Mississipi Valley-type (MVT) vs Sedimentary Exhalative (SEDEX) Ore Deposits
Info: 8328 words (33 pages) Dissertation
Published: 8th Sep 2021
Tagged: Geology
SECTION 1: SUMMARY
MVT vs SEDEX deposits
MVT deposits stands for Mississippi Valley-type (MVT) deposits while SEDEX deposits in the other hand, stands for Sedimentary exhalative deposits. Both are globally distributed, Sediment-Hosted Lead-Zinc mineralization, and are sub-classified as clastic-dominated or carbonate dominated. They form in clusters and are the richest global sources of lead and zinc. They are formed by the action of hydrothermal fluid but have no direct link with igneous bodies or igneous activities. However, igneous activities (Magma) could be a source of heat for the hydrothermal fluids in some of the deposits. According to many authors, Genetic, spatial and temporal relationship exists between some of the two deposit types.
Although MVT and SEDEX share some similarities, they also defer in some respects; the main difference between the two ore deposits is their depositional settings; SEDEX involves open space precipitation and sedimentation on or just below the seafloor while MVT deposits form in open spaces within carbonate platform sequences. SEDEX is hosted mainly in the siliciclastic or clastic sediments and classified as clastic-dominated lead-zinc deposit while the MVT deposits is hosted by dolomite (Carbonates) and classified as carbonate-dominated lead-zinc deposits. In terms of the deposition relationship with their host rocks, most SEDEX deposits are SYNGENETIC, while the MVT deposits are EPIGENETIC. More so, considering their ages and geologic settings, MVT deposits are Phanerozoic (
Studies of the characteristics, similarities and differences between MVT and SEDEX ore deposits over time has led to the proposal of several genetic and geologic models by different authors to enhance the understanding of the origin, process of formation and localities where SEDEX and MVT ore deposits are found world-wide and such models are tools for mineral exploration geologist to design exploration models in search of SEDEX and MVT ore deposits. For instance, a basin-ward search, adjacent to an MVT ore deposit, for a SEDEX ore deposit within a passive margin is likely to have a positive outcome.
SECTION 2: DESCRIPTION OF GEOLOGICAL CHARACTERISTICS OF MVT AND SEDEX ORE DEPOSITS
| Geological Characteristics | Sedimentary Exhalative (SEDEX) Ore Deposits | Mississippi Valley Type (MVT) Ore Deposits | |
| 1. | Depositional Environment/Host rocks |
– Mostly hosted by basinal marine, fine-grained rocks composed of mainly cherts, shales, siltstones and occasionally sandstones and conglomerates. – The rocks represent a range from pelagic to hemipelagic sediments. – Basin-wide turbiditic siltstone and sandstone interbedded with ambient low energy sediments. – limestone and dolomite could be present in some(1a). |
– Mainly hosted by dolostone (1b1) – Those formed in dolostones are larger and richer than those formed in limestone1b2). – MVT deposits are primarily formed in Carbonate platforms.
– mainly hosted in dolostone and limestone(1b). |
| 2 | Ore Texture/Controls |
– Layered and banded ores. – exhibiting parallel alternation with the host rock’s layers. – Sharp contacts between sulphides and sediments. – Chaotic and varied textures with massive zones, breccias, irregular veins and disseminated sulphides(2a) |
– Precipitation of sulphides, dissolution and replacement of host rocks, filling of open spaces and dissolution of collapsed breccias. – The host rock is leached and replaced by the acidified hydrothermal fluid. – The ore deposits are usually fine-grained but there could exist coarse-grained galena. – They are finely banded, massive sulphide lenses and others(2b). |
| 3 | Tectonic/Geological Setting | – occur within extensional tectonic setting
The tectonism usually leads to fault reactivation, intrabasinal clastic sedimentation, magmatism evidenced in volcanism and or sill emplacement may be present. – Reduced marine basins (3a) |
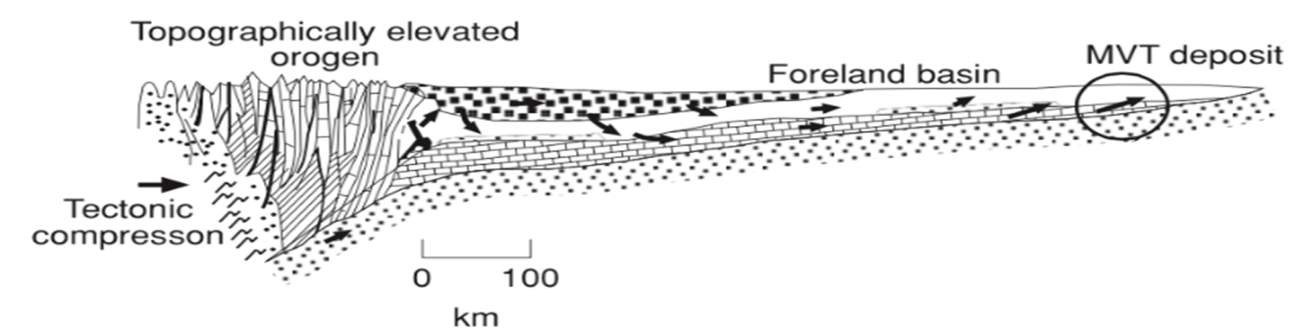
MVT are formed within the forelands as a result of crustal scale orogenic contraction, accompanied by reactivation of extensional faults, fractures, and fracture zones (which are well known to be responsible for the formation of the ores) (3b).. |
| 4 | Geographic and Age distribution |
– Found in North America, Australia and Asia. Age is between Proterozoic (1.4 to 1.8 Ga) and in Phanerozoic. – (4a). |
– MVT deposits have been found throughout the world but are most abundant in North America and in Europe. – They range in age from Devonian to Permian and Cretaceous to Tertiary(4b). |
| 5 | Deposition Description Mineralogy |
– The minerals contained in the SEDEX ore deposits include: Sulphides, Carbonates and quartz. – Pyrite is common, Pyrrhotite may be locally present in high concentration but not so common. -The major ore minerals are Sphalerite and Galena.
– Significant amount of silver may be present near the vents – Chalcopyrite is rare but could be present. – Common carbonates are; Calcite, Siderite, dolomite and ankerite (CaFe(Co3)2). – Concentration of manganese in the carbonates. – Barite may be present, Apatite may be present along with the calcite and quartz is the dominant gangue(5a) |
– The dominant minerals are Galena, Sphalerite, dolomite, marcasites and calcite, Minor to absent barite, Rare fluorite, incorporated silver in the crystal structure of galena. – Chalcopyrite is absent or have minor occurrence.
– Sulphides and sulphursalts of Cu, Ni, Co, Fe, Sb and Ag may be present in few deposits(5b). |
| 6 | Deposit’s Morphology/Geometry | – Exhibit a highly variable morphology and appear in form of mounds, lenses, tabular or shear-like bodies.
