Oxygen Reduction on Stressed Surface
Info: 11342 words (45 pages) Dissertation
Published: 11th Dec 2019
Tagged: Organic Chemistry
Recently, a paper published on Faraday Discussion “Mechano-electrochemistry effects due to deformation of copper oxide films” by Dr. Kramer and his colleagues, attempted to illuminate and clarify how the oxidation is happening on the stressed metal surface. By using cyclic voltammetry to study the single crystal surfaces of Cu (100) in perchloric acid, results came out identifying that different surface conditions behave uniquely to strain. And under any tensile strain will diminish the reconstructive surface oxidation. [1] In conclusion, the elastic strain represents to electrochemical sensitivity. Their experiment is of great significance to underlying the chemical response under applying mechanical changing situation. Opportunities for metal corrosion protections have been uncovered. This project aims to investigate how the oxygen reduction reaction acting on the surface in different environments and clarify the material oxidation by adding oxygen adsorbates on the surface. Simulation of Pt (111) model with several bond distances has been used to carry out the calculation for the total energy per unit cell in a vacuum and aqueous environment referred to the contact angle theory to analysis the surface free energy. Density Functional Theory (DFT) acted as a method that describes the realistic materials’ electrons and atoms will be used to determine the number of particles take part in the reaction and the chemical characteristic of oxygen.
Declaration
I, Zhengxu Wang declare that this article and any work presented from this paper is based on my own research and being my final study for my topic. I confirm that:
- This research was done wholly and mainly done by myself for a degree study at the University of Southampton.
- Any part of this topic been studied previously has been clearly stated for any other qualification at the university or other proposes.
- Any published work from others has been clearly stated in this report.
- Sources are already listed for any table or image obtained from others’ finding. Apart from that, thesis without resource listed is my own work.
- I have acknowledged all main sources of help.
- None of those works have been published before the final submission.
Acknowledgements
I sincerely present my great appreciate to Dr. Kramer for his help and guidance throughout my project. I truly send my great thanks to him for training me on learning computational languages and writing programs. I would express my gratitude for his time and patience to lead me and let me finish this project with my best. Contents Abstract Declaration Acknowledgements 1. Background information and objectives 1.1 Finding of Oxygen reduction reaction 1.2 Objective and motivation 2. Introduction 2.1 Mechanism of Oxygen Reduction Reaction (ORR) 2.2 Oxidation on the metal surface 2.3 Application of ORR: Fuel cell 2.4 Use of Supercomputer 2.5 Contact angle: 2.5.1 Solid contact observations 2.5.2 What is contact angle? 2.5.3 Application of contact angle 3. Platinum property 3.1 characteristics of Platinum 3.2 Manufacturing and extracting of Platinum 3.3 Why using Pt simulation? 3.4 Surface energy and Platinum surface energy 4. Computational methodology 4.1 Metal surface modelling 4.1.1 Relaxation 4.1.2 Reconstruction 4.2 Software usage 4.2.1 Use of Density Functional Theory (DFT) and VSAP 4.2.2 Use of VESTA 4.3 Geometry inputs and Experiments data 4.3.1 Vacuum condition 4.3.2 Changing of Kpoints 4.3.3 Aqueous environment 5. Results 5.1 Pt (111) vacuum environment data analysis 5.2 KPOINTs data analysis 5.3 Total energy per unit cell of Pt (111) in pure water 5.4 Surface energy and contact angle 5.5 Determine surface energy (using total energy and contact angle) of aqueous condition 6. Outlook and further analysis 6.1 Study of oxidation on stressed surface 6.2 Adding oxygen adsorbates 7. Discussion 8. Conclusion 9. References List of figures Figure 1. Chemical process of oxidation and reduction Figure 2. Rust formation process..................................................... Figure 3. Steel corrosion on the motorcycle.............................................. Figure 4. William Robert fuel cell Figure 5. Simulation of the contact angle Figure 6. Platinum ore Figure 7. Table of the physical properties of Platinum Figure 8. Platinum dissolves in aqua regia liquid Figure 9. Surface energy of Pt and Pd in different planes Figure 10. Comparing the unrelaxed surface and the relaxed surface Figure 11. A bulk structure without surface reconstruction.................................... Figure 12. The “missing row model” of a bulk structure under surface reconstruction.................. Figure 13. Modelling of Platinum cell with 60Å and grid size of 7x7.............................. Figure 14. General Output from VESTA (Pt_111_60.0, 60Å)................................... Figure 15. INCAR file of fundamental parameters submitted into Iridis4........................... Figure 16. POSCAR file, Pt_111_60.0, distance of 60Å....................................... Figure 17. KPOINTS file shows the initial values of the slab grid size.............................. Figure 18. New INCAR file for aqueous environment in Iridis4.................................. Figure 19. General OUTCAR file from Iridis4 Figure 20. Total energy per unit cell against cell unit distance Figure 21. The unit cell total energy against the slab grid Figure 22. OSZCAR file of aqueous environment........................................... Figure 23. OSZCAR file of vacuum environment........................................... Figure 24. Contact angle measuring instrument: DSA30 Drop Shape Analyser....................... Figure 25. Contact angle and the parameters Figure 26. Parallelogram shape of Platinum bulk body Figure 27. Cross-section view of the experimental cell set up Figure 28. Pourbaix diagram for long range order unstained Cu (100)............................ Figure 29. Surface reconstruction due to the compression force (left) and tension (right) force............. Figure 30. Comparison of the ORR activity of 10 and 5 nm Pt nanofilm under different strain types (kinetic rate constants)..... Figure 31. simulation modelling of adding oxygen adsorbates onto the platinum surface................ Figure 32. OSZCAR file of adding Oads in vacuum condition................................... Figure 33. OSZCAR file of adding Oads in pure water condition................................. List of tables Table 1. Analysis of OSZCAR outputs of different distance Table 2. Analysis of OSZCAR outputs of different grid size
1. Background information and objectives
1.1 Finding of Oxygen reduction reaction
Oxygen reduction reaction (ORR) happens at every minute, anywhere and happens in so many ways. [2] Surely, it can be simply explained as a process of exchanging electrons. But as the investigation going further, it showed that in different conditions, the mechanisms of ORR are different. Reactions can happen in the aqueous environment or non-aqueous. And under the first condition, the media could be acidic, alkaline, or neutral. The earliest human use of oxidation can be found from 7,500-4,500 years ago in the Copper/Bronze Age, by heating the copper ores to produce the copper metal. [3] The concept of oxidation reduction reaction was pointed out at the end of 18th centuries. After combining a large amount of materials reacting with oxygen, scientists found out that those reactions had a something in common and concluded the principle of ORR. When extracting the metal from metallic ore, there is a loss of weight during the heating process and Antoine Lavoisier (1743-1794) believed that those weight losses somehow related to the loss of oxygen. [4] After the establishment of valence theory in 19th centuries, reactions where the valence increased called oxidation and those valences dropped are reductions. G. N. Lewis pointed out in 1916 that “a chemical bond forms by the interaction of two shared bonding electrons, with the representation of molecules as Lewis structures”. At that stage, the new definition of oxidation and reduction risen: the half process of electrons loss is oxidation and another half process which gain the electrons is the reduction. [5]
1.2 Objective and motivation
Oxygen reduction reaction is often investigating computationally by simulating surfaces in vacuum. While in reality, both conditions of vacuum and aqueous need to be taken into consideration during the experiment and simulation. The software packages cannot describe the reactions directly and work out the answer. A method of predicting contact angle of H2O on the platinum surface (aqueous condition) as the first step to building the simulation, the value of the contact angle would be used in Young’s Equation to calculate the surface energy and underlying the oxidation process.
2. Introduction
2.1 Mechanism of Oxygen Reduction Reaction (ORR)
Oxygen is the most fundamental and common element on the earth. Also, oxygen reduction reaction as the most important and ordinary reaction happens in life processes. During the past decades, scientists have gained lots of interest in research and development due to ORR’s wide application range. ORR relates to various sections such as biological respiration, dissolution, and fermentation, energy convert system (i.e. fuel cell, air battery). There are a huge amount of research and investigation covered in the chemical range to indicate ORR. Nevertheless, the mechanism of ORR occurs on the stressed surface is still undercover. [6]
Academically explain oxygen reduction reaction as “Redox (short for reduction–oxidation reaction), a chemical reaction in which the oxidation states of atoms are changed. Any such reaction involves both a reduction process and a complementary oxidation process, two key concepts involved with electron transfer processes. Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between chemical species. The chemical species from which the electron is stripped is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. “[2]
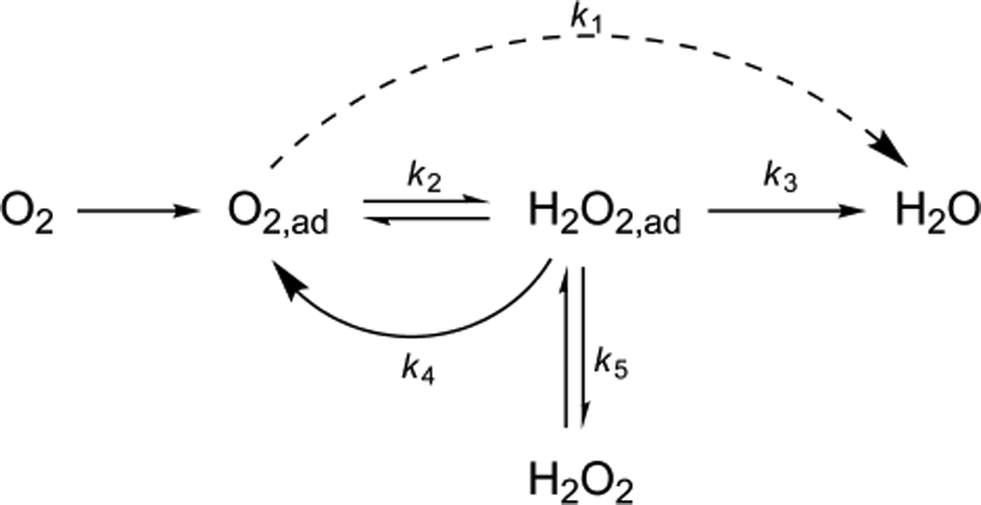 Figure 1. Chemical process of oxidation and reduction
(Image source: http://pubs.rsc.org/en/content/articlehtml/2014/cy/c3cy01049j)
To distinguish the ORR mechanisms’ difference, proton-coupled electron transfer precedes the step of O-O bonding breaking. At that situation, hydrogen peroxide in its second associative mechanism behaves as the intermediate of ORR. It has been proved that during ORR process, there is hydrogen peroxide exists but whether it is the key factor of ORR reaction or not is still unclear.
