Long-Acting Intraocular Delivery Strategies for Age-Related Macular Degeneration
Info: 10973 words (44 pages) Dissertation
Published: 21st Feb 2022
Tagged: Medical
Abstract
Age-related macular degeneration (AMD) is one of the leading causes of central vision loss in the elderly population. Treatment regimens for AMD, especially with biological agents, are complicated due to anatomical and physiological barriers, as well as administration of high doses and frequent regimens. Some clinical examples include monthly intravitreal administration of anti-VEGF antibody Lucentis® from Genentech and aflibercept (Eylea®) from Regeneron Pharmaceuticals. Long-acting sustained intraocular drug delivery provides promising solutions, such as Vitrasert® from Bausch & Lomb, an intravitreal implant made of a biodegradable polymer, poly (D,L-lactic co glycolic acid) (PLGA), and can be used as a guiding reference to formulate sustained delivery systems. In this review, we discuss the anatomy and physiology of the eye, barriers to delivery, pathology of AMD, opportunities for biological therapeutics, and future prospects of intraocular delivery strategies that are in development for treatment of AMD.
Keywords: Anatomy of the eye, age-related macular degeneration, intraocular delivery strategies, biological therapy, sustained delivery, PLGA microspheres
1. Anatomy and Physiology of the Eye
The orbit is a pyramidal, bony cavity in the facial skeleton, which consists of a base anterior and apex posterior. The eyeballs are present inside the orbit accompanied by the lacrimal apparatus. This enclosion protects the eyeballs along with ocular muscles, nerves and vessels. The major contents of the orbit are the eyeball, optic nerve, ocular muscles, fascia, nerves, vessels, fat, lacrimal glands and sac. (Figure 1) [1].
1.1 The Outermost Layer
Sclera and Cornea are present in the outermost layer of the eye that consist of fibrous tissues.
The sclera is the fibrous opaque part of the eyeball, which covers the posterior part of the eye. The white of the eye is referred to the anterior part of the sclera and can be seen through the transparent bulbar conjunctiva. The sclera, which continues as the cornea, originates from the limbus and mainly consists of collagen fibers and proteoglycans, and embedded in the extracellular matrix. The anterior most layer of the eye is the cornea, which is further divided into epithelium, stroma and endothelium, all of which have a different polarity for drug absorption. The cornea acts as a physical barrier for the eye and protects against entry of any exogenous substances. The corneal epithelium, which makes 90% of the cornea, is lipophilic in nature. Its’ superficial epithelial cells are joined together by desmosomes, which are surrounded by tight ribbon like junctions called zonula occludens. Stroma, comprised of collagen fibers arranged in a lamellar pattern forms the middle layer while the innermost layer is the endothelium, which is a monolayer of hexagonal shaped cells. The endothelium serves as a barrier between the stroma and the aqueous humor[1]. Most of the drugs delivered to the outermost layer of the eye are considered under the non-invasive route of administration [2] and explained in sections 2.1 and 2.2
1.2 The Middle Layer
The middle layer of the eye is vascular in nature and consists of the choroid, ciliary body and the iris. The choroid is a vascular dark brown membrane that is present between the sclera and the retina. It attaches firmly to the pigment layer of the retina and culminates anteriorly into the ciliary body. The choroid acts as a barrier for drug penetration into the neural retina. The second most important part of the middle layer of the eye is the ciliary body that connects the choroid with the iris. Anterior folds on the ciliary are called ciliary processes, which fill the anterior and posterior spaces of the eye with the aqueous humor, a fluid secreted by the ciliary processes that provides nutrients to the cornea and the lens. The iris is the third most important part of the middle layer of the eye. The iris is present in the anterior surface of the lens and is a thin contractile diaphragm with a central aperture called the pupil. The main role of the pupil is to transmit light. The size of the pupil varies depending on how much light enters the eye. The sphincter pupillae and the dilator pupillae help control the size of the pupil by closing and opening [1]. Intraocular and intravitreal deliveries of a therapeutic agent is administering the drug to the middle layer of the eye [2] and described in section 2.2
1.3 The Inner Layer
The inner layer the eye is called the retina and it governs the visual properties by receiving and absorbing light. The retina is composed of the optic, iridial and ciliary. The optic part of the retina is further divided into the neural layer and the pigmented layer. The neural layer helps receive light and the pigmented layer, consisting of single layer of cells, assists the choroid to absorb light and reduces the scattering of light in the eye. The ciliary and the iridial are pigmented layers of the retina and provide support to the cells over the ciliary body in the middle layer of the eye and the iris. The optic disc, also called optic papilla, is a circular disc, which is present in the fundus, the posterior part of the eye. The optic disc helps the optic nerves enter the eyeball. The optic disc is also referred to as the blind spot of the eye as it contains nerve fibers but no photoreceptors. As a result, it becomes insensitive to light and does not aid in visual properties. Lateral to the optic disc is a small oval area called macula lutea, also referred to as the yellow spot that consist of specialized photoreceptors responsible for acute vision. It alsoconsists of a central depression called the fovea centralis which is the area of most acute vision. The retina receives blood from the central artery of the retina that goes to all parts except the rods and cones. The neural layer of the optic part consists of rods and cones, which receive nutrients from the choriocapillaris [1]. Sub retinal delivery explained in section 2.2 is an example of delivery to the inner layer
2. Intra-Ocular Drug Delivery
Drug delivery has long been an area of interest for scientists due to its potential benefits of providing maximum efficacy with minimum risk to the patient. In order to provide maximum efficacy, a stable shelf life for the drug becomes of utmost importance. With large macromolecules such as proteins and peptides, this can be quite challenging since these therapeutic agents can undergo physical and chemical changes during storage. Need for high concentration drug product(typical for biotherapeutics) could potential cause issues such as viscosity, solubility and aggregation [3]. However, for ocular drug delivery, a formulation scientist not only has to deal with the shelf life stability of the bio therapeutic, but also assess the probable challenges that could likely come up because of delivery to a sensitive area. These challenges are mostly associated to the anatomy of the eye and we have classified them into non-invasive and invasive routes of administration in this review [4].
2.1 Non-Invasive Administration
Historically, routes of drug administration for small molecule therapeutics has typically been non-invasive, with a large majority of them being oral and topical administrations. Topical administrations include ocular, dermal, vaginal, nasal, and buccal applications[4]. Non-invasive route of administration provides complete bioavailability for a small molecule entity when compared to a biological entity. Non-invasive medications are also associated with higher patient compliance due to ease of administration. When comparing a pill to an injection, a pill taken at home is preferable to an injection requiring a hospital visit. However, as mentioned previously, non-invasive ocular drug delivery poses unique challenges. Precorneal and anatomical barriers restrict topical administration of eye drops for treatment of eye diseases. Factors such as blinking, tear film and tear turn-over wash off the topical drug administered into the eye causing reduced ocular bioavailability [4]. The lipophilicity of the corneal membranes helps the passage of moderately lipophilic small molecules but nonetheless hinders the permeation of biologic entities, which are typically hydrophilic and large molecular weight entities [5]. A study conducted by Nomoto, et.al showed a topical bevacizumab administration in the iris, choroid, vitreous of rabbits could not achieve therapeutic concentrations, despite aggressive dosing [6]. These undetectable concentrations of the therapeutics agents even after rigorous dosing of the drug is due to the physical barriers faced during topical administrations such as tear drainage [6].
