequence at the 3ʹ end. These cells were lysed and the soluble expression protein was purified using anti-his tag column; and the targeting ability to human CA6 was optimized in both cell culture and pre-clinical models [19] prior to clinical studies.
Cell Lines and Cell Culture
The human cell line CA6 positive-WISH (Originally derived from human amnion tissue; ATCC© CCL-25 TM ) was purchased from American Type Culture Collection (Manassas, VA), and the human ovarian carcinoma cell line A2780 (CA6 negative) was purchased from European Cell Culture Collection (Wiltshire, United Kingdom). WISH and A2780 cell were cultivated at 37°C in a humidified 5% CO2 incubator using Eagle’s minimum essential medium or Roswell Park Memorial Institute 1640 medium, respectively, and supplemented with 10% heat-inactivated fetal bovine serum, 2 mM (2 mmol/L) L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Invitrogen Life Technologies (Carlsbad, California), except for Eagle’s minimum essential medium, which was supplied by Lonza (Walkersville, MD).
Flow Cytometry
WISH or A2780 cells (2× 106) were resuspended in 0.1 mL of phosphate-buffered saline, 1% bovine serum albumin buffer (PBSA) containing 1:3 dilutions of 3 × 10−7 to 1 × 10−10 M of the BFab or its 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) conjugated DOTA-BFab [19] and kept for 1 hour on ice. Cells were washed twice with the PBSA and incubated for 1 hour on ice in the dark with either Alexa Fluor 488–conjugated mouse anti-human kappa mAb (1:50, Invitrogen Life Technologies) or fluorescein isothiocyanate–conjugated anti-6X His tag antibody (1:100, Abcam, Cambridge, Mass) in 0.1 mL PBSA. After three washes, cells were resuspended in 200µL of PBSA that contained 1% formaldehyde. After 30 mins formaldehyde was removed by washing of these cells with PBSA for three-times. Fluorescence-activated cell sorting assay (FACS) was performed to measure the binding affinity of the B-Fab, by collecting 104 events of live cells that are bound to CA6 antigen. Dead cells were identified with propidium iodide staining. Analysis was carried out on a flow cytometer (FACS Aria III, BD Biosciences, San Jose, CA, USA) at the Canary Center for Early Cancer Detection, a Stanford University School of Medicine Core Facility (Stanford, CA). The data were analyzed by FlowJo FACS analysis software version 10.1 (Tree Star, Ashland, OR) for Mean florescence intensity (MFI) associated with CA6-positive cells and subtracted with background MFI.
Synthesis of pharmaceutical grade 64Cu-BFab radiotracer
The synthesis and purification of DOTA-BFab and its labeling with 64Cu via the chelate DOTA, and the resultant product quality tests have been described previously [22].
Synthesis of DOTA-BFab precursor: DOTA-BFab was synthesized using the DOTA-NHS chelator (Macrocyclics, Dallas, TX). Precursor synthesis was performed according to established procedure using DOTA-NHS and BFab of 10:1 molar ratio [19, 22]
Radiolabeling: Labeling was performed with 64CuCl2 (approximately 1.7±0.08 GBq in 0.1 M (0.1 mol/L) HCl, (University of Wisconsin – Madison, Madison, WI) and the DOTA-BFab (1 mg in 15-20 mL) were incubated at 37°C in ammonium acetate (200–300 μL, 0.1 M (0.1 mol/L; pH = 5.5) for 1 hour. Sequestration of unlabeled radio metal was achieved by adding ethylenediaminetetraacetic acid (EDTA; 0.5 M; 0.5 mol/L), pH = 8, kept at room temperature for 15 mins) to attain a final concentration of 0.01 M (0.01 mol/L). This radiochemical mixture was purified via size exclusion chromatography (SEC) high-performance liquid chromatography (HPLC) (SEC-S2000; Phenomenex, Torrance, CA) in sterile condition as per the procedure published previously [22]. The final product of the purified tracer was formulated in phosphate buffer solution (0.1 M; 0.1 mol/L), pH = 7±0.5).
Quality assurance of the DOTA-BFab, 64Cu, 64Cu-BFab PET tracer
The ratio of mean DOTA chelate (c) per BFab (a) was assayed by Matrix-Assisted Laser Desorption Ionization (AB Sciex 5800 TOF/TOF machine; AB Sciex, Framingham, MA) connected to a CovalX high-mass detector, 1 pM (1 pmol/L) bovine serum albumin (used as an internal standard) divided by the mass of a single DOTA. Identity of the 64Cu was confirmed by the Multi-Channel Analyzer (MCA) analysis, 64Cu emits positrons with a main energy of 897 keV and an abundance of 22.7%. In addition, non-prompt 909-keV photons are emitted at an abundance of 99.9%. Radiochemical purity was determined by using both SEC-HPLC and instant thin-layer chromatography with Tec-Control Chromatography strips (Biodex Medical Systems, Shirley, NY) developed in saline. The radioimmuno-assay and the human serum stability was performed according to the established procedures [23]. The 64Cu-labeled fragments in phosphate buffer were mixed with a nine-fold volume of human serum (Equitech-Bio, Kerrville, TX) and incubated at 37°C for 24 hours to test the stability of the tracer.
