Polycystic Ovary Syndrome (PCOS) Pathophysiology, Diagnosis, Causes and Risks
Info: 4576 words (18 pages) Dissertation
Published: 16th Dec 2019
Polycystic ovary syndrome (PCOS) which impacts approximately 5–10 % of women of reproductive age, is a complicated endocrine disorder. Ovaries are stimulated to produce unnecessary quantities of male sex hormones (androgens) particularly testosterone, leading to development of polycystic ovaries. It generally presents with infrequent or irregular ovulation, usually defined as cycles of ≥36 days or <8 cycles a year and absence of ovulation, hirsutism (excessive body hair) and polycystic ovaries, together with a considerable dominance of insulin resistance. The follicles containing the oocyte usually do not mature and ovulate, causing an imbalance of hormones (Ndefo, Eaton, & Green, 2013).
In 1935, Stein and Leventhal first identified this endocrine disorder after noticing a pattern of uneven menstruation, obesity, and hirsutism, as well as cysts on the women’s ovaries. Although these symptoms were present prior to PCOS, it was not recognized and understood. Women were often looked upon as “freaks” and known as “the bearded lady,” synonymous with circus characters. (Kitzinger & Willmott, 2002). Although this condition is now known as the most common endocrine condition affecting women of reproductive age, its management and diagnosis has puzzled clinicians ever since the first time it was discovered. Various genetic, environmental and endocrinal factors make the diagnosis difficult. A universal agreement on diagnostic criteria for PCOS is yet to be determined.
Recently it was discovered that, more heart disease, diabetes, psychological issues, reproductive disorders, and cancer of the uterine lining were seen twice in women with PCOS than without the diagnosis (Hart & Doherty, 2015). The metabolic and reproductive abnormalities of PCOS have made women susceptible to infertility and endometrial cancer, demanding early diagnosis and proper treatment (Madnani, Khan, Chauhan, & Parmar, 2013). Due to the complexities of the syndrome, primary diagnosis and often needed interventions are often missed or delayed due to lack of definite diagnosis. (Dokras & Witchel, 2014).
To date, National Institutes of Health (NIH) criteria, Rotterdam criteria and Androgen Excess PCOS Society criteria have many definitions that are used for diagnosis of PCOS (Broder-Fingert, Shah, Kessler, Pawelczak, & David, 2009). Below is a table of the criteria’s for diagnosing PCOS.
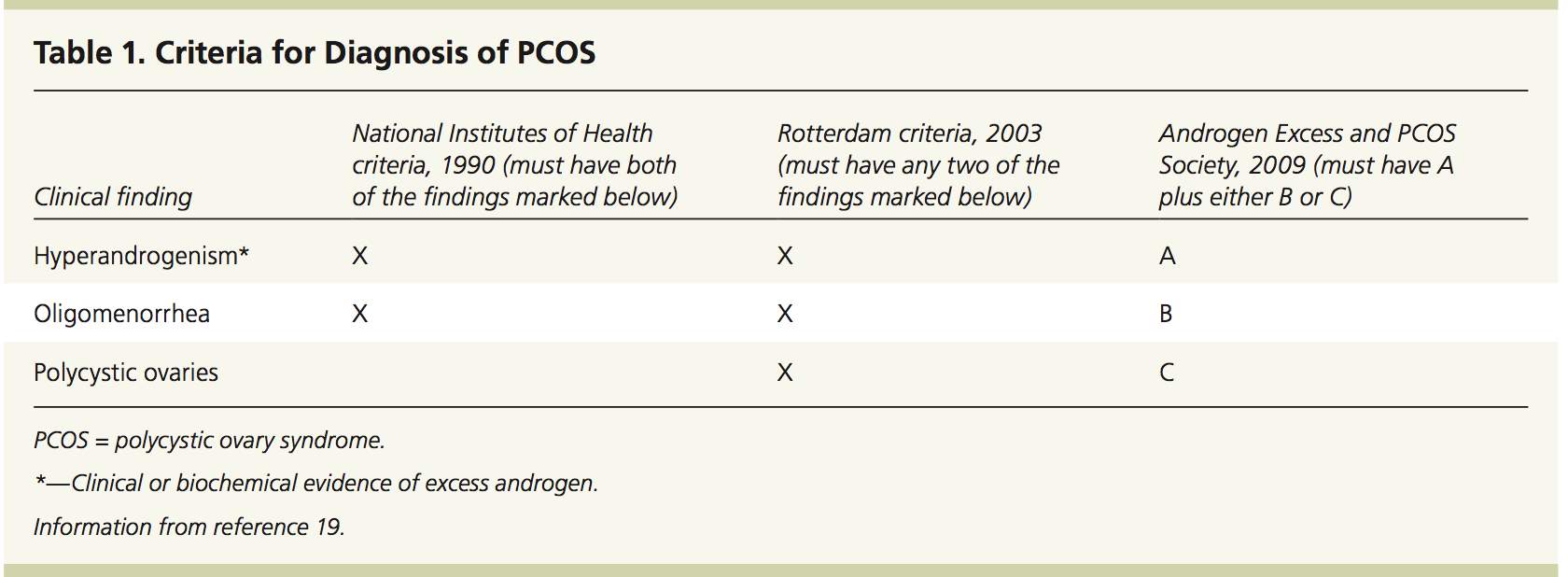