2.2 Invasive Administration
Due to the aforementioned low bioavailability issues with non-invasive therapies, invasive routes of drug administration are considered while working with biological entities such as proteins and peptides. During intra ocular delivery, the drug is directly targeted to the site of action in order to minimize the drug to travel through various ocular barriers as well as to decrease off target effects. However, ocular delivery is challenged by low patient compliance and increased risk of infection, since most of the drugs delivered to the eye requires multiple dosing. Invasive administration techniques, such as injectable, can be divided into periocular space, intra-ocular, and suprachoroidal [4]
Within the periocular space, drug delivery is possible through either the subconjunctivalor the sub tenon region [4]. An injection into the bulbar conjunctiva or superficially to the sclera helps achieve delivery directly into the subconjunctival space. However, limitations such as rapid clearance of the therapeutic agent, large pore diameter of the intracellular spaces of the scleral fiber could affect the drug delivery into this space. An injection into the fibrous membrane called the tenon’s capsule delivers the drug into the sub tenon space. Large volumes of drug formulations can be injected through this space, although the administration comes with complications such as pain, subconjunctival hemorrhage, optic nerve damage, retinal ischemia etc. [5]This space could be explored as an alternative to intravitreal injections [4]
Intrastromal delivery, which falls under the intra ocular space, is a delivery mechanism used to target the corneal stroma. Due to the densely packed collagen fibrils and proteoglycans of the stroma, large hydrophilic molecules such as proteins and peptides can easily diffuse through the stromal membrane [7]. Intracameral delivery is yet another delivery route used to inject drug solutions into the anterior segment of the eye. However, studies have shown that the concentration of the drug in the posterior segment of the eye is low when delivered intra-camerally [5]. Intravitreal administration is the most common form of administration when it comes to ocular drug delivery systems [7]. The therapeutic agents are delivered into the posterior segment of the eye, which has the capability to hold about 20-100ul of the solution without causing any visual impairment. Currently, the treatments available for wet AMD use intra vitreal drug delivery route [5].
Suprachorodial delivery helps to place the therapeutic agents right at the tissue site of action, which for most of the ocular diseases is either the choroid or the retina. Suprachoroidal injections delivers the drug in the suprachoroidal space, which can hold about 1ml of the drug formulation [5]. Recent studies have shown that sustained delivery systems ranging in a size of 20-100nm can be retained in the suprachoroidal space for a longer time as compared to small molecules and biopharmaceuticals [8]. Sub-retinal delivery is used to overcome the barriers of the retinal membrane and the retinal pigment epithelium (RPE). These injections are made either through transcleral route (passing through the pars plana and vitreous) or through the transcorneal route (passing through the iris, lens and vitreous [2]). This route can be used for the injection of macromolecules; however, the long term safety has not been fully studied [2].
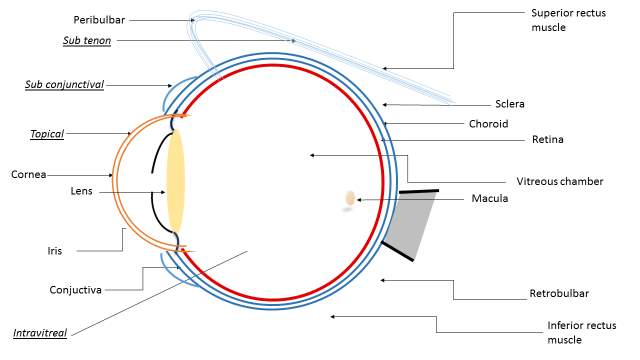
Figure 1: Eye anatomy withRoutes of administration for ocular drug delivery. Underlined: Routes of delivery
3. Pathophysiology of AMD
AMD is a degenerative retinal disease that damages the macula and causes permanent loss of central vision [9] . The macula is the most sensitive part of the retina located at the back of the eye. It consists of light sensitive cells that provide sharp central vision. For acute vision, the retina turns light into electrical signals and then sends these signals to the brain through the optic nerve These light signals are further translated into images by the brain that help an individual to see clearly. However, in patients with macular degeneration, the acuity of the vision is compromised and the center of the visual field may appear blurry or distorted
The risk of developing macular degeneration increases with age. According to the National Eye Institute, AMD is most widely seen in patients above the age of 70 [10]. VanNewkirk, et al. reported that as many as 20% of the patients suffering from macular degeneration are above the age of 60 and about 40% are above the age of 75. Early onset of the disease is mostly referred to as age related maculopathy, which then progresses into AMD [10].
The exact cause and underlying mechanism leading to AMD is still unknown, however the risk factors associated with AMD are multi-factorial which play a crucial role in the onset of the disease. Factors such as age, social class, gender, ethnicity, race and family history are connect to the pathophysiology of AMD. Recently, a study performed in a group of three racially similar populations from North America, Australia and Europe, suggested AMD was less prevalent in native African people and Aboriginal Australians, and statistically seemed more prevalent in European Americans [10].
AMD can be classified into atrophic or nonexudative, also referred to as “dry” form, and neovascular or exudative, also referred to as “wet” form. Visual loss of activity is associated with choroidal neovascularization (CNV) which includes complex pathways such as vascular endothelial growth factor (VEGF), hepatocyte growth factor, platelet derived growth factor (PDGF), tumor necrosis growth factor, chemokines, ephrins, and angiopoietins [10]. The phenomenon in which there is excessive growth of the blood vessels originating from the choriocapillaries and spreading through the retinal pigment epilthelium through the Bruch’s membrane is described as Choroidal Neovascularization. Upregulation of angiogenic factors such as VEGF is the key element responsible for the development and progression of CNV leading to AMD. Among AMD patients, the “dry” form is considered the most common form of AMD, occurring in almost 90% of patients. The progression of the dry form is very uncertain .The dry form of AMD tends to progress slowly, enabling early diagnosis advancing either into the wet form or sometimes progresses drastically and causes visual impairment without turning into the wet form. [10]. Statistically, patients showing symptoms of dry AMD have a 4-12% chance of developing choroidal neovascularization, which leads to the formation of wet AMD, and eventually complete blindness. Dry AMD is characterized by changes in the pigmentation of the retinal pigment epithelium (RPE) and Drusens, which are soft, round shaped deposits in the macula region that can be easily observed as yellow or white spots in the fundus of the eye. The size range of the drusen can be between 63 microns or even as high as 500 microns. The larger the size of these drusens, the greater chance the patient will progress from dry to wet AMD within 3-5 years. Pigment clumping inside the RPE is another factor that can lead to wet AMD. The “wet” form of AMD is rare and only seen in 10% of the AMD patient population [10]. However, the “wet” form is considered the most severe since it involves the formation of new blood vessels that grow and leak blood and fluid under the macula, potentially causing retinal detachment, scarring and impairment to the visual acuity. If the “wet” form of AMD is untreated, functional blindness may result within 2 years from the onset of disease [10]
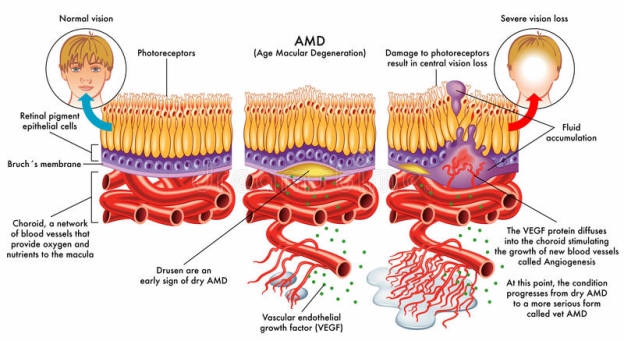
Figure 2: Age-related macular degeneration. Progression of the disease and comparison with normal vision. Image available online. No permission needed
4. Biological Therapies for AMD
4.1 Current Therapies for AMD
Current therapies prescribed for age related macular degeneration slow down the progression from dry form to wet form to avoid complete loss of visual acuity. Marketed therapies include treatment with one of the two Anti VEGF agents, namely, intravitreal administration of 2 mg of aflibercept (Eylea; Regeneron, Tarrytown, NY) monthly for the first month, followed by a dose once every two months and the second anti-VEGF agent, ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA), is dosed monthly at 0.5 mg via intravitreal administration
Historically, photodynamic therapy (PDT) was widely used for the management of AMD. This is achieved with laser therapy to either cause photocoagulation or along with a special medicine that works when exposed to light [11]. Augustin, et al., showed that the patients treated with PDT suffered from persistent neovascularization and about 47% of these patients relapsed within 5 years [12]. Unfortunately, due to the relapse of neo vascularization, even after treatment, these patients suffered from severe visual loss. To avoid choroidal neovascularization (CNV) relapse, verteporfin (Visudyne; Novartis) was administered to the patients starting in 2001[13]. Treatment with verteporfin and PDT helped to damage choroidal neovasculature, but the damaged neovascular would release coagulants and vasoactive factors resulting in the formation of fibrin clots and vasoconstriction. Due to these findings, treatments with PDT and verteporfin are used less frequently to treat AMD [13].