Cell Binding and Immunoreactive Fraction Analysis
The cell binding immunoreactive fraction was calculated as previously described [19, 22], WISH cells were used to determine the immunoreactivity. Briefly, WISH cells were grown in Eaglemedium in Earle’s BSS with non-essential amino acids, 1 mM sodium pyruvate, and 10% FBS. These cells were (> 90% viability) re-suspended in Hank’s Balanced Salt Solution (HBSS; Gibco, Mountain View, CA #14025-092) of 50 x 106 cells/mL. These cells were aliquoted at various concentrations (0.6 x 105 to 6 x 106; 10 concentrations) in 200 µL of HBSS, in two sets of duplicate Eppendorf tubes (2 mL volume). In one set of tubes (non-blocking cells), cells were received just HBSS (50 µL) and the other set of tubes (blocking of CA6+ antigen), cells were mixed with unmodified BFab in HBSS (50 µL; 1mg/mL). Both sets of tubes were incubated on ice for 30 mins and pelleted. These pelleted cells were further washed twice and resuspended in 150 µL of HBSS, followed by addition of 50 µL of tracer (64Cu-BFab; 1 µCi) in HBSS and kept at room temperature for 90-120 minutes. After incubation, and mild vortexing, remove 100 µL of aliquot from each of the cell suspension into a Falcon® Round-Bottom Polystyrene Tubes (12 x 75 mm) to count for total radioactivity. The remaining cells suspension (100 µL) in tubes were transfer into another Eppendorf tubes (Biopur, sterile, pyrogen-free) containing 200 µL of a 4:1 mixture of silicone oil (Catalogue #63148-52-7, Sigma Aldrich, St Louis, Missouri, MO) and mineral oil mixture (Catalogue #8042-47-5, Sigma Aldrich) and mixed well with addition of 200 µL of HBSS. These tubes were centrifuge at 16,000g for 5 minutes at room temperature. Freeze these tubes by submerging in liquid nitrogen and clip the bottom of Eppendorf tubes (a guillotine-style dog nail clipper) into a Falcon® counting tubes, which contains cell/oil mixture that bounds to the tracer. Both set of the tubes (total tracer activity and cell bound tracer activity) were counted in a gamma counter (Packard Cobra II Gamma counter, Ramsey, MN) using an energy window of 350-650 keV. From the radioactivity count, immunoreactivity (Kd) was computed by plotting total radioactivity/cell bound radioactivity ratio, against cell concentrations pelleted in each of the tubes. GraphPad software (Prism 6; San Diego,CA) was used to plot a non-linear curve fit with a “one site – total” binding model.
Patients Study
Both patients who participated in this study had histologically proven focal ovarian lesions or breast lesions. The first patient was a 62 y/o female BRCA negative widely metastatic high-grade serous Mullerian adenocarcinoma that was diagnosed November 2016. Her status after post three cycles of neoadjuvant carboplatin and paclitaxel, optimal interval debulking with robotic assisted hysterectomy, bilateral salpingo-oophorectomy, omentectomy, lymph node dissection, and 3 cycles adjuvant chemotherapy (carboplatin and paclitaxel regimen) completed. The second patient was a 47 y/o female with history of left intraductal breast carcinoma that was diagnosed in 2010. She was status-post lumpectomy, sentinel lymph node biopsy, adjuvant chemotherapy and radiation therapy. In 2014, she developed a low axillary recurrence (1 to 3 axillary lymph nodes (N1) that was treated with wide excision and a second round of chemotherapy. She was more recently diagnosed with a second recurrence in the low left axilla that was treated with a complete left axillary lymph node dissection on 12 April 2016 and post-operative radiation therapy in May 2016.
Safety
Before and up to 6 weeks after the administration of radiolabeled 64Cu-BFab, routine laboratory analyses were performed, including hemoglobin, hematocrit, mean corpuscular volume, red blood cell count, white blood cell count (including automated differential), platelet count, sodium, potassium, calcium, chloride, creatinine, urea, uric acid, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyl transferase, albumin, glucose, bilirubin, thyroid-stimulating hormone, and urine sediment. Vital signs including pulse rate, blood pressure, temperature, and respiratory rate were recorded before and up to 3 h after injection. In previous studies with anti-CA6 mAbs, no adverse effects were reported [19].
PET/CT Imaging of CA6 expression in cancer patients
Scans were analyzed using MIM Software (MIM, Cleveland, OH) and they were independently reviewed by two board certified nuclear medicine physicians. The first patient with ovarian cancer had her [18F]FDG PET/CT (dose : 10 mCi) scan performed on 13 January 2017 demonstrating no definitive evidence of active disease and a mildly FDG avid left paraortic lymph node that was likely reactive (negative biopsy on 31 January 2017). However, the patient had biopsy proven disease in omentum, myometrium, serosa, fallopian tube, and ovaries, bilateral. 64Cu B-Fab PET/CT scan was performed on 20 January 2017. Participant was intravenously injected with the tracer (5.5 mCi/0.25mg of DOTA-BFab) and imaged at 1 hr and 24 hrs post injection.
