Drug Eluting Stents for Coronary Heart Disease in Diabetics
Info: 13064 words (52 pages) Dissertation
Published: 2nd Nov 2021
Tagged: MedicalHealthcareDiabetes
Table of Contents
Introduction
Research Question, Aims and Objectives
Methodology
Study: Late clinical outcomes of diabetic patients treated with Sirolimus or Everolimus drug-eluting stents (DES): an analysis of the DESIRE registry
Literature Review
Critical Analysis
Study: Comparison of First- and Second-Generation Drug-Eluting Stents in an All-Comer Population of Patients with Diabetes Mellitus (from Katowice Zabrze Registry)
Literature Review
Critical Analysis
Study: Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes; Taxus Element versus Xience Prime in a Diabetic Population
Literature Review
Critical Analysis
Study: Clinical Impact of Second Generation Everolimus Eluting Stent Compared With First Generation Drug Eluting Stents in Diabetes Mellitus Patients; Insights from a Nationwide Coronary Intervention Register
Literature Review
Critical Analysis
Second- versus first-generation drug-eluting stents for diabetic patients: a meta-analysis
Literature Review
Critical Analysis
Discussion and Conclusion
References
Introduction
Coronary artery disease (CAD), also known as coronary heart disease (CHD) and ischaemic heart disease (IHD), is a primary cause of morbidity and mortality worldwide. It remains one of the utmost important main healthcare issue in the United States and around the world, in spite of a dramatic advancement in science and medicine. In spite of the fact that the mortality for this condition has progressively declined in the course of the most recent decades in western nations, regardless it causes around one third of all deaths in individuals older than 35 years (1). The explanation behind this are multifactorial and are generally because of the increasing age of our population. It is due to narrowing or stenosis of the coronary arteries as a consequence of deposition of the atherosclerotic plaque, which brings about an inadequate supply of oxygen to the heart muscle (2). CAD may influence one or more arteries of any different diameters (calibres) and the stenosis of arteries may be either partial or total, resulting in different severity of the disease. Coronary artery stenosis may be asymptomatic or may lead to angina – a chest pain that may be severe enough to hamper or prevent exertion. A critical reduction of the blood supply to the heart may result in myocardial infarction (MI) or even death (3) (4). A critical marker of CAD is angina pectoris, which is characterized as a syndrome of substernal chest discomfort, with a distinguishing quality and duration of discomfort, usually exacerbated by exertion or emotional stress. The discomfort is usually relieved by rest or the administration of Nitroglycerin, a drug that dilates the blood vessels (5). Some may even need a surgical intervention.
Cardiovascular disease (CVD) is a group of diseases that involves and may damage both the cardiac tissue, the heart and blood vessels. CVS includes CHD as well as CAD, and also acute coronary syndrome (ACS), amongst several other conditions. Although these terms are sometimes used interchangeably, they aren’t the identical. ACS is a subclass of CAD, whilst CHD is a result of CAD. In contrast, CAD is characterized by atherosclerosis in coronary arteries and can be asymptomatic, while ACS almost always presents with a symptom, such as unstable angina, and is frequently associated with MI whether the CAD is present or not (1).
One of the major established factor in causing CAD and MI is atherosclerosis. Two principal pathophysiologic phenomena that account for most clinical symptoms of CAD include: 1) progressive accumulation of plaque in the cardiac vessels, causing narrowing and stenosis of the lumen, eventually limiting the ability to increase blood flow in reaction to increments in myocardial demands, hence leading to myocardial ischemia and, in this manner, angina pectoris; and 2) plaque rupture that impedes the vessel lumen with ensuing end of myocardial flow – ultimately leading to the outcome of CAD and MI (6).
Lifestyle modification, pharmacological, fibrinolysis, and/or catheter based or surgical therapy are one of many options available as treatment for CAD (5).
There have been considerable advances in medicinal treatment to avert and treat CAD. Be that as it may, patients with prognostically noteworthy disease or manifestations of angina in spite of ideal therapeutic treatment or optimal medical therapy (OMT) require mechanical revascularization: either coronary artery bypass grafting or percutaneous coronary intervention. Coronary artery bypass grafting has remained the major method of revascularization amid the second half of twentieth century. However, PCI has turned into the favoured technique for revascularization in patients with single or double vessel disease without left main stem (LMS) disease (7).
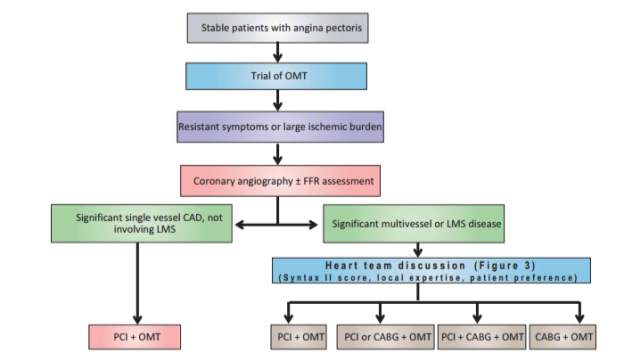
Figure 1: A potential revascularization pathway for patients with stable angina pectoris. OMT, optimal medical therapy; FFR, fractional flow reserve; CAD, coronary artery disease; LMS, left main stem; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft (7)
In 1968, Coronary-artery bypass grafting (CABG) was first launched and it quickly turned into the standard procedure for management in symptomatic patients with coronary artery disease. Improvements in coronary surgery (e.g., off-pump CABG, smaller incisions, enhanced myocardial preservation, use of arterial conduits, and improved postoperative care) have significantly diminished morbidity, mortality, and rates of graft occlusion. Wherein, in 1977, percutaneous coronary intervention (PCI) was first introduced and became the main competitor of the CABG procedure (8). CABG has been the dominating method of revascularization for the greater part a century and is the favoured technique for patients with multi-vessel disease, particularly those with diabetes mellitus, left ventricular systolic dysfunction or complex disease. Increased skill with the PCI approach, combined with enhanced innovation, has made it conceivable to treat progressively intricate lesions and patients with a background of clinically significant cardiac disease, risk factors for coronary artery disease, coexisting conditions, or anatomical risk factors (8). PCI involves both non-stent and stent procedures (7).
Stent innovation (type and platform, including the design, alloy used and the thickness of the stent) has grown quickly, and the advances in the technology is expected to decrease the probability of restenosis. Since restenosis is connected with the level of inflammation present at the time of the angioplasty, drug eluting stents (DES) were created. These are bare-metal stents (BMS) that are coated with a drug, such as an immune suppressant, to reduce the amount of inflammation or an antimitotic agent that is present to reduce number of cell proliferation (9).
The medication released by the DES reaches the therapeutic concentration only in the local tissue and is not at a significant level systemically, hence, evading the systemic adverse effects. A consequent advancement was the utilization of a drug polymer blend where the medication is held briefly inside a polymer “painted” onto the metallic stent, permitting the medication to elute gradually into encompassing tissues. In any case, not all stents are polymer based (9) (10).
While BMS are as yet utilized, DES are currently the favoured methodology and now offer clinicians the capacity to forestall restenosis by means of an alternate option. BMS forestalls restenosis by weakening blood vessel recoil force and contraction, which was seen in balloon angioplasty as well. Whereas DES supplies an anti-proliferative medication to the objectified target lesion and hence, hinders the exorbitant growth of the neo-intima. DES comprise of a standard metallic stent, a polymer covering, and an anti-restenotic drug that is blended inside the polymer and discharged over period of time.
Original DES, which is the first generation of DES, includes Sirolimus eluting stents (SES; 2003) and Paclitaxel eluting stents (PES; 2004). Zotarolimus and Everolimus, which are the second generation drug eluting stents (ZES, EES) were endorsed for use in the United States in 2008 (10). In spite of the fact that all DES have similar fundamental principle, each DES may offer disparities concerning deliverability (simplicity of placement), effectiveness (averting restenosis), and safety (thrombosis rates). The use of double antiplatelet treatment with stents has basically improved the outcomes and results in patients undergoing a PCI procedure and, thus, reduced procedure failure rate (9) (10).
The introduction of the original Sirolimus eluting (SES) and Paclitaxel eluting stents (PES) has promptly and notably lessened the restenosis rates and decreased the incidence of target injury revascularization, in contrast with the BMS, in diabetes mellitus patients and additionally in non-diabetic mellitus patients.
SES and PES are the 1st generation stents that were developed to prevent and slow the proliferation of the smooth muscle cells as well as other types of cell that can cause restenosis. These stents modernized the PCI procedure by reducing the rates of restenosis and stent thrombosis after the PCI procedure.
The FDA has approved SES and PES for use in patients with a newly diagnosed, previously untreated single lesions <28 mm to 30 mm in length and a vessel diameter between 2.5 mm and 3.75 mm. SES is composed of Sirolimus compound, which is a macrocytic triene antibiotic that releases slowly over 4 to 6 weeks and has immunosuppressive as well as anti-proliferative properties that helps reduces the rate of restenosis.