Following the use of PDT for management of AMD, researchers investigated an advanced approach for targeted therapy by synthesizing drugs directed at VEGF, since VEGF plays a crucial role in the onset of CNV resulting in AMD. Pegaptanib Sodium (Macugen; Pfizer) received food and drug administration (FDA) approval in 2001 as the first therapeutic RNA human aptamer targeting VEGF-165 isoform and exhibited an antiangiogenic effect [14]. However, Macugen injections slowed down the progression of the disease by decelerating CNV formation and improving visual acuity. [15]. Following the introduction of Macugen, Ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA) received FDA approval in 2004. Ranibizumab was considered more efficacious based on Phase III randomized clinical trials MARINA (646 patients recruited) and ANCHOR (379 patients recruited) in which ranibizumab showed improved safety and efficacy compared to Macugen [15]. Structurally, ranibizumab is a monoclonal antibody fragment with a molecular weight of 48kDa and specifically binds to VEGF-A isoforms and helps to minimize the severity of AMD [9]. However, patient compliance declined because ranibizumab required intravitreal administration [4], which increased the chances of site infection due to the need for multiple dosing therapy. Bevacizumab(Avastin,Genentech, Inc., South San Francisco, CA), a humanized monoclonal antibody with a molecular weight of 149kDa, received FDA approval in 2004 for treatment of colorectal cancer [9]. However, this anti angiogenic agent is used off-label to treat “wet” AMD and shows similar pharmacological properties when compared to ranibizumab. Due to the smaller size of ranibizumab, its vitreous half-life is shorter as compared to a larger size molecule like bevacizumab, which has a longer serum half-life and increasing the risk of systemic adverse events. One clinical study comparing PDT and bevacizumab found improved visual acuity and retina thickness after treatment with bevacizumab [16].
Later in 2011, a new drug, aflibercept, a recombinant fusion protein (Eylea; Regeneron, Tarrytown, NY), received FDA approval for treatment of AMD. The mode of action of this protein is to bind to all isoforms of VEGF-A and placental growth factor. Compared to bevacizumab, aflibercept has 10 times higher binding efficacy to VEGF [16]. VIEW I and VIEW II clinical trials of “wet” AMD patients, investigated the efficacy and safety of aflibercept[16]. In a clinical trial (NCT01958918) of patients who received aflibercept every 2 months for three subsequent months, researchers found it had similar efficacy when compared to ranibizumab. Based on these results, aflibercept is considered more beneficial, when compared to ranibizumab due to its low burden on the dosing regimen.
4.2 Emerging Treatment Strategies for AMD
Despite the availability of current anti VEGF treatments such as Ranibizumab, Eylea, researchers continue to work on better differentiating treatment strategies for AMD. One of the key challenges with the treatment of AMD is the need for multiple dosing as mentioned in section 4.1. In order to overcome this barrier, researchers are working to come up with long acting formulations of the existing anti VEGF molecules. Studies are also being conducted in exploring other angiogenic targets such as PDGF, C5 complement systems, integrin’s, gene therapy etc. in order to provide better treatments than the existing ones. In this section, we review different strategies that demonstrate promising potential for treatment of AMD in the near future.
4.2.1 New anti-VEGF therapies
Anti VEGF agents are currently the first line of treatment for AMD. However due to current treatment being limited to monthly intravitreal administration, it reduces patent compliance and increases the risk of serious infection.
Brolucizumab (RTH258) formerly ESBA1008, Alcon, Fort Worth, TX is a smaller humanized antibody fragment (scFv), and in clinical trials for the treatment of AMD caused by choroidal neovascularization [13]. The fragment is around 26kDa and due to its smaller size, exhibits an increase in ocular retinal space penetration as well as faster systemic clearance, thus reducing long exposure to systemic circulation [14]. A phase III clinical trial (NCT02307682) was initiated that compared Brolucizumab with aflibercept. The current recommended dose for aflibercept is 2mg (0.05ml or 50ul) administered by intravitreal injection monthly.[17] Most recent data released from this trial in Nov 2017 showed that brolucizumab could successfully provide a 12-week treatment frequency during the first 12-week cycle and could exhibit the effect of the drug in a quarterly interval up to week 48[44]. Positive Phase III data from brolucizumab helps establish a treatment regimen of intravitreal injection once every three months instead of current monthly injection thus increasing patient compliance and reducing the risk of exposing the patients to serious infection due to multiple dosing. Additional success in terms of sustained delivery for treatment of AMD is the most recent approval(August 2018) of a supplemental filing from Regeneron with respect to a 12-week dosing of aflibercept, actions by the FDA to be expected in the coming months.
Another anti-VEGF agent that binds to all VEGF-A isoforms is the designed ankyrin repeat proteins (DARPin) molecule from Allergan (Irvine, CA). AGN-150998, a type of DARPin, binds specifically to VEGF-A exhibiting high potency and shows a reduction in vascular leak during in vitro rabbit studies and reduced neovascularization in the anterior segment of the eye in rat models [18]. AGN-150998 is in Phase III clinical trials (NCT02462486) since 2015. Allergan announced via press release in July 2018, that the drug showed non-inferiority as compared to lucentis in Phase III trials.
Conbercept, a soluble receptor decoy blocks all forms of VEGF-A, VEGF-B, VEGF-C and platelet growth factor (PIGF), in contrast to VEGF-A only, is from Chengdu Khaghong Pharmaceutical Group in Chengdu, China is in multiple Phase III clinical trials(NCT03577899) [9]. The decoy is composed of the second Ig domain of VEGFR-1 and the third and the fourth domains of VEGFR-2 with an Fc region of human IgG[19]. In a phase I clinical trial of conbercept, the treatment was effective in improving visual acuity at 3 and 12 months at 0.5 and 2.0mg of intravitreal injections respectively [20].