– the sulphides are bedded and there exist sulphide stringer zone – Some are relatively thick, lenses or wedge shaped, either flat or tabular or occur as stratiform sheets(6a) |
– This deposit may be discordant but are stratabound on a district scale. – They appear as irregular bodies within one or more layers of their host rocks. – The deposits could be fault controlled and vary in shapes and forms. – They may be stratiform in some deposits(6b) |
| 7 | Alteration features and associated zonation |
– Feeder pipes and associated alterations are relatively subtle because there is a low sulphide content – The siliciclastic sediments are less reactive and the low permeability hemipelagic mud hosting most of the deposits restricts seawater recharge. – hydrothermal alteration is dependent on the mineral content and physical features of the sediment, temperature and chemical composition of the fluid. – Known alteration minerals are quartz, muscovite, siderite, chlorite ankerite, tourmaline and minor sulphides(7a). |
– There is no much alteration in this deposit type because the formation hydrothermal fluid temperature is low and acts on low temperature minerals and could not cause much changes. However, there are some little signs of alterations. – Sedimentary carbonates are recrystallised, incorporation of zinc into the hydrothermal carbonates. – Carbonates zones with traces of zinc may extend into several kilometres from the ore deposit. – Elevated Pb, Zn, Cu, Fe, Co and Ni have been found in non-regular zones within some of the deposits(7b). |
| 8 | Tonnage and Grade | – Accounts for 50% of Pb and Zn and 25% of global production(8a1)
– Large number of deposits worldwide with known grade and tonnage, – Grade is mostly greater than 20Mt – Highly variable metal grades Zn more abundant than Pb (8a4). |
– Produces 25% Pb and Zn resources worldwide and 25% of either Pb or Zn
– Grades and reserves are not well known. – > 80 MVT deposits worldwide – The grade varies between 3 to 20wt% of (Pb+Zn) with average of 7- 8 wt%. – The ore deposits contain more zinc than Pb. – Grade of Zinc is between 4-6 wt% and that of Lead is between 0-2 wt%(8b) |
References cited: 8a1 Goodfellow and Lydon (2007), 1a, 2a, 3a, 4a, 5a, 6a,7a 8a4 MacIntyre, (1995), 1b,2b, 3b,4b, 5b, 6b, 7b, 8b Paradis et al. (2007), 1b1 Leach and Sangster (1993), 1b2https://pubs.er.usgs.gov/publication/sir20105070A, 1a, 2a, 3a, 4a, 5a, 6a,7a 8a4 http://dx.doi.org/10.3133/sir20105070N
SECTION 3: A GENETIC (CONCEPTUAL) MODEL FOR MVT AND SEDEX ORE DEPOSIT
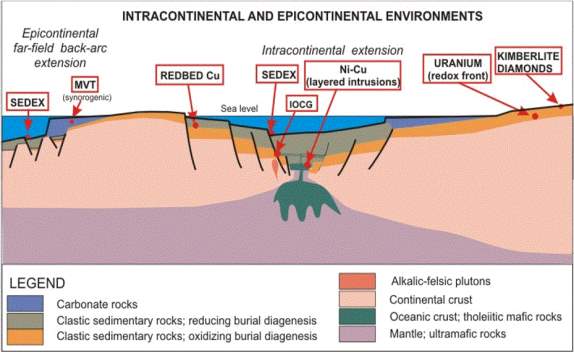
MVT and SEDEX ore deposits have almost the same process of formation and are both globally distributed (figure 1A & 1b) but the major difference between the two deposits is that they are formed in different geologic environments, which are distinguished by the orogenic setting in which they are formed (figures 2A & 2B). SEDEX ore minerals are formed within Continental rifting – intracratonic -back arc basins, while the MVT are mainly formed within a carbonate platform in foreland sedimentary basins by inflow of fluid due to topographic uplift during an orogenic process (Rob, 2005), see figure 2B.
For any of these ore deposit types to form, there will be a hydrothermal fluid of high salinity to leach out the metals, the fluid must be driven from its source to the depositional sites and conditions must be favourable at the deposition site for the minerals to be formed. Although different models have been put forward by different authors, they all include the description of fluid sources, Sources of ligands, what drives the fluid movement, the flow pathways of the fluid, Physical and chemical conditions that aid precipitation and formation of the ores (Emsbo, 2009).
SEDEX ore deposits (Carne and Cathro 1982), are finely laminated or bedded sulphide ores representing sea floor-exhaled chemical sediment precipitation from hydrothermal fluids. They are formed by 100o to 200 o C brines that are neutral or slightly acidic in pH with salinity (Ansdell et al., 1989; Bresser, 1992; Leach et al., 2004), while MVT ore deposits are formed from saline brines (similar to oil field brines) with 90°C – 200°C (Thom and Anderson, 2008), the brines of low temperatures from about 50oC – 150oC with few up to 300oC, but sometimes 90oC – 150oC (Leach et al., 2010), sourced from adjacent sedimentary basin containing zinc and lead. The hydrothermal fluid is heated by either rise in temperature due to increase in geothermal gradient or igneous activities in rifted volcano-sedimentary Basins, but not in direct contact with the fluid (Goodfellow and Lydon, 2007), see figure 3.
The salinities of hydrothermal fluids are contributed from different sources:
(1) evaporite dissolution by meteoric and marine waters(Goodfellow et al., 1993; Lydon, 1995),
(2) Sediment-trapped connate fluid(Garven et al., 2001; Large et al., 2005; Yang et al., 2006) and
(3) the down flow of residual brines from seawater (Davidson, 1998; Leach et al., 2004; Lydon, 2004)
The seawater evaporation source is widely considered, evidence abounds, as low paleolatitudes that favour evaporative environments are known in basins hosting the ores (Lydon, 2004; Goodfellow and Lydon, 2007, Leach et al., 2005a). Presence of ligands assists hydrothermal fluids in transporting or depositing metals, Pb and Zn have high solubilities in hydrothermal fluid above 100oC when salinity is high and H2S content is low (Kharaka et al., 1987; Moldovanyi and Walter, 1992; Hanor, 1996).
At depth or at the deposition sites, hydrogen sulphide is generated in sedimentary basins or within a carbonate platform by thermochemical sulphate reduction (TSR) and/or by thermal decomposition of organic materials (Hunt, 1996) and when metallic-rich hydrothermal fluid flowing within sedimentary basin mixes with H2S, it reduces the amount of metals carried in the fluid. However, if there is sufficient Fe in the sedimentary material, it will react with the H2S to form pyrite and hence allowing more of the metallic lead, zinc and Silver to remain concentrated in the fluid (Emsbo et al., 2009). The metals in both SEDEX and MVT ore deposits have their sources as oxidized clastic sedimentary rocks or basement rocks, because rocks with less organic carbon are likely to contain more metals as exemplified in red beds. Sulphur in form of sulphate, from marine sulphate or evaporites are carried in solution either together with metals or in a different solution, and when mixed together under favourable conditions, the ores are precipitated after the sulphate is reduced to sulphide by bacterial or thermogenic reduction.
