Cannabis and Adolescent Perceptions
Info: 10358 words (41 pages) Dissertation
Published: 1st Mar 2022
Tagged: CriminologyYoung People
Introduction
The age of initiation of cannabis use is changing with younger children and adolescents reporting daily cannabis use. According to the national institute on drug abuse (NIDA) (2014), 16.4% of individuals age 12–17 and 51.9% of individuals’ ages 18–25 years have used cannabis in their lifespan in the USA. While cannabis use appears to be increasingly recognised as a ‘safe’ recreational drug (Camchong et al 2016), it is imperative to comprehend what the consequences of chronic cannabis use are, during critical phases of development such as adolescence. Researchers are still uncertain as to precisely how cannabis use affects brain structure and function, but some studies indicate that long-term chronic use leads to fluctuations in both neurological function and physical brain structure (Battistella et al 2014). Further, cannabis’s relationship with psychological health remains unclear. Researchers have established that cannabis use, significantly influences adolescent psychosocial behavior, however, more research is required to corroborate accurately how cannabis use correlates to mental health (Shrivastava et al 2011).
Previous studies exploring the impact of cannabis on development, advocate that there is a tenacious effect on cognition and neuropsychological performance in individuals who initiate cannabis use during adolescence (Jacobus and Tapert 2014). Longitudinal data demonstrate that individuals with more persistent cannabis dependence have a distinct intelligence quotient (IQ) deterioration, with substantial impact on complete IQ (full-scale IQ) ( Meier et al 2012 ). Moreover, evidence proposes that overall IQ discrepancies do not completely improve after cessation of use (1 year), particularly in adolescent-onset cannabis users (Meier et al 2012). Further to its effects on intellectual aptitude, cannabis has been perceived to have a negative influence on neuropsychological test performance in tasks that assess executive function and psychomotor speed (Lane et al 2007). Even after 10 months of abstinence Schweinsburg et al (2008) propose that individuals that commenced using cannabis during adolescence have persistent neuropsychological deficits.
For the purpose of this assignment an overview of cannabis will be provided before focusing on more specific aspects of cannabis use pertinent to the adolescent population. This will include, gaining an insight into the perceptions and impact of legislative changes, among adolescents. Following on from this an overview of adolescent brain development will be discussed followed by an insight into the impact of cannabis use on the adolescent brain.
Finally the area of mental health and cannabis use among this cohort of society will be deliberated before concluding on the related points of the literature reviewed.
What is Cannabis?
Cannabis is the preferred title of the plant Cannabis Sativa, Cannabis Indica, and of minor significance, Cannabis Ruderalis (Gloss 2015). The usage of cannabis for medicinal, ceremonial or recreational reasons is resultant from the activities of cannabinoids in the cannabis plant and these compounds also produce the inadvertent adversarial consequences of cannabis (Madras 2015). It is estimated that the plant comprises of at least 750 chemicals, among which are some 104 different cannabinoids (Radwan et al 2015). Though cannabis comprises many chemical substances, it is delta-9- tetrahydrocannabinol (THC) that has been identified as the principal compound that creates the “high” that follows when smoking or ingesting the plant materials (Radwan et al 2015).
Chemical structure of THC
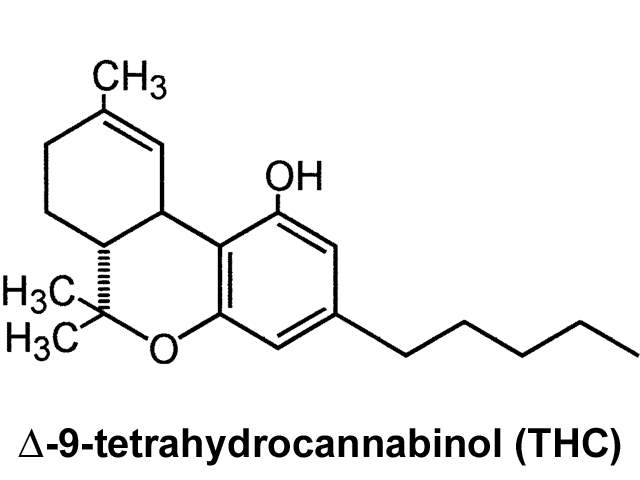
It is likely that other compounds in cannabis also contribute and interrelate with THC to produce its innumerable physical and psychological effects. Research has particularly concentrated on better comprehending the role of cannabidiol. Selected evidence proposes that cannabidiol may moderate the effects of cannabis, diminishing the potential anxiolytic and psychomimetic effects of THC, nevertheless other studies have not observed such effects (Karschner et al 2011). However, THC levels are fluctuating, as propagation of different strains are yielding plants and resins with striking growth in THC content over the previous decade, from ~ 3% to 12-16% or higher and differing in different countries (Swift et al 2013). THC levels in some cannabis preparations, have escalated even more drastically by using a concentrating process (butane hash oil) that produces levels approaching 80% THC (Stogner and Miller 2015).
Cannabis is consumed by numerous routes, with the most common route smoking, (Baggio et al 2014) followed by vaporization, and then by the oral route. Azorlosa et al (1995) have indicated that inhalation by smoking or vaporization discharges highest amounts of THC into blood within minutes, attaining its peak at 15-30 minutes, and decreasing within 2-3.
The active chemical in cannabis tetrahydrocannabinol (THC) strongly resembles the structure of cannabinoid chemicals that happen naturally in the body, called endogenous cannabinoids (NIDA 2014). These chemicals are neurotransmitters that direct communications between neurons in the brain and the rest of the nervous system (NIDA 2014). The areas of the brain most connected with the receptors for endogenous cannabinoids are the hippocampus, basal ganglia, amygdala, cerebellum, ventral striatum, neocortex, and the brain stem and spinal cord; these are the principal regions accountable for pleasure, concentration, memory, coordination, movement, and sensory and time perception (NIDA 2014).
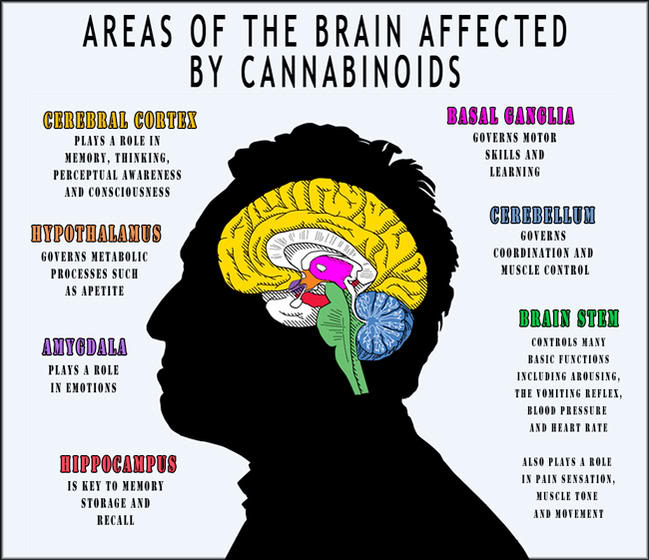
The areas closely linked with cannabinoid receptors also manage pain inflection and prompt higher-order processes (NIDA 2014). All of these areas feature in typical human brain function and effect the ways that we behave and connect to other people and stimuli (NIDA 2014). Resultant from THC, closely resembling endogenous cannabinoids in structure, it connects to the cannabinoid receptors in the brain and overwhelms the nervous system with communications to that portion of the brain (NIDA 2014). This stimulates the areas of the brain connected with endogenous cannabinoids, triggering instabilities to usual brain function (NIDA 2014). This produces the effects that cannabis users recognise, such as feelings of pleasure and relaxation, distorted perception of time, heightened sensory experiences, and slow motor function (NIDA 2014).
