Potential Therapeutic Effect of Ground Flaxseed on Begnin Prostatic Hyperplasia
Info: 8262 words (33 pages) Dissertation
Published: 9th Dec 2019
Tagged: Food and Nutrition
Research Assignment Part One: The Potential Therapeutic Effect of Ground Flaxseed on Begin Prostatic Hyperplasia
Question:
Is there a therapeutic effect of phytoestrogens, containing hydroxylated bicyclobenzylbutane diol, such as lignans, on begnin prostatic hyperplasia.
Target Population:
The target population of this therapeutic treatment would be for older males ranging from 40 years of age and older. The optimal population would include males with begnin prostatic hyperplasia rather than metastatic prostatic hyperplasia, in which a more aggressive form of treatment might be needed.
Intervention:
Administration of higher dosages of lignan extracts around 600 mg of lignin via flax seed extracts tablets. It has been shown in a particular study that the higher dosage of 600 mg of lignin extract resulted in a greater impact on the reduction begnin prostatic hyperplasia, as well as its lower urinary tract symptoms (LUTS).
Comparative Treatment/Population:
Ideally the study of interest should include a couple of study groups, with varying dosages of lignan extracts from flaxseed supplementation administered to the studies participants. There should be one treatment group that will act as a placebo comparative group, to which the treatment groups can be compared against to find any significance. There also needs to be a baseline to which all the groups need to be compared to, including the placebo group, to find if there is any significant difference from this baseline data, and to possible uncover if there is a placebo effect.
Defining Outcomes:
The resulting observational data will be quantified through clinical measurements which will hopefully include secondary measures such as prostate and bladder volumes, in which the residual volume would be calculated by the initial bladder volume minus the empty bladder volume 11. Secondary outcomes such as urine flow (Qmax) and changes in blood concentration of secoisolariciresinol (SECO), enterodiol (ED) and enterolactone (EL) 11. Primary therapeutic measures, such as the overall quality of life and decrease in the overall Begnin Prostatic Hyperplasia symptoms could be measured through international Prostate Symptom Scale (IPSS), quality of life (QOL), survey scores as well as the ratings in the lower urinary tract symptom questionnaire 11. These LUTS measurements would be compared between groups with the placebo, and within each group for individual variation 11.
Etiology, Prevalence, Justification:
The most common agreed upon mechanism of how benign prostatic hyperplasia occurs, is due to an imbalance in the synthesis of dihydrotestosterone from the conversion of testosterone via a 5-alpha reductase enzyme 11. This increases dihydrotestosterone levels within circulation or within the prostate tissue, which then binds alpha-adrenergic receptors, resulting in an increase in prostate tissue 11. I was unable to find clear statistics for the proportion of males with benign prostatic hyperplasia within Canada, which could possibly be due to the idea that because it is not a metastatic cancer, and usually required minimally invasive treatments, especially if it is has small to medium LUTS, maybe the male population is not reporting it to the system. However, multiple longitudinal and cross-sectional studies have been performed with a large range of males from different ethnicities and socioeconomic statuses and have found there to be an increase in the prostate size from 25g to 30g for males in their 40s, from 30g to 40g for males in their 50s and from 35g to 45g in males in their 60s 9. Pharmaceutical prostatic reductive agents such as Finasteride have been seen to be very effect at reducing not only the symptoms of begnin prostatic hyperplasia but also effective at reducing the prostate mass and volume. However they have been found to increase the rates of erectile dysfunction, decreased libido, depression and anxiety 10. Lignan extracts from flaxseed supplementation could be used as a natural alternative with similar reductive effects as its pharmaceutical counterparts all the while lacking the same potential adverse side effects and also having positive synergistic cardiovascular health benefits due to potential anti-oxidant components of the flaxseed supplementation besides the lignan extracts.
