Green Pharmacy in Australia: What Should We Be Doing?
Info: 9658 words (39 pages) Dissertation
Published: 6th Jan 2021
Abstract
Green Pharmacy is the study of the impacts of pharmaceuticals in the environment and devising methods to combat and reduce any possible consequences. Advancements in technology provide increasing knowledge and awareness that pharmaceutical residues in the environment are negatively affecting various ecosystems and impacting on global health.
This report will focus on four key aspects of Green Pharmacy: how pharmaceuticals enter the environment and their effects on nature, Australian sources of pharmaceutical waste and methods of disposal, current international initiatives and future prospects of Green Pharmacy in Australia.
A summary of Sweden’s Kloka Listan (Wise List) was produced to identify trends in the data. An in-depth analysis of fluoxetine was conducted to establish if there is a link between a molecule’s physicochemical properties and its environmental influence. From these findings, the applicability of Swedish initiatives in the Australian setting and the potential challenges involved were analysed. Other strategies such as patient and prescriber education and individualised lower-dose prescribing were also considered.
This report highlights the current lack of relevant evidence available, thus weakening the credibility and significance of any initiatives that may be implemented in Australia. As the significance of Green Pharmacy begins to emerge to the forefront, targeted research is required in order to address the limitations of past studies.
1. Introduction to Green Pharmacy
1.1 What is Green Pharmacy?
Green Pharmacy is the act of analyzing and studying the effect of pharmaceuticals on the environment and the possible strategies that could be employed to minimize their effect on nature.
Through the manufacturing, consumption and improper disposal of pharmaceuticals, residues of common drugs are being found in the environment. Concentrations have predominantly been found in sewage, surface water and wastewater treatment efflux; minute concentrations in soil, slurry and sediment and trace amounts in drinking and mineral water.1,2 Depending on the concentration of the residues and where they accrue, this could vastly impact the human life cycle. Drug metabolites can enter the aquatic or terrestrial environment to affect our food production; when concentrations accumulate into wastewater that is later recycled, plantation and crops can be unwholesome; pharmaceutical residues may even contribute to the current pandemic of antibiotic resistance.
1.2 Is it really an issue?
With the rapid development of technology, analytical research has proven that incredibly low concentrations of active drugs in pharmaceuticals are found in the environment. Concentrations are naturally measured in nanograms per litre in surface water.3 Thus, is pharmaceutical products in the environment a problem, or is it over-exaggerated?
To help uncover whether the impact of pharmaceuticals in the environment really is an issue; a recent study conducted in 2012 analysed the environmental risk of 582 active pharmaceutical ingredients (APIs) found in 50 of the most common drugs used in Sweden. It was concluded that of the 50 drugs, 95% were classified to have non-significant environmental impact.4 It was also mentioned that prioritization should not be based on sales statistics (mass of active drug sold per year); but instead biodegradation, removal of sewage treatment and the drug’s ability to bioaccumulate.5
There are many factors to be reviewed when calculating Predicted Environmental Concentrations (PEC) and Measured Environmental Concentrations (MEC), both of which are regularly used in determining the level of impact pharmaceuticals have on nature. Factors such as the movement of medications throughout different systems of the environment, other sources of contamination and undetectable means of degradation and bioaccumulation may not have been contemplated. This may contribute to the difference in number between the PEC and MEC of an API.
Although there has been increasing numbers of studies and analyses conducted to verify the significance of pharmaceutical impact on the environment, the majority of these papers explain how there are limitations to the current methods used to evaluate risk to human health and how different methodology could be employed.
1.3 How pharmaceuticals enter the environment
Depending on a drug’s physicochemical properties, degradation of different compounds means they can segregate into solids or water such as slurry or soil, and enter into the aquatic or terrestrial environment. Pharmaceuticals can disperse through the environment in many complex pathways, both directly and non-directly, as demonstrated in Figure 1.6 Yellow boxes show potential sources of pharmaceuticals in the environment. Red boxes indicate where concentrations of active drug can disperse. Blue and green boxes demonstrate where pharmaceuticals may bioaccumulate in the aquatic and terrestrial environment respectively.
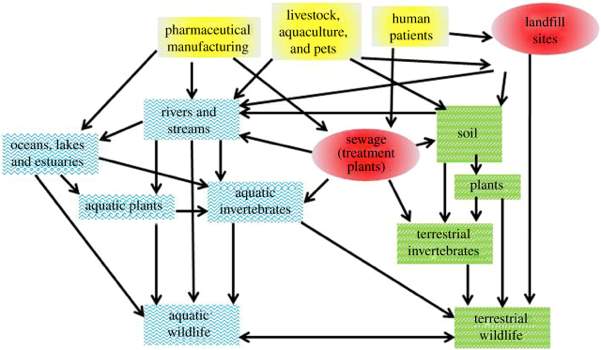
Figure 1: Potential pathways pharmaceuticals can disperse through the environment.6
There has been increasing detection of a range of pharmaceuticals such as synthetic hormones, anti-inflammatories and antidepressants in sediments, soil, surface and ground waters in the marine life.7 A recent experiment conducted by M.Yang et al, studied the effect of the antidepressant amitriptyline, at sublethal concentrations, on the growth inhibition and coordinated regulation of zebrafish embryos. Results showed that exposure to amitriptyline at sublethal concentrations significantly reduced the hatching time and length of embryos. Amitriptyline’s mode of action as a selective serotonin reuptake inhibitor (SSRI) has potential to disrupt important physiological processes and thus impact the hormone levels of fish. There appeared to be a correlation between the feminisation of male zebra fish to the early hatching and substantial growth inhibition of fish larvae as measured by the reduction in length of their embryos.8 Continuous exposure of marine life to pharmaceuticals, such as the commonly used amitriptyline, could cause vast decreases in population growth and result in possible extinction of this species.
1.4 Antibiotic resistance
The emergence of antibiotic resistance arises from complex reactions involving the mutation and procuration of mobile genes that have developed over time in bacteria. Resistance genes can develop naturally or through selective effects of pollutants such as certain pharmaceuticals.9 There is a current rise in the resistance of antibiotics such as carbapenems, especially in countries such as China, Greece and India where such antibiotics are heavily used to combat Klebsiella spp. Conditions caused by the Klebsiella species include: community acquired pneumonia, urinary tract infections such as acute pyelonephritis and epididymo-orchitis and chronic genital ulcerative disease.10 The emergence of the carbapenemase genes was linked to tourism, insanitation and the seepage of wastewater into drinking water.11
After use in human patients, antibiotics and their metabolites can be emitted into the environment by many means (Figure 1). This process can also be applied to the use of veterinary drugs used to treat conditions and maintain animal health. Plants have the capacity to absorb and convert antibiotics to less toxic metabolites and store them in their vacuoles and cell walls. This ability allows such resistance to antibiotics to be transferred to wild herbivores and invertebrates that ingest these plants as a means of energy.12 The proper implementation of discarding and the incineration of antibiotics can reduce the inclination of antibiotic resistance.