Furthermore, thermal pressure and geodynamic mechanisms are responsible drivers for the hydrothermal fluid flow and there are several models used to explain the processes. The models include:
(1) Sedimentary Compaction Model, which states that, sedimentation and compaction are responsible for driving and discharging the fluids to the sea (Sawkins, 1984; Lydon, 1986).
(2) Free Convection Models, which has it that, brines formed by halite dissolution or evaporation from sea water infiltrates into the underlain sediments and heated up as they descend deeper into the sedimentary basin. Subsequent sealing of the sediments by impermeable clastic sediments during the later stage of a basin development helps to retain the fluid for a long time, causing it to develop convection cells and building up pressure. The reservoir seal eventually ruptures when the hydrostatic pressure is exceeded, creating faults that triggers a recharge/discharge pathway, making way for the hot, metal-rich brines to flow upward and cold seawater to flow down along another fault to maintain a convention circuit (Russell et al., 1981; Garven et al., 2001; Large et al., 2005, Morrow, 1998) See figure 3.
(3) Topographically driven flow model, which proposed for flow of the fluid from high elevation to low elevation created by regional-scale orogeny (Grven, 1985, Bethke and Marsha, 1990, Garven and Raffensperger, 1997) and
(4) Density-Driven fluid flow as stated in USGS publication (Emsbo, 2009), it states that the deposits are formed from dense brine fluids sourced from evaporative seawater. In this model, dense residual brines produced by seawater evaporation will in terms of SEDEX ore deposits, permeate into a permeable basinal roccks and sink down deep into the sedimentary reservoir, leaching metals as it flows (Emsbo, 2009). It flows laterally through rock sequences at a depth toward the middle of the basin until exhaled unto the sea floor through fault pathways in the farther part of the basin, figure 3.
The hydrothermal brine fluid behaves in three different ways when discharged into the sea water (Sato, 1972), depending on its density, which in turn is controlled by salinity and temperature. It could either remains as a buoyant plume, goes through a density maximum during cooling by ambient waters or undergoes bottom hugging. Based on these behaviour, two types of SEDEX deposits occur, depending on where it is formed with respect to the feeding vent. If the hydrothermal fluid is buoyant, the deposit will form closer to the vent and is known as vent-proximal deposits and if denser than sea water it will accumulate and pool in depressions further away from the vents and the deposit formed is known as vent-distal deposits (Goodfellow and Lydon, 2007), see figure 4.
Moreover, sometimes, the fluids migrate upwards along faults to carbonate platform within a basin margins (Jones et al., 2002), forming the common small MVT-style deposits or dolomitizes the carbonate rocks. In the other hand, MVT deposits form from diagenetic interactions between a host carbonates and both metal-bearing and sulphate-bearing basinal brines forced into the region by orogenic squizzing and uplift (Rob, 2005).
See the chemical equations for the ore formation process: ZnCl + SO42– = ZnS (Sphalerite) + Cl– + 2O2 and PbCl+ + SO42– = PbS (Galena) + Cl– + 2O2
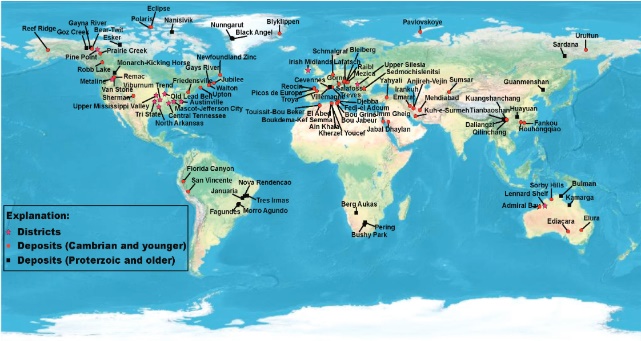
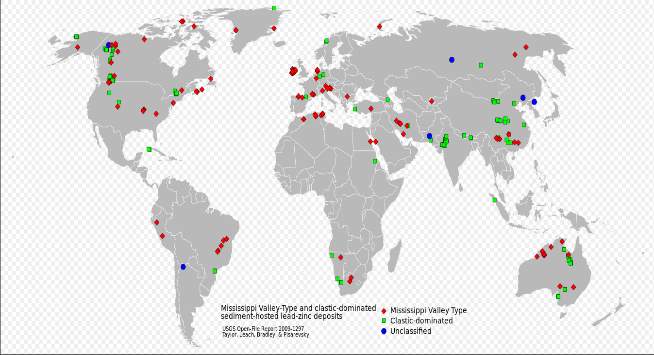
Figure 1: The global locations of SEDEX and MVT ore deposits (From USGS open file report, 2009 and Google)
2A
2A
Figure 2:(A) tectonic/depositional environment of different mineral types with Sedex and MVT deposit types included (modified from Soltan, 2017)(B) MVT Zn-Pb depositional environment (Robb, 2005).
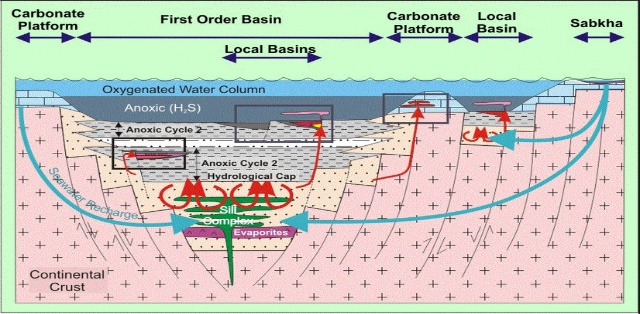
Figure 3: The free convection model of SEDEX and MVT deposits, with igneous intrusion as a source of heat (From Goodfellow and Lydon 2007)
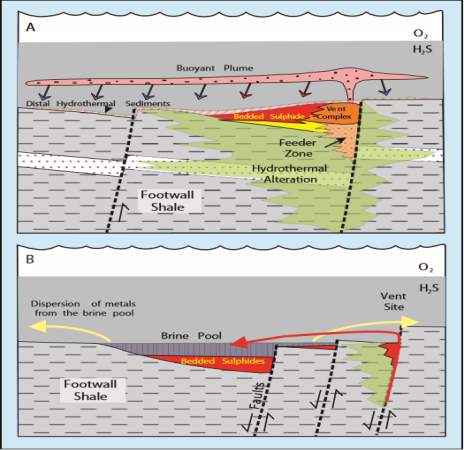
Figure 4: illustration of SEDEX deposit forming(A) closer to the vent and (B) farther from the vent (From Goodfellow and Lydon, 2007)
SECTION 4: EXPLORATION MODELS:
To explore for SEDEX and MVT ore deposits, exploration geologists will always look out for the important geologic characteristics and their possible formation models to make a quick and better decision on the prospectivity (Exploration potential) of an area, and his or her decisions are based on the exploration models:
SEDEX EXPLORATION MODEL
(1) Knowledge from already discovered SEDEX deposits show that they occur in clusters and in multiple horizons, hence, presence of one ore deposit is a strong indication that others would exist.
