Mixed Amine Impregnated Mesoporous Silica for Post Combustion CO2 Capture
Info: 6584 words (26 pages) Dissertation
Published: 13th Dec 2019
Tagged: Biology
Abstract
A high amine efficiency amine-modified silica adsorbent has been developed based on mixed amine of polyethyleneimine (PEI) and diethanolamine (DEA). Through tuning the ratio of PEI to DEA in amine mixture, the silica loaded with 50% DEA and 50% (S/P50/D50) exhibited the best adsorption performance. The CO2 capacity up to 2.93 mmol/g with amine efficiency of 0.39 was achieved for this newly synthesized adsorbent under dry CO2. Under simulated humid flue gas conditions, this adsorbent exhibited the breakthrough capacity of 2.4 mmol/g. The adsorption kinetics in S/P50/D50 was improved as demonstrated by sharp CO2 adsorption curves compared to typical PEI modified silica adsorbents. The addition of DEA in PEI impregnated silica play a key role of enhancing CO2 adsorption capacity and kinetics. The hydroxyl groups in DEA enable the PEI molecules well dispersed in silica pores, thereby increasing the amount of accessible sorption sites. The other promoting effects to CO2 adsorption arise from the secondary amine groups in DEA, which also act as efficiency adsorption sites. The CO2 diffusion resistance in S/P50/D50 is reduced as indicated by less kinetically controlled CO2 adsorption behavior at elevated adsorption temperature compared to pure PEI functionalized silica. This adsorbent showed good reversibility and the capacity was nearly stable after 2 adsorption-desorption cycles. This adsorbent offers great promise to capture CO2 from flue gas at a large scale. This strategy can be conveniently expanded to a wide range of material combinations. synergistic
Highlights
- The amine silica adsorbents with high amine efficiency up to 0.39 were developed in this work.
- The silica loaded with 50% DEA and 50% PEI exhibited a high adsorption capacity of 2.93 mmol/g with fast adsorption kinetics.
- The addition of DEA in PEI impregnated silica adsorbents boost the adsorption capacity and kinetics.
1 Introduction
In current highly carbon-dependent world, the consumption of fossil fuels mainly contributes to the anthropological CO2 emissions that cause the accelerating rise of atmospheric CO2 level and the global climate change[1, 2]. Considerable research effort has been devoted to exploring the efficient CO2 capture technology to reduce CO2 emission from major source points such as coal or gas-fired power plants[3, 4]. In most cases, CO2 chemical absorption by aqueous amine solutions which is still the most viable way for the reduction of CO2 emissions, remains the benchmark CO2 capture process [5-8]. However, this energy intensive process poses several disadvantages including low energy efficiency, corrosion problems, resulting in an increase in plant parasitic load on the plant [9, 10].
To date, many prominent examples of solid adsorbents for CO2 capture have been reported and extensively investigated to substitute aqueous amine scrubber techniques [11-15]. Most of these solid adsorbents were prepared by either impregnating organic amine molecules (class 1) on porous supports [11, 14, 16] or chemically grafting amino groups onto support surface (class 2)[12, 17, 18]. Both the classes of chemisorbents exhibits high adsorption capacity and selectivity. Moreover, the amine functionalized solid adsorbents with relatively lower adsorption enthalpy than amine aqueous solution present a new opportunity to reduce energy penalty in the regeneration step. Class 1 adsorbent with simple preparation procedure, is more commonly reported than class 2 adsorbents. Different organic amino polymers containing primary and secondary amine groups, such as polyethylenimine (PEI)[19], tetraethylenepentamine (TEPA)[20-22] etc. are often utilized to be impregnated onto porous support surface to prepare class 1 amine adsorbents.
Song et.al[11] reported the first PEI modified MCM-41 type silica with a adsorption capacity around 2.5 mmol/g at 50 oC with 50% PEI loading in 1 atm dry CO2. Later on, various types of porous supports such as mesoporous silica[19], alumina[23], as well as metal organic framework compounds[24] are modified with linear or branched PEI compounds with different molecular weights for CO2 adsorption. Most of these adsorbents have promising CO2 capacities in the range of 2.0-4.0 mmol/g, which is related to the quantities of accessible active adsorption sites by CO2 [11, 19]. The high CO2 capacity in these amine adsorbents was commonly achieved by increasing the amine loading in porous supports. However, the attempt to increase amine loading always comes at the expense of amine efficiency[1]. For example, PEI impregnated silica adsorbents with high amine loading is demonstrated to have high CO2 diffusion resistance and amino groups are unevenly distributed in the porous materials[22]. Less active adsorption sites are available led by agglomerated viscous PEI chains in silica pores. As a result, CO2 capacity increased with adsorption temperature (thermodynamically controlled adsorption process was observed) and amine efficiency is relatively low. An efficient way to reduce the diffusion resistance in class 1 adsorbents is to use low molecular weight amine species such as TEPA to improve the dispersion of amine[25, 26].
Zhu et al modified the SBA-15 with TEPA utilizing the micelle formed by templates to disperse amine molecules[21]. This study demonstrated that CO2 adsorption in TEPA-SBA-15 was a kinetically-controlled process with high adsorption capacity of 3.93 mmol/g, indicating well dispersion of TEPA inside pores. However, small molecule amine functionalized solid adsorbents have the disadvantage of unsatisfied cyclic stability due to high volatility of low molecular weight amines.
Another strategy to reduce the resistance of CO2 diffusion into supports is to improve the dispersion of amine molecule in the support pore surface. The uniform distribution of amines on silica surface enable more active adsorption site to be accessible by CO2. In Zhu’s study[21], they demonstrated the hydroxyl groups in templates (Pluronic P123) not only enhance the CO2 capacity but also enable the uniform distribution of TEPA on supports. In our recent study [3] , we found that with the presence of water in simulated flue gas, the CO2 diffusion resistance in amine adsorbents were significantly reduced. This result indicated that the hydroxyl groups in water molecules play a role of enhancing the CO2 adsorption capacity but also exhibit a “plasticizing effect”, thereby improving PEI dispersity and minimizing internal diffusion limitations. Other works [21, 27] added the additive of glycerol or polyethylene glycerol (PEG) into PEI to prepare PEI/silica adsorbents. The CO2 capacity increased as well with the addition of these components containing hydroxyl groups, which not only change the CO2–amine reaction mechanism[27], also contributes to better distribution of amine groups.