Figure 1. Chemical process of oxidation and reduction
(Image source: http://pubs.rsc.org/en/content/articlehtml/2014/cy/c3cy01049j)
To distinguish the ORR mechanisms’ difference, proton-coupled electron transfer precedes the step of O-O bonding breaking. At that situation, hydrogen peroxide in its second associative mechanism behaves as the intermediate of ORR. It has been proved that during ORR process, there is hydrogen peroxide exists but whether it is the key factor of ORR reaction or not is still unclear.
2.2 Oxidation on the metal surface
The process of metal oxide with oxygen, whether being exposed to air or from water, is called corrosion. [32] Oxidations of steel and copper are agreed to be the most common phenomenon of corrosion, which the surface iron and copper molecules are transferred into Fe2O3 and CuO. [33] These request the electrons travel between the metal surface and oxygen molecules.
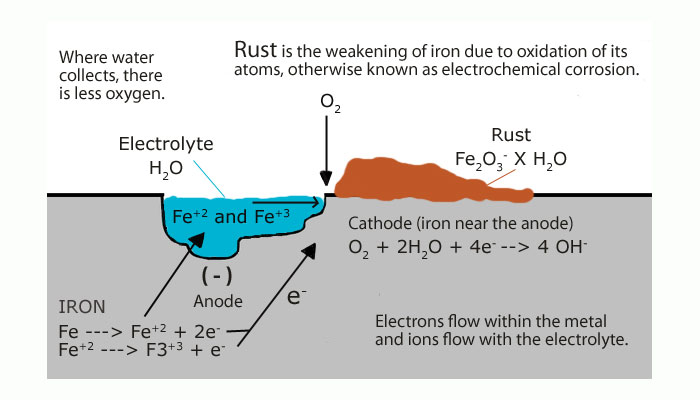 Figure 2. Rust formation process
(Image source from https://hocktools.wordpress.com/page/17/)
H. Over (2002) describe the reaction in his article Oxidation of Metal Surfaces as “the complex interaction of oxygen with the metal surface”. [34] Initially, an oxidation occurs must match up with two requirements: sufficient supply of oxygen atoms and surface metal atoms. The progress can be described that “electrons leap from the metal to the oxygen molecules, and the negative oxygen ions which are thus formed penetrate into the metal, causing the growth of an oxide surface”. [32] With the oxide surface layer becoming thicker, the speed rate for electron transformation is reduced. As a result, the process rate maybe gets slower and slower but always been continuing.
Figure 2. Rust formation process
(Image source from https://hocktools.wordpress.com/page/17/)
H. Over (2002) describe the reaction in his article Oxidation of Metal Surfaces as “the complex interaction of oxygen with the metal surface”. [34] Initially, an oxidation occurs must match up with two requirements: sufficient supply of oxygen atoms and surface metal atoms. The progress can be described that “electrons leap from the metal to the oxygen molecules, and the negative oxygen ions which are thus formed penetrate into the metal, causing the growth of an oxide surface”. [32] With the oxide surface layer becoming thicker, the speed rate for electron transformation is reduced. As a result, the process rate maybe gets slower and slower but always been continuing.
 Figure 3. Steel corrosion on the motorcycle.
(Image source from https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1992/illpres/oxidation.html)
Figure 3. Steel corrosion on the motorcycle.
(Image source from https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1992/illpres/oxidation.html)
2.3 Application of ORR: Fuel cell
The best and most popular to turn oxygen reduction reaction into use is the fuel cell. A fuel cell provides a mechanism to transfer chemical energy into electricity due to the oxidation and reduction reactions happen at its anode and cathode. The anode and cathode contain catalysts and building a connection between them two allows the irons moving.
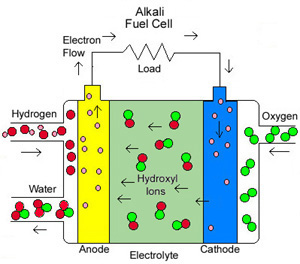 Figure 4. William Robert fuel cell
(Image source from http://americanhistory.si.edu/fuelcells/basics.htm)
Sir William Robert Grove (1839) used zinc and platinum electrodes invented the world’s first fuel cell. He based his experiment on the fact that sending an electric current through water splits the water into its component parts of hydrogen and oxygen. [9] So, Grove tried reversing the reaction - combining hydrogen and oxygen to produce electricity and water. This is the basis of a simple fuel cell.
During the 1950s to 1980s, scientists started to know better about fuel cell principles and they improved its functions to become stronger and more stable. At that stage, fuel cell been started put into space missions and energy technologies.
The common types of fuel cell we can find recently would be Proton exchange membrane fuel cells (PEMFCs), Direct methanol fuel cells and Alkaline fuel cells. The proton exchange membrane fuel cell (PEMFC) uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based electrodes. The direct methanol fuel cell is a variation of the PEMFC; it uses the same type of electrolyte. Instead of using hydrogen as fuel, methanol as a solution in water is directly oxidized to CO2. While the Alkaline fuel cells (AFC’s) have an alkaline electrolyte - commonly a liquid such as Potassium Hydroxide (KOH). In an AFC, hydroxide ions (OH-) travel from cathode to anode. This differentiates them from PEM Fuel Cells which have a solid polymer electrolyte which conducts protons. [10]
Figure 4. William Robert fuel cell
(Image source from http://americanhistory.si.edu/fuelcells/basics.htm)
Sir William Robert Grove (1839) used zinc and platinum electrodes invented the world’s first fuel cell. He based his experiment on the fact that sending an electric current through water splits the water into its component parts of hydrogen and oxygen. [9] So, Grove tried reversing the reaction - combining hydrogen and oxygen to produce electricity and water. This is the basis of a simple fuel cell.
During the 1950s to 1980s, scientists started to know better about fuel cell principles and they improved its functions to become stronger and more stable. At that stage, fuel cell been started put into space missions and energy technologies.
The common types of fuel cell we can find recently would be Proton exchange membrane fuel cells (PEMFCs), Direct methanol fuel cells and Alkaline fuel cells. The proton exchange membrane fuel cell (PEMFC) uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based electrodes. The direct methanol fuel cell is a variation of the PEMFC; it uses the same type of electrolyte. Instead of using hydrogen as fuel, methanol as a solution in water is directly oxidized to CO2. While the Alkaline fuel cells (AFC’s) have an alkaline electrolyte - commonly a liquid such as Potassium Hydroxide (KOH). In an AFC, hydroxide ions (OH-) travel from cathode to anode. This differentiates them from PEM Fuel Cells which have a solid polymer electrolyte which conducts protons. [10]
2.4 Use of Supercomputer
Calculations to determine the total energy per unit cell changing between particles with different bond distance are run by the supercomputer. University of Southampton supercomputing facility is called Iridis4, which is running two Iridis supercomputers at the same time to make a stronger supercomputing system and faster the calculating process. Results obtained from supercomputer are more accurate. By trying and trial to investigate the final answer, it provides the most realistic and reasonable values. Compared with normal desktop facilities, supercomputer runs a program with ten times more node numbers. Consequently, working on Iridis not only improves the results’ quality but also saves the amount of work and time.
2.5 Contact angle:
2.5.1 Solid contact observations
Hertz (1896) firstly estimated there was a contact zone between two smooth elastic solids. [11] He proved that bodies deformed elastically would lead to different shape and size of the contact zone. By pressing two spheres under a load of P0, the radiuses are R1 and R2, and the shape of contact zone is a circle with radius of a0
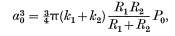 [11]
Where k1 and k2 are the sphere elastic constants, calculated using the following equations.
[11]
Where k1 and k2 are the sphere elastic constants, calculated using the following equations.
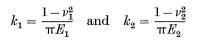 [11]
Where the Poisson ratio v and young’s modulus E are known from the materials’ properties.
Hertz was the first one presenting the idea of contact between two materials but recently some experiments reported contradictions to his theory.
Roberts and Kendall noticed that “at low loads contact areas between these bodies were considerably larger than those predicted by Hertz and tended towards a constant finite value as the load was reduced to zero”. [11] Their observations proved that with the load decreasing to zero, the contact forces became increasingly significant, however at a higher load value, the contact forces were of less importance.
[11]
Where the Poisson ratio v and young’s modulus E are known from the materials’ properties.
Hertz was the first one presenting the idea of contact between two materials but recently some experiments reported contradictions to his theory.
Roberts and Kendall noticed that “at low loads contact areas between these bodies were considerably larger than those predicted by Hertz and tended towards a constant finite value as the load was reduced to zero”. [11] Their observations proved that with the load decreasing to zero, the contact forces became increasingly significant, however at a higher load value, the contact forces were of less importance.