The second patient with breast cancer had her 18F-FDG PET/CT scan performed on 13 September 2017 outside Stanford demonstrating at least 4 FDG avid hepatic metastases, FDG avid bilateral hilar metastases, and an FDG avid osseous metastases at the T5 vertebra. Also, there were possible FDG avid lung metastases seen in lower left lobe. 64Cu B-Fab PET/CT scan was performed on 11 October 2017. Participant was injected with 11.7 mCi (0.38 mg of DOTA-B-Fab) and imaged at 1hr and 24hrs post injection. PET/CT image analysis was performed by using a picture archiving and communication system (PACS) software (Carestream Health, Inc, Rochester, NY).
Immunohistochemistry
For the ovarian cancer patient, immunohistochemistry staining for the CA6 receptor was performed on the peri-pancreatic lymph node tissue sample acquired on 3 November 2016 (prior to FDG PET scan) by endoscopic ultrasound guided fine needle aspiration that was initially positive for adenocarcinoma. Omentum from peritoneal tumor was acquired on 24 January 2017 (4 days after 64Cu-BFab immunoPET scan) after surgery and chemotherapy for IHC staining. For the breast cancer patient, IHC staining for the CA6 receptor was performed on the liver core biopsy (25 September 2017), after FDG-PET scanning on 13 September 2017. Both of these patient samples were fixed and processed into paraffin blocks according to hospital standard procedures. Blocks or 5-µm slides were sent to Sanofi for immunohistochemical evaluation. 5-µm thick sections were prepared from the blocks when needed. Immunohistochemical procedure was carried out on a Discovery XT platform (Roche Ventana, Tucson, AZ) by following method [24, 25]. CA6 expression was determined with SAR566658 (Internal Sanofi reagent) at 5.3 µg/mL as the primary antibody and a mouse biotinylated anti-DM4 antibody (internal Sanofi reagent) at 5.16 µg/mL as the secondary antibody. Detection was achieved with DABMap (reference: 760-124, Roche Ventana) and counterstaining included Hematoxylin (Catalog#: 790-2208, Roche Ventana) and bluing reagent (Catalog#: 760-2037, Roche Ventana). For each sample a set of three slides was prepared. The first slide was stained for CA6 using SAR566658, the second slide was incubated with an isotopic control reagent (HuIgG1-SPDB-DM4, Sanofi reagent) and the last slide did not receive any specific reagent (buffer only). Additional slides were hematoxylin-eosin-saffron stained to support evaluation. IHC slides were evaluated by a pathologist by light microscopy. Percentage of tumoral cells stained and intensity of staining for membrane and cytoplasmic staining were scored. Results were summarized as H-score calculated by following formula: H-score = [(1 * percentage of cells staining at 1) + (2 * percentage of cells staining at 2) + (3 * percentage of cells staining at 3)]
Statistical analysis
Analyses were performed in Prism 6 (GraphPad, version 6.02; San Diego, CA). Statistical significance was denoted for P
Results
Radiolabeling efficiency, specific activity, and overall quality (Table 1)
Synthesis and quality testing of the pharmaceutical grade 64Cu-BFab tracer (Figure 1) was established previously [19]. Radiolabeling efficiency of the 64Cu-BFab tracer was >90%, as measured by both TLC and HPLC. HPLC-purified tracer was >95% assayed by TLC and HPLC. Immunoreactivity and serum stability of the tracer were 69.0±6%, and >90%, respectively (Table 1). Overall tracer quality was summarized in Table 1. Also, the corresponding cold mass of BFab was less than 0.5 mg per patient.
Patients
Evaluation of 64Cu-DOTA-BFab immuno-PET was performed to measure the CA6 expression levels in two female (47 – 62 yrs) cancer patients (ovarian and breast). Patients received tracer intravenously (203.5 – 433 MBq or 5.5 – 11.7 mCi; 0.25 – 0.38 mg of DOTA-BFab) post-surgery and chemotherapy. Both patients were previously diagnosed with their malignancies, had initial resections of the primary tumor, and have undergone multiple chemotherapy treatments.
Safety
The 64Cu-DOTA-BFab tracer was well tolerated by both patients at the given doses with no safety concerns or adverse events. After administration of this novel immunoPET tracer, neither patients had adverse reactions nor significant changes in blood and urine parameters. 64Cu-BFab was found to be safe and well tolerated by both subjects at the administrated doses.
Tracer distribution and whole-body PET/CT imaging of 18FDGand 64Cu-DOTA-BFab
The 18FDGand 64Cu-DOTA-BFab tracers were utilized in both ovarian and breast cancer patients. The visual quality of the immunoPET images varied slightly between the two patients (Figs. 2F, and 3F). After 1 hour, 64Cu-BFab radioactivity was mostly confined to the blood pool, as might be expected with an antibody fragment-based radiotracer. After 24 hours post injection, 64Cu-BFab uptake could be appreciated in the liver, kidneys, spleen, mediastinum, and vascular structures.