Searches:
Pubmed (Limitations, Species: humans, randomized, prostatic hyperplasia)
Cochrane Library (Limitations: none, benign prostatic hyperplasia)
| Database | Search Terms | PubMed MeSH terms or MeSH classification tree | Limits Set | Number of Articles Search Retruned |
| PubMed | Prostatic Hyperplasia + Lignans | Benign Prostatic Hyperplasia
Lignans |
Language: English
Species: Human |
7 |
| Cochrane Library | Benign Prostatic Hyperplasia + phytoestrogens | N/A | None | 2 |
| Pubmed | Benign Prostatic Hyperplasia + Lignans | Benign Prostatic Hyperplasia
Lignans |
Language: English
Species: Human |
7 |
| Pubmed | Benign Prostatic Hyperplasia + phytoestrogens | Benign Prostatic Hyperplasia
Phytoestrogens |
Language: English
Species: Human |
12 |
To start the research process, I searched for systematic reviews on the Cochrane Database to gain a generalized understanding of whether or not lignan extract supplementation has been studied to a descent degree in regards to its potential reductive effects with begnin prostatic hyperplasia and whether or not there is a significant correlation. I did not find a systematic review within the Cochrane database. From here I decided to dig deeper and search archival article databases such as PubMed, Web of Science, and Practice-based Evidence in Nutrition due to the fact that I wanted to see if whether or not lignan extract supplementation administered to patients to combat Begnin Prostatic Hyperplasia utilized in the clinical setting, has yielded any therapeutic effects. I used the both the search terms, Begnin and Prostatic hyperplasia to cover both the cancerous form and not cancerous form in conjunction I used both the phytoestrogen and lignan MeSH terms to add to the search parameters. Lastly I used the Nutrition database to find different lignan extract supplementations that are available on the market currently, from which I could determine cost effectiveness of the therapeutic treatment as well as to gain an idea of what real-world supplementation exists. I searched for market supplementation and dosages that mimic the dosages administered in the given studies to replicate the obtained results from the observed studies.
CAP: Part 2
Possible Reductive Effects of Lignan Extracts from Flaxseed husks in Regards to Benign Prostatic Hyperplasia
Systems Involved: The human male Urinary and Reproductive system
Therapeutic Modality Used: Phytoestrogenic, flaxseed containing Lignan extracts.
Paper Title: Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia (BPH)
Authors: Zhang, W., Wang, X., Liu, Yi., Tian, H., Flickinger, B., Empie, W, M., Sun, Z, S.
Journal: Journal of Medicinal Food
Publication: 2008, 207-214
Aim: The aim of this research was to determine whether lignan extracts obtained from flaxseed will reduce the lower urinary tract symptoms (LUTS), reduce IPSS, increase the maximal urinary flow rate (Qmax) and the quality of life (QOL) of individuals containing Benign Prostatic Hyperplasia (BPH) 11.
Design: Double-blinded Randomized Control Study, with human subjects.
Setting: The article did not explicitly state the location at which this experiment was performed, however, the recruitment and experimental methods protocol were approved by board of the Tumor Hospital and Institute of Beijing China, the Peoples Republic 11. It is therefore safe to assume that the study did occur in Beijing China at the Tumor Hospital and Institute 11. It is also worth noting that the participants recruited for the study were comprised of Asian middle aged males 11.
Participants: 87 Chinese participants falling within the age range of 55-80, diagnosed with BPH, were recruited for this double-blinded randomly controlled study 11. Demographic information was also included during the inclusion process which included age, medical history, marriage status, occupation, education level and current medications 11. Participants needed to have a prostate volume of greater than or equal to 30 cm3, a IPSS score greater than or equal to 7, and a Qmax at around 5 to 15 mL/second 11. Other biochemical measurements included in the screening were blood serum levels of glucose, triglycerides, total cholesterol and creatine, Refer to Table1 11. Inclusion criteria for the participants also enforced the fact that the subjects were not to be sensitive to any flaxseed extracts. Blood serum levels of gamma-glutamyl transferase, aminotransferase, serum creatinine <110 micromol/L, and blood urea nitrogen <7.1 mg/dL were normal in the included patients Refer to Table 1 11. The clinical measurements that were performed on the participants included a genitourinary tract physical examination, digital prostate rectal examination, urine bacterial count, urinary and prostatic volume urinary information (Qmax and average flow rate) was recorded 11. Lastly the residual volume of the participants was calculated by the difference between the initial urinary volume and the voided bladder volume Refer to Table 1 11. All participants who met the study requirements and willingly accepted to participate in the study read and signed a consent form.