2.5.2 What is contact angle?
Sinha did an experiment in 2005 by modelling a (9,3) semi-infinite solid-liquid interaction to identify the contact angle. [12] They have been investigating the relation between contact angle and temperature. Referring to the equation below,
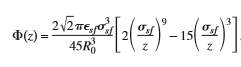 [13]
Where R0 is a constant number for FCC (111) solid surface, z is the distance to the wall, Ɛsf and Ơsf represent the Lennard-Jones factors. That study came to an output that by increasing the temperature, the contact angle would be decreased, no matter how the potential changes, the trend always remains the same. In 2003, Kimura and Maruyama first simulated a water droplet on a platinum surface. [13] They used two different potentials between water and platinum. By using Spohr–Heinzinger4 potential, they found that the contact angle is always 0°, which suggests that this potential is not suitable to predict the contact angle.
More recently, Vijay K. Dhir and his cos published “Molecular dynamics simulation of the contact angle of liquid on solid surface” in 2009 taking a further step to study contact angle. [13] They applied Particle-particle particle-mesh (PPPM) method to obtain a higher accuracy simulation model of various contact angles with different temperature and wall potential by reducing the error from the Coulombic and Lennard-Jones potentials in long term.
[13]
Where R0 is a constant number for FCC (111) solid surface, z is the distance to the wall, Ɛsf and Ơsf represent the Lennard-Jones factors. That study came to an output that by increasing the temperature, the contact angle would be decreased, no matter how the potential changes, the trend always remains the same. In 2003, Kimura and Maruyama first simulated a water droplet on a platinum surface. [13] They used two different potentials between water and platinum. By using Spohr–Heinzinger4 potential, they found that the contact angle is always 0°, which suggests that this potential is not suitable to predict the contact angle.
More recently, Vijay K. Dhir and his cos published “Molecular dynamics simulation of the contact angle of liquid on solid surface” in 2009 taking a further step to study contact angle. [13] They applied Particle-particle particle-mesh (PPPM) method to obtain a higher accuracy simulation model of various contact angles with different temperature and wall potential by reducing the error from the Coulombic and Lennard-Jones potentials in long term.
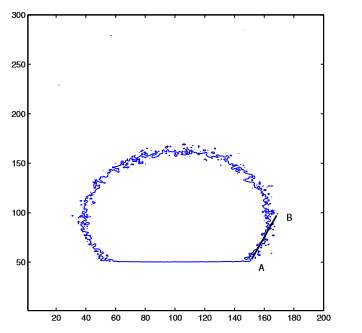 Figure 5. Simulation of the contact angle
(Image source from: Shi, B. and Dhir, V. (2009). Molecular dynamics simulation of the contact angle of liquids on solid surfaces)
To obtain the simulation, drop a small amount of liquid on a solid surface. When the system reaches its equilibrium, what the droplet density contour shows is exactly the contact angle of the model. Also, “molecular dynamics simulation was used to study the contact angle of Lennard-Jones liquid and water on the solid surface”. All the simulation processes make the experiment easier by simply consume that the density contour represents the liquid droplet’s contact angles. As a consequent, the relationship of system temperature and contact angle, as well as the interaction energy between solid and fluid can be determined. Vijay Dhir and co concluded that with the system temperature increasing, the contact angle would be decreased. The higher the solid-fluid potential is, the smaller contact angle will be. At a certain time that the temperature or the solid-fluid potential reach high enough, the contact angle is no longer exist. “The calculated contact angles for water platinum, argon-virtual solid wall, and the experimental value of water-aluminium cases were compared and found to show a similar trend.” [13]
This newest contact angle simulation experiment by Dhir and his co is a remarkable milestone for the surface chemical mechanism research. His finding is of significant importance as it allows academic and public to get better known about any influence on contact angle study.
Figure 5. Simulation of the contact angle
(Image source from: Shi, B. and Dhir, V. (2009). Molecular dynamics simulation of the contact angle of liquids on solid surfaces)
To obtain the simulation, drop a small amount of liquid on a solid surface. When the system reaches its equilibrium, what the droplet density contour shows is exactly the contact angle of the model. Also, “molecular dynamics simulation was used to study the contact angle of Lennard-Jones liquid and water on the solid surface”. All the simulation processes make the experiment easier by simply consume that the density contour represents the liquid droplet’s contact angles. As a consequent, the relationship of system temperature and contact angle, as well as the interaction energy between solid and fluid can be determined. Vijay Dhir and co concluded that with the system temperature increasing, the contact angle would be decreased. The higher the solid-fluid potential is, the smaller contact angle will be. At a certain time that the temperature or the solid-fluid potential reach high enough, the contact angle is no longer exist. “The calculated contact angles for water platinum, argon-virtual solid wall, and the experimental value of water-aluminium cases were compared and found to show a similar trend.” [13]
This newest contact angle simulation experiment by Dhir and his co is a remarkable milestone for the surface chemical mechanism research. His finding is of significant importance as it allows academic and public to get better known about any influence on contact angle study.
2.5.3 Application of contact angle
“Wetting refers to the study of how a liquid deposited on a solid (or liquid) substrate spreads out or the ability of liquids to form boundary surfaces with solid states.” [14] Contact angle provides a clear method to define the wettability of a surface or material. In a case of a complete wetting surface, the contact angle is fairly small, approaching 0o is the best. Where a good wetting condition, the contact angle is between 0o to 90o, and above that is not wettable. When the contact angle reaches 180o refers to ultra-hydrophobic materials with a unique effect call lotus effect. [15] The application of contact angle obtains a high importance in environment, technology, and bio-chemistry. The theory of imbibition of water into a porous solid plays a significant role in soil mechanics, geology study, food production and even more department. From a study of determining the permeability of the soil element, to rehydrate the dry food or compressed food, imbibition is extremely dependent on wetting. By investigating the liquid-solid angles, the imbibition of a liquid can be easily determined. [20] [14]
3. Platinum property
3.1 characteristics of Platinum
Platinum, symbol Pt, is an unreactive sliver coloured transition metal with excellent ductility. Because of its properties, dense and precious, platinum has been used major in jewelry as well as in catalytic converters for vehicles. In chemical field, platinum is also an ideal catalyst to help fuel cells work more effectively and accelerate reactions for nitric acid and benzene, etc. As one of the least reactive metals, platinum performs great on resisting corrosion, even at the extreme environment. [16]
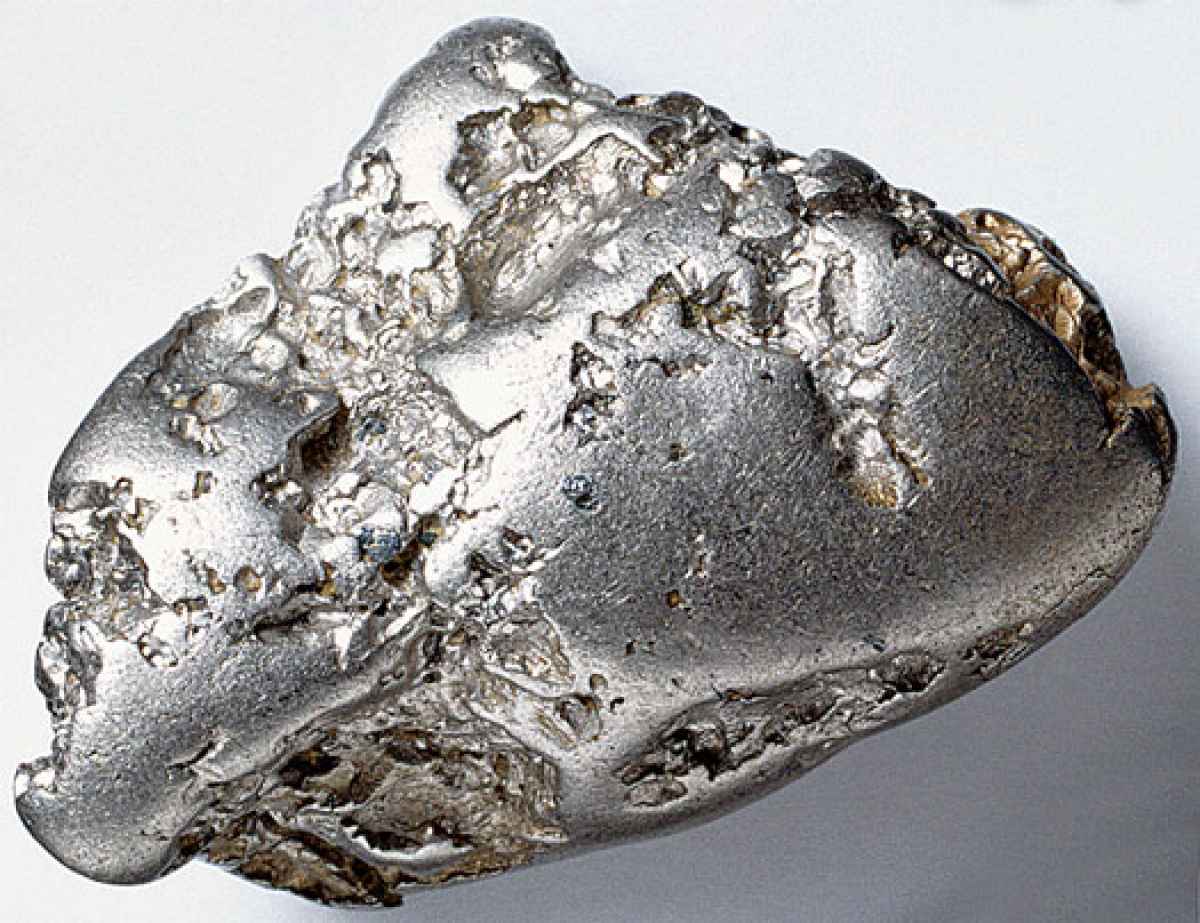 Figure 6. Platinum ore
(Image source: http://www.financialgazette.co.zw/gloomy-outlook-for-zim-platinum-mines/)
More often, platinum exists in oxidation states of +2 and +4, rarely in +1 and +3. Due to its chemical formation and properties, platinum stabilizes by metal bonding in polymetallic species, and “tetracoordinate platinum(II) compounds tend to adopt 16-electron square planar geometries.” [17] It only dissolute in aqua regia appearing a transparent orange colour. Platinum is of crystals, cubic formed shaped with rounded corners, and fairly easy to be distorted.