Representative images of immunoPET from both patients at 24 hrs post injection of 64Cu-BFab and 1h after 18FDG are presented in Figures 2-3 as the maximum intensity projection (MIP), CT, and PET/CT images of 18FDG-PET (A-C) and 64Cu-BFab-PET (D-F). Mid skull to thigh [18F]FDG-PET scan for the ovarian cancer patient (Figure 2A-C) was performed 1 week prior to the 64Cu-BFab PET (Figure 1D-F). Mid skull to thigh [18F]–FDG PET (Figure 3 A-C) scan for the breast cancer patient was performed 1 month prior to the 64Cu-BFab PET (Figure 2D-F). In Figure 2A, the [18F]FDG PET MIP image of ovarian cancer patient with mid skull to thigh scan shows no tumor uptake but with brown fat distribution. The same ovarian cancer patient, after chemotherapy treatment, had 64Cu-BFab PET scan performed at 24 hours after administration and showed no tracer uptake (Figure 2D) for tumor lesions that were seen in the [18F]FDG PET scan. In Figure 3A, MIP image of an 18FDG PET scan indicated avid metastases in the liver, bilateral hila, and thoracic spine in the breast cancer patient. The corresponding SUVmax values are identified by the serial numbers (1= 4.8, 2=10.9, 3=8.3, 4=12.7, 5=6.9 and 6=6.5) next to the arrows. A month after the 18FDG PET scan, the same breast cancer patient had 64Cu-BFab PET scan performed at 1 and 24 hours after administration. There was no tracer uptake (Figure 3D-F) for any tumor lesions, but tracer uptake was noted to be very high in kidneys (SUVmax =xx) compared to other organs (Figure 3D and F).
Tumor tissue staining pre- and post- tracer administration to evaluate the CA6 expression level in the ovarian cancer patient
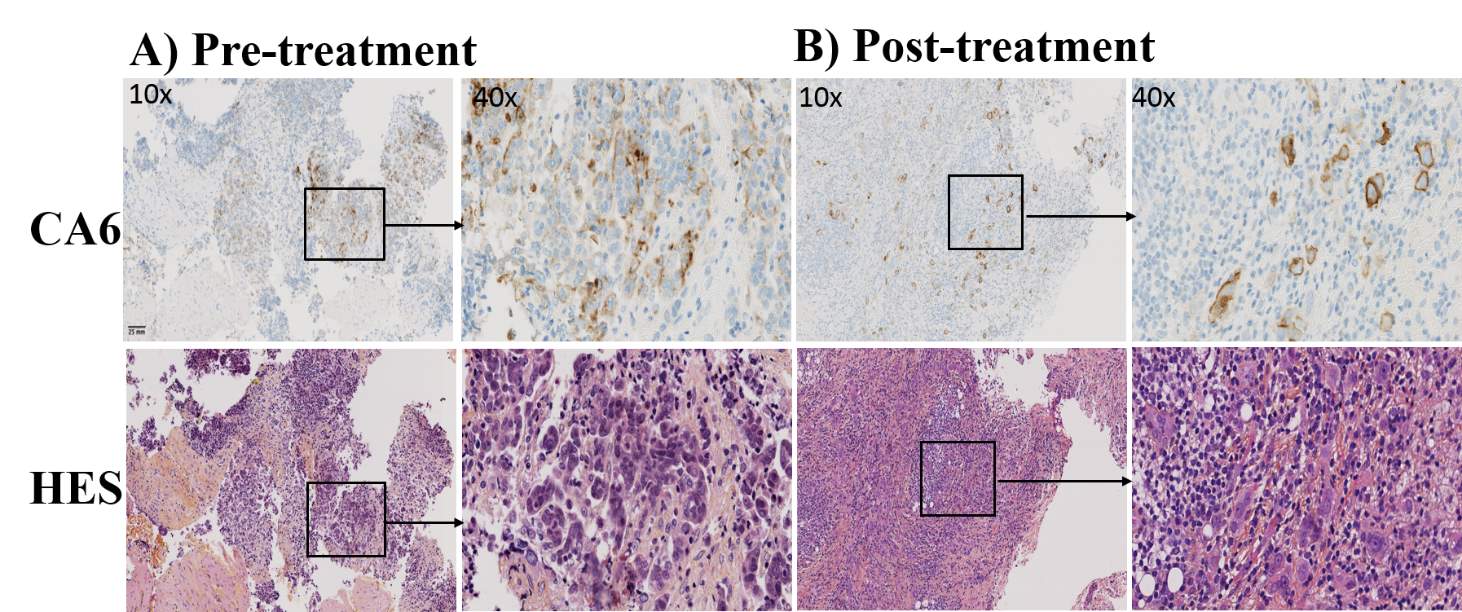
Figure 4 displays the IHC staining for CA6+ and HES of pre- and post- therapy of the ovarian patient. Ovarian patient tissues from pre- (lymph nodes) and post- therapy (omentum from peritoneal tumor) were analyzed for CA6 expression that indicated expression levels were intermediate and weak, with an IHC H-score of 80 and 35, respectively. IHC H-score results of the liver metastasis in the breast cancer patient was 35. These IHC data may represent weak expression of CA6.
