Application of Disposable Technologies for Upstream (Cell Culture) Bioprocessing
Info: 6269 words (25 pages) Dissertation
Published: 10th Dec 2019
Tagged: BiologyInformation Technology
- Introduction:
The commercial success of mammalian cell-derived recombinant proteins has allowed for an increase in demand for novel single-use bioreactor (SUB) systems and associate technologies that facilitate greater productivity, increased flexibility and reduced costs. (Odeleye et al., 2013) bearing this in mind it is necessary to examine the market for inroads that are being made in a rapidly developing single use technology (SUT) market. Due to the fragility of the cells and the strict regulation environment it must be determined that the SUT doesn’t have a detrimental effect that will affect productivity or cause any undesired effects on the product
There are many considerations that need to be explored before SUT can be adopted, It is not as simple as going to a single use provider and purchasing one of their off the shelf products and hope that the regulatory agencies will accept the manufacturers validation so that a product can be produced. The same stringent regulations apply in terms of validation and other factors that apply normally in the course of obtaining a licence to manufacture
Production has historically been carried out in stainless steel stirred tank reactors, but advances in single use bioreactors have provided the option of utilising single use technology with reduced capital cost. There has been a lot of interest recently in the use of single-use reactors for upstream processing this is because it results in faster installation and commissioning and reduces the effort associated with cleaning and sterilisation operations. (Gadgil, 2017)
The focus of this project is to examine the inroads being made by single use technology with a focus on the upstream manufacturing, as well as examining the technology that is available it is necessary to look deeper at other concepts that will have an impact such as engineering, processing and integrated single use options, economics, connection technology, sensors and PAT. Leachables and extractables are a disadvantage and are to be examined with a view to addressing it. As single use technology is still relatively new, standards or lack of are also to be considered as part of this study. This project takes a holistic approach to the use of single use technology and is broad based.
- Main Section
Engineering Considerations
In the traditional bioreactor all parameters have to be optimised for the cells, This applies to parameters such as KLA, pH, Dissolved Oxygen etc. Single use bioreactors are different these systems exhibit fluid flow regimes unlike those encountered in traditional glass or stainless steel bioreactors. “With such disparate mixing environments between SUBs currently on the market, the traditional scale-up procedures applied to stirred tank reactors (STRs) are not adequate.”(Odeleye et al., 2013).
The aim of a study was to conduct investigations into the hydrodynamics of single-use bioreactors at laboratory scale to understand its impact upon the, metabolic activity and protein productivity and growth of a mammalian cell culture.(Odeleye et al., 2013)
Particle Image Velocimetry (PIV) was used in the study The bioreactors used the PIV measurements are the 3L CellReady by Merck Millipore, The PBS Biotech’s PBS 3 bioreactor and the Sartorius 2L Biostat Cultibag. The Measurements were obtained at different impeller rates from 80 to 350 rpms. The PBS 3 is an air driven 3L bioreactor whose mixing is performed through the buoyancy of bubbles. The Sartorius Cultibag is a rocked bag bioreactor system with a 2 litre volume. Measurements were taken at different rocking speeds
A study into the impact of fluid dynamic characteristics on mammalian cell culture performance and behaviour was undertaken and the results are that the PBS exhibits a greater degree of fluid dynamic homogeneity when compared to the Cellready. Given the shifts seen in metabolic behaviour and cell specific productivity, the study concluded that the fluid dynamic environment will impact upon cellular performance. By determining the critical hydrodynamic parameters applicable to the different flow regimes that are party to single use bioreactors. It will enable greater cross compatibility and scalability across the range of single use bioreactors.(Odeleye et al., 2013) It is important that these types of studies are performed especially for the bioreactors as it impacts the scale up seed train and indeed production itself as the operation and control of bioreactors processes are critical to successful outcomes. This type of study gives a good scientific basis for further development.
Cell culture processing options
There are several market factors like production costs, multiproduct manufacturing that are flexible. The biosimilars market and the time to get to market because of competition, and multiple and smaller manufacturing plants that allow for multiple products.
The options available are the WAVE bioreactor where mixing takes place by formation of waves as a result of rocking motion within the reactor. The orbitally shaken single use bioreactors use rotating motion with no agitator in the vessel to provide mixing and KLA gas transfer. The stirred-tank bioreactor is used in single use systems by mounting plastic bags placed inside a cylindrical shell to support the bag. Mixing occurs by an agitator bringing the principle close to that of the traditional stainless steel bioreactors.
