Relationship Between Periodontal Disease and Diabetes – a Review Analysis
Info: 17647 words (71 pages) Dissertation
Published: 11th Dec 2019
THE RELATIONSHIP BETWEEN PERIODONTAL DISEASE AND DIABETES – A REVIEW ANALYSIS
ABSTRACT
As the most common chronic inflammatory disease in humans, periodontal disease affects up to 90% of the world’s population (Petersen, 2003). Periodontal disease (PD) is the most common oral disease for many adults. Its effect range from gum inflammation to serious damage to the soft tissue and bone that supports the teeth, which makes it both a prevalence and severe health problem in the U.S. Its treatment is generally a long term process which can make it costly and a fiscal burden to the patient who often lacks enough dental coverage to offset the expense. The onset of periodontal disease requires the presence of an anaerobic or acidic condition. Systemic pathologies often facilitate these pathogen-friendly and one such common and prevalent condition is diabetes mellitius. Diabetes mellitus (DM) is an endocrine disorder characterized by either cells resistance to insulin or a decreased ability of the pancreas beta cells to secrete endogenous insulin. DM is generally categorized into 2 types, Type I Diabetes and Type II Diabetes. The disorder can be the result of either of the characterized traits or a combination of them (American Diabetes Association, 2010). Outcome of either of this situation is the inability of cells to uptake and therefore utilize glucose, a factor in which exacerbate or is an indirect cause of the onset of periodontal disease in many patients. The correlations of the two diseases will be the focus of this review. Diabetes can create an acid environment (Baynes and Thorpe, 1999; Dandona et al., 1996), promote anaerobic activity (Ditzel, 1976) and the immune system cells’ functions (Salvi et al., 1997), thereby preventing the body’s defense mechanism. Current evidence proposes that insulin resistance may be a major shared metabolic abnormality linking the interaction of PD and Type II DM (Abhijit, 2012). This review proposes a causative model whereupon unstable glycemic control as a result of Type II DM promoting PD bacterial growth. In turn, chronic inflammation from PD impedes pancreatic functions by acting as a reservoir of inflammatory mediator (Li et al., 2000), which exacerbates and increases insulin resistance. Under these conditions, insulin resistance would then result in even more unstable glycemic control and thus results in a vicious cycle. Breaking this cycle is an essential component in the successful treatment of periodontal disease. Reducing insulin insensitivity and or improving glycemic control is required for treatment of these types of periodontal conditions. An understanding and analysis of the relationship of these to disease states is critical to best medical/dental practices is a focus of this review.
PERIODONTAL DISEASE
Periodontitis, a form of gum disease, affects 10-15% of adults worldwide (Petersen & Ogawa, 2005). According to the National Health and Nutrition Examination Survey (NHANES) between the years of 2009 to 2012, approximately 64.7 million people in the United States over the age of 30, have some form of periodontitis. The incidents of periodontitis were stratified across race and socioeconomic status. The most prevalence case was found greatest in the Hispanics population (63.5%), followed by non-Hispanic blacks (59.1%) and Non-Hispanic Asian Americans (50.0%). The lowest of the percentage rating were among non-Hispanic whites (40.8%). When compared, the prevalence distribution of PD between the different socioeconomic levels, those in the lowest status had double the rate in contrast to those of the highest status (Eke et al., 2015). Studies suggested the lack of dental education, funds, and access to dentists’ leads to poor oral hygiene care.
Although periodontal disease manifests as an infection in the oral cavity, increasing research over the past decade offers evidence that the pathways of inflammation links oral infection to the complete body health. Due to the link between periodontal disease and systemic health, the high prevalence of periodontal disease is a major public health concern (Seymour et al., 2007). Further research is needed because the educational value of the disease is important not just an individual’s health, but extends to the well-being of society and the economy.
The primary etiology of periodontal disease is the ineffective daily care of oral hygiene. Caused by a certain bacteria, periodontal disease is characterized by gingival inflammation due to the degradation of the fundamental supporting structures of the periodontium. The periodontium is the specialized tissues that both surround and support the teeth, maintaining them in the maxillary and mandibular bones. The periodontium consist of four major components, the gingiva (gum), the cementum, alveolar bone, and the periodontal ligament. Without proper oral treatment to save or restore the periodontium and its component, the result is tooth decay or lost.
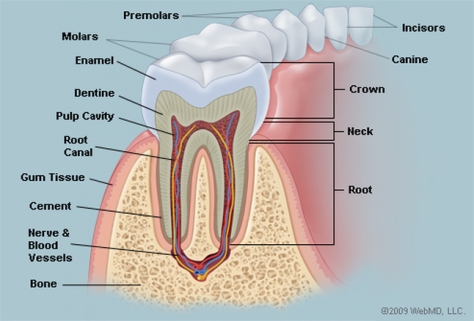
Source: http://www.webmd.com/oral-health/picture-of-the-teeth#1
The prognosis of the disease is dependent on several aspects including the profile of microorganisms, systemic background, genetic component of the host and their response (Kim & Amar, 2006). While there are other factors associated with periodontal disease, it is most commonly demonstrated through the buildup of subgingival plaque, which is a soft, adhesive, colorless film of bacteria that forms frequently on the surface of the teeth and gums (Novak & Novak, 2006). A hardened form of plaque is known as calculus or tartar. The process of plaque formation begins with a mass of bacterial growth on the surfaces of the teeth. After a period of manifestation, usually as a result of inadequate oral hygiene, the bacteria will form tartar, a brown or yellow hardening plaque, behind, in front, or between the teeth, or along the gum line. Dental plaque leads to caries, or periodontal diseases, if not treated properly.
Plaque and calculus both consist of bacteria known as periopathogens and virulence factors (Szkaradkiewicz & Karpiński, 2013). The inception of caries involves Streptococci mutans bacteria (Koo et al., 2013). These microorganisms adhere well to the surface of tooth and produce acid from glucose in much greater amounts when compared to other microorganisms in the mouth (Bowen & Koo, 2011). When the S. mutans levels in plaque are great, typically above 1%, a patient is at a high risk for caries (Loesche, 1986). The accumulation of these bacteria can cause the deterioration of periodontal tissues and surrounding alveolar bone structure leading to PD (Flemmig, 1999).
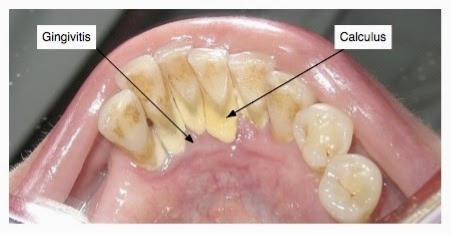
Source: http://www.thehealthyarchive.info/2015/04/remedies-to-remove-tartar-and-plaque.html
Periodontitis is frequently preceded by numerous stages of gingival inflammation commonly identified as gingivitis. Gingivitis is a mild form of periodontal disease that causes irritation, redness and inflammation around the gingiva, the part of the gum surrounding the base of the teeth (National Institute of Dental and Craniofacial Research, 2013). The most common cause of gingivitis is poor oral hygiene. Good oral hygiene habits can prevent gingivitis at the initial stage of inflammation where no bone loss has occurred (Goldstein, 2014). As observed in approximately 75% of adults living in the U.S, the primary cause of gingivitis is plaque (Albandar et al., 1999).
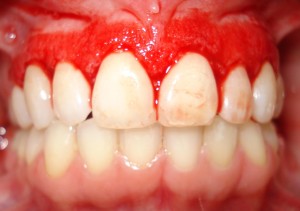
Source: http://www.romtd.com/gingivitis-causes-and-risk-factors-symptoms-diagnosis-treatment-and-natural-remedies/
When gingivitis progresses to periodontitis, the form of treatment becomes much more complicated. Treatment process of periodontitis involves specialize dental procedures and can require oral surgery. Although the advancement of periodontitis is accompanied by symptoms such as having a bad taste in the mouth and/or periodic halitosis (bad breath), individuals may also have these experience of symptoms without progression of the disease (Bosy et al., 1994). Numerous definitive signs and indicators of gingivitis include red, enlarged, tender gums that may result in bleeding upon brushing or flossing. During this disease stage, inflammation of the gingiva, more commonly known as the gums, occurs (Yamamoto & ebrary, 2011). This occurrence results from the accumulation and adherence of diverse microbes to the biofilm over time on the surface of the enamels. As the bacterial mass increases in size, there is a shift of the plaque’s microflora composition from primarily gram-positive facultative aerobes to gram-negative anaerobes. This shift in microflora composition is comprised of a complex, interacting communities that are more permanently established (Hasan & Palmer, 2014).
During the gingivitis stage, the deep tissues of the periodontium and alveolar bone are not yet affected. The connective tissue attachment to the tooth remains at its original level. Gingivitis may eventually advance to periodontal disease where the plaque-induced inflammation of the periodontal tissues is irreversible if not treated properly (Pihlstrom et al., 2005).
Subsequently, the infection leads to receding of the gum tissue around the teeth and along the root surface, revealing the roots and giving teeth an elongated, exterior arrangement (Southerland et al., 2005). As the progression of bacterial accumulation continues, formation of periodontal pockets between the teeth and gums occurs. The appearance of these periodontal pockets indicates degradation of the bone and periodontal structures that support the teeth. This process would eventually result in tooth loss (Loesche & Grossman, 2001).
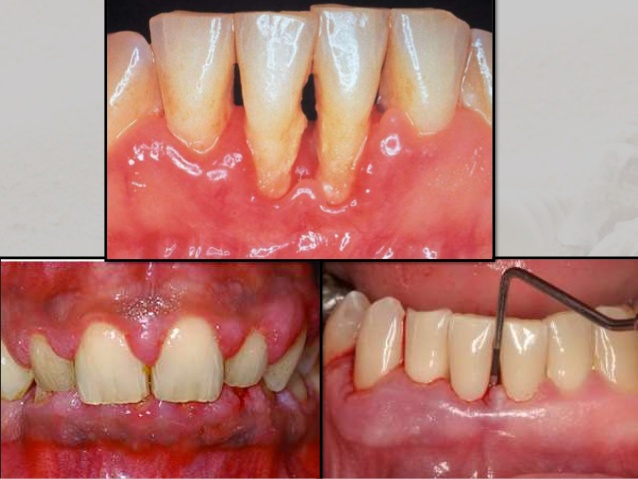
Source: https://www.google.com/search?q=chronic+periodontitis&source=lnms&tbm=isch&sa=X&ved=0ahUKEwia7InMyfrSAhWE1CYKHdrqBggQ_AUICCgB&biw=1517&bih=751#tbm=isch&q=periodontal+pocket&*&imgrc=NXeAv5jRdYNZmM:
While there are many forms of periodontitis, the two most distinctive that may occur are chronic periodontitis and aggressive periodontitis (Armitage, 1999). Chronic periodontitis is a moderate form of the disease. Some signs and symptoms of the disease may include, but not limited to, gum inflammation, deep pockets or even halitosis. This form is more commonly prevalent in adults. It is characterize by the presence of one or more teeth with a pocket formation no greater than 4 mm in depth (Brown & Loe, 1993; Brown et al., 1996). The disease involves a gradual loss of periodontal attachment (Lindhe et al., 1999). Chronic periodontitis has a slow to moderate rate of the disease progression. However, there can be periods of more rapid progression. Further complications of the moderate chronic periodontitis form can turn into aggressive periodontitis.