(2) Existence of intracratonic or epicratonic sedimentary basins is a good site to search for SEDEX deposit since all the known SEDEX deposit are found in them.
(3) A thick sedimentary basin is a good place to search for the ore since it has been established that fluid temperature of more than 100oC required to form SEDEX can only exist within a thick basin.
(a) Basins within sediment-filled continental rifts with extensional faults, varied thickness, unconformities, and chemically reduced seafloor environment indicating anoxic cycles in the basin’s development
(b) The evaporative environment indicator of paleoclimate under which the basin is developed and deposition of barite, apatite, pyrite, and Mn-Fe-Ca-Mg carbonates farther away from the basin.
(c) Presence of hydrothermal alteration such as quartz, chlorite, ankerite, siderite, and muscovite alteration which could be in association with vein and disseminated sulphides and
(d) Searching for geochemical anomalies within metal-rich shales, sediment and water samples for deposit-forming and their pathfinders.
MVT EXLORATION MODEL
1. Location of MVT deposits within shelf margins carbonate platforms.
2. Search within carbonate rocks with ages ranging from middle Proterozoic to carboniferous (Mostly from mid to late Proterozoic) since most of the known MVT deposits were formed within those periods.
3. Presence of faults through which fluids can migrate upward such as regional fault system that serves as controls on the formation of MVT ore deposits.
3.Paleoclimatic information about the conditions favourable for the formation and preservation of carbonate rocks and evaporites can give us clue about the presence of MVT ore deposits, since MVT deposits are mostly found in such environments.
4. Presence of disseminated sulphides within the carbonate rock, SEDEX deposits close to the carbonate platform, hydrothermal sediments away from the carbonate rocks that are rich in Mn-Fe-Ca-Mg carbonates and MVT ore deposit-forming elements in stream sediments and water.
5. Galena, pyrite, and marcasites are conductive minerals and can be detected by induced Polarization geophysical method.
Chargeability of carbonates rocks is low, hence, Ground electromagnetic (EM) methods may work for deposits with sufficient concentrations of iron sulphide minerals. Seismic, electromagnetic, and gravity methods of geophysics can be applied to delineating some of the geological features that indicate presence of SEDEX and MVT deposits.
SECTION 5: DISCUSSION ON REAL WORLD EXAMPLES OF SEDEX AND MVT
There are so many SEDEX and MVT ore deposits known globally but in this discussion, I will be taking the Howard Pass District deposit from the Selwyn Basin in the Yukon district of Canada as a real-life example of SEDEX ore deposit and the Polaris Pb-Zn District deposit as an example of MVT ore deposits and describe their genetic models as compared to the generalized model of the two deposit types. Starting with the Howard Pass District deposit first and secondly with the Polaris District.
5.1: HOWARD PASS DISTRICT ORE DEPOSIT
This district contains 14 Zn-Pb sedimentary exhalative (SEDEX) deposits and is located within Selwyn basin, Yukon, Canada (Gadd et al., 2016). It is one of the sediment-hosted lead-zinc mineralization in the Selwyn Basin (Goodfellow, 2007), a fault-controlled epicratonic marine Basin (aulacogen), formed by rifting [pulling apart of two continental blocks along Tintina fault (an intra-continental transform fault)], followed by subsidence and deposition of the lithologic units in which the sulphide minerals are formed. The basin contains one of the world-class sediment-hosted Zn-Pb mineralization and is located between Mackenzie, Casiar and MacDonald carbonate platforms and contains approximately 393 Mt grading 4.5% Zn and 1.5% Pb (Selwyn Chihong Mining Limited, 2012).
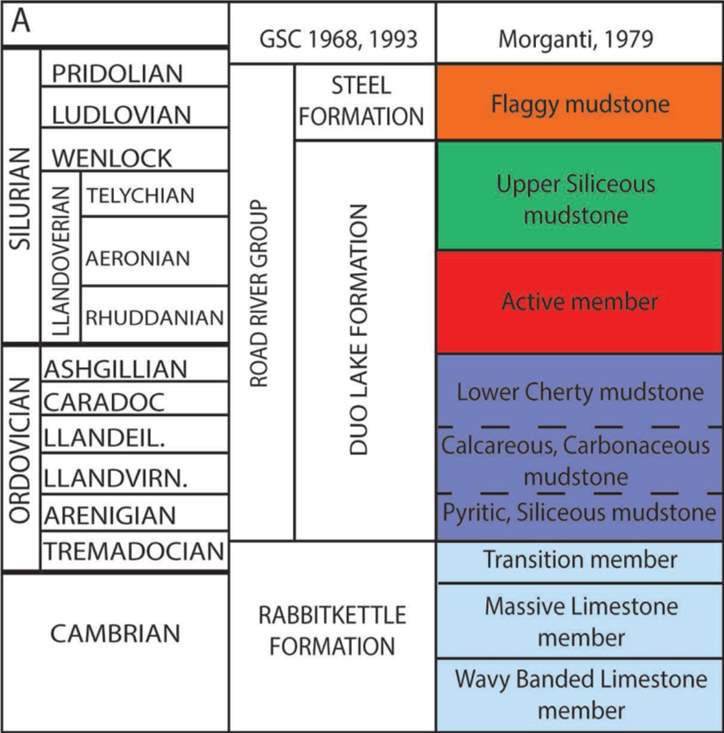
The host rock formation is Duo Lake Formation with Calcareous, siliceous and Carbonaceous mudstone. The Pb-Zn mineralization occurs within the Early Silurian active member (ACTM) figure 5 and is characterized by a sequence of carbonaceous pelagic and hemipelagic mudstone, shale and chert (Gad et al., 2016) as shown in figure 5. Where the mineralization is not deformed, it is bedded or laminated and currently, no evidence of vent complex and/or strong hydrothermal alteration exist in the district, (Goodfellow and Jonasson, 1986).