Issues with the use of single-use bioreactors is that the possibility of some media components binding to plastic bags. Leachables from the plastic bags adversely affecting cells is another problem. These interactions depend on the cell culture media composition and the bioreactor material, it needs to be evaluated for the specific system being adopted.(Gadgil, 2017).
Targeted therapy benefit from developments in the upstream cell culture manufacturing is T-cell immunotherapy of cancer it requires the harvesting of T-cells from a patient their manipulation and expansion in vitro it is then transferred back to the patient. These products require separate containment, making the use of some form of reasonably self-contained single-use manufacturing technology the only possible manufacturing strategy for such type of therapy. The use of single use bioreactors which were not available at the time the first such drugs were developed in this area and the drugs are very expensive. The use of single use can lower the capital cost requirement. The study refers to entire manufacturing trains comprising of single-use technologies might in the future enable mobile manufacturing units for recombinant protein production. (Gadgil, 2017) Think of this concept where your T-Cells may be grown in a room in close proximity and administered as a targeted medication specifically for your needs.
Connection Technology
Sterile single use disposable connector options are vitally important. They can be used in conjunction with traditional SS bioreactors and associated equipment or they can be used to facilitate a single use strategy. In recent times a full range of 1/8inch to 1 inch flow options have been developed, they can be used from upstream to downstream applications. Traditionally Male to Female connections would have been the norm but the new connection technology is Genderless. This have further simplified system design to enable use of single use technology that can be easily combined to provide a wide range of complex solutions.
The advances in single-use system technology allow bioprocess engineers to replace most fixed piping networks and storage vessels with single-use transfer lines and storage systems. As stated previously single-use eliminates the need for CIP validation for many components and it reduces maintenance and capital expense by eliminating vessels, valves, and sanitary piping assemblies. (Boehm, 2014)

Fig 1 Genderless Connections (Boehm, 2014)
When compared with traditional stainless plastic connectors have different characteristics valves, Consensus guidelines were developed a team of connection experts from various companies to address key performance attributes of single-use connection technology. The Bio-Process Systems Alliance (BPSA) Technical Committee has formed subcommittees to address best practices for major categories of single-use technologies.
Using a SIP connector allow personnel to make sterile connections between these pre-sterilised single-use systems and stainless steel bioreactors for aseptic transfer of media. Similarly, single-use transfer lines may be used to transfer inoculum between bioreactors using either a peristaltic pump or headspace pressure.(Boehm, 2014) The advantages are that it eliminates SIP and CIP operations associated validations and it guarantees sterility. Other examples of SIP connectors are outlined in (biopharminternational, 2017) website online here
Connection technology also extends to tubing welders and tube sealers, there are a variety of units on the market are currently used widespread in the industry.
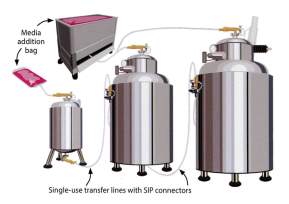
Fig 2: SIP Connectors on SS (Boehm, 2014) Fig 3: SIP Connectors on SU (Boehm, 2014)
By using pre-sterilized connectors and tubing, medium can be moved from the production suite to another suite without the need to sterilise stainless steel piping or equipment. Another advantage is that single-use systems gives the manufacturer greater flexibility when determining which process to run in each production suite.
Disposable technology implementation considerations.
The forces driving the demand for single use technologies are a mix of process efficiencies including cost reduction and sterility assurance. A cautionary approach is adopted, particularly with regard to the validation requirements and practical considerations when such technologies are implemented.(Sandle and Saghee, 2011) The use of single-use technology is still in its early stages and there are a number of validation steps that need to be undertaken before such technology is fully adopted by manufacturers. They include assessing any leachables or extractables that might arise when the product comes into contact with the single-use technology. This is considered the biggest disadvantage when using disposable technology and methods for detection are outlined in this project. The presence of extractables could lead to the failure of any microbial contamination to materialise or an adulterated product. Another disadvantages size limitations. Customisation and development costs for specific manufacturing is expensive. Single use disposable technologies are usually manufactured from plastic polymers involving processes of injection moulding. It is recommended that the assembly of components should be happen in an ISO Class 5 clean room. The plastic items are sterilised using gamma irradiation once assembled
Advantages of using Single Use
- Cross contamination can arise through the recycling of equipment. Disposable components are single use and eliminates the chance of cross-contamination or product carryover between process runs.