The efficacy of SES in preventing restenosis has been established in the SIRIUS (12), RAVEL (11), and SCANDSTENT trials (13) as well as the RESEARCH registry (14). The RAVEL (12) and SIRIUS (11) trial compares the SES with BMS in patients that had either stable or unstable angina. These patients received dual antiplatelet therapy for 6 to 9 months after the PCI procedure. The result of the trial showed that there was a significant reduction of in stent restenosis, late lumen loss, and target lesion revascularization over 1 to 5 years, after the PCI procedure, in the group where patient received SES.
Nonetheless, even though SES proved to be better in reducing the stent restenosis rate, there were no difference in the rate of deaths or MI. Another 1st generation DES that is frequently used is PES, which involves a bare metal stent coated with Paclitaxel, a drug that is released bimodally slowly over a two week period. It is an antineoplastic drug that works by interrupting the function of the microtubules that are accountable for appropriate chromosome segregation during cell division.
The usefulness of PES was proven in the TAXUS II and TAXUS IV trials, which inspected patients with generally low risk lesions or formerly untreated coronary stenosis who indiscriminately and randomly got BMS or PES with either a moderate or a slow medication release rate. All trials brought about lessening of in-stent restenosis and target lesion revascularization. Moreover, TAXUS IV exhibited that these advantages were kept up and retained in subgroups, including patients with vessels <2.5 mm in diameter, those with lesions >20 mm long, and those with renal failure or diabetes.
Pooled and long term study additionally uncovered a decrease in cardiovascular incidents (10). The most up to date member from the PES group is the TAXUS Liberté, which has more slender struts to enhance deliverability and has appeared to be non-inferior compared to its prototype model in the TAXUS ATLAS trial (10) (17).
In the course of the last 10 years, original 1st generation DES, particularly SES and PES, have turned into the most generally utilized device, as a stent worldwide, in the PCI procedure. Be that as it may, in spite of their reasonable predominance and advantage in preventing restenosis and reducing the need for repeat revascularisation due to the eluted anti-proliferative medications, information about the long term efficacy were still inconclusive. But more studies have been providing follow-up data that arose concerns as to their long haul safety profile, especially that the 1st generation DES can cause late and very late thrombotic events, which in turn associated them with a high rate of death and MI.
Such incidents have been accredited to the inadequate re-endothelialisation instigated by the drug induced inhibition of endothelial cell proliferation, stent malapposition, and accelerated neo-atherosclerosis and, most significantly, polymer instigated prolonged vessel wall inflammation.
Second generation DES were hence created, and were intended to conquer all these long term issues, utilizing new biocompatible polymer coatings, less toxic anti-proliferative medications and in the long run, best in class thin strut Co-Cr metal amalgams. They have all been widely studied and tried in a randomized clinical trials with proven positive significant result. The Everolimus eluting stent (EES) has been observed to be more secure than original and biodegradable DES; in contrast, Zotarolimus-eluting stent (ZES) was inferior as compared to the SES in regards to major adverse cardiovascular events but still better than PES as far as MI is concerned, in terms of recent meta-analysis of the randomized controlled trials (18).
As mentioned, the more current second generation stents, EES and ZES, are more slender and more adaptable with a cobalt-chromium composite ally platform, which makes them more deliverable than the original first generation stents. These stents may likewise be more biocompatible, along with producing less provocative inflammatory reaction with quicker vessel endothelialization. The EES stent is composed of Everolimus, which is a sirolimus derived material and is a semisynthetic, lipophilic, exceptionally absorbable macrolide immunosuppressant.
Everolimus elutes and releases after some time, with 80% absorbed within month and the rest of it over a 4-month term (10). The EES also showed effectiveness over BMS in the SPIRIT FIRST trial (19), with significantly lower in stent late lumen loss at 6 months (10). Additionally, EES also showed its superior functioning to PES in a meta-analysis studies. The four trials used in the meta-analysis showed that EES lessened the risk of stent thrombosis, MI, ischemic target lesion revascularization, and death. In the SPIRIT II–IV trials (20) (21) (22), EES and PES were evaluated in patients with simple straightforward and complex coronary disease. The group that utilized EES resulted in lower rates of in-stent late loss and target lesion failure (characterized via cardiac death, target-vessel MI, ischemic target lesion revascularization) up to 2 years after the procedure (10). Albeit no randomized trials have looked at EES and SES, the X-SEARCH registry (23) was utilized to assess the adequacy and safety of EES in higher risk complex patients. EES were looked at in three recorded population of patients who got BMS, SES, or PES. At 6 months, the rate of target lesion revascularization in EES were significantly lower in contrast with the patient that received BMS, and EES showed rates of target lesion revascularization practically identical to those patients that received SES or PES.
ZES, also a 2nd generation stent that utilizes Zotarolimus, a Sirolimus subsidiary, is a lipophilic immunosuppressant with the polymer that is utilized as a part of ZES, copies the cell layer’s phospholipid phosphorylcholine, hence, making it more effective with ninety five percent of the drug eluting within the initial 2 weeks. The adequacy of ZES has been analysed in the ENDEAVOR trials. In ENDEAVOR I (24) and II (25), which compared the efficacy and the safety of ZES and BMS, target lesion revascularization rate was lower with ZES at up to 2 years.
In ENDEAVOR III (26) and IV (27), which contrasted ZES with SES and PES, individually, angiographic in-segment late lumen loss — a surrogate for restenosis — was higher in the ZES population versus the SES population. In contrast with the PES group, however, ZES was non-inferior for the essential primary endpoint of target vessel failure. The SORT-OUT III (27) trial indicated comparable outcomes between the ZES and SES group in the cardiovascular death, MI, and target lesion revascularization, which are the primary endpoint of the study.
It also showed that the cardiac death, MI and target lesion revascularization happened more regularly with ZES than with SES (10). However, another study, the ZEST trial (28), which analysed ZES, SES, and PES, found no distinction in the essential primary endpoint of death, MI, and target lesion revascularization. In a recent trial evaluating EES and ZES in complex clinical or lesion characteristics (renal insufficiency, low ejection fraction, recent acute MI, multiple or long bifurcations, bypass grafts, in-stent restenosis, unprotected left main artery, thrombus, or total occlusion), there was no distinction in the essential endpoint of target-vessel failure. PES and SES were analysed in a few clinical trials, a large portion of which established that SES were related with lower rates of clinical restenosis and late lumen loss. The superiority of SES might be expected over other stent due to its mechanism of action, timing of drug eluting and delivery, and cellular inflammatory reaction in response to SES (10) (29).
The National Institute for Health and Care Excellence (NICE) guideline for clinicians in United Kingdom expresses that DES are suggested for use in PCI for the treatment of CAD, inside their directions for use, just if:
1) the artery with stenosis that is to be dealt with has calibre below 3 mm or the lesion is lengthier than 15 mm,
2) and the value distinction between DES and BMS is close to £300. NICE states that there were no measurably huge contrasts in outcomes such as deaths and MI, recognized between any types of DES subgroups. However, it states that there is a reduced revascularization rates, when DES is compared with BMS. The pooled DES investigation showed that revascularisation rates were lessened by around seventy five percent in contrast to the BMS group, predictable crosswise over most studies of the PES (Taxus (17)) and SES (Cyher; Endeavor (24) at 6–9 months) (9).
This dissertation will focus on diabetes mellitus and different drug eluting stents as well as their superiority when compared to one another.
Both insulin and non-insulin dependent diabetes mellitus, plays a major role in causing CAD; and are responsible for 14-50% of new cases of CVD. Moreover, the most widely recognized cause of death in adult with diabetes mellitus, is CAD. Interventional treatment of patients with CAD and diabetes remains a complex situation and requires an experienced expertise. This group of population is known to experience more side effects, quick advancement in disease development as well as a greater burden of coronary atherosclerosis, in contrast to the non-diabetic group. This is the consequence of numerous cardiovascular risk factors associated with diabetes mellitus, which make PCI more challenging and exacerbate the risk for unfavourable outcome (29).
The prevalence of CAD In the diabetic population correlates to the period of patient having diabetes as well as the level of glycaemic control. The worse the diabetic control, the higher the risk for CAD as diabetes pathogenesis involves endothelial dysfunction, augmented lipoprotein peroxidation, amplified inflammation, a pro-thrombotic state, and associated metabolic irregularities. Hence, these diabetic patients are more prone to MI, with higher prevalence of CAD, higher risk of greater infarct size, are more susceptible to post infarct complications including heart failure, shock and death; in contrast to their nondiabetic counterparts. Moreover, angina in diabetic patients are more likely to be missed as they can have atypical presentation of the ischemic symptoms; which includes nausea, dyspnoea and sweating, without the chest pain. This is called “silent ischaemia”, secondary to the autonomic nervous system dysfunction, which accounts for up to 90% of their ischemic episodes. They can also present with symptoms of pulmonary oedema, arrhythmias, heart block, or syncope as their angina equivalent (30).