Discussion
The purpose of this study was to assess the safety profile of the tracer (64Cu-BFab) in human patients and to evaluate the uptake in CA6 antigen positive tumors to select patients for future immunotherapy using ADC [20]. Overall, the tracer preparation had high quality with respect to purity, stability in human serum, and binding affinity towards CA6+ cells (Table 1). The mean whole-body radiation exposure to these patients was 11.1 mSv. The choice of using 64Cu radioisotope to label BFab (~71 kDa) was based on half-lives of each components. Since the half-life of 64Cu (12.7 hrs), and BFab (
Our tracer was administered into two patients diagnosed with ovarian or breast cancers. We feel compelled to report our results in this pilot safety study with these two patients because this study was closed due to non-availability of cancer patients at our center. In both patients, the tracer was found to be safe and well tolerated. After administration of the 64Cu-BFab tracer, it cleared from most normal organs after 24h. At the same time point, 64Cu-BFab did not show tumor uptake, compared to normal organs, which correlates well with IHC scoring, indicating no significant CA6+ tumors from ovarian (Figure 4), as well as breast cancer (data is not shown) patients. Albeit with respect to the FDG tracer, we were able to see tumor uptake in the breast cancer patient (Fig 3A), this scan was performed due to recurrence of tumor metastasis from breast to liver. Subsequently this patient received chemotherapy again, prior to 64Cu-BFab tracer imaging. Hence, we cannot directly compare between tracer uptakes images from [18F]FDG vs. 64Cu-BFab. Although the quality of the PET images was visually acceptable, the uptake of the individual organs was not appreciable (low 64Cu-BFAb signal compared to the background) to delineate individual organ ROIs.
Quantification of CA6 expression was assessed by IHC for ovarian patient both by immunoPET (24 hours after 64Cu-BFab injection), and tissue biopsies (five days after tracer injection). A good agreement was found between the overall low immunoPET signals and the weak IHC staining data (scoring for CA6+ staining from ovarian patient) obtained from biopsies. In these weak CA6+ lesions no 64Cu-BFab tracer uptake was observed due to lack of sensitivity, which may be due to below the detectable limits of the PET signals. Further, the detection limit of the immunoPET signal was low when compared to the IHC, it may be due to the partial-volume effects of PET, which is a known limitation of the PET technique for very small tumors [26]. Theoretically we can at least identify patients with low and high tumor uptake of targeted tracer; therefore, it is possible to select the patients based on the 64Cu-BFab PET image to assess the CA6 expression level and decide who may benefit from the ADC therapy. 64Cu-BFab immuno-PET has the potential to become an important tool in the detection and treatment monitoring of CA6 positive-cancer. Further clinical evaluation with this immunoPET tracer is warranted.
Conclusions
In this report, we presented the safety of 64Cu-BFab radiotracer in two human patients. Overall, our novel 64Cu-BFab tracer was found to be safe and well tolerated. There was no significant tracer uptake in both patients, probably because they were treated by chemotherapy prior to immunoPET. Further, quantitative analyses of the expression level of cell-surface CA6 levels in lesions by IHC staining (H-score) correlates with the overall weak immunoPET signals in both patients.
References
1. Hedrich WD, Fandy TE, Ashour HM, et al. (2018) Antibody-Drug Conjugates: Pharmacokinetic/Pharmacodynamic Modeling, Preclinical Characterization, Clinical Studies, and Lessons Learned. Clin Pharmacokinet 57(6):687-703
2. Thomas A, Teicher BA, Hassan R (2016) Antibody-drug conjugates for cancer therapy. Lancet Oncol 17(6):e254-e262
3. Blum RH, Wittenberg BK, Canellos GP, et al. (1978) A therapeutic trial of maytansine. Cancer Clin Trials 1(2):113-117
4. Senter PD, Sievers EL (2012) The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol 30(7):631-637
5. Lambert JM, Chari RV (2014) Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem 57(16):6949-6964
6. Flygare JA, Pillow TH, Aristoff P (2013) Antibody-drug conjugates for the treatment of cancer. Chem Biol Drug Des 81(1):113-121
7. Sievers EL, Senter PD (2013) Antibody-drug conjugates in cancer therapy. Annu Rev Med 64:15-29
8. Beck A, Goetsch L, Dumontet C, et al. (2017) Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 16(5):315-337
9. Mullard A (2013) Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov 12(5):329-332
10. Wu AM, Senter PD (2005) Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 23(9):1137-1146
11. Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, et al. (2019) Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J Med Biotechnol 11(1):3-23