4.2.2 Other anti-angiogenic targets
Platelet derived growth factor (anti-PDGF-BB)
Platelet derived growth factor (PDGF) is a group of four polypeptide chains of PDGF viz: PDGF-A, B, C and D. Each of its isoforms can combine to form a heterodimer such as PDGF-BB or its homodimer. Pericytes present on the endothelial cell surface plays a crucial role in the maturation cascade of angiogenesis. PDGF-BB binds to the PDGF receptor B found on the pericytes and plays an important role in regulation, survival and maturation of the cells [21]. By blocking PDGF-BB and administering a VEGF, inhibitor there is a potential to regress mature neovascularization thus making CNV available for a VEGF medication [21]. Studies have shown that a combination therapy of anti-PDGF and anti-VEGF can successfully regress CNV while an anti VEGF monotherapy cannot [19]. Opththotech, a New York based company is currently developing a 32-mer PEGylated DNA aptamer Fovista (formerly E10030) that binds and inhibits PDGF-BB. In a randomized, phase III clinical trial, Fovista was administered in combination with ranibizumab (NCT01944839). In late 2016, the drug company announced that the pre specified endpoints of the study were not achieved when the combination therapy of Fovista and ranibizumab was compared to the monotherapy of ranibizumab. The drug company also entered into another phase III trial comparing the combination therapy of Fovista with either aflibercept or bevacizumab. These results were also disappointing as the monotherapy of the two compounds showed much better improvement in visual acuity as compared to the combination therapy (clinicaltrails.gov NCT01940887). This molecule was terminated and withdrawn from clinical trials. REGN2176-3 (Regeneron, Tarrytown, NY) is also a combination therapy antibody targeting PDGF-B in combination with aflibercept. REGN2176-3 was evaluated in a Phase II clinical trial (NCT02418754) [22]. Most recent data also shows disappointing results from Phase II for this compound, which further lead to termination of the clinical study. No additional efficacy was seen as compared to aflibercept (clinicaltrials.gov)
Complement C5 protein
Complement proteins such as C3,C5b-9,CFB and CFH are high in the plasma levels in AMD patients and are also detected in drusens and AMD lesions[23] Studies have also shown genetic evidence which suggest that the complement system maybe dysregulated in AMD patients. Researchers are now trying either to inhibit the complement activation systemically or locally to suppress the formation of choroidal neovascularization (CNV)[24][25]. Inhibition of C3, C5b-9, CFB and MAC complement have shown to suppress CNV in animal models[26]. Novartis’s C5 target monoclonal antibody LFG316 has completed Phase II clinical trials in the treatment of wet AMD. The results from the trial are currently posted (NCT01535950).[23] Yehoshua et.al and Garcia Filho et. al have published and discussed Alexion pharmaceuticals C5 targeting monoclonal antibody eculizumab for the treatment of dry AMD (NCT00935883). Ophthotech C5 aptamer zimura is currently ongoing phase II clinical trial in combination with lucentis for neovascular AMD(NCT02397954)[23]
Integrin
Integrins’ are transmembrane proteins composed of a and b subunits that dimerize to form more than 20 variant receptors which mediate different responses such as control of gene expression, spreading and migration and growth and differentiation of the cells. These transmembrane proteins mediate the connection between a cell and its extracellular membrane. Integrin ligands are the fibronectin, vitronectin, laminin and collagen [27]. Studies suggests that some integrins such as αvβ1,3 and 5 are overexpressed in the macula of patients with AMD caused by CNV[28]. A
chimerical monoclonal antibody, volociximab (Ophthotech Corporation, Princeton, NY), is an agent that blocks the binding of fibronectin to the αvβ1 integrin [27]. Due to its lack of permeability into the retinal space, volociximab was administered along with ranibizumab in early phase I clinical trial. The trial showed a good improvement in visual acuity after 9 weeks of first administration [29].
Tissue Factor (TF)
In the presence of an inflammatory response, the vascular endothelial cells, monocytes and macrophages express tissue factor (TF). The presence of TF induces the expression of pro-inflammatory cytokines, which increases the inflammatory response and activates the coagulation cascade [30]. TF is highly expressed in new vessels of the choroidal neovasuclar membrane and is not present in normal vasculature [22]. ICON-I (Iconic Therapeutics, South San Francisco, CA) is a human fusion immunoprotein, which selectively antagonizes TF. ICON-I is currently being studied in a phase II clinical trial (NCT02358889) to evaluate the safety, bioactivity and pharmacodynamic response of combination therapy with ranibizumab and to compare the monotherapy of ICON-I with the monotherapy of ranibizumab [22]
Macrophages associated with Age- Related Macular Degeneration (AMD)
In some studies, there has been corroboration that the eyes of aged mice showed the presence of Rho associated kinase (ROCK) signaling and M2 like macrophage accumulation in comparison to normal eyes suggesting the possibility of macrophages being involved in macular degeneration [31] RhoA and RhoE are upstream of ROCK which forms two isoforms ROCK1 and ROCK2[31] Zandi et al showed that when a person ages, the ROCK2 signaling increasing which results in expression of pro angiogenic M2 like macular degeneration associated macrophages. Increased levels of IL4, CD163 were also found in diseased mice which could become potential biomarkers for the treatment of AMD[31] Selectively inhibiting ROCK2 isoform instead of ROCK1/2 showed an increase in M1 biomarkers such as CD80 and CCR7 which are essential to maintain healthy eyes[31] Questioning the existence of macrophages in the pathophysiology of Age Related macular Degeneration(AMD) can open new possibilities for an effective treatment
Gene therapy
Advances in gene therapy are making it possible to implant a protein and inhibit VEGF activity that leads to CNV. The Cas9 protein, with a guide RNA forming the ribonucleoproteins (RNP’s), was administered subretinally into an adult mouse eye and effectively reduced CNV [32]. Companies, such as Genzyme and Adverum (formerly Avalanche Therapeutics), are also developing an adeno-associated virus (AAV) to deliver long-lasting gene therapy to treat AMD. A genetically sequenced adenovirus containing the VEGF blocking tyrosine kinase inhibitor sFLT01, showed a reduced retinal thickness when compared to an intravitreal injection of anti-VEGF agents in an early phase clinical trial [33].