Figure 6. Platinum ore
(Image source: http://www.financialgazette.co.zw/gloomy-outlook-for-zim-platinum-mines/)
More often, platinum exists in oxidation states of +2 and +4, rarely in +1 and +3. Due to its chemical formation and properties, platinum stabilizes by metal bonding in polymetallic species, and “tetracoordinate platinum(II) compounds tend to adopt 16-electron square planar geometries.” [17] It only dissolute in aqua regia appearing a transparent orange colour. Platinum is of crystals, cubic formed shaped with rounded corners, and fairly easy to be distorted.
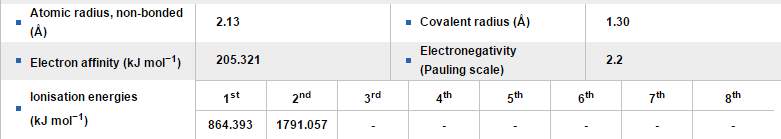 Figure 7. Table of the physical properties of Platinum
(Image source: http://www.rsc.org/periodic-table/element/78/platinum)
The earliest detected the using of platinum was in ancient Egypt. Platinum was found in the golden decorations. The mixture of platinum and gold somehow showed at that time, there was no clear knowledge of different metals and Egyptians could not have recognized the difference between platinum and gold. In 1557, an Italian humanist Julius Caesar Scaliger for the first time came up with a description of this unknown metal, as “which no fire nor any Spanish artifice has yet been able to liquefy". At that time, platinum was being defined as the impurity in gold and it was forbidden to adulterate any in gold. Charlies Wood found various forms of platinum samples in 1741 and tried to investigate the properties of platinum. In 30 years after that, people started discovering platinum mines and decided to learn about that new type of metal, but didn’t make any progress. Until 1772, Carl von Sickingen successfully alloyed platinum with gold and dissolved the new material in aqua regia liquid. Then platinum became a popular metal catalysis and considered a noble metal. [16] [30]
Figure 7. Table of the physical properties of Platinum
(Image source: http://www.rsc.org/periodic-table/element/78/platinum)
The earliest detected the using of platinum was in ancient Egypt. Platinum was found in the golden decorations. The mixture of platinum and gold somehow showed at that time, there was no clear knowledge of different metals and Egyptians could not have recognized the difference between platinum and gold. In 1557, an Italian humanist Julius Caesar Scaliger for the first time came up with a description of this unknown metal, as “which no fire nor any Spanish artifice has yet been able to liquefy". At that time, platinum was being defined as the impurity in gold and it was forbidden to adulterate any in gold. Charlies Wood found various forms of platinum samples in 1741 and tried to investigate the properties of platinum. In 30 years after that, people started discovering platinum mines and decided to learn about that new type of metal, but didn’t make any progress. Until 1772, Carl von Sickingen successfully alloyed platinum with gold and dissolved the new material in aqua regia liquid. Then platinum became a popular metal catalysis and considered a noble metal. [16] [30]
 Figure 8. Platinum dissolves in aqua regia liquid
(Image source: https://simple.wikipedia.org/wiki/Aqua_regia)
Figure 8. Platinum dissolves in aqua regia liquid
(Image source: https://simple.wikipedia.org/wiki/Aqua_regia)
3.2 Manufacturing and extracting of Platinum
Platinum mined from mine is an incredibly rare ore and it is necessary to be extracted through a series of complicated processes. Depending on the sophistication of the mines and the miner’s technological function, there are a variety of different ways to purify the platinum. It is rarely to seek for a large piece of pure platinum, so using a precise way to extract platinum from ore is the only method at the current stage. [19] To reduce the difficulty on extraction, during the exploiting, methods on controlling explosion or by taking an advanced mining techniques to obtain platinum is important. Once mined the platinum from the mountain, crush the ores into small pieces and simple to treat. Then using air as a tool to separate any impurity from platinum particles. It can be described that “Air particles then bubble through an aeration tank and adhere to the platinum particles. This brings the platinum particles, with the air particles, up to the top of the tank. The layer of particles that ultimately forms can be skimmed off and refined into platinum.” Nowadays, the platinum material becomes increasingly popular being used for vehicle emissions control, chemical reactions catalyst, and jewelry production. Using pure platinum metal for toxic gas treatment gives a better result in emission control, which helps to decrease the amount of carbon dioxide and global warming. As well as for chemical catalyst, higher the platinum percent catalyst content gives a better experiment procedure and result.
3.3 Why using Pt simulation?
Platinum has a particular high effectivity on oxidizing carbon monoxide(CO) so it is always the first choice of metal for diesel applications. Platinum (Pt) more often be used as the catalysis. Though the cost is not cheap to make a fuel cell of Pt-based material, in the experiment or simulation, using Pt would give out a more accurate result. At some stage, platinum and another Platinum Group Metal Palladium(Pd) obtain an equal effect as the catalyst, the cost of platinum to the cost of palladium is relatively lower. Platinum considered to be the better choice. Also, some other metals can be competent, however, platinum has advantages of a high melting points, which during the reaction process, it will hardly reach the temperature and provides a sign of its “overall thermal durability”. On the other hand, majorly recyclable and interaction with toxicants indicates that platinum to be a type of eco-environment material. Once it has less harm with a relative low budget, platinum would continually be agreed as the effective chemical catalyst and used more often in simulation.
3.4 Surface energy and Platinum surface energy
Surface energy, also called interface energy, is defined by the “work per unit area done by the force that creates the new surface”. [29] It refers to the bonds between molecules to form a surface. Physically speaking, initially, surfaces require less energy to let deformation happen to compare to a material bulk. In other words, the surface molecules contain a higher level of energy than the molecules in solid materials, otherwise, the work done to generate the surface would drive to remove the material bulk. Surface energy can also be expressed by the amount of work spent on cutting a solid into two parts. Metals with high surface energy are of great molecular interaction and vice versa.
Surface energy of (111) plane can be determined using the following equation
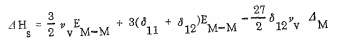 [26]
Where
[26]
Where 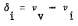 represents the i-th bond perturbation when forming the surface.
represents the i-th bond perturbation when forming the surface.
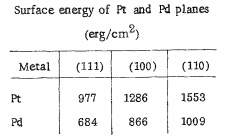 Figure 9. Surface energy of Pt and Pd in different planes
(Image resource: Galeev, T., Bulgakov, N., Savelieva, G. and Popova, N. (1980). Surface properties of platinum and palladium.)
Obviously, for platinum, the plane of (111) has less surface energy match with the others, which means Pt is stronger, more prevail and responsible along with the polycrystalline metal.
Figure 9. Surface energy of Pt and Pd in different planes
(Image resource: Galeev, T., Bulgakov, N., Savelieva, G. and Popova, N. (1980). Surface properties of platinum and palladium.)
Obviously, for platinum, the plane of (111) has less surface energy match with the others, which means Pt is stronger, more prevail and responsible along with the polycrystalline metal.