2. Green Pharmacy in Australia
2.1 Waste Classification
In Australia we currently have methods to deal with pharmaceuticals to encourage Green Pharmacy but this concept is not as recognized as it should be. There is legislation in place to help Australians with discarding medications and laws enforced to govern the eventual destruction of this waste. In accordance with Environment Protection Authority (EPA) regulations, there are two categories that waste can be classified under: clinical and related waste.13 Clinical waste is defined as industrial waste generated in clinical settings that has the possibility to cause injuries, disease or public offence; this comprises of sharps (syringes, scalpels or anything with the ability to cut and penetrate the skin), blood, animal and human tissue, urine, faeces, laboratory cultures and microorganisms.13 Related waste on the other hand includes waste contaminated or created with chemicals, cytotoxic substances, radioactive compounds and other pharmaceutical materials.14
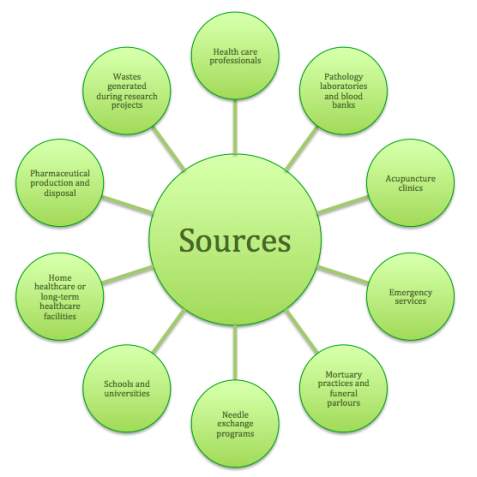
These wastes can be produced from numerous sources as depicted in Figure 2.
Figure 2: Sources of clinical and related wastes.13
2.2 Disposal of medication
Consumers have formed a habit of discarding out-of-date and unwanted medications via general waste and the sewerage. Unwanted medications are often tipped down the sink, dumped into the toilet or put out with the garbage, which can be critically detrimental to the environment because the waste can end up in waterways and landfill sites. Consumers need to be educated about the appropriate action for medication disposal and encouraged to use this option instead of just what is convenient, familiar and easy. The Return Unwanted Medicines (RUM) Project was developed in Australia and provides the safest and easiest way to dispose expired and unwanted medication in the community. The purpose of the project is to make the return of all household medicines to any participating pharmacy possible at any time, free of charge. All consumers need to do is separate expired medicines into a bag or container along with any unwanted medications for return to the pharmacy for disposal.15 Some expired medications can undergo chemical changes that may result in toxic products, thus should be returned to the pharmacy for disposal instead of being kept at home. Furthermore, medications stored at home can be a source of poisoning in children and a source of confusion in elderly patients.16 Following the Australian Pharmacy Collection Protocol, RUM bins or containers should be kept in an area of the dispensary or a room in the pharmacy where there is no public access. The container may be placed in an observable location but should be kept out of reach of the public to emphasise the significance of the message that expired and unwanted medicines can be returned to the pharmacy where they will not be recycled, but instead collected for destruction. Full RUM containers from pharmacies are delivered to the wholesaler depot where they are stored in a quarantine area and put onto pallets, ready for collection by the selected waste transport agent.17 The designated waste disposal company receives the containers from the transport agent and at the earliest opportunity uses the process of high temperature incineration to destroy the RUM containers and their contents.16
2.3 Clinical and related waste
Discarding clinical and related waste in everyday general waste is illegal as this could result in undesirable consequences for the community and the environment.13 Particular types of waste cannot be treated by common methods such as the autoclave system or processing through an alkaline oxidation plant as demonstrated in Table 1. Instead, this waste must be segregated and discarded of in a facility that has authorisation to treat this waste. This waste comprises of both clinical and related waste.18 For all related waste, elimination must be carried out via the process of incineration.13

Table 1: Waste treatment processes used in Victoria.13
2.4 Waste incineration
Incineration involves combustion of waste materials at high temperatures to result in many end products including an inert ash, carbon dioxide, water and as little pollutants as possible. Modern clinical waste incinerators come equipped with Air Pollution Control Equipment. This new equipment destroys medical waste components whilst reducing the volume of the waste material by 90% and controlling emissions released into the atmosphere. Unlike landfilling, incineration is a rapid oxidation process and has the added benefit that all stages of the process can be monitored and modified. To be a well-designed incinerator system, the equipment must use a combination of the techniques of a good mixing of gases under control for combustion, high temperatures and the appropriate amount of burn time (retention time) to destroy the waste.18
2.5 Wastewater treatment
The purpose of wastewater treatment is to remove possible pathogens and solids from wastewater. The systems at the treatment plants can be separated into three sections: primary, secondary and tertiary treatment. Primary treatment includes the screening, settling and removal of solids. Secondary treatment involves using bacteria as biological treatment. This step is where most pharmaceutical remains are eradicated, leaving only very minute traces to enter the environment. Finally, tertiary treatment includes further filtering, chemical and ultraviolet light treatment processes. The liquid effluent can be discharged into water systems or recycled while the solid sludge, also known as biosolids, is disposed of by incineration or used in landfill as a soil additive or fertiliser.19 Some pharmaceuticals contain microbeads which allow for modified release of APIs. Microbeads however, are so small that they are not filtered during the wastewater treatment process and end up in the effluent. This is problematic because these microbeads are not biodegradable and are found in aquatic environments where they are ingested by marine species. Manufacturers are slowly removing microbeads from their products and replacing them with naturally biodegradable alternatives, which is positively impacting the environment.20 Further research should be carried out to discover suitable alternatives so that use of microbeads is eventually eliminated.
In applying waste minimisation principles and improving waste management, Australians can work towards reducing waste disposal costs, whilst generating other positive economic, environmental and social advantages. This can be done by looking at initiatives already in place internationally and revising them to fit the Australian environment.