Figure 1:
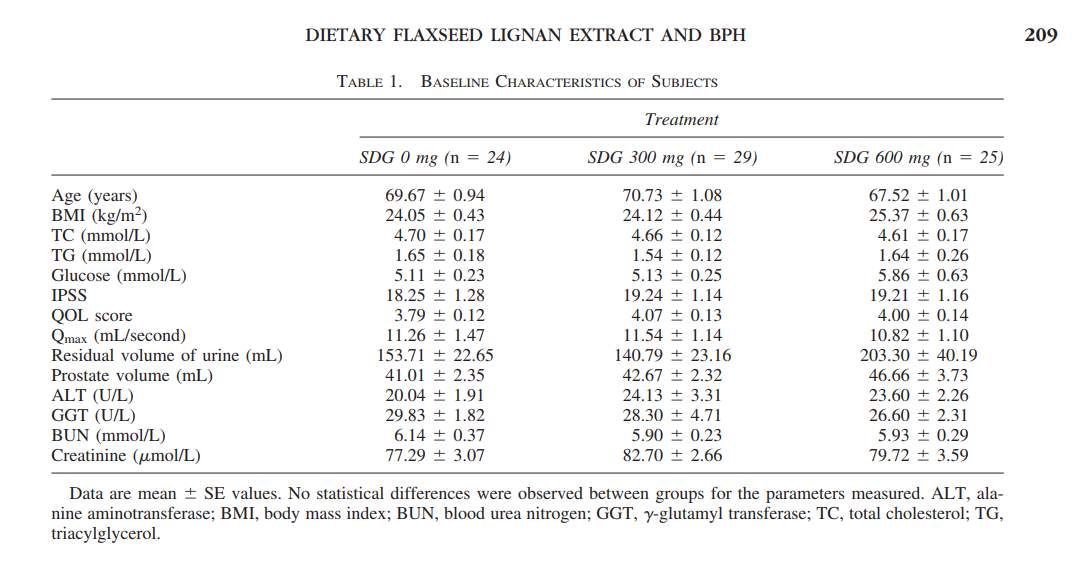 Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
Intervention:
Participants were assigned to three groups, two of the groups being the intervention treatment group and the last group being the comparative placebo group 11. The groups were determined based on the amount of seciosolariciresinol diglucoside (SDG), a principal lignan found in flax seed, was delivered to the subjects of each of the groups 11. The subjects allocated to the placebo comparative group received 0 mg of SDG whereas the two treatment groups received either 300 mg of SDG for one intervention group and 600 mg of SDG for the other intervention group 11. The prepared SDG supplemental intervention was administered in tablet form and contained 33.0% SDG, 15.1% coumaric acid glycoside, 8.1% of Ferulic acid glycoside, 15.6% hydroxylmethylglutaric acid and the rest constituents were water and other components 11. These tablets were designed to deliver the daily SDG dose twice a day, with two tablets given each time it was administered 11. The placebo intervention tablet was comprised of food-grade maltodextrin with food-grade coloring to give it a color of that of the flax seed extract (brownish in color) 11. In addition to flaxseed lignan extract or placebo material, tablets were composed using commonly used excipient ingredients 11 .
Outcome Measures:
The primary lignan SDG breaks down into the metabolites, enteroiol (ED), enterolactone (EL), and Secoisolariciresinol (SECO) which lacks the diglycoside 11. ED and EL have been documented to act as an inhibitor of the 5 alpha reductase enzyme which is known to catalyze the transformation between testosterone and dihydrotestosterone 11. This information is important because the researchers monitored the blood serum levels of SECO, ED and EL in the two intervention groups when SDG was being administered as well as in the participants in the placebo comparative group 11. A 20 mL blood sample was taken at the beginning of the study, at the two month mark and at the four month mark, after an overnight fast and for all three of the groups; Refer to Figure 2 11. Other physiological measures were taken for each of the subjects in the three groups which included their Qmax, their prostate volume residual volume of the bladder (as mentioned above), their International Prostate Symptom Score (IPSS), and their Quality of Life score (QOL) see Figure 3 11. The researchers also recorded the change in the subjects IPSS and QOL score from the recorded baseline through survey input, alongside measuring blood serum contents of the primary lignan SECO and its metabolites, ED and EL; See Figure 4 11. The experimental data obtained from the administration of the flaxseed lignan extracts were compared to pharmaceutical 5-alpha reductase inhibitors, alpha-reductase receptor inhibitors and other botanicals; see Figure 5 11.