4. Computational methodology
4.1 Metal surface modelling
Relaxation and reconstruction are the two main processes occur in surface atoms rearrangements. This approach is involved in the energetic changing of the system, for instance, minimize the surface free energy as the final target. Temperature is one of the main influence in this atoms reconstruction and low temperature has an effect on limiting the kinetic which hinder and forbid this process. [21]
4.1.1 Relaxation
Relaxation is a microcosmic and sensitive process of the surface layer rearrangement. However, at some points, it is a significant influence on the material properties. The main part of the relaxation refers to adjusting the spacing of the layer which is perpendicularly located to the surface. Nothing changed in both direction (parallel or perpendicular or symmetry to the surface). The following image is showing that particles from the first layer were pushed towards the second layer on a relaxed surface. (i.e. d1-2 < dbulk ) [21]
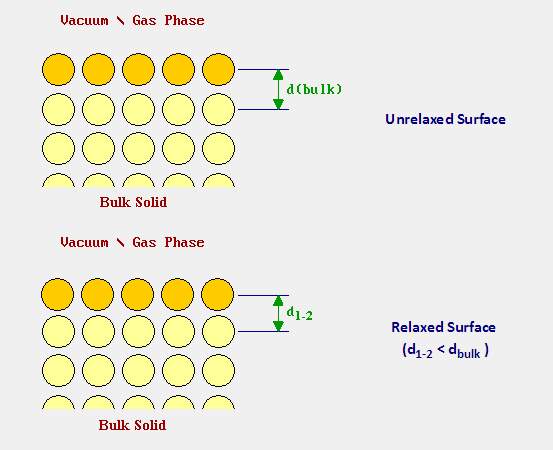 Figure 10. Comparing the unrelaxed surface and the relaxed surface
(Image resource: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm)
Figure 10. Comparing the unrelaxed surface and the relaxed surface
(Image resource: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm)
4.1.2 Reconstruction
The surface atoms relocation has a significant effect, especially in the layer’s reconstruction of large size atoms. It usually happens on the metal surface (the majority of the surface is the FCC (111)) which is considered of not stable enough, or on the semi-conductive surfaces. [21]
Reconstruction and relaxation have a common factor that all those processes involving the deformation of the surface structure or changes happen. The picture below shows the side view of the bulk structure without happened the reconstruction. And the following picture shows the bulk structure under reconstruction. Both structures are of FCC (111). [21]
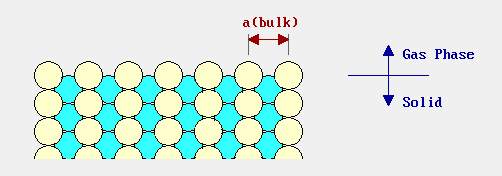 Figure 11. A bulk structure without surface reconstruction
(Image resource: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm)
Figure 11. A bulk structure without surface reconstruction
(Image resource: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm)
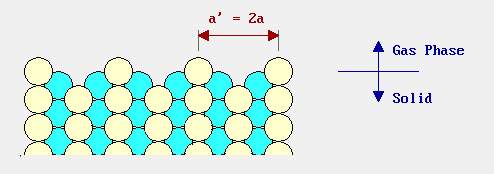 Figure 12. The “missing row model” of a bulk structure under surface reconstruction
(Image resource: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm)
Figure 12. The “missing row model” of a bulk structure under surface reconstruction
(Image resource: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm)
4.2 Software usage
4.2.1 Use of Density Functional Theory (DFT) and VSAP
Density Functional Theory was founded and created by Hohenberg and Kohn in 1964 in approach to compute the electronic structure of matter. DFT using in this project has proved to be highly successful in describing structural and electronic properties in a vast class of materials, ranging from atoms and molecules to simple crystals to complex extended systems (including glasses and liquids). Furthermore, using DFT to curry out the calculations, e.g. total energy per unit cell, make the calculating works computationally simple. For these reasons, DFT is considered to be a common tool in first-principles calculations aimed at describing – or even predicting – properties of molecular and condensed matter systems. In this project, Density functional theory had been applied to determine the changing of unit cell total energy and stabilities in both vacuum and aqueous environments. It has been finding that the use of DFT provides a reasonable highly accurate running process and results. [23] In Vienna ab initio Simulation Package (VASP) via using the wave function method, I generated the energies adsorption on Pt (111) surface. VASP has been found and recommended to be the simplest computer program to scale and model materials’ atomic structure. In real life, the majority of objectives are many-body structure and it is such a pain to obtain the solutions only by working on the physical equations. VASP does a great job by combining those equations with computing method to “computes an approximate solution to the many-body Schrödinger equation, either within density functional theory (DFT), solving the Kohn-Sham equations, or within the Hartree-Fock (HF) approximation, solving the Roothaan equations.” Though this simulation on Pt (111) only consider reactions happen between two Pt atoms, the use of VASP computing offers a high accuracy modelling. For instance, use VASP to draw the particle central quantities, “like the one-electron orbitals, the electronic charge density, and the local potential is expressed in plane wave basis sets. The interactions between the electrons and ions are described using norm-conserving or ultra-soft pseudopotentials, or the projector-augmented-wave method.” In order to investigate the groundstate of the electron, VASP can effectively use the “matrix diagonalization techniques”, just like “the residual minimisation method with direct inversion of the iterative subspace (RMM-DIIS) or blocked Davidson algorithms.” [24]
4.2.2 Use of VESTA
The software package used in the simulation is VESTA (Visualization for Electronic and STructural Analysis), a 3D visualization program to simulate the particle physical structure, locating electron, nuclear, and analysis volumetric data. When running the calculation on Iridis4, bond distance, the number of particle, and environmental settings need to be written as a program and submitted into job queue for analyzation. VESTA will automatically generate the multiply simulation structure. It assumes that data from INPUT file is the correct value for building the model. Simply drag the file into VESTA package and the software outputs the 3D virtual drawing. [25]
4.3 Geometry inputs and Experiments data
To investigate the total unit cell energy changes under different environment, two simulations have been set up. The first and simplest case is considering various bond distances reflected various numbers of energies under vacuum condition. Another case is to determine under aqueous environment, which is more complicated on setting out the input file.
4.3.1 Vacuum condition
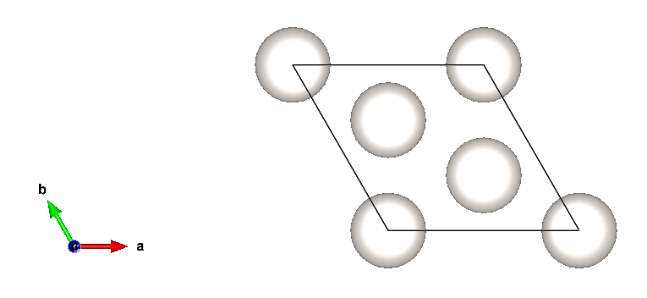
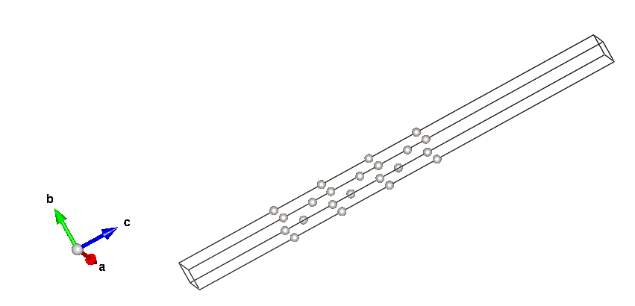
Figure 13. Modelling of Platinum cell with 60Å and grid size of 7x7
Left: plan view for Pt cell Right: 3D view of two layers Pt cell
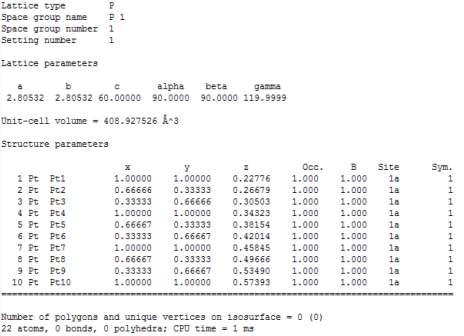 Figure 14. General Output from VESTA (Pt_111_60.0, 60Å)
The first experiment was to determine how the total energy per cell changing with different distance between particles. Only two platinum layers have been modelled in the simulation, but it supposed to be an infinite number of layers. The bulk Platinum (111) Face Centre Cubic (FCC) system is simulated in the vacuum environment. Factors have been generated and settled down before running the program. Because doing the platinum simulation has been the most general and simplest experiment, when setting the parameters, some of the numbers are very common and in wildly used. The picture below is the INCAR file which represents the properties of the platinum layer.
Figure 14. General Output from VESTA (Pt_111_60.0, 60Å)
The first experiment was to determine how the total energy per cell changing with different distance between particles. Only two platinum layers have been modelled in the simulation, but it supposed to be an infinite number of layers. The bulk Platinum (111) Face Centre Cubic (FCC) system is simulated in the vacuum environment. Factors have been generated and settled down before running the program. Because doing the platinum simulation has been the most general and simplest experiment, when setting the parameters, some of the numbers are very common and in wildly used. The picture below is the INCAR file which represents the properties of the platinum layer.
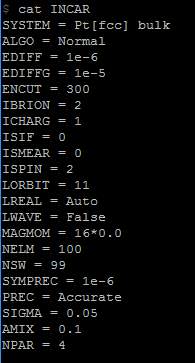 Figure 15. INCAR file of fundamental parameters submitted into Iridis4
Having a better look at the different distance influence on the total energy, 7 different simulations for the platinum plane have been taken into a test. The distance varies are 25Å, 30Å, 40Å, 50Å, 60Å, 70Å and 80Å. In POSCAR file, the first section is a 3x3 matrix, which represents the distance between platinum atoms and distance between layers. Only change the number locates at (3,3) into the value we need and keep rest of them the same. For example, when the test on the 60Å distance’s effect, rewrite the figure into 60.000000000 and save the change.
The first five calculations were run smoothly in Test Queue, required less node number and wall time. When the distance jumped above 60Å, it took a longer time to run the programmer and required a higher configuration. Jobs of 70Å, 80Å and 90Å were submitted into Phi Queue and used more nodes and wall time for analyzing.
Figure 15. INCAR file of fundamental parameters submitted into Iridis4
Having a better look at the different distance influence on the total energy, 7 different simulations for the platinum plane have been taken into a test. The distance varies are 25Å, 30Å, 40Å, 50Å, 60Å, 70Å and 80Å. In POSCAR file, the first section is a 3x3 matrix, which represents the distance between platinum atoms and distance between layers. Only change the number locates at (3,3) into the value we need and keep rest of them the same. For example, when the test on the 60Å distance’s effect, rewrite the figure into 60.000000000 and save the change.
The first five calculations were run smoothly in Test Queue, required less node number and wall time. When the distance jumped above 60Å, it took a longer time to run the programmer and required a higher configuration. Jobs of 70Å, 80Å and 90Å were submitted into Phi Queue and used more nodes and wall time for analyzing.

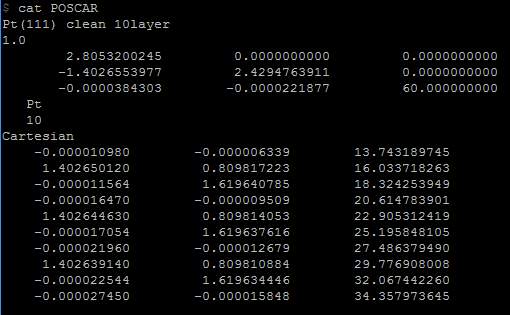 Figure 16. POSCAR file, Pt_111_60.0, distance of 60Å
Figure 16. POSCAR file, Pt_111_60.0, distance of 60Å
4.3.2 Changing of Kpoints
Initially, expect there are only two layers of platinum atom existing and calculate under this circumstance obtaining the most theoretical result. In the previous part of this process we kept the KPOINTS file unchanged (Gamma-centred slab grid 0, Gamma 7 7 1) and observe the energy varies with distance. The slab grid of 7x7 is the initial setting for the platinum slab. In the second section of the vacuum condition analysis, we aimed to investigate different slab grids affect the unit cell total energy.