3. Environmentally classified pharmaceuticals
3.1 International Green Pharmacy initiatives
On the international level, Sweden is currently leading the research into Green Pharmacy practices through environmental hazard and risk assessments of pharmaceuticals. This initiative was introduced in 2003 by the environmental Stockholm City Council. At present, such assessments are conducted by the Swedish Association of the Pharmaceutical Industry (LiF).21
LiF analyses the medications on the Wise List (Kloka Listan), which is a list of recommended pharmaceuticals for common diseases issued by the Drug and Therapeutics Committee (DTC) of the Stockholm County Council. LiF classifies these substances according to both their environmental hazard and environmental risk.21
Environmental hazard refers to the inherent, environmentally damaging characteristics of the active substance, with respect to three key aspects:
- Persistence, its ability to resist degradation (remain unchanged or undegraded) in the aquatic environment.21,22
- Bioaccumulation in the adipose tissue of aquatic organisms based on its partition coefficient. A substance with a log P ≥ 4 has potential for high bioaccumulation.22,23
- Toxicity, the concentration of the API required to kill, immobilise or inhibit growth of 50% of organisms present in three different trophic levels in the aquatic ecosystem; usually algae, crustaceans and fish. Those 100 mg/L are considered of lower toxicity.22,23,24,25,26
Overall, each of these characteristics are assigned a score from 0 ⎯ 3 by the LiF. The sum of these values equates to the substance’s Persistence-Bioaccumulation-Toxicity (PBT) index, which can range from 0 ⎯ 9. Thus, the higher the PBT index, the greater the threat to the environment.21
Environmental risk assesses the toxicity to the aquatic environment with respect to the total sales of active pharmaceutical ingredient, API/kg per year.23 It is calculated as a ratio between the predicted environmental concentrations (PEC) in µg/L in Swedish waterways and the highest concentration (µg/L) that does not have a harmful effect on the environment (PNEC). The ratios are categorised into 4 categories (Table 2).22
| Risk | PEC / PNEC ratio |
| Insignificant | PEC / PNEC ≤ 0.1 |
| Low | 0.1 |
| Moderate | 1 |
| High | PEC / PNEC > 10 |
Table 2: Risk classifications according to their PEC/PNEC ratio.21,22,23
Substances ‘exempt’ from classification are unlikely to pose any threat to the environment as deemed by the European Medicines Agency (EMA). This includes vitamins, electrolytes, carbohydrates, amino acids, peptides, lipids, vaccines and herbal medicinal products.27 Substances that ‘cannot be excluded’ lack sufficient data to calculate the PEC/PNEC ratio.21
Classifying environmental risk adheres to the ‘precautionary principle’, which is part of the European Union (EU) law and applicable to all member countries. It aims to ensure “measures can be taken if there is a reason to believe that a product or a method of production involves unacceptable risks to the health of human beings, animals, plants and the environment – even if there is no definitive scientific proof of such an effect.” 28
The 2014-2015 Environmentally Classified Pharmaceuticals list presented by Stockholm County Council assesses a total of 694 substances. Classification of these substances into their respective risk categories is presented in Figure 3. The female sex hormone, ethinylestradiol is the sole substance that possesses a high environmental risk.21 Of the substances where sufficient data is available to calculate the PEC/PNEC ratio, the overall impact to the environment appears to be insignificant.
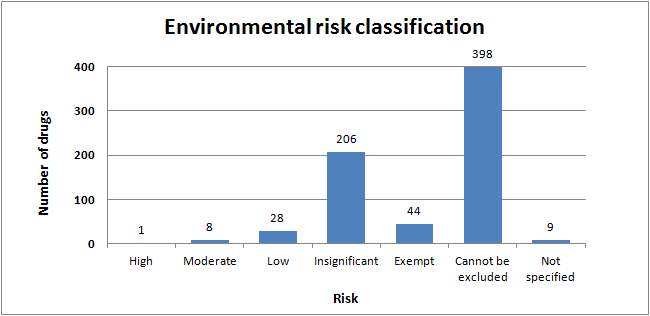
Figure 3: Environmental risk classification of the substances on the Wise List (Klokan Lista)21
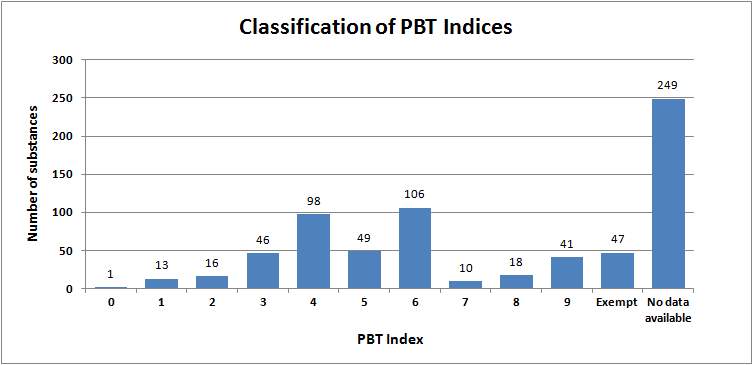
Figure 4 provides an overview of the drugs classified according to their PBT Index. Excluding the 249 substances where there is no ecotoxicity data and the 47 exempt substances, the remainder have a PBT index, which is situated around the 4 ⎯ 6 range. This can be interpreted as having a moderate environmental hazard. Substances with a PBT index of 9 were predominantly dermatologicals, sex hormones and CNS drugs.21
Figure 4: A breakdown of the proportion of substances in the Wise List (Klokan Lista) for each Persistence-Bioaccumulation-Toxicity (PBT) index21
As CNS drugs make up almost one-fifth of the substances with a PBT index of 9, this will form the basis of further discussion. Fluoxetine, with a PBT index of 6 and a low risk classification has been chosen as the representative drug to assess whether physicochemical properties can be used to predict environmental impact on the environment.21
3.2 Structural and chemical analysis of fluoxetine
Fluoxetine is a SSRI used to treat major depression and compulsive disorders.29 It has widespread use in Australia with over 1.44 millions prescriptions dispensed between 2012 ⎯ 2014.30The concentration of fluoxetine present in rivers and aquatic systems vary amongst different locations. In the UK, a study of 162 effluent wastewater treatments report median fluoxetine concentrations of 23 ng/L.31 In contrast, fluoxetine concentrations were as high as 104 ⎯ 119 ng/L in the effluent channels of Crabtree creek in North Carolina, USA.32 The effect of fluoxetine in various aquatic species includes changes to activity, behaviour and reproduction. Exposure to fluoxetine at concentrations as low as 0.3 ng/L, resulted in decreased cAMP levels in the mantle and gonads of Mediterranean mussels, altering signalling pathways in this species.33 Arabian Killfish swam 38% slower than controls following exposure to 0.3µg/L. Goldfish males of the Carassius auratus species released less milt (sperm and seminal fluid) and testosterone levels were reduced by 75% at fluoxetine concentrations of 54 µg/L.34 In most studies, physiological changes observed have resulted from fluoxetine exposure at concentrations higher than those present in water systems. However, as environmental exposure tends to be chronic rather than acute, the effects observed may still occur as a result of persistence or accumulation of the active substance.
To understand the effect of fluoxetine on the environment, it is important to consider the physicochemical properties that may contribute to its toxicity, bioaccumulation and/or persistence in aquatic systems. The structure of fluoxetine is provided in Figure 5.35
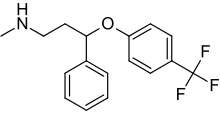
Figure 5: Chemical structure of fluoxetine35
Fluorines are often used in the manufacture of pharmaceutical substances due to their ability to enhance metabolic stability through an inductive effect.36 The C-F bond is extremely strong (485 kJ/mol compared to 416 kJ/mol for a C-H), making the compound more resistant to degradation.37 This can increase its bioavailability, producing a greater therapeutic effect in the patient. Fluoxetine has a trifluoro moiety para to the benzene ring. The increased number of fluorines can potentially contribute to its persistence in the environment and consequently have detrimental effects.