Diethanolamine (DEA) with one secondary amine group and two hydroxyl groups is often used as an efficient absorbents in amine scrubber process[27, 28]. The addition of DEA into PEI silica adsorbents is hypothesized to play a role of improving the dispersity of PEI also to enhance CO2 capacity with the presence of amine groups. This amine molecule is a promising component for preparing high efficiency adsorbents. The pure DEA and amine mixture of DEA and TEPA has been utilized to functionalize various silica supports in previous studies [23, 27, 29]. Though high adsorption capacities were achieved for these adsorbents, the considerable leaching of TEPA and DEA is a major drawback for the use of these amines. To circumvent this problem, we demonstrate the synthesis of amine-loaded silica sample by using mixture of much stable high molecular weight polymeric amine of PEI and the additives of DEA. Both the cyclic stability and adsorption capacity compared to pure DEA impregnated silica are improved as expected. Moreover, this methodology proves to be an efficient strategy to increase amine efficiency compared to typical PEI silica adsorbents.
Low-cost commercial silica gel with high surface area and porosity was chosen as the porous support in this work and the adsorbents were prepared through low cost and simple wet impregnation technology to deposit PEI and various additives with hydroxyl groups into silica. We successfully develop the PEI/DEA impregnated silica that demonstrated high CO2 adsorption capacity with high amine efficiency. The adsorption performance of the resulting adsorbents was characterized thoroughly in this work.
2 Experimental and Methods
2.1 Material Synthesis
The commercial amorphous silica gel, Gasil HP260®, from PQ Corporation, was used to synthesis the supported amine adsorbents. The solid amine adsorbents was prepared by impregnating desired amount of polyethylenimine (average Mn~600, Mw~800) and DEA with various ratio into the silica support. 1 g of amine mixture of PEI and DEA was dissolved in 20 ml of methanol under stirring. Then, 1 g of silica samples wad added and the mixture was stirred for an additional 12 h. The methanol solvent was later removed by rotary evaporator. The samples were further treated in vacuum oven at 90 oC for 3 h before testing. The ratio of PEI and DEA for preparing adsorbents was 10:0, 9:1, 7:3, 5:5, 3:7,1:9, and 0:10. To explore the impact of additive of polyethylene glycol ( PEG) on adsorbent performance , different adsorbents was prepared in the methanol solution of amine mixture and PEG (weight ratio of amine mixture to PEG=9:1). All reagents were used as received. The prepared samples are termed as S/Px/Dy with S representing silica support, where x and y denotes the mass fraction of the components of PEI and DEA.
2.2 Characterization Methods
Nitrogen physisorption experiments: Nitrogen physisorption experiments were performed on a Micromeritics ASAP 2020 at 77K. The surface area and pore volume were calculated from the isotherm data using the Brunauer–Emmett–Teller (BET) and Barrett-Joyner-Halenda (BJH) methods, respectively.
FTIR characterization: Infrared spectroscopy was used to characterize the chemical species in the fiber adsorbents after exposure to various gases. Infrared spectra (IR) were recorded on a Bruker TENSOR II FTIR spectrometer. The KBr pellets of silica adsorbents were prepared by mixing ~1.0 mg sample powder with 100 mg KBr.
Elemental analysis: The nitrogen contents of the resulting samples were determined by elemental analysis with an Vario EL Ⅲ element analyzer instrument.
2.3 Adsorption Experiments
The CO2 equilibrium capacity measurements were performed with a Netzsch STA409PG TG/DSC apparatus. The samples were first treated in flowing N2 at a flow rate of 60 mL/min at 90 oC for 30 min to remove the preadsorbed CO2 and H2O in the adsorbents, and then exposed to pure CO2 at desired adsorption temperature for 1h. The cyclic adsorption experiments were also performed using TGA at 35 oC for the continuous 10 cycles.
2.4 Breakthrough experiments under simulated flue gas
The breakthrough experiments were performed at custom-built fixed bed. The adsorption column is made of pyrex glass, with the length of 14 cm and an inner diameter of 1cm. Firstly, 200 mg sample was loaded into fixed bed. The sample was treated at 105 oC for 1 h under N2 flow at 60 mL/min and cooled to 35 oC. In the dry CO2 adsorption experiment, 10% CO2/N2 gas mixture was flowed to the bed for 1 h. In the humid adsorption experiments, the water-saturated N2 was flowed to the fixed bed for 30 min first and then dry CO2 was used to perform adsorption experiments. The adsorption time is 30 min in both dry and humid experiments. The effluent gas composition was monitored by Pfeiffer Omnistar Mass Spectrometer through the entire experiment.
3 Results and Discussion
3.1 Characterizations of the adsorbents
The amine species of DEA and PEI (Mn~600 g/mol), abbreviated as D and P respectively, was chosen as amine species for amine silica adsorbents synthesis. Commercial silica with pore volume of 1.75 mL/g and surface area of 391 m2/g was selected as the adsorbents supports, which is represented by S. Other additives with hydroxyl groups including PEG and glycerol were added with amine into silica as well. Their CO2 capacities are summarized in Table 1. Obviously, the amine silica adsorbents based on DEA and PEI was successfully synthesized as illustrated by high CO2 adsorption capacity. The 10% glycerol was added into PEI to investigate the hydroxyl group effects on CO2 capacity. The results show that CO2 capacity increased from 1.92 mmol/g to 2.17 mmol/g, indicating the positive effects of glycerol on CO2 adsorption. Although the amine species loading amount was fixed at 50% in silica, the adsorbent with 70% PEI and 30% DEA (S/P70/P30) showed a much higher adsorption capacity of 2.78 mmol/g than S/PEI and S/DEA. With both hydroxyl groups and amine groups present in DEA, the addition of DEA into S/PEI greatly enhance the CO2 capacity.