12. James ML, Gambhir SS (2012) A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 92(2):897-965
13. Carmon KS, Azhdarinia A (2018) Application of Immuno-PET in Antibody-Drug Conjugate Development. Mol Imaging 17:1536012118801223
14. Kearse KP, Smith NL, Semer DA, et al. (2000) Monoclonal antibody DS6 detects a tumor-associated sialoglycotope expressed on human serous ovarian carcinomas. Int J Cancer 88(6):866-872
15. Lopus M, Oroudjev E, Wilson L, et al. (2010) Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther 9(10):2689-2699
16. Smith NL, Halliday BE, Finley JL, et al. (2002) The spectrum of immunohistochemical reactivity of monoclonal antibody DS6 in nongynecologic neoplasms. Appl Immunohistochem Mol Morphol 10(2):152-158
17. Lu CY, Chen GJ, Tai PH, et al. (2016) Tetravalent anti-CD20/CD3 bispecific antibody for the treatment of B cell lymphoma. Biochem Biophys Res Commun 473(4):808-813
18. Olafsen T, Sirk SJ, Olma S, et al. (2012) ImmunoPET using engineered antibody fragments: fluorine-18 labeled diabodies for same-day imaging. Tumour Biol 33(3):669-677
19. Ilovich O, Natarajan A, Hori S, et al. (2015) Development and Validation of an Immuno-PET Tracer as a Companion Diagnostic Agent for Antibody-Drug Conjugate Therapy to Target the CA6 Epitope. Radiology 276(1):191-198
20. Gomez-Roca CA, Boni V, Moreno V, et al. (2016) A phase I study of SAR566658, an anti CA6-antibody drug conjugate (ADC), in patients (Pts) with CA6-positive advanced solid tumors (STs)(NCT01156870). Journal of Clinical Oncology 34(15_suppl):2511-2511
21. Smith NL, Halliday BE, Finley JL, et al. (2001) Immunohistochemical distribution of tumor-associated antigen CA6 in gynecological neoplasms as detected by monoclonal antibody DS6. Int J Gynecol Pathol 20(3):260-266
22. Natarajan A, Gowrishankar G, Nielsen CH, et al. (2012) Positron emission tomography of 64Cu-DOTA-Rituximab in a transgenic mouse model expressing human CD20 for clinical translation to image NHL. Mol Imaging Biol 14(5):608-616
23. Natarajan A, Arksey N, Iagaru A, et al. (2015) Validation of 64Cu-DOTA-rituximab injection preparation under good manufacturing practices: a PET tracer for imaging of B-cell non-Hodgkin lymphoma. Mol Imaging 14
24. Grosset AA, Loayza-Vega K, Adam-Granger E, et al. (2017) Hematoxylin and Eosin Counterstaining Protocol for Immunohistochemistry Interpretation and Diagnosis. Appl Immunohistochem Mol Morphol
25. Fedchenko N, Reifenrath J (2014) Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagn Pathol 9:221
26. Srinivas SM, Dhurairaj T, Basu S, et al. (2009) A recovery coefficient method for partial volume correction of PET images. Ann Nucl Med 23(4):341-348
Legends for Table and Figures
Table
Table 1: Characterization of 64Cu-BFab radiotracer. Table 1 provided the 64Cu-BFab tracer dose injected for the patient’s study. Tracer synthesis yielded high purity and specificity to CA6+ antigen expressed on tumor. Further, the key qualities of the tracer such as purity (>99%), serum stability (>90%), immunoreactivity (>60%), and sterility were met the specifications of the injectable dose that are approved by the FDA-IND. DOTA = 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid, HPLC = high performance liquid chromatography, TLC = thin-layer chromatography.
Figures
Figure 1: Schematic diagram of the synthesis of 64Cu-DOTA-BFab. 1= BFab {VH = heavy variable domain, VL = light variable domain}; 2 = NHS-DOTA; 3 = 64Cu; 4 = 64Cu-DOTA-BFab.
Figure 2: PET/CT images of the ovarian cancer patient for CA6 expression. Maximum intensity projection (MIP) images using [18F]FDG-PET (A) performed 1 week prior to the 64Cu-BFab PET (D) tracer. FDG-PET mid skull to thigh (A-C), and the whole body 64Cu-BFab PET (D- F) scans were performed 24 hours after administration of the tracers. The MIP-FDG-PET, CT, PET/CT (A-C) and MIP-BFab-PET, CT, PET/CT (D-F) images are showing no tumor uptake, Figure 2 (A) [18F]FDG-PET uptake was corresponded to brown fat distribution.
Figure 3: PET/CT images of the breast cancer patient for CA6 expression. Maximum intensity projection (MIP) images using [18F]FDG-PET (A) performed one month prior to the 64Cu-BFab PET (D) tracer. FDG-PET mid skull to thigh (A-C), and whole body 64Cu-BFab PET (D- F) scans were performed 24 hours after administration of the tracers. The images of MIP-FDG-PET, CT, PET/CT (A-C) and MIP-BFab-PET, CT, PET/CT (D-F) are corresponds to FDG-PET and B-Fab tracers, respectively., Figure 3 (A) FDG-PET uptake are indicated by arrows indicating the FDG avid metastases in the liver, bilateral hila, and thoracic spine. The corresponding SUVmax of FDG uptake were identified by the serial numbers (1= 4.8, 2=10.9, 3=8.3, 4=12.7, 5=6.9 and 6=6.5) next to arrow. Figure 3 (D) Same breast cancer patient with whole body 64Cu-BFab PET scan 24 hours after administration showing no tumor uptake.