If any individual had diabetes, which is an independent risk factor for CVD, their chance of suffering CVD increases by two to four fold, with worsened prognosis, no matter what the coronary revascularization modality (surgical or percutaneous) is. It is predicted that around 25% of patients treated with PCI are diabetics. Indeed, even with the introduction of DES, which, contrasted with bare metal stents, enormously diminished restenosis rates, these diabetic population still have more regrettable clinical results, with higher rates of restenosis, stent thrombosis, acute MI, and death, when compared to the non-diabetic counterparts (31).
Overall, even though DES has improved the outcomes as well as reduced the risk of restenosis after PCI procedure, there were high mortality rates in patients with diabetes who received DES, when compared to BMS. This made CABG the main preferred modality of treatment in diabetic patients with multi-vessel disease, for the past several years, as to reduce the risk of repeat revascularization, compared with stenting (32). However, due to recent advances and newer stent production, the second generation of DES is expected to reduce the mortality.
Research Question, Aims and Objectives
To guide this review, the following research question and aim were devised.
- What is more superior, the 1st or the 2nd generation DES in PCI, specifically performed on diabetic patients?
- To explore the literature that investigates the role of 2nd generation DES, specifically within the context of individuals with diabetes. The objectives of this review is to :
- Identify the studies that compare 1st and 2nd generation of DES
- Evaluate the significance and superiority of the different generation of DES
- Explore the clinical usefulness of the 2nd generation of DES
- Identify the gaps in the studies comparing the DES, in which future studies should address
- Formulate recommendations based on the studies used in the review
Methodology
A number of electronic databases were used to identify relevant studies for this review as well as to help answer the research question, with a published data. The electronic databases included New England Journal of Medicine as well as PubMed. They were selected on the basis that it is a database with vast information and is a commonly used medical literature source that includes extensive numbers of good standardized peer reviewed literature and journals that are relevant to the current review topic. Google search engine were also utilized as not to miss out studies not published in these databases. The keywords used included but not limited to “PCI in diabetic patients”, “DES in diabetic patients”, “1st generation vs 2nd generation DES”, “sirolimus in diabetic patients”, “everolimus in diabetic patients”, “zotarolimus in diabetic patients, “paclitaxel in diabetic patients”, “clinical outcomes of PCI with DES in diabetic patients”, “Comparison of First and Second Generation DES”, etc. The words “and” and “or” were applied either to broaden or narrow the search results using the Boolean operators. Search limiters restricted non diabetic patients and researches in other languages, were applied, when available. The search terms used for the database were constant, in order to ensure consistency of the search process. The population of interest were patients with diabetes, regardless of how long they have been diabetic. The exposure of interest were comparison in diabetic patients who underwent PCI either with first or second generation DES. The electronic database using the searched keywords yielded 14 studies, whereas, the reference lists of the identified key studies were also searched in order to find additional relevant studies, but none of the literature were found. The keywords were also searched using the internet through the Google search engine, returned 4 relevant studies.
| Source | Keywords | Database | Number of Studies |
| Electronic database | “PCI in diabetic patients”, “DES in diabetic patients”, “1st generation vs 2nd generation DES”, “Comparison of First and Second Generation DES”, etc. | PubMed, New England Journal of Medicine | 14 |
| Internet | “PCI in diabetic patients”, “DES in diabetic patients”, “1st generation vs 2nd generation DES”, “Comparison of First and Second Generation DES”, etc. | Google search engine | 4 |
| Reference list of studies | 0 |
Table 1: Source of literature
Multiple inclusion and exclusion criteria were employed to the search process, in order to refine the data set and ensure the relevance of selected literature. The inclusion criteria comprised of studies performed in English, published in the last 5 years, as to keep the literature studies database more up to date and more manageable. The type of study conducted was an important inclusion criterion. Certain study designs are considered to be more reliable and these are randomized controlled studies (RCTs) and meta-analysis. However, prospective studies, cohort studies, well designed epidemiological studies including non-randomized assessments may also be included in standard review processes, when necessary. In summary, inclusion criteria were restricted to the following:
- Studies that explored the role of 1st generation and 2nd generation of DES in diabetic patients
- Studies that aimed at comparing the 2 generation of DES in diabetic patients with clear end point
- Participants of the studies were diabetic patients
- Participants aged 18 years and above
- International studies that were published in English language
Exclusion criteria
- Studies that mainly focused on non-diabetic patients
- Studies that had patient population less than 100 , as that is considered quite a small population to be considered significant in a scientific studies
- Studies that only focused on either 1st or 2nd generation of DES with no comparison between the two
- Case reports and conference abstracts as their contribution to generating good quality evidence is of doubtful significance
After applying the inclusion and exclusion criteria, as well as removing duplicate studies, 5 quantitative number of studies remained and will be further used in this review.
The selected study were analysed using algorithm, critical appraisal tool and checklist obtained from Scottish Intercollegiate Guidelines Network (SIGN) and Critical Appraisal Skills Programme (CASP).
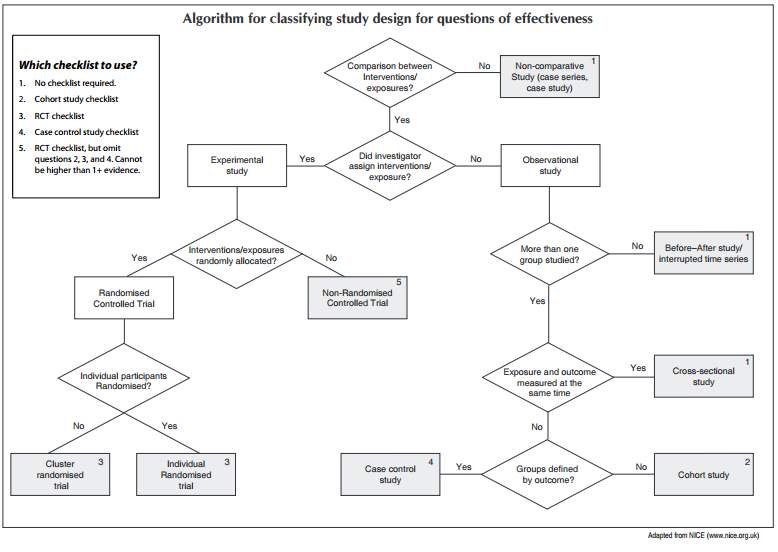 Figure 2: Algorithm for classifying study design for questions of effectiveness
Figure 2: Algorithm for classifying study design for questions of effectiveness
Study: Late clinical outcomes of diabetic patients treated with Sirolimus or Everolimus drug-eluting stents (DES): an analysis of the DESIRE registry
Literature Review
This DESIRE Registry is a prospective, non-randomized, single-arm study with sequential inclusion of patients receiving treatment. The study was performed at a single institution (Hospital do Coração, Associação do Sanatório Sírio) from São Paulo, and the objective of the study was to explore the long term clinical result of patients that received DES, during the PCI procedure.
Inclusion criteria were patient with minimal of one lesion with narrowing or stenosis of more than 50% and a suitable anatomy for PCI for stenting. There were no restrictions of how many lesions or vessels pathology as well as number of DES that can be implanted, in the protocol. Only diabetic patients, either insulin dependent or non-insulin dependent, were included in this study.
The study looked into patient either treated with 1st generation SES (CypherTM, Cordis – Warren, USA) and 2nd generation EES (XIENCETM, Abbott Vascular, Santa Clara, USA; or PromusTM, Boston Scientific – Natick, USA) were involved. The study focused on patients receiving treatment from January 2007 forward, as that is when there was a modification in the antiplatelet protocol at the institution. All the patients in the study received antiplatelet according to the protocol, that is, they received Acetylsalicylic Acid and Thienopyridines for minimal of 12 months following PCI.
The main purpose of the study was to compare the incidence of major adverse cardiac events (MACE) at follow up of diabetic patients, either receiving SES or EES. The collective endpoint of MACE encompassed of either cardiac death, acute MI, or ischemia driven target lesion revascularization. Angiographic success was defined as a Thrombolysis in Myocardial Infarction (TIMI) grade 3 final flow, no dissections, and residual stenosis of lesser than 20% by quantitative coronary angiography. Patient were asked to come for follow up at 1, 6, and 12 months after the PCI procedure, and then annually. The follow up were either clinic visits, or telephone appointments, done according to the pre-established protocol.
There were total of 3,856 patients included in the DESIRE registry, conducted from January of 2007 through to October of 2014. From the 3,856 patients, there were 1,280 patients that were diabetic (33.2%). 414 (62.3%) of these patients were assigned and treated with SES and 384 patients were implanted with EES. For the breakdown of the population group, most patients were male, with a mean age of 61.7 ± 4.3 years vs. 62.1 ± 4.6 years. There was no significant difference in population between the insulin dependent and non- insulin diabetes group. Most of the patients in the study that had undergone PCI, had a stable CAD. 99.4% of the study population had a follow up.