The District Zn-Pb deposits are mostly diagenetic in origin, the distribution of trace elements within the deposits was used for the proposition of a refined genetic model (Gad et al., 2016). From their Secondary Ion Mass Spectrometer analysis, the authors found a biogenically reduced sulphur with negative values of δ34S (in framboidal Pyrite) and thermogenically reduced sulphur with positive values of δ34S in sphalerite and galena (Gadd et al., 2016; Gadd et al., 2017). This model has it that dense brine was accumulated in a depression filled with hemipelagic sediments that are saturated with water. The marine environment was anoxic/or euxinic with a dynamic seafloor in terms of oxidation and reduction reactions.
A progressive increase in the porosity of silt-to-clay-sized mud from 50% at a depth of 100 metres below the seafloor and upward (Einsele, 2000), could be the case of the sediments in ACTM before the metalliferous fluid was introduced into the sediment. The high porosity and permeability of the sediments allowed some of the dense brine originally settled at the seafloor to infiltrated into the sediment, displacing the ambient sea water initially present in the pore spaces of the carbonaceous mud (Sangster and Hillary 2000). The brine carried both sulphate and metals in it; Some of the transported metals in the brine were not precipitated within the water column because the sulphate in it needed to be reduced to sulphide (Goodfellow, 1987). However, precipitation occurred after thermochemical sulphate reduction (TSR) by the interaction of the brine with the H2S produced originally by Biogenic sulphate reduction(BSR) in the sulphurdic porewater. The BSR was in operation before the introduction of the hot metalliferous brine, but after the fluid was introduced into the sediments, the thermochemical Sulphate Reduction replaced the Biogenic Sulphate Reduction at a temperature of (>100 °C) (Gadd et al., 2017). The calcite in the mud was produced when either TSR or [ methanogenesis [2CH2O + Microbes = Methane(CH4) + CO2,] oxidized the organic carbon, which resulted in precipitation of CO2 in the mud during sedimentation. This process caused the deposition of sulphide ores in the carbonaceous mud. Consequent to the deposition of the sulphides was the cementation of the sediments because of CO2 build-up that led to the high reduction in porosity and permeability and over-pressurized the pore fluid and lastly resulted in the upward migration of metal-rich hydrothermal fluid. The upward migration of the fluid created features known as dewatering pipes( Jonasson and Goodfellow 1986), that are present in the formation as discordant ZnS- and PbS-rich longitudinal structures known as stringers, cutting across laminated sediments. This is an example of SEDEX ore deposit formation from Sedimentary-Compaction and fluid mixing Model as shown in figure 6
11
figure 5: Stratigraphic section of the Howard’s Pass District: showing ACTM as the host for the deposit (modified form Gordey and Anderson 1993
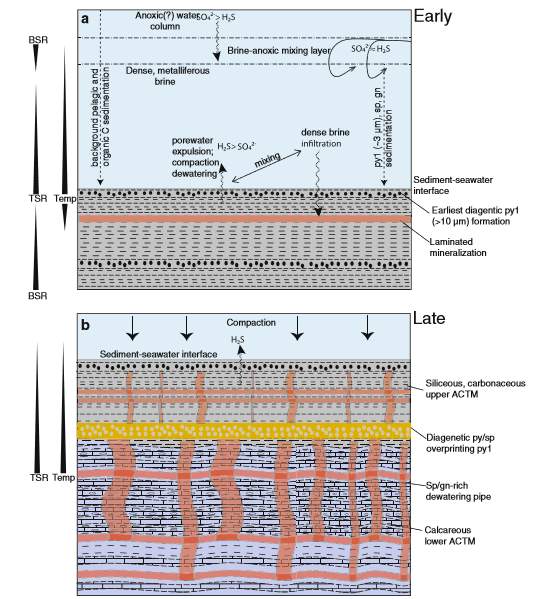
figure 6:A Illustration of how SEDEX deposits in the Howard Pass Deposit was formed(adapted and modified from Ireland et al. 2004; Gadd et al. 2016)
5.2: THE POLARIS MVT ORE DEPOSIT
The Polaris Pb-Zn District ore deposit is located on Little Cornwallis Island in the Canadian central Arctic Island, Nunavut and covers about 450 Km north-south by 130 Km east-west, containing a diluted in-place ore reserve of 26 million tonnes, having 3.7% Pb and 13.9% Zn prior to mining and total production at the end of mine was 20. 1 million tonnes, 3.6% Pb and 13.4% Zn (Dewing, 2007). The age of the deposit ranges between Late Devonian to Early Carboniferous (Christensen et al., 1995).
Dominant Early to Middle Devonian activities in the Arctic Island led to the deposition of shallow marine and non-marine syntectonc clastic rocks in the Foreland Basin, which developed during the Early carboniferous, forming the Sverdrup Basin that contains the Thumb Mountain Formation which the MVT ore is deposited. Ellesmerian contractional event caused local extensional faulting that permitted ore fluids to begging migrating upward, interacting with the host rock, causing dissolution, brecciation, collapsing and thinning of the Carbonate beds due to the excessive leaching of the carbonate material (Reid et al., 20013).
The ore deposit is hosted in the Thumb Mountain Formation (figure 7), comprising of 350 m thick carbonate rock, underlain by Bay Fiord Formation and overlain by Irene Bay Formation and Cape Phillips Formation (Dewing and Turner, 2003). The Polaris MVT ore deposit got its metallic ions from within its stratigraphic column and has no indication of basement or basal clastics involvement as indicated by strontium and lead isotopes, but were leached out deeper in the stratigraphic column, transported in sulphate-rich brine; the sulphate, probably was derived from the Bay Ford anhydrite and the flow was driven by either the Ellesmerian Orogeny or by the Orogenic process of the Mid-Carboniferous opening of Sverdrup Basin(Dewing et al., 2007). The hot basinal brine kept rising along faults until it reached the Thumb Mountain Formation, which contains limestone that is organic-rich, permeable and overlain by an aquitard (Irene Bay Formation shale) (figure 8).
The fluid temperature ranges between 80 to 105°C, which allowed for bacterial sulphate reduction. (Machel et al., 1995).
The H2S that helped in the precipitation of the metallic sulphides was produced by a process of Bacteria Sulphate Reduction (BSR), where sulphate was reduced to H2S using the high amount of algal material present in the upper Thumb Mountain Formation. The H2S, then reacted with zinc, lead, and iron ions to produce ore minerals (Sphalerite -ZnS2, Galena-PbS2 and Pyrrite-FeS2). The hydrogen ion released in this process made the fluid acidic and dissolved the carbonate resulting in the production of dissolution breccias and other fabrics. A hallow of barite was produced around the main ore by the remnant sulphate expelled from the system, figure 8.
