Study of Lidocaine Levels in Children after Airway Topicalisation During Direct Laryngotracheobronchoscopy (DLTB)
Info: 8473 words (34 pages) Dissertation
Published: 9th Dec 2019
Tagged: MedicineSurgical Studies
Study of lidocaine levels in children after airway topicalisation during direct laryngotracheobronchoscopy (DLTB)
Contents:
Declaration of originality ………………………………………………….. 3
Abstract ……………………………………………………………………………. 4
Introduction …………………………………………………………………….. 5
Literature review………………………………………………….. 5
Aims and standards ………………………………………………………….. 9
Search methods and metrics ……………………………………………. 9
Methodology …………………………………………………………………… 10
Results and analysis …………………………………………………………. 12
Discussion ………………………………………………………………………… 15
Strengths and weaknesses ……………………………………. 15
Implications of results ………………………………………….. 16
Areas of uncertainty and work for the future ……….. 18
Conclusions ……………………………………………………………………… 20
References ………………………………………………………………………. 21
Appendices ……………………………………………………………………… 2
Abstract
Introduction: Direct laryngotracheobronchoscopy (DLTB) requires prior topicalisation of the larynx with lidocaine spray. Prior studies have shown that a small number of patients may experience high plasma levels of lidocaine predisposing to toxicity. However, clinical trial evidence looking at the relationship between topical lidocaine dose and plasma lidocaine levels is scarce. In addition, variations in practice with regard to management of patients exist in lieu of formal guidance in spite of agreed standards of care by anaesthetists of patients undergoing DLTB.
Aims and methods: A retrospective audit of anaesthetic practice was carried out on all patients who underwent DLTB procedures at RMCH between February and April 2017. This ran alongside a single arm research study in which patients undergoing DLTB procedures had blood samples taken at set points in time to ascertain peak lidocaine levels.
Results: Audit results indicate a significant variation in lidocaine topicalisation practice. For example 15.4% of patients were documented as being allowed fluids <105 mins after topicalisation (less than the agreed 2 hour target). Key gaps in documentation have also been noted. Results for the research study will not be available until after the end of this project.
Conclusion: While no formal guidelines exist regarding lidocaine topicalisation, agreed standards derived from clinical studies are in place at RMCH. However, practice does not appear to conform to those increasing risks of complications. However, delineating the links between plasma concentration and complications is required to make an informed judgement on the appropriateness of current recommended practice.
Introduction
At the Royal Manchester Children’s hospital (RMCH) current practice when carrying out direct laryngotracheobronchoscopy (DLTB) procedures in paediatric ENT theatre involves prior topicalisation with either a 2% or 4% lidocaine spray to the supraglottic and subglottic region of the larynx via a laryngojet device at doses of 3-5 mg/kg. Prior studies have reported a small number of study participants experiencing plasma levels of lidocaine above the reported toxic levels. However these results have been conflicting and used different types of application devices. Consequently, it is unknown what peak plasma levels of lidocaine are achieved in our patients. We also do not currently have any formal guidance on how long resus nurses should be advised to wait until a patient can be allowed oral fluids after airway topicalisation, and there appears to be some variation in practice in the RMCH paediatric anaesthesia department with regards to this. Thus we have decided to complete a study which will look at the peak plasma levels of lidocaine intraoperatively after airway topicalisation by taking blood samples from patients at set five minute intervals up to twenty minutes after application. This will run alongside an audit of patients who have undergone DLTB at RMCH over a 3 month period which will look at the reported intra-operative and post-operative complications from lidocaine administration, doses administered, and the times that anaesthetists are recommending until oral fluids are restarted following topicalisation to assess current practice.
Literature review
During procedures in which there is mechanical stimulation of the respiratory tract, such as when there is tracheal intubation or during direct laryngotracheobronchoscopy, the risk of respiratory-cardiovascular reflex responses is increased. One of the ways in which this is experienced is in the development of laryngospasm. The respiratory tract contains a rich supply of receptors. The concentration of these receptors is highest in the larynx and the proximal respiratory tract. In addition the proximal receptors are more sensitive to mechanical stimulation than those of the distal respiratory tract which are more chemosensitive.1 Consequently, manipulation of the upper airways is more likely to evoke a laryngospasm response. Laryngospasm, which is frequently preceded by stridor, is a contraction of the muscles of the larynx after a forceful inspiration leading to a blockage of the glottis. Closure of the glottis occurs either due to adduction of the true vocal cords or with the addition of false vocal cord adduction. The supraglottic tissues may also block the glottis as they are pulled downwards due to the pressure gradient formed by the inspiratory air pressure. This is a primitive response with the aim of preventing pulmonary aspiration.2 This blockage prevents inspiration and expiration and can lead to oxygen desaturation, negative pressure pulmonary oedema and hypercapnia.3,4 If allowed to continue, laryngospasm can progress to death.3 Laryngospasm is more common in children than in adults and has been reported to occur in up to 25% of children undergoing general anaesthesia.4 Laryngospasm is most likely at light planes of anaesthesia and thus can be stopped by increasing the depth of anaesthetic or waking the patient. Other mechanisms of breaking the laryngospasm include placing the patient on CPAP or use or a muscle relaxants.2
Similarly, the instrumentation of the airway can lead to post-procedural pain. It may also cause vagal stimulation predisposing to raised intracranial pressure, increased risk of intracranial haemorrhage and haemodynamic changes.5 These all increase the risk of morbidity for the patient and lengthen recovery time. Thus mechanisms to avoid them where possible should be used.