Figure 4: CA6-expression of ovarian cancer patient tissue specimens. Immunohistochemistry was carried out to evaluate CA6 expression level of the ovarian adenocarcinoma patient (High-grade serous carcinoma= HGSC). Representative tissue specimens from the same patient of peripancreatic lympnode (A, pre-treatment) and omentum from peritoneal tumor (B, post-treatment of chemotherapy, surgery, and adjuvant-chemotherapy) for staining of CA6 positive expression. Tissues were stained for CA6 expression (top panels) using validated anti-human CA6 immunohistochemistry performed with SAR566658 (Internal reagent from Sanofi) and hematoxylin eosin saffron (HES) staining (bottom panels) for the evaluation of morphology. IHC analysis for pre- and post- treatment tissue specimens indicated H-scores were 80 (medium) and 35 (weak), respectively for CA6 expression. Original magnification indicated on images 10×, scale bar = 25 mm.
Table 1
64Cu-BFab Quality Assurance
|
Tests # |
Test Specification |
Results |
Release specification |
|
1 |
Immunoconjugate purity |
>95% |
>90% |
|
2 |
DOTA/B-Fab (c/a) |
~1 |
|
|
3 |
Appearance |
Clear solution |
Colorless solution |
|
4 |
pH |
6.8 |
6.5± 0.5 |
|
5 |
Radiopharmaceutical purity by HPLC |
>99% |
>90% |
|
6 |
Radio chemical Yield by TLC |
>99.5% |
>55% |
|
7 |
Specific Activity |
2.0 Ci/µmol |
1-5 Ci/µmol |
|
8 |
Human serum Stability |
>90% |
>90% |
|
9 |
Bacterial endotoxin |
|
|
|
10 |
Sterility |
Pass |
Pass |
|
11 |
Immunoreactivity |
>60% |
>60% |
Figure 1
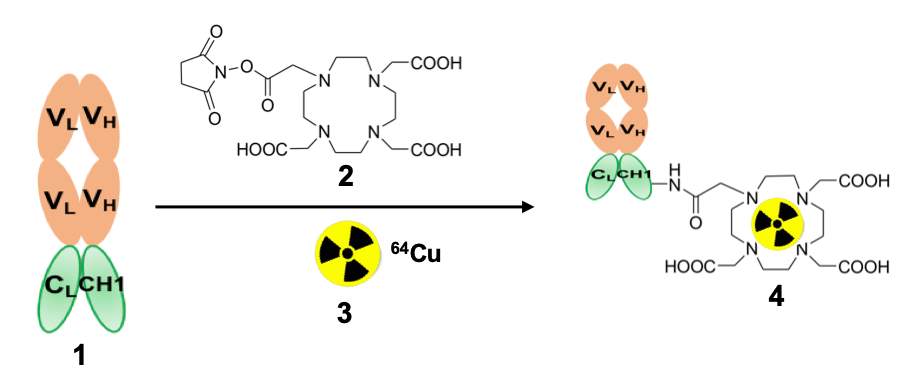
Figure 2
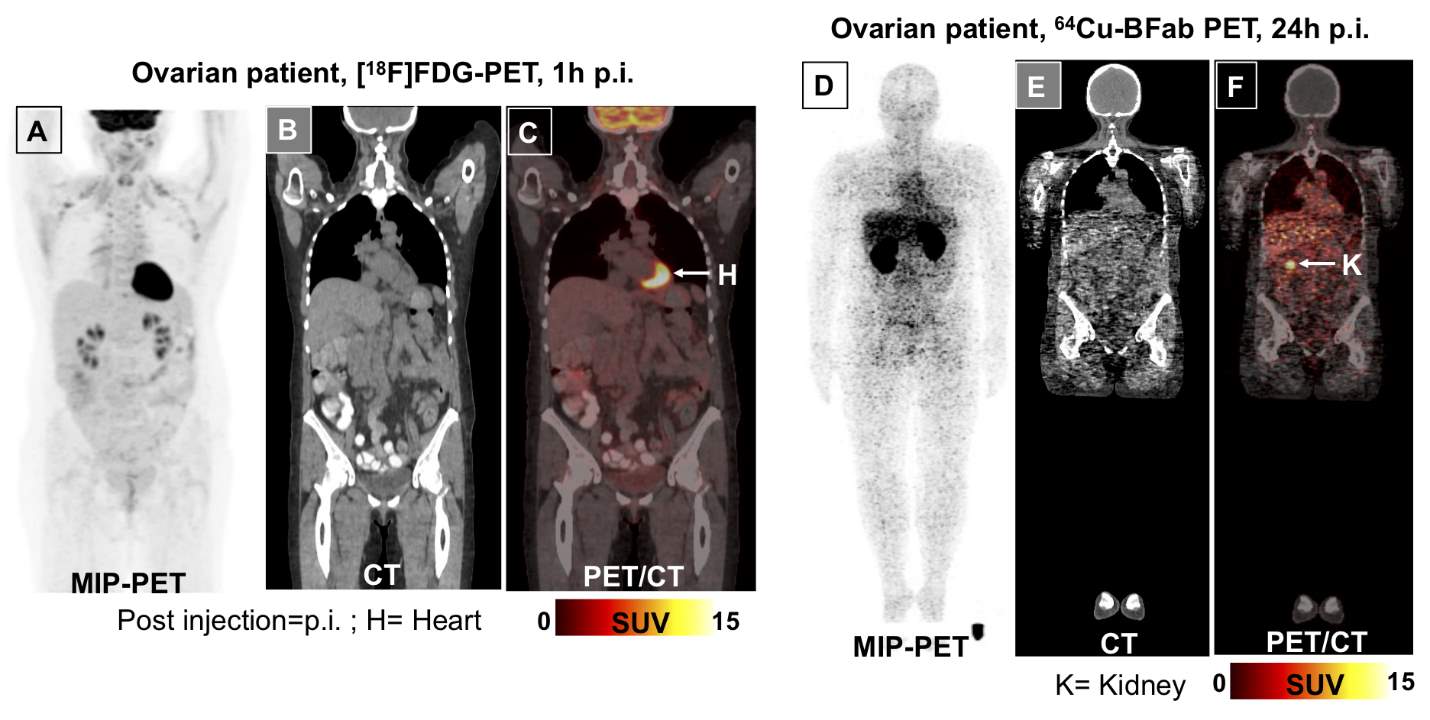
Figure 3
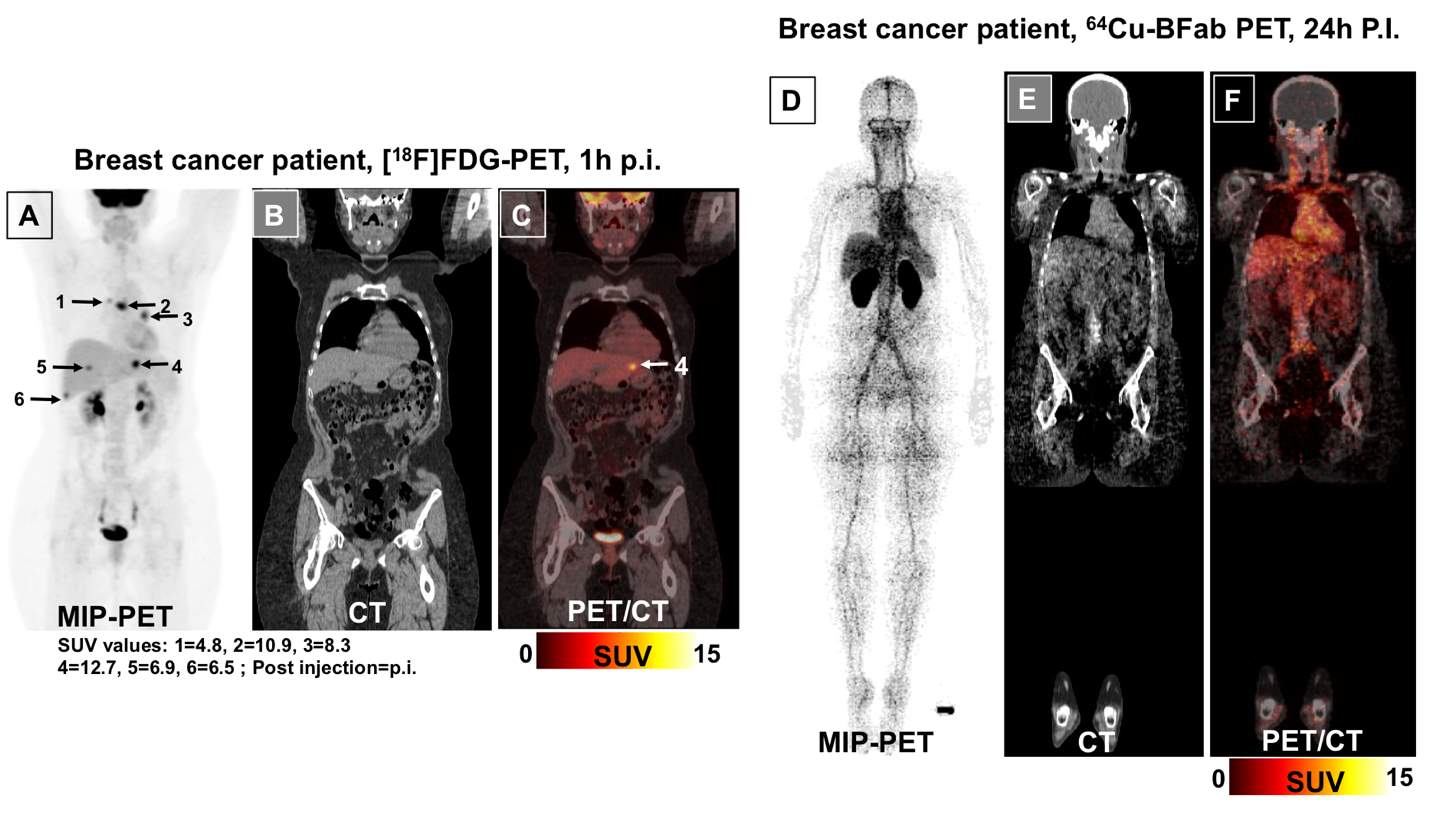
Figure 4
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biomedical Science"
Biomedical Science focuses on how cells, organs and systems function in the human body and underpins much of modern medicine. Biomedical Science applies parts of natural and/or formal sciences to help develop advances in healthcare.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