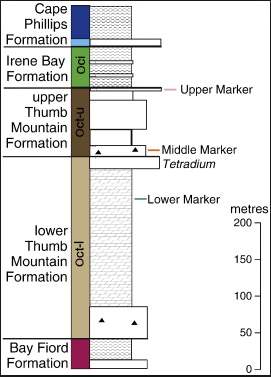
FIGURE 7: Stratigraphic column showing the Polaris Ore hosted in the Thumb Mountain Formation (Oct-1) (from Dewing et al., 2013 after Sharp et al., 1995a)
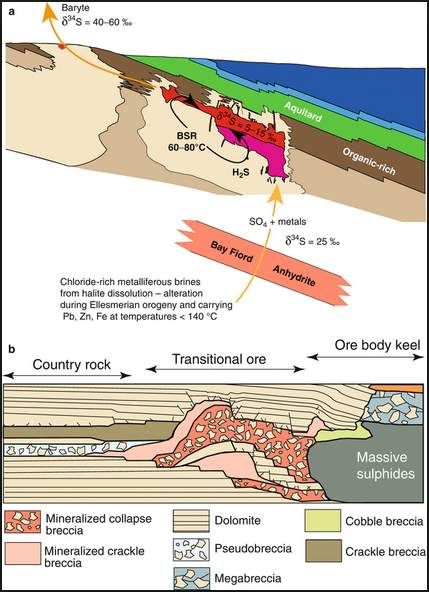
FIGURE 8: Fluid flow path for the formation of Polaris MVT ( obtained from Warren, 2016)
SECTION 6: CONCLUSION
It could be concluded from the information gathered while reviewing literatures on this two deposit types that; MVT and SEDEX ore deposit are globally distributed, the richest sources of Pb and Zn and are formed through hydrothermal processes that have no direct link with magmatic fluids.
The ore fluids are hot/warm saline basinal fluid either from evaporative sea water, connate water or dissolution of evaporites within a sedimentary basin. The basinal brine hydrothermal fluids known to be involved in the formation of the two deposit types leach out, transport and deposit metals depending on the temperature, pH, salinity, and the chemical conditions (Redox reaction) of the fluid. Pb and Zn are leached from the sedimentary basin by the fluid depending on the amount of the ligands (H2S and SO2) present. Based on literatures, H2S-deficient fluid will have high concentration of Pb and Zn and, the more the sulphate contained in a fluid, the less the amount of barium that it will dissolve and transport. H2S is generated at depths within basins by a process known as thermochemical sulphate reduction (TSR), in which organic materials are thermally decomposed or by the actions of micro-organisms (bacteria) through a process known as Biogenic sulphate reduction (BSR).
The SEDEX and MVT ore fluid flow within a basin are generally through thermal, pressure and geodynamic mechanisms. Three main models for the fluid flow, includes; Sedimentary Compaction Model, Convection Cell Model and Topographic Driven Model. An additional model, “Density Driven Model” has also been proposed by USGS to take care of the shortcomings in the other three models.
Although the formation of both MVT and SEDEX ore deposits could be explained using the same kinds of models, there are some remarkable features that distinguish each from the other. The SEDEX deposits are clastic-dominated, stratiform, syngenetic/diagenetic since they are laminated and have tabular morphology and are Proterozoic to Phanerozoic in age while MVT ore deposits are Carbonate-dominated, epigenetic in nature and show replacement textures and are Proterozoic in age. They form within carbonate platforms, are stratabound, formed from fluid similar to oil field brine and are mostly located within the foreland arc Basin. The discovery of one of these deposits can lead to the discovery of the other in some locations.
REFERENCES
Anderson GM, Thom J (2008) The role of thermochemical sulfate reduction in the origin of Mississippi Valley-type deposits. I. Experimental results. Geofluids (in press) (2008), 16–26.
Ansdell, K.M., Nesbitt, B.E., and Longstaffe, F.J., 1989, A fluid inclusion and stable isotopic study of the Tom Ba-Pb-Zn deposit, Yukon Territory, Canada: Economic Geology, v. 84, p. 841–856.
Berner RA, Raiswell R (1983) Burial of organic carbon and pyrite sulphur in sediments over Phanerozoic time: a new theory. Geochim Cosmochim Acta 47:855–862
Bethke, C.M., and Marshak, S., 1990, Brine migration across North America – the plate tectonics of groundwater: Earth and Planetary Science Letters, Annual Review, v. 18, p. 287-315.
Bresser, H.A., 1992, Origin of base metal vein mineralization in the Lawn Hill mineral field, north western Queensland: Townsville, James Cook University, B.Sc. thesis, 115 p.
Carne, R.C., and Cathro, R.J., 1982, Sedimentary exhalative (sedex) zinc-lead-silver deposits, northern Canadian Cordillera: CIM Bulletin (1974), v. 75, no. 840, p. 66–78.
Christensen, J.N., Halliday, A.N., Leigh, K.E., Randell, R.N., and Kesler, S.E., 1995, Direct dating of sulphides by Rb-Sr: A critical test using the Polaris Mississippi-Valley-type Zn-Pb deposit: Geochimica et Cosmochimica Acta, v. 59, p. 5191-5197.
Cooke, D.R., Bull, S.W., Large, R.R., McGoldrick, P.J., Lydon, J.W., and Anonymous, 2000, The importance of oxidized brines for the formation of Australian Proterozoic stratiform sediment-hosted Pb-Zn (sedex) deposits: Economic Geology, v. 95, no. 1, p. 1–18.
Davidson, G.J., 1998, Alkali alteration styles and mechanisms, and their implications for a “brine factory” source of base metals in the rift-related McArthur Group, Australia: Australian Journal of Earth Sciences, v. 45, no. 1, p. 33–49.
Dewing, k., and turner, e. 2003. Structural setting of the Cornwallis lead-zinc district, Arctic Islands, Nunavut. Geological Survey of Canada Current Research B-4:1 – 9.
Dewing, K., and Turner, E., 2003, Structural Setting of the Cornwallis Lead-Zinc District, Arctic Islands, Nunavut: Geological Survey of Canada, Current Research. B-4, 9 p.
Dewing, K., Sharp, R.J., Turner, E., 2007, Synopsis of the Polaris Zn-Pb District, Canadian Arctice Islands, Nunavut, in in Goodfellow, W.D., ed., Mineral Goodfellow, W.D., ed., Mineral Deposits of Canada: A Synthesis of Major Deposits of Canada: A Synthesis of Major Deposit-Ty Deposit-Types, District pes, District Metallogeny, the Evolution of Geological Provinces, Metallogeny, the Evolution of Geological Provinces, and Exploration Methods: Geological and Exploration Methods: Geological Association of Canada, Mineral Deposits Division, Special Publication No. 5, p. 655-672.
Dewing, K., Turner, E., and Harrison, J.C., 2007, Geological history, mineral occurrences and mineral potential of the sedimentary rocks of the Canadian Arctic Archipelago, in Goodfellow, W.D., ed., Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods: Geological Association of Canada, Mineral Deposits Division, Special Publication 5, p. 733-753
Einsele G (2000) Sedimentary basins: evolution, facies, and sediment budget, 2nd edn. Springer, Berlin, p 792
Emsbo, P. 2009, Geologic criteria for the assessment of sedimentary exhalative (sedex) Zn-Pb-Ag deposits: U.S. Geological Survey Open-File Report 2009−1209, 21 p.