Epidemiology of cannabis use
Patterns of drug use in Ireland have become increasingly diverse in terms of the type of drug used, the availability of drugs and the demographics of users (van Hout 2009a). Service providers consider that illicit drug and drug use has become a normal feature of society (van Hout 2009a). UNICEF Ireland, Changing the Future (2011) surveyed a significant number of 16-20 year olds, and established that over a third of participants had taken an illegal drug and that most had done so by the age of sixteen (UNICEF Ireland, Changing the Future 2011).
Widespread availability and ease of access to illegal substances has contributed to a degree of acceptance in communities and has facilitated with the normalization of drug use. In recent years Cannabis has become so obtainable and inexpensive, its use has amplified significantly (Bellrose 2012). Cannabis is regarded among young people as a ‘safe drug’ and van Hout (2010) argues that most young people estimate cannabis to be as safe as smoking cigarettes and were not concerned with any future health impact associated with use.
The United Nations Office on Drugs and Crime (UNOCD) World Drug Report (2012) identify that cannabis is the worlds’ most widely used substance with between 119 and 224 million users worldwide. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2011) lifetime prevalence figures for adult population (15-64 yr. old) show that 78 million European adults report using cannabis with Western Europe having the uppermost frequency for cannabis in the world. In line with the aforesaid Global and European statistics, it was observed that cannabis is the most extensively accessible and frequently used drug in Ireland (Long and Horgan 2012). van Hout (2010) has indicated that the social accommodation of recreational cannabis use is becoming more commonly established within Irish society.
Similar to figures from other countries; 32.2 % of young Irish adults aged 15-34 years, compared to 18% of older adults aged 35-64 years reported using cannabis with use more common in males National Advisory Committee on Drugs (NACD) ( 2011). Lifetime cannabis use was reported by 23.5% of 15 year olds in the 2006 Irish HBSC survey (Currie et al 2008). In 2006/07, cannabis was the most commonly used illegal drug with 21% reporting lifetime use, which was also a significant increase on 2002/03 rates (12.0%).
Prevalence rates for lifetime cannabis usage amongst young adults (15-34 yrs) were at least double those of older adults, 29.1% versus 14.5% respectively. Moreover, lifetime and preceding month frequency of cannabis use among young adults had significantly increased since 2002/03 (NACD 2008b).
However, Degenharth and Hall (2012) caution that since studies from different countries often use various methods for estimating the prevalence of substance use, comparison of results between countries could be misleading. The interpretations of reported estimates should therefore be made with awareness of these methodological limitations.
Perceived cannabis use norms and impact of legislative changes among adolescents
There is an emergent perception, predominantly in adolescents and young adults (Lopez-Quintero and Neumark 2010), that cannabis is ‘harmless’ particularly when compared to other abused substances such as nicotine and alcohol that are legal. Notwithstanding the latent consequences, the levels of perceived risk and disapproval of cannabis amid adults and adolescents are minimal and progressively diminishing ( Johnston et al 2012). Rationales allude to, for this perception include, the deliberation that cannabis-associated mortality is lower than tobacco and alcohol, which are linked with cancer and overdose/ vehicular accidents, correspondingly (Hurd et al 2014).
Friese and Grube (22013) suggest that from an American perspective, changes in state laws on medical or recreational cannabis use, may influence changes in adolescents’ access to cannabis and cannabis use (CU) norms. Information regarding medical cannabis legalization or discrimination of cannabis use may indicate more liberal community norms in relation to CU generally, and research data propose that CU norms in the community are related to adolescent cannabis use (Friese and Grube 2013).
Perceived parental or friends’ approval of cannabis use is allied with an elevated cannabis use prevalence among college students (Labrie et al 2011). Although cannabis use practices among adolescents are less researched, preceding studies suggest that adolescent’ perceptions of their parents’ or peers substance use or norms, may effect adolescents’ substance use (Iannotti et al 1996). Cannabis use norms may be impacted by community’s drug use level or norms, adolescents’ substance use, and the availability of cannabis (Bahr et al 2005). Drug use norms of close friends can be associated with adolescents’ intention towards substance use (Olds et al 2005). In a study comprising of 180 heavy CUs who participated in a treatment trial, the perceived approval of close friends’ to cannabis use was positively associated with cannabis use (Walker et al 2011). Therefore, when considered jointly, adolescent’s perceived CU norms appear to be an important correlate of cannabis use (Wu et al 2015).
With reference to the ‘Theory of planned behaviors’, a person’s attitudes, norms and perceived control may influence the intention of CU and actual use (Malmberg et al 2012).
The opposite association between adolescent’s discontentment or apparent disapproval for cannabis use by significant others, and lower CU, may be related to a higher level of adolescent’s confidence, in being able to decline or avoid CU in tempting circumstances (Malmberg et al 2012). Specifically, for those adolescents with negative attitudes towards cannabis use and experience disapproval of CU from those within their social environments, they may have a higher level of refusal or a lower intention of using cannabis, in comparison to those within their peers who perceive a higher level of cannabis use acceptability (Malmberg et al 2012).
In their critical review of the literature, Sznitman and Zolotov (2015) concluded that there was insufficient evidence to support medical cannabis’s negative influence on public health.
Other studies support some concerning trends, predominantly as it relates to adolescents. One American study found that adolescents had significantly higher rates of reported use in the past 30 days and a lower perception of risk in states with legalized medical cannabis (Wall et 2011). Significant differences in past-year use and prevalence of dependence/misuse were identified between states with medical cannabis laws and states without them (Cerda et al 2012). The probabilities of past-year use and dependence were virtually double for individuals living in states with legalized medical cannabis (Cerda´ et al 2012).
The literature on adolescent use and perceptions of cannabis in relation to legislative changes is in an embryonic state. Cerda´ et al (2012), hypothesize that ‘‘Future studies are needed . . .
on the particular impact of medical marijuana legalization on youth who bear a disproportionate burden of marijuana-related disorders, and are vulnerable to the advertising effects of other substances such as tobacco’’ (p. 26). The initial research appears to propose that both cannabis laws and adolescent use are attributable to lenient normative approaches.
However, absent from this debate are the opinions of those who may have the greatest perception into the individual and social consequences of cannabis use, substance misuse treatment providers (Sorbesky and Gorgens 2016). ).