Table 1: Clinically approved and emerging biological drug candidates from the treatment of AMD
| Product
Name |
Molecule Structure | Biological Target | Commercial
Source |
Clinical Trial | Current Status |
| Macugen (Pegaptanib sodium) | Single chain antibody Fv | VEGF | Alcon | NCT00406107 | Marketed product |
| Lucentis (ranibizumab) | Monoclonal antibody | VEGF | Novartis
/Genentech |
NCT01948830 | Marketed product |
| Avastin (bevacizumab) | Monoclonal antibody | VEGF | Roche
/Genentech |
NCT00593450 | Marketed product, off-label for the treatment of AMD |
| Eylea (aflibercept) | Fusion protein | VEGF | Regeneron | NCT02309281 | Marketed product |
| Brolucizumab (RTH258) | Single chain Fv | VEGF | Novartis | NCT02307682 | Phase III clinical trial |
| Abicipar Pegol (AGN-150998) | DARpin | VEGF | Allergan | NCT02859766 | Phase 1 clinical trial complete |
| Conbercept | Fusion protein | VEGF | Chengdu Kanghong | NCT03577899 | Phase III clinical trial |
| Fovista | PEGylated aptamer | Anti PDGF-BB | Ophthotech | NCT02214628 | Terminated after Phase II clinical trial |
| REGN2176-3 | Monoclonal antibody | Anti PDGF | Regeneron | NCT02418754 | Terminated after Phase II clinical trial |
| Retinostat | Lentiviral | Anti angiogenic | Oxford Biomedical/
Sanofi |
NCT01301443 | Phase I clinical trial is complete |
| NT-503 | Encapsulated cell technology | Implantation of ciliary neurotropic factor | Neurotech | NCT02228304 | Terminated after Phase 1 |
| LFG316 | Monoclonal antibody | C5 | Novartis | NCT01535950 | Completed Phase II |
| Eculizumab | Monoclonal antibody | C5 | Alexion | NCT00935883 | Completed Phase II |
| Zimura | Aptamer | C5 | Ophthotech | NCT02397954 | Completed Phase II |
5. Long-Acting Intraocular Formulations
Sustained delivery and or long acting formulations that came into existence in the early 70s, were mainly prescribed to enhance already existing therapeutic agents for improving efficacy and patient compliance. It is a mechanism by which the drug is delivered to the target site over a period, maintaining steady state levels. Sustained delivery helps in reducing adverse effects, which is generally exhibited as peak/trough kinetics when using systemic delivery. The need for multiple dosing is also minimized by developing sustained delivery formulations [7]
In order for a formulation to be long acting, the active agents is released with zero order kinetics. This release is possible by using either biodegradable or non-biodegradable polymers. In the early 80s, ganciclovir, produced by Roche, was used in acquired immunodeficiency syndrome (AIDS) patients who showed cytomegalovirus (CMV) retinitis. Intravitreal injection of ganciclovir, an anti-viral low molecular weight entity, was used to treat CMV retinitis. However, the half-life of this drug was as short as several hours, which in turn required multiple dosing. Later, Bausch created a second-generation drug called Vitrasert, which is a clinical effective intravitreal implant constructed with biodegradable polymers to release ganciclovir over a period [7].
For treatment of AMD, patients are prescribed with anti VEGF agents that needs to be intravitreally administered once every month to regress CNV and sustain efficacy. An exception is aflibercept treatment that requires intravitreal administration once in two months. This still poses the issue of shorter duration of effect and the need for multiple dosing to achieve maximum efficacy, which further reduces patient compliance.
Multiple dosing intravitreal administration comes with a risk for adverse events such as post injection infectious endophthalmitis, intraocular inflammation, retinal detachment and a rise of intraocular pressure. Due to the need of repeated injections of anti VEGF agents, high cost and associated economic burden falls onto the patient responsibility [22]. To overcome these challenges, researchers are working on sustained delivery agents in order to minimize repeated intravitreal injections of anti VEGF agents, which thereof reduces the risks of adverse events and decrease the economic burden on the patient population. In the following sections, we will discuss about long acting formulation strategies involving encapsulation of anti VEGF agents using biodegradable polymers such as poly lactic co glycolic acid (PLGA).
5.1 Types of Polymers
Non-Biodegradable Polymers
Non-Biodegradable polymers are advantageous over biodegradable polymers as they provide a well-controlled diffusive zero order kinetics, which helps to provide sustained delivery of the therapeutic agent from several weeks to months. Nondegradable polymers are used to fabricate either the matrix or the reservoir form of the drug formulation. A matrix is a homogeneous mixture of the polymer and the drug whereas the reservoir is a system in which the drug is encapsulated within the polymer. The drug release from both these systems is based on diffusion, polymer degradation by physical or chemical stimuli or solvent activation in which the polymer swells causing the release of the drug through osmosis. Different non – biodegradable polymers that have been tested in different studies include poly (vinyl alcohol) (PVA), ethylene vinyl acetate copolymer (EVA), etc. They are used to produce implants of various shapes such as rods, pellets, discs, filaments etc. using procedures such as solution casting, molding and extrusion. However, a major drawback with these polymers is low absorption and metabolism in the body. Consequently, a surgical procedure would be needed to implant and retract the drug delivery system. Hence, patient’s compliance using non-biodegradable implants is less due to the use of an invasive procedure. Commercially available non-biodegradable drug implants such as Vitrasert (Chiron Corporation with Hoffman-LaRoche) and Retisert (Bausch & Lomb) is for the delivery of small molecules. Most recent studies have shown that an osmotic pump, implanted in the subcutaneous space and connected to the sclera, can deliver IgG for 28 days. NCT02510794, a Phase II clinical trial, which is currently ongoing, has patients tested with a ranibizumab in a non-biodegradable port delivery system to deliver therapeutics concentrations of the drug into the vitreous over an extended period.
Biodegradable Polymers
Biodegradable polymers for drug delivery can be either from a natural or synthetic source. Natural polymers such as animal proteins, polysaccharides are often hydrophilic in nature whereas synthetic polymers are hydrophobic in nature. Due to the hydrophobic nature of these synthetic polymers, they are widely being studied for sustained delivery as they help retain the drug over an extended period as compared to hydrophilic polymers
PLGA, a copolymer of poly lactic acid and poly glycolic acid, is the most widely investigated polymer for microencapsulation and sustained delivery of therapeutic proteins and peptides, even though no biopharmaceutical products are available on the market[7]. Its excellent biocompatibility and biodegradability makes it the primary choice for sustained release formulations [34]. Extensive studies have been performed to understand the controlled release applications of various anti-cancer agents, antibacterial, hormone receptor agonists and antagonists using these biodegradable polymer [35] Currently PLGA is FDA approved and widely used in surgical sutures, vascular grafts and scaffolds for tissue engineering [35]. When used as a drug carrier, it encapsulates both lipophilic and hydrophilic molecules, which then could release drugs to the site of action; and when used as intraocular implants, it releases the therapeutic agent into the site of implantation. [36]. Since PLGA is a copolymer of polylactic acid (PLA) and polyglycolic acid (PGA), modulating the ratio of each of these polyesters helps to modify the release kinetics of the therapeutic agents. Use of PLGA as drug carriers has been demonstrated by fabricating anti angiogenic drugs encapsulated with the polymer and presented as a microsphere formulation. Ozurdex, an FDA approved PLGA based implant loaded with 0.7mg of dexamethasone surgically implanted into the vitreal cavity can provide delivery of dexamethasone for a period of 6 months. To date, there are no clinical trials initiated with a biodegradable polymer based implant for biotherapeutic agents.
5.2 Polymeric Microspheres
Microspheres range between 1 to 1000um in diameter and are produced by a simple solvent extraction technique. They can be defined as the drug loaded microspheres, which are homogenously distributed through the matrix, and microcapsules are the drug contained in a reservoir system encapsulated with a polymeric shell. PLGA is the most common biodegradable polymer used to produce microspheres. Its biocompatibile nature helps clear out any degradable products faster from the eye and the systemic circulation. The encapsulation process for proteins is much more challenging as compared to its small molecule counterparts, since proteins tend to degrade faster by losing their intact structure and bioactivity when treated with polymers. Even while fabricating the micro particles, harsher process related steps such as exposure to high temperature or organic solvents can destabilize the protein structure leading to physical instabilities such as aggregation and precipitation. Solvent evaporation methods are commonly used to generate micro particles. It involves the classical use of oil in water emulsion technique [7] in which the drug is first dissolved in an organic phase containing the PLGA polymer and then slowly mixed in an aqueous phase. The solvent is further evaporated using heat or applied vacuum resulting into a suspension containing the micro particles [37]. Recently, bevacizumab was encapsulated using PLGA and polyethylene glycol- polylactic acid (PEG-PLA) to form a microspheric formulation. Both these formulations release bevacizumab over a period of 90 days and showed encapsulation efficiency of more than 90% [38]. However, in vivo comparison of bevacizumab PLGA microsphere formulations, bevacizumab-PEG-PLA microsphere formulations with its unmodified liquid formulations revealed that all bevacizumab formulation irrespective of its presentation decreased the harmful CNV as compared to its placebo, but the study could not prove if the long acting formulation was statistically significantly better than the classical liquid formulation [39] . Over the years, we have witnessed several strategies to produce microsphere formulations for AMD reach the first phase of clinical trials, but none have been commercially approved yet.