Overall, diabetic patients receiving 2nd generation DES, EES, tend to show lower cardiac events, lower mortality and reduced target lesion revascularization (5.3% vs. 1.3%, p value of lesser than 0.01 for the rate of mortality and 3.4% vs. 1.3% with p value equal to 0.06, for the target lesion revascularization). In conclusion, the primary finding of the DESIRE registry revealed that the EES, which is a 2nd generation EES, proved to be a superior DES than the 1st generation DES, as it showed a better safety profile, lesser mortality, lower cardiac adverse events, lesser stent thrombosis and better efficacy when compared to SES, in a diabetic patient(31) .
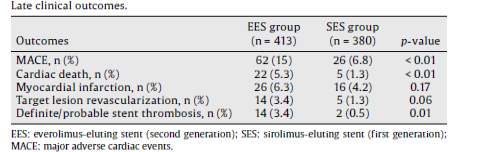
Table 2: Showing late clinical outcomes of the diabetic patients treated with Sirolimus or Everolimus DES: an analysis of the DESIRE registry
Critical Analysis
This was a prospective, non- randomized trial, performed for 7 years in 1,280 diabetic patients, at 1 centre. The aim of the study was to compare the 1st and the 2nd generation DES in PCI performed on diabetic patient. It had some limitations due to it being non-randomized hence it could be prone to pitfalls such as bias by the clinician performing the study. The study also didn’t compare with a BMS group.
However, it had a good inclusion criteria where all diabetic patients both insulin dependent and non-insulin dependent patients were included. The study group were well diverse and the end points of the study were well objected. Although there were minor flaw in this study, it is a very good, comprehensive and well-constructed study with a large study group, and a significant result showing that the diabetic patient that received the first generation of DES were more prone to thrombosis and adverse cardiac events.

Figure 2: Algorithm for classifying study design for questions of effectiveness
The risk ratio and odds of the study were then calculated using the Risk Calculator found on SIGN website, done via an Excel programme. From the study, there were total of 414 patients in the EES group with 62 patients suffering from MACE outcome and total of 384 patients in the SES group with 26 suffering from MACE outcome. Calculated absolute risk for the MACE endpoint outcome in the EES group was 0.15, absolute risk in the SES group was 0.07 with absolute risk reduction (ARR) of 0.08. The calculated relative risk (RR) and relative risk reduction were 0.45 and 0.55 respectively. The odds in the EES group was 0.18, odds in the SES group was 0.07 and the odd ratio was 0.41. The calculated number needed to treat was 12.19.
Other endpoints’ risks and odds were also calculated. In the study, there were total of 414 patients in the EES group with 22 patients suffering from cardiac death and total of 384 patients in the SES group with 5 suffering from cardiac death outcome. Calculated absolute risk for the cardiac death endpoint outcome in the EES group was 0.05, absolute risk in the SES group was 0.01, with an ARR of 0.04. The calculated RR and relative risk reduction were 0.25 and 0.75 respectively. The odds in the EES group was 0.06, odds in the SES group was 0.01 and the odd ratio was 0.24. The calculated number needed to treat was 24.93. For MI as endpoint, there were total of 414 patients in the EES group with 26 patients suffering from MI and total of 384 patients in the SES group with 16 suffering from MI. Calculated absolute risk for the MI endpoint outcome in the EES group was 0.06, absolute risk in the SES group was 0.04, with an ARR of 0.02.
The calculated RR and relative risk reduction were 0.66 and 0.34 respectively. The odds in the EES group was 0.07, odds in the SES group was 0.04 and the odd ratio was 0.65. The calculated number needed to treat was 47.31. For target lesion revascularization as endpoint, there were total of 414 patients in the EES group with 14 patients suffering from target lesion revascularization and total of 384 patients in the SES group with 5 suffering from target lesion revascularization. Calculated absolute risk for the target lesion revascularization endpoint outcome in the EES group was 0.03, absolute risk in the SES group was 0.01, with an ARR of 0.02. The calculated RR and relative risk reduction were 0.36 and 0.61 respectively. The odds in the EES group was 0.04, odds in the SES group was 0.01 and the odd ratio was 0.38. The calculated number needed to treat was 48.09. For definite/probable stent stenosis as endpoint, there were total of 414 patients in the EES group with 14 patients suffering from definite/probable stent stenosis and total of 384 patients in the SES group with 2 suffering from definite/probable stent stenosis. Calculated absolute risk for the target lesion revascularization endpoint outcome in the EES group was 0.03, absolute risk in the SES group was 0.01, with an ARR of 0.03. The calculated RR and relative risk reduction were 0.15 and 0.85 respectively. The odds in the EES group was 0.04, odds in the SES group was 0.01 and the odd ratio was 0.15. The calculated number needed to treat was 34.96.
In conclusion, in this one centre trial, the use of 2nd generation EES in all diabetic patients was effective and safe, with lower rates of cardiac mortality and stent thrombosis, significantly, in contrast to the 1st generation DES, SES.
Study: Comparison of First- and Second-Generation Drug-Eluting Stents in an All-Comer Population of Patients with Diabetes Mellitus (from Katowice Zabrze Registry)
Literature Review
This Katowice Zabrze Registry study matched the safety as well as the efficacy between the 1st generation DES and 2nd generation DES, in an unhampered, true, two centre population of diabetic patients, who were undergoing PCI. The study is a prospective as well as retrospective, non-randomized study performed at a two tertiary cardiac centres (Upper Silesian Medical Centre in Katowice and 2nd Department of Cardiology, Zabrze).
The study was performed from 1st January 2009 to 31st January 2010. The study also retrospectively included all patients in the enrolling centre whose medical records showed that they are diabetic and had undertaken a PCI procedure with the implantation of either 1st generation or a 2nd generation DES.
The main object of interest for this Katowice Zabrze Registry study were patients suffering from Diabetes Mellitus and CAD. Stents that were implanted in these diabetic patients weren’t randomized but were rather chosen according to the operator’s decision. This depended on what the current best practice was, the operator’s expertise with type of stent, their preferences as well as their knowledge into what type of stent would suit the lesion type best.
The option of the stent were either 1st generation DES, made from a durable polymer, such as PES (Taxus, Boston Scientific Corporation, Maple Grove, MN, USA; LucChopin1, LucChopin2, Balton, Poland) or SES (Cypher, Cordis, USA; Carlo, CarloS, Balton, Poland) or a 2nd generation DES, such as EES (Promus, Boston Scientific Corporation; Xience, Xience Prime, Abbott Vascular, Santa Clara, CA, USA), or ZES (Endeavor, Resolute, Medtronic, Minneapolis, MN, USA), or Biolimus-eluting stent (Biolimus A9, Biosensors International, Switzerland).
If there should be an occurrence of implantation of more than one stent in one patient, the DES embedded to the lesion with the more serious stenosis was considered as the principle stent and incorporated into the review.
Diabetic patients that underwent the PCI procedure were then recommended to receive a dual antiplatelet therapy consisting of both Acetylsalicylic acid and P2Y12 subtype of ADP receptor inhibitors for 12 months. Baseline information regarding the clinical data and the procedure were then collected retrospectively from medical records. Patients were then followed up at 1 year. The primary efficacy endpoint was a combination of major adverse cardiac and cerebrovascular events (MACCE), including all deaths, non-fatal MI, target vessel revascularization and stroke, in patients involved in this study. The secondary endpoints were singular segments of the primary endpoint, which again included all cause death, MI, target vessel revascularization, stroke, and patients that needed CABG. DES’s safety were characterized as gastrointestinal bleeding and a definite stent thrombosis, either acute, late or collective at the end of one year.
There were total of 1916 patients involved in the study, with 717 patients that were diabetic (37%). Of these diabetic patients, 36% or 257 patients were implanted with 1st generation DES (230 received PES and 27 patients received SES). The rest 64% or 460 patients received 2nd generation DES, with 46 patients implanted with Biolumus eluting stents, 253 patients implanted with EES and 171 patients implanted with ZES.
Both groups that received either the 1st or the 2nd generation DES, had a comparable standard demographic profiles, with similar cardiovascular risk factors. In view of diabetic patients from the Katowice Zabrze registry in a real life setting, the implantation of 2nd generation DES ended up being similarly effective and to be safer in contrast to the original 1st generation DES. The pattern for lower rates of stent thrombosis in 2nd generation DES was most articulated right on time after the stent implantation and was maintained for up to a period of 1 year.
Diabetes mellitus is known to heighten and increase the danger of stent thrombosis and restenosis after PCI, which already aggravates eosinophilia, by advancing neo-intimal hyperplasia, smooth muscle cell production, raised platelet reactivity, increase local inflammation, and plaque development. In this study, it is shown that 2nd generation DES were more secure with a better safety profile than original, 1st generation DES and essentially decreases the rate of stent thrombosis. This review affirms superiority of 2nd generation DES when compared to 1st generation DES, in patients with diabetes mellitus as far as stent thrombosis is concerned (29).