Cannabis as a ‘gateway drug’
Hurd et al (2014) deliberate that a chief characteristic of the debate concerning adolescent cannabis use is whether there is a possibility that it increases the usage of other addictive substances, such as heroin and cocaine later in life, a phenomenon recognised as the ‘Gateway Hypothesis’. However, epidemiological and clinical studies have chronicled an important connection between continual early cannabis exposure and the increased vulnerability to other illicit drug use (Fergusson and Boden 2008).
Overall, the data according to Hurd et al (2014) proposes, that use of ‘heavy’ drugs is virtually systematically preceded by the use of cannabis, and that susceptibility is correlated with the intensity of cannabis exposure. Moreover, the use of cannabis further emerges as more destructive, when its commencement arises in younger versus older adolescents, with regard to the adjustment for adolescent in transitioning to young adulthood, education achievement, employment, misbehaviour and capacity to adapt to adult role (Fergusson and Boden 2008). Notwithstanding these results, a principal warning regarding human studies, is the complexity of indicating a contributory relationship between adolescent cannabis use and consequent behavioural disturbances, particularly when considering the influence of genetic and environmental features in conjunction with other characteristics such as polysubstance use (Cleveland and Wiebe 2008).
Given these complexities, animal models are a respected method to attain direct understandings about the relationship between early cannabis exposure and behavioral disruptions (Bostwick 2012). Various rodent investigations discovering the latent gateway consequences of cannabis, have primarily considered synthetic cannabinoid agonists, however, these differ in pharmacological properties to THC (Fattore and Fratta 2011).
Nevertheless, studies exploring adolescent exposure to cannabinoid agonists or THC, deliver indications of heightened consumption and sensitivity later in life to opiate drugs (Tomasiewicz et al 2012).
Although animal studies demonstrate protracted behavioral and neurobiological consequences of adolescent THC exposure into adulthood, Hurd et al (2014) observe that not all adolescent cannabis users advance to future addictions or psychiatric disorders . In fact, notwithstanding its commonplace usage, only a subsection of teens and young adults using cannabis progress to abuse or dependence (SAMHSA 2011). Indeed, for the majority of teenagers, cannabis is a terminus with no further use in relation to that or other illicit drugs, as they mature into full adulthood, signifying that there are variances in individual susceptibility and there are remarkable differences in human beings in relation to their environment, behavioral traits, genetics, and cultural norms (Hurd et al 2014). While these and other influences contribute to substantial areas of complex disorders such as addiction, comprehension of the influence of particular factors is as equal a challenge as determining their connections to risk (Hurd et al 2014)
Adolescent Brain Development: Overview
The term adolescent originates from the Latin verb ‘adolescere’, meaning “to grow” and is understood as a cycle of change and is often considered the most transformative phase in an adolescents life. The period is more often said to extend from around 10 years of age to some years over 20. The definition is, nevertheless, still largely dependent on culture and context (Steinberg 2002). However, adolescence is a period of significant changes in the structure and function of the brain; other than the first three years of life, no other developmental phase is characterized by more intense transformations (Steinberg 2011).
While development of complete brain size is essentially complete by age five, explicit structural and functional changes endure during adolescence and contribute to more competent cognitive functioning (Tau and Peterson 2010). During adolescence, the brain experiences significant developmental changes, with the frontal lobe maturing in later adolescence and into early adulthood and both myelination and synaptic refinement continuing throughout adolescence (Arain et al 2013)). Studies in typically developing teens without heavy alcohol or drug use demonstrate that white matter volumes increase throughout the brain with continued myelination during adolescence (Jacobus and Tapert 2014.) Grey matter volumes peak around age 13 in males and age 11 in females and then decline as unnecessary neural connections are eliminated, resulting in a net volume loss during this time (Giedd 2012). Increases in myelination, detected as increases in white matter volumes, and in pruning of grey matter, detected as decreases in cortical grey matter, facilitate more effective communication among neurons in the brain. These changes allow specialized cognitive processing required for optimal cognition and performance (Brown & Tapert 2004).
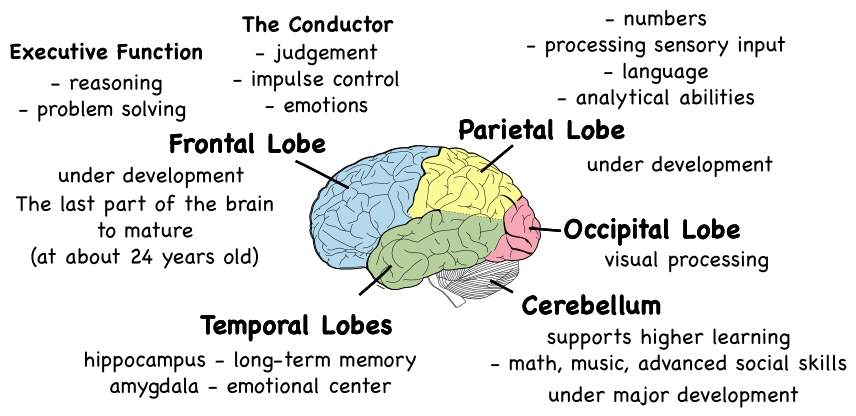
During adolescence, the chief function of the brain is to generate effective neuronal pathways through neuronal refinement (Tau and Peterson 2010). This process comprises substantial loss of synapses in neocortical regions, transformation of the prefrontal cortex, maturational changes in the hippocampus (Whitford et al 2007). Regions that experience significant synaptic pruning in adolescence are temporal and frontal regions and striatal areas, with prefrontal cortical areas being particularly late to mature (Casey et al 2008). Significantly, circuits concerning the prefrontal cortex and the striatum are fundamental to complex order cognitive skills which are reflective of decision making, risk and reward processing, and cognitive control (Geier and Luna 2009).
Cannabis Use and the Adolescent Brain
Cannabis impact on brain development is remarkable in adolescent users, having intense consequences on numerous regions of neurological and psychosocial growth (Volkow et al 2014). The human brain continues to experience rapid and dynamic expansion until approximately 21 years of age, in which the brain experiences physical changes (Volkow et al 2014). If the brain is subjected to cannabis during this development, particularly in adolescent years, reward and pleasure centres can be “recalibrated”, signifying that cannabis use throughout developmental years can create essential changes in learning and social behavior (Volkow et al 2014).
Discoveries of intensified risk linked with adolescent cannabis use, combined with research indicating a role of the endocannabinoid system in regulating neurodevelopmental processes, have led to assumptions that adolescent cannabis use may interrupt the standard course of neurodevelopmental processes and cause long-term changes in brain functioning (Viveros et al 2012). Additionally, it seems there are a number of influences (e.g., female sex, early trauma experience, genetics) that may decrease the consequences of adolescent cannabis use on brain development (Lubman et al 2015).