- Sterility assurance is heightened through and elimination of the way that connections are undertaken
- Single-use technologies allow for processes efficiencies, there are several advantages over standard reusable stainless steel systems,
- Turnaround equipment is eliminated therefore presenting opportunities to save on such energy use, waste disposal, and detergents usage and other cleaning chemicals used, and labour costs.
- Energy savings arise from the elimination of cleaning therefore the usage of costly to produce WFI is vastly reduced
Disadvantages of Single Use
- Time taken to implement a single-use system is delayed with the validation requirements.
- The cost of performing validation rises further if there are a range of different products that require different testing.
- Different products and the plastic material of a single use system will react in different ways.
- The requirement for the biopharmaceutical company to audit the sterilisation process of the single-use technologies and be satisfied that the materials supplied are suitable and are delivered sterile is an inconvenience for the manufacturer
- The reliability of the vendor will they stay in business and will their suppliers stay in business are all considerations and are disadvantages.
By following best validation practices, single use systems offer more advantages than disadvantages for the biopharmaceutical sector and represent the future direction that the industry is taking according to (Sandle and Saghee, 2011).
A test for the identification leachables and extractables.
As outlined in this project, Leachables and Extractables is the biggest disadvantage when it comes to adopting single use technology this is because they create new challenges in bioprocessing, as potentially toxic or inhibitory substances could leach from the equipment into the process stream. These substances threaten the bioprocess performance, due to cell growth inhibition that results in loss of cell lines and reduced yields. The risk of leachable materials entering drug substance and drug product has become one of the biggest concerns when adopting single-use technology.
In a study carried out at NIBRT Dublin Ireland. A method for the identification of leachables in chemically defined media for CHO cell culture using dispersive liquid to liquid micro extraction (DLLME) and UHPLC-MS is described. It was found that general extraction conditions for any group of leachables regardless of their specific chemical functionalities can be applied and similar optimum conditions were obtained with the two types of media used. (Dorival-García and Bones, 2017)
A rapid, sensitive and reliable method for untargeted identification and quantification of Leachables & Extractables compounds by DLLME followed by UHPLC- MS has been developed. The method was focused especially on the determination of the degradation products of Irgafos168. The performance of DLLME as extraction technique was studied by comparison of two commercial CHO cells media, resulting in similar optimum conditions for both, this suggests that the optimised experimental conditions could be applied for other cell culture media.. The method can be applied for the screening of untargeted leachables and also for the quantification of degradation products of Irgafos 168 in culture media. (Dorival-García and Bones, 2017) It is positive to see inroads being made in terms of rapid testing methods that are being developed in order to address the Leachables and Extractables problem.
Integrated single use technologies for biopharmaceuticals
Companies are developing integrated solutions for single use technologies. In this section examples are outlined of what’s available on the market.

Fig 4: Mobius FlexReady Solutions. L to R Tangential flow filtration; Buffer and media preparation; Virus filtration; and Clarification. (Valle, 2009)
.
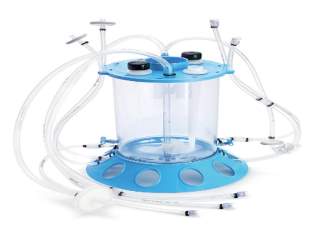
Fig 5: The Mobius CellReady 3L Bioreactor. (Valle, 2009)
The Mobius CellReady 3L Bioreactor used single-use processing, while incorporating standard design features it is designed to replace traditional glass bench top bioreactors.
In a study, the performance of the Mobius CellReady singleuse 3L bioreactor was compared to a 3L glass bioreactor. The Mobius CellReady 3L single-use bioreactor evaluated in this study represents a convenient alternative to traditional methods used to seed large-scale production runs. (Wood et al., 2011)
Manufacturing today have higher titer and single-use solutions complements this. There are smaller batch processes that reduce product costs, and it allows for greater process flexibility. There is also the potential for operation in the uncontrolled unclassified grey space outside the costly clean room.
On the market there are single-use Mobius FlexReady solutions for clarification, virus filtration, and tangential flow filtration and also for media and buffer preparation. The units in Figs 4 & 5 are based on a detailed understanding of biopharmaceutical manufacturing constraints.(Valle, 2009)
Recovery Centrifugation & Filtration.