A number of procedures using instrumentation to the airway are liable to increase the risk of laryngospasm and stridor. Direct laryngotracheobronchoscopy (DLTB) is one of those procedures. DLTB is a procedure in which the airway can be assessed from the oropharynx to the main bronchi. This can be used for diagnostic indications with the potential to add therapeutic interventions such as papillomata removal. DLTB in children often uses a rigid endoscope for visualisation of the airways which must be performed in children under general anaesthetic.6 Spontaneous breathing is maintained with a volatile anaesthetic via a nasopharyngeal or oropharyngeal tube.6,7 This allows the visualisation of the airways via the insertion of a rigid endoscope which is passed gradually as distal as the primary bronchi. However this is an especially stimulating procedure due to the use of instrumentation along the length of the trachea and bronchi via the larynx.6 The aim of DLTB is to diagnose conditions which can predispose to breathing difficulties such as sleep apnoea and stridor. These would include laryngomalacia, vocal cord palsy and subglottic stenosis.7 DLTB can also be combined with other procedures to allow therapeutic interventions. For example in foreign body inhalation, a forceps device can be used to allow retrieval of the item. In respiratory papillomatosis a microdebrider can be used to remove papillomata from the vocal cords and proximal trachea.6 In patients with subglottic cyst formation the cyst can be marsupialised using microinstrumentation to resolve the cyst.7 Consequently, DLTB has a dynamic role in the diagnosis and treatment of airway abnormalities.
Due to the risks associated with laryngospasm and the negative impact it can have upon the DLTB procedure, mechanisms to prevent it are desirable. The application of topical local anaesthetics are a commonly used technique to reduce the incidence of laryngospasm, reduce post-procedural sore throat, improve conditions for intubation of the trachea and minimise the vagal stimulation response to instrumentation of the airways.8 The effective use of local anaesthetics can also reduce the dose of volatile anaesthetic required by minimising stimulation of the larynx. Many local anaesthetics are available but the most commonly used for topical anaesthetisation of the larynx is lidocaine.9 Lidocaine is a tertiary amine which is an amide derivative of diethylaminoacetic acid. Lidocaine is extensively metabolised by the liver (~70%) and <10% is excreted in an unmetabolised state by the kidneys.10 Consequently, its metabolism is adversely affected by conditions affecting blood flow to the liver such as in cardiac failure.8 Increased volume of distribution of lidocaine and lower levels of hepatic enzymatic activity in younger children lead to slower clearance than in older children and adults.11 In addition greater vascularity of the respiratory tracts in young children will lead to more absorption of lidocaine.8 The excretion half-life of lidocaine is 90-110 minutes, which lengthens in individuals with sub-optimal metabolism.10 Duration of action of lidocaine has, however, been shown to be much shorter when applied topically to the oral mucosa. Application of 2% and 4% lidocaine to the lower lip results in a local anaesthetic effect for 12 and 15 minutes respectively.8 The degree of absorption of lidocaine is also influenced by the degree of dryness of the oral mucosa with higher peak plasma levels of lidocaine reported in patients with dryer mucosa.9 Lidocaine’s therapeutic effect is due to its blockade of voltage gated Sodium channels. By preventing the inward flow of Sodium ions through the channel pore, neurons and muscles cells are unable to generate and propagate action potentials. Thus, pain receptors in the skin are unable to transmit signals away from the area of injury.12 Both IV and topical use of lidocaine are effective at preventing laryngospasm. However, when used topically the effect is more localised, total dose required is lower and plasma concentration is lower, leading to a superior effect.13
The question of peak plasma levels of lidocaine after topical administration is pertinent due to the relationship between peak plasma levels and toxicity induced by lidocaine. The BNFc recommends a maximum lidocaine dose for topicalisation of 3mg/kg.14 However, the use of doses higher than that is widely reported with some anaesthetists using up to 10 mg/kg.8 The plasma level of lidocaine at which toxicity starts to occur is above 5 µg/ml, but it is unclear how likely the maximum dose of topical lidocaine is to result in plasma levels of lidocaine greater than that. 11 In a study by Eyres et al in 1978 topical administration of lidocaine hydrochloride 4%, 4 mg/kg resulted in some children experiencing plasma lidocaine levels of greater than 5 µg/ml.15 A similar follow up study by Eyres et al in 1983 using the same strength and dose of lidocaine applied topically to the larynx, resulted in a number of children randomly distributed throughout the age ranges experiencing serum lidocaine levels of >8 µg/ml.16 However no children in either of these studies experienced any symptoms of toxicity. In contrast a study by Sitbon et al in 1996 used lidocaine doses of 8-16mg applied topically to the larynx (i.e. at lower doses than the Eyres group studies). In none of the children included in this study was a Cmax of >2.3 µg/ml detected.9 Consequently, it is unclear how likely plasma levels above the maximum recommended are truly experienced in children undergoing lidocaine topicalisation of the vocal cords.