Table 1 CO2 capacity of various amine silica in dry pure CO2
| Name | qe |
| mmol/g | |
| S | 0.15 |
| S/P100 | 1.92 |
| S/D100 | 2.24 |
| S/P100+10% Glycerol | 2.17 |
| S/P70/D30 | 2.78 |
Here, the synthesis procedure of S/P/D adsorbents was further optimized by tuning the ratio of PEI and DEA while the amine amount loaded in silica was fixed at 50 wt%. And their CO2 capacities were measured in a flow of pure CO2 using TGA. Figure 1 presents the equilibrium adsorption capacity with various DEA and PEI ratio. It can be seen that the maximum CO2 capacity of 2.93 mmol/g is achieved with the ratio of DEA to PEI of 5:5. The following work chose this ratio as optimized synthesis procedure to prepare silica adsorbents impregnated with DEA and PEI.
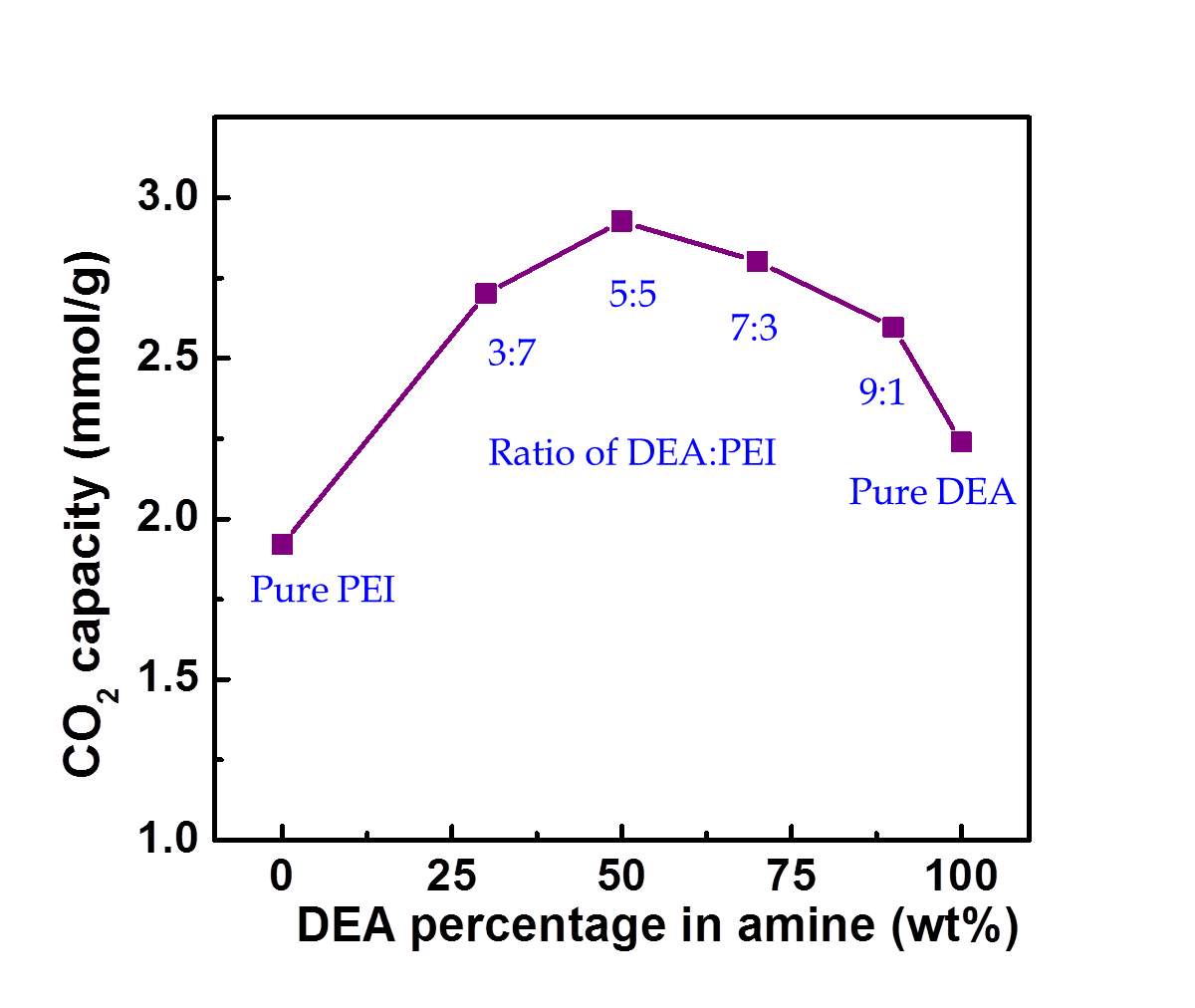
Figure 1 DEA percentage on CO2 capacity of S-P/D
The adsorbents of S/P50/D50 was fully characterized to investigate their textual properties , CO2 adsorption capacities in practical flue gas conditions and cyclic stability. Here, the typical S/P adsorbents were included to compare with S/P50/D50 to illustrate the positive effects of DEA on adsorption performance of S/P adsorbents.