Adolescent cannabis use might be connected with protracted changes in brain functioning in part through the interruption of synaptic pruning processes in those regions that are simultaneously growing (Lubman et al 2015). With regard to adolescence being a predominantly significant period for the development of prefrontal brain areas, more recent animal studies have revealed that altering endocannabinoid neurotransmission in adolescent female rats causes enduring fluctuations in prefrontal brain regions reflective of disrupted synaptic pruning (Rubino et al 2015). Therefore, research reinforces the suggestion that, at some level, particular cognitive and behavioural deficiencies linked with early adolescent cannabis use in the studies related to humans, may possibly be connected to the disturbance of endocannabinoid-mediated neurodevelopmental procedures by cannabis (Albertella and Copeland 2016). It is plausible to hypothesize that adolescent exposure to cannabinoids might somehow interrupt these maturational proceedings, thus leading to an adult brain with transformed network connectivity in these very brain areas (Rubino and Parolaro 2014)
Cognitive Impairment
Adolescents who use cannabis frequently, characteristically exhibit a number of non-acute cognitive deficits in contrast to adolescents who do not use cannabis or use it occasionally (Pardini et al 2015). For instance, a study by Hanson et al (2010) found enduring discrepancies in the area of selective attention within a group of adolescent heavy cannabis users, compared to a control group who had used cannabis less than 5 times in their lifetime.
Fluctuations in verbal learning and memory amongst this group, nonetheless, improved following a period of abstinence (Schweinsburg et al 2008). Furthermore, some studies have indicated that adults who started using cannabis early in adolescence display more substantial cognitive impairment than adults who started using cannabis later (Koenders et al 2016).
Volkow (2014) has utilised the findings of non-acute cognitive deficits in adolescent cannabis users, to support the idea that adolescence may be a particularly sensitive phase for the adverse consequence of cannabis use. Albertella and Copeland (2016) contend that there is also research, to suggest that pre-existing factors connected to cognition may provide justification for some of the differences found between adolescent cannabis users and non-users. Some group differences in cognition may not be the consequence of cannabis use but may be due to pre-existing group differences leading to earlier inception of cannabis use (Jacobus and Tapert 2014). In particular, measures of selective attention undertaken in early adolescence have been established to predict greater cannabis use by late adolescence (Squeglia et al 2014). Correspondingly, impulsivity-related personality traits have also been shown to predict regular adolescent cannabis use (van Leeuwen et al 2011)
Studies have also found differences in brain structure between those who commence cannabis use in adolescence and non-users (Jacobus and Tapert 2014). These include differences in orbitofrontal cortex volume which is a prefrontal brain area related to impulsivity, reward processing, and cognitive control (Casey et al 2008) — at age 13, which predicted subsequent onset of cannabis use by age 16 (Cheetham et al 2012). Thus, some of the cognitive deficits seen in adolescent cannabis users as reported by Hanson et al (2010) may in fact have preceded cannabis use.
Long-term cannabis use has been connected with cognitive impairments in a diversity of investigations, however, there is incongruity concerning the timeframe of the harmful effects.
Meier et al. (2012) undertook a 38-year follow up study using the 1,037 participants from the ‘Dunedin Longitudinal Study’. Participants were tracked from birth to age 38 and data on cannabis use was established through interviews at ages 18, 21, 26, 32, and 38. Further to this baseline neuropsychological testing was conducted at age 13, before cannabis use had been commenced, and follow up neuropsychological testing was conducted at age 38. The investigators established that persistent cannabis use was allied to comprehensive cognitive declines, affecting multiple domains of neurocognitive functioning. Furthermore, participants with adolescent onset of cannabis use had cognitive deficits that persisted more than a year after cessation of cannabis use (Meier et al 2012). Results suggested that persistent use of cannabis that is initiated while the brain is still developing might have a comprehensive enduring influence on cognition even after end of cannabis use.
Executive functioning
Studies on adolescents with cannabis use histories identified worse performance on perseverative responding and flexible thinking in contrast to controls (Lane et al 2007).
Inferior performance on executive functioning amid adolescent cannabis users was correlated to more days of cannabis use in the preceding month (Harvey et al 2007). In a recent, large-scale, longitudinal exploration, individuals with persistent cannabis dependence showed decline in their intelligence quotient with time, predominantly in executive functioning (Meier et al 2012).
Even after one month cannabinoids are measureable in the blood of chronic daily cannabis smokers during continued abstinence (Bergamaschi et al 2013). Cannabis continues to impair executive functions, with the chronic, heavy cannabis users showing the most enduring deficits (Shrivastava et al 2011). Decision-making, organization and concept creating are the most prominent and robust deficits, but verbal fluency may or may not persevere at this point (Madras 2015).
Learning and Memory
Verbal and spatial working memory aptitudes develop throughout adolescence, with older adolescents countering more accurately and more quickly (Brown et al 2009), and cannabis use during this time appear to interfere with those improvements. Comparable to studies examining the influence of alcohol on learning and memory, numerous studies have revealed similar discrepancies among cannabis using youth. In one of the initial investigations of the influence of cannabis on adolescent cognition, Schwartz et al (1989) identified that short-term memory impairment persisted even after six weeks of abstinence in cannabis-dependent adolescents (ages 14-16) compared to matched controls. Studies in the past two decades have consistently recognised discrepancies in instant and delayed recall among adolescent and young adult (ages 13-24) cannabis users (Gonzalez et al 2012). In a study of adolescent cannabis users ages 16-18, cannabis users displayed inferior verbal learning and memory, even after one month of abstinence (Medina et al 2007). However, memory deficits identified among young adult ,(ages 20-24) cannabis users with recent use showed improvement with abstinence over the course of eight years (Tait et al 2011). Notably, diminished performance on learning and recall among adolescent cannabis users has been connected to severity, frequency, and age of initiation of cannabis use (Solowij et al 2011).
Processing Speed and Attention
In the literature related to cannabis, deficits in attention and processing speed have also been consistently recognised (Schweinsburg 2008). Adolescent cannabis users who smoke more than once per week were found to have inferior performance on attention tasks (Harvey et al 2007). In a longitudinal study exploring neuropsychological performance amid heavy cannabis using youth, compared to non-using youth, between-group differences in attention were identified at baseline and across 3 weeks of observed abstinence, with attention differences persevering with time (Hanson et al 2010). Reduced processing speed has also been identified among heavy cannabis using youth (ages 16- 18), even after one month of monitored abstinence (Medina et al 2007).
The neurological changes identified in long-term users that initiated use during adolescence also factor into the lifetime education and achievements of users (Volkow et al 2014).
Dougherty et al (2012) report that adolescent-onset users are more likely to leave high school and experience adulthood difficulties in achievement (Dougherty et al 2012).
Adolescents that regularly consume cannabis experience more learning problems due to changes in brain structure and function, and are less likely to seek assistance in improving poor school performance (Dougherty et al 2013). Cannabis use is a reliable and significant gauge of poor grades in school, particularly as the age of first use decreases (Palamar et al 2014). Furthermore, long-term cannabis use correlates with unemployment, lack of higher education, dependence on social welfare programs, and low socioeconomic status (Volkow et al 2014).