Lidocaine toxicity is a rare but serious complication of treatment with Lidocaine. Diagnosis after topicalisation for endoscopic procedures is very uncommon and this is believed to be a result of the protective influence provided by general anaesthetics meaning that some adverse events such as seizures will only occur at a higher threshold plasma level when used with volatiles such as sevoflurane. 17 In non-anaesthetised adult patients toxic effects have been reported to be seen from 5 µg/ml but once anaesthetised toxic effects are unlikely to be seen at less than 10 µg/ml.18 Lidocaine toxicity, which is more generally seen with IV use, has a variety of troubling effects and is proportional to concentration. Lidocaine is known to induce convulsions, and transient neuropathic symptoms have been reported.10 However in anaesthetised patients these symptoms are rarely seen. Often the first signs of toxicity visible will be effects on the cardiovascular system. Arrhythmias and ventricular fibrillation have been reported to precede cardiac arrest.8,10 Although haemodynamic changes occur less frequently in children and infants than in adults due to a reduced influence of the sympathetic nervous system on baseline cardiovascular rate, severe toxicity can lead to hypotension and bradycardia.10 Although toxic effects are more likely to be seen with intravenous or epidural use, there have been case reports of these effects occurring after topical use. Case studies have been published of adult patients suffering from convulsions soon after receiving lidocaine topicalisation for maxillofacial surgery and bronchoscopy.18, 19
The primary intention of lidocaine topicalisation is to numb the vocal cords and to prevent the laryngeal reflexes. However, this introduces an area of risk due to the potential to interfere with the process of swallowing. Swallowing involves a complex dynamic of muscle action and reflex. The larynx must be pulled upwards to shut the airway thus preventing the aspiration of fluids into the lungs. However, the anaesthetisation of the sensory nerves of the airways can interfere with the process of swallowing increasing the risk of aspiration and subsequent aspiration pneumonia.8 Although some reviews note that no cases of aspiration post-topical anaesthetisation have been reported in children, studies in adults have shown that lidocaine topicalisation leads to unsafe swallowing and aspiration events have been reported. 8,20,21 Consequently, it is common practice to withhold oral fluids from patients for a period after the surgery until normal laryngeal reflexes have returned.8 However, there is a wide variety of practice with different anaesthetists recommending varying time periods. While the excretion half-life and the time period of anaesthetic effect on the lower lip has been noted earlier, it is unclear how low lidocaine concentration needs to fall for sufficient reflex activity to be returned to the larynx and how long to wait after topicalisation to achieve that. One article described a research study which aimed to assess the effect of lidocaine on the upper airway reflexes. 4% lidocaine was applied topically to the larynx of a number of adult study participants at a dose at the lower end of the clinical range. Ammonia was administered to the vocal cords to elicit a chemosensitive response and this response was repeated at set time intervals of 20 minutes. This study found that it took at least around 100 minutes for airway reflexes to return to normal and in many patients took up to 120 minutes. Although the mechanism of stimulation in DLTB patients is mechanostimulatory rather than chemostimulatory the effect is quite similar. This study would indicate that it can take up to 2 hours for a full return to normal airway reflexes.22 However, what remains unknown is what degree of minimum reflex activity is required for safe swallowing. 8
Aims and standards
This project combines a research study arm and an audit arm, and thus had a variety of aims specific to those project arms.
The aim of the audit is to assess the practice among anaesthetists working at RMCH in relation to their dosing of lidocaine during topicalisation and their advice to nurses regarding time until first fluids post-procedurally. Although there are no national standards for these practices, there has been agreement within the paediatric anaesthesia department at RMCH on standards related to dosing and waiting time to first oral fluids post-topicalisation. This audit will assess whether practice is broadly similar and whether all clinicians are following agreed practices within the department. The results of this project will allow clinical leads within the department to decide on the need for further awareness-raising of agreed practices within the department and the possible need to introduce formal guidance. The audit is also aiming to review the frequency of intraoperative and postoperative complications following lidocaine topicalisation of the larynx prior to DLTB procedures, and dosing of lidocaine during those procedures.
The research study arm of this project is aiming to assess what the peak lidocaine levels achieved after airway topicalisation prior to DLTB procedures are. By understanding what peak levels are achieved in comparison to doses administered, it will facilitate the understanding of what the relationship between doses and Cmax are allowing for more flexible dosing of lidocaine if necessary. This will also show whether current practice at RMCH is safe. The primary endpoint will be peak plasma lidocaine levels. Secondary endpoints will include time to peak plasma levels, signs of lidocaine toxicity (only ECG changes and seizures once the patient is anaesthetised will be included), and episodes of post-procedural laryngospasm, coughing and oxygen desaturation. Data from this study will facilitate a review of practice within the department and the possible generation of clinical guidelines at RMCH.
Search methods and metrics
A search of relevant literature was conducted across a number of relevant databases including PubMed, EMBASE and Medline. Articles in languages other than English were excluded as were conference abstracts. A number of searches were completed which combined a number of search terms. The first search combined terms including, “laryngoscopy”, “DLTB”, “bronchoscopy”, “lidocaine”, and “paediatrics”. The second search combined terms including, “lidocaine”, “topical”, “adverse effects”, and “complications”. The third search combined the terms “DLTB”, and “procedure”. Titles and abstracts of all papers were reviewed and relevant articles chosen for inclusion. In addition relevant reference sources identified from the reference sections of the trial protocol and other papers identified through the previous search were included.