Aggressive periodontitis, in general, occurs more commonly in patients who are young, clinically healthy. The disease is often presented with a secondary feature form, such as inconsistency of microbial deposits on the periodontal tissues, elevated proportions of aggregatibacter actinomycemcomitans,a form of bacteria, or progression of pathogenesis (Henderson, Ward and Ready, 2010). This is typically identified by the manifestation of the gum pocket measuring greater than 6 mm (Brown & Loe, 1993; Brown et al., 1996). This can possibly lead to tooth loss in the early part of the infected individual’s life (Roshna & Nandakumar, 2012).
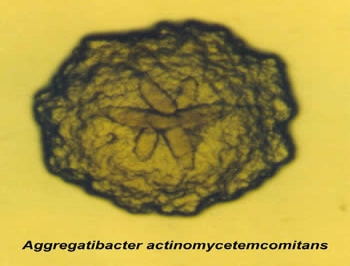
Source: https://dentistry.temple.edu/departments/periodontology-and-oral-implantology/omts-laboratory
Aggressive periodontitis is also characterized by a more advanced and destructive course. There are two different types of aggressive periodontitis, localized aggressive periodontitis and generalized aggressive periodontitis. Localized aggressive periodontitis (LAP) commonly appears around an individual’s adolescent stage of their life, more commonly during their puberty stage. LAP, signs and symptoms include, but are not limited to, how the antibody response to the infective agents. There’s the first presentation of the molar, gingival inflammation and then pocket depths. Generalized aggressive periodontitis (GAP) usually affects individuals under the age of 30. Signs and symptoms include, but are not limited to, antibody response to the infective agents, progression of periodontal destruction, and periodontal abscess. Consequently, prosthetic treatment is recommended for individuals with aggressive periodontitis (Di Febo et al., 2015).
Although both forms of periodontal disease demonstrate symptoms similar to those of gingivitis, symptoms are greater in severity due to higher accumulations of bacteria and inflammatory responses. Thus, the progression of gingivitis to periodontitis involves the aggregation of inflammation in the gingival connective tissue (Hodges 1998, p.21). However, it is important to note that not all types of periodontal disease are caused by periodontopathogenic bacteria or is distinguished by an apparent inflammatory process. This type of periodontal disease is known as non-inflammatory destructive periodontal disease (NIDPD). This result from periodontal tissue destruction associated with periodontal pocket formation. Other types of periodontal diseases are analogous to NIDPD, which shares many similar characteristics. However, there are no pathognomonic signs of inflammation (Repeke et al., 2012).
PATHOGENESIS OF PERIODONTAL DISEASE:
While it is commonly acknowledged that possible pathogenic microorganism involved in PD belongs to the normal bacteria found in the mouth (Papaioannou et al., 2009). Rescala et al. could not find any variances in the bacteriological profile between individuals with moderate chronic and aggressive periodontitis (Rescala et al., 2010). The majority of species, such as Porphyromonas gingivalis, appear to be associated with probing depth, as oppose to the aggressive or moderate chronic periodontitis specifically (Riep et al., 2009). Conversely, other researchers have indicated associations between diverse pathogens such as P. gingivalis, T. forsythia, T. denticola and chronic periodontitis (Bodet et al., 2007). As a result, the microbiological dissimilarities between the moderate chronic periodontitis and the aggressive periodontitis forms are a subject of widespread dispute (Armitage, 2010).
Nonetheless, the increased accumulation of bacteria or their products, such as lipopolysaccharides (LPS) mediates the inflammatory response associated with periodontitis through the production and release of cytokines, such as interleukins (IL) and tumor necrosis factor (TNF) (Chiang et al., 1999; Baqui et al., 1998). Cytokines are small soluble proteins synthesized by cells in order to alter the behavior or properties of another cell locally or systemically. It is essential for a variety of biological activities ranging from proliferation, to differentiation, homeostasis, regeneration, inflammation, and development, as well as repair (Okada & Murakami, 1998).
The presence of cytokines began with the pathogenesis of fever. LPS has been observed to enhance pro-inflammatory cytokine production in resident cells of periodontal tissue, such as gingival fibroblasts (Cekici et al., 2014). Furthermore, researchers have demonstrated that liposaccharides from oral microorganisms induced increases the levels of pro-inflammatory cytokines (IL-1/TNF), which resulted in aggravated recruitment of inflammatory cells (Baqui et al., 1998). This subsequent leukocytes recruitment to the diseased gingiva has been shown to induce higher rates of apoptosis in gingival fibroblast of patients with periodontitis (Koulouri et al., 1999).
Although cytokines are consisted of a broad range, there are three pro-inflammatory cytokines believed to play a crucial part in the destruction of periodontal tissue: IL-1, IL-6 and TNF-α (Palmqvist et al., 2008). The occurrence of these cytokines has been shown to greatly diminish cellular structure and function through inflammation by inducing apoptosis and impeding fibroblasts’ ability to repair damaged tissue (Graves et al., 2006).
Primarily produced by macrophages after infection, injury, or antigenic challenge, IL-1 is a polypeptide that induces a broad spectrum of systemic changes ranging from immunity and inflammation to tissue breakdown and homeostasis (Dinarello, 1988; Havemose-Poulsen & Holmstrup, 1997; Mizel, 1989; Nguyen et al., 1991; Stashenko et al., 1987; Tatakis, 1993). Under normal conditions, IL-1 is known to stimulate the proliferation of keratinocytes, fibroblasts, and endothelial cells. In addition, it enhances fibroblast synthesis of type 1 procollagen, collagenase, hyaluronate, fibronectin, and prostaglandin E2 (Okada & Murakami, 1998). However, in the presence of bacterial LPS, unrestricted production of IL-1 induces the synthesis of matrix-degrading enzymes, known as matrix metalloproteinases (MMPs), which are responsible for facilitating connective tissue destruction, leading to loss of attachment (Birkedal-Hansen et al., 1993).
Similar to IL-1, TNF-α also stimulates bone resorption and inhibit bone formation (Nguyen et al., 1991; Tatakis, 1993). The role of IL-1 and TNF in periodontal disease was investigated using the Macaca fascicularis monkey (Assuma et al., 1998). Periodontal bone reduction was induced by securing silk laces around the posterior teeth of the monkeys, thus causing an immediate inflammatory response (Assuma et al., 1998).
It has been proven that both IL and TNF play an essential part in the regulation of inflammatory progressions in the body by stimulating the production of secondary pro-inflammatory mediators to exaggerate inflammatory response (Page, 1991). An application of IL-1 and TNF blockers in lab animals had demonstrated an estimated 80% decrease in the gingival connective tissue due to inflammatory cell recruitment (Delima et al., 2002).
On the contrary, IL-6 is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. The purpose of IL-6 is to stimulate the immune system response during infections to the periodontal tissues. In addition, IL-6 can also stimulate preventive infections after a trauma, tissues burns, and damages leading to inflammation of the immune system.
Consequently, bacterial products and the subsequent inflammatory cascade stimulate osteoclastogenesis, leading to the destruction of alveolar bone (Sharma et al., 2016). Researchers also have demonstrated that gingival fibroblast exert its local osteotropic effects through production of IL-6 mediated by activation of MAP kinases. The expression of IL-6 in gingival fibroblast is further enhanced by IL-1B and TNF-α (Palmqvist et al., 2008).
Although the main cause of periodontal disease is plaque, there are numerous of other factors that can influence the healthiness of the gum. These factors includes age, education level, gender, tobacco smoking, genetics, stress, medications, environmental risk factors, clenching or grinding of the teeth, as well as other immunological diseases such as HIV infection (Imai & Ochiai, 2011; Timmerman & Van Der Weijden, 2006; Van Der Weijden & Slot, 2011). Among these influences, diabetes mellitus has been one of the greatest effects on disease progression in the study of periodontal disease (Preshaw et al., 2012).
DIABETES MELLITUS
Diabetes mellitus (DM) affects more than 18 million individuals in the United States and has extended to an endemic status affecting countless millions of people worldwide (Wild et al. 2004). According to the World Health Organization, it is currently estimated that there are 347 million people over the age of 18 who are suffering from diabetes. This number is projected to rise to approximately 439 million by 2030 (Diabetes, 2015). Base on the 2014 National Diabetes Statistics Report by The Center for Disease Control and Prevention, the estimated cost associated with diabetes in the United States in 2012 totaled $245 billion. The breakdown results being $176 billion in direct medical costs and the remaining $69 billion are from indirect costs related to disability, loss in work productivity and premature mortality (2014 National Diabetes Statistics Report, 2015).
Diabetes mellitus is classified in a group of metabolic disease in which an abnormally high concentration of sugar (glucose) levels in the blood affects bodily health over a prolonged period of time. As a result of metabolic syndrome, a condition which alter the biochemical processes in the body’s normal functioning, DM is caused by hyperglycemia (or elevated blood sugar) resulting from defective cellular activity or limited secretion of insulin (Loghmani, 2005). DM may be further complicated through poor regulation of lipid metabolism and blood protein content (Tan et al., 1997). Diabetes mellitus can also be characterized into two different types, Type I diabetes and Type II diabetes. Type I diabetes is known as insulin-dependent diabetes or juvenile diabetes. Type II diabetes is regarded as non-insulin dependent diabetes or adult onset diabetes (American Diabetes Association, 2010).
In Type I diabetes, the insulin generating beta cells in the islets of Langerhans of the pancreas are annihilated by abnormal antibodies (Loghmani, 2005). This condition, believed to be genetically inherited, leads to absolute insulin deficiency (Dean & McEntyre, 2004). In addition, Type I diabetes mellitus usually develops in childhood to young adulthood and constitutes about 5% to 10% of all cases of diabetes (Hainsworth, 2004). Type I diabetes is also responsible for greater than 90% of diabetic cases in young individual below the age of 25 (Hainsworth, 2004). Importantly to note, the older an individual is, the more likely they are to develop this form of diabetes (Kobayashi et al., 1993). This subgroup is referred to as latent autoimmune diabetes in adults (LADA) (Pollak & Vasquez, 2012). LADA is a slow, progressive form of Type I diabetes (Pollak & Vasquez, 2012).
Individuals with Type I diabetes are dependent on the supply of insulin for glycemic control and survival (Loghmani, 2005). Although the cause of diabetes may be due to interference in the autoimmune sequence, it can also be idiopathic, implicating that it can develop without a known cause (Umpierrez et al., 1995). Though genetic predisposition is the leading risk factor for the development of Type I diabetes, there appears to be an environmental influence as well (Purohit & Sharma, 2015). Nutritional intake and pathological infections may serve as a trigger to abnormal antibody responses in the body, resulting in damaging to the beta cells of the pancreas (Toeller et al., 2001; Wagenknecht et al., 1991). The onset of Type I diabetes is often abrupt as individuals become more prone to ketoacidosis and large fluctuations in plasma glucose levels (Wojcik et al., 2015). Furthermore, an acute onset of Type I diabetes manifest itself in definitive signs and symptoms to the human body. Signs and symptoms includes polyuria (excessive urine output), polydipsia (excessive thirst), polyphagia (excessive appetite), and pruritus (irritating sensation). The ideal management for Type I diabetes is control insulin injection to help maintain proper glucose balance in the body. Individuals could develop weakness and fatigue if it remains untreated (Morris, 2014).