Cannabis and Mental health issues in adolescents
The capacity of cannabis to induce paranoia was first noted in 1845 by French psychiatrist Moreau de Tours. He was a personal user of cannabis however, he also who also studied the impact of cannabis on his students. He observed “acute psychotic reactions, generally lasting but a few hours, but occasionally as long as a week” (Moreau 1973). While the acute effects of cannabis have been extensively observed, there are further long-term consequences that can have considerable impact on otherwise healthy controls (Renard et al 2014). Researchers have been able to establish that it is practically impossible to overdose on THC to the point of death, however, THC consumption can create erratic or psychotic behavior that in extreme circumstances could lead to accidental death (NIDA 2014).
Psychotic disorders
Epidemiological studies advocate that cannabis use throughout adolescence presents an amplified risk for the emergence of psychotic symptoms later in life (Evins et al 2012). In a 4 year study by Henquet et al (2005) of cannabis use in 2,437 14-24 year old participants, identified as at risk and not at risk, for psychosis, a dose-response, with more frequent cannabis use associated with a higher risk for psychosis was identified. However, the converse was not ascertained in that, risk for psychosis was not found to be a predictor of future cannabis use. Therefore, the results of Henquet et al (2005) study proposed that cannabis usage was a risk factor for psychotic disorders, rather than psychotic disorders being a risk factor for future cannabis use.
Bossong and Niesink (2010) undertook a literature search that comprised of various neurobiological disciplines, which ultimately converged into a model that may clarify the neurobiology of cannabis-induced schizophrenia. Bossong and Niesink (2010) contend that cannabis use during adolescence intensifies the possibility of developing psychotic disorders later in life. However, they acknowledge that the neurobiological processes underlying this relationship are unidentified. Their model postulates that adolescent exposure to D9-tetrahydrocannabinol (THC), the primary psychoactive substance in cannabis, transitorily interrupts physiological control of the endogenous cannabinoid system over glutamate and GABA release. As a result, THC may negatively have an impact on adolescent experience-dependent development of neural circuitries contained within prefrontal cortical areas.
Contingent on the dose, precise time window and duration of exposure, the development of psychosis or schizophrenia may be the consequence (Bossong and Niesink 2010).
Though particular cognitive deficits may be a fundamental characteristic of schizophrenia, introducing cannabis use into the equation has stimulated remarkable findings. The paramount findings have indicated that participants with schizophrenia and adolescent cannabis use actually have better cognitive functioning than those without adolescent cannabis use (Lesson et al 2012). However, only a minor amount of people who use cannabis go on to develop psychotic symptoms. This had directed Golgberger et al (2010) to suggest that it is probable that both environmental factors and genetic predisposition feature in influencing the psychotomimetic impact of cannabis, which is associated with early cannabis exposure and a family history of psychosis. Furthermore, a recent study of a large sample of students established that sensitivity to the psychotomimetic effects of cannabis is suggestive of an inherent characteristic present subsequent to their first exposure to cannabis (Krebs et al 2014).
Anxiety disorders
Anxiety disorders are the most common difficulties that result from chronic heavy cannabis use (Renard et al 2014). Kedzior and Laeber (2014) report that the lifetime prevalence for anxiety disorder within the general population is estimated around 6–17%, and this frequency is increased in cannabis users with a prevalence up to 20% (Reilly et al 1998). A comprehensive and recent meta-analysis (Kedzior and Laeber 2014) directs that anxiety is significantly linked with the consumption and misuse of cannabis. However, only a few studies have monitored the association concerning adolescent cannabis use and enduring anxiety disorders. The use of cannabis throughout adolescence, can magnify the possibility of creating anxiety-related symptoms in adulthood, predominantly if the inception of use was initiated before the age of 15 (Renard et al 2014). Moreover, girls are at greater probability than boys to for these symptoms to develop (Hayatbakhsh et al 2007).
A recent study by Degenhardt et al (2013) observed the relationship between cannabis use and mental health of people between the ages of 15 and 29. The authors established that substantial cannabis use throughout adolescence was associated with an augmented risk of adulthood mental health challenges including anxiety disorders.
Depressive disorders
In many countries, an emergent body of evidence corroborates a correlation between cannabis use and depression among young people (Renard et al 2014). Amongst people who use cannabis the frequency of depressive disorders is twenty five per cent with approximately half of these depressive disorders presenting as major depression, and the other half are severe mood disorders (Chabrol et al 2008). A study conducted among Australians adolescents, between 13 and 17 years of age established that those who use cannabis, have three times the probability of encountering the criteria for depression in later life, in comparison to adolescents who never used cannabis (Rey et al 2002). Similarly, Hayatbakhsh et al (2007) established that those who frequently use cannabis are more likely to display symptoms of anxiety and depression in early adulthood, predominantly when the researchers took into account cumulative exposure to cannabis, and potentially considerable confounding characteristics such as maternal smoking and alcohol consumption. Further to this, a longitudinal study that was undertaken in young Norwegians and followed over a 13-year period demonstrated a dose-dependent relationship between chronic cannabis consumption and suicidal propensities later in life (Pedersen 2008). Taken together, these longitudinal studies propose that early commencement and recurrent cannabis use, increases the vulnerability to depression later in life (Renard et al 2014).
Among people with psychosis, van Gastel et al (2013) report elevated use of cannabis and it has been further recognized by Ferdinand et al (2005), that psychotic symptoms are established in people who have never used cannabis, before the onset of psychotic symptoms, which also predicts future cannabis use. This proposes that existing cannabis-dependent subjects may have underlying psychiatric disorders that contributed to self-medication and that through repeated use, led to dependence (Renard et al 2014). Thus, while cannabis may itself increase drug addiction and psychiatric vulnerability, preceding prodromal conditions may initially promote the initiation and continuation of cannabis use (Hurd et al 2014).
Conclusion
The exposure of adolescent to cannabis is connected with a variety of enduring adverse consequences, including heightened vulnerability to addiction, cognitive impairment, and psychosis-related illness, which may be correlated to cannabis-induced interruptions in neurodevelopmental processes. These effects are moderated by a number of influences, with adolescents exposed to early life trauma and/or those who are exposed to certain genetic and personality vulnerabilities being at a greater risk of experiencing adverse long term effects.
Further research is required to understand precisely the manner in which various risk characteristics interact with the use of cannabis and how such interactions may influence neurodevelopmental processes within susceptible individuals.
REFERENCES
Albertella, L. and Copeland, J. (2015) Cannabis use and the adolescent brain, National cannabis prevention and information centre (28).
Arain, M., Haque, M., Johal, L., Mathur, P., Nel, W., Rais, A., Sandhu, r. and Sharma, S. (2013) Maturation of the adolescent brain, Neuropsychiatric Disease and Treatment, 9, 449–461. http://doi.org/10.2147/NDT.S39776
Azorlosa, J., Greenwald, M. K .and Stitzer, M. (1995) Marijuana smoking: effects of varying puff volume and breath hold duration, Journal of Pharmacology and Experimental Therapeutics, 272(2), 560-569.