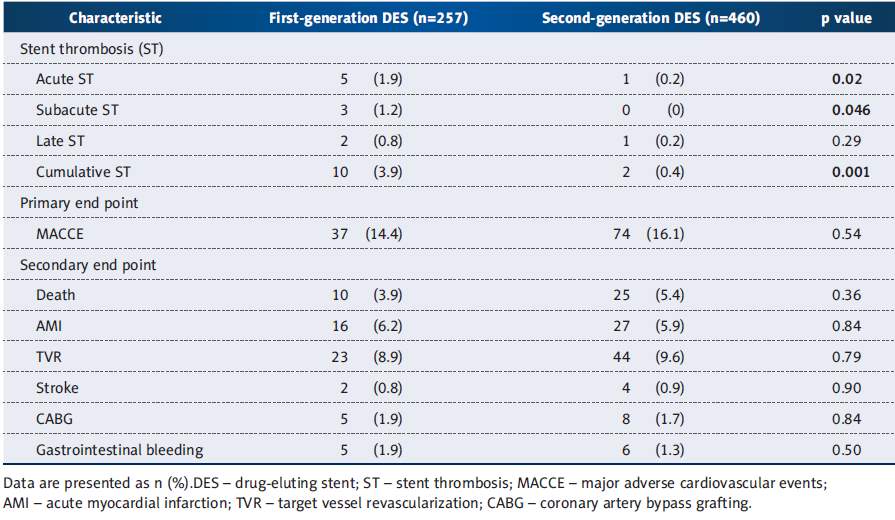
Table 3: Clinical Outcomes at one year (10)
Critical Analysis
This was a prospective as well as a retrospective, non- randomized trial, performed for 1 year in which 717 patients were diabetic, performed at 2 centres. The aim of the study was to compare the 1st and the 2nd generation DES in PCI performed on diabetic patient. The result showed that the rate of major adverse cardiac event were equal in both groups (p value = 0.54). The 2nd generation DES were safer than the 1st generation DES (log-rank for cumulative stent thrombosis at 1 year p value lesser than 0.001).
The study had some limitations due to it being non-randomized hence it could be prone to pitfalls such as bias by the clinician performing the study. The study also didn’t compare with a BMS group. However, it had a good inclusion criteria where all diabetic patients both insulin dependent and non-insulin dependent patients were included. The study group were well diverse and the end points of the study were well objected. Although there were minor flaw in this study, it is a very good, comprehensive and well-constructed study with a large study group, and a significant result showing that the diabetic patient that received the 1st generation of DES were more prone to thrombosis.
The limitations of the study was that there could have been more patients with insulin dependent diabetes. Accepting that insulin dependent diabetes incites more weakened vessels and has more negative in situ impacts and plaque load on the vessels due to the insulin resistance, the separation between insulin dependent and non-insulin dependent diabetes patients could advance the review and give extra direction on ideal decision amongst first and second generation stent for PCI in each setting. In any case, this was not done on the grounds that the trial wanted as many diabetic patients as possible with a diverse group of patients.
In light of the sub-analysis of diabetic patients from the Katowice-Zabrze registry in a real life setting, the patient that received 2nd generation DES during PCI procedure, ended up being similarly efficient and to have better safety profile when compared with the original 1st generation DES. The trend for lower rates of stent thrombosis in 2nd generation DES was most distinct right after stent were implanted and the trend was sustained for up to 1 year (10).
In conclusion, this present trial adds to the available data on safety and efficacy of various types of stents used for PCI in Diabetes Mellitus patients, leading to the conclusion that the usage of the second generation DES, all things considered, in the setting of diabetes, is invaluable and beneficial in terms of safety of the procedure, especially early after stent placement. In light of this, patients with diabetes, after implantation of 1st generation DES should undergo a consistent follow up, especially early on after the procedure, focused on signs and symptoms that are suggestive of stent thrombosis, as the study concludes that they are at higher risk. This review likewise proposes that the implantation of 2nd generation of DES ought to be considered for each case of PCI in diabetic patients so as to decrease the rate of stent thrombosis.
Therefore, in this two centre trial, the use of second-generation EES in unselected diabetic patients was effective and safe, with lower rates of stent thrombosis when compared to first-generation of DES, significantly (10).
Study: Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes; Taxus Element versus Xience Prime in a Diabetic Population
Literature Review
The Taxus Element versus Xience Prime in a Diabetic Population (TUXEDO)–India study was performed to see if Paclitaxel, a 1st generation DES, is non inferior to Everolimus, a 2nd generation DES. The TUXEDO study was published in New England Journal of Medicine on October 14, 2015. It is an investigator initiated, multicentre, randomized clinical trial. Patients were thought to be qualified on the prospect that they had diabetes mellitus and are either having symptomatic coronary artery diseases or a silent ischemia.
The angiographic criteria vital for meeting the criterion to be included in this study were lesions 34 mm or less in length and a reference vessel diameter between 2.25 mm and 4.0 mm (determined by visual estimation from angiograms). Although it was a randomized trial, the staff performing the procedure had knowledge about what type of stent would be implanted. All patients received some medications before the PCI. This included oral aspirin of 350 mg dose as well as a loading dose of clopidogrel of 600 mg, prasugrel of 60 mg, or ticagrelor of 180 mg, either one of those. Patients were asked to continue to take aspirin of 75 to 150 mg daily plus clopidogrel (at least 75 mg daily), prasugrel (10 mg daily), or ticagrelor (90 mg twice daily) for a minimal of 1 year after the stent were implanted in the PCI procedure. The decision of the second antiplatelet medication and the specifics of the PCI procedure were left to the circumspection of the clinician. Follow up for these patients that underwent DES implantation were arranged at 30 days, 6 months, 1 year and at 2 years.
There were total of 1830 patients with diabetes and CAD in the study with the study taking place between June 23, 2011, and March 12, 2014, with 46 centres participating in the study. The primary end point of this TUXEDO trial was target vessel failure at 1 year and the target vessel failure was defined as a combination of cardiac death, MI, or target vessel revascularization. The secondary end points were ischemia driven target lesion revascularization, target vessel revascularization, the combination of cardiac death or target vessel MI, major adverse cardiac events (a combination of cardiac death, MI, or target lesion revascularization), cardiac death, non-cardiac death, all death from any cause, and stent thrombosis. The end point of the procedure were the rate of success of the PCI procedure. The study was powered to detect non inferiority with respect to the primary end point of target vessel failure at the end of 1 year. A total of 1783 patients (97.4%) completed the 1 year follow up.
In the intention to treat analysis, PES did not meet the standard for non-inferiority to EES with respect to target vessel failure at 1 year (p value = 0.38 for non-inferiority). This study was performed to test the superiority of EES to PES, in respect to the primary end points, and EES was found to be superior to PES, in regards to the target vessel failure (relative risk, 0.53; 95% CI, 0.33 to 0.83; P value= 0.005). At 1 year, patients assigned to receive a PES had significantly higher rates of ischemia driven target vessel revascularization and target lesion revascularization than did patients that were implanted with EES (3.4% vs. 1.2% for both end points, p value equal to 0.002), as well as significantly higher rates of spontaneous MI (3.2% vs. 1.2%, p value equal to 0.004), stent thrombosis (2.1% vs. 0.4%, p value equal to 0.002), cardiac death or MI (4.0% vs. 2.3%, p value equal to 0.03), and major adverse cardiac events (5.9% vs. 3.4%, p value equal to 0.01). No vast contrast were detected between the two treatment groups that received different DES, with respect to other outcomes. However, from this study, the degree of technical success and procedural accomplishment were equal between the two groups (33).
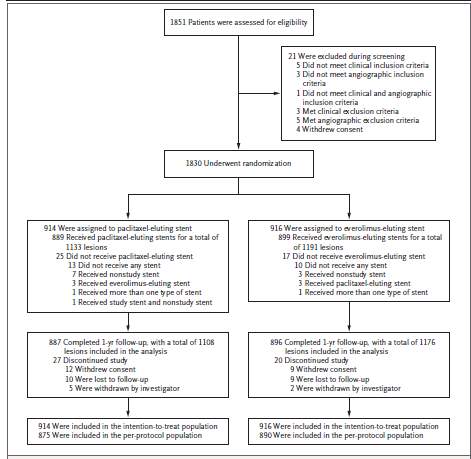
Figure 3: Screening, Randomization and Follow-up in TUXEDO study
Critical Analysis
This was a prospective, randomized trial, performed from June 23, 2011, to March 12, 2014 in which 1851 diabetic patients were included, performed at forty six sites. From the 1851 patient group, 20 were excluded as they did not meet the inclusion criteria group or withdrew their consent. The remaining 1830 were randomly assigned to two groups, one receiving Paclitaxel and the other group receiving Everolimus. The aim of the study was to compare the 1st and the 2nd generation DES in PCI performed on diabetic patient. The result revealed that PES was not non inferior to the EES in respect to the primary outcome of target vessel failure (p value 0.005). The results for secondary outcomes were constant with the result of the primary outcome, with higher rates of MI, stent thrombosis, target vessel revascularization, target lesion revascularization, cardiac death and major adverse cardiac events in the PES group than in the EES group.