Figure 2:
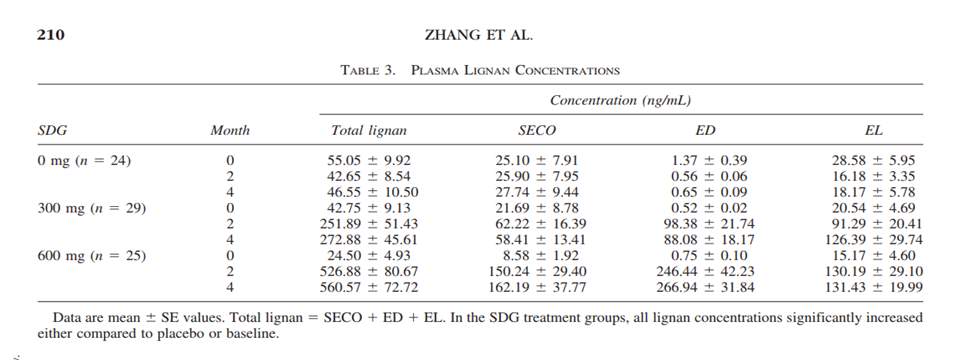
Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
Summary of Findings:
79 out of the 87 participants completed the study, with 9 subjects dropping out of the study before completion 11. Four of the nine patients who dropped out, required alternate treatment, two of the subjects had a significantly large in magnitude travel schedule, one participant needed to be relocated and the last two participants did not give a reason as to why they dropped of the study before completion 11. The researchers observed a changed within the Qmax, IPSS, QOL, residual volume, prostatic volume and in the number of subjects containing mild LUTS 11. There were significant reductions in the IPSS score in the subjects of all groups at the two month mark, with reductions in the placebo group of -6.0+/-1.55, reductions within the 300mg SDG group of -4.97+/-1.32 and reductions in the 600mg SDG treatment group of -7.0+/-1.41; Please refer to Figure 3 11. However only the participants allocated to the two SDG intervention groups (300mg, 600mg) displayed a significant decrease in their IPSS at the four month interval mark, -7.33 +/- 1.18, -6.88 +/-1.43 respectively; Please refer to Figure 3 11. The degree of significance in the decrease in the IPSS of the SDG groups at both the two month and four month marks compared to the baseline was greater than the significance found at the two month mark of the placebo comparative group 11. The significance found at the two month mark for the placebo comparative group had a p-value of less than 0.05, whereas the p-value for the 300mg and 600mg SDG groups was less than 0.01; Please refer to Figure 3 11. This is to say there is less of a probability that the data obtained from the SDG treatment groups is less likely due to chance. There was no significant difference found between groups however for the reduction in the IPSS’s obtained from the subjects in all of the groups 11. There was a significant difference found between groups, between the SDG intervention groups and the comparative placebo group, for the QOL score only for the 600 mg SDG intervention group, at both the two and four month time intervals 11. There was no significance found between the three study groups, one placebo comparative group and two intervention groups, for either the 0mg SDG or 300mg SDG groups, at either the two month or four month time interval; Refer to Figure 3 11. Within groups there was a significant difference found at months two and four, for both of the SDG intervention groups 11. This significance was found between each of the data points found in the three groups compared to the baseline, and occurred at both of the two month and four month intervals for the two intervention groups, with a p-value less than 0.01 for the 300mg SDG group at the two month mark 11. The p-value attributed to the 300mg SDG group at the four month mark and for the 600mg SDG group at both the two and four month time intervals, was less than 0.001; Refer to Figure 3 11. The within group significance for the 0mg SDG group however, only occurred at the two month mark, with a p-value less than 0.05, losing this significance at the four month mark with a p-value greater than 0.05 11.