Methodology
Research Study
This is a single-arm study with no changes made to standard clinical care provided to the trial participants. In this study 50 children who have been listed for DLTB procedures have been identified and their parents/guardians have been provided with trial information at the pre-operative anaesthetic review. They are approached again on the day of the procedure and recruited to the study if agreed. During the procedure a 2% lidocaine spray will be applied to the larynx supraglotically and subglotically via a mucosal atomisation device. The lidocaine will be administered at a dose of 3-5 mg/kg as clinically required. The procedure will only deviate from standard practice in that a second cannula will be fitted once the child is anesthetised to allow blood samples to be taken. This will be attempted up to a maximum of 3 times and if unable to site the 2nd cannula the child will be excluded from the study. A 1 ml blood sample will then be taken at five minute intervals up to 20 minutes post topicalisation. The samples will be taken for freezing and storage at the biobank at CMFT and once all of the samples are collected will be transferred to Nottingham University hospital’s department of clinical biochemistry for analysis. The samples will be assessed for their lidocaine concentration and will be analysed collectively to indicate the peak plasma level and time to achieve peak plasma level.
Inclusion criteria will be children between the ages of 0-8 years undergoing DLTB for diagnostic or therapeutic purposes, who have been appropriately consented for inclusion in this study. Exclusion criteria will include exposure to lidocaine before the procedure (for example the use of local anaesthetic at the cannula site), >3 attempts needed to site the second cannula, use of medications which alter the metabolism of lidocaine (for example some anti-convulsant medications such as phenytoin or anti-retroviral medication such as darunavir).
A limitation of this arm of the study is that the plasma lidocaine level results will not be available until after this review is completed and thus cannot be analysed as part of this review. However, other endpoints which have been recorded on the day of the procedure e.g. frequency of complications, will be analysed using the results available during the period that this review is being generated.
This study has received appropriate local and national ethical approval and registration:
CMFT Ref.: R03818, Cost Code: IRAS Reference: 156416, REC Reference: 16/NW/0004, Research Study: Lidocaine levels In DLTBs Study, EudraCT: 2014-005207-25
Audit
There are no official guidelines which make recommendations on the duration of time between airway topicalisation with lidocaine and taking oral fluids. However, a number of case studies indicate that it can take up to 120 minutes for full laryngeal reflexes to return. Consequently, the guidance at RMCH is that patients should remain nil by mouth for 2 hours after airway topicalisation. In an effort to assess practice, the medical notes for all patients who had received DLTB procedures over a three month period were obtained and reviewed. Inclusion criteria were all patients who had undergone a DLTB procedure between February and April 2017 at RMCH requiring lidocaine topicalisation. No exclusion criteria were identified. This time period was chosen to facilitate obtaining a sufficient quantity of data to make the results statistically significant while also being feasible to obtain for secretarial staff.
A data collection form had been devised and this was reviewed by the project tutor (see Appendix 1). This form sought information on patient demographics, dose administered, complications and documented time to first oral fluids. After collection the information was collated and analysed.
A limitation of the data collection for complications is that it is unclear if all experienced complications have been recorded. It is also unclear when there have been complications either intra-operatively or post-operatively whether those have occurred due to the effects of Lidocaine or due to other post-surgical factors such as secretion accumulation in the pharynx.
Results and analysis
As part of the audit the notes for all patients who were admitted to RMCH for DLTB procedures between February and April 2017, were reviewed. This amounted to 69 sets of patient notes. Using the notes, required data was collected using the data collection form (see Appendix 1).
The start time for anaesthetics and the time that the patient could have fluids following the procedure were recorded. For the first fluid time the anaesthetic chart was reviewed to see if it was documented by the anaesthetist. The operative notes were also reviewed to see if the nurse had documented the time after the information being handed over by the anaesthetist. The anaesthetist had documented the time to restart oral fluids 61 times (88%). The time had not been documented by the anaesthetist 8 times (12%). Of those occasions where it was undocumented by the anaesthetist, it had been handed over to the nurse and recorded 4 times. This means that on 4 occasions, the time to restart oral fluids was left unrecorded.
When looking at the 65 occasions when the time to restarting oral fluids was documented, the time period has been grouped into 4 time categories; ≤75 mins, 76-105 mins, 106-135 mins and ≥136 mins (see Fig. 1)
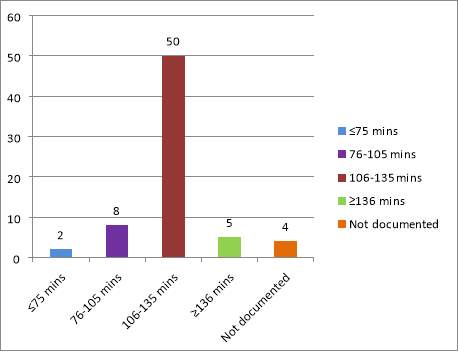
Figure 1. Recommended time periods until oral fluids are restarted after topicalisation with lidocaine pre-DLTB
While on the majority of occasions a time period of 106-135 mins (close to the recommended time period of 2 hours), was documented (76.9% of notes in which time was documented), there was some variance in what different anaesthetists were recommending. On 8 occasions a time of 76-105 minutes was recommended (12.3%), on 5 occasions a time of ≥135 minutes was recommended (7.7%), and on 2 occasions a time of less than 75 minutes was recommended (3.1%).