Type II diabetes arises in individuals with the impairment of insulin function, accompanied by insulin resistance, a condition in which cells fail to responds to insulin properly (Loghmani, 2005). Non-insulin-dependent diabetes mellitus (NIDDM), or commonly known as adult-onset diabetes, is initiated by the lack of insulin development. The effect of Type II diabetes in adulthood is the progression of excessive body weight due to lack of exercise. In many circumstances of Type II diabetes, the pancreas produces abnormally large quantities of insulin (Loghmani, 2005). A key characteristic of Type II diabetes is the absence of insulin sensitivity by the cells of the body (predominantly muscle cells and lipids) (DeFronzo & Tripathy, 2009). The body produces these larger quantities of insulin in an effort to assist cells to establish and distinguish the presence of insulin (Cefalu, 2001).
In addition to the absence of insulin sensitivity leading to the increase of insulin resistance, the pancreas may malfunction, which also lead to the discharge of insulin, and causes an increase in glucose levels (Ferrannini et al., 2005). Furthermore, the liver may possibly continue to maintain glucose production in diabetic patients despite elevated glucose levels which can perpetuate the disease state (Ferrannini et al., 1990; Stone & Van Thiel, 1985).
The onset of Type II diabetes is more gradual than Type I diabetes and is often associated with obesity. Type II diabetes constitutes approximately 90-95% of all diabetes case. Several accompanying factors may include, but not limited to, an individual choice of lifestyle, such as the lack of exercise and nutritional diets. In some cases, sometimes there are genetic risk factors that may cause Type II diabetes (American Diabetes Association, 2010). The likelihood of having Type II diabetes occurs with the increase in age and lack of physical activity (American Diabetes Association, 2010). Furthermore, it is more prevalent among individuals with hypertension or dyslipidemia (Grossman & Messerli, 2008; Pardina et al., 2016). Moreover, African Americans, Hispanics, and Aboriginal individuals may have an increased 90% genetic risk of developing Type II diabetes (Egede & Dagogo-Jack, 2005; Harris et al., 1998; O’Dea, 1991; Stern & Mitchell, 1993). The management of Type II diabetes usually involves a combination of exercise and diet that includes implementation of workout routine that results in weight reduction and consumption of hypoglycemic drugs (Tuomilehto et al., 2001). However, in severe cases of Type II diabetes, insulin injections are necessary (Ohkubo et al., 1995).
Gestational diabetes mellitus (GDM) is another subcategory of diabetes with its onset or first detection occurs during pregnancy due to carbohydrate intolerance (American Diabetes Association, 2010). In a case-control study by Woman’s Hospital, Baton Rouge, studies indicate 77.4% women with gestational diabetes mellitus have periodontitis compared to 57.5% pregnant women with non-GDM. Additionally, women with GDM have a higher chance of developing Type II diabetes later in life (Kim et al. 2002).
GDM also significantly increase the risk of maternal and infant morbidity, including fetal macrosomia (significantly larger than average baby weight), preeclampsia (high blood pressure during pregnancy), preterm birth, and the need for cesarean section (Xiong et al., 2009). The fetus of GDM mother also have a greater risk of obesity and diabetes as a young adult (Catalano, 2010).
Additionally, the classification of diabetes can be under that of “other specific types” (American Diabetes Association, 2010). These consist mainly of specific genetically defined forms of diabetes and quantify diabetes associated with other diseases, such as pancreatitis or drug use (American Diabetes Association, 2010).
Complications of diabetes are associated with long-term progression of blood glucose concentrations or hyperglycemia (Hyperglycemia in Diabetes, 2015). Subsequently, diabetes results in the development of advanced glycation end-products (AGEs) (Brownlee, 2005). The accumulation of AGEs in blood plasma and in the tissues of diabetic individuals has been linked to diabetic complications (Kilpatrick et al., 2009). AGEs formed as the result of non-enzymatic glycation to proteins or lipids under hyperglycemic conditions. It primes endothelial cells and monocytes to increase sensitivity in order to manufacture more pro-inflammatory mediators (Kirsten et al., 1990).
AGE-enriched gingival tissue has superior vascular permeability, increasing the likelihood of greater collagen fiber degradation and demonstrated accelerated destruction of both bone and non-mineralized connective tissue (Lalla & D’Ambrosio, 2001). During this process, migration and phagocytic activity are also altered due to vascular obstruction resulting from the thickened endothelial basement membrane, which triggers an ‘infection-mediated’ pathway of cytokine upregulation and endorse exacerbation of the pro-inflammatory cytokines concentration (Southerland et al., 2005).
Apart from the buildup of AGEs, diabetes mellitus also shares a similar pathophysiology involving that of periodontal disease (Matthews, 2002). The accelerated development of atherosclerosis of larger blood vessels caused by diabetes mellitus creates conditions including peripheral vascular disease, coronary artery disease, and cerebrovascular disease due to insufficient blood supply (Fowler, 2008). Microvascular complication of diabetes mellitus includes retinopathy, nephropathy, and neuropathy (Control & Group, 1993). Peripheral neuropathy may be an indication of loss of neural sensation functioning in the limbs by dysesthesias or burning sensations (American Diabetes Association, 2010). Retinopathy is a disease of the retina in the eye and may lead to blindness. Nephropathy being a progressive renal disease could possibly lead to kidney failure (American Diabetes Association, 2010).
PATHOGENESIS OF DIABETIC VASCULAR DISEASE:
The human body maintains vascular homeostasis by sustaining adequate blood flow and nutrient delivery through blood vessels and anatomical tissue junctions, while preventing thrombosis and leukocyte diapedesis (Kinlay et al., 2001). Nitric oxide (NO) is one of the most important molecules synthesized by the endothelial cell to maintain this balance (Moncada & Higgs, 1993). NO synthase (eNOS) produces NO through a 5-electron oxidation of the guanidine-nitrogen terminal of L-arginine (Moncada & Higgs, 1993). Nitric oxide functions to protect the blood vessel from endogenous injury like atherosclerosis, by facilitating molecular signaling that downregulates vascular smooth muscle cell proliferation and migration (Napoli et al., 2006). In addition, NO also functions to inhibit leukocyte and platelet interaction with the vascular wall (Kubes et al., 1991; Radomski et al., 1987).
Defects of endothelium-derived NO enable the function of pro-inflammatory transcription factor nuclear factor kappa B (NF-kB) resulting in production of chemokines and cytokines and expression of leukocyte adhesion molecules (Zeiher et al., 1995). These activities stimulate vascular smooth muscle cell degradation by monocyte migration into the intima establishing resident macrophage foam cells (Grover & Luthra., 2013). This process is the initial morphological changes of atherosclerosis (Collins et al., 2001; Libby, 2000).
The bioavailability of NO represents a vital indication of vascular health, reflecting stability between tissue degradation and its production via NOS (predominantly by oxygen-derived free radicals) (Arnal et al., 1999). Many of the metabolic imbalances known to occur in diabetes such as hyperglycemia, insulin resistance, and excess free fatty acid release facilitate irregularities in endothelial cell function by affecting the degradation or synthesis of NO (King, 1996).
DISCUSSION
A bidirectional relationship occurs when two or more conditions promote one another. While there are several risk factors for periodontal disease, currently, diabetes mellitus has been recognized as the only systemic disease linked through biochemical mechanisms with periodontal disease (Grover & Luthra., 2013). Epidemiological studies have consistently shown that diabetes is associated with increased risk of periodontitis (Mealey, 2007). Type I and Type II diabetes mellitus affect the immune system and inflammatory reaction which can be detected at the indigenous and general level (Nishimura et al., 2005; Schmidt et al., 1999). Type II diabetes patients tend to have a higher propensity for periodontal disease because of the patients’ high blood glucose level, which effects glucose secretion in saliva (Aydin, 2007). This would exacerbate plaque development on the teeth, leading to gum disease.
The effect of periodontitis during pregnancy is significantly important. In order for the mother to maintain healthy oral care during pregnancy, her intake of insulin has to be properly balance. With periodontitis, the systemic inflammatory response may have a negative result to insulin resistance. Such an infection-induced insulin resistance to periodontal infection could exacerbate the preexisting pregnancy-induced insulin resistance and may cause impaired glucose tolerance and the manifestation of gestational diabetes. As a subcategory of diabetes, GDM occurs during pregnancy due to glucose intolerance, it’s the first indication of predetermined Type II diabetes later on in life, and may not affects patients during pregnancy. Although the possibilities are present for developing Type II diabetes, it does not necessarily develop after pregnancy. Additionally, there is a difference between developing periodontitis before or after the presences of GDM, but does not necessarily mean the disease is untreatable or there is a significant risk factor in treatment.
While the majority of research has concentrated on Type II diabetes, Type I diabetes however appears to have an identical correlational risk for periodontitis (Van Dyke & Dave, 2005). The magnitude of the increased risk for periodontitis is known to be dependent on the level of glycemic control, as it is with, risk of all complications of diabetes (El-Shinnawi & Soory, 2013). Thus, in well controlled diabetes with HbA1c of around 7% or lower, there appears to be little effect of diabetes on risk for periodontitis (Irani et al., 2015). Nonetheless, the risk increases exponentially as glycemic control deteriorates (Tan et al., 2015). Overall, the increased risk of periodontitis in individuals with diabetes is estimated to be between 2 to 3 fold, thereby, increases the risk for periodontitis by 2-3 times (Mealey & Ocampo, 2007; Tsai et al., 2002).
Periodontal disease is often regarded as the sixth complication of diabetes, with its incidence of severity found to be between 39% to 59.6% increase when compared to non-diabetics (“The pathogenesis of periodontal diseases,” 1999). In terms of oral manifestations, patients may experience delayed wound healing and xerostomia (dryness of the mouth syndrome), as well as an increased susceptibility to periodontal disease (Loe, 1993).
In normal salivary, its function is mediated by the muscarinic M3 receptor (Nakamura et al., 2004). Other efferent nerve signals also play a critical role in salivation. These nerves are mediated by acetylcholine, and an increase in this molecule will stimulate salivary glandular epithelial cells to increase glandular secretions in order to assist the breakdown of carbohydrates (Sharma et al., 2002). However, xerostomia occurs when there is a malfunction of the secretory capability of the salivary glands leading to a degeneration or absence of saliva in the mouth (Jellema et al., 2007; Langendijk et al., 2008; Salvi et al., 1997).
In patients with xerostomia, there can be a noticeably increased in parotid gland enlargement, inflammation and cheilitis (fissuring of the lips), tenderness or abscesses of the tongue and buccal mucosa, sialadenitis (salivary gland infection), oral candidiasis, cracking and fissuring of the oral mucosa, and halitosis (bad breath) (Bauroth et al., 2003; Mandel, 1994). In addition, the combination of microbes in a dehydrated mouth coupled with glucose consumption could also result in an elevated dental caries risk (Southerland et al., 2005). If left untreated, expansion of dental caries can be severe and widespread, which can result in infection of the tooth swelling and dental pulp (Southerland et al., 2005).
The etiology of xerostomia is associated with a non-inflammatory, non-neoplastic development of the parotid gland and is assumed to take place in 25% of patients with moderate to severe diabetes, particularly in individuals with Type I diabetes with poor metabolic control. Examination of caries and history of xerostomia may ascertain upon acquiring patients’ medical history and during examination of the oral cavity. Xerostomia would be assumed if a tongue depressor adheres to the buccal mucosa. Additionally, in females, xerostomia is presumed if lipstick sticks to the anterior of the teeth. Furthermore, the oral mucosa will appear erythematous or be sticky and dry, leading to the hypothesis that an overgrowth of Candida albicans may be present. Candida albicans, a form of yeast infection, can be white or red patches or it can be a combination of both. This fungi is frequently found on the dorsal surface of the tongue and on the soft or hard roof of the mouth (Russotto, 1981).
Patients with xerostomia frequently complain of complications with speaking, eating, swallowing, or wearing dentures (Greenspan, 1996). Dehydrated, brittle foods, such as crackers and cereals, may be predominantly problematic when it comes to chewing and swallowing. Individuals who use dentures may have difficulty with denture lesions, the tongue sticking to their palate, and denture retention. Furthermore, individuals with xerostomia often complain of a throbbing tongue (glossodynia), taste disorders (dysgeusia), and an increased requirement to drink water, particularly in the evening (Southerland et al., 2005).
Although the mechanisms that link diabetes and periodontitis are not entirely understood, it involves numerous aspects of inflammation, immune functioning, neutrophil activity, and cytokine mobility (Taylor et al., 2013). Both Type I and Type II diabetes are associated with elevated inflammation (Dandona et al., 2004). Diabetes increases inflammation in periodontal tissues with higher levels of inflammatory mediators such as interleukin-1β (IL-1β), IL-6, interferon gamma (IFNγ), tumor necrosis factor-α (TNF-α) (Engebretson et al., 2004; Salvi et al., 1997). IL-1β, IL-6, and TNF-α, known as cytokine proteins, act as insulin antagonists with the addition of TNF-α hindering lipid metabolism, while IFNγ induces apoptosis of pancreatic β cells (Souza et al., 2008). In addition, there is an increased level of C-reactive protein (CRP), a marker of inflammation which can lead to insulin resistance (Festa et al., 2000; Frohlich et al., 2000). As such, periodontal disease has been associated with higher levels of inflammatory mediators such as TNF-α in people with diabetes (El-Shinnawi & Soory, 2013). Accumulation of such reactive oxygen species (ROS), oxidative stress, the interactions between progressive glycation end products (AGEs) due to the prolonged hyperglycemic state in the periodontal tissues and their receptor end products (RAGE, the receptor for advanced glycation end products), all contribute to increased inflammation in the periodontal tissues of people with diabetes (El-Shinnawi & Soory, 2013).
High glucose concentration in oral fluids may assist microscopic organisms to propagate and establish the platform for gum disease (Preshaw & Bissett, 2013). Microbial products such as lipopolysaccharide (LPS) or endotoxin also perform a role in the propagation of the inflammatory reaction pathway via Toll-like receptors proteins (TLRs) of the human body, subsequently, promoting an inflammatory cascade as a result (Wittebole et al., 2005). These TLRs play an essential part in the innate immune response, predominantly in the early communication between phagocytic cells of the tissue macrophages, and the contaminating microorganisms, such as Porphyromonas gingivalis (Kirschning et al., 1998).
Chromosomal and biological studies have established that the toll-like receptor proteins perform a crucial role in the instantaneous reaction to infection (Akira & Sato, 2003; Takeda et al., 2003). Although LPS monocyte interactions demonstrate one of the best premeditated models of distinctive resistance, as in bacterial endotoxin and gram-negative bacteria, LPS regulates TLR protein expression (Guha & Mackman, 2001; Li et al., 2014). The underlying mechanism behind periodontal disease and this LPS-TLRs pathway is not completely understood (Cario et al., 2000; De SOUZA et al., 2012).
Firatli followed Type I diabetic individuals for healthy controls for 5 years, found that those with diabetes mellitus were twice as likely to have clinical attachment loss of the periodontal structure as nondiabetic subjects (Firatli, 1997). In an alternative cross-sectional study, diabetes proved to exaggerate all periodontal deficits, including bleeding scores, loss of attachment, and probing depth to missing teeth (Bridges et al., 1996). One study has even revealed that diabetic individuals are 5 times more likely to have moderate edentulous (a missing tooth or no teeth) than those of nondiabetic subjects (Moore et al., 1998). Thus, people with Type I and Type II diabetes appear correspondingly susceptible to tooth loss and periodontal disease.
While there are many other factors that correlate to the high prevalence of periodontal diseases in diabetic patients, this association between the two disease states is strengthened when certain populations with a genetic component such as Aboriginal and Native Americans are observed (Roberts-Thomson et al., 2014; Skrepcinski & Niendoriff, 2000). In recent study, patients with diabetes who smoked are 10 times likely to develop periodontal disease (Moore et al., 1999). The severity of periodontal disease associated to the extent of diabetes appears to have little to no association amongst both Type I and Type II diabetes (Moore et al., 1998; Sandberg et al., 2000).
THE EFFECT OF PERIODONTITIS ON DIABETES
Numerous studies have been undertaken to uncover the relationship between periodontal disease and the regulation of diabetes (Grossi et al., 1997; Faria-Almeida et al., 2006; Kiran et al., 2005). It is believed that periodontitis increases resistance and result in poor glycemic control (Genco et al., 2005). These studies state that periodontal rehabilitation could increase metabolic regulators of diabetes with good evidence to support this theory (Salto et al., 2003). A 10-year cohort study with subjects ranging from normal glucose tolerance, impaired glucose tolerance, to no glucose tolerance, was given the most support to the correlation between these two diseases (Lagervall et al., 2003; Saito et al., 2004).
An increase in mean dental pocket depth was more closely accompanied with the development of glucose intolerance from standard rank than the past glucose tolerance position itself. Thirty-three percent of the subjects with diabetes or impaired glucose tolerance at the conclusion of the study downgraded their glucose status to normal. Moreover, the percentage with normal glucose tolerance was lower in subjects with deeper pocket depths than in those with shallower pockets. Further analysis of the research shows that mechanical periodontal treatment alone improves periodontal health, but had little effect on the level of glycosylated hemoglobin, i.e. diabetes development (Lagervall et al., 2003; Saito et al., 2004). Nonetheless, the degree and interval of the development may not be clinically substantial.
Grossi and others have proposed that diminished levels of serum AGEs effect mechanisms of periodontal septicity (Grossi et al., 1997). The level of glycemic control is the greatest determining factor for periodontal impact (Teeuw et al., 2010). Non-surgical periodontal treatment (root surface debridement) eliminates bacterial plaque accumulation and reduces gingival inflammation, which could enhance the glycemic level (Faria-Almeida et al., 2006; Kiran et al., 2005; Miller et al., 1992). When diabetic individuals with good glycemic control and nondiabetic individuals were followed over the course of 3 years, it was discovered that their periodontal health was comparable to each other (Tervonen & Karjalainen, 1997). Still, research concludes that diabetic patients with poor glycemic control had additional gingival attachment loss and were more likely to demonstrate periodontal disease (Stewart et al., 2001; Westfelt et al., 1996). Thus, the irrefutable conclusion is that prevention and management of periodontal disease must be regarded as an essential part of diabetes control and vice versa (Oral health in America, 2000).
The philosophies on the management of periodontitis in diabetic individuals are analogous to those of nondiabetic individuals and consistent with the treatment standards for all high-risk individuals who already have periodontal disease (Katz et al., 1991). Foremost efforts should be centered on identifying periodontitis in individuals who are at risk for diabetes as early detection is essential in preventing possible future complications (Holm et al., 2016). Diabetic individuals with poor metabolic control should visit a medical professional more often, especially if periodontal disease is already present. Individuals with healthy controlled diabetes who have proper oral hygiene and who are on a regular periodontal maintenance schedule have the same risk of severe periodontitis as nondiabetic subjects (Mansour & Abd-Al-sada, 2005). Furthermore, all individuals should take precaution and eliminate environmental risk factor, such as smoking, to maintain healthy oral hygiene (Obradovic et al., 2012).
PERIODONTAL MANAGEMENT OF THE DIABETIC PATIENT:
There’s a weak indication from experimental trials that diabetics require more comprehensive and antagonistic periodontal treatment than non-diabetics with periodontal disease. Nonetheless, once periodontal disease is diagnosed, the diabetic patient should continue on a routine dental maintenance schedule for strict plaque control at three-month intermissions so that the periodontal conditions will remain healthy (Teeuw et al., 2010). Initial treatment of plaque removal during the early stages of PD will give the gum more of a successful chance to reattach to the teeth, therefore restoring oral health to reduce the rate of more serious periodontitis. Periodontal well-being may deteriorate more hastily in poorly regulated diabetics than in other patients, and possibly be as significant to customary treatment (Taylor et al., 1998; Tsai et al., 2002). As a result, awareness of patients’ metabolic control is imperative for diagnosis and recollection of intermissions of the disease.
For patients who do not respond well to initial treatment based on the results of microbial analysis, it may be necessary to utilize an appropriate antibiotic (Martorelli de Lima et al., 2004). Doxycycline is generally the prescription of choice due to its antimicrobial effects coupled with its ability to inhibit metalloproteinase activity and non-enzymatic glycosylation (Martorelli de Lima et al., 2004). Non-surgical periodontal therapy in conjunction with 100 mg doxycycline is linked with a mean 0.6% reduction of HbA1c in well-controlled Type II diabetic individuals (Engebretson & Hey-Hadavi, 2011). However, non-surgical periodontal therapy combined with systemic doxycycline has been shown to have no effect on HbA1c of poorly-controlled Type I diabetic patients (Llambés et al., 2012). While the improvement of HbA1c appear to be comparatively modest, nonetheless, it has a very substantial influence, as every 1% reduction in HbA1c is associated with a significantly reduced risk for diabetes complications (Stratton et al., 2000). In some cases, due to the severity of periodontitis, non-surgical treatment is deemed unsuccessful. Periodontist may then recommend surgery to help cleanse and preserve the gingival and teeth.
To avoid an episode of hyperglycemia, it is necessary for diabetic patients to schedule appointments at the time of their highest insulin activity (Lalla & D’Ambrosio, 2001). This also depends on the type of insulin used and may vary from 30 minutes to eight hours post-injection (Insulin Basics, 2015). In addition to scheduling appointments, a diabetic patient should not change their insulin regimen or diet prior to their treatment.
CONCLUSION
The association between periodontal disease and diabetes mellitus appears to be a bidirectional relationship in which they exacerbate one another’s conditions. The presentation of the elevated levels of glucose in the blood is due to the impairment of secretion or utilization of insulin. This results in hyperglycemia that affects various tissues and their functions throughout the body. The pathogenesis of periodontal disease is multifaceted. Periodontal disease is propagated by its effect on the initiation and preservation of the inflammatory process and by microbial flora and other abundant bacterial products within the oral cavity (Offenbacher et al., 2008; Van Dyke, 2008).
As previously stated, the successive host response to any bacterial or fungal infection facilitates an intricate cascade of tissue-destructive pathways. Diabetes mellitus can overstress the host reaction to the local microbial influences, such as endotoxin, causing an unusually destructive periodontal cessation (Ryan et al., 2003). Periodontal disease is correlated to hyperglycemia, in which with poorer control of the diabetic state the greater the threat of developing periodontal disease.
Various epidemiological studies have demonstrated that both Type I diabetes and Type II diabetes are predictors of periodontal disease when poorly regulated (Loe, 1993). The five indicators of diabetes are neuropathy, nephropathy, retinopathy, peripheral vascular disease and cardiovascular disease. Regular health maintenance should be monitored closely which in turns helps to identify and treat periodontal disease in susceptible patients in the early stages of the disease (Gurav, 2016). Indications show that control of periodontal infection has an influence on improvement of glycemic regulation demonstrated by a decrease in hemoglobin A-1c levels and a reduction in insulin demand (Iwamoto et al., 2001).
In addition to periodontal infection and gingival enlargement, accompanying complications of the mouth have frequently been reported in patients with diabetes such as dental caries, xerostomia, burning mouth syndrome, Candida infection, lichen planus, and even delayed wound healing (Lamster et al., 2008; Saini et al., 2010; Sandberg et al., 2000). Therefore, it can be acknowledged that DM negatively influences soft tissues, such as the microvasculature beds of the gingiva and bones, and thus affects the stability of the oral cavity.
Diabetic patients with poor metabolic control should be monitored more often, particularly those already diagnosed with periodontal disease. Patients with well-controlled diabetes who are on a regular periodontal maintenance schedule, and also have good oral hygiene care have a comparable threat of severe periodontitis as non-diabetic patients.
Overwhelming evidence supports that periodontal disease is connected with increased cardiovascular indisposition in patients with diabetes mellitus (Deshpande et al., 2008). Additional rigorous, systematic study is necessary to establish that treating periodontal disease can be an adjunct therapy to glycemic regulation, and possibly diminish the encumbrance of diabetes mellitus complications (Taylor & Borgnakke, 2008). Conclusively, it is critical to note that the regulation of periodontal disease must be reflected as a fundamental part of diabetes control. Major efforts should be made to diagnose periodontitis in patients who are at risk or diagnosed with diabetes.
REFERENCES
2014 National Diabetes Statistics Report. (2015). Retrieved April 09, 2016, from http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html
Gurav, A. N. (2012). Periodontitis and Insulin Resistance: Casual or Causal Relationship? Diabetes & Metabolism Journal, 36(6), 404–411. http://doi.org/10.4093/dmj.2012.36.6.404
Akira, S. and S. Sato (2003). “Toll-like receptors and their signaling mechanisms.” Scand J Infect Dis 35(9): 555-562.
Albandar, J. M., J. A. Brunelle and A. Kingman (1999). “Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994.” J Periodontol 70(1): 13-29.
Amar, S., N. Gokce, S. Morgan, M. Loukideli, T. E. Van Dyke and J. A. Vita (2003). “Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation.” Arterioscler Thromb Vasc Biol 23(7): 1245-1249.
American Diabetes Association. (2010). Diagnosis and Classification of Diabetes Mellitus. Diabetes Care, 33(Suppl 1), S62–S69. http://doi.org/10.2337/dc10-S062
Arigbede, A., Babatope, B., & Bamidele, M. (2012). Periodontitis and systemic diseases: A literature review. Journal of Indian Society of Periodontology J Indian Soc Periodontol, 16(4): 487-491.
Armitage, G. C. (1999). “Development of a classification system for periodontal diseases and conditions.” Ann Periodontol 4(1): 1-6.
Armitage, G. C. (2010). Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontol 2000, 53, 70-88. doi:10.1111/j.1600-0757.2010.00357.x
Arnal, J. F., A. T. Dinh-Xuan, M. Pueyo, B. Darblade and J. Rami (1999). “Endothelium-derived nitric oxide and vascular physiology and pathology.” Cell Mol Life Sci 55(8-9): 1078- 1087.
Assuma, R., T. Oates, D. Cochran, S. Amar and D. T. Graves (1998). “IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis.” J Immunol 160(1): 403- 409.
Atkinson, J. C., M. Grisius and W. Massey (2005). “Salivary hypofunction and xerostomia: diagnosis and treatment.” Dent Clin North Am 49(2): 309-326.
Aydin, S (2007). A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. J Biochem Mol Biol Jan 31 40(1): 29–35.
Bauroth, K., C. H. Charles, S. M. Mankodi, K. Simmons, Q. Zhao and L. D. Kumar (2003). “The efficacy of an essential oil antiseptic mouthrinse vs. dental floss in controlling interproximal gingivitis: a comparative study.” J Am Dent Assoc 134(3): 359-365.
Baqui, A. A. M. A., Meiller, T. F., Chon, J. J., Turng, B.-F., & Falkler, W. A. (1998). Granulocyte- Macrophage Colony-Stimulating Factor Amplification of Interleukin-1β and Tumor Necrosis Factor Alpha Production in THP-1 Human Monocytic Cells Stimulated with Lipopolysaccharide of Oral Microorganisms. Clinical and Diagnostic Laboratory Immunology, 5(3), 341–347.
Baynes, J. W. and Thorpe S. R. (1999). “Role of oxidative stress in diabetic complications: a new perspective on an old paradigm,” Diabetes, vol. 48, no. 1, pp. 1–9.
Bethin, K. E., Vogt S. K. and Muglia L. J. (2000). “Interleukin-6 is an essential, corticotropin- releasing hormone-independent stimulator of the adrenal axis during immune system activation.” Proc Natl Acad Sci U S A 97(16): 9317-9322.
Birkedal-Hansen, H., Moore, W. G., Bodden, M. K., Windsor, L. J., Birkedal-Hansen, B., DeCarlo, A., & Engler, J. A. (1993). Matrix metalloproteinases: a review. Crit Rev Oral Biol Med, 4(2), 197-250.
Bodet, C., Chandad, F., & Grenier, D. (2007). [Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis]. Pathol Biol (Paris), 55(3-4), 154-162. doi:10.1016/j.patbio.2006.07.045
Bowen, W. H., & Koo, H. (2011). Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Research, 45(1), 69–86. http://doi.org/10.1159/000324598
Bosy, A., Kulkarni, G. V., Rosenberg, M., & McCulloch, C. A. (1994). Relationship of oral malodor to periodontitis: evidence of independence in discrete subpopulations. J Periodontol, 65(1), 37-46. doi:10.1902/jop.1994.65.1.37
Bridges, R. B., J. W. Anderson, S. R. Saxe, K. Gregory and S. R. Bridges (1996). “Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors.” J Periodontol 67(11): 1185-1192.
Brown, L. J., Brunelle, J. A., & Kingman, A. (1996). Periodontal status in the United States, 1988-1991: prevalence, extent, and demographic variation. J Dent Res, 75 Spec No, 672-683.
Brown, L. J., & Loe, H. (1993). Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000, 2, 57-71.
Brownlee, M. (2005). The pathobiology of diabetic complications: a unifying mechanism. Diabetes, 54(6), 1615-1625.
Cario, E., Rosenberg, I. M., Brandwein, S. L., Beck, P. L., Reinecker, H. C., & Podolsky, D. K. (2000). Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol, 164(2), 966-972.
Catalano, P. M. (2010). The impact of gestational diabetes and maternal obesity on the mother and her offspring. J Dev Orig Health Dis, 1(4), 208-215. doi:10.1017/S2040174410000115
Cefalu, W. T. (2001). Insulin resistance: cellular and clinical concepts. Exp Biol Med (Maywood), 226(1), 13-26. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11368233
Cekici, A., Kantarci, A., Hasturk, H., & Van Dyke, T. E. (2014). Inflammatory and immune pathways in the pathogenesis of periodontal disease.Periodontology 2000, 64(1), 57–80. http://doi.org/10.1111/prd.12002
Chen, I. (2000). “The Surgeon General’s report on oral health: implications for research and education.” N Y State Dent J 66(9): 38-42.
Chiang, C. Y., Kyritsis, G., Graves, D. T., & Amar, S. (1999). Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun, 67(8), 4231-4236.
Collins, T. and M. I. Cybulsky (2001). “NF-kappaB: pivotal mediator or innocent bystander in atherogenesis?” J Clin Invest 107(3): 255-264.
Control, T. D., & Group, C. T. R. (1993). The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine, 329(14), 977-986. doi:10.1056/NEJM199309303291401
Cosentino, F., K. Hishikawa, Z. S. Katusic and T. F. Luscher (1997). “High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells.” Circulation 96(1): 25-28.
Dandona, P., A. Aljada and A. Bandyopadhyay (2004). “Inflammation: the link between insulin resistance, obesity and diabetes.” Trends Immunol 25(1): 4-7.
Dandona, P., K. Thusu, S. Cook, et al. (1996). “Oxidative damage to DNA in diabetes mellitus,” The Lancet, vol. 347, no. 8999, pp. 444–445.
De SOUZA, J. A. C., ROSSA JUNIOR, C., GARLET, G. P., NOGUEIRA, A. V. B., & CIRELLI, J. A. (2012). Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. Journal of Applied Oral Science, 20(2), 128–138. http://doi.org/10.1590/S1678- 77572012000200002
Dean L, McEntyre J. The Genetic Landscape of Diabetes [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004. Chapter 2, Genetic Factors in Type 1 Diabetes. 2004 Jul 7.Available from: http://www.ncbi.nlm.nih.gov/books/NBK1662/
DeFronzo, R. A., & Tripathy, D. (2009). Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care, 32(Suppl 2), S157–S163.
Delima, A. J., S. Karatzas, S. Amar and D. T. Graves (2002). “Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists.” J Infect Dis 186(4): 511-516.
Deshpande, A. D., Harris-Hayes, M., & Schootman, M. (2008). Epidemiology of Diabetes and Diabetes-Related Complications. Physical Therapy, 88(11), 1254–1264. http://doi.org/10.2522/ptj.20080020
Di Febo, G., Bedendo, A., Romano, F., Cairo, F., & Carnevale, G. (2015). Fixed prosthodontic treatment outcomes in the long-term management of patients with periodontal disease: a 20-year follow-up report. Int J Prosthodont, 28(3), 246-251. doi:10.11607/ijp.3995
Diabetes. (2015). Retrieved March 25, 2016, from http://www.who.int/mediacentre/factsheets/fs312/en/
Dinarello, C. A. (1988). Biology of interleukin 1. Faseb j, 2(2), 108-115.
Ditzel, J. (1976). “Oxygen transport impairment in diabetes.” Diabetes 25(2 SUUPL):832-8.
Dotson, S., R. Freeman, H. J. Failing and G. K. Adler (2008). “Hypoglycemia increases serum interleukin-6 levels in healthy men and women.” Diabetes Care 31(6): 1222-1223.
Egede, L. E., & Dagogo-Jack, S. (2005). Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am, 89(5), 949-975, viii. doi:10.1016/j.mcna.2005.03.004
Eke, P. I., Dye, B. A., Wei, L., Slade, G. D., Thornton-Evans, G. O., Borgnakke, W. S., . . . Genco, R. J. (2015). Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol, 86(5), 611-622. doi:10.1902/jop.2015.140520
El-Shinnawi, U. and M. Soory (2013). “Associations between periodontitis and systemic inflammatory diseases: response to treatment.” Recent Pat Endocr Metab Immune Drug Discov 7(3): 169-188.
Elter, J. R., C. M. Champagne, S. Offenbacher and J. D. Beck (2004). “Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease.” J Periodontol 75(6): 782-790.
Engebretson, S. P. and J. Hey-Hadavi (2011). “Sub-antimicrobial Doxycycline for Periodontitis Reduces Hemoglobin A1c in Subjects with Type II Diabetes: a Pilot Study.” Pharmacological research: the official journal of the Italian Pharmacological Society 64(6): 624- 629.
Engebretson, S. P., J. Hey-Hadavi, F. J. Ehrhardt, D. Hsu, R. S. Celenti, J. T. Grbic and I. B. Lamster (2004). “Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and Type II diabetes.” J Periodontol 75(9): 1203-1208.
Faria-Almeida, R., A. Navarro and A. Bascones (2006). “Clinical and metabolic changes after conventional treatment of Type II diabetic patients with chronic periodontitis.” J Periodontol 77(4): 591-598.
Ferrannini, E., Gastaldelli, A., Miyazaki, Y., Matsuda, M., Mari, A., & DeFronzo, R. A. (2005). Beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab, 90(1), 493-500. doi:10.1210/jc.2004-1133
Ferrannini, E., Lanfranchi, A., Rohner-Jeanrenaud, F., Manfredini, G., & Van de Werve, G. (1990). Influence of long-term diabetes on liver glycogen metabolism in the rat. Metabolism, 39(10), 1082-1088.
Festa, A., D’Agostino, R., Jr., Howard, G., Mykkanen, L., Tracy, R. P., & Haffner, S. M. (2000). Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation, 102(1), 42-47.
Firatli, E. (1997). “The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years.” J Periodontol 68(2): 136-140.
Flemmig, T. F. (1999). “Periodontitis.” Ann Periodontol 4(1): 32-38.
Foss-Freitas, M. C., N. T. Foss, E. A. Donadi and M. C. Foss (2006). “In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from Type I and Type II diabetic patients evaluated according to the metabolic control.” Ann N Y Acad Sci 1079: 177-180.
Fowler, M. J. (2008). Microvascular and Macrovascular Complications of Diabetes. Retrieved April 16, 2016, from http://clinical.diabetesjournals.org/content/26/2/77.full#ref-20
Frohlich, M., Imhof, A., Berg, G., Hutchinson, W. L., Pepys, M. B., Boeing, H., . . . Koenig, W. (2000). Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care, 23(12), 1835-1839.
Genco, R. J. and S. G. Grossi (1998). “Is estrogen deficiency a risk factor for periodontal disease?” Compend Contin Educ Dent Suppl (22): S23-29.
Genco, R. J., S. G. Grossi, A. Ho, F. Nishimura and Y. Murayama (2005). “A proposed model linking inflammation to obesity, diabetes, and periodontal infections.” J Periodontol 76(11 Suppl): 2075-2084.
Goldstein, M. V. (2014). Periodontal disease and treatment Proceedings of the NAVC Conference, 18-22 January 2014, Orlando, Florida, USA. Volume 28, Veterinary Technician and Practice Manager (pp. un-un). Gainesville; USA: North American Veterinary Community (NAVC).
Graves, D. T., Liu, R., Alikhani, M., Al-Mashat, H., & Trackman, P. C. (2006). Diabetes-enhanced inflammation and apoptosis–impact on periodontal pathology. J Dent Res, 85(1), 15-21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16373675
Greenspan, D. (1996). “Xerostomia: diagnosis and management.” Oncology (Williston Park) 10(3 Suppl): 7-11.
Grossi, S. G., F. B. Skrepcinski, T. DeCaro, D. C. Robertson, A. W. Ho, R. G. Dunford and R. J. Genco (1997). “Treatment of periodontal disease in diabetics reduces glycated hemoglobin.” J Periodontol 68(8): 713-719.
Grossman, E., & Messerli, F. H. (2008). Hypertension and diabetes. Adv Cardiol, 45, 82-106. doi:10.1159/0000115189
Grover, H. S., & Luthra, S. (2013). Molecular mechanisms involved in the bidirectional relationship between diabetes mellitus and periodontal disease. Journal of Indian Society of Periodontology, 17(3), 292–301. http://doi.org/10.4103/0972-124X.115642
Guha, M., & Mackman, N. (2001). LPS induction of gene expression in human monocytes. Cell Signal, 13(2), 85-94.
Gurav, A. N. (2016). Management of diabolical diabetes mellitus and periodontitis nexus: Are we doing enough? World Journal of Diabetes, 7(4), 50–66. http://doi.org/10.4239/wjd.v7.i4.50
Hainsworth, T. (2004). “NICE guidance on diagnosis and management of Type I diabetes.” Nurs Times 100(32): 28-29.
Harris, M. I., Klein, R., Cowie, C. C., Rowland, M., & Byrd-Holt, D. D. (1998). Is the Risk of Diabetic Retinopathy Greater in Non-Hispanic Blacks and Mexican Americans Than in Non-Hispanic Whites With Type 2 Diabetes?: A U.S. population study. Retrieved April 07, 2016, from http://care.diabetesjournals.org/content/21/8/1230.short
Hasan, A., & Palmer, R. M. (2014). A clinical guide to periodontology: pathology of periodontal disease. Br Dent J, 216(8), 457-461. doi:10.1038/sj.bdj.2014.299
Havemose-Poulsen, A., & Holmstrup, P. (1997). Factors affecting IL-1-mediated collagen metabolism by fibroblasts and the pathogenesis of periodontal disease: a review of the literature. Crit Rev Oral Biol Med, 8(2), 217-236.
Henderson, B., Ward, . J. M. and Ready, D. (2010), Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen?. Periodontology 2000, 54: 78–105. doi:10.1111/j.1600- 0757.2009.00331.x
Hodges, K. (1998). Introduction to Nonsurgical Periodontal Therapy. In Concepts in Nonsurgical Periodontal Therapy (p. 21). Cengage Learning.
Holm, N. C., Belstrom, D., Ostergaard, J. A., Schou, S., Holmstrup, P., & Grauballe, M. B. (2016). Identification of Individuals with Undiagnosed Diabetes and Pre-Diabetes in a Danish Cohort Attending Dental Treatment. J Periodontol, 87(4), 395-402. doi:10.1902/jop.2016.150266
Hujoel, P. P., M. Drangsholt, C. Spiekerman and T. A. DeRouen (2002). “Periodontitis-systemic disease associations in the presence of smoking–causal or coincidental?” Periodontol 2000 30: 51-60.
Imai, K. and K. Ochiai (2011). “Role of histone modification on transcriptional regulation and HIV-1 gene expression: possible mechanisms of periodontal diseases in AIDS progression.” Journal of Oral Science 53(1): 1-13.
Irani, F. C., R. R. Wassall and P. M. Preshaw (2015). “Impact of periodontal status on oral health- related quality of life in patients with and without Type II diabetes.” J Dent.
Iwamoto, Y., Nishimura, F., Nakagawa, M., Sugimoto, H., Shikata, K., Makino, H., . . . Murayama, Y. (2001). The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol, 72(6), 774-778. doi:10.1902/jop.2001.72.6.774
Jellema, A. P., B. J. Slotman, P. Doornaert, C. R. Leemans and J. A. Langendijk (2007). “Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer.” Int J Radiat Oncol Biol Phys 69(3): 751-760.
Katz, P. P., Wirthlin, M. R., Jr., Szpunar, S. M., Selby, J. V., Sepe, S. J., & Showstack, J. A. (1991). Epidemiology and prevention of periodontal disease in individuals with diabetes. Diabetes Care, 14(5), 375-385.
Kilpatrick, E. S., Rigby, A. S., & Atkin, S. L. (2009). The Diabetes Control and Complications Trial: the gift that keeps giving. Nat Rev Endocrinol, 5(10), 537-545. doi:10.1038/nrendo.2009.179
Kim C, Newton KM, Knopp RH. (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care, 25:1862–1868.
Kim, J., & Amar, S. (2006). Periodontal disease and systemic conditions: A bidirectional relationship. Odontology, 94(1), 10-21.
Kimble, R. B., A. B. Matayoshi, J. L. Vannice, V. T. Kung, C. Williams and R. Pacifici (1995). “Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period.” Endocrinology 136(7): 3054-3061.
King, G. L. (1996). “The role of hyperglycaemia and hyperinsulinaemia in causing vascular dysfunction in diabetes.” Ann Med 28(5): 427-432.
Kinlay, S., P. Libby and P. Ganz (2001). “Endothelial function and coronary artery disease.” Curr Opin Lipidol 12(4): 383-389.
Kiran, M., N. Arpak, E. Unsal and M. F. Erdogan (2005). “The effect of improved periodontal health on metabolic control in Type II diabetes mellitus.” J Clin Periodontol 32(3): 266-272.
Kirschning, C. J., H. Wesche, T. Merrill Ayres and M. Rothe (1998). “Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide.” J Exp Med 188(11): 2091-2097.
Kirstein, M., Brett, J., Radoff, S., Ogawa, S., Stern, D., & Vlassara, H. (1990). Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A, 87(22), 9010-9014. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2247477
Kobayashi, T., Tamemoto, K., Nakanishi, K., Kato, N., Okubo, M., Kajio, H., . . . Kosaka, K. (1993). Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care, 16(5), 780-788.
Kontani, M., Ono, H., Shibata, H., Okamura, Y., Tanaka, T., Fujiwara, T., … Hamada, S. (1996). Cysteine protease of Porphyromonas gingivalis 381 enhances binding of fimbriae to cultured human fibroblasts and matrix proteins.Infection and Immunity, 64(3), 756–762.
Koo, H., Falsetta, M. L., & Klein, M. I. (2013). The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res, 92(12), 1065-1073. doi:10.1177/0022034513504218
Koulouri, O., Lappin, D. F., Radvar, M., & Kinane, D. F. (1999). Cell division, synthetic capacity and apoptosis in periodontal lesions analysed by in situ hybridisation and immunohistochemistry. J Clin Periodontol, 26(8), 552-559.
Kubes, P., M. Suzuki and D. N. Granger (1991). “Nitric oxide: an endogenous modulator of leukocyte adhesion.” Proc Natl Acad Sci U S A 88(11): 4651-4655.
Lagervall, M., L. Jansson and J. Bergstrom (2003). “Systemic disorders in patients with periodontal disease.” J Clin Periodontol 30(4): 293-299.
Lalla, R. V. and J. A. D’Ambrosio (2001). “Dental management considerations for the patient with diabetes mellitus.” J Am Dent Assoc 132(10): 1425-1432.
Lamster, I. B., E. Lalla, W. S. Borgnakke and G. W. Taylor (2008). “The relationship between oral health and diabetes mellitus.” J Am Dent Assoc 139 Suppl: 19S-24S.
Langendijk, J. A., P. Doornaert, I. M. Verdonck-de Leeuw, C. R. Leemans, N. K. Aaronson and B. J. Slotman (2008). “Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy.” J Clin Oncol 26(22): 3770-3776.
Levato, C. M. (2005). “Caries management: a new paradigm.” Compend Contin Educ Dent 26(6A Suppl): 448-454.
Li, C., Li, B., Dong, Z., Gao, L., He, X., Liao, L., … Jin, Y. (2014). Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll-like receptor 4 mediated nuclear factor κB pathway. Stem Cell Research & Therapy, 5(3), 67. http://doi.org/10.1186/scrt456
Li, X., Kolltveit, K. M., Tronstad, L., & Olsen, I. (2000). Systemic Diseases Caused by Oral Infection. Clinical Microbiology Reviews, 13(4), 547–558.
Libby, P. (2000). “Changing concepts of atherogenesis.” J Intern Med 247(3): 349-358.
Lindhe, J., R. Ranney, I. Lamster, A. Charles, C.-P. Chung, T. Flemmig, D. Kinane, M. Listgarten, H. Löe, R. Schoor, G. Seymour and M. Somerman (1999). “Consensus Report: Chronic Periodontitis.” Annals of Periodontology 4(1): 38-38.
Llambés, F., Silvestre, F. J., Hernández-Mijares, A., Guiha, R., Bautista, D., & Caffesse, R. (2012). Efect of periodontal disease and nonsurgical periodontal treatment on C-reactive protein. Evaluation of type 1 diabetic patients. Medicina Oral, Patología Oral Y Cirugía Bucal, 17(4), e562–e568. http://doi.org/10.4317/medoral.17793
Loe, H. (1993). “Periodontal disease. The sixth complication of diabetes mellitus.” Diabetes Care 16(1): 329-334.
Loesche, W. J. (1986). Role of Streptococcus mutans in human dental decay. Microbiological Reviews, 50(4), 353–380.
Loesche, W. J., & Grossman, N. S. (2001). Periodontal Disease as a Specific, albeit Chronic, Infection: Diagnosis and Treatment. Clinical Microbiology Reviews, 14(4), 727–752. http://doi.org/10.1128/CMR.14.4.727-752.2001
Loghmani, E. (2005). Chapter 14 Diabetes Mellitis: Type 1 and Type 2 (M. Story, Ed.). In J. Stang (Ed.), Guidelines for Adolescent Nutrition Services (pp. 167-182). Minneapolis, MN.
Luo, D., Luo, Y., He, Y., Zhang, H., Zhang, R., Li, X., . . . Min, W. (2006). Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol, 169(5), 1886-1898. doi:10.2353/ajpath.2006.060603
Mandel, I. D. (1994). “Antimicrobial mouthrinses: overview and update.” J Am Dent Assoc 125 Suppl 2: 2S-10S.
Mansour, A., & Abd-Al-sada, N. (2005). Periodontal Disease among Diabetics in Iraq. Medscape General Medicine,7(3), 1-16.
Matthews, D. C. (2002). The relationship between diabetes and periodontal disease. J Can Dent Assoc, 68(3), 161-164.
Martorelli de Lima, A. F., C. C. Cury, D. B. Palioto, A. M. Duro, R. C. da Silva and L. F. Wolff (2004). “Therapy with adjunctive doxycycline local delivery in patients with Type I diabetes mellitus and periodontitis.” J Clin Periodontol 31(8): 648-653.
Mealey, B. L. and G. L. Ocampo (2007). “Diabetes mellitus and periodontal disease.” Periodontology 2000 44(1): 127-153.
Meltzer, S., L. Leiter, D. Daneman, H. C. Gerstein, D. Lau, S. Ludwig, J. F. Yale, B. Zinman and D. Lillie (1998). “1998 clinical practice guidelines for the management of diabetes in Canada. Canadian Diabetes Association.” Cmaj 159 Suppl 8: S1-29.
Miller, L. S., Manwell, M. A., Newbold, D., Reding, M. E., Rasheed, A., Blodgett, J., & Kornman, K. S. (1992). The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol, 63(10), 843-848. doi:10.1902/jop.1992.63.10.843
Mizel SB (1989). The interleukins. FASEB 1 3:2379-2388.
Mohamed, H. G., S. B. Idris, M. F. Ahmed, O. E. Boe, K. Mustafa, S. O. Ibrahim and A. N. Astrom (2013). “Association between oral health status and Type II diabetes mellitus among Sudanese adults: a matched case-control study.” PLoS One 8(12): e82158.
Moncada, S. and A. Higgs (1993). “The L-arginine-nitric oxide pathway.” N Engl J Med 329(27): 2002- 2012.
Moore, P. A., R. J. Weyant, M. B. Mongelluzzo, D. E. Myers, K. Rossie, J. Guggenheimer, H. M. Block, H. Huber and T. Orchard (1999). “Type I diabetes mellitus and oral health: assessment of periodontal disease.” J Periodonto l70 (4): 409-417.
Moore, P. A., R. J. Weyant, M. B. Mongelluzzo, D. E. Myers, K. Rossie, J. Guggenheimer, H. Hubar, H. M. Block and T. Orchard (1998). “Type I diabetes mellitus and oral health: assessment of tooth loss and edentulism.” J Public Health Dent 58(2): 135-142.
Morris, J. (2014). Type 1 Diabetes – Causes, Symptoms & Treatment | Diabetes Zone. Retrieved April 06, 2016, from http://www.diabeteszone.org/type-1-diabetes.
Nakamura, T., M. Matsui, K. Uchida, A. Futatsugi, S. Kusakawa, N. Matsumoto, K. Nakamura, T. Manabe, M. M. Taketo and K. Mikoshiba (2004). “M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice.” The Journal of Physiology 558(Pt 2): 561- 575.
Napoli, C., de Nigris, F., Williams-Ignarro, S., Pignalosa, O., Sica, V., & Ignarro, L. J. (2006). Nitric oxide and atherosclerosis: an update. Nitric Oxide, 15(4), 265-279. doi:10.1016/j.niox.2006.03.011
National Institute of Dental and Craniofacial Research. (2013). Periodontal (gum) disease: Causes, symptoms, and treatments. Retrieved Mar. 09, 2017 from https://www.nidcr.nih.gov/oralhealth/Topics/GumDiseases/PeriodontalGumDisease.htm#gingiv itis.
Nguyen, L., Dewhirst, F. E., Hauschka, P. V., & Stashenko, P. (1991). Interleukin-1 beta stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res, 10(1-2), 15-21.
Nishimura, F., Soga, Y., Iwamoto, Y., Kudo, C., & Murayama, Y. (2005). Periodontal disease as part of the insulin resistance syndrome in diabetic patients. J Int Acad Periodontol, 7(1), 16-20.
Noh, M., Jung, M., Kim, S., Lee, S., Park, K., Park, D., . . . Park, Y. (2013). Assessment of IL‑6, IL‑8 and TNF‑α levels in the gingival tissue of patients with periodontitis. Experimental and Therapeutic Medicine Exp Ther Med 6: 847-851.
Novak JM, Novak KF. Chronic periodontitis. In: Carranza FA, editor. Clinical periodontology. Philadelphia: Saunders Elsevier; 2006. pp. 494–499
Oana A. Velea, Caroline Kralev, Dan Onisei, Doina Onisei, Luminita M. Nica, Iulian P. Velea (2013). “Diabetes mellitus and periodontal disease – A two-way road: Current concepts and future considerations (Literature Review).” ESJ 9(9)
Obradovic, R., Kesic, L. J., Gasic, J., Petrovic, M., & Zivkovic, N. (2012). Role of smoking in periodontal disease among diabetic patients. West Indian Med J, 61(1), 98-101.
O’Dea, K. (1991). Westernisation, insulin resistance and diabetes in Australian aborigines. Med J Aust, 155(4), 258-264.
Offenbacher, S. and G. E. Salvi (1999). “Induction of prostaglandin release from macrophages by bacterial endotoxin.” Clin Infect Dis 28(3): 505-513.
Offenbacher, S., Barros, S. P., & Beck, J. D. (2008). Rethinking periodontal inflammation. J Periodontol, 79(8 Suppl), 1577-1584. doi:10.1902/jop.2008.080220
Ogawa, H., T. Damrongrungruang, S. Hori, K. Nouno, K. Minagawa, M. Sato and H. Miyazaki (2014). “Effect of periodontal treatment on adipokines in Type II diabetes.” World J Diabetes 5(6): 924-931.
Ohkubo, Y., Kishikawa, H., Araki, E., Miyata, T., Isami, S., Motoyoshi, S., . . . Shichiri, M. (1995). Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract, 28(2), 103-117. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7587918
Okada, H., & Murakami, S. (1998). Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med, 9(3), 248-266.
Oral health in America: a report of the surgeon general. Rockville: US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000.
Page, R. C. (1991). The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res, 26(3 Pt 2), 230-242.
Palmqvist, P., Lundberg, P., Lundgren, I., Hanstrom, L., & Lerner, U. H. (2008). IL-1beta and TNF-alpha regulate IL-6-type cytokines in gingival fibroblasts. J Dent Res, 87(6), 558-563.
Pardina, E., Ferrer, R., Rossell, J., Baena-Fustegueras, J. A., Lecube, A., Fort, J. M., . . . Peinado- Onsurbe, J. (2016). Diabetic and dyslipidaemic morbidly obese exhibit more liver alterations compared with healthy morbidly obese. BBA Clin, 5, 54-65. doi:10.1016/j.bbacli.2015.12.002
Papaioannou, W., S. Gizani, A. D. Haffajee, M. Quirynen, E. Mamai-Homata and L. Papagiannoulis (2009). “The microbiota on different oral surfaces in healthy children.” Oral Microbiol Immunol 24(3): 183-189.
Petersen, P. E. and H. Ogawa (2005). “Strengthening the prevention of periodontal disease: the WHO approach.” J Periodonto l76(12): 2187-2193.
Pihlstrom, B. L., Michalowicz, B. S., & Johnson, N. W. (2005). Periodontal diseases. The Lancet, 366(9499), 1809-1820.
Pollak, F. and T. Vasquez (2012). “[Latent autoimmune diabetes in adults].” Rev Med Chil 140(11): 1476- 1481.
Preshaw, P. M., A. L. Alba, D. Herrera, S. Jepsen, A. Konstantinidis, K. Makrilakis and R. Taylor (2012). “Periodontitis and diabetes: a two-way relationship.” Diabetologia 55(1): 21-31.
Preshaw, P. M. and S. M. Bissett (2013). “Periodontitis: oral complication of diabetes.” Endocrinol Metab Clin North Am 42(4): 849-867.
Purohit, S. and A. Sharma (2015). “Luminex and other multiplex high throughput technologies for the identification of, and host response to, environmental triggers of Type I diabetes.” 2015: 326918.
Radomski, M. W., R. M. Palmer and S. Moncada (1987). “The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium.” Biochem Biophys Res Commun 148(3): 1482-1489.
Repeke, C.E., Cardoso, C.R., Claudino, M., Silveira, E.M., Trombone, A.P.F,., Campanelli. A.P.,… Garlet, G.P. (2012). Non-inflammatory destructive periodontal disease: a clinical, microbiological, immunological and genetic investigation. Journal of Applied Oral Science, 20(1), 113–121. http://doi.org/10.1590/S1678-77572012000100020
Rescala, B., W. Rosalem, Jr., R. P. Teles, R. G. Fischer, A. D. Haffajee, S. S. Socransky, A. Gustafsson and C. M. Figueredo (2010). “Immunologic and microbiologic profiles of chronic and aggressive periodontitis subjects.” J Periodontol 81(9): 1308-1316.
Riep, B., L. Edesi-Neuss, F. Claessen, H. Skarabis, B. Ehmke, T. F. Flemmig, J. P. Bernimoulin, U. B. Gobel and A. Moter (2009). “Are putative periodontal pathogens reliable diagnostic markers?” J Clin Microbio l 47(6): 1705-1711.
Roberts-Thomson, K. F., Do, L. G., Bartold, P. M., Daniels, J., Grosse, A., & Meihubers, S. (2014). Prevalence, extent and severity of severe periodontal destruction in an urban Aboriginal and Torres Strait Islander population. Aust Dent J, 59(1), 43-47. doi:10.1111/adj.12138
Roshna, T., & Nandakumar, K. (2012). Generalized Aggressive Periodontitis and Its Treatment Options: Case Reports and Review of the Literature. Case Reports in Medicine, 2012, 1-17.
Russotto, S. B. (1981). “Asymptomatic parotid gland enlargement in diabetes mellitus.” Oral Surg Oral Med Oral Pathol 52(6): 594-598.
Ryan, M. E., O. Carnu and A. Kamer (2003). “The influence of diabetes on the periodontal tissues.” J Am Dent Assoc134 Spec No: 34s-40s.
Saini, R., S. A. Al-Maweri, D. Saini, N. M. Ismail and A. R. Ismail (2010). “Oral mucosal lesions in non oral habit diabetic patients and association of diabetes mellitus with oral precancerous lesions.” Diabetes Res Clin Pract 89(3): 320-326.
Saito, T., Y. Shimazaki, Y. Kiyohara, I. Kato, M. Kubo, M. Iida and T. Koga (2004). “The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study.” J Dent Res 83(6): 485-490.
Salvi, G. E., J. G. Collins, B. Yalda, R. R. Arnold, N. P. Lang and S. Offenbacher (1997). “Monocytic TNF alpha secretion patterns in IDDM patients with periodontal diseases.” J Clin Periodontol 24(1): 8- 16.
Salvi, G.E., J.G. Collins, B. Yalda, et al (1997). “Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus populations.” J Periodontol. 68:127–135.
Sandberg, G. E., H. E. Sundberg, C. A. Fjellstrom and K. F. Wikblad (2000). “Type II diabetes and oral health: a comparison between diabetic and non-diabetic subjects.” Diabetes Res Clin Pract 50(1): 27-34.
Sarkar, R., E. G. Meinberg, J. C. Stanley, D. Gordon and R. C. Webb (1996). “Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells.” Circ Res 78(2): 225-230.
Schmidt, M. I., Duncan, B. B., Sharrett, A. R., Lindberg, G., Savage, P. J., Offenbacher, S., . . . Heiss, G. (1999). Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet, 353(9165), 1649- 1652.
Seppala, B., T. Sorsa and J. Ainamo (1997). “Morphometric analysis of cellular and vascular changes in gingival connective tissue in long-term insulin-dependent diabetes.” J _ Periodontol 68(12): 1237-1245.
Seymour, G. J., Ford, P. J., Cullinan, M. P., Leishman, S., & Yamazaki, K. (2007). Relationship between periodontal infections and systemic disease. Clin Microbiol Infect, 13 Suppl 4, 3-10. doi:10.1111/j.1469-0691.2007.01798.x
Sharma, N. C., Galustians, H. J., Qaqish, J., Cugini, M., & Warren, P. R. (2002). The effect of two power toothbrushes on calculus and stain formation. Am J Dent, 15(2), 71-76.
Sharma, M., Jindal, R., Siddiqui, M., & Wangnoo, S. (2016). Diabetes and Periodontitis: A medical perspective. Journal of the International Clinical Dental Research Organization J Int Clin Dent Res Organ 8(1): 3-7.
Silvestre, F. J., L. Miralles, F. Llambes, D. Bautista, E. Sola-Izquierdo and A. Hernandez-Mijares (2009). “Type I diabetes mellitus and periodontal disease: relationship to different clinical variables.” Med Oral Patol Oral Cir Bucal 14(4): E175-179.
Skrepcinski, F. B. and W. J. Niendorff (2000). “Periodontal disease in American Indians and Alaska Natives.” J Public Health Dent 60 Suppl 1: 261-266.
Smith, M., G. J. Seymour and M. P. Cullinan (2010). “Histopathological features of chronic and aggressive periodontitis.” Periodontol 2000 53: 45-54.
Souza, K. L., E. Gurgul-Convey, M. Elsner and S. Lenzen (2008). “Interaction between pro- inflammatory and anti-inflammatory cytokines in insulin-producing cells.” J Endocrinol 197(1): 139-150.
Stewart, J. E., K. A. Wager, A. H. Friedlander and H. H. Zadeh (2001). “The effect of periodontal treatment on glycemic control in patients with Type II diabetes mellitus.” J Clin Periodontol 28(4): 306-310.
Stashenko, P., Dewhirst, F. E., Rooney, M. L., Desjardins, L. A., & Heeley, J. D. (1987). Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res, 2(6), 559-565. doi:10.1002/jbmr.5650020612
Stern, M. P., & Mitchell, B. D. (1993). Chapter 32: Diabetes in Hispanic Americans. In Diabetes in Hispanic Americans: Current research and educational programs (2nd ed., pp. 631-659). Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Health.
Stratton, I. M., A. I. Adler, H. A. Neil, D. R. Matthews, S. E. Manley, C. A. Cull, D. Hadden, R. C. Turner and R. R. Holman (2000). “Association of glycaemia with macrovascular and microvascular complications of Type II diabetes (UKPDS 35): prospective observational study.” Bmj 321(7258): 405-412.
Szkaradkiewicz, A. K., & Karpiński, T. M. (2013). Microbiology of chronic periodontitis. Journal of Biology and Earth Sciences, 3(1), M14-M20.
Takeda, K., T. Kaisho and S. Akira (2003). “Toll-like receptors.” Annu Rev Immunol 21: 335-376.
Tan MH, Daneman D, Lau DC, MacLean DR, Ross SA, Yale JF (1997). Diabetes in Canada: strategies towards 2000. Toronto, Ontario Canadian Diabetes Advisory Board, pp. 1- 3.
Tan, N. C., Barbier, S., Lim, W. Y., & Chia, K. S. (2015). 5-Year longitudinal study of determinants of glycemic control for multi-ethnic Asian patients with type 2 diabetes mellitus managed in primary care. Diabetes Res Clin Pract, 110(2), 218-223. doi:10.1016/j.diabres.2015.07.010
Tatakis, D. N. (1993). Interleukin-1 and bone metabolism: a review. J Periodontol, 64(5 Suppl), 416-431.
Taubitz, A., Schwarz, M., Eltrich, N., Lindenmeyer, M. T., & Vielhauer, V. (2013). Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS One, 8(7), e68167. doi:10.1371/journal.pone.0068167
Taylor, G. W., & Borgnakke, W. S. (2008). Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis, 14(3), 191-203. doi:10.1111/j.1601- 0825.2008.01442.x
Taylor, G. W., Burt, B. A., Becker, M. P., Genco, R. J., Shlossman, M., Knowler, W. C., & Pettitt, D. J. (1998). Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol, 69(1), 76-83. doi:10.1902/jop.1998.69.1.76
Taylor, J. J., P. M. Preshaw and E. Lalla (2013). “A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes.” J Clin Periodontol 40 Suppl 14: S113-134.
Teeuw, W. J., Gerdes, V. E. A., & Loos, B. G. (2010). Effect of Periodontal Treatment on Glycemic Control of Diabetic Patients: A systematic review and meta-analysis. Diabetes Care, 33(2), 421–427. http://doi.org/10.2337/dc09-1378
Tervonen, T. and K. Karjalainen (1997). “Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in Type I diabetes.” J Clin Periodontol 24(7): 505-510.
Tesseromatis, C., A. Kotsiou, H. Parara, E. Vairaktaris and M. Tsamouri (2009). “Morphological changes of gingiva in streptozotocin diabetic rats.” Int J Dent2009: 725628.
The pathogenesis of periodontal diseases. (1999). J Periodontol, 70(4), 457-470. doi:10.1902/jop.1999.70.4.457
Timmerman, M. F. and G. A. van der Weijden (2006). “Risk factors for periodontitis.” International Journal of Dental Hygiene 4(1): 2-7.
Toeller, M., Buyken, A. E., Heitkamp, G., Cathelineau, G., Ferriss, B., & Michel, G. (2001). Nutrient intakes as predictors of body weight in European people with type 1 diabetes. Int J Obes Relat Metab Disord, 25(12), 1815-1822. doi:10.1038/sj.ijo.0801816
Tsai, C., C. Hayes and G. W. Taylor (2002). “Glycemic control of Type II diabetes and severe periodontal disease in the US adult population.” Community Dentistry and Oral Epidemiology 30(3): 182-192.
Tuomilehto, J., Lindstrom, J., Eriksson, J. G., Valle, T. T., Hamalainen, H., Ilanne-Parikka, P., . . . Uusitupa, M. (2001). Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med, 344(18), 1343-1350. doi:10.1056/nejm200105033441801
Umpierrez, G. E., Casals, M. M., Gebhart, S. P., Mixon, P. S., Clark, W. S., & Phillips, L. S. (1995). Diabetic ketoacidosis in obese African-Americans. Diabetes, 44(7), 790-795. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7789647
Van Der Weijden, F. and D. E. Slot (2011). “Oral hygiene in the prevention of periodontal diseases: the evidence.” Periodontology 2000 55(1): 104-123.
Van Dyke, T. E. (2008). The management of inflammation in periodontal disease. J Periodontol, 79(8 Suppl), 1601-1608. doi:10.1902/jop.2008.080173
Van Dyke, T. E., & Dave, S. (2005). Risk Factors for Periodontitis. Journal of the International Academy of Periodontology, 7(1), 3–7.
Wagenknecht, L. E., Roseman, J. M., & Herman, W. H. (1991). Increased incidence of insulin-dependent diabetes mellitus following an epidemic of Coxsackievirus B5. Am J Epidemiol, 133(10), 1024-1031. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1852097
Westfelt, E., H. Rylander, G. Blohme, P. Jonasson and J. Lindhe (1996). “The effect of periodontal therapy in diabetics. Results after 5 years.” J Clin Periodontol 23(2): 92-100.
Wicht, M. J., R. Haak, S. Kneist and M. J. Noack (2005). “A triclosan-containing compomer reduces Lactobacillus spp. predominant in advanced carious lesions.” Dent Mater 21(9): 831-836.
Wild, S., G. Roglic, A. Green, R. Sicree and H. King (2004). “Global prevalence of diabetes: estimates for the year 2000 and projections for 2030.” Diabetes Care 27(5): 1047-1053.
Wittebole, X., S. M. Coyle, A. Kumar, M. Goshima, S. F. Lowry and S. E. Calvano (2005). “Expression of tumour necrosis factor receptor and Toll-like receptor 2 and 4 on peripheral blood leucocytes of human volunteers after endotoxin challenge: a comparison of flow cytometric light scatter and immunofluorescence gating.” Clin Exp Immunol 141(1): 99-106.
Wojcik, M., Sudacka, M., Wasyl, B., Ciechanowska, M., Nazim, J., Stelmach, M., & Starzyk, J. B. (2015). Incidence of type 1 diabetes mellitus during 26 years of observation and prevalence of diabetic ketoacidosis in the later years. Eur J Pediatr, 174(10), 1319-1324. doi:10.1007/s00431-015-2537-1
Xiong X, Elkind-Hirsch KE, Vastardis S, Delarosa RL, Pridjian G, Buekens P. PERIODONTAL DISEASE IS ASSOCIATED WITH GESTATIONAL DIABETES MELLITUS: A CASE-CONTROL STUDY. Journal of periodontology. 2009; 80(11):1742-1749. doi:10.1902/jop.2009.090250.
Yamamoto, S. L., & ebrary, I. (2011). Periodontal disease: Symptoms, treatment, and prevention. Hauppauge, NY: Nova Science.
Zeiher, A. M., B. Fisslthaler, B. Schray-Utz and R. Busse (1995). “Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells.” Circ Res 76(6): 980-986.
Zhang, Y. H., Heulsmann, A., Tondravi, M. M., Mukherjee, A., & Abu-Amer, Y. (2001). Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem, 276(1), 563-568. doi:10.1074/jbc.M008198200
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Diabetes"
Diabetes is a metabolic disorder that results in an abnormally high blood glucose level. Blood glucose levels are controlled by insulin produced by the pancreas. In diabetics, the pancreas either doesn’t produce enough (or any) insulin, or the body does not respond sufficiently to the insulin that the pancreas produces.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