Centrifugation has not escaped the single use technology market. In clarification technology, single-use systems can adapt to changing surface area and media grade requirements they eliminate the need for significant capital investment in a centrifuge. The Mobius FlexReady Solution for clarification is an option and it uses Millipore’s Millistak depth filtration media for cell-culture clarification directly from a bioreactor. (Valle, 2009)
In 2016 Sartorius Stedim Biotech has signed a contract to acquire US centrifuge specialist kSep Holdings Inc. kSep has developed and markets single-use, fully automated centrifugation systems used for manufacturing biopharmaceuticals, such as vaccines, cell-based therapeutics and monoclonal antibodies (‘News: Sartorius Stedim Biotech to acquire kSep Systems,’ 2016) Sartorius Stedim Biotech is a big player in the single use market so this acquisition should help with more innovations going forward as they continue to excel with their single use technology offerings.
In a 2008 study of the environmental footprint (Valle, 2009) “A comparison of stainless steel based bioprocessing facility versus single-use technologies found that single-use systems required:
- 38% less space
- 21% less labour, primarily by reducing CIP activities
- 29% less energy to run
- 87% less water
The savings from the above are huge as WFI is extremely expensive to produce, more space will allow opportunities for expansion and energy savings and labour are all high costs. This will help to lower the price point of the medication so it will ultimately lead to a competitive advantage.”
Sensors
In a study current and next generation sensors such as pH, dissolved oxygen and temperature sensors that will help drive the use of single-use bioreactors in industry are reviewed. Many of the sensors still in use today rely on technology created in the 1960s. There is a reluctance to change due to the strict regulatory requirements of sensors to monitor bioprocesses therefore well understood methods are used making it difficult to incorporate new sensor technology into industry but single use technology is relatively new so sensor technology will have to adapt. Clark dissolved oxygen sensor or glass pH probes, which rely on technology from the 1960’s are commonly used and it is difficult for new technology to get to market. New Fibre optic probes that are light sensitive are used with some of the single use technology, This was evident on the wave bioreactor on a recent visit to Nibrt in Dublin for upstream practical work.
Capsule technology is an area that shows some real promise. The technology uses a multi parametric sensor chip that is combined with a capsule. It allows it to move throughout a bioreactor with the mechanical motion of the bioreactor impellors, The nature of this device means that it could link in with existing instrumentation to allow easier integration into industry. It is very advances and will allow for measurements at every point of the bioreactor, giving accurate representations of key parameters such as pH, dissolved oxygen and temperature and of the homogeneity there can be many devices placed in the bioreactor at once. They are also PAT compliant. The PATsule is a novel capsule for real-time, multi-parametric monitoring of critical parameters for biopharmaceutical production (O’Mara et al., 2018)

Fig 6: PATsule (O’Mara et al., 2018)
PAT (Process Analytical Technology)
In the bioprocessing industry, critical quality attributes or critical process parameters that are frequently monitored include temperature, pH, dissolved oxygen, cell density, viable cell density nutrient media content and metabolite concentration. The bulk of PAT equipment that are currently in use are sensor-based technology including probes that measure the above parameters. In this journal despite recent efforts outlined to develop PAT tools for bioprocessing, the authors outline that increased innovation is needed to permit the development of new accurate, specific and robust PAT instrumentation. It is noted that it is especially true for single use technology applications as new innovative bioreactor technology must be matched with new novel disposable sensor technology so that its full potential is realised. Dielectric probes positioned within a bioreactor have been used to monitor bulk capacitance of mammalian cell cultures and have shown sensitivity to early apoptotic changes in cells this is crucial for monitoring, This technology has great potential for use as a PAT tool for monitoring physiological changes in cells. (O’Mara et al., 2018)
Affordable Biologics with Single Use
Increasing competition for new indications and the launch of biosimilars for the monoclonal antibody market is putting pressure on the industry to produce drugs at lower cost. Single-use technologies and continuous upstream processes are proven to be cost efficient to increase biomass production. The cost of medications is rising from expensive to unaffordable. In Ireland for example recently it has been highlighted on the news that certain drugs are unaffordable and patients are being denied the medications based on cost alone. Some governments have passed legislation aimed at reducing expenditures on follow on biologics such as biosimilars they have helped the market by shortening the approval pathway
In the last decade, there has been a dramatic increase in expression yield from progress in cell lines, expression systems, and culture. Increased competition from biosimilars and other follow-on biologics is forcing established manufacturers to find or develop and integrate new ways to stay competitive and conserve market share. There are many improved technologies emerging, the stage is now set for the industry to reduce the costs of bio therapeutics.