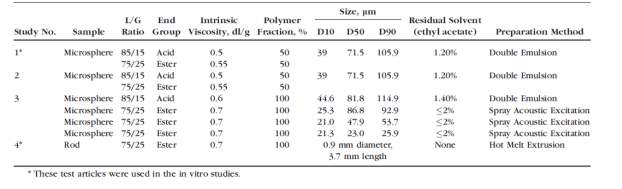
Table 2: Characterization of PLGA microspheres using different lactic/glycolic acid ration and different preparation method. Permission obtained from author [39]
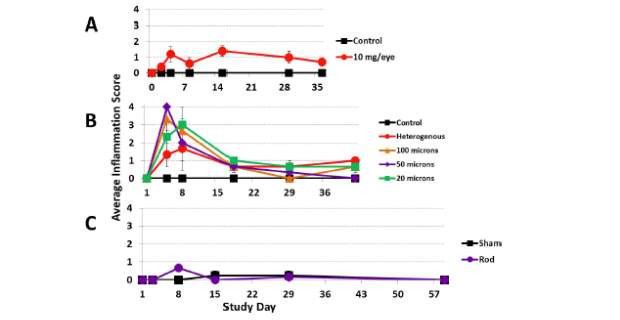
Figure 3: Impact of (A) dosing, (B) size of microparticle, and (C) delivery carrier on inflammation score in non-human primate. Permission obtained from author [39]
5.3 Polymeric Nanoparticles
The size range of a nanoparticle is between 1-100nm [10]. Similar to micro particles, nanoparticles are also composed of biodegradable polymers. They act as an active carrier for targeted delivery of proteins and peptides. Nanoparticles can be further classified into liposomes (non-covalent aggregates mainly composed of a lipid bilayer), micelles (Composed of amphiphilic polymers, which self-assemble to form organized structures), dendrimers and nanowafers [2]. Studies have demonstrated that intravitreal injections of nanoparticles tends to create clouding of the vitreous due to the light scattering property of polymeric particles. Unlike micro particles, which sink into the lower part of the vitreous due to the high molecular mass, nanoparticles, tends to float in the vitreous causing haze formation in the eye [2]. In vitro studies of an anti angiogenic PEDF formulated as PLGA nanoparticles showed the release of the drug even after 40 days of administration. However, 75% of the drug was released within the first 10 days of administration [40].
5.4 In Situ Gel Formulations
Another popular matrix that has been studied for controlled drug delivery systems are hydrogels. These are chemically or physically cross-linked polymers that form a honeycomb like structures used in various biomedical applications such as tissue engineering and controlled delivery of large molecules. [41] Hydrogels can be fine tuned for a drug release profile from weeks to months improving the drug bioavailability and reducing the side effects[41] However, historically these hydrogels needed surgical procedures to implant and also remove them from the targeted area after use. [41]. This limitation can be enable by using the in situ forming smart hydrogels.
They are low viscous polymeric solutions that form a gel by external stimuli such as temperature, pH and light [42]. These formulations are composed of substances such as chitosan, poloxomers and hydroxypropylmethlycellulose that have been studied to be administered as ocular depot systems [43]. Studies have demonstrated the use of large macromolecules in these types of formulations. Light sensitive gel systems of bevacizumab has shown 60 days of drug release post suprachoroidal administration in rats. [44] More recently in vivo studies conducted with PLGA based reversible thermal gel systems shows the release of bevacizumab over 9 weeks [44].
Another study shows the effect of polyelectrolyte complexes(PEC’S), a type of hydrogels produced using alginate or chitosan solutions that exhibits sustained delivery over a period of one month.[41] Nathan et.al studied the extended release of Anti VEGF agent bevacizumab using hydrogel systems that form by electrostatic interactions between alginate and chitosan with the antibody. They could show a sustained delivery over a period of 28 days with an initial slow release of bevacizumab as compared to a model IgG. The net negative charge of bevacizumab has a strong electrostatic interaction with the positively charged chitosan chains resulting into a slower release as compared to a model IgG.[41]
5.5 Implants and Drug Rings
Biodegradable implants using PLGA are capable of sustaining the release of the drug from weeks to several months. Higher drug loading is possible with these systems due to the larger size and smaller surface to volume ratio [45]. However, one of the main challenges with respect to PLGA implants is the use of a surgical procedure to place the implant in the vitreous or the sclera of the infected eye[2]. Similar to micro and nanoparticles, these implants also suffer poor stability, controlled release rate and duration of release from the polymeric matrix [2]. Ozurdex is a PLGA based implant releasing dexamethasone has been approved by the FDA for macular edema and noninfectious uveitis [46]. Biodegradable implants containing large macromolecules are still not available commercially [46].
Non-biodegradable implants are usually composed of a core drug reservoir, which releases the drug continuously through the semi permeable membrane. These implants tends to release drug following a zero order kinetics, however they need to be surgically removed from the patients’ eye since the non-biodegradable material will not be cleared naturally through the body [47]. Vitrasert and Retisert are two commercially approved non-biodegradable implants for the delivery of small molecules [48]
The effect of two-year delivery of ciliary neutrophic factor has been studied as a novel drug delivery method for implant based technology. An encapsulated cell technology, NT-501, was developed to deliver a therapeutic agent directly into the retinal space by circumventing the blood retinal barrier [49]. Briefly, the delivery system consists of a humanized cell line, which is genetically engineered to produce and express a selected therapeutic protein, over an extended period. First, a stable expressing, long term viable cell line is selected and encapsulated into a semi-permeable membrane. Next, this matrix is implanted into the vitreous cavity. The calculated half-life of ciliary neurotrophic factor through the NT-501 system in the vitreous is roughly 51 months [9]. In developmental studies, drug capsule rings were implanted inside the periphery of the retinal space following cataract surgery, to deliver anti-VEGF treatments inside the eye for a sustained period of time [9]. Studies suggest that this ring device could deliver ranibizumab continuously over a period of 90 days [9]
6. Conclusions
AMD is one of the leading causes of irreversible loss of visual acuity in patients above the age of 70 years, affecting and influencing their quality of life. Due to the complexity of ocular therapy, it is challenging to find an efficacious and safe formulation that also ensures patient compliance. Systemic administration, especially with biologics, is often hindered due to therapeutic agent’s inability to cross the blood retinal barrier, leading to low bioavailability in the retinal space. Topical administration is restricted by precorneal barriers such as blinking tear film, tear turnover and the possibility of the therapeutic agent being washed away by these factors. The only current viable choice for ocular drug delivery is through intravitreal administration, which successfully delivers the therapeutic agent into the retinal space and helps to regress choroidal neovascularization. Currently, line of treatment for AMD includes biologics such as Ranibizumab, Aflibercept, and bevacizumab (off-label). While the monthly intravitreal injection of ranibizumab does show improvement in visual acuity by 15 letters [44], it is rather cumbersome for the patients and there is an urgent need to develop long acting formulations that do not require frequent dosing. The 2018 phase III results showed that Novartis’s brolucizumab maintained efficacy upto 12 weeks as compared to the standard dose of monthly intravitreal administration of aflibercept, enabling quarterly injections instead of monthly dosing. Regeneron’s Aflibercept also showed similar results enabling a 12-week dosing interval for the treatment of AMD. Novel technologies such as gene therapy, angiogenic targets, and combination therapy using Anti-PDGF-BB and anti VEGF, agents are currently under investigation. Different drug delivery routes such as sustained delivery implants, drug carriers, and encapsulated cell technology are also gaining significance amongst formulation scientists to reduce the cost and economic burden of multiple dosing needed to maintain efficacy of anti angiogenic agents. These novel drug delivery systems such as hydrogels, micro particles, implants and drug rings not only help target the drug in an effective manner minimizing side effects, but also improve sustainability of the drug in the ocular regions. With more research moving in this direction, there is hope to obtain superior treatments for AMD patients in the decade to come.