Figure 3:

Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
At the four month mark there was an increase in the Qmax measure from the comparative baseline data which resulted in the production of the ranges of 0.43 +/- 1.57, 1.86+/- 1.08, and 2.7+/-1.93 mL/second Qmax measures in the placebo comparative group, the 300mg SDG and 600mg SDG groups respectively; Refer to Figure 3 11. The large magnitude of response range and variance as well as the small population size of each of the groups, could have led to a lack of significance for the between group data and for the within group data 11. There was no significant difference between the residual bladder volume for either within group data or between group data 11. There was however a significant reduction of the prostate volume from the base line in the placebo group and both of the intervention group, however there was no significant difference in the between group data, when compared to the placebo, 0mg SDG group; Refer to Figure3 11. It was reported that there was a reduction in the LUTS for patients in the treatment groups from moderate/sever to mild after four months 11. Lastly the total lignan, SECO, ED and EL all lead significant reductions in the IPSS and QOL but only the total lignan concentrations and EL were correlated with an increase in the Qmax ; Refer to Figure 4 11. Referring to Figure 4, all of the correlations with the change in IPSS, QOL and Qmax are negatively correlated with the concentrations of SECO, ED and EL indicating, the reciprocal affect the metabolites of the lignin extract have on the IPSS, QOL and Qmax measures 11. Lastly, no adverse were reported, and the blood serum levels of the urea nitrogen, creatine, alanine aminotransferase and gama-glutamyl transferase were normal 11.
Figure 4:
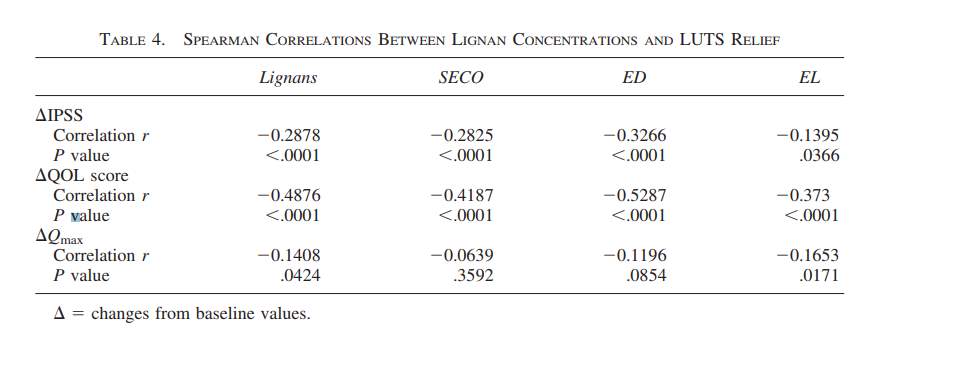
Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
Cost Effectiveness/Conflict of Interest: Ground concentrated lignan powder dietary supplementation, can be found through many companies, and is available through Heartland Natural. The concentrated lignan concentration dietary supplementation powder supplied by Heartland is available a tub unit of 150 grams 5. According to Heartland there is 223 mg of SDG per serving, however this could be adjustable to mimic the quantities found to have a significant effect on Begnin Prostatic Hyperplasia according to the target study, which would be 300 and 600mg serving sizes 5. It was calculated that at the serving size of 300mg tablets, two tables, twice a day, the volumetric tub containing 150 grams of concentrated lignan would last 125 days. The individual would have to purchase the volumetric tub approximately 2-3 times per year if they were to ingest the correct servings outlined as significant by the current study. At a cost of 19.95 per tub, an individual would spend up to 40-60 dollars per year on this dietary supplementation at a serving size of 300 mg four times per day. If the serving size is 600mg, twice in the morning and twice at night, to replicate the studies parameters, then the individual would have to obtain a new tub roughly six times a year if they are following the serving parameters every single day. The individual would as a result spend roughly around 120 dollars per year on the concentrated lignan extract powder a year from Heartland Natural.