Some processing examples are that longer run duration and the ability to sustain biomass levels enable perfusion bioreactors to offer a productivity advantage of at least 4-fold as compared to a fed-batch unit with the same reactor volume for upstream processing. In recovery the authors state that for clarification, centrifuges are not the right solution and are becoming outdated because they are difficult to scale and complicated to operate. They favour filtration as an alternative to centrifugation due to much simpler implementation in flexible single use continuous processes. New disposable filtration designs offer more flexibility and scalability. (Jacquemart et al., 2016)
Replacing traditional facilities where disposable technologies are implemented with a flexible concept design allows for easy and rapid scale up by increasing the number of cost-efficient production units. Flexible facilities will experience minimum expenses during clinical phases and grow with market demand, hence minimising risks while optimising time to market and global distribution without the challenges of complex worldwide distribution logistics. (Jacquemart et al., 2016) this can be achieved with single use technology.

Fig 7: Univercells modular concept for combining USP and DSP in a small-footprint cabinet (Jacquemart et al., 2016)
Example of continuous processing the output from the perfusion bioreactor in the left chamber is continuously feeding into the downstream purification train right chamber. The productivity of the perfusion bioreactor is matched with the downstream process for efficient biologic manufacturing.
Standards for single-use
There seems to be a lack of standards and standard setting
In a 2012 survey it was revealed that (69.1%) (Lopes, 2015)of the industry are restricting the adoption of single use technology due to concerns about leachables and extractables. In the same survey the industry continues to find selection, qualification and validation of single-use components a challenge. It outlines that “Standardisation would help reduce the need to assess different materials and designs.”(Langer, 2012)
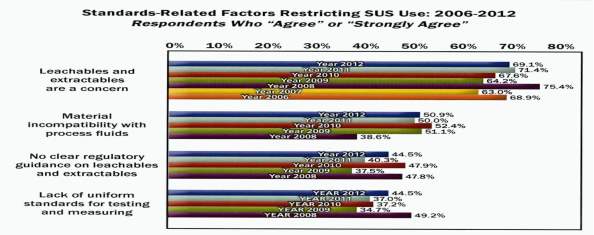
Fig 8: SU Standards Survey (Langer, 2012)
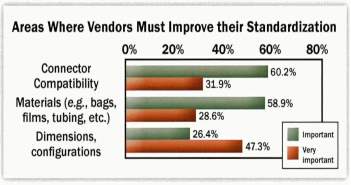
Fig 9: SU Standards Improvement Considerations (Langer, 2012)
Single use technology has multiple systems available, its components and assemblies lack standardisation aswell as harmonised tests and procedures among suppliers. Investment in novel technologies, research and standardisation and training is vitally important for further development and implementation of the technology across all sectors of the biopharma industry. (Lopes, 2015).
The analysis of challenges and limitations of single-use technology according to (Lopes, 2015) can be categorised in future developments necessary in four key areas:
- Novel future technologies required.
- Independent research of single use technology.
- Standardisation and modularity.
- Testing and training on single use technology.
It is projected that the majority of commercial-scale biologics manufacturing will be done in disposable equipment in the near future because of improvements in single use design and innovation. The industry suppliers are seeking guidance from regulatory authorities to better define expectations regarding single-use technology.(Langer, 2011)
The transfer of a CHO fed-batch process to single-use
In this study entitled the successful transfer of a modern CHO fed-batch process to different single use an industrial CHO fed-batch process is established in different single-use and reusable bioreactors.
Single use bioreactors involved in this study were the stirred tank reactor BIOSTAT STR 50 L with a CultiBag STR 50 L and the Rocking BIOSTAT RM 50 optical with CultiBag RM 50 L.
Due to different agitation and gassing principles present in the BIOSTAT STR and Cultibag RM the kLa and mixing times were chosen as a scale-up criteria. The process transfer is considered successful, if comparable cellular proliferation activities and product titers are obtained.