Emsbo, Poul, 2000, Gold in sedex deposits: Reviews in Economic Geology, v. 13, p. 427–437.
Emsbo, Poul, Seal, R.R., Breit, G.N., Diehl, S.F., and Shah, A.K., 2016, Sedimentary exhalative (sedex) zinc-lead-silver deposit model: U.S. Geological Survey Scientific Investigations Report 2010–5070–N, 57 p., http://dx.doi.org/10.3133/sir20105070N.
Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR, Cullen D, Dauphin P, Hammond D, Hartman B, Maynard V (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta 43:1075–109
Gadd MG, Layton-Matthews D, Peter JM, Paradis S (2016) The worldclass Howard’s Pass SEDEX Zn-Pb district, Selwyn Basin, Yukon. Part I: trace element compositions of pyrite record input of hydrothermal, diagenetic and metamorphic fluids to mineralization. Miner Deposita 51:319–342
Gadd MG, Layton-Matthews D, Peter JM, Paradis S, Jonasson IR (2017) The world-class Howard’s Pass SEDEX Zn-Pb district, Selwyn Basin, Yukon. Part II: the roles of thermochemical and bacterial sulfate reduction in metal fixation. Open Acess, Springer.com.
Gardner, H.D., and Hutcheon, I., 1985, Geochemistry, mineralogy, and geology of the Jason Pb-Zn deposits, Macmillan Pass, Yukon, Canada: Economic Geology, v. 80, p. 1257–1276.
Garven, G., 1985, The role of regional fluid flow in the genesis of the Pine Point deposit, Western Canada Sedimentary Basin: Economic Geology, v. 80, p. 307-324.
Garven, G., and Freeze, R.A., 1984, Theoretical analysis of the role of groundwater flow in the genesis of stratabound ore deposits. 2. Quantitative results: American Journal of Science, v. 284, p. 1125-1174
Garven, G., and Raffensperger, J.P., 1997, Hydrogeology and geochemistry of ore genesis in sedimentary basins, in Barnes, H. L., ed., Geochemistry of hydrothermal ore deposits: Wiley, New York, p. 125-189.
Garven, G., Bull, S.W., and Large, R.R., 2001, Hydrothermal fluid flow models of stratiform ore genesis in the McArthur Basin, Northern Territory, Australia: Geofluids, v. 1, no. 4, p. 289–311.
Garven, G., Bull, S.W., and Large, R.R., 2001, Hydrothermal fluid flow models of stratiform ore genesis in the McArthur Basin, Northern Territory, Australia: Geofluids, v. 1, no. 4, p. 289–311
Garven, G., Raffensperger, J.P., Dumoulin, J.A., Bradley, D.C., Young, L.E., Kelley, K.D., and Leach, D.L., 2003, Coupled heat and fluid flow modeling of the Carboniferous Kuna Basin, Alaska— Implications for the genesis of the Red Dog Pb-Zn-Ag-Ba ore district: Journal of Geochemical Exploration, v. 78–79, no. 2003, p. 215–219.
Goodfellow WD, Jonasson I (1986) Environment of formation of the Howards Pass (XY) Zn-Pb deposit, Selwyn Basin, Yukon. In: Morin JA (ed) Mineral deposits of Northern Cordillera: Canadian Institute of Mining and Metallurgy: Special Volume 37:19–50
Goodfellow, W.D., and Lydon, J.W., 2007, Sedimentary exhalative (SEDEX) deposits, in Goodfellow, W.D., ed., Mineral deposits of Canada—A synthesis of major deposit-types, district metallogeny, the evolution of geological provinces, and exploration methods: Geological Association of Canada, p. 163–184.
Goodfellow, W.D., Lydon, J.W., and Turner, R.J.W., 1993, Geology and genesis of stratiform sediment hosted (SEDEX) zinc-lead-silver sulphide deposits, in Kirkham, R.V., Sinclair, W.D., Thorpe, R.I., and Duke, J.M., eds., Mineral deposit modeling, p. 201–251.
Goodfellow, W.D., Lydon, J.W., and Turner, R.J.W., 1993, Geology and genesis of stratiform sediment hosted (SEDEX) zinc-lead-silver sulphide deposits, in Kirkham, R.V., Sinclair, W.D., Thorpe, R.I., and Duke, J.M., eds., Mineral deposit modeling, p. 201–251.
GoodfellowWD(1987)Anoxicstratifiedoceansasasourceofsulphurin sediment-hosted stratiform Zn-Pb deposits (Selwyn Basin, Yukon, Canada). Chem Geol: Isot Geosci Sect 65:359–382
Hanor, J.S., 1994, Origin of saline fluids in sedimentary basins, in Parnell, J., ed., Geofluids—Origin, migration and evolution of fluids in sedimentary basins: Geological Society [London] Special Publications, p. 151–174
Hanor, J.S., 1996, Controls on the solubilization of lead and zinc in basin brines, in Sangster, D.F., ed., Carbonate-hosted lead-zinc deposits: Society of Economic Geologists Special Publication 4, p. 483–500.
Hunt, J.M., 1996, Petroleum geochemistry and geology: New York, Freeman, 743 p.
Jones, G.D., Whitaker, F.F., Smart, P.L., and Sanford, W.E., 2002, Fate of reflux brines in carbonate platforms: Geology, v. 30, p. 371–374.
Kharaka, Y.K., Maest, A.S., Carothers, W.W., Law, L.M., Lamothe, P.J., and Fries, T.L., 1987, Geochemistry of metal-rich brines from central Mississippi Salt Dome Basin, U.S.A: Applied Geochemistry, v. 4, p. 543–561
Kreuzer, O.P., Etheridge, M.A., Guj, P., McMahon, M.E., and Holden, D.J., 2008, Linking mineral deposit models to quantitative risk analysis and decision-making in exploration: Economic Geology, v. 103, no. 4, p. 829–850.
Large, R.R., Bull, S.W., McGoldrick, P.J., Walters, S., Derrick, G.M., and Carr, G.R., 2005, Stratiform and stratabound Zn-Pb-Ag deposits in Proterozoic sedimentary basins, northern Australia: Society of Economic Geologists, 931–963 p.
Large, R.R., Bull, S.W., McGoldrick, P.J., Walters, S., Derrick, G.M., and Carr, G.R., 2005, Stratiform and stratabound Zn-Pb-Ag deposits in Proterozoic sedimentary basins, northern Australia: Society of Economic Geologists, 931–963 p.
Lawrence, R. (2005). Introduction to Mineral Processes: The Movement of hydrothermal fluid in the earth crust (pp. 138 – 139). Victoria, Australia: Blackwell
Leach, D., Marsh, E., Bradley, D., Gardoll, S., and Huston, D., 2005a, The distribution of sedex Pb-Zn deposits through Earth history: Proceedings of the Biennial SGA Meeting, v. 8, Vol. 1, p. 145–148.
Leach, D.L., Bradley, D.C., Huston, D., Pisarevsky, S.A., Taylor, R.D., and Gardoll, S.J., 2010, Sediment-hosted leadzinc deposits in Earth history: Economic Geology, in press.
Leach, D.L., Marsh, E., Emsbo, P., Rombach, C.S., Kelley, K.D., and Anthony, M., 2004, Nature of Hydrothermal Fluids at the Shale-Hosted Red Dog Zn-Pb-Ag Deposits, Brooks Range, Alaska: Economic Geology, v. 99, no. 7, p. 1449–1480.
Lydon, J.W., 1983, Chemical parameters controlling the origin and deposition of sediment-hosted stratiform lead-zinc deposits, Short Course Handbook v. 8: Mineralogical Association of Canada, p. 175–250.
Lydon, J.W., 1986, Models for the generation of metalliferous hydrothermal systems within sedimentary rocks, and their applicability to the Irish Carboniferous Zn-Pb deposits, in Andrew, C.J., Crowe, R.W.A., Finlay, S., Pennell, W.M., and Pyne, J.F., eds., Geology and genesis of mineral deposits in Ireland: Dublin, Irish Association for Economic Geology, p. 555–577.
Lydon, J.W., 1995, Sedimentary exhalative sulphides (SEDEX), in Eckstrand, O.R., Sinclair, W.D., and Thorpe, R.I., eds., Geology of Canadian mineral deposit types, p. 130–152.
Lydon, J.W., 2004, Geology of the Belt-Purcell Basin and Sullivan Deposit, in Deb, M., and Goodfellow, W.D., eds., Sediment-hosted lead-zinc sulphide deposits; attributes and models of some major deposits in India, Australia and Canada: New Delhi, Narosa Publishing House, p. 100–148.
Machel, H.G., Krouse, H.R., and Sassen R., 1995, Products and distinguishing criteria of bacterial and thermochemical sulfate reduction: Applied Geochemistry, v. 10, p. 373-389.
MacIntyre, Don (1995): Sedimentary Exhalative Zn-Pb-Ag, in Selected British Columbia Mineral Deposit Profiles, Volume 1 – Metallics and Coal, Lefebure, D.V. and Ray, G.E., Editors, British Columbia Ministry of Energy of Employment and Investment, Open File 1995-20, pages 37-39.
Magnall J.M., Gleeson S.A, Stern R.A, Newton R.J, Poulton S.W and Paradis S. (2016) Open system sulphate reduction in a diagenetic environment – Isotopic analysis of barite and pyrite from the TOM and Jasson Late Devonian Zn-Pb-Ba deposits, Selwyn Basin, Canada: 2016, Elsevier Ltd (an open access article)
Moldovanyi, E.P., and Walter, L.M., 1992, Regional trends in water chemistry, Smackover Formation, Southwest Arkansas: Geochemical and physical controls: American Association of Petroleum Geologists Bulletin, v. 76, p. 864–894.
Morrow, D., 1998, Regional subsurface dolomitization; Models and constraints: Geoscience Canada, v. 25, p. 57-70.
Polito, P.A., Kyser, T.K., Golding, S.D., and Southgate, P.N., 2006, Zinc Deposits and Related Mineralization of the Burketown Mineral Field, Including the World-Class Century Deposit, Northern Australia: Fluid Inclusion and Stable Isotope Evidence for Basin Fluid Sources: Economic Geology, v. 101, no. 6, p. 1251–1273.
Poul, E. (2009). Geologic Criteria for the Assessment of Sedimentary Exhalative (Sedex) Zn-Pb-Ag Deposits: Theory of Deposit Formation (pp. 3 – 14). Department of Interior, U.S. Geological Survey Open-File Report.
Reid, S., Dewing, K., Sharp, R., (2013). Structural and diagenetic origin of breccias in the carbonate-hosted Polaris Zn-Pb deposit, Nunavut, Canada: Ore Geology Reviews, Volume 55, Issue C, November 2013
Russell, M.J., Solomon, M., and Walshe, J.L., 1981, The genesis of sediment-hosted exhalative zinc and lead deposits: Mineralium Deposita, v. 16, p. 113–127.
Sangster D, Hillary E (2000) SEDEX lead-zinc deposits: proposed subtypes and their characteristics. Explor Min Geol 7:341–357
Sawkins, F.J., 1984, Ore genesis by episodic dewatering of sedimentary basins—Application to giant Proterozoic lead-zinc deposits: Geology, v. 12, p. 451–454.
Selwyn Chihong Mining Limited, 2012, Selwyn announces updated mineral resource for XY West deposit: Vancouver, British Columbia, Canada, Press Release, 5 p.
SHARP, R.J., STE-MARIE, C.P., and LORENZINI, C. 1995a.Field study of Polaris Mine area, NWT, Canada. In: Misra, K.C., ed. Carbonate-hosted lead-zinc-fluorite-barite deposits of North America. Guidebook Series 22:38 – 41
Soltan A.M.M (2017) Economic Geology – Metallogeny and plate tectonics. Retrieved from https://www.researchgate.net/publication/312189113_Economic_Geology__Metallogeny_and_plate_tectonics
Taylor, R.D., Leach, D.L., Bradley, D.C., and Pisarevsky, S.A., 2009, Compilation of mineral resource data for Mississippi Valley-type and clastic-dominated sediment-hosted lead-zinc deposits: U.S. Geological Survey Open-File Report 2009–1297, 42 p.
Warren J.K. (2016) Lower Temperature Metals in an Evaporitic Framework. In: Evaporites. Springer, Cham
Yang, J., Bull, S., and Large, R., 2004, Numerical investigation of salinity in controlling ore-forming fluid transport in sedimentary basins—Example of the HYC Deposit, northern Australia: Mineralium Deposita, v. 39, no. 5–6, p. 622–631.
Yang, J., Large, R.R., Bull, S., and Scott, D.L., 2006, Basin-scale numerical modeling to test the role of buoyancy-driven fluid flow and heat transfer in the formation of stratiform Zn-Pb-Ag deposits in the northern Mount Isa Basin: Economic Geology, v. 101, no. 6, p. 1275–1292.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Geology"
Geology is an “Earth science” or “geoscience” concerned with the study of the physical structure of the Earth (or other planetary body) and the rocks of which it is made, the processes that shaped it and its physical, chemical and biological changes over time.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