The study was quite well designed as it was randomized hence it was not prone to pitfalls such as bias by the clinician performing the study. It had a good inclusion criteria where all diabetic patients both insulin dependent and non-insulin dependent patients were included. The study group were well diverse and the end points of the study were well objected. Although there were minor flaw in this study, it is a very good, comprehensive and well-constructed study with a large study group, and a significant result showing that the diabetic patient that received the first generation of DES were more prone to stent thrombosis. However, the study didn’t compare with a BMS group, missing out on a major opportunity to increase the significance to the study. Also, this study was funded by Boston Scientific which is the manufacturer of the Paclitaxel eluting stent. Although the study was designed to have no bias between the two, there still maybe some bias due to the conflict in interest.
The study also tried to compare between the insulin dependent diabetes group and non-insulin dependent diabetes group as insulin resistant is a risk factor for damaged vessels and has more negative in situ impacts and plaque load on the vessels due to the insulin resistance. In the subgroup analysis between the insulin dependent (forty percent of the patient population in the study group) and non-insulin dependent study (sixty percent of the patient population in the study group), there were significant results between the two groups. This result differs from all the other meta-analysis, such as the SPIRIT V study (34), which shows trend that patients who are diabetic on insulin tends to do worse with worse outcome in Everolimus eluting stents group compared to the Paclitaxel eluting stents group.
In previous studies, as well as meta-analysis of other studies, the results were conclusive that there were lower rates of death, myocardial infarction, stent thrombosis, and ischemia driven target lesion revascularization with Everolimus eluting stents than with Paclitaxel-eluting stents in patients without diabetes mellitus. However, in this study involving the diabetic patients, there was no significant difference in regards to any efficacy or safety outcome. Other meta-analysis involving diabetic patients also showed Everolimus stent to be most efficacious with lower rate of restenosis, myocardial infarction, and stent thrombosis.
In conclusion, this TUXEDO trial in India, involving 46 sites and 1831 population group of patients with diabetes mellitus, did not show any non-inferiority of Paclitaxel eluting stents to the Everolimus eluting stents in patients with coronary artery disease and diabetes mellitus. Everolimus eluting stents were superior to paclitaxel-eluting stents in regards to several end points, including target vessel failure, myocardial infarction, and stent thrombosis. (33).
Study: Clinical Impact of Second Generation Everolimus Eluting Stent Compared With First Generation Drug Eluting Stents in Diabetes Mellitus Patients; Insights from a Nationwide Coronary Intervention Register
Literature Review
Although there has been many studies that focuses on the significant advantage of 2nd generation DES when compared to the 1st generation, all these studies were focused on non-diabetic population. Limited data are available regarding the comparison of first generation and second generation DES. Hence, this study was performed to compare the second generation EES to first-generation SES and PES in diabetes mellitus patients.
This trial analyses all diabetic patients that received PCI from January 18, 2007, up to July 27, 2011. The information was gathered from the Swedish Coronary Angiography and Angioplasty Register (SCAAR database) that has information on 29 centres where PCI are performed, in Sweden. The PCI performed in Sweden, with 13,830 (19.3 %) being diabetic patients out of a total of 71,639 patients. There were total of 110,610 stents implanted. Out if these 21,962 (19.8%) were stents implanted in diabetic patients. Out of the 21,962 stents implanted in diabetic patients, 11,493 were BMS, 10,422 were DES and 47 were not classified as either one of them or had missing data. From the 10,422 implanted stents, 2,836 were PES with 1,370 implanted SES, and 3,929 were EES. The remaining 2,258 stents represent Biolimus eluting stent and ZES, which is not analysed in this study.
All the PCI procedure, follow up and adverse outcomes were logged into the web based nationwide SCAAR database, that made the follow up more continuous with greater quality of care. The primary end point of this study was a combined safety and efficacy of the stent, mortality, stent thrombosis and restenosis at 1 year. For this present study, the EES was compared with PES and SES in diabetes mellitus patient. Diabetes was either confirmed by patient who has been previously diagnosed, or if the patient was using antidiabetic drug before the PCI procedure.
This study defines restenosis as more than 50% stenosis estimated visually during angiogram by fractional flow reserve value of 0.80 in a previously stented patient. The clinical significance of re-stenosed lesions were identified by symptoms, routine non-invasive functional testing which includes an exercise test or nuclear scan and/or an invasive functional evaluation by fractional flow reserve, may be needed. Other studies used target lesion revascularization as their endpoint, however, this study used the above method to detect restenosis. The target lesion revascularization endpoint can cause bias as it pools revascularizations performed due to restenosis as well as stent thrombosis, especially in high risk patients receiving 1st generation DES. In SCAAR registry, stent thrombosis is described as occlusion seen on angiogram or non-occlusive thrombus in a stent previously implanted. All data regarding the mortality were obtained from Nation Population Death Registry, Sweden. In total there were 4,751 diabetic patients in this study. In total these patients received 8,134 DES with 3,928 being EES, 2,836 as PES, and 1,370 being SES.
This study showed that patient that received EES had significantly lower rates of adverse cardiac event when contrasted with SES. A comparable trend were also seen when EES were compared with PES. There was no substantial variances seen in the insulin dependent diabetes compared with the non-insulin dependent diabetes but an inclination towards better outcomes was seen in these non- diabetic stented with SES and PES.
This study concluded that patients stented with EES had lower mortality in contrast to patients implanted with SES or PES. This trend was maintained irrespective of whether the patient was an insulin treated diabetic patient or not. However, there were no significant differences seen in rate of restenosis in the EES, SES or PES group in all diabetic patients. There was tendency for improved outcome in non-insulin diabetic patient receiving EES compared to SES (35).
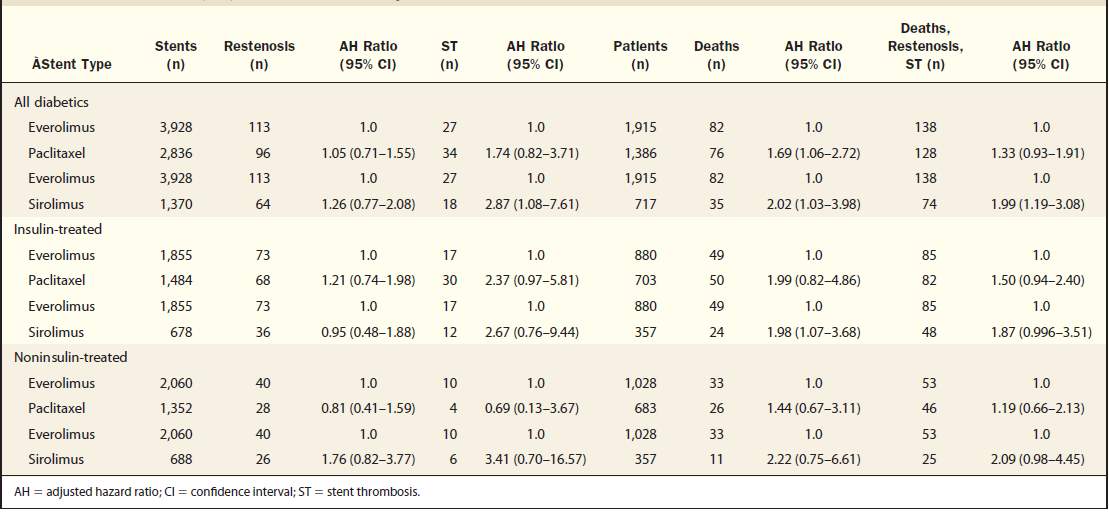
Table 4: Result from the clinical impact of second generation Everolimus eluting stent compared with first generation drug eluting stents in diabetes mellitus patients; insights from a nationwide coronary intervention register
Critical Analysis
This was a retrospective qualitative study where data were collected from the SCAAR registry in Sweden for patients that underwent PCI. The study was conducted for a period of 5 years and the study had 4,751 diabetic population with a total of 8,134 stents. 3,928 of the patients received EES with 2,836 receiving PES and 1,370 receiving SES. This was the largest study that compared the 1st generation DES to the 2nd generation DES. It revealed that all diabetic patients receiving EES, a 2nd generation DES, had a lower rate of restenosis, lower rate of mortality, and lower rate of stent thrombosis significantly, especially when compared with SES, a 1st generation DES. However, even with the large population of the study, it couldn’t detect any significant difference between EES and PES.