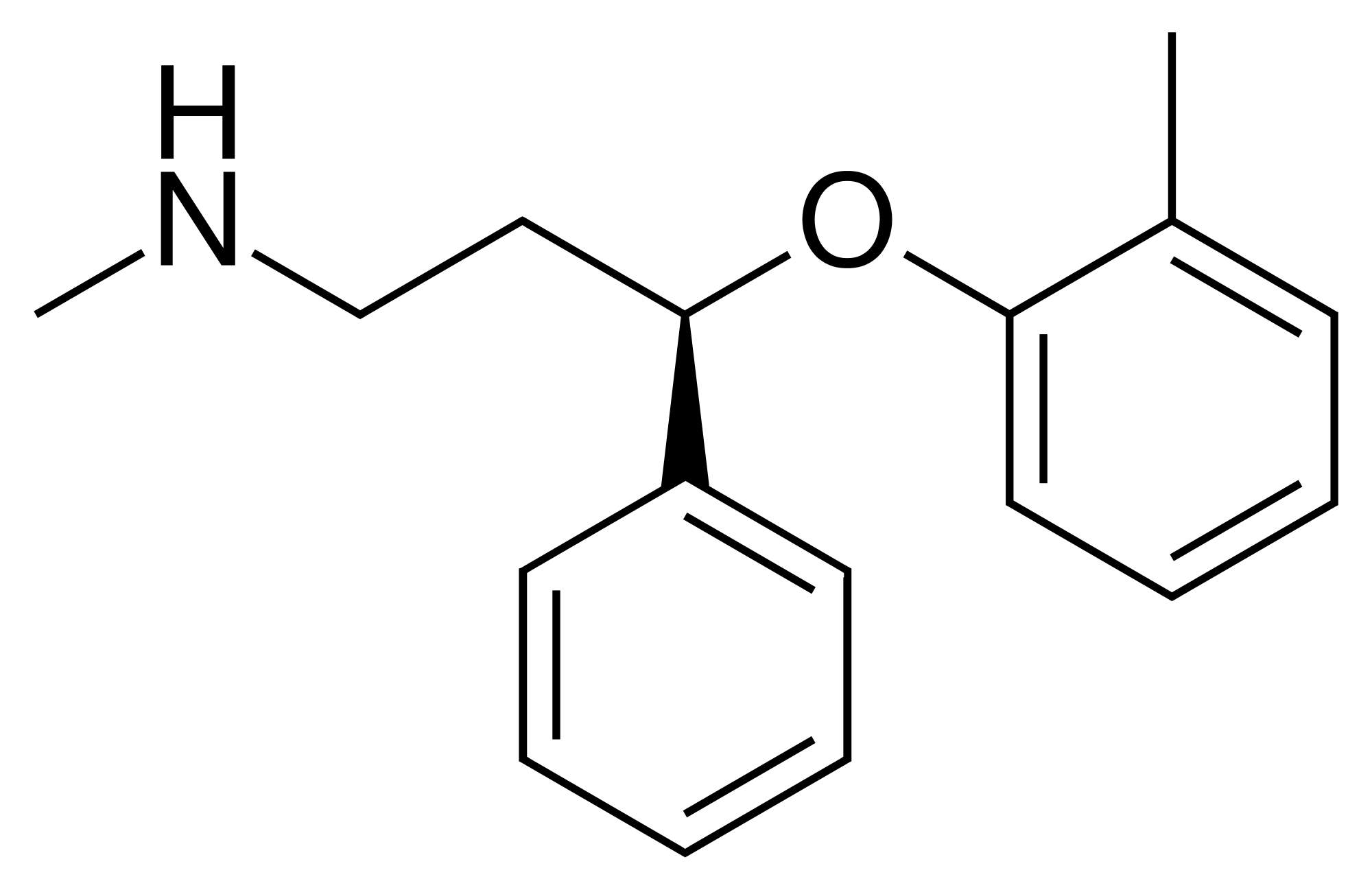
The structure of atomoxetine is almost identical to fluoxetine (Figure 6) with the exception of the methyl substituent, which replaces the trifluoro moiety in fluoxetine. Atomoxetine poses an overall insignificant risk, despite having the same PBT index (PBT index = 6) as fluoxetine, which has a low risk. This raises the question of whether the presence of fluorines actually contributes to a molecule’s toxicity in aquatic systems. The difference may be attributed to the variances in sales volume, ~ 371,000 prescriptions for atomoxetine compared to ~ 4.1 million for fluoxetine.
Figure 6: Chemical structure of atomoxetine38
Fluoxetine is a weak base with a pKa of ~9.8.35 In the ocean which is slightly basic at pH 8.1, the majority of fluoxetine is present as the ionised conjugate acid, whilst a small amount is deprotonated to its neutral form.39 Given the unionised species is more lipophilic, there is speculation that this small portion of the unionised species can potentially cross the blood-brain-barrier (BBB), or equivalent in aquatic organisms, and be of sufficient concentration to elicit either a therapeutic or adverse effect.40 Furthermore, temperature increases in water increase the pH and thus can result in a greater portion of unionised species which can potentiate the aforementioned effects.40 This may contribute to the toxicity score of 3 for its PBT Index.
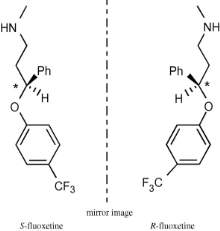
Structurally, fluoxetine exhibits chirality and is administered as a racemic mixture (Figure 7). In-vitro studies reveal enantiospecific toxicity whereby S-fluoxetine is more toxic to Pimephales promelas fish in regards to growth, survival and reproduction than the R enantiomer.30,42
Figure 7: Structure and configuration of R- and S-fluoxetine. * indicates chiral centres.42
Lipophilicity can be measured using the LogP value. With a LogP value of 4, fluoxetine is a rather lipophilic molecule and can potentially permeate through the BBB to exert its effect. LiF states that substances with LogP ≥ 4 may potentially be bioaccumulating.23 It also fulfills one criteria of Lipinski’s Rule of 5 (log P
Fluoxetine undergoes hepatic metabolism and demethylation produces the active metabolite norfluoxetine.45 Both substances exhibit long half-lives. Fluoxetine has a half-life of 2 ⎯ 6 days, whilst norfluoxetine’s half-life can range from 4 ⎯ 16 days.35 Combined with chronic exposure, fluoxetine is likely to reside in aquatic environments for lengthy periods of time. This can contribute to its persistence score of 3 as assigned by LiF.21
Although fluoxetine has a low environmental risk rating, it can be seen that it has adverse effects on aquatic wildlife. Further research should be conducted on other SSRIs to find an alternative with lower environmental impact. This research can help influence future prescribing practices.
4. Future prospects for Australia
4.1 Applying Swedish practices in Australia
After analysing the data available from Sweden and the practices already being implemented in Australia, the question of whether Australia should implement a preferred medicines list similar to the “Wise List” can be posed. Firstly, the effectiveness of Sweden’s list needs to be considered to gauge if such a list will have a significant influence on prescribing if introduced in Australia.