Baggio, S., Deline, S., Studer, J., Mohler-Kuo, M., Daeppen, J. and Gmel, G. (2014) Routes of administration of cannabis used for nonmedical purposes and associations with patterns of drug use, Journal of Adolescent Health, 54(2), 235-240.
Bahr, S., Hoffmann, J. and Yang, X. (2005) Parental and peer influences on the risk of adolescent drug use, Journal of Primary Prevention, 26(6), 529-551.
Battistella, G., Fornari, E., Annoni, J., Chtioui, H., Dao, K., Fabritius, M., Favrat, B., Mall, J.F., Maeder, P. and Giroud, C. (2014) Long-term effects of cannabis on brain Structure, Neuropsychopharmacology, 39(9), 2041-2048. http://doi.org/10.1038/npp.2014.67
Bellerose, D. (2012) Changing patterns of drug use impact on service and communities, Drug net Ireland, 41 (22).
Bergamaschi, M., Karschner, E., Goodwin, R., Scheidweiler, K., Hirvonen, J., Queiroz, R. and Huestis, M. (2013) Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws, Clinical chemistry, 59(3), 519-526.
Bossong, M. G. and Niesink, R. J. (2010) Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia Progress in neurobiology, 92(3), 370-385.
Bostwick, J. M. (2012) Blurred Boundaries: The Therapeutics and Politics of Medical Marijuana, Mayo Clinic Proceedings, 87(2), 172–186. http://doi.org/10.1016/j.mayocp.2011.10.003
Brown, S., McGue, M., Maggs, J., Schulenberg, J., Hingson, R., Swartzwelder, S., Martin, C., Chung, T., Tapert, S., Sher, K., Winters, K., Lowman, C.and Murphy, S. (2008) A developmental perspective on alcohol and youths 16 to 20 years of age, Pediatrics, 121, 290-310.
Brown, S. and Tapert, S. 2004) Adolescence and the trajectory of alcohol use: Basic to clinical studies, Annals of the New York Academy of Sciences, 1021, 234-244.
Camchong, J., Lim, K. O.and Kumra, S. (2016) Adverse effects of cannabis on adolescent brain development: a longitudinal study, Cerebral Cortex, bhw015.
Casey, B. J., Jones, R. M. and Hare, T. A. (2008) The Adolescent Brain. Annals of the New York Academy of Sciences, 1124, 111–126. http://doi.org/10.1196/annals.1440.010
Cerdá, M., Wall, M., Keyes, K. M., Galea, S. and Hasin, D. (2012) Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence, Drug and alcohol dependence, 120(1), 22-27.
Chabrol H., Chauchard E. and Girabet J. (2008) Cannabis use and suicidal behaviours in high-school students, Addictive Behaviours, 33, 152–155.
Cheetham, A., Allen, N., Whittle, S., Simmons, J., Yücel, M. and Lubman, D.(2012) Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study, Biological psychiatry, 71(8), 684-692.
Cleveland, H. H. and Wiebe, R. P. (2008) Understanding the association between adolescent marijuana use and later serious drug use: Gateway effect or developmental trajectory?, Development and psychopathology, 20(02), 615-632.
Currie, C., Nic Gabhainn, S., Godeau, E., Roberts, C., Smith, R.and Currie, D. (Eds.). (2008) Inequalities in young people’s health: HBSC international report from the 2005/2006 Survey, Copenhagen: WHO Regional Office for Europe.
Degenhardt, L., Ferrari, A.J., Calabria, B., Hall, W.D., Norman, R.E., McGrath, J., Flaxman, A.D., Engell, R.E., Freedman, G.D., Whiteford, H.A. and Vos, T. (2013) The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study, PLoS One, 8(10), e76635.
Degenhardt, L. and Hall, W. (2012) Extent of illicit drug use and dependence, and their contribution to the global burden of disease, Lancet, 7, 55-70.
Dougherty, D.M., Mathias, C.W., Dawes, M.A., Furr, R.M., Charles, N.E., Liguori, A., Shannon, E.E. and Acheson, A. (2013) Impulsivity, attention, memory, and decision-making among adolescent marijuana users, Psychopharmacology, 226(2), 307-319.
European Monotoring Centre for Drugs and Drug Addiction (EMCDDA), (2010) The state of the Drugs Problem in Europe, Luxemburg: Office for Official Publications of the European Communities, Available http://www.emcdda.eu/publications/annual-report/2010 [accessed: 23 rd March 2017]
Evins, A. E., Green, A. I., Kane, J. M. and Murray, R. M. (2012) The effect of marijuana use on the risk for schizophrenia, Journal of Clinical Psychiatry, 73(11), 1463-1468.
Ferdinand, R.F., Sondeijker, F., van der Ende, J., Selten, J.P., Huizink, A. and Verhulst, F.C. (2005) Cannabis use predicts future psychotic symptoms, and vice versa, Addiction, 100, 612-618.
Fergusson, D. M. and Boden, J. M. (2008) Cannabis use and later life outcomes, Addiction, 103(6), 969-976.
Fattore, L. and Fratta, W. (2011) Beyond THC: The New Generation of Cannabinoid Designer Drugs, Frontiers in Behavioral Neuroscience, 5, 60. http://doi.org/10.3389/fnbeh.2011.00060
Friese, B. and Grube, J. (2013) Legalization of medical marijuana and marijuana use among youths, Drugs: Education, Prevention and Policy, 20(1), 33–39.
Geier, C. and Luna, B.(2009)The maturation of incentive processing and cognitive control, Pharmacology Biochemistry and Behavior, ;93(3):212-21.
Giedd, J. N. (2012) The Digital Revolution and Adolescent Brain Evolution, The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 51(2), 101–105. http://doi.org/10.1016/j.jadohealth.2012.06.002
Gloss, D. (2015) An Overview of Products and Bias in Research, Neurotherapeutics, 12(4): 731-4.
Goldberger, C., Dervaux, A., Gourion, D., Bourdel, M. C., Lôo, H., Laqueille, X. and Krebs, M. O. (2010) Variable individual sensitivity to cannabis in patients with Schizophrenia, International Journal of Neuropsychopharmacology, 13(9), 1145-1154.
Gonzalez, R., Schuster, R., Mermelstein, R., Vassileva, J., Martin, E. and Diviak, K. (2012) Performance of young adult cannabis users on measures of impulsive behavior and their relationship to symptoms of cannabis use disorders, Journal of Clinical and Experimental Neuropsychology, 34(9), 962-976.
Hanson, K., Winward, J., Schweinsburg, A., Medina, K., Brown, S. and Tapert, S.F. (2010) Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence, Journal of Addictive Behaviors, 35, 970-976
Harvey, M., Sellman, J., Porter, R. and Frampton, C. (2007) The relationship between non-acute adolescent cannabis use and cognition, Drug and Alcohol Review, 26, 309-319.