The study was quite well designed with a good inclusion criteria where all diabetic patients both insulin dependent and non-insulin dependent patients were included. The study group were well diverse and the end points of the study were well objectified. Although there were minor flaw in this study, it is a very good, comprehensive and well-constructed study with a large study group, and a significant result showing that the diabetic patient that received the 2nd generation of DES were of lesser chance of having stent thrombosis, or cardiac adverse event. The study also made a point of comparing the insulin dependent and non-insulin dependent group. Even though it was not a randomized trial and may be prone to clinical technician bias, it collected data from a very thorough and large database.
The main limitation of this study was that it was a retrospective study, lacking randomization, hence prone to bias. It also mainly involved only Swedish population, therefore, lacking ethnicity diversity (35).
Second- versus first-generation drug-eluting stents for diabetic patients: a meta-analysis
Literature Review
This study is a meta-analysis, systemic literature review study that looks into 1st generation DES compared with 2nd generation DES, in diabetic population. The study explored the PubMed, EMBASE and Cochrane databases, up to July 2013 using these keywords; “everolimus-eluting stent, zotarolimus-eluting stent, second-generation eluting stent, sirolimus-eluting stent, paclitaxel-eluting stent, first-generation eluting stent, diabetes, diabetic, human, random”.
The inclusion criteria composed of randomized controlled trials, with studies that had a follow up of at least 6 months period. All studies comparing BMS to DES were excluded as well as all the retrospective studies, even if randomized, were excluded (36).
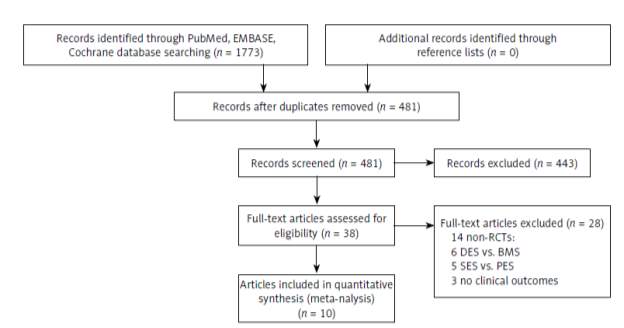
Figure 4: Flowchart of selection of studies for inclusion
From the results yielded, this meta-analysis looked into 10 trials, with a total of 4,503 patients. This meta-analysis mainly looked into EES compared to SES, and the meta-analysis concludes that EES significantly lowered mortality (p value = 0.01), lowered the risk of stent thrombosis (p value = 0.03). Furthermore, the 2nd generation DES, EES, also showed a trend in reducing the rate of recurrence in MI in contrast to the PES (p value = 0.08), a 1st generation DES. However, ZES, a 2nd generation DES, was observed to have higher rates of stent thrombosis, and greater risk of target lesion revascularization, when compared to SES (both with p value less than 0.05) (36).
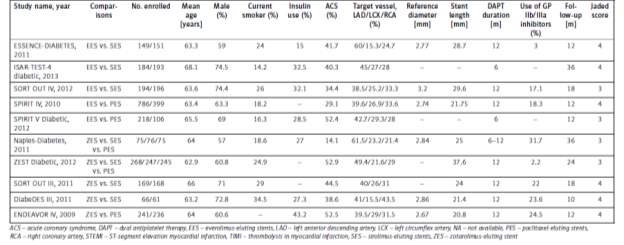
Table 5: Baseline characteristics of randomized controlled trials included in the meta-analysis
Critical Analysis
This meta-analysis studied 10 trials, with a total of 4,503 patients, using PubMed, Cochrane and EMBASE database. The aim of the meta-analysis was to compare the 1st and the 2nd generation DES in PCI performed on diabetic patients. All studies confirmed that second generation DES had a better safety profile than first generation DES. It had a good inclusion criteria where all diabetic patients both insulin dependent and non-insulin dependent patients were included as well as inclusion criteria of only studies that compared DES with one another, excluding the BMS group. The meta-analysis also only included randomized controlled trials, thus reducing clinician bias that can occur is studies with non-randomization. From this meta-analysis, it is concluded that 2nd generation EES, has a better efficacy as well as significantly reduced rate of mortality, when compared to SES, a 1st generation DES. EES also showed a trend in reducing stent thrombosis, as well as rate of re-infarction. However, in this meta-analysis, it is revealed that ZES, another 2nd generation DES, is associated with greater risk of target lesion revascularization as well as stent thrombosis, in contrast to 1st generation DES, especially SES. Therefore, this meta-analysis suggests that EES should be the recommended DES implanted primarily in all diabetic patients, when undergoing a PCI procedure (36).
Discussion and Conclusion
Diabetes is one of the risk factor for developing CAD, with increasing epidemics in the first world country. Diabetes also increases the risk of stent thrombosis and target lesion revascularization, which eventually leads to stent failure concluding in either another stent placement, cardiac death, MI or CABG. However, there is still a relatively small number of studies done to compare between the 1st generation and the 2nd generation DES in a diabetic population. Even with studies available, there seems to be a conflicting results. The NICE guideline that is the basis of clinical guidance in United Kingdom, still does not address this issue and does not specify which DES to be used, but rather when a DES should be considered over a BMS. Hence, most diabetic patients are at risk of having an under optimal treatment when undergoing PCI. The current dissertation addresses this issue and helps as well as update the clinicians on recent studies done comparing the 1st generation and 2nd generation DES, in the diabetic population. This will help in making more informed decision of what DES should be considered in this sub-group of population. This dissertation looks into 5 studies, 3 non-randomized, 1 randomized and 1 meta-analysis.
The first study, DESIRE registry, is a non- randomized trial, performed for 7 years in 1,280 diabetic patients, at 1 centre. 414 of the patients were implanted with SES and 384 patients were implanted with EES. The study revealed a significant result showing that the diabetic patient that received the 1st generation of DES were more prone to thrombosis and adverse cardiac events. 2nd generation EES also had a better safety profile, lesser mortality, lower cardiac adverse events, lesser stent thrombosis and better efficacy. From the study, 62 patients in the EES group suffered from MACE outcome and 26 in the SES group suffered from MACE outcome.
The second study, Katowice Zabrze Registry, was a prospective non-randomized trial performed at a two tertiary cardiac centres and occurred from 1st January 2009 to 31st January 2010. The study involved 1916 patients, with 717 patients that were diabetic. Of these, 257 patients were implanted with 1st generation DES (230 received PES and 27 patients received SES) with the rest 460 patients receiving 2nd generation DES, (46 received Biolumus eluting stents, 253 received EES and 171 received ZES). In this study, it is shown that 2nd generation DES had a better safety profile than 1st generation DES and essentially decreases the rate of stent thrombosis. The result showed that the rate of major adverse cardiac event were equal in both groups.
The third study, TUXEDO registry, was performed to see if Paclitaxel, is non inferior to Everolimus. It is an investigator initiated, multicentre, randomized clinical trial with total of 1830 patients (914 implanted with PES and 916 implanted with EES), taking place between June 23, 2011, and March 12, 2014, with 46 centres participating in the study. At 1 year, patients implanted with PES had significantly higher rates of ischemia driven target vessel revascularization and target lesion revascularization than did patients that were implanted with EES as well as significantly higher rates of spontaneous MI, stent thrombosis, cardiac death and major adverse cardiac events. The result revealed that PES was inferior to the EES in respect to the primary outcome of target vessel failure (p value 0.005). The results for secondary outcomes were constant with the result of the primary outcome, with higher rates of MI, stent thrombosis, target vessel revascularization, target lesion revascularization, cardiac death and major adverse cardiac events in the PES group than in the EES group.
The fourth study, an Insights from a Nationwide Coronary Intervention Register, was the largest study, done to compare the EES to SES and PES in diabetes mellitus patients, and performed from January 18, 2007, up to July 27, 2011. It was a retrospective study that collected data from the SCAAR database that had information on 29 centres in Sweden. In total there were 3,928 implanted EES, 2,836 as PES, and 1,370 being SES. This study showed that patient that received EES had significantly lower rates of adverse cardiac event when contrasted with SES. This study concluded that patients stented with EES had lower mortality in contrast to patients implanted with SES or PES. It revealed that all diabetic patients receiving EES, a 2nd generation DES, had a lower rate of restenosis, lower rate of mortality, and lower rate of stent thrombosis significantly, especially when compared with SES, a 1st generation DES. However, even with the large population of the study, it couldn’t detect any significant difference between EES and PES.
The fifth study was a meta-analysis that looked into 10 trials, with a total of 4,503 patients. This meta-analysis mainly looked into EES compared to SES, and the meta-analysis concludes that EES significantly lowered mortality, lowered the risk of stent thrombosis. Furthermore, the 2nd generation DES, EES, also showed a trend in reducing the rate of recurrence in MI in contrast to the PES, a 1st generation DES. However, ZES, a 2nd generation DES, was observed to have higher rates of stent thrombosis, and greater risk of target lesion revascularization, when compared to SES concluded that 2nd generation EES, has a better efficacy as well as significantly reduced rate of mortality, when compared to SES, a 1st generation DES. EES also showed a trend in reducing stent thrombosis, as well as rate of re-infarction. However, in this meta-analysis, it is revealed that ZES, another 2nd generation DES, is associated with greater risk of target lesion revascularization as well as stent thrombosis, in contrast to 1st generation DES, especially SES.