7. References
[1] Keith L Moore, No Title, 1999.
[2] A. Manuscript, Ocular delivery of macromolecules, (2015) 172–181. doi:10.1016/j.jconrel.2014.06.043.Ocular.
[3] M. Pindrus, S.J. Shire, R.F. Kelley, R. Wong, Y. Xu, S. Yadav, Solubility Challenges in High Concentration Monoclonal Antibody Formulations: Relationship with Amino Acid Sequence and Intermolecular Interactions, (2015). doi:10.1021/acs.molpharmaceut.5b00336.
[4] R. Gaudana, H.K. Ananthula, A. Parenky, A.K. Mitra, Ocular Drug Delivery, AAPS J. 12 (2010) 348–360. doi:10.1208/s12248-010-9183-3.
[5] A. Mandal, D. Pal, V. Agrahari, H.M. Trinh, M. Joseph, A.K. Mitra, Ocular delivery of proteins and peptides: Challenges and novel formulation approaches, Adv. Drug Deliv. Rev. (2018). doi:10.1016/j.addr.2018.01.008.
[6] H. Nomoto, F. Shiraga, N. Kuno, E. Kimura, S. Fujii, K. Shinomiya, A.K. Nugent, K. Hirooka, T. Baba, Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits, Investig. Ophthalmol. Vis. Sci. 50 (2009) 4807–4813. doi:10.1167/iovs.08-3148.
[7] B.A. Booth, L. Vidal Denham, S. Bouhanik, J.T. Jacob, J.M. Hill, Sustained-Release Ophthalmic Drug Delivery Systems for Treatment of Macular Disorders, Drugs Aging. 24 (2007) 581–602. doi:10.2165/00002512-200724070-00006.
[8] U.D.J.P. Rai, S.A. Young, T.R. Thrimawithana, H. Abdelkader, A.W.G. Alani, B. Pierscionek, R.G. Alany, The suprachoroidal pathway: A new drug delivery route to the back of the eye, Drug Discov. Today. 20 (2015) 491–495. doi:10.1016/j.drudis.2014.10.010.
[9] V.M. Villegas, L.A. Aranguren, J.L. Kovach, S.G. Schwartz, H.W. Flynn, Current advances in the treatment of neovascular age-related macular degeneration, Expert Opin. Drug Deliv. 14 (2017) 273–282. doi:10.1080/17425247.2016.1213240.
[10] D.G. Birch, F. Qi Liang, Age-related macular degeneration: a target for nanotechnology derived medicines, Int. J. Nanomedicine. 2 (2007) 65–77. doi:http://dx.doi.org/10.2147/nano.2007.2.1.65.
[11] D.K. Newman, Photodynamic therapy : current role in the treatment of chorioretinal conditions, 30 (2016) 202–210. doi:10.1038/eye.2015.251.
[12] A.J. Augustin, S. Puls, I. Offermann, Triple Therapy for Choroidal Neovascularization Due To Age- Related Macular Degeneration, Retina. 27 (2007) 133–140.
[13] A.J. Abd, R.K. Kanwar, J.R. Kanwar, Aged macular degeneration: current therapeutics for management and promising new drug candidates, Drug Discov. Today. 22 (2017) 1671–1679. doi:10.1016/j.drudis.2017.07.010.
[14] E.W.M. Ng, D.T. Shima, P. Calias, E.T. Cunningham, D.R. Guyer, A.P. Adamis, Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease, Nat. Rev. Drug Discov. 5 (2006) 123–132. doi:10.1038/nrd1955.
[15] E.B. Rimm, D. Sc, D.I. Chasman, D. Ph, F.B. Hu, D. Ph, L. Qi, D. Ph, New England Journal, N. Engl. J. Med. 367 (2012) 1387–1396. doi:10.1056/NEJMoa1203039.
[16] V. Chong, P.K. Kaiser, P. Mitchell, Posterior Segment Age-related Macular Degeneration Safety and Efficacy of Ranibizumab and Bevacizumab for the Treatment of Neovascular Age-related Macular Degeneration, (2012) 34–42. doi:10.17925/EOR.2012.06.01.34.
[17] P.U. Dugel, G.J. Jaffe, P. Sallstig, J. Warburton, A. Weichselberger, M. Wieland, L. Singerman, Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial, Ophthalmology. 124 (2017) 1296–1304. doi:10.1016/j.ophtha.2017.03.057.
[18] A. Stahl, M.T. Stumpp, A. Schlegel, S. Ekawardhani, C. Lehrling, G. Martin, M. Gulotti-Georgieva, D. Villemagne, P. Forrer, H.T. Agostini, H.K. Binz, Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications, Angiogenesis. 16 (2013) 101–111. doi:10.1007/s10456-012-9302-0.
[19] C. Cui, H. Lu, Clinical observations on the use of new anti- VEGF drug , conbercept , in age-related macular degeneration therapy : a meta-analysis, Clin Interv Aging. (2018) 51–62. doi:10.2147/CIA.S151225.
[20] X. Li, G. Xu, Y. Wang, X. Xu, X. Liu, S. Tang, F. Zhang, J. Zhang, L. Tang, Q. Wu, D. Luo, X. Ke, Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 Study: AURORA study, Ophthalmology. 121 (2014) 1740–1747. doi:10.1016/j.ophtha.2014.03.026.
[21] P. Choudhury, W. Chen, R.C. Hunt, Production of platelet-derived growth factor by interleukin-1 beta and transforming growth factor-beta-stimulated retinal pigment epithelial cells leads to contraction of collagen gels., Invest. Ophthalmol. Vis. Sci. 38 (1997) 824–833.
[22] K.G. Falavarjani, S.R. Sadda, Hot Topic in Pharmacotherapy for Neovascular Age-Related Macular Degeneration, (2017) 1–7. doi:10.2174/13816128226661612.
[23] H. Xu, M. Chen, Targeting the complement system for the management of retinal inflammatory and degenerative diseases, Eur. J. Pharmacol. 787 (2016) 94–104. doi:10.1016/j.ejphar.2016.03.001.
[24] E. Lipo, S.M. Cashman, R. Kumar-Singh, Aurintricarboxylic acid inhibits complement activation, membrane attack complex, and choroidal neovascularization in a model of macular degeneration, Invest. Ophthalmol. Vis. Sci. 54 (2013) 7107–7114. doi:10.1167/iovs.13-12923.
[25] M. Nozaki, B.J. Raisler, E. Sakurai, J. V. Sarma, S.R. Barnum, J.D. Lambris, Y. Chen, K. Zhang, B.K. Ambati, J.Z. Baffi, J. Ambati, Drusen complement components C3a and C5a promote choroidal neovascularization, Proc. Natl. Acad. Sci. 103 (2006) 2328–2333. doi:10.1073/pnas.0408835103.