As part of the audit the lidocaine dosing and its documentation were also reviewed. When documenting how much lidocaine was given anaesthetists could either document the specific dose, or the volume and strength of lidocaine applied. The dose in one of these two forms was specified in 59/69 (85.5%) of the anaesthetic records. On three of these occasions (4.3%) the strength of lidocaine sprayed used was recorded but the volume used was not. Conversely on one occasion (1.4%), the volume was recorded but the strength was not.
Of the 59 sets of notes in which the dose administered was recorded, the dose given in mg/kg was calculated. As can be seen in Fig. 2 a range of doses has been used. Of the children administered >5 mg/kg some quite high doses used were observed. One child was administered a dose of 10 mg/kg, other received doses of 7.92 mg/kg and 6.19 mg/kg for example. Conversely other children were seen to receive very low doses with one child receiving a dose equating to 1.6 mg/kg
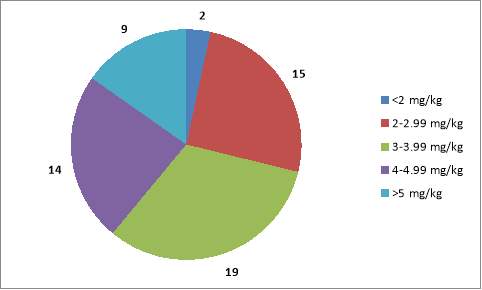
Figure 2. Numbers of patients administered lidocaine within a number of ranges of concentrations.
The rate of complications observed when using lidocaine for DLTB were also recorded as part of the audit. These were separated into intraoperative and postoperative complications. As frequency of possible adverse effects of lidocaine use was desired, the noted side effects of lidocaine were recorded as noted in the summary of product characteristics for the 4% Lidocaine laryngojet.23 Other adverse effects were not included. See Fig. 3. Oxygen desaturations have been reported frequently in 14.5% of the patients (although in all but one of these cases they responded rapidly to oxygen. In one case admission to HDU was required). Laryngospasm has also been observed in 2 of the patients, 2.9%, in the audit.
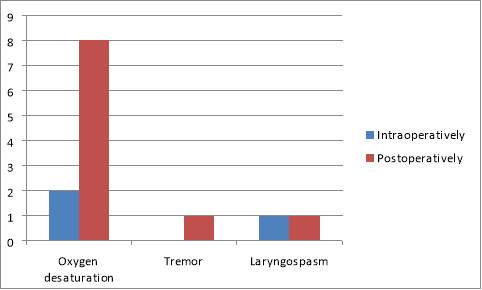
Figure 3. Frequency of reported adverse effects in patients who have received lidocaine topicalisation
Discussion
Strengths and weaknesses
The research section of the project is ongoing and consequently there are no final results from the project, unfortunately. A weakness of this project has been the difficulties in recruiting patients during the period of my involvement with the trial. Due to the relatively small numbers of patients undergoing DLTB procedures during the period of the project there have been few opportunities to engage in the recruitment and data collection part of the trial. In addition, during the delivery of the blood to the Biobank (who are responsible for preparing and then freezing the blood samples), it was noted that the original blood bottles which had been used for sample collection were not suitable for the preparation process. As a result, it was decided that alternative blood bottles would be used. These bottles are currently being ordered and while they are awaited it is not possible to recruit any further patients onto the trial. This has resulted in the trial taking longer to recruit patients to, and thus results longer to generate. It has also been difficult to recruit sufficient numbers of patients onto the trial. Due to the previously mentioned delays with the trial and the necessity for a member of the anaesthetic team to be present at the procedure in addition to the procedural anaesthetist it has not always been possible to recruit all potential candidates onto the trial. In addition on one occasion one candidate had to be excluded from the trial after it was not possible to site a cannula in the required number of attempts. This could prove a limitation of this trial as if this was repeated again, it could lead to children with chronic health conditions who may demonstrate different drug handling characteristics to well children but who may have poor venous access due to repeated procedures being excluded from the trial, thus biasing the results.
This research project however has a variety of strengths. The intervention required for the trial is relatively non-invasive, requiring only a second cannula, and thus it is more likely that parents will assent to their children taking part than if a more invasive procedure was required. The trial procedure follows standard practice closely and thus the results will enable the findings to be mapped to what occurs in practice closely. Due to fact that the study will provide results which show a clear relationship between dose administered, plasma levels and complications experienced it should be possible to make clear decisions about clinical practice within the department using the unambiguous results of the study. Statistical analysis has informed us of how many participants are required to ensure that results are statistically significant, which is 50 children. This cohort is a realistic figure to recruit and thus will ensure that the trial produces statistically valid and useful data.