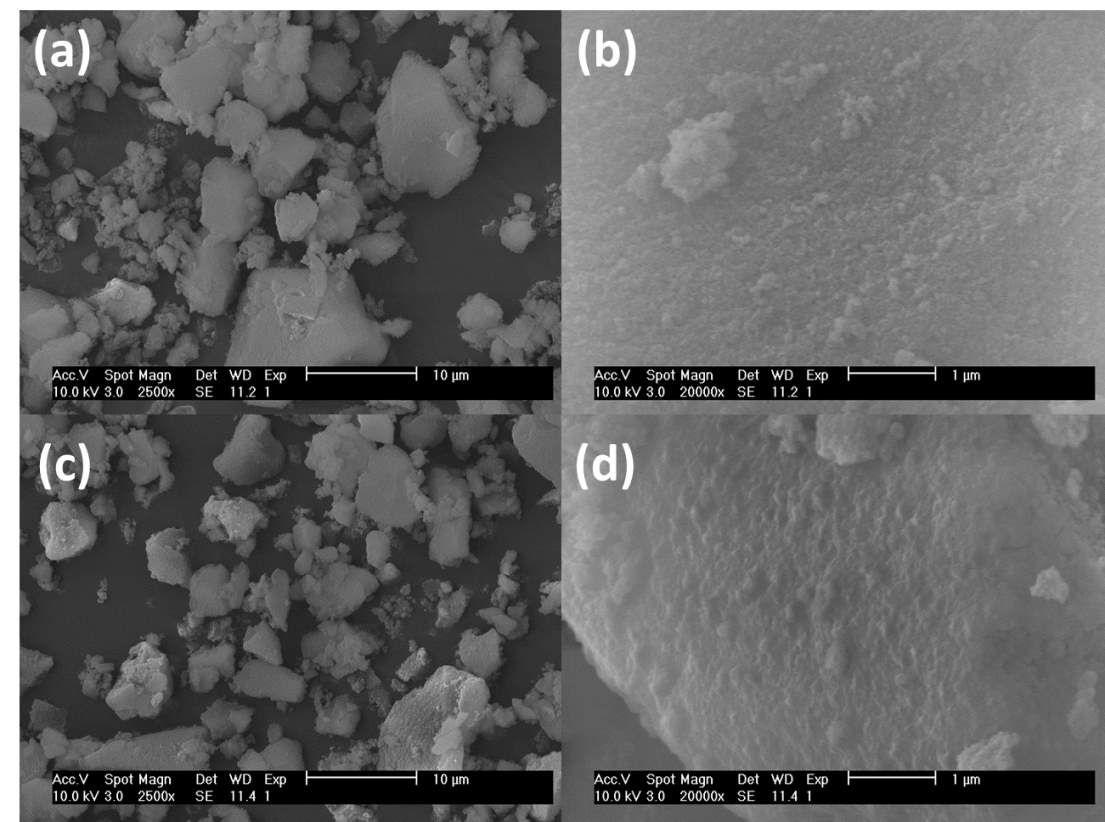
The SEM results, as shown in Figure 2, demonstrated that the silica supports are amorphous and the average particle size is around 7.1 µm. The impregnation of PEI/DEA onto silica surface did not have obvious visible impacts on silica morphology. N2 adsorption/desorption isotherms of both pure silica samples and synthesized amine silica adsorbents exhibited typical IV adsorption-adsorption shape (IUPAC classification) with a hysteresis loop, which is characteristic of mesoporous materials. The corresponding adsorbents properties such as surface area, pore volume and amine loading are shown in Table 2. The pore volumes of the adsorbents were calculated from physisorption data using the BJH method .The surface area and pore volume were decreased after the impregnation of amine molecules, confirming that PEI and DEA were successfully loaded into the pores of support. It can be found that the pore volume and surface area for amine silica adsorbents is related to amine loading. The S/P with high amine loading possess the lower pore volume and surface area as most of the pores are occupied by amine molecules. Large percentage decrease of pore volume and surface area was observed in S/P than S/P/D, which is a result of that higher amine loading in former adsorbents.
Figure 2 SEM images of Silica (a, b) and S/P/D 5-5 (c,d)
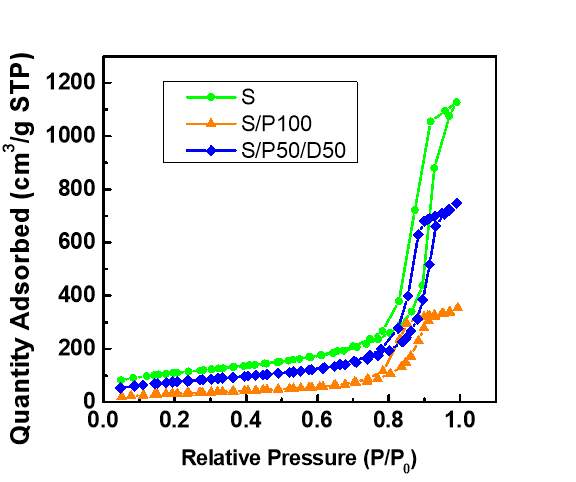
Figure 3 Nitrogen adsorption-desorption isotherm at 77K for S, S/P100 and S/P50/D50 samples
Assuming the amine amount loaded in silica is 50%, the theoretical amine loading in adsorbents is estimated as 11.6 mmol N/g and 8.19 mmol N/g in S/P100 and S/P50/D50 adsorbents respectively. The amine loading was experimentally measured using elemental analysis. As seen from results in Table 2, experimental amine loading is close to theoretical estimation, indicating amine species during impregnation step is fully attached inside silica pores. The amine efficiency is estimated by dividing CO2 adsorption capacity by amine loading. It is evident that the amine efficiency in S/P50/D50 is more than doubled compared with S/P100. Based on the proposed reaction mechanism of the amine-CO2 in dry conditions in the previous report[27], the theoretical amine efficiency is 0.5. Here, S/P50/D50 approaches this value. Although the analysis in this work cannot demonstrate the CO2 –DEA/PEI mechanism, this high amine efficiency indicates the addition of DEA increases the accessible sorption sites in supported amine system. On the other hand, the hydroxyl group may help disperse amino groups inside pore more uniformly therefore reducing CO2 diffusion resistance. Another reason of amine efficiency increase may be ascribed to the hydroxyl group promoted the formation carbamate type zwitterions, so that one amine plus hydroxyl group can react with one CO2 molecule[27].
Table 2 Textual properties of amine silica adsorbents
| Name | Surface area | Pore volume | Amine loading | Amine efficiency | Adsorption enthalpy |
| m2 /g | mL/g | mmol N/g | kJ/mol | ||
| S | 390.6 | 1.75 | 0 | 0 | — |
| S-P100 | 123.4 | 0.55 | 11.4 | 0.16 | 87.5 |
| S-P50/D50 | 278.4 | 1.15 | 7.4 | 0.39 | 85.7 |
3.2 Equilibrium adsorption performance of S/P50/D50 and S/P100
The adsorption capacity of amine silica samples in a flow of pure CO2 curves were probed via TGA. The CO2 adsorption curves as a function of adsorption time are displayed in Figure 4. The equilibrium CO2 adsorption capacity of S/P100 and S/P50/D50 are 1.92 and 2.93 mmol/g, respectively. The adsorbents of S/P50/D50 achieved around 90% equilibration in less than 1 minute whereas the CO2 weight gain for S/100 is at a relatively slow rate as illustrated by the reduced slope of uptake curves. Evidently, the adsorption performance of S/P50/D50 is superior to that of S/P100 kinetically. More rapid CO2 adsorption kinetics in S/P50/D50 is also demonstrated by the shaper exothermic peaks observed in the DSC curves than S/P100. This is not surprising as S/P50/D50 had the much large pore volume. More spaces in the adsorbents could enhance the CO2 adsorption[30]. The addition of DEA improves the mass transfer rate during adsorption process likely attributed to the increased gas-PEI interface with well dispersed amino groups inside silica pores. The rapid adsorption kinetics is essential for shortening the operational cycle time in practical application. The adsorption enthalpy was estimated from the integration of exothermic peaks. The adsorption enthalpies are calculated as 87.5 kJ/mol and 85.7 kJ/mol, respectively, indicating close interaction strength for CO2 with amine species of PEI and DEA.