Hayatbakhsh, M., Najman, J., Jamrozik, K., Mamun, A., Alati, R. and Bor, W.(2007. Cannabis and anxiety and depression in young adults: a large prospective study Journal of the American Academy of Child & Adolescent Psychiatry, 46(3), 408-417.
Henquet, C., Krabbendam, L., Spauwen, J., Kaplan, C., Lieb, R., Wittchen, H. and Van Os, J. (2004) Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Bmj, 330(7481), 11.
Hurd, Y., Michaelides, M., Miller, M. and Jutras-Aswad, D. (2014) Trajectory of adolescent cannabis use on addiction vulnerability, Neuropharmacology, 76, 416-424.
Iannotti, R., Bush, P. and Weinfurt, K. (1996) Perception of friends’ use of alcohol, cigarettes, and marijuana among urban schoolchildren: a longitudinal analysis, Addictive Behaviours, 21(5):615-32.
Jacobus, J. and Tapert, S. (2014) Effects of Cannabis on the Adolescent Brain, Current Pharmaceutical Design, 20(13), 2186–2193.
Johnston, L., O’Malley, P., Bachman, J. and Schulenberg, J. (2012) Demographic subgroup trends for various licit and illicit drugs, 1975–2011, Institute for Social Research:,University of Michigan. Retrieved from: http://www.monitoringthefuture.org/pubs/occpapers/mtf-occ77.pdf
Karschner, E.L., Darwin, W.D., McMahon, R.P., Liu, F., Wright, S., Goodwin, R.S. and Huestis, M.A. (2011) Subjective and physiological effects after controlled Sativex and oral THC administration, Clinical Pharmacology & Therapeutics, 89(3), 400-407.
Kedzior, K. and Laeber, L. (2014) A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population- a meta-analysis of 31 studies, Bio Med Central Psychiatry, 14, 136. http://doi.org/10.1186/1471-244X-14-136.
Krebs M. O., Morvan Y., Jay T., Gaillard R. and Kebir O. (2014) Psychotomimetic effects at initiation of cannabis use are associated with cannabinoid receptor 1 (CNR1) variants in healthy students, Molecular Psychiatry 19, 402–403. 10.1038/mp.2013.188
Koenders, L., Cousijn, J., Vingerhoets, W.A., van den Brink, W., Wiers, R.W., Meijer, C.J., Machielsen, M.W., Veltman, D.J., Goudriaan, A.E. and de Haan, L. (2016) Grey matter changes associated with heavy cannabis use: a longitudinal sMRI study, PloS one, 11(5), p.e0152482.
LaBrie, J. W., Hummer, J. F. and Lac, A. (2011) Comparing injunctive marijuana use norms of salient reference groups among college student marijuana users and nonusers, Addictive Behaviors, 36(7), 717-720.
Lane, S., Cherek, D., Tcheremissine, O., Steinberg, J. and Sharon, J. (2007) Response perseveration and adaptation in heavy marijuana-smoking adolescents, Addictive behaviors, 32(5), 977-990.
Leeson, V., Harrison, I., Ron, M., Barnes, T. and Joyce, E. (2011) The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia, Schizophrenia Bulletin, sbq153.
Lopez-Quintero, C.and Neumark, Y. (2010) Effects of risk perception of marijuana use on marijuana use and intentions to use among adolescents in Bogotá, Colombia Drug and alcohol dependence, 109(1), 65-72.
Long, J. and Horgan, J. (2012) Drug use among the general population, by regional drugs task force area, Drug net Ireland , 43, 24-27.
Lubman, D. I., Cheetham, A. and Yucel, M. (2015) Cannabis and adolescent brain development, Pharmacology and Therapeutics, 148, 1-16.
Madras, B. K. (2015) Update of cannabis and its medical use, Report to the WHO Expert on Drug Dependence (http://www. who. int/medicines/access/controlled-substances/6_2_cannabis_update. pdf.
Malmberg, M., Overbeek, G., Vermulst, A., Monshouwer, K., Vollebergh, W. and Engels, R. (2012) The theory of planned behavior: precursors of marijuana use in early adolescence? Drug and Alcohol Dependence, 123(1), 22-8.
Medina, K. L., Hanson, K. L., Schweinsburg, A. D., Cohen-Zion, M., Nagel, B. J. and Tapert, S. F. (2007) Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence, Journal of the International Neuropsychological Society, 13, 807-820.
Meier, M.H., Caspi, A., Ambler, A., Harrington, H., Houts, R., Keefe, R.S., McDonald, K., Ward, A., Poulton, R. and Moffitt, T.E. (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife, Proceedings of the National Academy of Sciences, 109(40), 2657-2664.
Moreau, J. J. (1973) Hashish and mental illness, New York: Raven Press.
National Advisory Committee on Drugs and Public (NACD) Health Information and Research Branch,(2011) Drug use in Ireland and Northern Ireland: First results from the 2010/11, Drug Prevalence Survey, Dublin, National Advisory Committee on Drugs (NACD) and Public Health Information and Research Board (2008b). Drug use in Ireland and Northern Ireland 2006/2007 Drug Prevalence Survey: Cannabis Results. Dublin and Belfast: NACD and PHIRB
National Institute on Drug Abuse. (2014) Marijuana: How does marijuana produce its effects? Retrieved March 2017, from drugabuse.gov: http://www.drugabuse.gov/publications/research-reports/marijuana/how-doesmarijuana-produce-its-effects
Olds, R. S., Thombs, D. L. and Tomasek, J. R. (2005) Relations between normative beliefs and initiation intentions toward cigarette, alcohol and marijuana, Journal of Adolescent Health, 37(1), 75.
Palamar, J. J., Ompad, D. C. and Petkova, E. (2014) Correlates of Intentions to Use Cannabis among US High School Seniors in the Case of Cannabis Legalization, The International Journal on Drug Policy, 25(3), 424–435. http://doi.org/10.1016/j.drugpo.2014.01.017
Pardini, D., White, H., Xiong, S., Bechtold, J., Chung, T., Loeber, R. and Hipwell, A. (2015) Unfazed or Dazed and Confused: Does Early Adolescent Marijuana Use Cause Sustained Impairments in Attention and Academic Functioning? Journal of Abnormal Child Psychology, 43(7), 1203–1217. http://doi.org/10.1007/s10802-015-0012-0
Pedersen, W. (2008) Does cannabis use lead to depression and suicidal behaviours? A population‐based longitudinal study, Acta Psychiatrica Scandinavica, 118(5), 395-403.
Radwan, M., ElSohly, M., El-Alfy, A., Ahmed, S., Slade, D., Husni, A., Manly, S., Wilson, L., Seale, S., Cutler, S. and Ross, S. (2015) Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis Sativa, Journal of Natural Products, 78(6): 1271-6.
Reilly, D., Didcott, P., Swift, W. and Hall, W. (1998), Long‐term cannabis use: characteristics of users in an Australian rural area, Addiction, 93(6), 837-846.