PCOS is considered a diagnosis of elimination and multiple factors have to be ruled out prior, even though the criteria offered by the different groups seem to have similarity. Various findings, such as congenital adrenal hyperplasia, nonclassic adrenal hyperplasia, Cushing syndrome, androgen-secreting tumor, idiopathic hyperandrogenism, idiopathic hirsutism, hyperprolactinemia, and thyroid disorders must be carefully looked at before the diagnosis. Insulin-resistant situations such as acromegaly have had reported case of PCOS (Teede, Deeks, & Moran, 2010). The presence of polycystic ovaries alone was not considered enough by any group as 20%–30% of otherwise normal women have sign of multiple cysts on their ovaries (Sirmans & Pate, 2013).
A huge inconsistency still exists when it comes to diagnosis, research and support. An Australian survey highlighted that PCOS takes multiple physician visits which delays the process and timely diagnosis (M. E. Gibson-Helm, Lucas, Boyle, & Teede, 2014). PCOS is one of the most serious, underserved, underfunded conditions affecting the health of women which leads it be often overlooked or a missed diagnosis. There is a lack of one agreed upon test; different sets of diagnostic criteria are still being used which needs clarity. Also, PCOS features have similarity with normal pubertal development making it hard to diagnose. Most women may seek care for their presenting symptoms from different disciplines and if a woman’s care is not coordinated, the accurate diagnosis of PCOS may not be made. Given these challenges, comprehensive care for PCOS in a multidisciplinary setting is widely advocated, but not received by most women (M. Gibson-Helm, Teede, Dunaif, & Dokras, 2017).
Early diagnosis and interventions are important to optimize treatment, improve quality of life and timely management. Awareness of the condition is not widespread and many physicians do not perform the necessary diagnostic tests or recognize that PCOS has broad and potentially devastating consequences. Proper diagnosis and management of PCOS is essential as PCOS has many potential metabolic and cardiovascular risks if not managed appropriately.It is clear that the underlying pathophysiology of PCOS is not fully understood.As a result, it is often focused on individual symptoms, not the syndrome itself. However, as the understanding of the pathophysiology of PCOS improves, so does its management. Although it should be individualized, focusing on all metabolic consequences and decreasing future complications is crucial. More extensive research and understanding of the pathophysiology of PCOS will help improve managements success (Sirmans & Pate, 2013).
According to the non-profit support organization, PCOS Challenge, Inc. (2017), PCOS awareness and support organizations receive less than 0.1 percent of the government, corporate, foundation, and community funding that other health conditions receive. Given that the number of women affected worldwide by PCOS is significant, more needs to be done to increase funding for research, identify targeted treatments and make diagnosing simple.
MCDB Biology Literature Review-
Background
Polycystic ovary syndrome (PCOS) is a common hormonal disorder among girls and women during their reproductive years. Normally, women make small amounts of “male” hormones (called androgens), such as testosterone, but women with PCOS produce slightly higher amounts. This hormone imbalance causes an assortment of health problems, such as irregular menstrual periods, too much hair on the body and face (hirsutism), and a very large number of follicles (small fluid-filled sacs where eggs develop) on the ovaries. These many follicles look like cysts, which is where the term “polycystic” comes from (Hoeger, Legro & Welt, 2014).
Other comorbidities include obesity, insulin resistance, and a higher risk for diabetes, cardiovascular disease, gestational diabetes, infertility, and metabolic syndrome (Gourgari et al., 2015).
Pathophysiology
The pathophysiology of PCOS appears to be ‘multi- factorial and polygenic’. Both nature and nurture seem to play a role in contributing to PCOS. It has been found to cluster in families, particular in those with history of polycystic ovaries (PCO), non-insulin dependent diabetes mellitus, cardiovascular disease (CVD) and breast cancer. Meanwhile, excessive intake of excitatory amino acids that can affect the pituitary regulation of the ovary cycles such as monosodium glutamate (MSG), exposure to endocrine-disrupting chemicals such as pesticides, and sedentary lifestyle are also believed to result in PCOS. The potential pathophysiologic mechanism leading to the classic PCOS phenotypes in adolescents appear to coincide with normal physiological changes seen in the transition to puberty, which are ‘maturation of luteinizing hormone (LH) secretion pattern, onset of an adult pattern of insulin resistance, increase in adrenal androgen production and body mass (Yii et al., 2009).
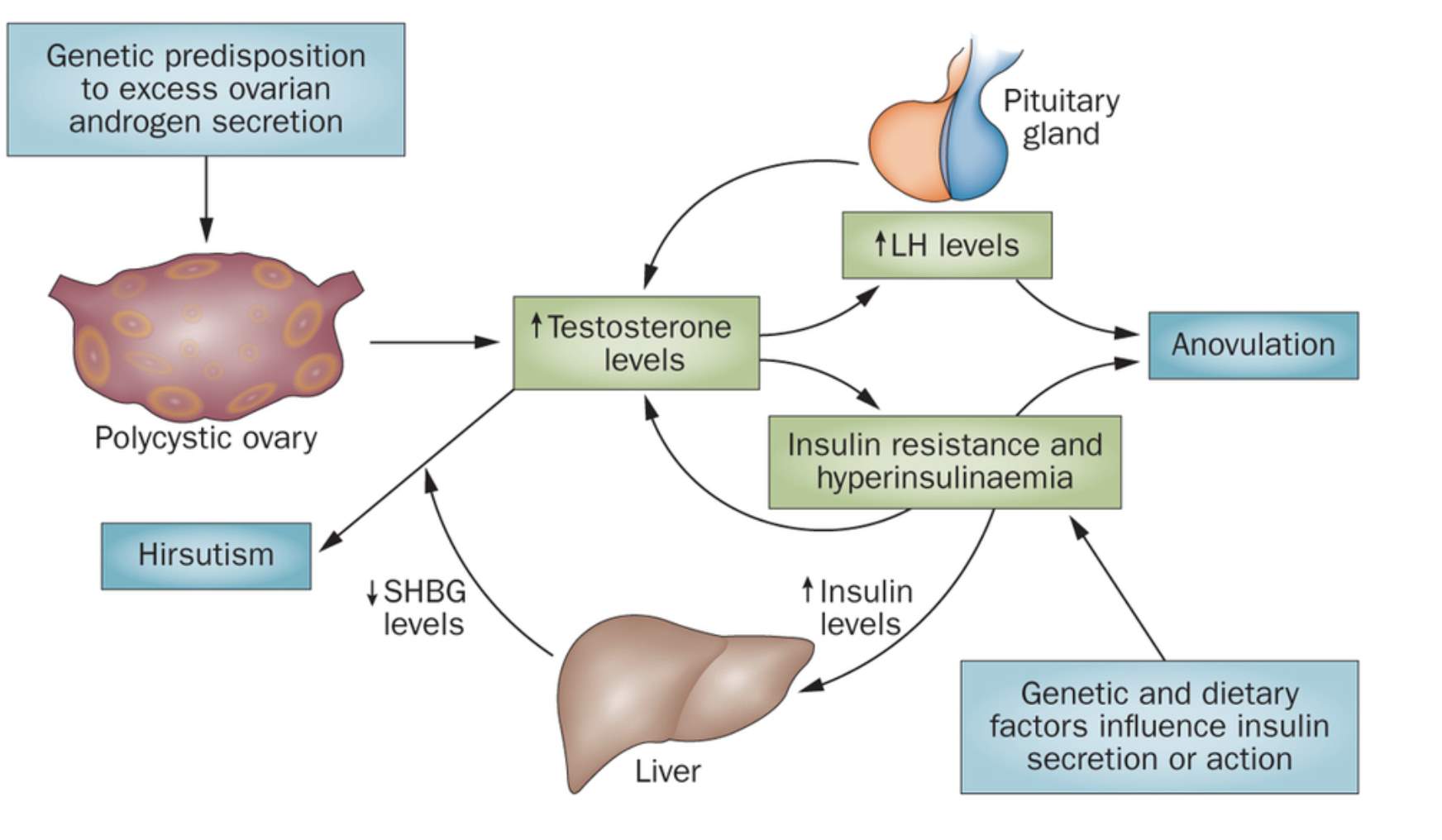
In normal women, the adrenal glands and ovaries secrete androgens in response to ACTH and LH, respectively. Approximately half of the androgen production stems from direct secretion and half from enzymes peripherally converting 17-ketosteroids into androstenedione (predominantly) in skin, liver, and adipose tissue. The hypothalamic-pituitary axis does not directly regulate androgen production in the adrenal glands, and intraglandular autocrine and paracrine factors also influence androgen production throughout the body. In women with PCOS, the ovaries are the main source of androgen, but in some 30–50% of individuals who show enhanced 17-ketosteroid responses to ACTH the adrenal gland contribute (Dumesic et al., 2015). Androgens are usually associated with “male hormones” because they are seen in greater quantities in males, although a small amount of these are needed by women in order to carry out certain vital tasks such as building and maintaining bone and muscle mass. Biochemical features of PCOS include elevated androgens, particularly testosterone, luteinizing hormone (LH), estrogen, insulin levels and decreased Sex-hormone binding globulin (SHBG) levels (Tsilchorozidou, Overton, & Conway, 2004)
Feedback disturbances in the hypothalamus-hypophysis-ovary axis (HHOA) are another typical feature of PCOS, with increased frequency and amplitude of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) pulsatile secretion. Higher levels of this hormone induce greater androgen synthesis in ovarian theca cells (TC). In turn, hyperandrogenemia induces a decrease in feedback sensitivity to both estradiol and progesterone in gonadotropic hypothalamic cells, reinforcing GnRH and LH hypersecretion. This represents the first of many self-perpetuating pathophysiologic cycles in which hyperandrogenemia plays a pivotal role in the development and progression of PCOS, while simultaneously warranting the presence of the clinical manifestations. The constant growth of follicles, along with nonselection of a dominant unit, leads to the hyperstimulation of several of these structures, hence the alternative proposed denomination of “polyfollicular ovary syndrome”, which maintains all the characteristic hormonal imbalances (Rojas et al., 2014).
Diagnosis
Despite the high prevalence, PCOS is an under recognized condition, and many women remain undiagnosed (Gibson-Helm et al., 2016). Diagnosis commonly requires at least 2 of the 3 following features: polycystic ovaries on ultrasound, biochemical/clinical hyperandrogenism, and oligo/amenorrhea, with exclusion of other etiologies. Undiagnosed women with PCOS may seek care from the dermatologist for hirsutism and acne; from the primary care physician for isolated dyslipidemia, obesity, or impaired glucose tolerance; and from the mental health specialist for anxiety and depression. In the absence of communication among these healthcare providers, PCOS may remain undetected, and although some symptoms are treated, the patient may not receive counseling or screening for gynecological, metabolic, or psychiatric problems. Incomplete disclosure from the patient compounded with targeted evaluation by the healthcare provider may lead to PCOS diagnosis getting missed (Dokras & Witchel, 2014).
Not all women with PCOS have polycystic ovaries, nor do all women with ovarian cysts have PCOS. Although pelvic ultrasound is a major diagnostic tool, it is not the only one. Many definitions are used for diagnosis of PCOS such as National Institutes of Health (NIH) criteria, Rotterdam criteria and Androgen Excess PCOS Society criteria.
NIH criteria: In 1990, a workshop sponsored by the NIH suggested that a patient has PCOS if she has oligoovulation, signs of androgen excess (clinical or biochemical) and other entities are excluded that would cause polycystic ovaries.
Rotterdam criteria: In 2003, a consensus workshop held in Rotterdam indicated PCOS to be present if any 2 out of 3 criteria are met including oligoovulation and/or anovulation, excess androgen activity and polycystic ovaries (By gynecologic ultrasound).
The Rotterdam definition is wider, including many more patients, most notably patients without androgen excess. Critics say that findings obtained from the study of patients with androgen excess cannot necessarily be extrapolated to patients without androgen excess.
Androgen excess PCOS Society criteria: In 2006, the Androgen Excess PCOS Society suggested a tightening of the diagnostic criteria to all of the following including excess androgen activity, oligoovulation/ anovulation, polycystic ovaries and other entities are excluded that would cause excess androgen activity (Kabel, 2016).
The goal of further evaluation of suspected PCOS is twofold: to exclude other treatable conditions that can mimic PCOS and to detect and treat long-term metabolic complications. Anovulation is common after menarche, so it is reasonable to delay workup for PCOS in adolescents until they have been oligomenorrheic for at least two years (Carmina, Oberfield, & Lobo, 2010).
Simple tests can be performed to exclude disorders such as hyperprolactinemia (causing anovulation), nonclassic congenital adrenal hyperplasia (causing excess androgen production), Cushing’s syndrome (causing all three diagnostic criteria as well as insulin resistance), and the presence of any androgen-secreting tumors (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop, 2003).
Accurate diagnosis of PCOS is of critical importance to public health, given the chronic nature of the disorder and its association with multiple health consequences. Currently the ESHRE/ASRM or Rotterdam criteria are the agreed international diagnostic criteria for PCOS, although further research is needed for its unclear etiology and mechanisms of insulin resistance. It can be seen that despite all of these various methods of assessing PCOS, there is a lack of any solid, objective test that that can provide an absolute affirmative diagnosis at this time. More research is needed to clarify the complex pathophysiology of PCOS.
Cause
Although the genetic etiology of PCOS remains unknown, a family history of PCOS is relatively common. However, none of the existing family studies of PCOS convincingly establishes a mode of inheritance either because the number of families studied was too small; the parental phenotypes could not be firmly established; and/ or the male phenotype is uncertain. Despite the heterogeneity in study design and the inability to obtain comprehensive phenotype information to permit a formal segregation analysis, collectively the existing literature strongly suggests the clustering of PCOS in families with a mode of inheritance suggestive of an autosomal dominant pattern (Diamanti-Kandarakis, Kandarakis, & Legro, 2006).
While increasing evidences over many years point to familial aggregation of women with PCOS, the model of inheritance of PCOS has not yet been defined. The main candidate genes are those encoding for factors involved in the synthesis, transport, regulation and effects of androgens. Other candidate genes are those encoding for factors involved in insulin metabolism, such as insulin receptors, signaling cascade proteins responsible for binding of insulin to its receptor, IGF system, other growth factors and the gene encoding for Calpain-10 enzyme, responsible for insulin secretion and action (Franks, McCarthy, & Hardy, 2006).
There is increasing evidence suggesting that PCOS affects the whole life of a woman, can begin in utero in genetically predisposed subjects, it manifests clinically at puberty, continues during the reproductive years. It can also expose patients to increased risk of cardiovascular disease, hypertension, diabetes and other metabolic complications, especially after menopause. During the fertile period, it may cause anovulatory infertility and could be associated with increased prevalence of gestational complications, such as miscarriage, gestational diabetes and preeclampsia (De Leo, La Marca, & Petraglia, 2003). Early diagnosis is therefore fundamental by enabling close follow-up and in an attempt to reduce the risk of such gestation complications.
Environmental factors have been shown to play a role in the pathogenesis of PCOS. There have been several studies observing the role of socio-economic status (SES) and unhealthy behavior, including smoking, poor diet, and lack of exercise (Graham & Der, 1999; Barkley, 2008). Although the prevalence of PCOS is similar in all countries, ethnic factors influence the phenotypic manifestations of the syndrome. The prevalence of PCOS among Caucasian women, varies from 4.7 % in Alabama, to 6.5 % in Spain and 6.8 % in Greece (Leo et al., 2016).
There is a fundamental need for more research regarding the pathogenesis of PCOS in order to identify the underlying causes. In addition to the challenges inherent to gene discovery for any common disease, there are several notable factors that further complicate research into the genetic basis of PCOS. These include the multiple diagnostic criteria and heterogeneity of PCOS and the fact that PCOS can be diagnosed only in women of reproductive age. As noted above, diagnosing premenarchal girls, adolescent girls, and menopausal women is problematic. Furthermore, there is no accepted male phenotype, although males do appear to have androgen-related and metabolic dysfunction. Also, PCOS impairs fertility or delays fertility, which reduces family size. Therefore, the availability of relatives for family-based genetic studies is limited (Dumesic et al., 2015).
The genetic evaluation of PCOS could be the gateway to many other novel areas of research. Since researchers are perplexed by the rapid evolution of the disease, the identification of genomic loci would give considerable insight. The connection between PCOS and male relatives, a contentious topic, could be better understood with the advancement of genetic analysis. These two areas require a fundamental basis upon which to build theories in order to expand our knowledge on the etiology of the disease. These discoveries would also help create a novel treatment or cure. The indefinite diagnostic criteria in addition to its immense intricacy make PCOS a challenging area of research (Barthelmess & Naz, 2014).
Risks
Women with PCOS may therefore present with a variety of serious clinical complications including psychological problems (reduced quality of life, poor self-esteem, depression, anxiety), reproductive manifestations (hirsutism, infertility and pregnancy complications), and metabolic implications (insulin resistance, metabolic syndrome, impaired glucose tolerance, diabetes mellitus 2 and potentially cardiovascular disease). Given the heterogeneous nature of PCOS and the spectrum of clinical features, presentation can vary across the life cycle. PCOS is a chronic condition with psychological and reproductive manifestations usually beginning in adolescence then transitioning to include infertility and increasing metabolic complications over time. However, when combined with obesity, metabolic implications of PCOS such as impaired glucose tolerance, DM2 and the metabolic syndrome can present in adolescence (Teede et al., 2010).
Women with PCOS have been presented a greater risk of endometrial cancer, which is related to the estrogen increase and the high prevalence of anovulatory cycles which favor the endometrial hyperplasia, as it is already known. The overweight is also linked to this type of cancer and represents, therefore, an additional risk factor for endometrial cancer in these patients (Kaaks, Lukanova, & Kurzer, 2002).
After PCOS is diagnosed, studies show that more than 50% of patients develop prediabetes or diabetes, and there is an increased risk of myocardial infarction (MI), dyslipidemia, hypertension, anxiety, depression, endometrial cancer, and sleep apnea.Moreover, pregnant women with PCOS should be informed of the increased rates of miscarriage, gestational diabetes, pre-eclampsia, and premature delivery (Ndefo et al., 2013).
References:
Barthelmess, E. K., & Naz, R. K. (2014). Polycystic ovary syndrome: current status and future perspective. Frontiers in Bioscience (Elite Edition), 6, 104–19. https://doi.org/10.2741/E695
Broder-Fingert, S., Shah, B., Kessler, M., Pawelczak, M., & David, R. (2009). Evaluation of adolescents for polycystic ovary syndrome in an urban population. JCRPE Journal of Clinical Research in Pediatric Endocrinology, 1(4), 188–193. https://doi.org/10.4008/jcrpe.v1i4.50
Carmina, E., Oberfield, S. E., & Lobo, R. A. (2010). The diagnosis of polycystic ovary syndrome in adolescents. American Journal of Obstetrics and Gynecology, 203(3). https://doi.org/10.1016/j.ajog.2010.03.008
De Leo, V., La Marca, A., & Petraglia, F. (2003). Insulin-Lowering Agents in the Management of Polycystic Ovary Syndrome. Endocrine Reviews, 24(5), 633–667. https://doi.org/10.1210/er.2002-0015
Diamanti-Kandarakis, E., Kandarakis, H., & Legro, R. S. (2006). The role of genes and environment in the etiology of PCOS. Endocrine, 30(1), 19–26. https://doi.org/10.1385/ENDO:30:1:19
Dokras, A., & Witchel, S. F. (2014). Are Young Adult Women With Polycystic Ovary Syndrome Slipping Through the Healthcare Cracks? The Journal of Clinical Endocrinology & Metabolism, 99(5), 1583–1585. https://doi.org/10.1210/jc.2013-4190
Dowdy, D. (2012). Emotional Needs of Teens With Polycystic Ovary Syndrome. Journal of Pediatric Nursing, 27(1), 55–64. https://doi.org/10.1016/j.pedn.2010.08.001
Dumesic, D. A., Oberfield, S. E., Stener-Victorin, E., Marshall, J. C., Laven, J. S., & Legro, R. S. (2015). Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocrine Reviews, 36(5), 487–525. https://doi.org/10.1210/er.2015-1018
Franks, S., McCarthy, M. I., & Hardy, K. (2006). Development of polycystic ovary syndrome: involvement of genetic and environmental factors. International Journal of Andrology, 29(1), 278–285. https://doi.org/10.1111/j.1365-2605.2005.00623.x
Gibson-Helm, M. E., Lucas, I. M., Boyle, J. A., & Teede, H. J. (2014). Women’s experiences of polycystic ovary syndrome diagnosis. Family Practice, 31(5), 545–549. https://doi.org/10.1093/fampra/cmu028
Gibson-Helm, M., Teede, H., Dunaif, A., & Dokras, A. (2017). Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism, 102(2), 604–612. https://doi.org/10.1210/jc.2016-2963
Gourgari, E., Lodish, M., Shamburek, R., Keil, M., Wesley, R., Walter, M., … Stratakis, C. A. (2015). Lipoprotein particles in adolescents and young women with PCOS provide insights into their cardiovascular risk. Translational Endocrinology and Metabolism, 100(11), 4291–4298. https://doi.org/10.1210/jc.2015-2566
Hahn, S., Janssen, O. E., Tan, S., Pleger, K., Mann, K., Schedlowski, M., … Elsenbruch, S. (2005). Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. European Journal of Endocrinology. https://doi.org/10.1530/eje.1.02024
Hart, R., & Doherty, D. A. (2015). The Potential Implications of a PCOS Diagnosis on a Woman’s Long-Term Health Using Data Linkage. The Journal of Clinical Endocrinology & Metabolism, 100(3), 911–919. https://doi.org/10.1210/jc.2014-3886
Kaaks, R., Lukanova, A., & Kurzer, M. S. (2002). Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiology Biomarkers and Prevention, 11(12), 1531–1543. https://doi.org/10.1016/j.jmig.2012.05.009
Kabel, A. M. (2016). Polycystic Ovarian Syndrome : Insights into Pathogenesis , Diagnosis ,prognosis,pharmalogical and non-pharmalogical treatment. Journal of Pharmacological Report, 1(1), 1–3.
Kitzinger, C., & Willmott, J. (2002). “The thief of womanhood”: Women’s experience of polycystic ovarian syndrome. Social Science and Medicine, 54(3), 349–361. https://doi.org/10.1016/S0277-9536(01)00034-X
M.M., N., B., H., S., B., A., S., B., B., M.M., A., & A., S. (2013). Animal models of human PCOS, valuable tools for the diagnosis and treatment. Iranian Journal of Reproductive Medicine, 11, 35. Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L72055590%5Cnhttp://www.ssu.ac.ir/ijrm/index.php/ijrm/article/view/61/42%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=16806433&id=doi:&atitle=Animal+models+of+human+PCOS%2C+va
Madnani, N., Khan, K., Chauhan, P., & Parmar, G. (2013). Polycystic ovarian syndrome. Indian Journal of Dermatology, Venereology, and Leprology. https://doi.org/10.4103/0378-6323.110759
Mobeen, H., Afzal, N., & Kashif, M. (2016). Polycystic Ovary Syndrome May Be an Autoimmune Disorder. Scientifica. https://doi.org/10.1155/2016/4071735
Ndefo, U. A., Eaton, A., & Green, M. R. (2013). Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P & T : A Peer-Reviewed Journal for Formulary Management, 38(6), 336–55.
Rojas, J., Chávez, M., Olivar, L., Rojas, M., Morillo, J., Mejías, J., … Bermúdez, V. (2014). Polycystic Ovary Syndrome, Insulin Resistance, and Obesity: Navigating the Pathophysiologic Labyrinth. International Journal of Reproductive Medicine, 2014, 1–17. https://doi.org/10.1155/2014/719050
Sirmans, S. M., & Pate, K. A. (2013). Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical Epidemiology, 6(1), 1–13. https://doi.org/10.2147/clep.s37559
Teede, H., Deeks, A., & Moran, L. (2010). Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Medicine. https://doi.org/10.1186/1741-7015-8-41
Tsilchorozidou, T., Overton, C., & Conway, G. S. (2004). The pathophysiology of polycystic ovary syndrome. Clinical Endocrinology. https://doi.org/10.1046/j.1365-2265.2003.01842.x
Yii, M. F., Lim, C. E. D., Luo, X., Wong, W. S. F., Cheng, N. C. L., & Zhan, X. (2009). Polycystic ovarian syndrome in adolescence. Gynecological Endocrinology. https://doi.org/10.1080/09513590903015551
Kathleen M. Hoeger, Richard S. Legro, Corrine K. Welt; A PATIENT’S GUIDE: Polycystic Ovary Syndrome (PCOS), The Journal of Clinical Endocrinology & Metabolism, Volume 99, Issue 1, 1 January 2014, Pages 35A–36A, https://doi.org/10.1210/jc.2014-v99i1-35A
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