[26] S.J. Kim, J. Kim, J. Lee, S.Y. Cho, H.J. Kang, K.-Y. Kim, D.-K. Jin, Intravitreal human complement factor H in a rat model of laser-induced choroidal neovascularisation., Br. J. Ophthalmol. 97 (2013) 367–70. doi:10.1136/bjophthalmol-2012-302307.
[27] M. Ishikawa, D. Jin, Y. Sawada, S. Abe, T. Yoshitomi, Future Therapies of Wet Age-Related Macular Degeneration, 2015 (2015). doi:10.1155/2015/138070.
[28] S.C. Finnemann, V.L. Bonilha, A.D. Marmorstein, E. Rodriguez-Boulan, M.M. Dyson, Phagocytosis of rod outer segments by retinal pigment epithelial cells requires ␣v5 integrin for binding but not for internalization, Cell Biol. Commun. by Torsten N. Wiesel. 94 (1997) 12932–12937. doi:10.1073/pnas.94.24.12932.
[29] H. Quiroz-Mercado, Integrin peptide therapy in choroidal and retinal neovascularization, Retin. Today. SEP (2013) 30–31.
[30] G.-F. Wang, X.-L. Zou, Tissue factor with age-related macular degeneration., Int. J. Ophthalmol. 5 (2012) 609–13. doi:10.3980/j.issn.2222-3959.2012.05.13.
[31] S. Zandi, S. Nakao, K.-H. Chun, P. Fiorina, D. Sun, A. Hafezi-Moghadam, ROCK-Isoform Specific Polarization of Macrophages Associated with Age-Related Macular Degeneration, Cell Rep. 57 (2015) 742–768. doi:10.1002/dev.21214.Developmental.
[32] K. Kim, S.W. Park, J.H. Kim, S.H. Lee, D. Kim, T. Koo, K.E. Kim, J.H. Kim, J.S. Kim, Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration, Genome Res. 27 (2017) 419–426. doi:10.1101/gr.219089.116.
[33] D.P. Barakat MR, New Developments for the Treatment of Exudative and Nonexudative AMD, Retin. Physician. 12 (2015) 26–32.
[34] A.A. Ignatius, L.E. Claes, In vitro biocompatibility of bioresorbable polymers: poly (L, DL-lactide) and poly (L-lactide-co-glycolide), Biomaterials. 17 (1996) 831–839. http://www.sciencedirect.com/science/article/pii/0142961296814219.
[35] L.J. Suggs, S.A. Moore, A.G. Mikos, Synthetic Biodegradable Polymers for Medical Applications, 2007. doi:10.1007/978-0-387-69002-5_55.
[36] Y.-S. Rhee, M. Sohn, B.H. Woo, B.C. Thanoo, P.P. DeLuca, H.M. Mansour, Sustained-Release Delivery of Octreotide from Biodegradable Polymeric Microspheres, AAPS PharmSciTech. 12 (2011) 1293–1301. doi:10.1208/s12249-011-9693-z.
[37] D.T. O’Hagan, D. Rahman, J.P. McGee, H. Jeffery, M.C. Davies, P. Williams, S.S. Davis, S.J. Challacombe, Biodegradable microparticles as controlled release antigen delivery systems., Immunology. 73 (1991) 239–42. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1384472&tool=pmcentrez&rendertype=abstract.
[38] C.K. Pan, C. Durairaj, U.B. Kompella, O. Agwu, S.C.N. Oliver, H. Quiroz-Mercado, N. Mandava, J.L. Olson, Comparison of Long-Acting Bevacizumab Formulations in the Treatment of Choroidal Neovascularization in a Rat Model, J. Ocul. Pharmacol. Ther. 27 (2011) 219–224. doi:10.1089/jop.2010.0158.
[39] E.A. Thackaberry, C. Farman, F. Zhong, F. Lorget, K. Staflin, A. Cercillieux, P.E. Miller, C. Schuetz, D. Chang, A. Famili, A.L. Daugherty, K. Rajagopal, V. Bantseev, Evaluation of the toxicity of intravitreally injected PLGA microspheres and rods in monkeys and rabbits: Effects of depot size on inflammatory response, Investig. Ophthalmol. Vis. Sci. 58 (2017) 4274–4285. doi:10.1167/iovs.16-21334.
[40] H. Li, V. V Tran, Y. Hu, W.M. Saltzman, C.J. Barnstable, J. Tombran-tink, A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres, 83 (2006) 824–833. doi:10.1016/j.exer.2006.04.014.
[41] E. Ruel-Gariépy, G. Leclair, P. Hildgen, A. Gupta, J.C. Leroux, Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules, J. Control. Release. 82 (2002) 373–383. doi:10.1016/S0168-3659(02)00146-3.
[42] M.H. Turabee, T. Thambi, H.T.T. Duong, J.H. Jeong, D.S. Lee, A pH- and temperature-responsive bioresorbable injectable hydrogel based on polypeptide block copolymers for the sustained delivery of proteins: In vivo, Biomater. Sci. 6 (2018) 661–671. doi:10.1039/c7bm00980a.
[43] V.H.G. Phan, T. Thambi, H.T.T. Duong, D.S. Lee, Poly(amino carbonate urethane)-based biodegradable, temperature and pH-sensitive injectable hydrogels for sustained human growth hormone delivery, Sci. Rep. 6 (2016) 1–12. doi:10.1038/srep29978.
[44] R.M. Wazen, S. Kuroda, C. Nishio, K. Sellin, J.B. Brunski, A. Nanci, NIH Public Access, 8 (2014) 1385–1395. doi:10.2217/nnm.12.167.Gene.
[45] S.S. Lee, P. Hughes, A.D. Ross, M.R. Robinson, Biodegradable Implants for Sustained Drug Release in the Eye, (2010) 2043–2053. doi:10.1007/s11095-010-0159-x.
[46] M. Zhao, E. Rodríguez-Villagra, L. Kowalczuk, M. Le Normand, M. Berdugo, R. Levy-Boukris, I. El Zaoui, B. Kaufmann, R. Gurny, I. Bravo-Osuna, I.T. Molina-Martínez, R. Herrero-Vanrell, F. Behar-Cohen, Tolerance of high and low amounts of PLGA microspheres loaded with mineralocorticoid receptor antagonist in retinal target site, J. Control. Release. 266 (2017) 187–197. doi:10.1016/j.jconrel.2017.09.029.
[47] J. Driot, G.D. Novack, K.A.Y.D. Rittenhouse, C. Milazzo, P.A. Pearson, Ocular Pharmacokinetics of Fluocinolone Acetonide After Retisert TM Intravitreal Implantation in Rabbits Over a 1-Year Period, 20 (2004) 269–275.
[48] J.L. Bourges, C. Bloquel, A. Thomas, F. Froussart, A. Bochot, F. Azan, R. Gurny, D. Benezra, F. Behar-cohen, Intraocular implants for extended drug delivery : Therapeutic applications ☆, 58 (2006) 1182–1202. doi:10.1016/j.addr.2006.07.026.
[49] K. Kauper, C. McGovern, S. Sherman, P. Heatherton, R. Rapoza, P. Stabila, B. Dean, A. Lee, S. Borges, B. Bouchard, W. Tao, Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases, Investig. Ophthalmol. Vis. Sci. 53 (2012) 7484–7491. doi:10.1167/iovs.12-9970.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical"
The word Medical refers to preventing or treating injuries or illnesses, relating to the study or practice of medicine. Medical care involves caring for a patient and helping them through their journey to recovery.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