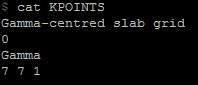 Figure 17. KPOINTS file shows the initial values of the slab grid size
Additional slab size of 3x3 (Gamma 3 3 1), 5x5 (Gamma 5 5 1), 9x9 (Gamma 9 9 1) and 11x11 (Gamma 11 11 1) were considered and to obtain the most critical value. In Iridis4, copied the file of Pt_111_60 (a distance of 60Å) into a new folder. Only changed the KPOINTS file to control the variables.
Figure 17. KPOINTS file shows the initial values of the slab grid size
Additional slab size of 3x3 (Gamma 3 3 1), 5x5 (Gamma 5 5 1), 9x9 (Gamma 9 9 1) and 11x11 (Gamma 11 11 1) were considered and to obtain the most critical value. In Iridis4, copied the file of Pt_111_60 (a distance of 60Å) into a new folder. Only changed the KPOINTS file to control the variables.
4.3.3 Aqueous environment
The second experiment was to determine in aqueous condition. It is more complicate compare with the first one due to there are more elements involved in the simulation affecting the results. Using different types of liquid in the test would give you the different results. As a member of negative metal, platinum ion acts extremely stable in any chemical reaction. It can hardly dissolve in acid or alkaline solution unless in aqua regia. The contact angle was used to estimate the solution viscosity. A larger contact angle reflects the solution is viscose. The contact angle was also being taken into consideration.
From the experimental point of view, any factor could be the key point influences the result. In my simulation, I picked up to simulate in a clean water condition. Modelled a droplet on a platinum plane and observed the total energy change compared with the vacuum condition.
The data of 60Å distance continually being used as the initial value. To write a new program and let it do the work in aqueous condition, new commands were written in INCAR file: ‘LSOL = .TRUE.’ ‘EB_K = 80’.
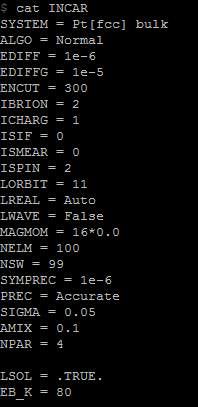 Figure 18. New INCAR file for aqueous environment in Iridis4
Figure 18. New INCAR file for aqueous environment in Iridis4
5. Results
5.1 Pt (111) vacuum environment data analysis
If the job runs correctly, the OUTCAR file formed to record the general timing and accounting information for this job. It memorized the total CPU time used, system time used, and maximum memory and volume used to during the calculating.
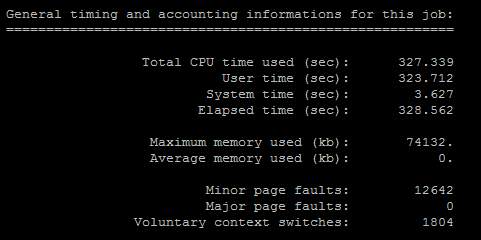 Figure 19. General OUTCAR file from Iridis4
To obtained the most accurate result, Iridis4 ran the calculation three times. After each calculation finished, the supercomputer would record each calculation running process in one file. Three calculations, three files: relax1, relax2 and static. The first calculation running, Iridis4 did 55 times deviations and take the most reliable value into the second computation, and so on until getting the closest number to the final answer.
Figure 19. General OUTCAR file from Iridis4
To obtained the most accurate result, Iridis4 ran the calculation three times. After each calculation finished, the supercomputer would record each calculation running process in one file. Three calculations, three files: relax1, relax2 and static. The first calculation running, Iridis4 did 55 times deviations and take the most reliable value into the second computation, and so on until getting the closest number to the final answer.
| Distance (Å) | Total Energy (eV) | Change in energy | F |
| 25 | -4.5258E+01 | -3.17E-02 | -4.53E+01 |
| 30 | -5.2746E+01 | -3.11E-02 | -5.27E+01 |
| 40 | -5.8476E+01 | -2.69E-02 | -5.85E+01 |
| 50 | -5.9237E+01 | -2.67E-02 | -5.92E+01 |
| 60 | -5.8832E+01 | -2.79E-02 | -5.92E+01 |
| 70 | -5.8386E+01 | -3.24E-02 | -5.84E+01 |
| 80 | -5.8147E+01 | -3.87E-02 | -5.82E+01 |
Table 1. Analysis of OSZCAR outputs of different distance
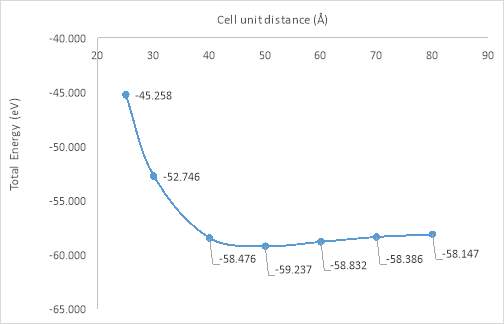 Figure 20. Total energy per unit cell against cell unit distance
The results showed the reasonable tendency and are relative good as expectation. From the Iridis4 output, it is obvious that the total energy changing relays on different distance. The first three points from the plot (a distance of 25Å, 30Å and 40Å), energy changes dramatically and tend to be mildly at the end. After reached a value, for instance, unit cell total energy at 60Å is the most realistic value in this experiment, no matter how the distance varies, it has an only tiny influence on the energy change. In real life, there is a boundary that at a certain distance which influences the unit cell total energy to its maximum value.
Figure 20. Total energy per unit cell against cell unit distance
The results showed the reasonable tendency and are relative good as expectation. From the Iridis4 output, it is obvious that the total energy changing relays on different distance. The first three points from the plot (a distance of 25Å, 30Å and 40Å), energy changes dramatically and tend to be mildly at the end. After reached a value, for instance, unit cell total energy at 60Å is the most realistic value in this experiment, no matter how the distance varies, it has an only tiny influence on the energy change. In real life, there is a boundary that at a certain distance which influences the unit cell total energy to its maximum value.
5.2 KPOINTs data analysis
After plotting the graph of total energy per cell against distance, the point of 60Å distance presented to be the most reliable number. Then I fixed the distance, and change the grid size of the platinum model. The grid size from the previous file was 7x7. Then after setting the lattices into 3x3, 5x5, 9x9 and 11x11, different unit cell energies calculated from Iridis showed in the table below.
| Grid Size | Total Energy (eV) |
| 3x3 | -56.908 |
| 5x5 | -57.930 |
| 7x7 | -59.235 |
| 9x9 | -59.406 |
| 11x11 | -59.292 |
Table 2. Analysis of OSZCAR outputs of different grid size
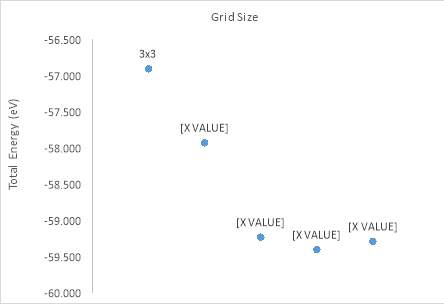 Figure 21. The unit cell total energy against the slab grid
The grid size of 7x7 tends to be the best result. By increasing the lattices size, the graph of the total energy per unit cell against grid size shows a shape closes to the periodic wave function. The size varies from 3x3 to 7x7, the energy raises up sharply while from 7x7 to 11x11, there is not much difference occurring. The larger grid size would be more expensive, so in realistic, budget consideration somehow is the basic and first factor has an effect on the experiment.
Figure 21. The unit cell total energy against the slab grid
The grid size of 7x7 tends to be the best result. By increasing the lattices size, the graph of the total energy per unit cell against grid size shows a shape closes to the periodic wave function. The size varies from 3x3 to 7x7, the energy raises up sharply while from 7x7 to 11x11, there is not much difference occurring. The larger grid size would be more expensive, so in realistic, budget consideration somehow is the basic and first factor has an effect on the experiment.
5.3 Total energy per unit cell of Pt (111) in pure water
Calculating the cell total energy change in pure water needed a longer time and more process of steps until the result came out. Results (OSZCAR) file of pure water condition compared with vacuum condition showed below.
 Figure 22. OSZCAR file of aqueous environment
Figure 22. OSZCAR file of aqueous environment
 Figure 23. OSZCAR file of vacuum environment
Comparing those two results, the cell total energy of aqueous condition is 59.229eV while of the vacuum condition is 59.235eV. The difference is tiny.
Figure 23. OSZCAR file of vacuum environment
Comparing those two results, the cell total energy of aqueous condition is 59.229eV while of the vacuum condition is 59.235eV. The difference is tiny.
5.4 Surface energy and contact angle
The relationship of the contact angel and the surface energy can be represented using Young’s equation.
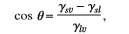 [13]
Where ϒ sv refers to the solid-vapor interfacial tension difference, ϒ sl is the solid-liquid interfacial tension while the liquid-vapor interfacial tension is represented by ϒ lv.
It is believed that measuring the contact angle can drive to the surface energy measurement. The most common way is the sessile drop method: by measuring the edge of the droplets on the surface to investigate the contact angle and so on to determine the surface free energy. That is not the only way, also there are several different approaches measuring the surface energy. F. Hejda, P Solar, and J. Kousal studied a comparison of a various approach of determining surface free energy by measuring contact angle in 2010. [28] They made a compare of the commonly used methods and listed out the advantages and disadvantages of them.
[13]
Where ϒ sv refers to the solid-vapor interfacial tension difference, ϒ sl is the solid-liquid interfacial tension while the liquid-vapor interfacial tension is represented by ϒ lv.
It is believed that measuring the contact angle can drive to the surface energy measurement. The most common way is the sessile drop method: by measuring the edge of the droplets on the surface to investigate the contact angle and so on to determine the surface free energy. That is not the only way, also there are several different approaches measuring the surface energy. F. Hejda, P Solar, and J. Kousal studied a comparison of a various approach of determining surface free energy by measuring contact angle in 2010. [28] They made a compare of the commonly used methods and listed out the advantages and disadvantages of them.
 Figure 24. Contact angle measuring instrument: DSA30 Drop Shape Analyser
(Image resource: http://www.physics.gla.ac.uk/igr/index.php?L1=capabilities&L2=equipment&L3=contact_angle)
Therefore, they confirmed that “methods using a pair of liquids give results dependent on the liquids chosen”. And they also found out “using a pair of non-polar and polar liquid yielded most reliable results. This is even more clear when a two-liquid method is transformed into a multiple-liquid method”. [28]
Figure 24. Contact angle measuring instrument: DSA30 Drop Shape Analyser
(Image resource: http://www.physics.gla.ac.uk/igr/index.php?L1=capabilities&L2=equipment&L3=contact_angle)
Therefore, they confirmed that “methods using a pair of liquids give results dependent on the liquids chosen”. And they also found out “using a pair of non-polar and polar liquid yielded most reliable results. This is even more clear when a two-liquid method is transformed into a multiple-liquid method”. [28]
5.5 Determine surface energy (using total energy and contact angle) of aqueous condition
The surface energy of liquid-vapor, ϒ lv, is 0.0728Pa which equals to 72.8 mN/m2. Converting into the unit obtained from Iridis4 and get the result of 4.5 meV/Å2.
As mentioned before, Young’s equation represents the relationship between contact angle and the surface energy.
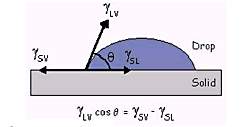 Figure 25. Contact angle and the parameters
(Image resource: http://www.cpc.tu-darmstadt.de/research_8/ongoing_projects/fluid_solid_interfaces/wettability/wettability.en.jsp)
Theoretically, the contact angle θ between liquid and gas varies in the range of 20°to 30°. [13] [31] Assumed the average value 25° to be taken into the first calculation and worked out that
ϒ sv - ϒ sl = cos25° ϒ lv
ϒ sv - ϒ sl = 0.906 x 4.5 meV/Å2
ϒ sv - ϒ sl = 4.1 meV/Å2
Figure 25. Contact angle and the parameters
(Image resource: http://www.cpc.tu-darmstadt.de/research_8/ongoing_projects/fluid_solid_interfaces/wettability/wettability.en.jsp)
Theoretically, the contact angle θ between liquid and gas varies in the range of 20°to 30°. [13] [31] Assumed the average value 25° to be taken into the first calculation and worked out that
ϒ sv - ϒ sl = cos25° ϒ lv
ϒ sv - ϒ sl = 0.906 x 4.5 meV/Å2
ϒ sv - ϒ sl = 4.1 meV/Å2

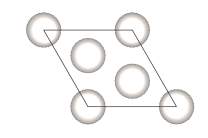
Figure 26. Parallelogram shape of Platinum bulk body The platinum simulation is of two surfaces and in parallelogram shape with 4 equal side length of 2.805Å. The plane area, A is A = 2 x 2.805Å2 x sin120° = 13.63 Å2 The number of total energy change from Iridis4, is 59.229 meV and it applies to both sides of the platinum particle. So, the unit surface energy converted is △EA=ϒsv- ϒsl= 59.229 meV13.63Å2= 4.35 meV/Å2 Comparing the theoretical value 4.1 meV/Å2and the experimental value of 4.35 meV/Å2, those two numbers are reasonable closed. Therefore, the experiment process achieved a high accuracy on data analyzing and proved to be correct.
6. Outlook and further analysis
6.1 Study of oxidation on stressed surface
It is believed that there is a connection between mechanical forces and chemical corrosion. At the macro level, applying a load to metal surface destroys it original stability and operates the deformation between atoms. Once the balance has been broken, oxygen atoms have a chance to cut into chains and act with metal producing oxides. Dr. Kramer and his co did a similar experiment attempted to investigate the oxidation on the deformed copper films. [1]
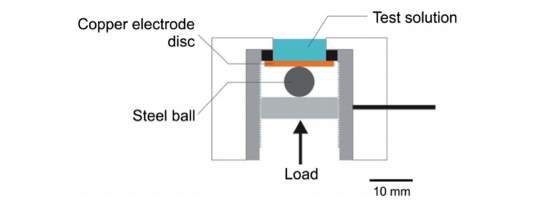 Figure 27. Cross-section view of the experimental cell set up
(Image source from Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films.)
At the same time, they used the same terminations to determine the strained surface and unstrained surface, and interestingly that the Pourbaix diagram was similar.
Figure 27. Cross-section view of the experimental cell set up
(Image source from Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films.)
At the same time, they used the same terminations to determine the strained surface and unstrained surface, and interestingly that the Pourbaix diagram was similar.
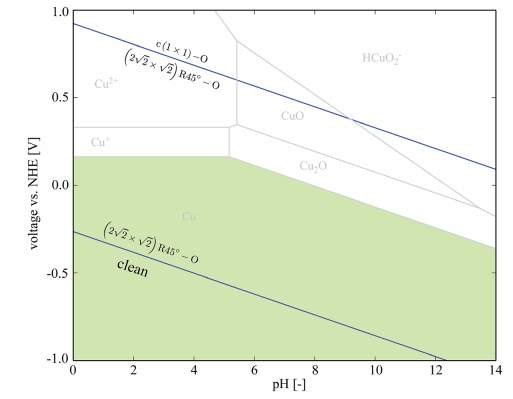 Figure 28. Pourbaix diagram for long range order unstained Cu (100)
(Image source from Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films.)
It has been agreed that the compressive stress acting on the surface is operated by the oxygen. They confirmed that “as the missing row reconstruction relieves at least part of the induced surface stress, it stands to reason that this termination benefit less from superimposing a tensile strain than the unreconstructed surface.” The energy due to the reconstruction of missing row, nevertheless, cannot fully reflect the compensate benefit to the surface stress.
Figure 28. Pourbaix diagram for long range order unstained Cu (100)
(Image source from Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films.)
It has been agreed that the compressive stress acting on the surface is operated by the oxygen. They confirmed that “as the missing row reconstruction relieves at least part of the induced surface stress, it stands to reason that this termination benefit less from superimposing a tensile strain than the unreconstructed surface.” The energy due to the reconstruction of missing row, nevertheless, cannot fully reflect the compensate benefit to the surface stress.
 Figure 29. Surface reconstruction due to the compression force (left) and tension (right) force.
(Image source from Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films.)
More recently, Jing Liu and her colleagues (2014) investigated the corrosion behaviour of T91 C-shaped martensitic steel ring under a static LBE condition and at 480 degrees. They came out to a conclusion: the oxidation of these components will be accelerated by stress in oxygen saturated LBE. And to control the oxygen corrosion, the simplest way is by controlling the oxygen content of LBE. [8]. And later, Minshu Du, Lishan Cui, Yi Cao and Allen J. Bard (2015) tested how different types of strain influence the ORR activity on Pt nanofilm with various thickness of 10nm and 5nm. They deduced that the Pt film under compressive strain reveals a more active ORR reaction then that of tensilely strained Pt nanofilm. They also explained their finding numerically that “the compressive strain of the Pt nanofilm improves the ORR activity, which reflects in a 52% enhancement of the kinetic rate constant and a 27-mV positive shift of the half-wave potential for the compressively strained 5 nm Pt compared to the pristine Pt. Conversely, the tensile strain leads to a 35% decrease of the rate constant and a 26-mV negative shift of the half-wave potential for the 5 nm Pt.” [7] As a consequence, they proved the mechanical forces does have effect on the chemical reaction mechanism.
Figure 29. Surface reconstruction due to the compression force (left) and tension (right) force.
(Image source from Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films.)
More recently, Jing Liu and her colleagues (2014) investigated the corrosion behaviour of T91 C-shaped martensitic steel ring under a static LBE condition and at 480 degrees. They came out to a conclusion: the oxidation of these components will be accelerated by stress in oxygen saturated LBE. And to control the oxygen corrosion, the simplest way is by controlling the oxygen content of LBE. [8]. And later, Minshu Du, Lishan Cui, Yi Cao and Allen J. Bard (2015) tested how different types of strain influence the ORR activity on Pt nanofilm with various thickness of 10nm and 5nm. They deduced that the Pt film under compressive strain reveals a more active ORR reaction then that of tensilely strained Pt nanofilm. They also explained their finding numerically that “the compressive strain of the Pt nanofilm improves the ORR activity, which reflects in a 52% enhancement of the kinetic rate constant and a 27-mV positive shift of the half-wave potential for the compressively strained 5 nm Pt compared to the pristine Pt. Conversely, the tensile strain leads to a 35% decrease of the rate constant and a 26-mV negative shift of the half-wave potential for the 5 nm Pt.” [7] As a consequence, they proved the mechanical forces does have effect on the chemical reaction mechanism.
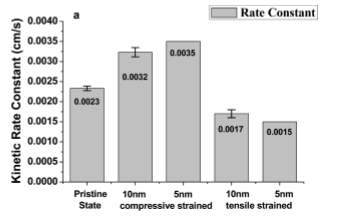 Figure 30. Comparison of the ORR activity of 10 and 5 nm Pt nanofilm under different strain types (kinetic rate constants)
(Image source from Du, M., Cui, L., Cao, Y. and Bard, A. (2015). Mechanoelectrochemical Catalysis of the Effect of Elastic Strain on a Platinum Nanofilm for the ORR Exerted by a Shape Memory Alloy Substrate.)
Figure 30. Comparison of the ORR activity of 10 and 5 nm Pt nanofilm under different strain types (kinetic rate constants)
(Image source from Du, M., Cui, L., Cao, Y. and Bard, A. (2015). Mechanoelectrochemical Catalysis of the Effect of Elastic Strain on a Platinum Nanofilm for the ORR Exerted by a Shape Memory Alloy Substrate.)
6.2 Adding oxygen adsorbates
Figure 31. simulation modelling of adding oxygen adsorbates onto the platinum surface
The last part of this project was to verify how total energy changes when adding adsorbates onto the surface. A total number of eight oxygen atoms (four on one surface) been attached to platinum and tested in both vacuum and aqueous environment.
 Figure 32. OSZCAR file of adding Oads in vacuum condition
Figure 32. OSZCAR file of adding Oads in vacuum condition
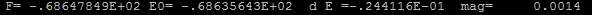 Figure 33. OSZCAR file of adding Oads in pure water condition
Total energy per unit cell of vacuum condition is 68.635671eV while of pure water condition is 68.35643eV. Interesting to find that the difference between them is ignorable. Comparing those two results with the previous results without adding adsorbates (the cell total energy of aqueous condition is 59.229eV while of the vacuum condition is 59.235eV), a dramatic jump of total energy changing happened.
Figure 33. OSZCAR file of adding Oads in pure water condition
Total energy per unit cell of vacuum condition is 68.635671eV while of pure water condition is 68.35643eV. Interesting to find that the difference between them is ignorable. Comparing those two results with the previous results without adding adsorbates (the cell total energy of aqueous condition is 59.229eV while of the vacuum condition is 59.235eV), a dramatic jump of total energy changing happened.
7. Discussion
Generally speaking, Density Functional Theory (DFT) and other ab initio methods allow the results to be highly accurate and reliable. During the first section of this project, the use of DFT method produced a series of reasonable values of total energy per unit cell with different distance between platinum layers. The tendency showed that by increasing the distance will enhance the total energy and consequently after reached the boundary value, the changing of total energy tends to be stable. Convert the total energy per unit cell into unit surface energy using contact angle produce a simple but effective method to check the theoretical value and tentative value. Obtained the contact angle of water on the platinum surface from the recent study [31] and used for converting the experimental result (the total energy per unit cell) to the surface free energy. Comparing the theoretical value 4.1 meV/Å2and the experimental value of 4.35 meV/Å2, the difference between these values are acceptable. The experimental process is believed to be the correct way of working on the calculation of total energy. Nevertheless, some of the experimental results are various from the expecting values at some stage. Last part was to determine how total energy been influenced by adding oxygen adsorbates on the surface. Supervised to fine that there was no significant change between the vacuum condition and aqueous condition. Unfortunately, not many research can indicate and explain what leads to this result. One of the main reason, this might indicate that calculations in the vacuum are transferable to the aqueous environment but further work would be needed.
8. Conclusion
Understanding the surface and structure state of platinum in different environments represents a basic way to investigating how it preforms under corrosion. Additionally, this performance indicates the importance of catalysis and in electronics. Previous literature discussed the characteristics of the oxidation and reduction happen on the platinum interface. There is a highly chance that the stress on the oxidative surface related to the metal surface reconstruction. This work produces a new determination of oxidation and reduction induced by the mechanical forces impose on a metal surface as well as in different environments. By working on calculating the surface energies, it clearly investigates the influence due to different conditions on the metal surface. Adding adsorbates of Oads acts as a considerable factor in understanding the process as well as the electrochemical behaviour of the material. Further research also needs to be involved providing alternative method identify how metal performs physically and chemically.
9. References
- Kramer, D., Wang, Y. and Wharton, J. (2015). Mechano-electrochemistry effects due to deformation of copper oxide films. Faraday Discuss., 180, pp.137-149.
- En.wikipedia.org. (2017). Redox. [online] Available at: https://en.wikipedia.org/wiki/Redox
- Science.jrank.org. (2016). Oxidation-Reduction Reaction - History. [online] Available at: http://science.jrank.org/pages/4959/Oxidation-Reduction-Reaction-History.html
- American Chemical Society. (2017). Antoine Laurent Lavoisier The Chemical Revolution - Landmark - American Chemical Society. [online] Available at: https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/lavoisier.html
- En.wikipedia.org. (2017). Valence bond theory. [online] Available at: https://en.wikipedia.org/wiki/Valence_bond_theory#History
- Mpie.de. (2017). Understanding the Mechanism of the Oxygen Reduction Reaction on Pt. [online] Available at: http://www.mpie.de/2973216/Oxygen-Reduction-Reaction-_Mechanism_
- Du, M., Cui, L., Cao, Y. and Bard, A. (2015). Mechanoelectrochemical Catalysis of the Effect of Elastic Strain on a Platinum Nanofilm for the ORR Exerted by a Shape Memory Alloy Substrate. Journal of the American Chemical Society, 137(23), pp.7397-7403.
- Liu, J., Jiang, Z., Tian, S., Huang, Q. and Liu, Y. (2016). Stress corrosion behaviour of T91 steel in static lead–bismuth eutectic at 480 °C. Journal of Nuclear Materials, 468, pp.299-304.
- Corrosion-doctors.org. (2017). Sir William Grove. [online] Available at: http://corrosion-doctors.org/Biographies/GroveBio.htm
- Nedstack.com. (2017). Fuel Cell Types. [online] Available at: http://www.nedstack.com/technology/fuel-cell-types
- Johnson, K., Kendall, K. and Roberts, A. (1971). Surface Energy and the Contact of Elastic Solids. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 324(1558), pp.301-313.
- S. Sinha, Ph.D. dissertation, University of California, 2005
- Shi, B. and Dhir, V. (2009). Molecular dynamics simulation of the contact angle of liquids on solid surfaces. The Journal of Chemical Physics, 130(3), p.034705.
- Chemistry LibreTexts. (2017). Contact Angles. [online] Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Liquids/Contact_Angles
- Chem.qmul.ac.uk. (2017). An Introduction to Surface Chemistry. [online] Available at: http://www.chem.qmul.ac.uk/surfaces/scc/
- En.wikipedia.org. (2017). Platinum. [online] Available at: https://en.wikipedia.org/wiki/Platinum
- Rsc.org. (2017). Platinum - Element information, properties and uses | Periodic Table.
- Anon, (2017). [online] Available at: https://www.reference.com/paramagnetic/platinum-extracted-da4a18e80c7e613e#
- Reference. (2017). How is platinum extracted? [online] Available at: https://www.reference.com/science/platinum-extracted-da4a18e80c7e613e
- Kruss.de. (2017). Contact angle. [online] Available at: https://www.kruss.de/services/education-theory/glossary/contact-angle/
- Chem.qmul.ac.uk. (2017). 1.6 Relaxation & Reconstruction of Surfaces. [online] Available at: http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6.htm
- S.E.Golunski. (2007). Why Use Platinum in Catalytic Converters? Platinum Metals Rev., 2007, 51, (3), 162
- Giannozzi, P. (2002). DENSITY FUNCTIONAL THEORY FOR ELECTRONIC STRUCTURE CALCULATIONS. Lecture Notes per il Corso di Struttura della Materia (Dottorato di Fisica, Universit`a di Pisa, 2002).
- Vasp.at. (2017). About VASP. [online] Available at: https://www.vasp.at/index.php/about-vasp/59-about-vasp.
- Jp-minerals.org. (2017). VESTA. [online] Available at: http://jp-minerals.org/vesta/en/
- Galeev, T., Bulgakov, N., Savelieva, G. and Popova, N. (1980). Surface properties of platinum and palladium. Reaction Kinetics and Catalysis Letters, 14(1), pp.61-65.
- Eyland, P. (2017). Lecture 8 (Surface Tension and Surface Energy). [online] Insula.com.au. Available at: http://www.insula.com.au/physics/1279/L8.html [Accessed 10 Apr. 2017].
- F. Hejda, P. Solaˇr, J. Kousal. (2010). Surface Free Energy Determination by Contact Angle Measurements – A Comparison of Various Approaches. WDS'10 Proceedings of Contributed Papers, Part III, pp.25–30.
- En.wikipedia.org. (2017). Surface energy. [online] Available at: https://en.wikipedia.org/wiki/Surface_energy
- En.wikipedia.org. (2017). Aqua regia. [online] Available at: https://simple.wikipedia.org/wiki/Aqua_regia
- KANDLIKAR, S., MARUYAMA, S., STEINKE, M. and KIMURA, T. (2001). MOLECULAR DYNAMICS SIMULATION AND MEASUREMENT OF CONTACT ANGLE OF WATER DROPLET ON A PLATINUM SURFACE. In: International Mechanical Engineering Congress and Exposition. New York: ASME.
- Nobelprize.org. (2017). The Nobel Prize in Chemistry 1992. [online] Available at: https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1992/illpres/oxidation.html
- The Balance. (2017). Which Metals Oxidize and Corrode?. [online] Available at: https://www.thebalance.com/what-is-the-definition-of-oxidized-metal-2340018
- Over, H. (2002). SURFACE CHEMISTRY: Oxidation of Metal Surfaces. Science, 297(5589), pp.2003-2005.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Organic Chemistry"
Organic chemistry is a branch of chemistry that studies the chemical composition, properties, and reactions of organic compounds that contain carbon.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