The audit looked at all the children who had undergone DLTB procedures from February until April 2017. Thus it provided a good picture of what practice was like during a set time period throughout all of the anaesthetics team.
However, there were a number of difficulties with the audit. The audit looked at the rate of complications experienced intra-procedurally and post-procedurally. However, it was not possible to understand whether the complications being experienced were as a result of the use of lidocaine or due to other factors such as the procedure itself. It was also unclear whether all of the adverse effects that the patient experienced were recorded by the recovery staff, e.g. were all instances of oxygen desaturations recorded onto the recovery notes? Another difficulty in mapping the complication to the dose administered is that it is not possible to understand how much lidocaine was truly absorbed. Previous studies have shown significant variation in the plasma lidocaine levels obtained between different individuals administered the same dose of lidocaine in mg/kg.15 Unless it is possible to understand what plasma levels of lidocaine are experienced after a specific dose applied and what factors influence that level, the link between dose administered and complications experienced is opaque. This is something which will be better understood once the results of the research study are available.
The audit also looked at the time recommended by the anaesthetist until oral fluids should be recommenced post-topicalisation. This required good documentation on behalf of the anaesthetist as it was assumed that topicalisation occurred at the recorded anaesthetic start time. If incorrect timings are recorded for the anaesthetic start time then the time until fluids are recommenced will be incorrect. Aspects of documentation have been looked at however and will be commented upon.
Implications of results
One of the focuses within the audit was the time recommended by anaesthetists until patients can recommence oral fluids post-topicalisation. Although no specific guidelines exist, it is standard practice within the RMCH anaesthetic department that 2 hours should be the requisite time between topicalisation and restarting fluids. This measure was developed in response to clinical findings in a variety of clinical studies.22 However, it became clear that a variety of timings were being recommended. As see in Fig. 1, it was documented that 2 patients could have fluids less than an hour and fifteen minutes after topicalisation with lidocaine, and 8 patients could have fluids after between 76 and 105 minutes post-topicalisation; significantly shorter than the time advised. While it has been found that topical lidocaine applied to the lower lip loses effect within 12-15 minutes, some studies have suggested that it can take up to 2 hours for the vocal cords to regain full reflex activity after topicalisation.8,22 It is unknown how much laryngeal reflex activity is required to prevent fluid aspiration, and thus it would seem that applying caution would be judicious. Consequently, the two hour period until oral fluids are recommenced would seem a prudent practice within the RMCH department.
These differences seen in the duration of time between the application of topical lidocaine and restarting oral fluids have clinical implications. The risk of fluid aspiration, which has been previously noted in case studies of adult patients, is increased if an insufficient amount of time is allowed to elapse after lidocaine topicalisation, particularly in this patient population who may have a higher pre-existing rate of laryngeal abnormalities.8,20,21 Fluid aspiration can lead to a chemical or bacterial pneumonia or the development of laryngospasm, and thus must be avoided if possible.
Similarly, it was documented in the notes of 5 patients that they should remain nil by mouth until over 2 hours and fifteen minutes post-topicalisation. Due to the general anaesthetic that patients receive prior to this procedure most will have been starved for several hours before the procedure and consequently may be dehydrated. DLTB procedures are often quite emetogenic and thus administration of oral fluids can help to ameliorate that effect. Consequently, leaving children without oral fluids longer than is necessary is unkind and inadvisable. The rationale for specific anaesthetists to recommend a shorter or longer time period until fluids can be recommenced is unclear. It would be advisable for the views of these anaesthetists to be sought before clinical guidelines are developed.
When the dose of lidocaine applied to the larynx has been reviewed, it is clear that there are a range of doses of lidocaine being used. While the BNFC recommends that a dose of no more than 3mg/kg should be applied, we can see that in the majority of cases doses greater than this are being used.14 Of the 59 cases reviewed, only 28.8% of patients received a dose of less than 3 mg/kg. 32.3% received a dose of 3-4 mg/kg, 23.7% received a dose of 4-5 mg/kg and 15.25% received a dose of >5 mg/kg. In one case a patient received a dose of 10 mg/kg. This would serve to show that it is common practice to prescribe lidocaine in an unlicensed fashion within the department. While the relationship between the dose of lidocaine administered and the plasma concentration experienced as result is unclear, the frequent high dosing should be borne in mind in cases where patients experience adverse effects such as oxygen desaturation and laryngospasm. Previous studies have shown that even with low dosing across a number of subjects, some individuals will experience high plasma lidocaine levels predisposing them to lidocaine toxicity.15 The most striking aspect of the dosing of lidocaine however is the clear range of doses of lidocaine being used between anaesthetists. While in many cases licenced or just above licenced doses are being used, it is clear that many anaesthetists are using doses far in excess of that. It is however unclear why higher doses are being used, given that there was only one reported case of laryngospasm during the topicalisation process reported. Higher doses are likely to increase the risk of adverse effects and thus their use should be minimised where possible. To truly understand the risks associated with this excess application of lidocaine, it will be useful to better understand the relationship between dose applied and plasma concentration. High plasma concentrations would serve to indicate that high doses of lidocaine being used are not prudent. However, lower plasma concentrations would show that it may be possible to use higher doses of lidocaine spray to achieve a better effect.