In comparison if an individual were to take Finasteride, one of the most popular pharmaceutical 5 alpha reductase inhibitors on the market, the price hike in comparison to the lignin dietary supplement is significant. This price hike in comparison to the lignin dietary supplementation is true for all of the quantity options available 5 mg tablets of Finasteride, including the 30 tablet, 60 tablet and 180 tablet options 3. Individuals are instructed to take one 5mg tablet a day, daily, to reduce the prostatic volume that accompanies begnin prostatic hyperplasia 7. If an individual to purchase the 30 tablet option for $29.00 dollars, and take one tablet a day, every day, the individual would have to purchase a new batch of tablets ever month and spend roughly 348 dollars per year 4. If that individual would purchase the 60 tablet option for $41.00, that individual would have to refill on tablets six times a year if purchase behavior is constant and spend roughly $249.28 per year. Lastly if an individual were to purchase the 180 tablet option for $92.00, they would have to refill approximately twice a year if they purchase behavior and tablet intake is constant and spend roughly around 184 dollars on Finasteride a year. Taken all together even with the smallest price hike with the 180 tablet option for Finasteride, dietary supplementation of lignan is cheaper per year even at its higher dosage of 600mg at four times a day and could be considered to be cost effective and economically feasible. However, although the lignin dietary supplementation is significantly more cost effective the available benign prostatic hyperplasia pharmaceuticals, it might be less feasible in the sense that it requires more discipline to self-administer the dietary lignin supplementation compared to take Finasteride. This is due to the fact that self-administration of finasteride requires only one tablet to be taken a day, in comparison to the dietary lignin supplementation in which an individual is required to take it four times a day in order to reproduce the reductive effects found in the study mentioned above.
Authors Conclusion: The conclusion that the authors stated, was one in which dietary lignan extracts from flax seeds seems to be culpable in the reduction of LUTS of individuals with BPH, as well as increasing the quality of life with these individuals 11. Lastly it was observed that the lignan SDG extracts from the flax seeds seemed to produce a reduction in subjects prostate volume comparable to that of other botanical medicines as well as 5 alpha-reductase and alpha-adrenergic receptor pharmaceutical blockers which has been seen by other studies to have a significant decrease in the prostatic volume Refer to Figure 5 11.
Figure 5:
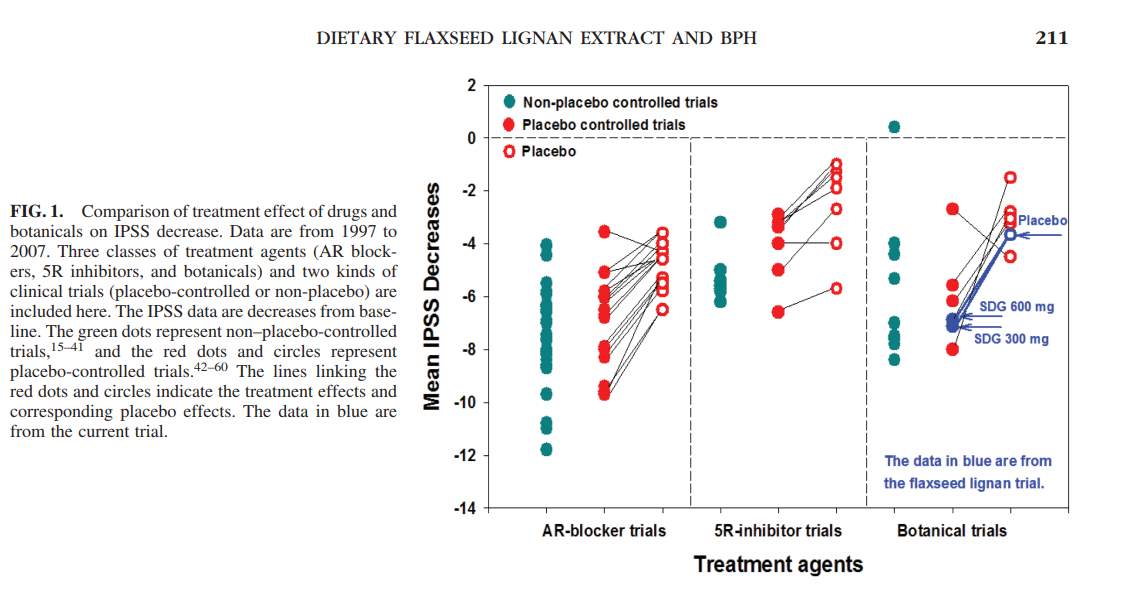
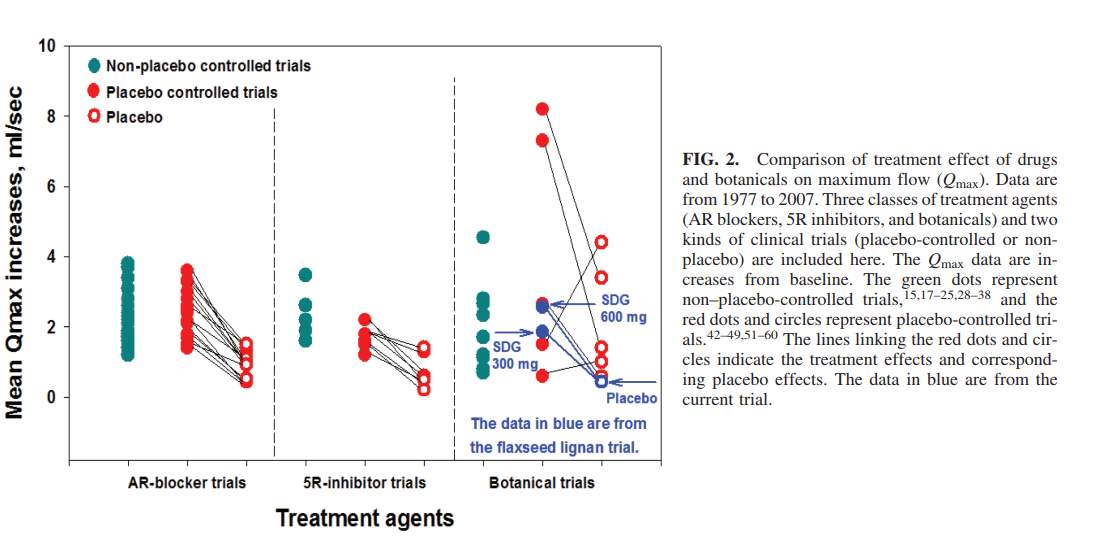
Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
Quality of Evidence: The evidence relayed by this study should receive a CAP grade B, as a result that there is more than one well designed RCT in which the data agrees with the double blinded RCT study analyzed above. However the reason why it should not receive an A grade is because the sample subject size analyzed in these studies is small and the time period over which it was performed is not very long, with a maximum of four months. This could have resulted in the lack of between group significance found in the primary study and in other RCT’s indicating that the studies power could have been more significant.