In conclusion, there is a significant trend that 2nd generation of DES, especially EES, has improved outcome compared to 1st generation DES, in reducing stent thrombosis, major adverse cardiac outcomes, MI and cardiac death, in the diabetic population. Hence, it should be the DES of choice in diabetic patients.
References
Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Annals of Translational Medicine. 2016;4(13):256-256.
What causes coronary heart disease? [Internet]. BBC Science. 2017 [cited 19 April 2017]. Available from: http://www.bbc.co.uk/science/0/21686950
Stable Angina Pectoris – Doctor’s Information [Internet]. Patient.info. 2017 [cited 19 April 2017]. Available from: https://patient.info/doctor/stable-angina-2
Heart Attack information. Signs of a heart attack at Patient [Internet]. Patient.info. 2017 [cited 19 April 2017]. Available from: https://patient.info/in/health/heart-attack-myocardial-infarction-leaflet
Thames M, Sease D, Damien A. Ischemic Heart Disease: an overview. Johns Hopkins Advanced Studies in Medicine. 2004;4 (10B):794-802.
Epstein S, Waksman R, Pichard A, Kent K, Panza J. Percutaneous Coronary Intervention Versus Medical Therapy in Stable Coronary Artery Disease. JACC: Cardiovascular Interventions. 2013;6(10):993-998.
Iqbal J, Serruys P. Revascularization strategies for patients with stable coronary artery disease. Journal of Internal Medicine. 2014;276(4):336-351.
Head S, Kieser T, Falk V, Huysmans H, Kappetein A. Coronary artery bypass grafting: Part 1–the evolution over the first 50 years. European Heart Journal. 2013;34(37):2862-2872.
National Institute for Health and Care Excellence. Drug-eluting stents for the treatment of coronary artery disease. NICE Guidance; 2008.
Cassagnol M, Saad M. Drug-eluting Stents. 1st ed. Medscape. Medscape; 2012.
Holmes D. Analysis of 1-Year Clinical Outcomes in the SIRIUS Trial: A Randomized Trial of a Sirolimus-Eluting Stent Versus a Standard Stent in Patients at High Risk for Coronary Restenosis. Circulation. 2004;109(5):634-640.
Morice M, Serruys P, Costantini C, Wuelfert E, Wijns W, Fajadet J et al. Two-year follow-up of the RAVEL study: A randomized study with the sirolimus-eluting Bx VELOCITY stent in the treatment of patients with denovo native coronary artery lesions. Journal of the American College of Cardiology. 2003;41(6):32.
Kelbæk H, Thuesen L, Helqvist S, Kløvgaard L, Jørgensen E, Aljabbari S et al. The Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) Trial. Journal of the American College of Cardiology. 2006;47(2):449-455.
Gruberg L. RESEARCH Registry: Sirolimus-Eluting Stents for Treatment of In-Stent Restenosis in the Real World — Preliminary Results. 1st ed. Medscape.
Silber S, Colombo A, Banning A, Hauptmann K, Drzewiecki J, Grube E et al. Final 5-Year Results of the TAXUS II Trial: A Randomized Study to Assess the Effectiveness of Slow- and Moderate-Release Polymer-Based Paclitaxel-Eluting Stents for De Novo Coronary Artery Lesions. Circulation. 2009;120(15):1498-1504.
Stone G. One-Year Clinical Results With the Slow-Release, Polymer-Based, Paclitaxel-Eluting TAXUS Stent: The TAXUS-IV Trial. Circulation. 2004;109(16):1942-1947.
Ormiston J, Charles O, Mann T, Hall J, McGarry T, Cannon L et al. Final 5-year results of the TAXUS ATLAS, TAXUS ATLAS Small Vessel, and TAXUS ATLAS Long Lesion clinical trials of the TAXUS Liberté paclitaxel-eluting stent in de-novo coronary artery lesions. Coronary Artery Disease. 2013;24(1):61-68.
Navarese E, Kowalewski M, Kandzari D, Lansky A, Górny B, Kołtowski Ł et al. First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients. Open Heart. 2014;1(1):e000064.
SPIRIT FIRST Clinical Trial of the Abbott Vascular XIENCE V® Everolimus Eluting Coronary Stent System – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 19 April 2017]. Available from: https://clinicaltrials.gov/ct2/show/NCT00180453
SPIRIT II: A Clinical Evaluation of the XIENCE V® Everolimus Eluting Coronary Stent System – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 19 April 2017]. Available from: https://clinicaltrials.gov/ct2/show/NCT00180310
SPIRIT III Clinical Trial of the XIENCE V® Everolimus Eluting Coronary Stent System (EECSS) – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 19 April 2017]. Available from: https://clinicaltrials.gov/ct2/show/NCT00180479
SPIRIT IV Clinical Trial: Clinical Evaluation of the XIENCE V® Everolimus Eluting Coronary Stent System – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 19 April 2017]. Available from: https://clinicaltrials.gov/ct2/show/NCT00307047
Steinberg D, Townsend J, Rideout P. Everolimus-eluting stents in interventional cardiology. Vascular Health and Risk Management. 2012;:393.
Kandzari D, Leon M, Meredith I, Fajadet J, Wijns W, Mauri L. Final 5-Year Outcomes From the Endeavor Zotarolimus-Eluting Stent Clinical Trial Program. JACC: Cardiovascular Interventions. 2013;6(5):504-512.
The ENDEAVOR II Clinical Trial: The Medtronic Endeavor Drug Eluting Coronary Stent System in Coronary Artery Lesions – Full Text View – ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2017 [cited 19 April 2017]. Available from: https://clinicaltrials.gov/ct2/show/NCT00614848
Eisenstein E, Leon M, Kandzari D, Mauri L, Edwards R, Kong D et al. Long-Term Clinical and Economic Analysis of the Endeavor Zotarolimus-Eluting Stent Versus the Cypher Sirolimus-Eluting Stent. JACC: Cardiovascular Interventions. 2009;2(12):1199-1207.
Maeng M, Tilsted H, Jensen L, Kaltoft A, Kelbæk H, Abildgaard U et al. 3-Year Clinical Outcomes in the Randomized SORT OUT III Superiority Trial Comparing Zotarolimus- and Sirolimus-Eluting Coronary Stents. JACC: Cardiovascular Interventions. 2012;5(8):812-818.
Jang S, Park D, Kim W, Kim Y, Yun S, Kang S et al. Differential long-term outcomes of zotarolimus-eluting stents compared with sirolimus-eluting and paclitaxel-eluting stents in diabetic and nondiabetic patients: Two-year subgroup analysis of the ZEST randomized trial. Catheterization and Cardiovascular Interventions. 2013;81(7):1106-1114.
Kawecki D, Morawiec B, Dola J, Wańha W, Smolka G, Pluta A et al. Comparison of First- and Second-Generation Drug-Eluting Stents in an All-Comer Population of Patients with Diabetes Mellitus (from Katowice-Zabrze Registry). Medical Science Monitor. 2015;21:3261-3269.
Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison’s Principle of Internal Medicine. 19th ed. McGraw-Hill Medical; 2015.
Costa J, Sousa A, Moreira A, Costa R, Maldonado G, Cano M et al. Late clinical outcomes of diabetic patients treated with sirolimus or everolimus drug-eluting stents: an analysis of the DESIRE registry. Revista Brasileira de Cardiologia Invasiva (English Edition). 2015;23(1):17-21.
Mak K. Drug eluting stents for patients with diabetes. BMJ. 2012;345(sep12 1):e5828-e5828.
Kaul U, Bangalore S, Seth A, Arambam P, Abhaichand R, Patel T et al. Paclitaxel-Eluting versus Everolimus-Eluting Coronary Stents in Diabetes. New England Journal of Medicine. 2015;373(18):1709-1719.
Grube E, Chevalier B, Smits P, Džavík V, Patel T, Mullasari A et al. The SPIRIT V Study. JACC: Cardiovascular Interventions. 2011;4(2):168-175.
Kedhi E, Gomes M, Lagerqvist B, Smith J, Omerovic E, James S et al. Clinical Impact of Second-Generation Everolimus-Eluting Stent Compared With First-Generation Drug-Eluting Stents in Diabetes Mellitus Patients. JACC: Cardiovascular Interventions. 2012;5(11):1141-1149.
Yan P, Dong P, Li Z. Systematic review/Meta-analysis Second- versus first-generation drug-eluting stents for diabetic patients: a meta-analysis. Archives of Medical Science. 2014;2:213-221.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Diabetes"
Diabetes is a metabolic disorder that results in an abnormally high blood glucose level. Blood glucose levels are controlled by insulin produced by the pancreas. In diabetics, the pancreas either doesn’t produce enough (or any) insulin, or the body does not respond sufficiently to the insulin that the pancreas produces.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