Results: The high cell density CHO fed-batch process with industry relevant titers was successfully transferred from a reference bioreactor to a variety of single-use bioreactor systems. (Ruhl et al., 2013)
Single-use disposable technologies for biopharmaceutical manufacturing: Existing technology on the market for upstream Processing (Shukla and Gottschalk, 2013)
Table: 1 Single use technology that are available for upstream processing.
| Bag cultures | 20–300 litre WaveTM Bioreactors (GE Healthcare)
<300 litre Biostat Cultibag (Sartorius Stedim) <500 litre BioWave (Sartorius Stedim) <25 litre Appliflex (Applicon) <160 litre Tsunami Bioreactor (Tsunami Bio) |
| Production bioreactors | Single Use Bioreactor (SUB) < 2000 litre (Hyclone/ThermoFisher Scientific)
XDR Disposable Stirred Tank Reactor < 2000 litre (Xcellerex) Biostat Cultibag STR Plus < 200 litre (Sartorius Stedim Biotech) |
| Bench-top bioreactors
(laboratory scale, but could also be used for seed train) |
CelligenBLU Single Use Bioreactor (New Brunswick) < 14 litre
CELLtainer Single Use Microbial Bioreactor (Lonza) < 15 litre Mobius Cellready (Applikon and Millipore) < 2.4 litre XDR-10 (Xcellerex) 4.5–10 litre |
| Centrifugation | kSep1 400 and 6000 (for 1–6000 litres) (kSep Systems)
Unifuge (Carr Centritech) for up to 1000 litres |
| Depth filtration | POD (Millipore)
Stax (Pall) Zeta Plus (Cuno) Sartoclear P (Sartorius-Stedim) |
| In-process microfiltration | Ready To Process HF (GE)
KrosFlo (Spectrum) |
| Mixing (bag mixing with rotating stirrer) | 6–2000 litre LevmixerTM (ATMI Life Sciences)
6–2000 litre Magnetic Mixer (ATMI Life Sciences) 100–1000 litre MobiusTM (Millipore) 100–1000 litre XDM QuadTM (Xcellerex) 50–1000 litre Flexel1 3D LevMix (Sartorius-Stedim Biotech) 50–2000 litre Single Use Mixer (Hyclone/ThermoFisher Scientific) |
| Mixing (bag mixing with rocking) | 20–1000 litre WaveTM (GE Healthcare)
30–5000 litre HyNetics (HyNetics Corp) 5–2000 litre SALTUS (Meissner) |
| Connections/tubing | C-flex weldable tubing
Bioquate DAC (Bioquate) Ready-mate DAC (GE) KleenPak (Pall) Quick-connect, MPC, Saniquik, AseptiQuik, sanitary TC (Colder) |
| Bulk drug substance cold storage | CX5-14 HDPE Labtainer (Hyclone)
Bioeaze PE (SAFC) Flexel Sartorius |
| Bulk drug substance freeze-thaw | Celsius Pak (Sartorius)
Platinum Ultra Pak Lonza PETG or polycarbonate bottles (Nalgene) |
Table Listing.
Table 1: Single use technology that is available for upstream processing (Shukla and Gottschalk, 2013)
Figures Listing.
Fig 1: Genderless Connections (Boehm, 2014)
Fig 2: SIP Connectors on SS (Boehm, 2014)
Fig 3: SIP Connectors on SU (Boehm, 2014)
Fig 4: Mobius FlexReady Solutions. (Valle, 2009)
Fig 5: Mobius CellReady 3L Bioreactor. (Valle, 2009)
Fig 6: PATsule Sensor (O’Mara et al., 2018)
Fig 7: Univercells’ modular concept (Jacquemart et al., 2016)
Fig 8: SU Standards Survey (Langer, 2012)
Fig 9: SU Standards Improvement Considerations (Langer, 2012)
- Discussion & Conclusion
The single use market is still in it’s infancy in relative terms, production capacity issues have been offset somewhat by developments in increased cell densities and titres due to efficiencies in production cycles so capacity is becoming less of a problem. Combine that with increases in capacity with single use equipment itself bridges the gap. Novel genderless connection technology give manufacturer’s greater flexibility and there are many options that can be used for both traditional and single use applications. However sensor technology does lag behind in terms of development. Even with sensor technology the innovations in the pipeline such as the capsule technology are game changers as they will allow for flexible enhanced monitoring that will be PAT compliant.
There appears to be a disjoint however because in the reluctance of the industry due to tight regulations to move from dated sensor technology to newer innovations, the regulatory authorities encourage PAT and these innovations are essential for a PAT strategy but the regulations themselves appear to be an obstacle to embracing the technology for the industry .