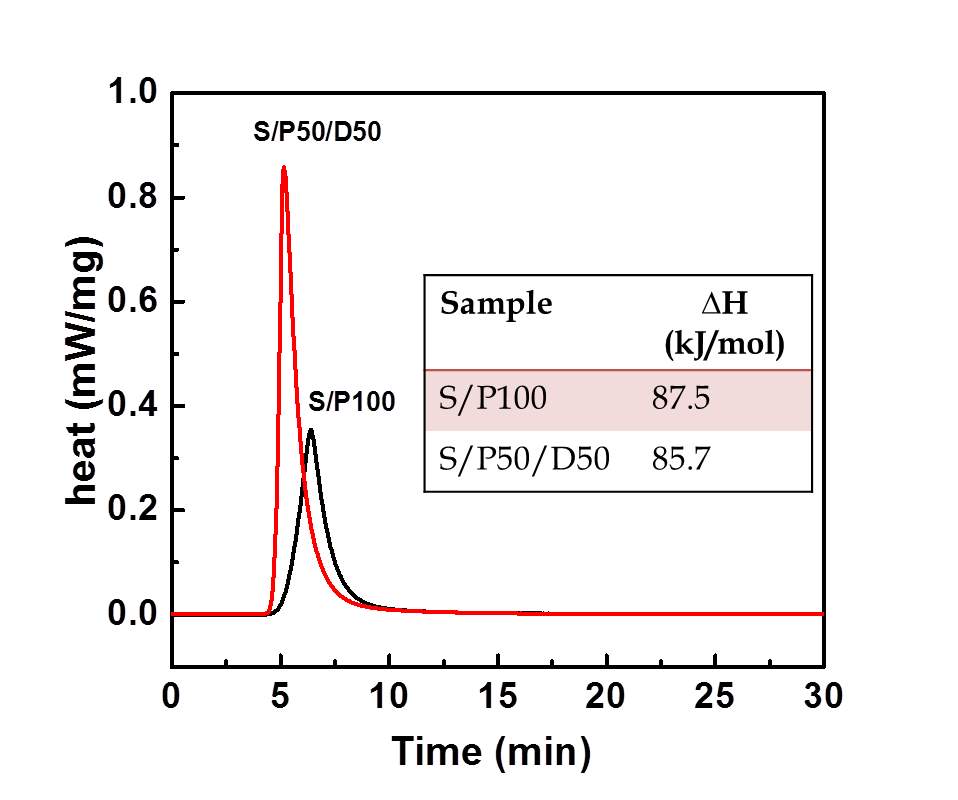
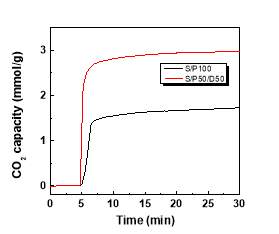
Figure 4 CO2 adsorption curve and DSC signals during adsorption in S/P100 and S/P50/D50
The adsorbents were exposed to the pure CO2 at six different temperatures: 35 oC, 45 oC and 55 oC, 65 oC, 75 oC and 85 oC to investigate the adsorption temperature effects on adsorbents performance. The equilibrium capacity qe as a function of the adsorption temperature is present in Figure 5. Evidently, S/P100 samples demonstrated the increased CO2 adsorption upon elevating the temperature from 35 to 85 C, indicating the CO2 adsorption in S/P100 is a kinetically controlled process as reported in early previous study[9, 11]. As the adsorption temperature increases, more accessible CO2 active sites are available to react with CO2, thereby enhancing adsorption capacities. For S/P50/D50 samples, the adsorption capacity exhibited a slight increasing trend over the temperature range of 35-65 oC, whereas the adsorption capacity slightly reduced over 65 oC due to competing kinetic and thermodynamic effects. These observations suggest that PEI and DEA are well dispersed in the S/P50/D50 compared to S/P100. In the PEI infused silica samples, the viscous PEI with large molecules size cannot disperse very well inside silica pore. Only limited quantities of amine groups (yellow dotes in the inset graph of Figure 5) in PEI was exposed to adsorbates and be accessible by CO2 at low temperature. With the increase of temperature, PEI chains become more flexible[31] and the PEI cluster inside silica pores has enlarged volume with active more adsorption site available (represented by blue dots in the inset graph of Figure 5). As a result, the CO2 capacities in S/P100 increase with the adsorption temperature.
For the amine sample of S/P50/D50, this diffusion limitation induced by agglomerated PEI is reduced. First, small molecule size of DEA did not block CO2 diffusion path in the porous silica as indicated by higher pore volume. Secondly, the hydroxyl groups in DEA are favorable for increasing PEI chain flexibility upon the formation of hydrogen bonding thereby weakening intramolecular interaction in PEI[3] so that more active sites can be assessable even at low temperature, as illustrated by higher CO2 capacities in S/P50/D50 than S/P100 although the amine loading represented by the mole content of nitrogen elements is lower in S/P50/D50 than S/P100. Thus, the addition of DEA into PEI impregnated silica offers not only high adsorption capacities also high amine efficiency.
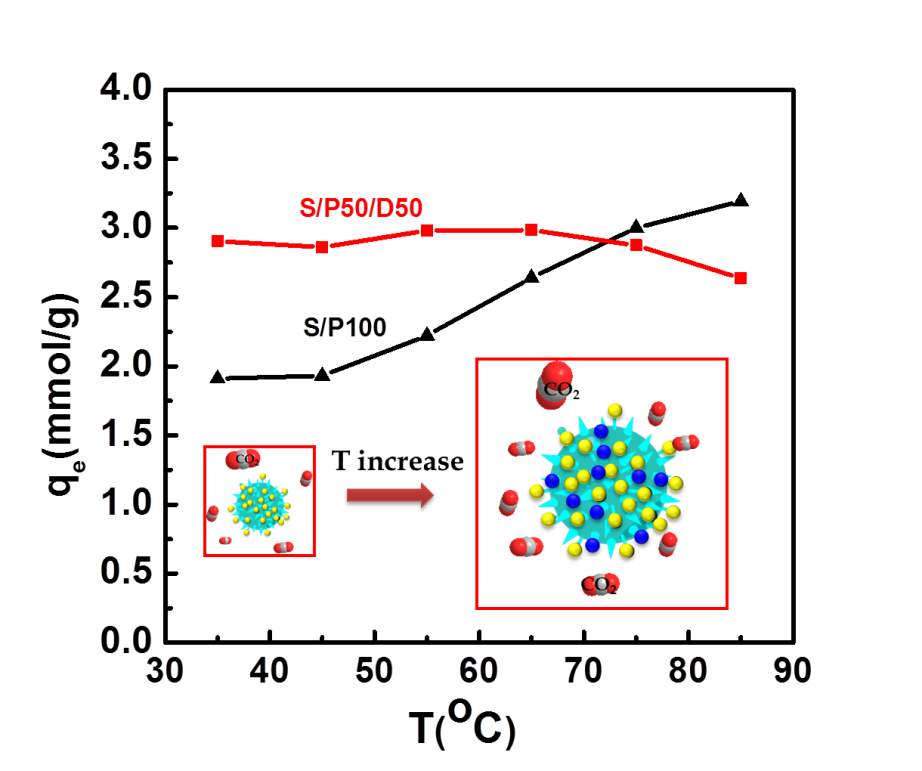
Figure 5 Temperature effects on adsorption performance of silica amine adsorbents. The yellow and blue ball symbols represent active sites for CO2 adsorption. The inset graph shows the accessible adsorbents sites in PEI species represented by yellow and blue dots.
The structural information of S/P100 and S/P50/D50 are characterized using IR spectrum. The FTIR spectrum of the amine-modified silica is compared with that of the silica sample. As shown in Figure 6,the silica framework of silica have a IR adsorption band at 467 cm-1 and a strong IR adsorption band at 1100 cm-1, which are ascribed to silica main structure motions [22, 32]. These bands are still present upon modification by amine molecules, indicating the intact silica structure after functionalization. The OH region of all samples display a broad band at 3388 cm-1 as a result of the hydroxyl groups (silanol groups or OH group in DEA) with hydrogen bonding [32, 33]. The new IR bands in amine modified silica are attributed to the characteristic vibration of NH2 and CH2 group. The IR bands at 2857 cm-1 , 2956 cm-1 and 1306 cm-1 are attributed to asymmetric stretch of methylene groups[22]. As both DEA and PEI molecule contain –CH2- and –NH- group, IR spectrum of S/P100 and S/P50/D50 did not exhibit the visible difference. The bands at 1475 cm-1 and 1571 cm-1 are assigned to N-H deformation in ionic carbamates [32, 34], which are the product from the reaction of CO2 with amine groups. To note here, the peak 1571 cm-1 was not detected in the IR spectrum of S/D100 instead that the new peak at 1545 cm-1 ascribed to carbamate or carbonates [34]was found. We speculate that this appearance of this new peak arises from differences of chemical environments caused by the DEA. The group frequencies for carbamate groups changes to low energy region. Perhaps the hydroxyl group in DEA changes the interaction mechanism of CO2 with amine groups.
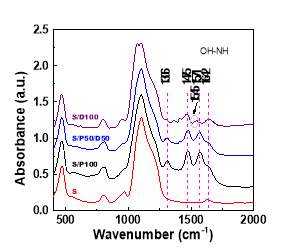
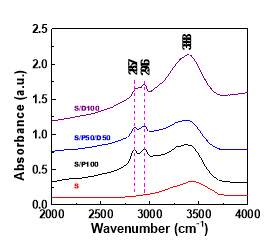
Figure 6 The FTIR spectrums of silica functionalized with pure PEI, PEI/DEA and pure DEA compared with that of silica support
3.3 Cyclic stability of S/P100 and S/P50/50 adsorbents
To obtain preliminary insight into the cyclic stability of S/P50/D50, cyclic adsorption-desorption experiments were performed via TGA under the flow of pure CO2. Figure 7 show the equilibrium CO2 adsorption capacities versus the numbers of adsorption-desorption cycles. In first 2 cycles, the CO2 capacity in S/P50/D50 decreased from 2.93mmol/g to 2.56 mmol/g. After the first 2 cycles, the capacity of S/P50/D50 dropped slightly and this adsorbent is fairly stable. The final capacity after 10 cycles is 2.40 mmol/g, which is even higher than the initial capacity of fresh S/P100. The significant capacity loss at the first 2 cycles could be attributed to the loss of small molecule amine species upon heating during desorption step. Compared to S/P50/D50, the sample of S/P100 exhibited less capacity loss in 10 cycles. The CO2 capacities after 10 cycles are 1.79 mmol/g. However, its capacities are lower than that of S/P50/D50 over the entire cyclic operation. As demonstrated in previous studies [14, 19], the capacity loss led by the slow amine species evaporation is commonly found in the class 1 adsorbents due to a lack of chemical bond between PEI and sorbent supports, which has the adverse effects on the performance of fiber sorbents.
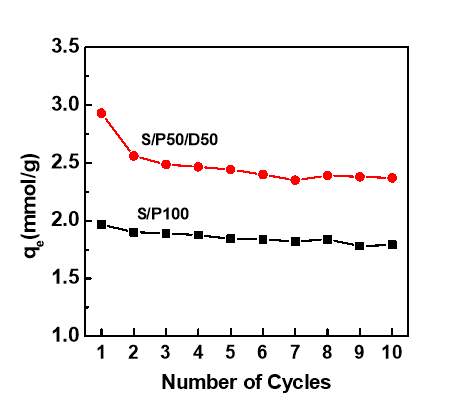
Figure 7 Cyclic capacity of silica impregnated with various amine molecules
3.4 Breakthrough experiments under dry and humid conditions
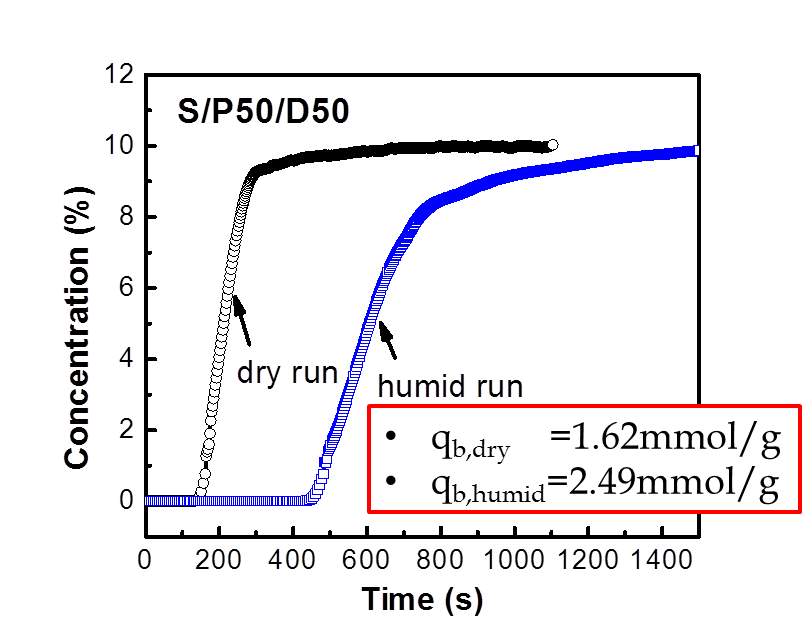
Figure 8 Breakthrough curves of S/P50/D50 under dry and humid conditions at 35 C.
The breakthrough capacity, qb, is a more important parameter in evaluating adsorbent utility in a practical industrial process. The breakthrough experiments were performed in a custom –built fixed bed column. Breakthrough capacity is defined as where CO2 concentration reached 50% of inlet composition of CO2. The simulated flue gas of 10% CO2/90% N2 was used during breakthrough experiments under both dry and humid conditions. The flow rate of flue gas is 40 mL/min. Under humid CO2 adsorption experiments, the adsorbents were prehydrated with humid nitrogen first and then CO2 was flew into fixed bed. From the breakthrough curve profile, CO2 broke through the fixed bed at 180 s under dry conditions while breakthrough time increased to 400s under humid conditions. This fact indicates the CO2 adsorption capacity increased dramatically upon the presence of moisture. And the qb was estimated as 1.62 mmol/g under dry conditions and the qb increased by ~54% under humid conditions. Many research works have studied the CO2-amine reaction mechanism with/without the moisture. Basically, one CO2 molecule requires two amine groups in the dry condition, where the main reaction is the formation of carbamate. [35-37] When the moisture is present in the flue gas, carbamate may further react with water to generate the bicarbonate or CO2 directly reacts with water and amine group with the formation of bicarbonate. Thus, the theoretical efficiency increased from 0.5 to 1. In the S/P50/D50 samples, the hydroxyl group in DEA may influence reaction mechanism, which can not be elucidated so far based on the current experimental evidences. The high qb value of 2.49 mmol/g under humid conditions for S/P50/D50 demonstrates this type material is potentially attractive for CO2 capture. The addition of DEA in PEI impregnated silica adsorbents is beneficial for the better distribution of amine in the pores, thereby reducing the diffusion resistance and boosting the adsorption capacity and kinetics.
DSC[17]
It has also been suggested
that the presence of hydroxyl groups in PEG could change to
some extent the absorption mechanism by forming bicarbonates.
25
5. Conclusion
4 Conclusions
Tuning The mixed amine of DEA and PEI was successfully impregnated onto mesoporous silica to prepare the new amine silica adsorbents named as S/P50/D50. When DEA PEI ratio is 5:5, the capacity is maximumThis adsorbent exhibits the attractive CO2 adsorption capacities of 2.93 mmol/g. Under humid conditions, the breakthrough capacity is 2.4 mmol/g, exhibiting promising application for CO2 capture. More important, amine efficiency for this adsorbent is significantly enhanced contributed by the addition of DEA. Compared to low amine efficiency of 0.19 for typical S/PEI adsorbents, the amine efficiency for S/P50/D50 is 0.39. S/P50/D50 demonstrated faster adsorption kinetics as illustrated by shaper co2 uptake curves than S/P100. After 10 adsorption-desorption cycles, the capacity of S/P50/D50 is around 2.4 mmol/g, even higher than S/P100. This work shows the positive effects of dea on S/P50/D50. The hydroxyl group could favor the dispersion of Pei and on the other hand, secondary amine groups also interact with co2 to promote the co2 adsorption in p/d/s samples.
Acknowledgments
The authors gratefully acknowledge the financial support of the Nation Natural Science Foundation of China (Grant No.21506252 ),the Fund of China University of Petroleum (Grant No. 2462015YJRC017) . we also thank the financial support from Key National Lab of heavy petroleum in China University of Petroleum (Beijing).
1. Gadipelli, S., H.A. Patel, and Z. Guo, An Ultrahigh Pore Volume Drives Up the Amine Stability and Cyclic CO2 Capacity of a Solid-Amine@Carbon Sorbent. Advanced Materials, 2015. 27(33): p. 4903-4909.
2. Booth, B. and N. Bellouin, Climate change: Black carbon and atmospheric feedbacks. Nature, 2015. 519(7542): p. 167-168.
3. Fan, Y., et al., Dynamic CO2 adsorption performance of internally cooled silica-supported poly(ethylenimine) hollow fiber sorbents. AIChE Journal, 2014. 60: p. 3878-3887.
4. Labreche, Y., et al., Poly(amide-imide)/Silica Supported PEI Hollow Fiber Sorbents for Postcombustion CO2 Capture by RTSA. ACS Applied Materials & Interfaces, 2014. 6(21): p. 19336-19346.
5. Choi, S., J.H. Drese, and C.W. Jones, Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem, 2009. 2(9): p. 796-854.
6. Rubin, E.S., et al., The outlook for improved carbon capture technology. Progress in Energy and Combustion Science, 2012. 38(5): p. 630-671.