In 2000, the first list of common drugs used in general practice was created in Stockholm. Subsequently, the first edition of Sweden’s “Wise List” was published in 2001 by the DTC for the public and has since undergone various changes annually. A study conducted over a period of 10 years (2000 ⎯ 2010) monitored the effects of the list’s implementation. During this period, surveys and telephone interviews were conducted to analyse prescriber and public attitudes towards the list. All prescribers surveyed were familiar with the list and 81% found the recommendations trustworthy. Prescribers reported the reliability of the list was attributed to the high quality of the information, which lacked bias and was void of influence from commercial interest. The principles of evidence-based medicine and the prescriber’s confidence in the DTC’s expertise also contributed to positive reports. The main reasons reported for distrust were insufficient quantity of information provided and the strong focus on drug expenditure when recommending drugs.46 Most prescribers appreciated that the patient version of the list helped stimulate and facilitate conversation between the prescriber and patient regarding the most appropriate treatment. A majority of the patients interviewed had positive attitudes towards asking their doctors to utilise the recommendations on the list. Approximately 90% of participants of all surveys wanted access to the list.46 This positive response from both prescribers and patients in Sweden may also reflect the attitudes of the Australian population and indicate the potential success of a similar list in Australia. It also highlights benefits of improved prescriber-patient relationships and increased patient involvement in the management of their own health, in addition to the environmental benefits of such a list.
Adherence to the recommendations was also tracked in this study through evaluation of dispensing data to assess how Sweden’s list was utilised. In primary healthcare centres, adherence to the list’s recommendations increased from 83% to 87% from 2003 to 2009. The strongest adherence was seen for proton pump inhibitors and antibiotics for urinary tract infections due to the short-term nature of the treatment course and the high rate of treatment initiation. The study also speculates that the observed changes to drug utilisation was the result of economic incentives for healthcare centres that displayed high adherence to the list.46 These results suggest that Australia could achieve comparable rates of adherence to an Australian “Wise List” if prescribers are given appropriate incentives.
An Australian “Wise List” could potentially reduce Australia’s contribution to pharmaceutical contamination in the environment. However, there are many gaps in the data available that impedes the implementation of a comprehensive and fully reliable recommendations list. Approximately 65% of the drugs included in the Environmentally Classified Pharmaceuticals list (as shown in Figure 2) do not have definitive risk classifications and of these, approximately 57% of them had insufficient data available to complete a risk assessment. Many commonly used drugs in Australia have a risk classification of “cannot be excluded” and the significance of their effects are still unknown. For example, atenolol and cephalexin are present in the list of top 10 drugs dispensed on the PBS in 2014 in the community setting, but lack the ecotoxicity data required to assign them a risk classification. Perindopril, from the PBS list, does not even appear in Sweden’s collated data.21,47 This deficiency in available research hinders the adaptation of a suitable list of recommendations for the Australian population using Sweden’s data.
Additionally, environmental risk assessment (ERA) based on acute toxicity tests does not accurately indicate the potential for chronic environmental effects as a result of long-term continuous release and exposure to subacute levels. Compounds with low persistence can potentially cause chronic effects if there is continuous release into the environment.43 Therefore, current ERA data is not reliable for analysing long-term environmental risk. Continual research is required so that Australia’s “Wise List” can be regularly updated and stays relevant and reliable in the long run.
4.2 Pharmaceutical cocktails
It is important to consider that pharmaceuticals are not presented in the environment as a single contaminant but instead, as a complex mixture of multiple drugs and other contaminants such as pesticides and drug excipients. This alters the environmental characteristics of the drug in question.48 A study was conducted on Daphnia magna (a freshwater water flea) to determine the combined ecotoxicological effects of residues from a mix of pharmaceuticals where the endpoint measured was the immobilisation of the flea. Clofibrinic acid (a lipid-lowering agent) and carbamazepine (an anti-epileptic) displayed 95% immobilisation as a mixture in comparison to the 1% and 16% immobilisation for the sole agents respectively (Figure 8). A similar trend was observed with a mixture of diclofenac and ibuprofen.49 This study illustrates the complexity of the aquatic environment and reinforces the need for more research in order to fully understand the impact of pharmaceuticals on the environment. Future experimental studies should aim to mimic the mixture of contaminants found in the area of interest to more accurately determine the effects seen in the real environment. This data can also contribute and improve the reliability of an Australian preferred medicines list.
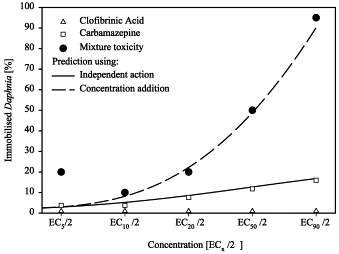
Figure 8: Toxicity of clofibrinic acid and carbamazepine as a single agent and as a mixture on Daphnia at varying concentrations.49
Overall, despite the benefits of a preferred medicines list that considers environmental impact in combination with safety, efficacy and cost-effectiveness, a great deal of research still needs to be done before Australia can implement a list that becomes a valuable part of prescribing practices.
4.3 Patient and prescriber education
In contrast to the lengthy process of adapting a “Wise List” for Australia, reviewing and updating current practices can produce more immediate effects. A nationwide audit of the medicines returned in RUM bins was conducted in 2013. In this study, the top 20 medicines from the audit were compared to the top 20 medicines dispensed on the PBS in 2012. Of these 20 drugs, only six drugs appeared on both lists. Some of the drugs that are commonly dispensed but not commonly returned to RUM bins include atenolol, tiotropium and latanoprost.50 Despite the significance of the environmental risk of these drugs being undetermined, it is still important to minimise their exposure to the environment by encouraging patients to return unused or expired packs to be disposed of safely.21 This can be done through adequate patient education.
Pharmacists and prescribers are in a prime position to provide additional education and counselling to patients to minimise medicine wastage and unsafe disposal. Pharmacists should particularly be encouraged to promote and increase patient awareness of the availability of the RUM program and the associated benefits such as improved public health and safety and reduced environmental pollution. Patients on new medicines can be directed to return the unused portion of a pack if their dose changes. This strategy is also useful for medicines that have a limited shelf life once opened such as antibiotic suspensions and eye drops. Patients should also be counselled on how and when to take medicines appropriately as improving adherence can reduce wastage. Adherence is particular important for anti-infectives as incomplete courses can increase the risk of antimicrobial resistance if discarded inappropriately.50 Oestrogen patches, vaginal rings and inhalers should also be returned after use as they can still contain APIs.21
Prescribers can also be heavily involved in moving towards a more efficient use of medicines. Prescriber should be educated on the potential environmental impact of pharmaceuticals. They should be encouraged to reflect on their own prescribing practices and consider the environmental effects of the drugs they commonly prescribe and whether there are alternatives with less impact. Another strategy is to only prescribe the quantities needed for the course of treatment (e.g. antibiotics) in order to reduce medicine wastage. If in doubt, prescribing repeats is preferred instead of whole packs. 21 This way, costs to the health care system and the patient are reduced and the volume of medicines that needs disposal is minimised. Lastly, prescribers, like pharmacists, are also in a suitable position to educate patients and remind them to return unused and expired medicines to pharmacies for appropriate disposal.
Although discussions regarding Green Pharmacy practices are often considered on a national level, it is important to remember that the contribution to environmental risk first begins on the level of the patient and healthcare professionals. Therefore, education is of the utmost importance in these two demographics to minimise the scale of environmental impact.