Renard, J., Krebs, M.-O., Le Pen, G. and Jay, T. (2014) Long-term consequences of adolescent cannabinoid exposure in adult psychopathology, Frontiers in Neuroscience, 8, 361. http://doi.org/10.3389/fnins.2014.00361.
Rey, J., Sawyer, M., Raphael, B., Patton, G. and Lynskey, M. (2002) Mental health of teenagers who use cannabis, The British Journal of Psychiatry, 180(3), 216-221.
Rubino, T. and Parolaro, D. (2014) Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence Progress in Neuro-Psychopharmacology and Biological Psychiatry, 52, 41-44.
Rubino, T., Prini, P., Piscitelli, F., Zamberletti, E., Trusel, M., Melis, M., Sagheddu, C., Ligresti, A., Tonini, R., Di Marzo, V. and Parolaro, D. (2015) Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex, Neurobiology of disease, 73, 60-69.
Schwartz, R.H., Gruenewald, P.J., Klitzner, M.and Fedio, P. (1989) Short-term memory impairment in cannabis-dependent adolescents, American Journal of Disorders in Childhood, 143, 1214-1219
Schweinsburg, A., Brown, S. and Tapert, S. (2008) The influence of marijuana use on neurocognitive functioning in adolescents, Current Drug Abuse Reviews, 1, 99-111.
Shrivastava, A., Johnston, M. and Tsuang, M. (2011) Cannabis use and cognitive dysfunction, Indian Journal of Psychiatry, 53(3), 187–191. http://doi.org/10.4103/0019-5545.86796
Sobesky, M. and Gorgens, K. (2016) Cannabis and adolescents: Exploring the substance misuse treatment provider experience in a climate of legalization, International Journal of Drug Policy, 33, 66-74.
Solowij, N., Jones, K.A., Rozman, M.E., Davis, S.M., Ciarrochi, J., Heaven, P.C., Lubman, D.I. and Yucel, M. (2011) Verbal learning and memory in adolescent cannabis users, alcohol users, and non-users, Psychopharmacology, 216, 131-144.
Steinberg, L. (2002) Adolescence, New York: McGraw Hill Companies.
Steinberg, L. (2008), A social neuroscience perspective on adolescent risk taking, Developmental Review, 28, 78–106.
Squeglia, L. M., Jacobus, J., Nguyen-Louie, T. T. and Tapert, S. F. (2014) Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology, 28(5), 782.
Stogner, J. and Miller, B. (2015) Assessing the Dangers of “Dabbing”: Mere Marijuana or Harmful New Trend? Paediatrics, 136(1): 1-3.
Substance Abuse and Mental Health Services Administration (SAMHSA) (2011) Results from the 2010 National Survey on Drug Use and Health:National Findings. Office of Applied Studies, NSDUH Series H-41, HHS PublicationNo. (SMA) 11e4658, Rockville, MD.
Swift, W., Wong, A., Li, K., Arnold, J. and McGregor, I. (2013) Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile, PLoS One, 8(7): e70052.
Sznitman, S. R. and Zolotov, Y. (2015) Cannabis for Therapeutic Purposes and public health and safety: A systematic and critical review, International Journal of Drug Policy, 26(1), 20–29.
Tait, R.J., Mackinnon, A. and Christensen, H. (2011. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort, Addiction, 106, 2195-2203.
Tau, G., and Peterson, B. (2010) Normal development of brain circuits, Neuropsychopharmacology, 35:147–68.
Tomasiewicz, H. C., Jacobs, M. M., Wilkinson, M. B., Wilson, S. P., Nestler, E. J.and Hurd, Y. L. (2012) Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability, Biological psychiatry, 72(10), 803-810.
United Nations International Children’s’ Emergency Fund (UNICEF) Irelans- CHANGING The Future (2011) Expereincing adolescent in contemporary Ireland: Alcohol and Drugs 2011. Available at http://www.unicef.ie/Downloads/ UNICEF-change –the future-A5-Report-Alcohol-DRUGS-Report-Web.pdf {accessed 23 March 2017}
UN Office on Drugs and Crime. (2012) World drug report 2012. Vienna, Austria: United Nations International Drug Control Research and Trend Analysis Branch. Retrieved from http:// www.unodc.org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf
Van Gastel, W. A., MacCabe, J. H., Schubart, C. D., Vreeker, A., Tempelaar, W., Kahn, R. S. and Boks, M. P. M. (2013) Cigarette smoking and cannabis use are equally strongly associated with psychotic-like experiences: a cross-sectional study in 1929 young adults, Psychological medicine, 43(11), 2393-2401.
Van Hout, M.C. (2009a) ‘Drug and alcohol use among rural Irish adolescents – a brief exploratory study’, Drugs and Alcohol Today, 9 (1), 20–26.
Van Hout, MC (2010) ‘Differentiated Normalisation and Drug Transitions among Rural Youth in Ireland, Drugs: Education, Prevention and Policy, 18 (2), 142–151.
Van Leeuwen, A. P., Creemers, H. E., Verhulst, F. C., Ormel, J.and Huizink, A. C. (2011) Are adolescents gambling with cannabis use? A longitudinal study of impulsivity measures and adolescent substance use: The TRAILS study, Journal of studies on alcohol and drugs, 72(1), 70-78.
Viveros, M., Llorente, R., Suarez, J., Llorente-Berzal, A., López-Gallardo, M. and Rodriguez de Fonseca, F.(2012) The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications, Journal of Psychopharmacology, 26(1):164-76.
Volkow, N. D., Baler, R. D., Compton, W. M. and Weiss, S. R. B. (2014) Adverse Health Effects of Marijuana Use, The New England Journal of Medicine, 370(23), 2219–2227. http://doi.org/10.1056/NEJMra1402309
Walker, D. D., Neighbors, C., Rodriguez, L. M., Stephens, R. S. and Roffman, R. A. (2011) Social norms and self-efficacy among heavy using adolescent marijuana smokers, Psychology of Addictive Behaviors, 25(4), 727.
Wall, M. M., Poh, E., Cerda´ , M., Keyes, K. M., Galea, S. and Hasin, D. S. (2011) Adolescent marijuana use from 2002 to 2008: Higher in states with medical marijuana laws, cause still unclear, Annals of Epidemiology, 21(9), 714–716.
Whitford, T. J., Rennie, C. J., Grieve, S. M., Clark, C. R., Gordon, E. and Williams, L. M. (2007) Brain maturation in adolescence: concurrent changes in neuroanatomy and Neurophysiology, Human brain mapping, 28(3), 228-237.
Wu, L.-T., Swartz, M. S., Brady, K. T. and Hoyle, R. H. (2015) Perceived Cannabis Use Norms and Cannabis Use among Adolescents in the United States, Journal of Psychiatric Research 64, 79–87. http://doi.org/10.1016/j.jpsychires.2015.02.022
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Young People"
The term Young People often refers to those between childhood and adulthood, meaning that people aged 17-24 are often considered to be a young person.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