When the complications experienced by the children in the audit were reviewed a pattern of common adverse effects which may be due to the lidocaine became apparent. In total there were 10 cases of oxygen desaturation including one in which the child needed to be transferred to HDU. The expectation would be that there was a correlation between the doses of lidocaine administered and the complications experienced. However in reality this was not the case. Of the 10 children who experienced oxygen desaturation, a range of lidocaine doses had been administered. These ranged from 2.92 mg/kg to 10 mg/kg but with the majority being near 3-4 mg/kg (just above the BNF recommended maximum of 3 mg/kg). Consequently, this would show that inferring a simple relationship between the dose administered and the likelihood of a complication occurring is not possible. Differences in drug handling between different children due to individual metabolic factors are likely to play a part. Understanding what the relationship between plasma lidocaine levels and the development of complications will be useful. This will enable us to understand how likely the adverse effects of lidocaine toxicity are to occur. However due to fact that even lower doses of lidocaine may be responsible for adverse lidocaine effects, (the child who was admitted to HDU after suffering from oxygen desaturation and respiratory distress received a lidocaine dose of 1.93mg/kg), careful thought may need to be given to the risks of giving greater doses of lidocaine topically. The difficulty of forming a clear plan for lidocaine dosing is compounded by the fact that it can be challenging to understand whether lidocaine or other independent factors are responsible for complications experienced and further study of the links between lidocaine and postoperative complications would be necessary.
One of the other focuses of the audit is documentation practice and how well this is complied with. Good documentation is vital to ensure that in the event of any adverse effects to the patient it is clear what occurred during the intraoperative and postoperative period, both for clinical and pharmacolegal reasons. However, important information was not always documented on the anaesthetic records during the procedure. In only 59/69 (85.5%) of the sets of notes was a dose for the lidocaine documented, meaning that it is difficult to assess a relationship between the dose of lidocaine and any complications which may occur as a result of topicalisation in nearly 15% of the case notes. Similarly, the time until fluids can be restarted was not documented by the anaesthetist 8 times, and of those 8 times, was then documented by the recovery nurse only 4 times. Although this information may have been handed over verbally by the anaesthetist, it is clear that if fluids were given early resulting in aspiration there would have been difficulties in assessing whether proper procedures were followed.
Areas of uncertainty and work for the future
The research study is still running and results will not be available for several months. Once available it will be possible to understand more fully the relationship between dose administered, plasma levels achieved, duration of plasma lidocaine and complications experienced. This will help to inform the findings of the audit more fully, understand whether current practice is safe and make recommendations easier to generate in the longterm. While it is important to understand the relationship between dose and plasma level, it is also important to try to elucidate how frequently the complications experienced are due to lidocaine rather than independent factors. This could be an area of focus for future work.
This audit has flagged up significant variations in practice in relation to time between topicalisation and restarting oral fluids. It is unclear what underlies the differences in practice and thus it would be helpful to engage with the anaesthetists within the team to understand the rationale behind their varying practices. This could help to either devise awareness raising sessions or new departmental clinical guidelines to encourage similar approaches in practice.
Deficiencies in documentation practice have been identified within this study. This has resulted in key information such as dosing and information handed over to nursing staff in recovery not being documented, raising clinical and pharmacolegal issues. Consequently, measures to raise awareness of the importance of this documentation for example with awareness-raising at departmental meetings.
Measures devised to improve compliance with agreed standards within the department and documentation should be reaudited. This should ideally happen once the results of the research study are available.
Conclusion
- Although results from the research study are not available, the data will be valuable in assessing the safety and appropriateness of agreed standards within the department in regard to dosing of lidocaine. Local guidelines can be updated and audit results reviewed in light of these results once available.
- There are significant variations in practice in regard to dosing of lidocaine for DLTB procedures and the time specified nil by mouth after topicalisation. These frequently do not follow agreed clinical standards within the paediatric anaesthesia department at RMCH. In some cases patients have been given doses far above BNFc recommended maximum and time until first oral fluids has been far shorter than local clinical standards recommend. Consequently, it would be advisable for awareness of local standards to be raised among anaesthetists to reinforce these.
- Deficiencies in documentation have been noted. This raises issues from a clinical and pharmacolegal point of view and good practice when documenting should be encouraged.
- Future projects could include working with anaesthetists to understand the variations in practice. Standards of care could be harmonised by awareness raising sessions at departmental training events. Reaudit should be performed after these interventions have taken place to assess their success. It may be wise to carry this out in light of the results of the research study.
References
- Hamaya, Y. and Dohi, S. (2000). Differences in Cardiovascular Response to Airway Stimulation at Different Sites and Blockade of the Responses by Lidocaine. Anesthesiology, 93(1), pp.95-103.
- Gavel, G. and Walker, R. (2013). Laryngospasm in anaesthesia: Table 1. Continuing Education in Anaesthesia, Critical Care & Pain, 14(2), pp.47-51.
- Koç, C., Kocaman, F., Aygenç, E., Özdem, C. And Çekiç, A. (1998). The use of preoperative lidocaine to prevent stridor and laryngospasm after tonsillectomy and adenoidectomy. Otolaryngology – Head and Neck Surgery, 118(6), pp.880-882.