4.4 Individualised lower-dose prescribing
Another idea proposed to reduce exposure of APIs to the environment is to reduce the initial doses given to patients through individualised lower-dose prescribing. Patients can be dosed with drug concentrations at the lower end of the dose-response curve to minimise the dose given while still achieving therapeutic goals. When lower doses are administered, less residues are excreted by the body into the environment, which also reduces the need for waste treatment to remove these residues. Additionally, lower-dose prescribing can lower patient costs and reduce the risk of side effects. Giving patients the option to gradually titrate to the lowest effective dose can also allow for greater control over their treatment and health, enabling for improved adherence and potentially better health outcomes.51 However, there is limited concrete data available illustrating that lower-dose prescribing is as effective as higher-dose prescribing. For example, in a particular study, colchicine dosed at 1.8mg was as effective as 4.8mg for acute gout. However, as the study was poorly designed with a small sample size, the results are unreliable.52 There are also mixed results and opinions from studies conducted on the same drug. In one study demonstrated ranitidine dosed at 25mg and 75mg, only displayed a difference of
5. Conclusion
Overall, it is becoming increasingly evident and undeniable, that the presence of pharmaceuticals in the environment is posing a threat to the welfare of animals, particularly aquatic species, as well contributing to global health issues such as the emergence of antibiotic resistance. Although there are measures in place in Australia such as RUM bins, wastewater treatment and incineration; sustainable practices are often overlooked in Australia. In contrast, this issue is of greater concern to the Swedish government who have developed a preferred medicines list with reference to environmental impact. Mirroring these practices in the Australian context would require further research on drugs commonly prescribed in Australia as well as additional study on long-term effects of pharmaceutical cocktails in the environment and lower-dose prescribing. However, prescribers and pharmacists can take a proactive approach in reducing the environmental impact by providing patient education on medication adherence and disposal as well as adopting appropriate prescribing practices.
More Pharmacy Content
- Evaluating Programs Offered for Pharmacy Residents
- Wyeth Pakistan Limited Business and Financial Analysis
References:
- Aus der Beek T, Weber F A, Bergmann A, Global Occurrence of Pharmaceuticals in the Environment: Results of a Global Database of Measured Environmental Concentrations (MEC) [Internet]. 2014 April [cited 2015 Aug 31]; slide 5, 15. Available from: http://www.pharmaceuticals-in-the-environment.org/files/en/bereich_2/application/pdf/weber_aus-der-beek_i.pdf
- Mudgal S, De Toni A, Lockwood S, Sales K, Backhaus T, Halling Sorenson B, Study on the environmental risks of medicinal products. Executive Agency for Health and Consumers [Internet]. 2013 Dec [cited 2015 Aug 31]; 110, 156, 157. Available from: http://ec.europa.eu/health/files/environment/study_environment.pdf
- Oldenkamp R, Juijibregts M, Hollander A, Versporten A, Goossens H, Ragas A, Spatially explicit prioritization of human antibiotics and antineoplastics in Europe. Environment International [Internet]. 2012 September [cited 2015 Aug 31];51:13-26. Available from: http://ac.els-cdn.com.ezproxy.lib.monash.edu.au/S0160412012002085/1-s2.0-S0160412012002085-main.pdf?_tid=d5a8a4b6-508f-11e5-8038-00000aacb35e&acdnat=1441101620_6fe783b0666e0d0e815ee99fb259480f
- Roos V, Gunnarsson L, Fick J, Larsson D, Ruden C, Prioiritising pharmaceuticals for environmental risk assessment: Towards adequate and feasible first-tier selection. Science of the Total Environment [Internet]. 2012 Jan [cited 2015 Aug 31]; 421-422:102-110. Available from: http://ac.els-cdn.com.ezproxy.lib.monash.edu.au/S0048969712000824/1-s2.0-S0048969712000824-main.pdf?_tid=7046cf98-5090-11e5-948f-00000aab0f6c&acdnat=1441101879_f1a5cefdbb554c7d9a62355669a16b6b DOI: 10.1016/j.scitotenv.2012.01.039
- Caldwell D, Mastrocco F, Margiotta-Casaluci L, Brooks B, An integrated approach for prioritizing pharmaceuticals found in the environment for risk assessment, monitoring and advanced research. Chemosphere [Internet]. 2014 Jan [cited 2015 Aug 31];115:4-12. Available from: http://ac.els-cdn.com.ezproxy.lib.monash.edu.au/S0045653514000873/1-s2.0-S0045653514000873-main.pdf?_tid=b0f51a4e-5091-11e5-b591-00000aacb35d&acdnat=1441102417_a584362b07447544167a4918f02eb5a0 DOI: 10.1016/j.chemosphere.2014.01.021
- Arnold K, Brown R, Ankley G, Sumpter J, Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Philosophical transactions B [Internet]. 2014 [cited 2015 Aug 31]; 396(1656). Available from: http://rstb.royalsocietypublishing.org.ezproxy.lib.monash.edu.au/content/369/1656/20130569 DOI: 10.1098/rstb.2013.0569
- Gaw S, Thomas K, Hutchinson T, Sources, impacts and trends of pharmaceutical in the marine and coastal environment. Philosophical transactions B [Internet]. 2014 [cited 2015 Aug 31]; 369(1656). Available from: http://rstb.royalsocietypublishing.org.ezproxy.lib.monash.edu.au/content/369/1656/20130572?ijkey=68855ead362eb3d018d9ec03faf0203a93868702&keytype2=tf_ipsecsha DOI 10.1098/rstb.2013.0572
- Yang M, Qui W, Chen J, Zhan J, Pan C, Lei X, Wu M, Growth inhibition and coordinated physiological regulation of zebrafish (Danio rerio) embryos upon sublethal exposure to antidepressant amitriptyline. Aquatic Toxicology [Internet]. 2013 Dec [cited 2015 Aug 31]; 151: 68-76. Available from: http://ac.els-cdn.com.ezproxy.lib.monash.edu.au/S0166445X13003792/1-s2.0-S0166445X13003792-main.pdf?_tid=6d232f6a-5094-11e5-9585-00000aacb362&acdnat=1441103592_d91b19e3a1b1345339955fc7d378f0fb DOI: 10.1016/j.aquatox.2013.12.029
- Wellington E, Boxall A, Cross P, Feil E, Gaze W, Hawkey O, Johnson-Rollings A, Jones D, Lee N, Otten W, Thomas C, Williams A, The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. The lancet [Internet]. 2013 Feb [cited 2015 Aug 31];13(2): 155-165. Available from: http://ac.els-cdn.com.ezproxy.lib.monash.edu.au/S1473309912703171/1-s2.0-S1473309912703171-main.pdf?_tid=0490a3fa-5095-11e5-99d8-00000aacb362&acdnat=1441103846_3e1af50aec39bb7004b1df93e1f9dfec DOI: 10.1016/S1473-3099(12)70317-1
- Qureshi.S. Klebsiella infections clinical presentation [Internet]. WebMD LLC; 2015 [updated October 6; cited 2012 October 17]. Available from: http://emedicine.medscape.com/article/219907-clinical
- Walsh R, Weeks J, Livermore D, Toleman M, Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. The Lancet [Internet]. 2011 May [cited 2015 Aug 31]; 11(5):355-362. Available from: http://ac.els-cdn.com.ezproxy.lib.monash.edu.au/S1473309911700597/1-s2.0-S1473309911700597-main.pdf?_tid=ab72f4d4-5095-11e5-8b1a-00000aab0f6b&acdnat=1441104126_a9af1211c14de4b8575e94620050cbc3
- Bartikova H, Skalova L, Stuchlikova L, Vokrai I, Vanek T, Podlipna R, Xenbiotic-metabolising enzymes in plants and their role in uptake and biotransformation of veterinary drugs in the environment. Drug Metabolism Reviews, Early Online. 2015 July [cited 2015 Aug 31;1-14:2,4.
- EPA Victoria. Clinical and Related Waste – Operational Guidance [Internet]. Victoria: State Government of Victoria, 2009 [updated 2009 Sep; cited 2015 Aug 31]. Available from: http://www.epa.vic.gov.au/~/media/Publications/IWRG612%201.pdf
- Environment Division. Approved Management Method for Clinical and Related Waste [Internet]. Tasmania: Department of Tourism, Arts and the Environment, 2007 [updated 2007; cited 2015 Aug 31]. Available from: http://epa.tas.gov.au/documents/amm_clinical_and_related_waste_companion.pdf
- Return Unwanted Medicines. Consumers – Return Unwanted Medicines [Internet]. 2015 [cited 31 Aug 2015]. Available from: http://www.returnmed.com.au/consumers/
- Return Unwanted Medicines. FAQ – Return Unwanted Medicines [Internet]. 2015 [cited 1 Sept 2015]. Available from: http://www.returnmed.com.au/faq/
- Return Unwanted Medicines. Pharmacists – Return Unwanted Medicines [Internet]. 2015 [cited 1 Sept 2015]. Available from: http://www.returnmed.com.au/pharmacists/
- Waste Management Association of Australia. Industry code of practice for the management of clinical and related wastes. 6th ed.NSW: Australia; 2010.
- Murdoch, K. Pharmaceutical Pollution in the Environment: Issues for Australia, New Zealand and Pacific Island countries [Internet].NSW: Australia; 2015 [cited 1 Sept 2015]. 36p. Available from: http://www.ntn.org.au/wp/wp-content/uploads/2015/05/NTN-Pharmaceutical-Pollution-in-the-Environment-2015-05.pdf
- Beat the Microbead. International campaign against microbeads [Internet]. Amsterdam: Plastic Soup Foundation; 2015 [updated 2015; cited 2012 Oct 15]. Available from: http://www.beatthemicrobead.org/en/in-short
- Stockholm läns landsting (Stockholm County Council). 2014-2015 Environmentally classified pharmaceuticals [Internet]. Stockholm: Stockholm läns landstring; [cited 2015 Aug 31]. 29p. Available from: http://www.janusinfo.se/Global/Miljo_och_lakemedel/Miljobroschyr_2014_engelsk_webb.pdf
- FASS. Fakta för förskrivare [Internet].Stockholm: FASS; 2015 [updated 2015 Feb 6; cited 2015 Aug 31]. Available from: http://www.fass.se/LIF/healthcarefacts?docId=76074&userType=0
- Janusinfo Stockholm läns landsting. About classification [Internet]. Stockholm: Janusinfo Stockholm läns landsting; 2014 [updated 2014 Apr 10; cited 2015 Aug 31]. Available from: http://www.janusinfo.se/Beslutsstod/Miljo-och-lakemedel/About-the-environment-and-pharmaceuticals/About-classification/
- OECD. OECD Guidelines for the Testing of Chemicals, Section 2. Test no. 203: Fish, Acute Toxicity Test [Internet]. Paris: OECD Publishing; 1992 [1992; cited 2015 Aug 31]. Available from: http://www.oecd-ilibrary.org/environment/test-no-203-fish-acute-toxicity-test_9789264069961-en
- OECD. OECD Guidelines for the Testing of Chemicals, Section 2. Test no. 202: Daphnia sp. Acute Immobilisation Test [Internet]. Paris: OECD Publishing; 2004 [2004 Nov 23; cited 2015 Aug 31]. Available from: http://www.oecd-ilibrary.org/environment/test-no-202-daphnia-sp-acute-immobilisation-test_9789264069947-en
- OECD. OECD Guidelines for the Testing of Chemicals, Section 2. Test no. 201: Freshwater Algae and Cyanobacteria, Growth Inhibtion Test [Internet]. Paris: OECD Publishing; 2011 [2011 July 28; cited 2015 Aug 31]. Available from: http://www.oecd-ilibrary.org/environment/test-no-201-alga-growth-inhibition-test_9789264069923-en
- European Medicines Agency. Guideline on the environmental risk assessment of medicinal products for human use [Internet]. London: EMA; 2006 [updated 2006 Jun 1; cited 2015 Aug 31]. 12p. Doc. Ref.: EMEA/CHMP/SWP/4447/00. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003978.pdf
- Janusinfo Stockholm läns landsting. Impact of pharmaceuticals on the environment [Internet]. Stockholm: Janusinfo Stockholm läns landsting; 2014 [updated 2014 Feb 24; cited 2015 Aug 31]. Available from: http://www.janusinfo.se/Beslutsstod/Miljo-och-lakemedel/About-the-environment-and-pharmaceuticals/Impact-of-pharmaceuticals-on-the-environment/
- Australian Medicines Handbook 2015 [Internet]. Adelaide: Australian Medicines Handbook Pty Ltd; 2015. [data version 2015 July; cited 2015 Aug 31]; [about 1 screen]. Available from: https://amhonline.amh.net.au.ezproxy.lib.monash.edu.au/chapters/chap-18/antidepressants/ssris/fluoxetine
- Pharmaceuticals Benefit Scheme. Australian Statistics on Medicines 2014. Canberra (AU): Australian Government Department of Health and Ageing; 2014 [cited 2015 Aug 31]. 335 p. Available from: http://www.pbs.gov.au/info/statistics/asm/asm-2014
- Gardner M, Comber S, Scrimshaw MD, Cartmell E, Lester J, Ellor B. The significance of hazardous chemicals in wastewater treatment works effluents. Sci Total Environ [Internet]. 2012 [cited 2015 Sept 1];437:363-372. Available from: http://www.sciencedirect.com.ezproxy.lib.monash.edu.au/science/article/pii/S004896971201039X DOI: 10.1016/j.scitotenv.2012.07.086
- Bringolf RB, Heltsley RM, Newton TJ, Eads CB, Fraley SJ, Shea D et.al Environmental occurrence and reproductive effects of the pharmaceutical fluoxetine in native freshwater mussels. Environ Toxicol Chem [Internet]. 2010 Jun [cited 2015 Sept 1];29(6):1311-1318. Available from: http://onlinelibrary.wiley.com.ezproxy.lib.monash.edu.au/doi/10.1002/etc.157/epdf DOI: 10.1002/etc.157
- Barry MJ. Effects of fluoxetine on the swimming and behavioural responses of the Arabian killifish. Ecotoxicology [Internet]. 2013 Mar [cited 2015 Sept 1];22(2):425-32. Available from: http://download.springer.com.ezproxy.lib.monash.edu.au/static/pdf/484/art%253A10.1007%252Fs10646-012-1036-7.pdf?originUrl=http%3A%2F%2Flink.springer.com%2Farticle%2F10.1007%2Fs10646-012-1036-7&token2=exp=1441161286~acl=%2Fstatic%2Fpdf%2F484%2Fart%25253A10.1007%25252Fs10646-012-1036-7.pdf%3ForiginUrl%3Dhttp%253A%252F%252Flink.springer.com%252Farticle%252F10.1007%252Fs10646-012-1036-7*~hmac=a0b33ca3626e054ecb421a36a938454fc7bce114bdbb02f9559bd946c451c1d9 DOI: 10.1007/s10646-012-1036-7
- Menningen JA, Lado WE, Zamora JM, Duarte-Guterman P, Langlois VS, Metcalfe CD et al.
Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus. Aquat Toxicol [Internet]. 2010 Nov [cited 2015 Sept 1];100(4):354-64. Available from: http://www.sciencedirect.com.ezproxy.lib.monash.edu.au/science/article/pii/S0166445X10003528%20. DOI: 10.1016/j.aquatox.2010.08.016 - DrugBank 4.3. Fluoxetine [Internet]. DrugBank; 2005 [updated 2013 June 13; cited 2015 Sept 1]. Available from: http://www.drugbank.ca/drugs/DB00472
- Muller K, Faeh C, Diederich F. Fluorine in Pharmaceuticals: Looking beyond Intuition. Science [Internet]. 2007 Sept [cited 2015 Sep 1];317(5846):1881-6. Available from: http://www.jstor.org.ezproxy.lib.monash.edu.au/stable/20048468?seq=1#page_scan_tab_contents
- Wakefield B. Fluorinated Pharmaceuticals. Innovations in Pharmaceutical Technology [Internet]. 1998 [cited 2015 Sept 1]. Available from: http://www.iptonline.com/articles/public/IPTFOUR74NP.pdf
- United States National Library of Medicine. Atomoxetine [Internet]. Maryland: United States National Library of Medicine; 2015 [updated 2015 Sep 30; cited 2015 Oct 15]. Available from: http://livertox.nih.gov/Atomoxetine.htm
- National Geographic. Ocean Acidification [Internet]. USA: National Geographic; 2015 [updated 2015, accessed 2015 Oct 15]. Available from: http://ocean.nationalgeographic.com/ocean/explore/pristine-seas/critical-issues-ocean-acidification/
- Brooks BW. Fish on Prozac (and Zoloft): ten years later. Aquat Toxicol. [Internet]. 2014 Jun [cited 2015 Sept 1];151:61-7. Available from: http://www.sciencedirect.com.ezproxy.lib.monash.edu.au/science/article/pii/S0166445X14000113 DOI: 10.1016/j.aquatox.2014.01.007
- Melbourne Water. What We Monitor [Internet]. Victoria: Melbourne Water; 2015 [updated 2015; accessed 2015 Oct 15]. Available from: http://www.waterwatchmelbourne.org.au/content/volunteer_monitoring/what_we_monitor/what_we_monitor.asp#3
- Stanley JK, Ramirez AJ, Chambliss CK, Brooks BW. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere [Internet]. 2007 Aug [cited 2015 Sept 1];69(1):9-16. Available from: http://www.sciencedirect.com.ezproxy.lib.monash.edu.au/science/article/pii/S0045653507006066 DOI: 10.1016/j.chemosphere.2007.04.080
- Cambridge MedChem Consulting. Lipophilicity [Internet]. Cambridge: Cambridge MedChem Consulting; 2015 [updated 2015; cited 2015 Oct 15]. Available from: http://www.cambridgemedchemconsulting.com/resources/physiochem/logD.html
- Cerep. Partition Coefficient – Log D [Internet]. Cerep: France; 2013 [updated 2013 Jul; cited 2015 Oct 15]. Available from: http://www.cerep.fr/cerep/users/pages/Downloads/Documents/Marketing/Pharmacology%20&%20ADME/Application%20notes/2013/Partition%20coefficient-LogD.pdf
- PharmGKB. Fluoxetine pathways, pharmacokinetics [Internet]. Stanford: PharmGKB; 2015 [updated 2015; cited 2015 Sept 1]. Available from: https://www.pharmgkb.org/pathway/PA161749012#PGG
- Gustafsson LL, Wettermark B, Godman B, Andersén-Karlsson E, Bergman U, Hasselström J et al. The ‘Wise List’ – A Comprehensive Concept to Select, Communicate and Achieve Adherence to Recommendations of Essential Drugs in Ambulatory Care in Stockholm. Basic Clin Pharmacol Toxicol [Internet]. 2011 April [cited 2015 Sept 1];108(4):224-33. Available from: http://www.janusinfo.se/Global/In_English/wise_list_meet_2012/background_information.pdf
- The Pharmaceutical Benefits Scheme. Australian Statistics on Medicines 2014 [Internet]. Canberra ACT: Department of Health; 2015 [cited 2015 Sept 2]. Available from: http://www.pbs.gov.au/info/statistics/asm/asm-2014/#_Toc425339266
- Carlsson C, Johansson A, Alvan G, Bergman K, Kühler T. Are pharmaceuticals potent environmental pollutants?: Part I: Environmental risk assessments of selected active pharmaceutical ingredients. Sci Total Environ [Internet]. 2006 July [cited 2015 Sept 1];364(1-3):67-87. Available from: http://www.sciencedirect.com.ezproxy.lib.monash.edu.au/science/article/pii/S0048969705005516
- Cleuvers M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett [Internet]. 2003 May [cited 2015 Sept 1];142(3):185-194. Available from: http://www.sciencedirect.com.ezproxy.lib.monash.edu.au/science/article/pii/S0378427403000687?np=y
- Bergen P, Kong D, George J, Hussainy S, Kirkpatrick C, Dooley M, Charman B. (Report prepared by: Sudhakaran S). The National Return and Disposal of Unwanted Medicines (NatRUM) Project Audit. Report provided to the Department of Health and Ageing, and the National Return and Disposal of Unwanted Medicines (NatRUM) Program Ltd. 2013 Dec [cited 2015 Sept 1]. 20,42 p. Available from: http://auspharmacist.net.au/images/rumrep.pdf
- Daughton CG, Ruhoy IS. Lower-dose prescribing: Minimising “side effects” of pharmaceuticals on society and the environment. Sci Total Environ [Internet]. 2013 Jan [cited 2015 Aug 20];443:324-37
- Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomised, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010 Apr;62(4):1060-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20131255?dopt=Abstract
- Pappa KA, Buaron K, Payne JE, Sirgo MA, Giefer EE. An evaluation of increasing doses of ranitidine for treatment of heartburn. Aliment Pharmacol Ther. 1999 Apr;13(4):475-81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10215731?dopt=Abstract
- Pappa KA, Gooch WM, Buaron K, Payne JE, Giefer EE, Sirgo MA et.al. Low-dose ranitidine for the relief of heartburn. Aliment Pharmacol Ther.1999 Apr;13(4):459-65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10215729?dopt=Abstract
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Sustainability"
Sustainability generally relates to humanity living in a way that is not damaging to the environment, ensuring harmony between civilisation and the Earth's biosphere.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