Leachable and extractables are still considered to be the biggest problem when implementing single use technology. Validation of the technology presents challenges. The sector appears to be crying out for guidance on standards, everyone is looking to the regulatory authorities for standard setting. In the surveys conducted over a period of 6 years there has been no real progress in dealing with some of these fundamental issues as can be seen in the surveys conducted. Inroads are being made in terms of rapid testing methods to detect the presence of leachables and extractables and this is major step forward.
Economically in a challenging market where there is huge competition and major pressures to reduce the costs of the medication, Single use can present a viable flexible alternative to embrace compared to the traditional. The savings on operational costs alone and the sheer flexibility that comes with single use technology present major competitive advantages to the manufacturer.
The Single use market will continue to grow and present new innovative technologies in the future. The market wants it and demands it and with that demand will surely come a pipeline of innovation.
- References
Boehm, J. (2014) Connection Technology Advances Closed System Processing: Single-Use Connectors Create Improved Flexibility and Reliability; and Drive Cost Savings for Biopharmaceutical Manufacturers. BioProcessing Journal, 13(1), 50-55.
Dorival-García, N. & Bones, J. (2017) Monitoring leachables from single-use bioreactor bags for mammalian cell culture by dispersive liquid-liquid microextraction followed by ultra high performance liquid chromatography quadrupole time of flight mass spectrometry. Journal of Chromatography A, 1512, 51-60.
Editors, B. I. (2017) Integrated Single-Use Sterile and SIP Connector Provide Flexibility. Available at: http://www.biopharminternational.com/integrated-single-use-sterile-and-sip-connector-provide-flexibility.
Gadgil, M. (2017) Cell culture processes for biopharmaceutical manufacturing. Current Science (00113891), 112(7), 1478-1488.
Jacquemart, R., Vandersluis, M., Zhao, M., Sukhija, K., Sidhu, N. & Stout, J. (2016) A Single-use Strategy to Enable Manufacturing of Affordable Biologics. Computational and Structural Biotechnology Journal, 14, 309-318.
Langer, E. (2012) Standards-Setting for Single-Use Equipment in Biopharmaceutical Manufacturing: An Update on Progress; and the Impact of Standards Implementation. BioProcessing Journal, 11(3), 20-26.
Langer, E. S. (2011) Disposable/Single-Use Equipment in Biopharmaceutical Manufacturing: Global Standardization Is An Absolute Necessity. BioProcessing Journal, 10(2), 36-41.
Lopes, A. G. (2015) Single-use in the biopharmaceutical industry: A review of current technology impact, challenges and limitations. Food & Bioproducts Processing: Transactions of the Institution of Chemical Engineers Part C, 93(1), 98-114.
Odeleye, A., Lye, G. J. & Micheletti, M. (2013) Engineering characterisation of single-use bioreactor technology for mammalian cell culture applications. BMC Proceedings, 1-3.
O’Mara, P., Farrell, A., Bones, J. & Twomey, K. (2018) Staying alive! Sensors used for monitoring cell health in bioreactors. Talanta, 176, 130-139.
Ruhl, S., Husemann, U., Jurkiewicz, E., Dreher, T. & Greller, G. (2013a) The successful transfer of a modern CHO fed-batch process to different single-use bioreactors. BMC Proceedings, 1-3.
Ruhl, S., Husemann, U., Jurkiewicz, E., Dreher, T. & Greller, G. (2013b) The successful transfer of a modern CHO fed-batch process to different single-use bioreactors. BMC Proceedings, 1-3.
Sandle, T. & Saghee, M. R. (2011) Some considerations for the implementation of disposable technology and single-use systems in biopharmaceuticals. Journal of Commercial Biotechnology, 17(4), 319-329.
Shukla, A. A. & Gottschalk, U. (2013) Single-use disposable technologies for biopharmaceutical manufacturing. Trends in Biotechnology, 31(3), 147-154.
Valle, C. (2009) Biotechnology drugs: Integrated single-use technologies for biopharmaceuticals. Filtration & Separation, 46(6), 18-21.
(2016) News: Sartorius Stedim Biotech to acquire kSep Systems. Filtration Industry Analyst, 2016, 5.

Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Information Technology"
Information Technology refers to the use or study of computers to receive, store, and send data. Information Technology is a term that is usually used in a business context, with members of the IT team providing effective solutions that contribute to the success of the business.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