- Mihara, T., Uchimoto, K., Morita, S. and Goto, T. (2014). The efficacy of lidocaine to prevent laryngospasm in children: a systematic review and meta-analysis. Anaesthesia, 69(12), pp.1388-1396.
- Mussavi, M., Asadollahi, K., Abangah, G., Saradar, S., Abbasi, N., Zanjani, F. and Aminizade, M. (2015). Application of Lidocaine Spray for Tracheal Intubation in Neonates – A Clinical Trial Study. Iranian Journal of Pediatrics, 25(1).
- Wilkinson, S., Sudarshan, P., Smyth, A. and Daniel, M. (2015). The role of airway endoscopy in children. Paediatrics and Child Health, 25(4), pp.182-186.
- Watson, G., Malik, T., Khan, N., Sheehan, P. and Rothera, M. (2007). Acquired paediatric subglottic cysts: A series from Manchester. International Journal of Pediatric Otorhinolaryngology, 71(4), pp.533-538.
- Roberts, M. and Gildersleve, C. (2016). Lignocaine topicalization of the pediatric airway. Pediatric Anesthesia, 26(4), pp.337-344.
- Sitbon, P., Laffon, M., Lesage, V., Furet, P., Autret, E. and Mercier, C. (1996). Lidocaine Plasma Concentrations in Pediatric Patients After Providing Airway Topical Anesthesia from a Calibrated Device. Anesthesia & Analgesia, 82(5), pp.1003-1006.
- Donald, M. and Derbyshire, S. (2004). Lignocaine toxicity; a complication of local anaesthesia administered in the community. Emergency Medicine Journal, 21(2), pp.249-250.
- Gunter, J. (2002). Benefit and Risks of Local Anesthetics in Infants and Children. Pediatric Drugs, 4(10), pp.649-672.
- Cummins, T. (2007). Setting up for the block: the mechanism underlying lidocaine’s use-dependent inhibition of sodium channels. The Journal of Physiology, 582(1), pp.11-11.
- Beringer, R., Skeahan, N., Sheppard, S., Ragg, P., Martin, N., McKenzie, I. and Davidson, A. (2010). Study to assess the laryngeal and pharyngeal spread of topical local anesthetic administered orally during general anesthesia in children. Pediatric Anesthesia, 20(8), pp.757-762.
- Joint Formulary Committee (2017). LIDOCAINE HYDROCHLORIDE | Drug | BNFc Provided by NICE. [online] Bnfc.nice.org.uk. Available at: https://bnfc.nice.org.uk/drug/lidocaine-hydrochloride.html#indicationsAndDoses [Accessed 15 Jun. 2017].
- Eyres, R., Kidd, J., Oppenheim, R. and Brown, T. (1978). Local anaesthetic plasma levels in children. Anaesthesia and Intensive Care, VI(3), pp.243-247.
- Eyres, R., Bishop, W., Oppenheimer, R. and Brown, T. (1983). Plasma lignocaine concentrations following topical laryngeal application. Anaesthesia and Intensive Care, 11(1), pp.23-26.
- Karasawa, F. (1991). The effects of sevoflurane on lidocaine-induced convulsions. Journal of Anesthesia, 5(1), pp.60-67.
- Mehra, P., Caiazzo, A. and Maloney, P. (1998). Lidocaine toxicity. Anesthesia Progress, 45, pp.38-41.
- Wu, F.-L., Razzaghi, A. and Souney, P. F. (1993), Seizure After Lidocaine for Bronchoscopy: Case Report and Review of the Use of Lidocaine in Airway Anesthesia. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 13: 72–78
- Lester, S., Langmore, S., Lintzenich, C., Wright, S., Grace-Martin, K., Fife, T. and Butler, S. (2013). The Effects of topical anesthetic on swallowing during nasoendoscopy. The Laryngoscope, 123(7), pp.1704-1708.
- Bastian, R. and Riggs, L. (1999). Role of Sensation in Swallowing Function. The Laryngoscope, 109(12), pp.1974-1977.
- Raphael, J., Stanley, G. and Langton, J. (1996). Effects of topical benzocaine and lignocaine on upper airway reflex sensitivity. Anaesthesia, 51(2), pp.114-118.
- Medicines.org.uk. (2017). Lidocaine Hydrochloride BP Laryngojet 4% – Summary of Product Characteristics (SPC) – (eMC). [online] Available at: http://www.medicines.org.uk/emc/medicine/4891 [Accessed 30 Jun. 2017].
Appendices
Appendix 1- Data collection form for audit
Data collection form DLTB project audit
Date of collection: _____________ Patient identifier:________________
Date of operation: _____________
Patient age: _______________ Patient weight: ________________
Time of topicalisation: ____________
Dose/ volume/ strength of Lignocaine:_______________________
Documented time until first post-op oral fluid: __________________
Intraoperative complications: __________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Postoperative complications:
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Surgical Studies"
Surgery is the branch of medicine that involves the treatment of injuries, diseases and other conditions by operative methods, i.e. by cutting open the body and removing or repairing a damaged part. Technological advances mean that many surgeries can now be performed without large incisions using what is known as keyhole surgery.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: