Relationship Between Neural Functioning During Stroop Task and Risky Sexual Behavior
Info: 11311 words (45 pages) Dissertation
Published: 28th Feb 2022
Tagged: PsychologyNeurology
Abstract
Background: Research suggests that deficits in both executive functioning and trait impulsivity may play a role in risky sexual behavior. At the neural level, differences in regulation of the prefrontal cortex have been linked to impulsivity, measured neurocognitively and through self-report. The relationship between neurocognitive measures of executive control and trait impulsivity in predicting risky sexual behavior has not been investigated.
Purpose: To investigate the relationship between neural functioning during the Stroop task and risky sexual behavior, as well as the effect of individual differences in urgent (positive and negative) impulsivity on this relationship.
Methods: 105 sexually active men who have sex with men (MSM)completed the Stroop task during functional magnetic resonance imaging scanning. They also completed impulsivity inventories and self-reported their risky sexual behavior (events of condomless anal sex in the last 90 days).
Results: Risky participants had greater activation than safe participants during the color congruent condition of the Stroop task in anterior cingulate cortex/dorsomedial prefrontal cortex, dorsolateral prefrontal cortex, left frontal pole, and right insula. Across these regions, this neural activation mediated the link between (positive and/or negative) urgent impulsivity and risky sexual behavior.
Conclusions: Findings suggest that the brains of men who engage in risky sexual behavior may be less adept at distributing cognitive resources efficiently during tasks of executive functioning than men who practice safe sex, and that this may relate to differences in the prefrontal cortical/fronto-insular system responsible for impulse control.
Key words: impulsivity, executive functioning,Stroop task, negative urgency, positive urgency
Introduction
Risky sexual behavior (i.e. condomless anal sex) among men who have sex with men (MSM) remains prevalent (1), despite its role in increasing the risk of sexually transmitted infections (STI), including HIV infection (2). Many social, cultural and psychological factors contribute to risky sexual decision-making in HIV-negative MSM (3-6). However, neurobiological contributions to risky sexual decision-making are not well understood (7). One neurobiological theory of risky decision-making (8, 9) posits that risky behavior emerges from the interaction of three neural systems: an amygdala-striatal system that promotes habitual appetitive behaviors, a prefrontal cortex system facilitating executive functioning and impulse control, and an insular cortex system that responds to homeostatic and interoceptive signals and facilitates switching among neural networks (default mode and executive control) (10). Although this model was developed in the context of addiction, it has been applied to other types of decision-making that pit appetitive drives against self control, such as overeating high-calorie foods (11). Like desirable foods and addictive substances, sexual stimuli have also been shown to reduce impulse control (12). Furthermore, neural activity in the prefrontal cortex during response inhibition correlates with both substance use and sexual risk in adolescents (13, 14) suggesting the efficacy of the neurobiological model in the domain of risky sex as well. Similarly, the amygdala and striatum have been implicated in the processing of sexual stimuli (15, 16). Stronger striatal reactivity in response to sexual cues has been shown to predict subsequent sexual behavior (17) and striatal sensitivity to sexual cues has been observed in individuals with compulsive sexual behavior relative to controls (16, 18). Based on this model and the existing work on the neurobiology of sexual risk, one contributor to risky sexual behavior in MSM may be individual variability within the prefrontal cortex system, leading to individual differences in executive functioning.
The Stroop task (19, 20) is commonly used as a measure of executive functions that are mediated by the prefrontal cortex (21). The classic Stroop task presented participants with a series of color words printed in various ink colors, and observed an interference effect such that naming the ink color of incongruent word-ink stimuli (e.g., the word “blue” printed in red ink) was slower and less accurate than naming the ink color of congruent stimuli (e.g., the word “red” printed in red ink) or neutral stimuli (e.g., a series of Xs printed in red ink). Stroop performance depends on successful response inhibition, interference resolution, and behavioral conflict resolution (22), because the incongruent condition of the task requires the suppression of a habitual prepotent response (i.e., word reading) in favor of a more difficult response (i.e., ink color naming of an incongruent color word). In an event-related Stroop design, which we employ here, participants are asked to switch among the conditions of the task (color naming or word reading for congruent, neutral or incongruent combinations) on a trial-by-trial basis, such that performance also depends on other components of executive function (e.g. task monitoring and switching, cognitive control).
Despite its prevalence in cognitive psychology and neuroscience, relatively little neuroimaging research has applied the Stroop task to risk-taking populations or those with impulse control deficits. Among the studies that have done so, results have been mixed. Abstinent methamphetamine abusers, pathological gamblers and adults with attention deficit hyperactivity disorder (ADHD) have been found to have less activation in prefrontal cortical regions than controls during incongruent Stroop conditions (23-25). Other studies have found that adolescents with ADHD (26), abstinent cocaine users (27), and patients with schizophrenia (28) showed greater activation than controls in similar regions during incongruent Stroop conditions. In two studies examining the relationship between risky sexual behavior and neuropsychological assessment of executive functioning, Stroop interference reaction time (reaction time on incongruent trials minus reaction time on neutral trials) was unrelated to sexually risky decision-making for drug-dependent men who were HIV+ (29) or HIV+ and HIV- men (30) for whom sexual preference is unknown. None of these studies, however, examined whether any patterns of neural activation exhibited during the Stroop task are associated with sexual risk-taking.
Other research has explored relationships between impulsivity, executive functioning and risky sexual behavior from a personality trait perspective. Self-report scales measuring impulsivity (31-33) have been shown to correlate positively with one another but not with behavioral task measures of impulsive disinhibition, including the Stroop task, suggesting that laboratory tasks and self-report scales may capture distinct facets of impulse control and impulsivity (34). Among self-report inventories of impulsivity, positive urgency (35), negative urgency and sensation seeking (36) correlated positively with risky sexual behavior in adults. Thus, a comprehensive investigation of the role of impulse control in risky sexual behavior should incorporate personality measures as well as neural and behavioral ones. One relevant study (37) has integrated the measurement of negative urgency with behavioral and neural responses to the Go/No-Go response inhibition task and found that neural activity during response inhibition predicted subsequent substance abuse in individuals high in negative urgency, but no research has synthesized all of these components in relation to real-world sexual risk taking behavior.
The heterogeneity of the extant neuroimaging and neuropsychological findings, and the gaps in the prior literature, underscore the exploratory nature of the current study, which is the first to investigate the relationship between neural correlates of the Stroop task, individual differences in impulsivity, and risky sexual behavior. In this study, we employed a large sample size (N = 105) of risky (i.e. engaging in condomless anal sex over the past 90 days) and safe (i.e. never engaging in condomless anal sex) MSM to investigate whether the neural signature of executive function elicited by the Stroop task differs on the basis of real-world sexual risk taking behavior, in keeping with the theorized role of the prefrontal cortex’s executive control system in risky decision-making. Specifically, we hypothesized that risky participants would display differential recruitment of the prefrontal cortex relative to safe participants during the Stroop task condition requiring the greatest inhibition, and that behavioral differences would parallel these neural differences, with risky participants exhibiting lower accuracy or longer reaction times. We further sought to understand the extent to which individual differences in impulsivity influence real-world sexual risk taking in this population, in the hope that understanding the relationship between neural executive control and underlying trait impulsivity can produce a clearer picture of which individuals may be more inclined towards sexual risk-taking and why. We hypothesized that risky participants would self-report higher impulsivity than safe participants, and theorized that impulsivity would correlate positively with neural activity in the prefrontal cortex during the Stroop task.
Methods
Participants
The current analysis is part of a larger study that used Internet advertisements to recruit a community sample in Southern California of 177 sexually active non-monogamous men who have sex with men (MSM). The research protocol was approved by the Institutional Review Board at the University of Southern California, and informed consent was obtained from all participants included in the study. All qualified participants were between the ages of 18 and 30 and self-reported engaging in anal sex in the last 90 days, being HIV negative, were free of neurological diagnoses, were not binge drinkers, and met all safety requirements for magnetic resonance imaging (MRI) scanning. Data collection took place between January 2012 and April 2014. Participants completed the tasks and surveys reported here as part of a two-session data collection procedure that comprised two 1.25-hour MRI scan sessions and approximately 1 hour of self-report measures. Sessions were typically separated by no more than one week; testing was divided to avoid fatigue and discomfort during extended MRI data collection. Participants were paid $100 following each session, with an additional payment of $1-$20 based on performance on certain tasks.
To categorize participants based on their sexual risk for data analysis, participants self-reported the number of times they had engaged in anal sex with and without a condom in the past 90 days; individuals who reported more than zero events of condomless anal sex (CAS) were categorized as “risky”, while those with zero CAS were categorized as “safe”. From this larger sample, 146 participants ended the study with complete behavioral and functional MRI (fMRI) data for two runs of the Stroop task. We excluded 27 participants on the basis of poor task performance using two metrics: those who scored less than 70% in any run of the congruent or neutral Stroop conditions, which reflects two standard deviations below the mean accuracy for congruent and neutral conditions (M = 90.5%, SD = 9.3%), and those who scored 0% in any run of the incongruent Stroop conditions, because neuroimaging data analysis of the incongruent condition would not be possible for these participants. These restrictions attempt to control for participants who failed to understand the task (despite prior training), who fell asleep, or who chose to respond at random, while still including participants who attempted to complete the task correctly but produced errors. These cutoffs were chosen specifically for this version of the Stroop, which is more difficult than the classic version of the task (which uses verbal responses) because it requires the prior memorization of a color-key mapping in addition to the task itself; the thresholds were selected to maximize the number of participants in analyses while ensuring enough trials to facilitate fMRI data analysis for the incongruent conditions. An additional 14 participants were excluded for exceeding motion of 3mm in any direction on any run of the fMRI task. A total of 105 participants (33 safe, 72 risky) were included in the analysis. Risky participants were over-sampled relative to safe participants in the larger study to facilitate subdivision of this population on the basis of lifetime methamphetamine (MA) use for future analyses (of the initial 177 participants, 56 reported zero CAS, 69 reported CAS but no MA use, 44 reported CAS and lifetime MA use, and 8 reported zero CAS and lifetime MA use). However, no analyses reported here were significant when participants were grouped on the basis of MA use versus non-use (33 MA, 72 non-MA), suggesting that neither the imbalance in sample size nor heterogeneity within the risky population drives our findings. Demographic characteristics of the sample are reported in Table 1.
fMRI Stroop Task
Our version of the Stroop task, adapted from the original (19) for use in the fMRI scanner, asked participants to press one of four buttons (red, blue, yellow or green) in response to one of two conditions, which were cued prior to the presentation of stimuli. In the “color” condition, participants responded to the ink color of stimuli, which were congruent, incongruent, or neutral (a series of Xs). In the “word” condition, participants responded to the text of the word stimuli, which were congruent, incongruent, or neutral (white text). In both conditions, participants responded within 2500 ms with a jittered inter-trial interval (mean = 5310 ms; range 5000-8000 ms). Participants completed two runs of the task, with 16 pseudo-randomized stimuli from each of the six conditions in each run, for a total of 192 trials.
Personality Measures
Participants completed a battery of personality inventories, including the UPPS-P impulsivity scale (38). The UPPS-P consists of five impulsivity subscales: negative urgency (engaging in impulsive behavior in response to negative affect), lack of premeditation, lack of perseveration, sensation-seeking, and positive urgency (engaging in impulsive behavior in response to positive affect). Because attrition from the study between the two sessions of data collection led to some instances of missing data, UPPS-P scores were collected for 90 of the 105 participants.
Functional imaging procedure
Participants completed the Stroop task as part of a larger battery of structural and functional MRI data collection, which included a Go/No-Go task measuring response inhibition, reversal learning tasks measuring learning from reward and punishment, a cups gambling task measuring risk-taking, and a virtual interactive narrative game exploring naturalistic sexual behavior; results from these tasks are being analyzed and reported separately. Neuroimaging data collection totaled approximately 2.5 hours, divided across two sessions, such that the Stroop task was completed during a 1.25-hour scan session. The order of tasks was counterbalanced across participants.
Participants lay supine in the scanner, and viewed visual stimuli back-projected onto a screen through a mirror attached to the head coil. Foam pads were used to minimize head motion. Stimulus presentation and timing of all stimuli and response events were controlled by Matlab with Psychtoolbox (http://www.psychtoolbox.org) extensions on a MacBook Pro. Participants’ responses were collected online using an MRI-compatible button box.
MRI data for the Stroop task were collected in one session in a 3T Siemens MAGNETOM Tim/Trio scanner. A T1-weighted anatomical image, a set of diffusion-weighted images, and several task-related functional image sequences were collected. Task-related fMRI data were acquired using T2*-weighted (TR=2000 ms, TE=25 ms, 64×64 matrix size with a resolution of 3 mm2, using 41 3.0-mm axial slices) imaging.
fMRI data preprocessing and statistical analysis
Image preprocessing and statistical analysis were carried out using FEAT (FMRI Expert Analysis Tool) version 6.00, part of the FSL package (FMRIB software library, version 4.1.8, www.fmrib.ox.ac.uk/fsl). The data analysis pipeline was managed using the XFSL package (http://xfsl.fmri.cn). The data were temporally filtered using a non-linear high pass filter with a 100s cut-off, and spatially smoothed using a 5mm full-width-half-maximum (FWHM) Gaussian kernel. A two-step registration procedure was used whereby images were first registered to the MPRAGE structural image, and then into the standard MNI (Montreal Neurological Institute) space, using affine transformations with FLIRT (39, 40) to the MNI-152 T1 template brain. Registration from MPRAGE structural images to standard space was further refined using FNIRT nonlinear registration (41). Statistical analyses were performed in the native image space, with the statistical maps normalized to the standard space prior to higher-level analysis. Melodic ICA was used to de-noise the preprocessed functional data (42). The FIX software package was used to automatically identify noise components (43, 44).
Data were modeled at the first level using a general linear model within FSL’s FILM module. Correct trials of the six conditions (color congruent [CC], color neutral [CN], color incongruent [CI], word congruent [WC], word neutral [WN], and word incongruent [WI]) were modeled separately. Error trials from the six conditions were also added to the model as separate nuisance covariates. We modeled the main effects of each individual condition as well as several contrasts, including comparisons of incongruent > congruent and incongruent > neutral (collapsing across color and word condition).
At the group level, we tested whether any lower-level effects differed on the basis of risky sex condition (risky versus safe). In this model, we used a random-effects model for group analysis using the FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 simple mixed effect model (45-47). Group images were thresholded using cluster detection statistics with a height threshold of z > 2.3 and a cluster probability of p
Results
Behavioral Results
Participants did not differ significantly in accuracy or reaction time on any of the task conditions on the basis of sexual risk-taking category. Across risk categories, performance on both the color and word conditions of the Stroop task differed on the basis of task difficulty. Comparisons of accuracy results by condition are presented in Table 2. Based on these behavioral results we focused subsequent fMRI analyses on the color congruent and color incongruent trials, because they represent the least and most difficult conditions of the Stroop task.
Risky and safe participants differed on several dimensions of the UPPS-P scale. Risky participants had higher scores than safe participants on negative urgency (t(88) = -3.837, p
fMRI Results
Activation across task conditions
We observed a consistent pattern of significant activation during the color congruent, color neutral and color incongruent conditions in the dorsal anterior cingulate cortex (dACC)/dorsomedial prefrontal cortex (DMPFC), dorsolateral prefrontal cortex (DLPFC), anterior insula, superior parietal lobule and occipital cortex. Peak voxel activation in these regions from nonparametric analyses for each of the color conditions is shown in Table 3.
We next investigated whether there were differences in activation across the task conditions by identifying regions where activation was greater in incongruent than congruent conditions and where activation was greater in incongruent than neutral conditions. These contrasts overlapped in several regions identified previously, including the dACC/DMPFC, left DLPFC, bilateral insula, and left frontal pole (Figure 1; Table 3). Thus, activation in these regions appears to increase with increasing task difficulty.
Neural differences by sexual risk category
We next investigated whether the increases in neural activation on the basis of task difficulty differed on the basis of sexual risk category. In order to explore this, we created a “task difficulty” mask of the overlap between the incongruent > congruent and incongruent > neutral contrasts (as shown in Figure 1). We then created five discrete regions of interest (ROIs) for each of the major anatomical regions contained in that mask (ACC/DMPFC, DLPFC, right insula, left insula, and left frontal pole), by selecting the overlap of the task difficulty mask with anatomically-defined masks from the Harvard-Oxford cortical and subcortical atlas for each region (Figure 2A). Finally, we extracted the percent signal change from each ROI, and compared percent signal change values for risky and safe participants using independent-samples t-tests.
Risky participants had greater activation than safe participants during color congruent trials in DMPFC (t(103) = 2.712, p = .008, d = .55), DLPFC (t(103) = 2.431, p = .017, d = .51), right insula (t(103) = 3.220, p = .002, d = .67) and left frontal pole (t(103) = 2.687, p = .008, d = .54). Risky participants also had greater activation than safe participants during color neutral trials in right insula (2.689, p = .008, d = .56) and left frontal pole (t(103) = 2.191, p = .031, d = .47). The patterns of activation in each of these ROIs are shown in Figure 2B-2F. No other differences between risky and safe participants were significant for the color neutral trials, and no differences were significant for color incongruent trials in any of the ROIs.
Across all participants, activation was greater during the color incongruent than the color congruent condition (as seen in the whole-brain contrast); however, the increase in activation from the color congruent condition to the color incongruent condition was greater for safe participants than risky participants in DMPFC (t(103) = 2.524, p = .013, d = .52), DLPFC (t(103) = 2.237, p = .027, d = .46), and right insula (t(103) = 2.220, p = .029, d = .47).
Mediational analyses
Because both positive and negative urgency significantly predicted whether our participants were risky or safe, we investigated whether patterns of neural activation during the Stroop mediate the link between urgency and having engaged in risky sexual behavior. We focused on the color congruent condition, controlling for activation during the color incongruent condition, to explore what might be driving the observed difference between risk groups in this condition shown in the ROI analyses. We assessed this in the five ROIs (ACC/DMPFC, DLPFC, Left Frontal Pole, Right Insula, Left Insula) during the color congruent condition, controlling for activation during the color incongruent condition. To do this we tested a series of bias-corrected, bootstrapped (at 10,000 samples) mediation models using logistic regressions with model 4 of the PROCESS macro for SPSS (50). In these mediational models, the a path denotes the effect of the trait (x) on brain activations (m), the b path denotes the effect of brain activations (m) on risk category (y), and the c’ path denotes the effect of the trait positive or negative urgency (x) on risk category (y), controlling for the indirect effect. A lack of a zero in the confidence interval reported in Table 4 indicates a significant indirect effect. As indicated in Table 4, for negative urgency, the indirect (mediational) paths were significant for the dACC/DMPFC, DLPFC, the left frontal pole, and the right insula. For positive urgency, the indirect (mediational) paths were significant for the ACC/DMPFC, DLPFC, and left frontal pole. No other indirect effects were significant. Figure 3 shows the mediational relationships of negative urgency, dACC/DMPFC and risk category, and of negative urgency, DLPFC, and risk category, for illustrative purposes. The same structure is also used for the remaining eight analyses shown in Table 4.
The direct effect of negative urgency on risk category, controlling for the indirect effect, was significant for each brain region: (1) dACC/ DMPFC= 1.485 (SE = .469), CI: .5648, 2.406; (2) DLPFC=1.2837 (SE = .4397), CI: .4219, 2.1454; (3) left Frontal Pole=1.4429 (SE = .4612), CI: .4391, 2.3468; (4) Right Insula =1.3887 (SE = .4561), CI: .4948, 2.2827; (5) Left Insula= 1.51626 (SE = .460), CI: .6609, 2.4643. For positive urgency, the direct effect was only significant for the left insula and the left frontal pole: (1) dACC/ DMPFC =.7714 (SE=.403), CI: -.0178,1.5606; (2) DLPFC =.6874 (SE = .399), CI: -.0961, 1.4710; (3) left Frontal Pole= .7938 (SE =.402), CI: .0059, 1.5817); (4) Right Insula=.7728 (SE=.399), CI: -.0097, 1.5554); (5) Left insula= .9114 (SE = .392), CI: .1435, 1.6792.
Discussion
The pattern of neural activation elicited by the Stroop task in this study is consistent with other Stroop neuroimaging research. An automated meta-analysis of 113 studies categorized by the term “Stroop task” from the NeuroSynth database (neurosynth.org) (51, 52) shows activation in ACC, DMPFC, DLPFC, insula, and parietal cortex, matching the regions we identified. These regions are active during Stroop performance not only in healthy control studies but also in studies of populations associated with behavioral risk and impulsivity, such as pathological gamblers (24), methamphetamine users (23), marijuana users (53), and adolescents with ADHD (54). This indicates that even though the Stroop task has not previously been used with sexually risky participants, our subjects engaged in the expected cognitive processing when performing the task.
We observed no behavioral differences in Stroop performance between participant groups in terms of accuracy and reaction time, contrary to our initial expectations, despite observing individual differences in impulsivity and differences at the neural level. However, findings of neural group differences in the Stroop task in the absence of behavioral differences have been shown in other studies, even those with impulsive/high-risk populations (24, 26, 27). This suggests that performance effects are separable from processing efficiency or neural recruitment in the context of the Stroop task, and individual differences at the neural level may alter only the latter while still producing equivalent task performance.
Across several regions where the Stroop task elicits activation (ACC/DMPFC, DLPFC, insula), the magnitude of activation increased between the color congruent (easy) condition and the color incongruent (difficult) condition. However, this increase was driven by safe participants, with risky participants’ elevated activation during the color congruent condition leading to less change on the basis of task difficulty. These results deviate from our hypothesis, as we expected neural differences to be more prevalent in the color incongruent condition, which necessitates the most pronounced inhibitory control. These findings suggest that risky men may modulate their recruitment of the neural resources necessary for inhibitory control less than safe men do in the face of changing task difficulty. A similar pattern of activation was seen in a Stroop study of methamphetamine (MA) abusers, wherein control participants showed increased DLPFC activation during incongruent trials that followed other incongruent trials versus those that followed congruent trials, while MA participants did not (55). It is worth noting, however, that the pattern reported by Salo and colleagues was due to a reversed pattern of activation on the part of MA participants (greater activation during incongruent trials that followed congruent trials), as opposed to the consistently elevated activation we observe. This difference may relate to neurological effects of methamphetamine dependence that are not present in our risky sample, despite the fact that both groups are theorized to have impaired cognitive control. It has also been noted that unlike neutral trials, color congruent trials still provide a competing response-eligible stimulus (e.g. participants must still process color information and word information) and that participants may engage in an attempt to suppress the word as distracting information, leading to increased recruitment of the left DLPFC during congruent trials (56). The fact that we observed this increased recruitment in risky but not safe participants opens up the possibility that they are engaging in different cognitive strategies in order to successfully execute the task.
The pattern of elevated cortical activity insensitive to task difficulty that we observed in risky participants may be explained by cortical inefficiency. A study of unipolar depressed individuals (57) found that they displayed hyperactivity in the ACC and DLPFC during incongruent Stroop conditions, and theorized that the altered affective state produced by depression elevates neural activity, leading to inefficiencies in cognitive processing during tasks. Similar work contrasting emotional and non-emotional Stroop conditions found deactivation in ACC during the non-emotional conditions, suggesting that this deactivation allocates resources for effective cognitive performance in healthy participants (58). Based on this theory, risky participants’ hyperactivity relative to safe participants in the color congruent condition represents an inability to reduce activation during easy trials, leading to altered neural activity but not necessarily impaired behavioral performance. Several studies (23, 24) have observed a blunted neural Stroop effect (i.e., smaller difference in activation between incongruent and congruent conditions) in high-impulsivity populations (gamblers, abstinent methamphetamine users) relative to control populations and have attributed this finding to neural inefficiency. It is worth noting, however, that other studies (59, 60) have observed opposite findings – a larger neural Stroop effect in patient populations relative to control populations – and have also attributed these results to neural inefficiency in patients. This highlights the need for studies of the Stroop task to investigate not only the interaction Stroop effect contrast, but also activation during individual conditions (e.g. color congruent, color incongruent) to disentangle differences in the myriad underlying cognitive processes utilized in Stroop performance. More broadly, caution should be exercised in conclusively interpreting larger and smaller magnitudes of activation as representing either efficiency or inefficiency without supporting evidence of differences in underlying processing or network engagement (61).
Several of the regions identified here have been associated with trait impulsivity as measured by psychometric inventories. Larger gray matter volumes in MPFC and DLPFC, as well as subcortical regions, correlated positively with the Barratt Impulsiveness Scale 11 (24) in healthy adults (62), and functional DLPFC activation correlated positively with the BIS in MDMA users and controls (63). Glucose metabolism (a proxy measure of neural activity) in the fronto-insular network, including the right insula, ACC and orbitofrontal cortex, correlated positively with impulsivity as measured by the BIS-11 in patients with Parkinson’s Disease (64). Similarly, BIS has been correlated with functional activation in the ACC (65) and middle frontal gyrus (66) elicited by a Go/No-Go task. These studies suggest increased fronto-insular brain activity independent of task performance may be a marker of impulsivity, which is consistent with our finding that risky participants are higher in behavioral impulsivity (negative and positive urgency and lack of premeditation) than safe participants, and could also explain the elevated levels of neural activity across task conditions observed for risky participants on the Stroop task.
Further support for the role of fronto-insular activity in impulsivity comes from the mediation relationship that we observed, which suggests that neural responsiveness during low-difficulty cognitive control underlies the relationship between urgent impulsivity and risky sexual behavior. This parallels the model put forth by Chester and colleagues (37) of negative urgency’s relationship with response inhibition (as measured by neural responsiveness during an emotionally valenced Go/No-Go task) and real-world substance abuse. They observed greater recruitment of PFC regions during negatively-valenced response inhibition for participants high in negative urgency (without behavioral performance differences), and this same pattern observed in the anterior insula predicted greater substance abuse measured one month and one year after scanning. Chester et al. suggest that elevated fronto-insular activity may be a compensatory mechanism to achieve satisfactory response inhibition for impulsive individuals, but that such demands may contribute to real-world impulse control failures such as substance use. Similarly, the elevated neural response during the Stroop task observed in our participants may reflect a need for greater cognitive control recruitment among more impulsive individuals even during less challenging control tasks; this elevated neural effort may prove insufficient under real-world circumstances requiring greater impulse control, leading to real-world risk-taking including risky sexual behavior. This interpretation is predicated on the idea that condom use is desirable from a cognitive control perspective (i.e., that using condoms during sex prevents the spread of HIV and STIs) but not from an affective perspective (i.e., that condomless sex is more pleasurable or desirable for oneself and/or one’s partner) and that the high-arousal state of a sexual encounter interferes with cognitive control mechanisms.
This study faces several limitations. The lack of behavioral or neural differences between the risky and safe group on the color incongruent condition make it difficult to interpret these results in the context of response inhibition, since accurate performance on congruent conditions does not require inhibition per se. Moreover, the Stroop task captures only some aspects of interference resolution, which may not reflect the cognitive processes most salient to real-world sexual situations. For example, Stroop is a less apt measure of the ability to restrain an inappropriate response during response execution than the Go/No-Go and Stop Signal tasks, although all tasks share some common circuitry (56). Subsequent analyses from this research project will be able to address this question more thoroughly, as participants also completed a Go/No-Go task as part of the larger study. Integrating individual responses across executive function tasks requiring various aspects of inhibition will paint a clearer picture of the role of various neurocognitive constructs on risky sexual behavior.
Taken together, our findings suggest that the brains of men who engage in risky sexual behavior may distribute cognitive resources in a distinct (and possibly less efficient) manner during tasks of executive function compared to men who practice safe sex, and that this may be due to differences in the prefrontal cortical/fronto-insular system responsible for aspects of executive functioning, task monitoring, and cognitive control. Although the observed neural differences between risky and safe men did not negatively impact behavioral performance on the Stroop task, this may be due to the demand effects of experiment participation or the non-arousing nature of the laboratory setting. In a real-world sexual situation, individual differences in executive functioning may interact with other neural systems, such as elevated urge states or habitual/reward-seeking behavior that are not present in a laboratory context, to increase the likelihood of engaging in actual risky sexual behavior. Subsequent research exploring the relationship between risky sexual behavior and cognitive control under more affectively arousing contexts can explore this theory. Furthermore, the mediational relationship with urgent impulsivity suggests that this self-report scale could be useful in identifying individuals at higher likelihood of engaging in condomless anal sex (with its concomitant risk of contracting HIV and other STIs), who could then receive targeted interventions such as pre-exposure prophylaxis. These findings offer a first look into the ability of the Stroop task to distinguish risky and safe participants on the basis of their neural patterns during a complex executive function task.
References
- Centers for Disease Control and Prevention. HIV testing and behaviors among gay, bisexual, and other men who have sex with men – United States. Morbidity and Mortality Weekly Report, 2013; 62: 958-962. Retrieved from: http://www.cdc.gov/mmwr/pdf/wk/mm6247.pdf.
- Bearinger LH, Sieving RE, Ferguson J, Sharma V. Global perspectives on the sexual and reproductive health of adolescents: patterns, prevention, and potential. Lancet. 2007; 369(9568): 1220–1231.
- Dudley MG, Rostosky SS, Korfhage BA, Zimmerman RS. Correlates of high-risk sexual behavior among young men who have sex with men. AIDS Educ Prev. 2004; 16(4): 328-340.
- Frye V, Latka MH, Koblin B, Halkitis PN, Putnam S, Galea S, Vlahov D. The urban environment and sexual risk behavior among men who have sex with men. J Urban Health. 2006; 83(2): 308-324.
- Mustanski BS. HIV in young men who have sex with men: A review of epidemiology, risk, and protective factors, and interventions. J Sex Res. 2011; 48: 218-253.
- Myers HF, Javanbakht M, Martinez M, Obediah S. Psychosocial predictors of risky sexual behaviors in African American men: Implications for prevention. AIDS Educ Prev. 2003; 15(1 Suppl): 66.
- Ross JM, Duperrouzel J, Vega M, Gonzalez R. The neuropsychology of risky sexual behavior. Int Neuropsychol Soc. 2016; 22(6): 586-594.
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;,8(11): 1458-1463.
- Noël X, Brevers D, Bechara A. (2013). A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013; 23(4): 632-638.
- Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015; 19(7): 414-420.
- He Q, Xiao L, Xue G, Wong S, Ames SL, Schembre SM, Bechara A. Poor ability to resist tempting calorie rich food is linked to altered balance between neural systems involved in urge and self-control. Nutr J. 2014; 13(1): 1.
- Macapagal KR, Janssen E, Fridberg DJ, Finn PR, Heiman JR. The effects of impulsivity, sexual arousability, and abstract intellectual ability on men’s and women’s Go/No-Go task performance. Arch Sex Behav. 2011; 40(5): 995-1006.
- Feldstein Ewing SW, Ryman SG, Gillman AS, Weiland BJ, Thayer RE, Bryan AD. Developmental cognitive neuroscience of adolescent sexual risk and alcohol use. AIDS Behav. 2016; 20: S97-S108.
- Goldenberg D, Telzer EH, Lieberman MD, Fulgini A, Galván A. Neural mechanisms of impulse control in sexually risky adolescents. Dev Cogn Neurosci. 2013; 6: 23-29.
- Cyders MA, Dzemidzic M, Eiler WJ, Kareken DA. An fMRI study of responses to sexual stimuli as a function of gender and sensation seeking: A preliminary analysis. J Sex Res. 2016; 53(8): 1020-1026.
- Voon V, Mole TB, Banca P, Porter L, Morris L, Mitchell S, Lapa TR, Karr J, Harrison NA, Potenza MN, Irvine M. Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLos One. 2014; 9(7): e102419.
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012; 32(16): 5549-5552.
- Seok JW, Sohn JH. Neural substrates of sexual desire in individuals with problematic hypersexual behavior. Front Behav Neurosci. 2015; 9: 206.
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935; 18: 643–62.
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991; 109: 163-203.
- Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd D. Stroop performance in normal control subjects: An fMRI study. NeuroImage. 2002; 16: 349-360.
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss, AL. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002; 16(1): 61-75.
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011; 194(3): 287-295.
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An fMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003; 160(11): 1990-1994.
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biol Psychiatry. 1999; 45(12): 1542-1552.
- Fan LY, Gau SSF, Chou TL. Neural correlates of inhibitory control and visual processing in youths with attention deficit hyperactivity disorder: a counting Stroop functional MRI study. Psychol Med. 2014; 44(12): 2661-2671.
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004; 16(4): 456-464.
- Nordahl TE, Carter CS, Salo RE, Kraft L, Baldo J, Salamat S, Robertson L, Kusubov N. Anterior cingulate metabolism correlates with Stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001; 25(1): 139-148.
- Wardle MC, Gonzalez R, Bechara A, Martin-Thormeyer EM. Iowa gambling task performance and emotional distress interact to predict risky sexual behavior in individuals with dual substance and HIV diagnoses. J Clin Exp Neuropsychol. 2010; 32: 1110-1121.
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Nunnally G, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. J Int Neuropsychol Soc. 2005; 11(2): 121-131.
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995; 51: 768-774.
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Pers Individ Dif. 1985; 6: 613-619.
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychol Assess. 2002; 14: 150-163.
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Pers Individ Dif. 2006; 40: 305-315.
- Zapolski TCB, Cyders MA, Smith GT. Positive urgency predicts illegal drug use and risky sexual behavior. Psychol Addict Behav. 2009; 23(2): 348-354.
- Deckman T, DeWall NC. Negative urgency and risky sexual behaviors: A clarification of the relationship between impulsivity and risky sexual behavior. Pers Individ Dif. 2011; 51(5): 674-678.
- Chester DS, Lynam DR, Milich R, Powell DK, Andersen AH, DeWall CN. How do negative emotions impair self-control? A neural model of negative urgency. NeuroImage. 2016; 132: 43-50.
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior. Technical report. West Lafayette, IN: Purdue University; 2006.
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002; 17(2): 825-841.
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001; 5(2): 143-156.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2. 2010.
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004; 23(2): 137-152.
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014; 95: 232-247.
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014; 90: 449-468.
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003; 20(2): 1052-1063.
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008; 41(2): 286-301.
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004; 21(4): 1732-1747.
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PNAS. 2016; 113(28): 7900-7905.
- Winkler A, Ridgway G, Webster M, Smith S, Nichols T. Permutation inference for the general linear model. NeuroImage. 2014; 92: 381-397.
- Hayes AF. Introduction to mediation, moderation and conditional process analysis: A regression-based approach. Guilford Press; 2013.
- Yarkoni T. Neurosynth core tools v0.3.1 [Data set]. Zenodo. 2014; http://doi.org/10.5281/zenodo.9925
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager, TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011; 8: 665-670.
- Sagar KA, Dahlgren MK, Gönenç A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cog Neurosci. 2015; 16: 84-92.
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An fMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009; 166: 1286-1294.
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: A functional magnetic resonance imaging study. Biol Psychiatry. 2009; 65(8): 706-709.
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007; 7(1): 1-17.
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RGM. Cortical inefficiency in patients with unipolar depression: An event-related fMRI study with the Stroop task. Biol Psychiatry. 2006; 59(10): 958-965.
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998; 44: 1219-1228.
- Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED. Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a Stroop task. Neuropsychopharmacology. 2010; 35: 775-782.
- Basten U, Stelzel C, Fiebach CJ. Trait anxiety modulates the neural efficiency of inhibitory control. J Cogn Neurosci. 2011; 23(10): 3132-3145.
- Poldrack RA. Is “efficiency” a useful concept in cognitive neuroscience? Dev Cog Neurosci. 2015; 11: 12-17.
- Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, Strafella AP. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2013; 26: 479-487.
- Valdes IH, Steinberg JL, Narayana PA, Kramer LA, Dougherty DM, Swann AC, Barratt ES, Moeller FG. Impulsivity and BOLD fMRI activation in MDMA users and healthy control subjects. Psychiatry Res Neuroimaging. 2006; 147(2-3): 239-242.
- Tahmasian M, Rochhausen L, Maier F, Williamson KL, Drzezga A, Timmerman L, Van Eimeren T, Eggers C. Impulsivity is associated with increased metabolism in the fronto-insular network in Parkinson’s Disease. Front Behav Neurosci. 2015; 9: 317.
- Brown SM, Manuck SB, Flory JD, Hariri A. Neural basis of individual differences in impulsivity: Contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006; 6(2): 239-245.
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: An fMRI study. Neuropsychologia. 2003; 41(14): 1959-1966.
| Table 1. Participant demographics. | ||||||||
| Age
M (SD) |
White
N (%) |
Black
N (%) |
Latino
N (%) |
CAS90
M (SD) |
AS90
M (SD) |
MA90
M (SD) |
||
| N | ||||||||
| Safe | 33 | 23.1 (2.7) | 8 (24.2%) | 15 (45.5%) | 10 (30.3%) | 0 (0) | 8.3 (13.4) | 0.1 (0.5) |
| Risky | 72 | 24.9 (3.3) | 26 (36.1%) | 18 (25%) | 28 (38.9%) | 7.3 (9.3) | 14.3 (12.2) | 1.5 (4.4) |
| Note. M = mean. SD = standard deviation. N = number of participants. CAS90 = Events of condomless anal sex in the past 90 days. AI90 = all events of anal sex in the past 90 days. MA90 = uses of methamphetamine in the past 90 days. | ||||||||
| Table 2. Stroop Task Percent Accuracy by Condition | |||||||
| Condition | M | SD | Contrast | t | p | Cohen’s d | |
| Color Congruent (CC) | 94.97% | 5.49% | CC vs. CN | 4.88 | <.001> | 0.48 | |
| Color Neutral (CN) | 91.85% | 6.58% | CI vs. CN | 18.52 | <.001> | 2.56 | |
| Color Incongruent (CI) | 62.95% | 19.16% | CI vs. CC | 18.93 | <.001> | 2.54 | |
| Word Congruent (WC) | 93.21% | 6.43% | WC vs. WN | 0.67 | 0.508 | 0.06 | |
| Word Neutral (WN) | 92.86% | 6.79% | WI vs. WN | 9.83 | <.001> | 1.12 | |
| Word Incongruent (WI) | 82.14% | 13.36% | WI vs. WC | 10.16 | <.001> | 1.19 | |
| CC vs. WC | 3.00 | 0.003 | 0.30 | ||||
| CN vs. WN | 1.65 | 0.103 | 0.16 | ||||
| CI vs. WI | 10.77 | <.001> | 1.09 | ||||
| Note. M = mean percent accuracy. SD = standard deviation. Significance threshold = p | |||||||
| Table 3. Coordinates (in MNI space) and Maximum intensity (z-statistic) of peak voxel activation for nonparametric whole-brain contrasts. | ||||||
| mm | ||||||
| Condition/Contrast | Location | Z-Max | X | Y | Z | |
| Color Congruent | dACC/DMPFC | R | 12.3 | 4 | 16 | 46 |
| L | 12.0 | -4 | 14 | 48 | ||
| DLPFC | R | 8.39 | 22 | 2 | 52 | |
| L | 9.77 | -26 | 6 | 52 | ||
| Insula | R | 9.39 | 34 | 26 | 6 | |
| L | 9.96 | -30 | 22 | 6 | ||
| Sup. Parietal Lobule/ Supramarginal Gyrus | R | 9.73 | 34 | -46 | 42 | |
| L | 12.6 | -38 | -48 | 44 | ||
| Occipital Cortex | R | 10.5 | 32 | -92 | 2 | |
| L | 11.2 | -38 | -84 | -10 | ||
| Cerebellum | L | 5.87 | -28 | -60 | -28 | |
| Color Neutral | dACC/DMPFC | R | 12.3 | 4 | 16 | 46 |
| L | 12.3 | -2 | 14 | 50 | ||
| DLPFC | R | 9.27 | 36 | 0 | 64 | |
| L | 10.5 | -26 | 2 | 62 | ||
| Insula | R | 10.1 | 34 | 24 | 6 | |
| L | 9.91 | -30 | 18 | 8 | ||
| Sup. Parietal Lobule/ Supramarginal Gyrus | R | 9.55 | 42 | -46 | 56 | |
| L | 12.2 | -46 | -34 | 42 | ||
| Occipital Cortex | R | 11.9 | 32 | -92 | 2 | |
| L | 11.8 | -30 | -94 | -4 | ||
| Cerebellum | L | 6.35 | -28 | -62 | -28 | |
| Color Incongruent | dACC/DMPFC | R | 14.6 | 8 | 16 | 42 |
| L | 14.5 | -4 | 14 | 48 | ||
| DLPFC | R | 10.5 | 28 | -4 | 60 | |
| L | 13.4 | -48 | 10 | 32 | ||
| Insula | R | 13.7 | 34 | 26 | 0 | |
| L | 14.7 | -32 | 24 | 0 | ||
| Sup. Parietal Lobule/ | R | 11.5 | 36 | -52 | 42 | |
| Supramarginal Gyrus | L | 14.4 | -32 | -58 | 44 | |
| Occipital Cortex | R | 11.4 | 32 | -90 | -8 | |
| L | 13.0 | -36 | -84 | -10 | ||
| Cerebellum | L | 6.35 | -28 | -62 | -28 | |
| Incongr. > Congruent | dACC/DMPFC | R | 9.22 | 8 | 30 | 26 |
| L | 6.31 | -4 | 32 | 38 | ||
| DLPFC | R | 4.99 | 40 | 10 | 32 | |
| L | 7.33 | -50 | 22 | 30 | ||
| Insula | R | 3.79 | 46 | 12 | -6 | |
| L | 6.74 | -32 | 24 | -2 | ||
| Frontal Pole | R | 5 | 46 | 42 | -10 | |
| L | 6.53 | -40 | 60 | 8 | ||
| Angular Gyrus | R | 5.9 | 56 | -44 | 26 | |
| L | 7.04 | -32 | -56 | 40 | ||
| Caudate | R | 6.0 | 10 | 12 | 2 | |
| L | 5.68 | -12 | 14 | 6 | ||
| VLPFC | R | 7.42 | 42 | 22 | -4 | |
| L | 7.52 | -42 | 20 | 0 | ||
| Cerebellum | R | 5.91 | 8 | -78 | -28 | |
| Incongr. > Neutral | dACC/DMPFC | R | 9.44 | 6 | 34 | 28 |
| L | 8.75 | -2 | 20 | 50 | ||
| DLPFC | L | 8.36 | -50 | 22 | 28 | |
| Insula | R | 7.79 | 34 | 24 | -4 | |
| L | 8.31 | -30 | 24 | -2 | ||
| Frontal Pole | L | 6.23 | -44 | 52 | 0 | |
| Angular Gyrus | L | 7.37 | -34 | -54 | 38 | |
Figure 1. Significant activation (p <.05 corrected for multiple comparisons the contrasts incongruent> Congruent (in yellow) and Incongruent > Neutral (in blue), overlaid on MNI-152 template brain. The overlapping region between the two contrasts is shown in green.
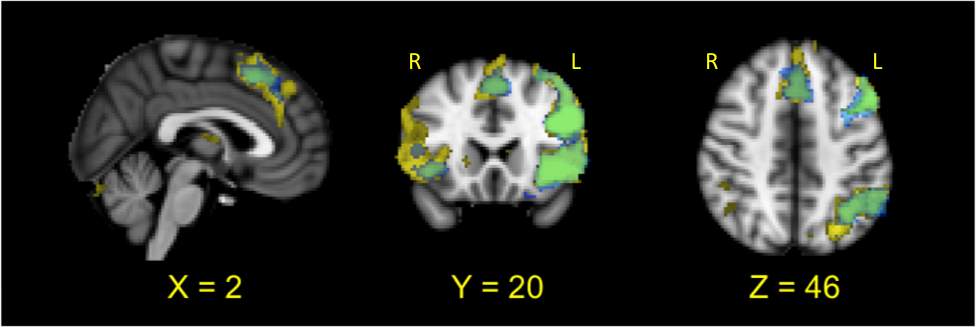
Figure 2. A. ROI masks for ACC/DMPFC (yellow), left DLPFC (red), left and right insula (blue) and left frontal pole (green). B-F. Difference in percent signal change for the CC, CN and CI conditions between safe and risky participants in ACC/DMPFC (B), left DLPFC (C), right insula (D), left insula (E), and left frontal pole (F). *p
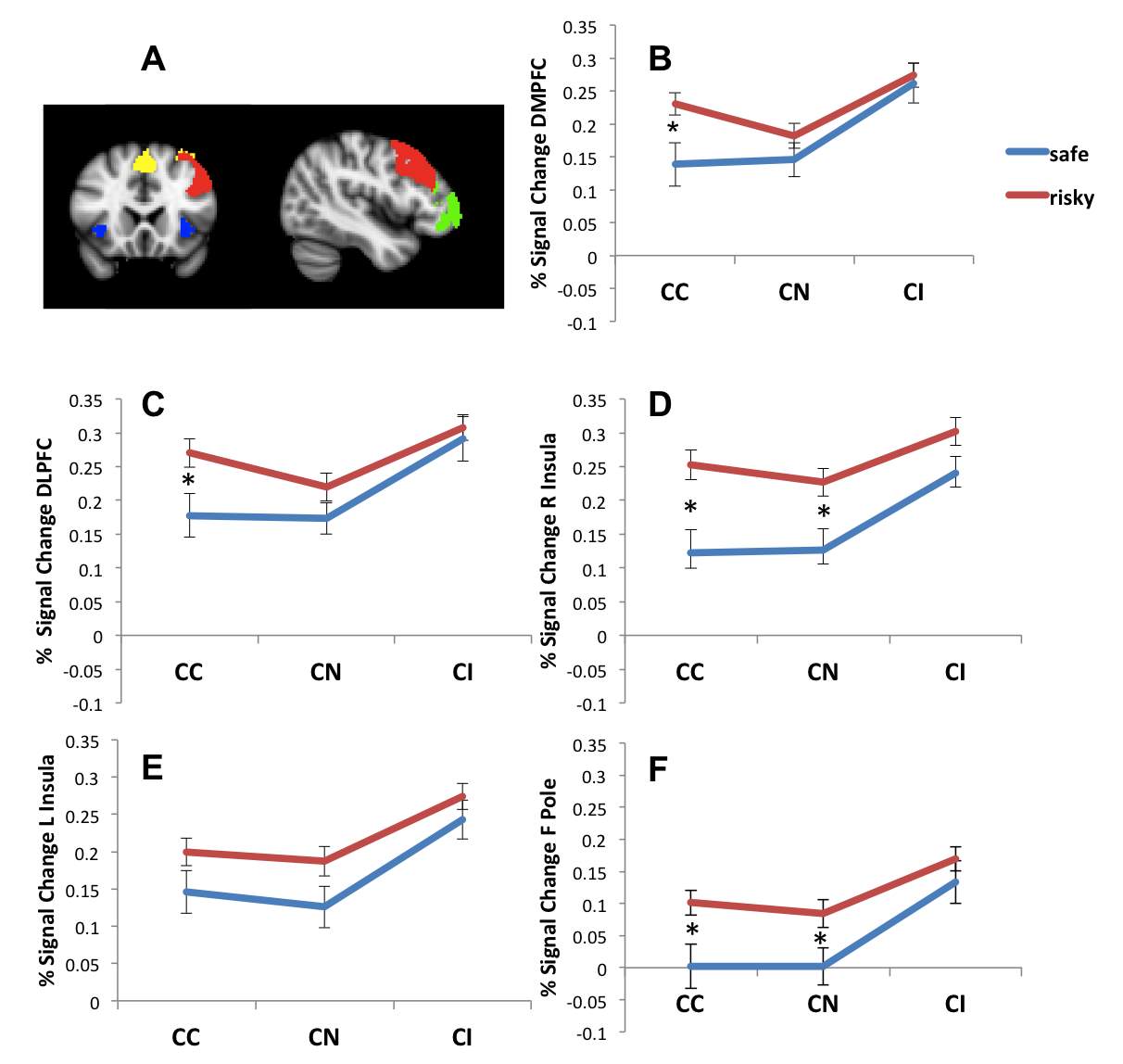
Table 4. Bootstrapped mediation effects of Negative and Positive Urgency on sexual risk-taking category. Assessed mediators are neural activity per brain location during Stroop color congruent task (controlling for activity during color incongruent task).
| Mediational Component | 95% CI
Indirect Effect |
||||
| Trait | Location | a | b | Boot
LLCI |
Boot
ULCI |
| Negative Urgency | dACC/DMPFC | .0436 | 5.648** | .0009 | .8127 |
| DLPFCa | .0703* | 4.22* | .0253 | .8915 | |
| Left Frontal Pole | .0563* | 3.6149* | .0004 | .6543 | |
| Right Insula | .0514* | 5.0525* | .0190 | .8096 | |
| Left Insula | .0031 | 3.171 | -.1278 | .2210 | |
| Positive Urgency | dACC/DMPFC | .0485* | 5.348** | .0247 | .7270 |
| DLPFCb | .0644* | 4.477* | .0406 | .8286 | |
| Left Frontal Pole | .0494 | 3.872* | .0018 | .6079 | |
| Right Insula | .0406 | 5.122* | -.0045 | .6322 | |
| Left Insula | .0104 | 2.7703 | -.0709 | .2793 | |
Note: Mediational component “a” is effect from trait to brain location, “b” is from brain location to dichotomous risk (safe (0), risky (1)). Values listed under the indirect effect are 95% confidence intervals of each indirect effect. *p<.05>p<.01>p<.001.> aAfter accounting for multivariate outliers via Mahalanobis distance, one outlier was removed. This had little influence on the mediation effect (a = .0649, p b = 3.949, p
bAfter accounting for multivariate outliers via Mahalanobis distance, one outlier was removed. This had little influence on the mediation effect (a = .0587, p p
Figure 3. Diagram of the mediational relationship among negative urgency, DMPFC brain activation and risk category (risky vs. safe) (A) and the mediational relationship among negative urgency, DLPFC brain activation and risk category (B). The same structure is also used for the remaining eight analyses shown in Table 4.

Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Neurology"
Neurology is the specialist branch of medicine that deals with the treatment of disorders of the nervous system. This means that neurologists concern themselves with issues affecting the brain, the nerves, and the spinal cord.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