7. Song, C., Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catalysis Today, 2006. 115(1–4): p. 2-32.
8. Rochelle, G.T., Amine Scrubbing for CO2 Capture. Science, 2009. 325(5948): p. 1652-1654.
9. Rezaei, F., et al., Aminosilane-Grafted Polymer/Silica Hollow Fiber Adsorbents for CO2 Capture from Flue Gas. ACS Applied Materials & Interfaces, 2013. 5(9): p. 3921-3931.
10. Mafra, L., et al., Structure of Chemisorbed CO2 Species in Amine-Functionalized Mesoporous Silicas Studied by Solid-State NMR and Computer Modeling. Journal of the American Chemical Society, 2017. 139(1): p. 389-408.
11. Xu, X., et al., Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy & Fuels, 2002. 16(6): p. 1463-1469.
12. Franchi, R.S., P.J.E. Harlick, and A. Sayari, Applications of Pore-Expanded Mesoporous Silica. 2. Development of a High-Capacity, Water-Tolerant Adsorbent for CO2. Industrial & Engineering Chemistry Research, 2005. 44(21): p. 8007-8013.
13. Lively, R.P., W.J. Koros, and J.R. Johnson, Enhanced cryogenic CO2 capture using dynamically operated low-cost fiber beds. Chemical Engineering Science, 2012. 71(0): p. 97-103.
14. Fan, Y., et al., Evaluation of CO2 adsorption dynamics of polymer/silica supported poly(ethylenimine) hollow fiber sorbents in rapid temperature swing adsorption. International Journal of Greenhouse Gas Control, 2014. 21(0): p. 61-71.
15. Wang, X. and C. Song, New Strategy To Enhance CO2 Capture over a Nanoporous Polyethylenimine Sorbent. Energy & Fuels, 2014. 28(12): p. 7742-7745.
16. Satyapal, S., et al., Performance and Properties of a Solid Amine Sorbent for Carbon Dioxide Removal in Space Life Support Applications. Energy & Fuels, 2001. 15(2): p. 250-255.
17. Wei, J., et al., Adsorption of carbon dioxide on organically functionalized SBA-16. Microporous and Mesoporous Materials, 2008. 116(1–3): p. 394-399.
18. Harlick, P.J.E. and A. Sayari, Applications of Pore-Expanded Mesoporous Silicas. 3. Triamine Silane Grafting for Enhanced CO2 Adsorption. Industrial & Engineering Chemistry Research, 2006. 45(9): p. 3248-3255.
19. Xu, X., et al., Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous and Mesoporous Materials, 2003. 62(1–2): p. 29-45.
20. Song, F., et al., Mesoporous TiO2 as the support of tetraethylenepentamine for CO2 capture from simulated flue gas. RSC Advances, 2013.
21. Yue, M.B., et al., CO2 Capture by As-Prepared SBA-15 with an Occluded Organic Template. Advanced Functional Materials, 2006. 16(13): p. 1717-1722.
22. Qi, G., et al., High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy & Environmental Science, 2011. 4(2): p. 444-452.
23. Auta, M. and B.H. Hameed, Adsorption of carbon dioxide by diethanolamine activated alumina beads in a fixed bed. Chemical Engineering Journal, 2014. 253: p. 350-355.
24. Lin, Y., et al., Enhanced selective CO2 adsorption on polyamine/MIL-101(Cr) composites. Journal of Materials Chemistry A, 2014. 2(35): p. 14658-14665.
25. Yue, M.B., et al., Efficient CO2 Capturer Derived from As-Synthesized MCM-41 Modified with Amine. Chemistry – A European Journal, 2008. 14(11): p. 3442-3451.
26. Liu, Y., et al., Carbon Dioxide Capture by Functionalized Solid Amine Sorbents with Simulated Flue Gas Conditions. Environmental Science & Technology, 2011. 45(13): p. 5710-5716.
27. Yue, M.B., et al., Promoting the CO2 adsorption in the amine-containing SBA-15 by hydroxyl group. Microporous and Mesoporous Materials, 2008. 114(1–3): p. 74-81.
28. Goeppert, A., et al., Nanostructured silica as a support for regenerable high-capacity organoamine-based CO2 sorbents. Energy & Environmental Science, 2010. 3(12): p. 1949-1960.
29. Sayari, A., Amine modified adsorbent, its preparation and use for dry scrubbing of acid gases. 2004, Google Patents.
30. Yan, X., et al., Amine-Modified SBA-15: Effect of Pore Structure on the Performance for CO2 Capture. Industrial & Engineering Chemistry Research, 2011. 50(6): p. 3220-3226.
31. Wang, X., et al., Infrared Study of CO2 Sorption over “Molecular Basket” Sorbent Consisting of Polyethylenimine-Modified Mesoporous Molecular Sieve. The Journal of Physical Chemistry C, 2009. 113(17): p. 7260-7268.
32. Danon, A., P.C. Stair, and E. Weitz, FTIR Study of CO2 Adsorption on Amine-Grafted SBA-15: Elucidation of Adsorbed Species. The Journal of Physical Chemistry C, 2011. 115(23): p. 11540-11549.
33. Huang, H.Y., et al., Amine-Grafted MCM-48 and Silica Xerogel as Superior Sorbents for Acidic Gas Removal from Natural Gas. Industrial & Engineering Chemistry Research, 2002. 42(12): p. 2427-2433.
34. Bacsik, Z., et al., Temperature-Induced Uptake of CO2 and Formation of Carbamates in Mesocaged Silica Modified with n-Propylamines. Langmuir, 2010. 26(12): p. 10013-10024.
35. Xu, X., et al., Influence of Moisture on CO2 Separation from Gas Mixture by a Nanoporous Adsorbent Based on Polyethylenimine-Modified Molecular Sieve MCM-41. Industrial & Engineering Chemistry Research, 2005. 44(21): p. 8113-8119.
36. Caplow, M., Kinetics of carbamate formation and breakdown. Journal of the American Chemical Society, 1968. 90(24): p. 6795-6803.
37. Serna-Guerrero, R., E. Da’na, and A. Sayari, New Insights into the Interactions of CO2 with Amine-Functionalized Silica. Industrial & Engineering Chemistry Research, 2008. 47(23): p. 9406-9412.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: