Research on Probiotics as Therapy for Crohn's Disease
Info: 12822 words (51 pages) Dissertation
Published: 11th Jun 2021
Abstract
Prebiotics are non-digestible ingredients of the diet that selectively promote the growth and activity of lactobacilli gold bifid bacteria in the colon.Current evidence indicates that the immune system in the intestinal mucosa reacts aggressively against the normal flora and does not favor the existence of alterations of the flora in intestines. The identification of NOD2, a member of the family NOD1 / apoptotic protease activation factor forms a clear association between immune recognition enteric bacteria and the development of the disease. Inclines are foods such as garlic, onion, artichokes and cereals, and these are also produced on a commercial basis in the food industry. The management of the seriously ill patient is complex, since many things have to be taken into account like multiple pathophysiological variables, metabolic, immunological and nutritional variables, to achieve homeostasis and achieve Proinflammatory / anti-inflammatory balance. Inflammation is regulated by the production of hydrolytic enzymes. There is also a production of anti-inflammatory cytokines and NK cells that destroy a larger number of infected cells. Although Saccharomyces boulardii has several pharmacobiological properties however, approaches to possible efficiency have been possible so far with no effect in small studies. Reduction of serum saturated fat, better-quality lactose digestion along with protection in contradiction of colon cancer between the effects claimed intended for probiotics are helpful immunomodulation. py.
Keywords: Crohn’s Disease, Randomized Controlled Trial, Probiotics, Lactobacillus
Contents
Acknowledgment…………………………………………………………………………………………………………………………………….2
Declaration..……………………………………………………………………………………………………………………………………………3
1.1 Background
Chapter Two: Literature Review
2.1 Specific Infectious agents
2.2 Lack of tolerance of flora
2.3 Probiotics, Prebiotics and Symbiotic
2.4 Bacterial Flora in Inflammatory Bowel Disease
2.5 Probiotics and Inflammatory Bowel Disease
2.6 Probiotics in patients with inflammatory bowel disease
2.7 Antibiotics in inflammatory bowel disease ……………………..……………………………………………………24
2.6 Probiotics in animal studies…………..………………………………………………………………………………………..25
Chapter Three: Methodology…………………………………………….21
3.1 Database search…………………………………………………21
3.2 Key words and descriptors………………………………………….21
3.3 Eligibility criteria………………………………………………….21
3.4 Research strategey……………………………………………….21
3.5 Sources……………………………………………………….21
Chapter Four: Critical analysis……………………………………………22
CHAPTER 05: CONCLUSION…………………………………………….25
Probiotics in Inflammatory Bowel Disease
Chapter One: Introduction
Background
Ulcerative colitis and Crohn’s disease are diseases of the gastrointestinal tract of chronic inflammatory character and the etiology is unknown. At the moment, although different mechanisms involved in the pathogenesis of the lesions are known, there is no single agent or cellular or molecular alteration that can explain all aspects of inflammatory bowel disease (Howarth, 2006 pp.nd).
Crohn’s disease and ulcerative colitis is increasing, causing a significant handicap in the patient and cause health issues (Bai and Ouyang, 2006 pp.381-382). Responsibility for initiation and sustainability of the inflammatory process is unknown but after few years of research, it was mediocre that was paid attention to the possible role of bacteria in the pathogenesis of IBD. However, a considerable number of clinical and laboratory support for the importance of bacterial flora was seen, so the most recent assumptions suggest that intestines abnormal immune response of the individual, probably with a genetic basis could be the cause (Jonkers and Stockbrügger, 2003 pp.170-171). Various studies performed in experimental animals, as well as in patients with IBD, so it is possible to manipulate the flora with the administration of probiotics and / or effects, producing an immunomodulatory response effect (Kwon and Farrell, 2003 pp.185-186). Normal flora has got an important influence on the integrity, functional status and in the development of the intestinal mucosa, modulating the epithelial responses to proinflammatory stimuli and exchanging regulatory signals with sub epithelial cells and the mucosal immune system.
The knowledge of the normal flora is still scarce; half of the Bacterial species are not cultivable (Campieri and Gionchetti, 1999 pp.1248-1249). Today there are several studies using molecular biology techniques with promising results, so it is possible that in a few years, progress can be seen in this field. The most recent hypotheses suggest that inflammatory bowel disease (IBD) originates from an abnormal immune response of the individual, probably on a genetic basis, to some antigens from the bacterial infection of flora (Dotan and Rachmilewitz, 2005 pp.429-430). Several studies, carried out both in animals of experimentation as in patients with IBD indicate that it is possible to manipulate the bacterial flora with probiotics and / or prebiotics producing an immunomodulatory effect in response to inflammation. Probiotics are living microorganisms which alter the intestinal micro flora and possess a beneficial effect on health (Sartor, 2004 pp.1632-1633).
Taking into account that the intestine is the organ most directly exposed to the action of the multiple microorganisms that make up the bacterial flora, it is not surprising that much of the research on the etiology of these diseases has focused on the influence of bacteria on The initiation and perpetuation of the inflammatory process. The concept that intestinal content contributes to the pathogenesis of inflammatory bowel disease is supported by some studies in patients with Crohn’s disease undergoing ileal resection and ileocolonic anastomosis (Ricci, Et.al, 2014 pp.112-113). The absence of contact of the mucosa with the intestinal contents through an ileostomy prevents the recurrence that reappears when the intestinal transit is restored. These observations raise a number of issues yet to be resolved (Matsumoto, Et.al, 2005 pp.425-426):
Is there a specific infectious agent responsible for inflammatory bowel disease? Does the inflammatory disease have its origin in a primary defect of the immune system that conditions an exaggerated response to a nonpathogenic intestinal flora (lack of immunological tolerance)?
the disease can be treated by modifying the intestinal flora through antibiotics or probiotics? This review intends to gather the scientific, clinical or experimental information that allows addressing these three issues.
Research Question
Do Probiotics have a therapeutic option for patients with Crohn’s disease, either in the acute phase or for maintenance?
Chapter Two: Literature Review
SPECIFIC INFECTIOUS AGENTS
Although traditional methods have not been able to demonstrate a specific pathogen causing inflammatory bowel disease, new molecular biology methods currently available have opened new possibilities for study, since the infectious etiology of inflammatory bowel disease is still a possible option (Sheil, Et.al, 2007 pp.823S-824S).
During the eighties and early nineties the possible relationship of Crohn’s disease with mycobacterial infection was postulated because of the discovery of a microorganism not classified but related to Mycobacterium paratuberculosis in patients with this disease. However, the results of subsequent investigations have been very contradictory and inconclusive (Tamboli, Et.al, 2003 pp.819-820). Some studies have demonstrated the presence of high titers of antibodies against M. paratuberculosis in patients with Crohn’s disease, but this has not been confirmed in subsequent studies. The detection of M. paratuberculosis in the tissues of patients has also been complicated and unconventional both by immunohistochemical techniques and by DNA analysis of the microorganism. On the other hand, the results of clinical studies with antituberculosis drugs have been contradictory (Schultz and Sartor, 2000 pp.S20-S21).
Escherichia coli is the most abundant gram-negative aerobic bacterial species in the normal intestinal flora in humans; For this reason, several studies have evaluated its role in the pathogenesis of inflammatory bowel disease (Gionchetti, Et.al, 2006 pp. 124-125). More than 20 years ago an increase in anti-E antibody titres was demonstrated. Coli in patients with Crohn’s disease. More recently, a group of French researchers demonstrated that E. coli is found in 65-100% of ileal lesions in patients with Crohn’s disease and that it is also the predominant flora (50-100% of the total number of Aerobic and anaerobic) (Campieri and Gionchetti, 1999 pp.1248-1249).
The same group of researchers, in a subsequent study, has attempted to determine whether Crohn’s disease is associated with the presence of a specific E. coli strain. The results obtained by molecular biology studies in tissues affected by Crohn’s disease suggest that, although a single strain of E. coli can not be isolated, some specific genotypes appear more frequently. In addition, most of them have the ability to adhere to the epithelium, which gives them the ability to colonize it and, therefore, to be virulent (Shanahan, 2000 pp.114-115).
Another of the infectious agents related to inflammatory bowel disease, which has elicited various studies, is the measles virus. Wakefield et al. Demonstrated that in the intestinal resection pieces of patients with Crohn’s disease there were vascular lesions caused by a granulomatous vasculitis (Shanahan, 2004. pp.817-818). In the study by electron microscopy of these lesions, we detected particles compatible with the presence of measles virus. In addition, it appears that perinatal exposure to measles virus increases the risk of Crohn’s disease and that vaccination increases the risk of developing an inflammatory bowel disease (Sartor, 2004 pp.1632-1633). However, although it seems clear that there is a component of vascular disease in Crohn’s disease, it is difficult to prove that it is exclusively induced by infection with the measles virus (Sartor, 2004 pp.1632-1633).
Involvement of other bacteria such as Salmonella, Yersinia, Clostridium difficile, etc., has also been investigated, but these bacteria are probably more related to exacerbations of the disease than to the underlying etiopathogenic mechanism. In a recent study, using conventional cultures and new techniques of molecular biology, the flora associated with the mucosa in biopsies of 305 patients with inflammatory bowel disease and in 40 controls has been investigated. In patients with inflammatory disease, especially with Crohn’s disease, high concentrations of faecal bacteria adhered to the mucosa were found (Dotan and Rachmilewitz, 2005 pp.429-430). Interestingly, the concentrations were higher in the non-inflamed than in the inflamed areas and there was no evidence of a translocation in the lamina propria or in the macrophages. When bacterial concentrations exceeded a certain value, bacterial inclusions could be observed by electron microscopy in enterocytes located near the lamina propria (non-superficial). However, no difference could be found between the control group and the group with inflammatory bowel disease in relation to the different isolated species, most of them of the genus Bacteroides between the anaerobic or E. coli between the aerobias (Shanahan, 2004. pp.817-818). The results of this study contradict the hypothesis of a single microorganism as an etiological agent of inflammatory bowel disease, with an abnormal immunological response in the host being more likely to occur, perhaps determined by some genetic defect, that would lead to a global alteration in the interaction Host-bacteria (Iizuka, Takaishi and Hibi, 2005 pp.779-780).
LACK OF TOLERANCE TO FLORA
Tolerance of the intestinal mucosa can be defined as an active process by which a potentially aggressive immune response is suppressed by recognizing a particular component of the intestinal tract as non-pathogenic. The intestine is in permanent contact with bacteria of normal intestinal flora, proteins and antigens of alimentary origin or potentially pathogenic bacterial products, and has to discriminate and execute a selective action against each other (Howarth, 2006 pp.nd).
The possibility that they are the components of the normal intestinal flora that initiate or perpetuate inflammatory bowel disease has been widely studied. The experimental results obtained in animal models of intestinal inflammation demonstrate that intestinal lumen bacteria are related to the mechanisms of intestinal inflammation (Kruis, 2004 pp.77-78). The research team observed in an animal model that broad-spectrum antibiotics that reach adequate concentrations in the intestinal lumen get an anti-inflammatory effect. In contrast, the same parenteral antibiotics lacked anti-inflammatory or therapeutic effect. In addition, we verified that the living elements of the flora, rather than food antigens or bacterial endotoxin, stimulate the inflammatory activity of the lesioned intestinal mucosa (Prantera, Et.al, 2002 pp.408-409).
Some predominant genera such as Bacteroides contribute particularly to the formation of deep and chronic mucosal lesions. In addition, lesions contaminated with certain species of anaerobes evolve easily into intestinal fibrosis (Ghosh, Van Heel and Playford, 2004 pp.621-622). On the contrary, other elements of the flora, like Lactobacillus, do not contaminate the lesions of the mucosa nor do they induce inflammation in the intestinal wall. In an animal model in mice it was observed that they lost tolerance to the autologous flora while colitis persisted. However, tolerance was restored when mice were treated with IL-10 or anti-IL-12 antibodies. In addition, mice selectively deficient in IL-10 or IL-2 (anti-inflammatory and tolerance-promoting cytokines), healthy under germ-free conditions, spontaneously develop colitis when colonized with bacteria (Fedorak and Madsen, 2004 pp.298-299).
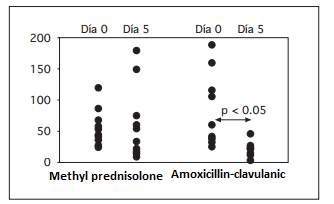
Fig. 1. Estimated release of interleukin-8 (pg / ml)
The intestinal flora is not part of the host and, therefore, immunological tolerance prevents an immune response against the “own bacteria”, which if it occurs would lead to a situation of chronic inflammation. Duchmann et al. (1997) demonstrated that the mononuclear cells of the inflamed intestinal mucosa of patients with inflammatory bowel disease proliferated when exposed in vitro to bacterial antigens from the autologous flora. However, cells from the unaffected mucosal areas of the same patients, patients in remission, and healthy controls did not proliferate to autologous bacteria. This study suggests that there is a loss of tolerance to the flora itself in the mucosa affected by active inflammatory bowel disease (Shanahan, 2001 pp.634-635).
The lack of tolerance to the bacterial flora probably plays an important role in the pathogenesis of the immunoinflammatory process, which ultimately results in lesions of the mucosa. Immunosuppressive drugs would inhibit immunoinflammatory mechanisms against a bacterial population, in principle non-invasive or pathogenic. On the other hand, reducing the bacterial load with broad-spectrum antibiotics would reduce the stimulation of the immunoinflammatory process (Kruis, 2004 pp.77-78). A study from same center investigated the effects of corticosteroid treatment (methylprednisolone) on the inflammatory response in patients with ulcerative colitis in the outbreak compared to amoxicillin-clavulanic treatment given with enteric coating to prevent its absorption in the small intestine. The study demonstrated a significant reduction in the metabolic activity of the flora, evaluated in vivo by the elimination of hydrogen in the breath after the administration of lactulose, only in the patients treated with the broad spectrum antibiotic with enteric cover (Marteau, 2005). A significant inhibition of the release of inflammatory eicosanoids in the rectal mucosa of patients from both groups was observed 5 days after treatment. However, the most striking response was suppression in the release of interleukin-8, which was virtually annulled in patients treated with antibiotics, whereas it was not modified in patients treated with corticosteroids (Figure 1). These data suggest that the presence of flora plays an important role in the pathogenesis of inflammatory lesions, so that the reduction of bacterial load has a deeper anti-inflammatory effect than the usual steroidal anti-inflammatory drugs (Jones, and Foxx-Orenstein, 2006 pp.49-50).
Taking into account the results of these and other investigations, the most likely hypothesis for the development of chronic intestinal inflammation is that of an abnormal immune response to the usual flora. This loss of tolerance could be due to a lack of mediators or regulatory cells, or to an alteration in the mucosal barrier that would allow the contact of the local immune effector cells with the bacterial products (Iizuka, Takaishi and Hibi, 2005 pp.779-780).
Prebiotics are non-digestible ingredients of the diet that selectively promote the growth and activity of lactobacilli gold bifid bacteria in the colon. The viability, transit and survival of many of the probiotic preparations which are available in the market, in so-called “Healthy foods” have not been demonstrated (Shanahan, 2004. pp.817-818). It is too early to recommend using this which is prepared in the usual clinical practice, that it is necessary to carry out scientific studies that demonstrate their real effectiveness. Data supporting a role of intestinal bacterial flora in the pathogenesis of IBD are increasingly persuasive (Iizuka, Takaishi and Hibi, 2005 pp.779-780). The most notable evidence from many experimental studies of spontaneous colitis in transgenic Knock-out mice; where these animals do not develop colitis when reared in germ-free conditions. It also highlighted the possibility of transferring colitis by T cells activated against bacterial antigens in a model of colitis in mice (Howarth, 2006 pp.nd). In humans, it is well known that the disease occurs in areas with the highest bacterial content. On the other hand, Crohn’s disease can work with antibiotics such as metronidazole and ciprofloxacin. A rate of clinical remission has also been described increasing following fecal transit bypass in Crohn’s disease, suggesting a fecal transit contributes towards the pathogenesis of inflammation entity (Kruis, 2004 pp.77-78). In this sense, it has shown that this luminal content (bacteria or their products) is able to trigger postoperative recurrence early to instill directly into the terminal ileum (Marteau, 2005). Current evidence indicates that the immune system in the intestinal mucosa reacts aggressively against the normal flora and does not favor the existence of alterations of the flora in ITNs. Thus, there is no significant difference in flora composition between patients with IBD and healthy controls (Jones, and Foxx-Orenstein, 2006 pp.49-50). However, high concentrations of bacteria in non-inflamed mucosa among patients with IBD was found, but not in healthy controls, suggesting that these changes are not secondary to inflammation, but the result of specific responses of the individual, either by special features of immunity or by a provision genetic or symbiotic interactions (Karthik, 2003 pp.369-370). The identification of NOD2, a member of the family NOD1 / apoptotic protease activation factor, as disease susceptibility Crohn’s disease, which is associated with localized inflammation forms a clear association between immune recognition enteric bacteria and the development of the disease (Lomangino, 2013 pp.8-9). This genetic response towards presence of bacterial components is believed to function as a cytosolic receptor for pathogenic components derived from bacteria, regulating signal paths dependent on nuclear factor NF. Probiotics and IBD Probiotics are living microorganisms that alter the micro flora intestinal and have got beneficial effect on health (Shanahan, 2001 pp.634-635). The bacteria that was used in general, lactobacilli or bifid bacteria, although others such as strains of escherichia coli, enterococci and Non-bacterial organisms, such as saccharomyces boulardii have been used previously (Fedorak and Madsen, 2004 pp.298-299). Prebiotics are non-digestible dietary ingredients that selectively, grows some bacterial colon strains. In general, the trafficking of oligosaccharides (fructose, such as inulin) and low molecular weight carbohydrates such as lactulose, this favors the growth of lactobacilli and bifid bacteria. Inclines are foods such as garlic, onion, artichokes and cereals, are also produced on a commercial basis and it is used in the food industry. Data from experimental models of spontaneous colitis suggest that there are more pathogenic luminescent bacteria than other (Gionchetti, Et.al, 2006 pp. 124-125).
Bactericides are Pathogens seen in many experimental models and probiotic treatments with lactobacilli appeared to have a beneficial effect. Probiotics, similarly appear to decrease the rate of progression from inflammation to dysplasia and colon cancer in the experimental animal (Ghosh, Van Heel and Playford, 2004 pp.621-622). Recently, in addition, a strain of Lactococcus Lactis secrete the cytokine IL-10, an active anti-inflammatory in the intestinal lumen, in two models experimental studies of colitis showed good results (Sheil, Et.al, 2007 pp.823S-824S). Controlled studies in humans are rare. In two studies it has been suggested that a non-pathogenic strain of E. coli has an efficacy similar to that of meclizine in patients with ulcerative colitis (Tamboli, Et.al, 2003 pp.819-820). On the other hand, the results obtained using a probiotic cocktail in patients with spectacular mixture contains 4 strains of lactobacillus, 3 strains of bifid bacteria and 1 strain of Streptococcus salivaris Sbsp thermophiles. Although the results of these studies and other uncontrolled experimental studies are promising, it is still too early to recommend common use of probiotics in clinical practice (Prantera, Et.al, 2002 pp.408-409). It is important to take into account that viability, transit and survival in probiotic preparations that are available in the market, in so-called “healthy foods” have not been shown. Data on the use of prebiotics are even rarer. Inulin or lactulose supplements are effective in models of experimental colitis (Ricci, Et.al, 2014 pp.112-113). On the other hand, preparations of dietary fiber containing sprouted barley can be effective in colitis models in patients with ulcerative colitis. Another type of dietary fiber, the seeds of plant ago ovate appear to be effective similar to meclizine to maintain remission in ulcerative colitis (Matsumoto, Et.al, 2005 pp.425-426). Complement with these two dietary fiber preparations resulted in concentrations of feces, known as promoting healing of the mucosa and preventing development cancer of the colon. Finally, the joint administration of probiotics and prebiotics may have a synergistic effect studying at depth while all of these results are provocative and hopeful, it is very early to recommend the use of these preparations in routine clinical practice, since rigorous scientific studies clarify the type of bacteria or combinations of organisms to be used, their isolated use or combined with prebiotics, the optimal dose diet and standard pharmacological therapy, frequency duration of treatment (Schultz and Sartor, 2000 pp.S20-S21). The management of seriously ill patients is complex, since it has to take into account multiple pathophysiological variables, metabolic, immunological and nutritional to achieve homeostasis and to achieve proinflammatory / anti-inflammatory balance (Bai and Ouyang, 2006 pp.381-382). For the previous several years nutritional and metabolic strategies were achieved not only the energy balance, but also the biological balance, among which are the probiotics. Probiotics are living microorganisms that are when administered in appropriate quantities, confer to the guest. Health experimental and clinical studies impact the utility as part of the therapeutic armament, which has favored its introduction and application in different scenarios such as: diarrhea, infections by clostridium, cancer, pneumonia associated with mechanical ventilation, pancreatitis, sepsis and inflammatory, immunological and hepatic Ticas (Jonkers and Stockbrügger, 2003 pp.170-171). The objective to provide scientific evidence, current concepts related to probiotics and its use in the seriously ill attended in the units of intensive therapy (Kwon and Farrell, 2003 pp.185-186).
Probiotics, Prebiotics and Symbiotic
The term probiotic is relatively new, in favor of life, it is used to designate bacteria which have beneficial effects for humans and animals. It was until 1960 when this word was used for the substances produced by microorganisms that promote growth of other microorganisms (Shanahan, 2000 pp.114-115). They must be able to survive gastric juices and develop in the presence of bile or be consumed in a food. probiotics are gram-positive bacteria and classified mainly in lactobacilli, bifid bacteria, and different strains of enterococci, streptococci, and molds like Saccharomyces boulardii (Campieri and Gionchetti, 1999 pp.1248-1249). Probiotics have beneficial effects its growth and / or activity in the digestive tract, what are the specificity of action and not the source of the microorganism. These micro-organisms have several action by which they have different effects on health, such as the production of lactic acid, acetic acid, ethanol, carbon dioxide, hydrogen peroxide, substances low molecular weight antimicrobial agents pyro glutamic acid, and bacteriocins (Dotan and Rachmilewitz, 2005 pp.429-430). One of particularly important is the decrease in pH because they synthesize substances that acidify the intestine, which produces the detachment of pathogenic bacteria and adherence of non-pathogenic bacteria, consume specific nutrients and compete with competitors intestinal receptors, thus allowing regeneration from the intestinal barrier (Sartor, 2004 pp.1632-1633).
Research in this field and methodological concepts need to be improved for more scientific evidence and not doubts about its use, effectiveness and safety part of the integral treatment of different diseases (Campieri and Gionchetti, 1999 pp.1248-1249). Inflammation similar to that of Crohn’s disease, used under normal conditions can be applied. The animals remain healthy objective when there is livestock and sterile conditions. In the patients with acute ulcerative colitis a reduced number of lactobacilli in biopsies is seen and active Crohn decreases the excretion of bifid bacteria in stool versus patients in remission (Dotan and Rachmilewitz, 2005 pp.429-430). During disease changes are increasingly Mucosa adherent and in the mucosa translocated bacteria (main pathogens are anaerobacter [bacteroides] and aerobier [enterobacteria]. A disturbed balance of bacteria, the intestinal micro flora, a genetic predisposition are decisive in the development of DEC Be (Sartor, 2004 pp.1632-1633). The disease manifests itself, If for not known an imbalance between luminal the flora, the intestinal epithelium and the controlled immune response intestinal wall (Shanahan, 2004. pp.817-818). There are some clues, that a loss of immunology tolerance to intestinal micro flora is essential. Persistence of antigens in the intestinal tract mucosa leads to an activation of specific T lymphocytes and macrophages, which brings about the formation of prion flammatory Cytokines (IL1, IL6, TNF-, INF-) inflammatory changes (Iizuka, Takaishi and Hibi, 2005 pp.779-780). This will make a series of Pro-inflammatory cytokines, which then have different mechanisms to cause known inflammation changes in ulcerative colitis and Crohn (Kruis, 2004 pp.77-78).In recent years, it has been shown that prebiotics and probiotics have different ways of influencing CED (Marteau, 2005).
Some commercial presentations have these microorganisms are as follows (Kruis, 2004 pp.77-78):
• Saccharomyces boulardi: It is marketed by Bio codex laboratories, Mont rouge.
• Commonly are given in doses of two capsules (250 mg) in the morning, which is equivalent for 1×1010 living organisms per day (Marteau, 2005).
• Lactobacillus GG. T c a i G they are marketed by Valois, Helsinki, Finland, the administered dose oscillation between 1 and 5×109 lactobacilli per day (Jones, and Foxx-Orenstein, 2006 pp.49-50).
• Lactobacillus acidophilus LA-1. Ac to -1 i A marketed By Nestle, Vevey, Switzerland. Generally given Doses less than 5×109 CFU and in some cases Less than 1×109 (Karthik, 2003 pp.369-370).
Some studies have used mixtures of strains of bacteria, which have been used more frequently and was studied is the cocktail called VSL-3, who contains strains of lactobacillus (lactobacillus acidophilus, lactobacillus casei, Lactobacillus delbrueckii subspecies bulgaricus and plantarum) three strains of bifid bacteria (Bifid bacterium lignum, infant, short) and streptococcus Saliva us subspecies thermophiles (Lomangino, 2013 pp.8-9). This cocktail contains 3×1012 live bacteria per gram. It produces and markets sigma-Tau, Pomezia, Italy and VSL Pharmaceuticals Fort Lauderdale, United States. They are administered in high doses, from 1.8×1012 to 3.6×1012 per day (Shanahan, 2001 pp.634-635).
Prebiotics
Prebiotics are no digestible components of a food which, when ingested, promote growth and establishment of beneficial flora germs Intestinal. The most commonly used prebiotics in practice are as follows:
• Fructooligosaccharides (FOS) (Fedorak and Madsen, 2004 pp.298-299).
• Galactooligosaccharides (GOS).
• Inulin.
• Trans-galacto-oligosaccharides (TOS).
• Lactulose (Gionchetti, Et.al, 2006 pp. 124-125).
• Oat fiber.
• Sprouted barley.
• Hydrolyzed guar gum.
• Sturdy starch.
• Plantago ovata.
• Betaglucan (Ghosh, Van Heel and Playford, 2004 pp.621-622).
Pectin
Symbiotic the mixture of probiotics is defined as symbiotic and prebiotics that have an effect on the host. The handful symbiotic used in clinical studies are:
• Lactobacillus plantarum 299 and 10 g of fiber L c i o 9 and y1 d of oats. It is produced and marketed by AB Probi, Lund, Sweden, the common dose is 1 to 2×109 bacteria per day Up to 5×109 (Sheil, Et.al, 2007 pp.823S-824S).
• Lactobacillus sporongens with more fructooligoic acid Saccharides.
• Synbiotic 2000. i c t That is composed of a mixture of four lactobacilli (Lactobacillus pediacoccus Pentosaceus, Leuconostoc mesenteroides, Lactobacillus Paracasei, Lactobacillus plantarum) more a blend of four bioactive plant fibers (Beta glucan, inulin, pectin and resistant starch) in total 10 g, is marketed by Medipharm, Kageröd, Sweden and Des Moines Iowa, United States United (Tamboli, Et.al, 2003 pp.819-820).
• Oligofructose plus inulin (SYN1) more Lactobacillus Rhamnosus GG and Bifidobacterium Lactis Bb12.
Bacterial Flora in Inflammatory Bowel Disease
The inflammatory reaction is confined to the mucosa as well as sub mucosa of the colon by means of clear differentiations in ulcerative colitis (UC) (Jones, and Foxx-Orenstein, 2006 pp.49-50). The entire GI tract can be fully involved and the inflammation can eventually extend over and with the colonic valve involvement from mucosa to serosa in Crohn’s disease (CD). By means of relatively normal mucosa, areas of swelling might be interspersed (Karthik, 2003 pp.369-370). In CD, time and again it is accompanied by rectal hemorrhage. The predominant indications are diarrhoea, abdominal pain along with weight loss though in UC, diarrhea is the key important symptom (Lomangino, 2013 pp.8-9). By means of highest occurrences in North America as well as Northern Europe together, diseases are common in the developed world. In a recent study, the usual annual occurrence was 5.4/100 000 on behalf of CD and 11.3/100 000 for UC (Shanahan, 2001 pp.634-635). With a second minor peak among 54 and 85 years the peak age of beginning for both diseases is between 14 to 30 years. As compare to males females are showing much higher occurance of CD. With an affinity to a male dominance UC seems more alike distributed among the sexes (Fedorak and Madsen, 2004 pp.298-299).
Both genetic as well as environmental factors are supposed to contribute but then again the etiology of IBD is unidentified. Habits of nutritional, as conditioning issues oral contraceptives along with breastfeeding have similarly come under suspicion(Ghosh, Van Heel and Playford, 2004 pp.621-622). With CD smoking is completely related. In the development along with recurrence of IBD the intestinal microbial flora is supposed to be a significant factor, but then again exactly how has not yet been clarified. Regarding IBD current treatment of it depends on anti-inflammatory medications, immune modulators, nutritious add-ons and surgery (Sheil, Et.al, 2007 pp.823S-824S).
Among some of the other positive effects, in contradiction of pathogens the intestinal microbial flora contributes towards digestion of nutrients along with metabolism of (pro) chemicals and that has a significant barrier function (Prantera, Et.al, 2002 pp.408-409). For instance, the colonic epithelial cells depend on short-chain full of fat acids (particularly butyrate) as energy basis short-chain fatty acids are formed by anaerobic bacterial fermentation of luminal biological compound along with proteins;. In addition to that, products of colonic microbial flora for instance peptidoglycan and lipopolysaccharides are immune stimulants (Ricci, Et.al, 2014 pp.112-113).
As far as the triggering effect of chronic intestinal inflammation is concerned it seems to depend one way or the other on the flora. IBD-like colonic inflammation will ultimately not occur deprived of the occurrence of an intestinal microscopic flora in laboratory animals for instance interleukin (IL)-12 lacking in mice or chemically preserved rats (Matsumoto, Et.al, 2005 pp.425-426). In health, towards the resident microorganisms will generally be limited by 3 issues, the colonic immune response homoeostasis in the microbial flora, comparative impermeability of the mucosal obstacle, along with a suppressive immune response (Schultz and Sartor, 2000 pp.S20-S21).
In patients with IBD a defective oppressive protected response has been described. The Th2 lymphocytes produce II-3, IL-4, IL-5 and IL-40 which are fully related by means of hypersensitivity reactions along with increased IgG1 production (Jonkers and Stockbrügger, 2003 pp.170-171). On behalf of an effective oppressive immune reaction a balance among these cytokine outlines is important. It has been indicated that the pattern of cytokine production seen in CD is a Th1-condition (Kwon and Farrell, 2003 pp.185-186).
This is a consensus the CED working group (Chronic inflammatory diseases) for nutrition to CED for DEC and weight loss (> 5 per cent in 3 months), symptoms of deficiency, after long-term intestinal resections might be due to malnutrition (in case of Morbus Crohn in 20 per cent – 70 per cent) and this should be treated (Shanahan, 2000 pp.114-115). Particularly, the emergence of a limited quality of life, opportunistic infections, osteopenia / osteoporosis, prolonged hospitalization and mobidity due to malnutrition should be treated In case of malnutrition, iron state, Vitamin B12 and folic acid levels, vitamin D and zinc in the blood are checked. Primary nutrition therapy with enthraller lipid diets are primarily given only in children ,but in adults only in special situations (Campieri and Gionchetti, 1999 pp.1248-1249). Patients with ileostomy should be on specific dietary advice. Although in recent years the detection of nutritional status and diet for chronic inflammatory (DEC) has been published, always has subordinate importance. These are consensual reports, evidence-based recommendations for nutrition at CED to create clinical access to simplify this issue for treating physicians (Sartor, 2004 pp.1632-1633).
Probiotics and Inflammatory Bowel Disease
A number of attempts have been made in order to modify the flora by means of probiotics, for the reason the evidence involving the intestinal microbial flora in IBD is made. In animals with new colitis orally or rectal lactobacilli have shown some developments (Ricci, Et.al, 2014 pp.112-113). In IL-10 lacking mice, Lactobacillus plant arum 299v prohibited beginning of disease along with reduction in well-known colitis. The onset and severity of colitis in methotrexate-treated rats was eventually reduced when L. plantarum 299v was given orally but then again this lactobacillus had not a single effect on recognized colitis persuaded in rats by an additional method (TNBS/E) (Matsumoto, Et.al, 2005 pp.425-426).
With probiotics use in patients with UC or CD, it gives an impression of intervention studies. Oral administration of L. casei GG (L. GG) in an open study was finally judged in order to have increased mucosal IgA in children by means of either active or quiet CD (Bai and Ouyang, 2006 pp.381-382). When L. To 4 children with temperately active CD GG was agreed intended for twelve months getting steady doses of corticosteroids, all better-quality clinically in addition to 3 were able to have their steroids reduced (Jonkers and Stockbrügger, 2003 pp.170-171). In amounts reaching from 105 to 106 colony-forming units/g 35 L. GG was recovered as of faecal samples. Out of 2 4 patients received metronidazole in addition to the probiotic in addition this may have prejudiced the clinical consequence. It has been studied with 10 patients by means of inactive CD of ileum, colon or together, free as of treatment by means of steroids for single month or additional immunosuppressive agents aimed at 3 months. In combination by means of Saccharomyces boulardii for a total of six months he prescribed meclizine 1 gram twice daily; only single patient reverted (Kwon and Farrell, 2003 pp.185-186).
The use of bioelectrical impedance analysis can lead to loss of body mass even to normal or elevated levels BMI are recorded on the change of non-grease where mass can be found. In future, the evaluation the force with a measuring device of the handle has valuable help. Food allergies and / or intolerances are caused by many patients with CED (Shanahan, 2000 pp.114-115). Only for lactose intolerance in Crohn’s disease with intestinal infestation there are positive studies. Otherwise, there are no secure notes increased intolerance or allergies to CED, incompatibilities but affected by those affected (Campieri and Gionchetti, 1999 pp.1248-1249).
Food allergies or intolerances knowledge is not due to disease genesis / onset involved, but often discussed. There is only that theory without corresponding proof. Food intolerances should be included in the treatment plan among patients with CED. However, the problem of malnutrition and malnutrition since many foods are avoided to become. Among the test methods food allergies / intolerances are respiratory tests H2 on lactose and fructose, as well as the maintenance of a nutritional journal best possible (Dotan and Rachmilewitz, 2005 pp.429-430). Other methods IgE test, food allergy challenge and elimination regimens should be in the centers in close collaboration with allergists and dieticians. Individual disposal schemes they have shown their effect, especially in the management of remissions (Sartor, 2004 pp.1632-1633). Respiratory testing to determine lactose and fructose intolerance should not be performed in an acute phase, since this is not the case, and can aggravate the complaints. They should only be based on a clear medical history. The nutritional journal can be used for both the patient, also important support for the team (doctor, dietician) for the elimination of symptom-causing foods. IgG tests are problematic because It is non-specific, but also IgE tests do not give much information (Shanahan, 2004. pp.817-818).
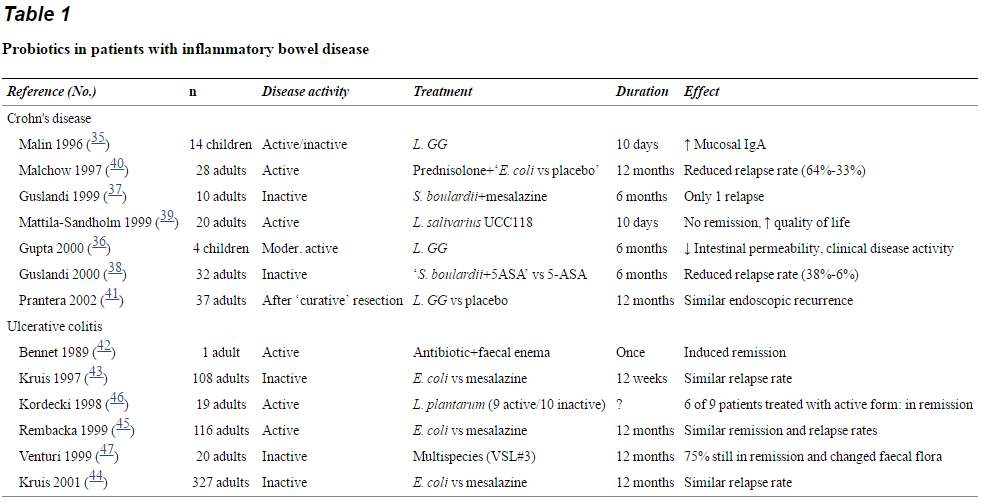
Probiotics in patients with inflammatory bowel disease
Consequently, in mixture with S. boulardii (quantity unreported) for 6 months likened 2 regimens in 34 patients of either meclizine 1 g 3 times per day or mesalazine 1 gram twice daily. In patients treated by meclizine and S. boulardii the relapse rate was considerably lower (6 per cent) than in those preserved by means of mesalazine alone (38 per cent) (Karthik, 2003 pp.369-370). Assessment of this importance is delayed by the change in amount of mesalazine. In an impression, it has been reported on an open study in which L. salivarius UCC118 have been given intended for 6 weeks to a total of 20 patients with relapsed CD (Lomangino, 2013 pp.8-9). Detailed general information on this study is not obtainable clinical reduction could not be encouraged in all patients but then again an overall increase in overall superiority of life was noted;. In CD patients only 2 studies where placebo measured (Shanahan, 2001 pp.634-635). For 1 year 32 patients through active colonic CD Malchow likened the non-pathogenic Escherichia coli by way of placebo that were similarly treated through a reliable prednisolone agenda. In the 2 groups remission rates were comparable, but then again consequently a lower revert rate was seen in the E. coli preserved group (33 per cent versus 64 per cent); numerical analyses were missing. 37 well-defined patients were studies after a ‘curative’ resection on behalf of CD who were randomized towards receive L. GG or palliative for 1 year. Clinical reappearance was detected in about the similar quantities (13 per cent versus 14 per cent, respectively) (Fedorak and Madsen, 2004 pp.298-299).
The environment to which the immune system is exposed to determines DC phenotype and state of activation and eventually drives the balance between effector and regulatory T cells In 1989, it was reported that the case of an adult regarding active UC, towards standard management regimens, followed by an purgative of ‘faecal bacteria’ as of a healthy contributor who was symptom-free intended for six months subsequently treatment through a short course of antibiotics. With mesalazine in 3 studies of UC patients E. coli Nissle 1913 has been associated (Ghosh, Van Heel and Playford, 2004 pp.621-622). In order to receive meclizine 500 mg 3 times each day or E. coli for around 12 weeks as well as 43 included 103 patients in reduction who were randomized, by means of a double-dummy design, establish similar relapse rates intended for the 2 regimens (Sheil, Et.al, 2007 pp.823S-824S). A total of 24 patients were similarly treated by means of corticosteroids but then again this was not further debated. It was not very clear why 32 patients didn’t complete the whole protocol even though the per-protocol examines along with intention-to-treat analyses gave related results (Tamboli, Et.al, 2003 pp.819-820). Also, used for a maintenance study this study period was eventually short. intended for 12 months in a similar design comparable results were far ahead reported (in abstract) on behalf of 323 patients treated. In addition to that, all patients were treated by means of corticosteroids in another double-dummy study 42 clinically dynamic UC patients (n=114) were fully treated intended for 1 week by means of gentamicin in addition to that then randomized to obtain E. coli Nissle 1917 or else mesalazine 800 milligram 3 times daily for 12 months;. In the two groups remission rates as well as subsequent waning rates were the same (Prantera, Et.al, 2002 pp.408-409). In active UC patients a slight open study has been achieved. Compared through none of 10 patients preserved by means of inactivated microorganisms 6 of 9 patients specified viable L. plantarum 299v reached reduction. Various studies performed in experimental animals, as well as in patients with IBD, it is possible to manipulate the flora with the administration of probiotics and / or effects, producing an immunomodulatory response effect inflammatory (Ricci, Et.al, 2014 pp.112-113). Data supporting a role of intestinal bacterial flora in the pathogenesis of IBD are increasingly persuasive. Thus, there is no significant difference in flora composition between patients with IBD and healthy controls. Bactericides are Pathogens in many experimental models, that probiotic treatments with lactobacilli appeared have a beneficial effect (Matsumoto, Et.al, 2005 pp.425-426).
In children and adolescents, two meta-analyzes have the same effectiveness of primary exclusive nutritional therapy and the steroid therapy. Therefore, due to the more unfavorable side effects of steroid therapy Nutritional therapy in children with active Crohn’s disease (Schultz and Sartor, 2000 pp.S20-S21). Furthermore, the use of the primary Nutritional therapy in children threatening growth and pubertal developmental delays be treated (Bai and Ouyang, 2006 pp.381-382). Almost 90 per cent of young people show growth deficits before the initial diagnosis and often before the occurrence other symptoms an Australian study showed higher response rates for newly diagnosed Crohn’s disease in childhood and adolescence; even if longer disease was a remission induction of 60 per cent, as well as preservation of the remission achieved above average 15 months (Jonkers and Stockbrügger, 2003 pp.170-171). In another study in children with Morbus Crohn could perform an endoscopically and histologically verified mucosal healing by enteral nutritional therapy in 76 per cent of cases compared with only 33 per cent in the steroid-treated patient group and a reduction of pro inflammatory cytokines (Kwon and Farrell, 2003 pp.185-186). Between nutrient-defined high-molecular (polymeric) diets, chemically defined peptides and elementary systems no significant difference in remission could be detected are, but a significantly higher weight gain In the case of a polymeric diet. In case of insufficient oral intake, intolerance of nasogastric Probes or Crohn infestation of the oropharynx or esophagus the diet is carried out via PEG probes (Shanahan, 2000 pp.114-115). Increased risk of fistula By PEG probe implantation does not exist. Partially enteral and parenteral nutrition the partial enteral nutrition shows, compared with the primary exclusive enteral nutrition significantly lower rates (12 versus 43 per cent, p = 0.025) (Campieri and Gionchetti, 1999 pp.1248-1249). A remission induction by parenteral diet with active Crohn’s disease is possible in principle, but the enteral diet is not superior. Has a number of studies showed that the complete immobilization of the intestine by parenteral nutrition no advantage in the control of a CED. Parenteral nutrition is especially in complex, distillatory Crohn’s disease, short-bow syndrome, in acute thrust without possibility of adequate oral diet, intestinal obstruction or perioperative situations as a therapy option (Dotan and Rachmilewitz, 2005 pp.429-430).
Probiotics, prebiotics and other nutrients concerning possible pathogenic factors of the intestinal flora, as well as their effects and CED was a series of studies for induction of remission with prebiotics such as inulin and oligo fructose and probiotics for instance E. coli Nissle as well as VSL # 3. The corresponding results are unpredictable. Unlike the management of remissions E. coli Nissle in ulcerative colitis not for induction recommend (Sartor, 2004 pp.1632-1633).
ANTIBIOTICS AND INTESTINAL INFLAMMATORY DISEASE
For many years, clinical and experimental studies have been conducted to clarify the role of antibiotic treatment in inflammatory bowel disease, not only because of its antibacterial action but also because some of them are also attributed immunomodulatory effects, such as in The case of metronidazole and ciprofloxacin. However, the data can not be considered conclusive, probably because many of the studies include few patients, are very heterogeneous from the clinical point of view and the criteria of effectiveness of the treatment, as well as their duration are very variable (Marteau, 2005).
Studies with vancomycin, metronidazole, tobramycin, rifaximin and ciprofloxacin have been performed in ulcerative colitis. Oral vancomycin was not effective in a study of patients with active ulcerative colitis, although it showed a tendency to decrease the need for surgery. Metronidazole, active against anaerobic bacteria, was also not effective intravenously associated with the conventional treatment of acute outbreak of ulcerative colitis (Bai and Ouyang, 2006 pp.381-382). Tobramycin administered orally for one week (a nonabsorbable antibiotic active against gram-negative bacteria) was more effective than placebo for controlling the outbreak of ulcerative colitis. However, tobramycin has not been shown to be effective in maintaining remission, or when administered intravenously associated with metronidazole in cases of acute outbreak. Administration of rifaximin, a broad-spectrum, non-absorbable antibiotic, has only been investigated in a study with few patients, in which it induces clinical and endoscopic improvement when compared to placebo (Lomangino, 2013 pp.8-9). Ciprofloxacin is the drug most widely used in recent clinical studies, given its broad spectrum for both gram-positive and gram-negative bacteria. Although short-term treatment, either orally or intravenously, has not demonstrated its efficacy in active ulcerative colitis, a study in which the antibiotic was administered for 6 months demonstrated the superiority of ciprofloxacin over placebo to decrease the rate Of relapse (Shanahan, 2001 pp.634-635).
Several studies have been performed in Crohn’s disease with metronidazole, ciprofloxacin and clarithromycin. Metronidazole has been shown to be effective in clinical studies in the treatment of mild or moderate active Crohn’s disease, in the treatment of perianal disease, and as a preventive treatment for postoperative recurrence (Gionchetti, Et.al, 2006 pp. 124-125). Ciprofloxacin, alone or in combination with metronidazole, has also been effective in the treatment of active Crohn’s disease, especially of a colonic condition and with a remission rate similar to that achieved with methylprednisolone. Finally, an open-label, uncontrolled study with clarithromycin, an antibiotic selected for its broad spectrum and its ability to penetrate macrophages has recently been published. Clarithromycin, administered for 4-12 weeks to patients with active Crohn’s disease, showed very promising results to control activity and maintain remission (Jonkers and Stockbrügger, 2003 pp.170-171).
Taking into account the results of all previous studies, the success of antibiotic treatment in inflammatory bowel disease is probably due to the choice of broad spectrum drugs, active intracellularly and with few side effects to maintain them over a prolonged period, and in the Careful selection of the groups of patients to be treated to assess the actual efficacy of the treatment (Ghosh, Van Heel and Playford, 2004 pp.621-622).
Probiotics in animal studies
The results of animal studies in experimental colitis models treated with probiotics have been encouraging. In the acetic acid-induced rat colitis model, administration of Lactobacillus reuteri R2LC immediately after induction prevented the onset of colitis. However, if administration was more delayed or with lower concentrations of bacteria, the effect was less prominent. Similarly, in the rat enterocolitis model induced with intraperitoneal methotrexate, ingestion of lactobacilli, especially Lactobacillus plantarum, decreased the severity of colitis, improving weight loss and intestinal permeability and reducing local myeloperoxidase, translocation Bacterial and plasma levels of endotoxin. The model of mice deficient in IL-10 is interesting to study the role of bacteria in the onset of inflammation of the colon mucosa (Karthik, 2003 pp.369-370). The spontaneous chronic colitis that develops these mice requires the presence of bacteria in the intestinal lumen (they do not develop it in germ-free conditions), it is prevented and treated with antibiotics and presents characteristics similar to human Crohn’s disease (Kruis, 2004 pp.77-78). In the Madsen et al (2000) study, IL-10-deficient mice present decreased levels of lactobacilli in the colon in the neonatal period and an increase of bacteria adhered and translocated in the colonic mucosa, developing a colitis at 4 weeks of age. The repopulation of the colon with Lactobacillus sp. Attenuates colitis and decreases the translocation and adhesion of other bacteria. Treatment of these mice with VSL # 3, a lyophilized preparation with 8 different bacteria, induces a normalization of epithelial function, mucosal permeability and a reduction in the secretion of TNF-α and IFN-γ. In the same model, the ingestion of the probiotic Lactobacillus salivarius ssp. Salivarius UCC118 inhibits not only the development of colitis but the onset of colon cancer. In contrast, ingestion of Lactobacillus plantarum 299 by rats that had been induced with colitis in the TNBS model did not induce an improvement in inflammation or increase in mucosal permeability (Marteau, 2005).
A recently published study describes the therapeutic effect of a genetically modified bacterium (Lactococcus lactis) to secrete IL-10, a cytokine with anti-inflammatory properties that promotes immune tolerance. The anti-inflammatory effect of the bacterium, used up until now in the production of fermented foods, was tested in the treatment of chronic colitis induced in mice by DSS and in the prevention of spontaneous colitis that develops mice deficient in IL-10 (Matsumoto, Et.al, 2005 pp.425-426). Intragastric administration of the bacterium after induction of colitis produced a significant reduction in intestinal inflammation measured by histology comparable to that of other more conventional treatments, such as corticoids. In the model of mice deficient in IL-10, the daily administration of the bacterium prevented the spontaneous development of colitis (Shanahan, 2000 pp.114-115). The advantages of this therapeutic modality are, on the one hand, the need for a lower effective dose of IL-10 than that required parenterally and, secondly, allows local administration in situ avoiding the drawbacks associated with systemic exposure Of cytokines with high biological activity. The study is promising as it introduces a new concept in the treatment of inflammatory bowel disease by integrating treatment with cytokines and modification of the local microenvironment in the intestine. However, more studies are needed, mainly related to safety, before applying these novel techniques in humans (Kwon and Farrell, 2003 pp.185-186).
Another possibility that has been explored is the use of non-absorbable polysaccharides that, administered orally, increase the saccharolytic activity of the colon flora favoring the overgrowth of native species of the genera Bifidobacteria and Lactobacillus (Campieri and Gionchetti, 1999 pp.1248-1249). An experimental study demonstrated that oral administration of inulin has a very significant anti-inflammatory effect in a model of distal colitis similar to human ulcerative colitis (Figure 2). Several conclusions can be drawn from the results of the animal experiments. On the one hand, the onset of inflammation of the intestinal mucosa is probably related to an alteration in the normal balance of the flora and this situation can be antagonized by manipulating the flora through the administration of ‘probiotic’ bacteria (Kruis, 2004 pp.77-78). Second, the variability of the efficacy of different probiotics, according to the animal model used, suggests that in the case of human inflammatory bowel disease, characterized by its great heterogenicity, it will be necessary to establish indications with specific bacteria according to the subgroup of patients (Dotan and Rachmilewitz, 2005 pp.429-430).
Chapter Three: Methodology
DATABASE SEARCH
In order to find scientific articles that could answer the research question, two databases will be used.
CINHAL (Cumulative Index to Nursing and Allied Health Literature) will be used as the primary database. It is a database specific to scientific publications in the field of health.
PubMED (UK National Library of Medicine, National Institutes of Health), a scientific database of medicine and biomedical sciences, will also consulted.
KEY WORDS AND DESCRIPTORS
In a first step, a list of key words related to the concepts of the research question will be established. This list will be developed using the PICOT tool, which includes the following concepts: population under study, interventions, context, results, and time. The synonyms of the key words found were also retained. They will be transformed, in a second step, into subject-descriptors (MeSH Terms) using the previously described scientific databases.
ELIGIBILITY CRITERIA
Inclusion and exclusion criteria will be used to find scientific articles relevant to the research question.
The articles must be primary scientific articles of total access, in English.
RESEARCH STRATEGY
A review of the literature in CINHAL and PubMed of reviews and Meta-analyzes published until December 2016, which included only adults. The search was limited to English, including combinations of the following terms: “Probiotics, Inflammatory Bowel Disease”.
SOURCES
This research strategy made it possible to retain six primary articles, which could bring useful elements and interesting ideas for research. In order to judge the relevance and quality of the results, these articles will be analysed from the critical reading grid of Fortin (2010).
Chapter Four: CRITICAL ANALYSIS
Probiotics are living microorganisms that, ingested in an adequate amount, exert beneficial effects on health beyond their purely nutritional properties. Although the concept of probiotic has traditionally been associated with lactobacilli and bifidobacteria, we can now include non-pathogenic bacterial strains such as E. coli or other non-bacterial microorganisms such as Saccharomyces boulardii (Tamboli, Et.al, 2003 pp.819-820).
The basic strategy of treating inflammatory bowel disease has so far been to modulate or suppress inflammatory responses and, except for antibiotic treatment studies, little attention has been paid to modifying the intestinal microenvironment in terms of flora . Although the use of antibiotics seems to be the most obvious method of manipulating the flora, its disadvantages, in terms of lack of specificity, risk of pathogen overgrowth, and development of resistance, have led to more subtle and controlled forms being investigated, such as administration Of prebiotics and probiotics, what has recently been called functional food (Ghosh, Van Heel and Playford, 2004 pp.621-622). In addition, the modification of the intestinal flora through probiotics offers the opportunity to act not only from the microbiological point of view, but probably from the immunological point of view. Studies suggest that, in addition to the purely competitive effect with other bacteria and the production of antimicrobial substances, probiotics can modify the epithelial- intestinal immune system by exerting an immunomodulatory effect, since some of them may have anti-inflammatory properties when interacting with Intestinal mucosa. In a study carried out in laboratory, the effect of certain bacteria on the release of TNF-α by the intestinal mucosa of patients with Crohn’s disease has been investigated. Surgical ileum pieces were obtained from patients with Crohn’s disease who had not responded to conventional treatment (Ricci, Et.al, 2014 pp.112-113). After dissecting the mucosa, it was cultured for 24 h with different bacteria with known probiotic properties. The release of TNF-α by the inflamed mucosa of patients with Crohn’s disease was significantly reduced when cultured with Lactobacillus casei. In addition, incubation with L. casei reduced the number of CD4 cells and the expression of TNF-α in intraepithelial lymphocytes. These findings suggest that some bacteria are able to interact with immunocompetent mucosal cells and locally modulate the production of proinflammatory cytokines (Jones, and Foxx-Orenstein, 2006 pp.49-50).
Although in recent years research has been done on the effect of probiotics in patients with inflammatory disease, data are still lacking based on controlled studies to determine its clinical efficacy (Jonkers and Stockbrügger, 2003 pp.170-171).
In two studies (Guarner and Schaafsma,1998; Shanahan, 2001) in children with Crohn’s disease, Lactobacillus GG was shown to have beneficial effects. Administered in the short term (10 days), it induced an increase in IgA secretory cells against lactoglobulin and casein, suggesting a stimulation of the intestinal immune response. When the probiotic was administered in the longer term, for 6 months, it induced a decrease in the permeability of the intestinal mucosa in addition to a reduction in the index of clinical activity.
In two pilot studies (Milner, 1999; Shanahan; 2001), the effect of Saccharomyces boulardii, a yeast that has been shown to be effective in the prevention and treatment of Clostridium difficile-associated diarrhea, has been investigated in patients with Crohn’s disease. Plein et al, in a double-blind controlled study, studied the effect of this probiotic associated with conventional treatment on the control of symptoms in patients with active Crohn’s disease. In the Saccharomyces group, a reduction in the frequency of stools was shown when compared to the placebo group. The study by Guslandi et al assessed the efficacy of S. boulardii as a maintenance treatment associated with mesalazine for 6 months in patients with Crohn’s disease in remission. The rate of relapse in the placebo group was significantly higher than in the yeast treated group (37.5 vs 6.25% at 6 months).
Two studies comparing the efficacy of a non-pathogenic E. coli strain (Nissle 1917) with mesalazine on induction and maintenance of remission have been published in patients with ulcerative colitis. In a first study, 120 patients with ulcerative colitis in remission were enrolled, who were randomized to receive mesalazine at a dose of 1,500 mg / day, or an oral preparation of E. coli Nissle 1917 for 12 weeks. At the end of the study period, the remission rate was similar in both groups (11.3 and 16%, respectively) suggesting that probiotic treatment may be an alternative as maintenance of remission in patients with ulcerative colitis. In a subsequent study, using the same bacteria, probiotic efficacy was investigated in long-term maintenance, 12 months, in 120 patients with active ulcerative colitis. The addition of the bacterium to the standard treatment of active ulcerative colitis did not modify either the rate of remission or the time to obtain it. On the other hand, treatment with non-pathogenic E. coli demonstrated an efficacy equivalent to treatment with mesalazine (relapse, 67% versus 73%, respectively) in maintenance of remission. According to some authors, this study presents some peculiarities that limit the importance of the findings, such as heterogeneity in severity of the outbreak, differences in treatment regimens with corticosteroids and high rate of relapse at the end of the study period. However, the results are interesting and encourage the development of new studies to investigate which bacterial strain, at what dose and for how long it should be used in the treatment of patients with inflammatory bowel disease.
The results obtained with a mixture of probiotics in patients with pouchitis have been more striking. Pouchitis is one of the most frequent long-term complications of patients with ileoanal reservoir. Although the cause is not known, the response to antibiotic treatment suggests that it probably originates from an alteration in bacterial balance, since it is associated with a decrease in the concentration in the feces of lactobacilli and bifidobacteria. The double-blind, placebo-controlled study included 40 patients with an ileoanal reservoir anastomosis with a history of recurrent chronic pouchitis (more than 3 outbreaks per year) who were in remission at the time of inclusion. Patients were randomized to receive probiotic or placebo preparation for 9 months. The probiotic preparation (VSL # 3) included 4 strains of lactobacilli, 3 of bifidobacteria and one strain of Streptococcus salivarius ssp. Thermophillus in the form of lyophilizate containing 5 × 1011 bacteria per gram. The aim of the study was to evaluate the efficacy of the probiotic and to compare it with that of placebo as a maintenance treatment for recurrent chronic pouchitis. At the end of the study period, only 15% of patients in the VSL # 3 group had recurrence, compared to 100% of patients in the placebo group. In addition, all patients who responded to probiotic treatment had a relapse within 4 months after discontinuation (Karthik, 2003 pp.369-370) .
The same mixture of probiotics, in an open study with 20 patients with ulcerative colitis in remission, allergic or intolerant to 5-ASA, proved to be effective as maintenance treatment. The authors justify the use of a mixture of different species of bacteria for two reasons. On the one hand, high concentrations of probiotic bacteria are administered and, on the other hand, the presence of different bacterial species favors a theoretical synergistic effect among them for the inhibition of pathogens. However, it would be highly desirable to determine whether the different strains of probiotics actually have this synergistic effect if all the strains are active and necessary to achieve the anti-inflammatory effect or if the use of the mixture is superior to that of a single bacterial strain.
Even though the pathology of IBDs are related to enteral bacteria and immunity, there is a need for further trials to prove the efficacy of pro and prebiotics in their management. As shown by many studies that probiotics can be handy in IBDs management, but there is a need for further evaluation. Since there is discrepancy between various studies, we need to do further studies and research in order to prove the efficacy of probiotics in the active management of IBDs i.e. in remission and maintenance.
CHAPTER 05: CONCLUSION
Bacteria of the commensal flora seem to play an important role in the mechanisms of inflammation involved in the pathogenesis of chronic diseases of the intestine. There is probably a primary defect in the host that reacts abnormally against a bacterial population with little or no virulence. The investigation of the interaction of the intestinal immune system with the diversity of floral bacteria opens up a wide range of possibilities to improve our knowledge about the pathophysiology of chronic inflammatory diseases of the intestine. Although we have few clinical studies at present, it is likely that in a few years the use of probiotics will occupy an important place in the therapeutic arsenal to prevent and combat inflammatory bowel disease.
The Benefits of retention In Crohn’s disease was examined several times with only one study with a randomized controlled trial design. It could relapse with the use of an enteral regimen. Studies on larger scale areas of expertise in support of endorsement diet in Crohn’s disease for remission support (Jones, and Foxx-Orenstein, 2006 pp.49-50). A combination of enteral nutrition with medication treatment with a small number of falls prospectively, but not randomly, combination with infliximab. Immunomodulation agents in previous studies on the use of omega-3 fatty acids for remission in Crohn’s disease are too heterogeneous to recommend their use (Karthik, 2003 pp.369-370). No effect with respect to remission , administration of omega-3- fatty acids in ulcerative colitis seen. A recommendation to the supplementation of amino acids glutamine and arginine can be dup. There is currently no probiotic for which an effect can be seen in Crohn’s disease (Lomangino, 2013 pp.8-9). While for remission induction with probiotics ECR, there are data from 5 RCTs with postoperative Crohn’s disease, of which 3 with Lactobacilli, one with VSL # 3 and one with a mixture of pro and Prebiotic called synbiotic 2000, which is constantly negative were used (Fedorak and Madsen, 2004 pp.298-299). Although Saccharomyces boulardii has several pharm cobiological properties however, approaches to possible efficiency have been possible so far with no effect in small studies. Probiotics are better at managing remissions in ulcerative colitis as placebo and equivalent to meclizine. Both E. coli Nissle and E. coli were effective also Bifidobakterien (N
All in all, there is a need for further research in this aspect of treatment of IBDs.
References
Bai, A.P. and Ouyang, Q., 2006. Probiotics and inflammatory bowel diseases. Postgraduate medical journal, 82(968), pp.381-382.
Campieri, M. and Gionchetti, P., 1999. Probiotics in inflammatory bowel disease: new insight to pathogenesis or a possible therapeutic alternative?. Gastroenterology, 116(5), pp.1248-1249.
Dotan, I. and Rachmilewitz, D., 2005. Probiotics in inflammatory bowel disease: possible mechanisms of action. Current opinion in gastroenterology, 21(4), pp.429-430.
Fedorak, R.N. and Madsen, K.L., 2004. Probiotics and the management of inflammatory bowel disease. Inflammatory bowel diseases, 10(3), pp.298-299.
Ghosh, S., Van Heel, D. and Playford, R.J., 2004. Probiotics in inflammatory bowel disease: is it all gut flora modulation?. Gut, 53(5), pp.621-622.
Gionchetti, P., Rizzello, F., Morselli, C., Tambasco, R. and Campieri, M., 2006. Probiotics in Inflammatory Bowel Diseases. In Inflammatory Bowel Disease and Familial Adenomatous Polyposis (pp. 124-125). Springer Milan.
Howarth, G.S., 2006. Probiotics and inflammatory bowel disease. Emerging Issues in Inflammatory Bowel Diseases. pp.nd
Iizuka, H., Takaishi, H. and Hibi, T., 2005. Probiotics for inflammatory bowel disease. Nihon rinsho. Japanese journal of clinical medicine, 63(5), pp.779-780.
Jones, J.L. and Foxx-Orenstein, A.E., 2006. Probiotics in inflammatory bowel disease. Pract Gastroenterol, 30(3), pp.49-50.
Jonkers, D. and Stockbrügger, R., 2003. Probiotics and inflammatory bowel disease. Journal of the Royal Society of Medicine, 96(4), pp.170-171.
Karthik, S.V., 2003. Probiotics in inflammatory bowel disease. Journal of the Royal Society of Medicine, 96(7), pp.369-370.
Kruis, W., 2004. Antibiotics and probiotics in inflammatory bowel disease. Alimentary pharmacology & therapeutics, 20(s4), pp.77-78.
Kwon, J.H. and Farrell, R.J., 2003. Probiotics and inflammatory bowel disease. BioDrugs, 17(3), pp.185-186.
Lomangino, K., 2013. Probiotics and Inflammatory Bowel Diseases. Clinical Nutrition Insight, 39(9), pp.8-9.
Marteau, P., 2005. Probiotics and inflammatory bowel disease. Colitis: Diagnosis and Therapeutic Strategies.
Matsumoto, S., Hara, T., Hori, T., Mitsuyama, K., Nagaoka, M., Tomiyasu, N., Suzuki, A. and Sata, M., 2005. Probiotic Lactobacillus‐induced improvement in murine chronic inflammatory bowel disease is associated with the down‐regulation of pro‐inflammatory cytokines in lamina propria mononuclear cells. Clinical & Experimental Immunology, 140(3), pp.425-426.
Prantera, C., Scribano, M.L., Falasco, G., Andreoli, A. and Luzi, C., 2002. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut, 51(3), pp.408-409.
Ricci, A., Tagliacarne, S.C., Valsecchi, C., Boggini, T., Cattaneo, F., Licari, A., Caimmi, S. and Castellazzi, A.M., 2014. PROBIOTICS AND INFLAMMATORY BOWEL DISEASES. Journal of biological regulators and homeostatic agents, 29(2 Suppl 1), pp.112-113.
Sartor, R.B., 2004. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology, 126(6), pp.1632-1633.
Schultz, M. and Sartor, R.B., 2000. Probiotics and inflammatory bowel diseases. The American journal of gastroenterology, 95(1), pp.S20-S21.
Shanahan, F., 2000. Probiotics and inflammatory bowel disease: is there a scientific rationale?. Inflammatory bowel diseases, 6(2), pp.114-115.
Shanahan, F., 2001. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology, 120(3), pp.634-635.
Shanahan, F., 2004. Probiotics in inflammatory bowel disease—therapeutic rationale and role. Advanced drug delivery reviews, 56(6), pp.817-818.
Sheil, B., Shanahan, F. and O’Mahony, L., 2007. Probiotic effects on inflammatory bowel disease. The Journal of nutrition, 137(3), pp.823S-824S.
Tamboli, C.P., Caucheteux, C., Cortot, A., Colombel, J.F. and Desreumaux, P., 2003. Probiotics in inflammatory bowel disease: a critical review. Best Practice & Research Clinical Gastroenterology, 17(5), pp.819-820.
Guarner F, Schaafsma G. Probiotics. Int J Food Microbiol 1998;39:237-8.
Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology 2001; 120:622-35.
Milner JA. Functional foods and health promotion. J Nutr 1999;129:S1395-7.
Shanahan F. Probiotics in inflammatory bowel disease. Gut 2001;48:609.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