Discussion:
This study has many positive strengths because it met the gold standard of research in regards to the fact that it was a double blind randomized controlled study which largely eliminates both selection and assessment biases. The experiment also contained two intervention groups, which led to greater exploration depth of the potential remedying effects of the lignan flaxseed extracts. The fact that the researchers performed a plethora of biochemical and demographical screenings at the beginning of and before the study had begun, resulted in the authors being able to control for certain possible external confounding variables in regards to the participants.
The study also contained a few limitations in regards to the validity of the data obtained in the study. The first confounding variable is that the participant population size was quite small, which could have been due a lack of access to adequate funds 11. If there is minimal to little funding of a study, it is very hard to acquire and retain participants for the study in question, and therefore with a smaller population size of participants it can lead to issues regarding external validity. The issue regarding external validity is that a small population size is not representative of the greater population which exists in society. Smaller population sizes for studies as well amplifies individual variation, which can result in an increased size of a confidence interval, increase data variation and therefore reducing the possible significance of these data points, in contrast to a larger population. Studies with a larger population base would have minimized individual variation which would lead to a more balanced data spread and a more reliable correlation with a narrower confidence interval. The smaller the population size, the less data points, which leads to individual data point variance to be amplified, resulting in a greater amount of outliers and a less reliable correlation. The second external confounding variable is that for all three of the study groups, the maximum time span studied was up to four months 11. If the researchers were able to examine the patients for a longer period, the data and conclusions stated in this study may have been different in many ways. Only a few more studies of been performed in regards to determining a possible correlation between Lignan extracts and Begnin Prostatic Hyperplasia, where the subjects were human, and these other studies, had time lines of either 30 days (one month) or eight weeks (two months); performed by Simons, R., Sonawane, N., Verbruggen, M., & Chaudary, J. and Demark-Wahnefried et al respectively 1,2,8. It seems as though there has not yet been a study that has tried to investigate the effects of supplemental and dietary Lignan extracts on begnin prostatic hyperplasia or prostatic hyperplasia for a longer period than four months, in which this target study by Zhang et al has touched upon. The observed data might have been different if a longer longitudinal time period was allocated, due to the fact that the confounding placebo effect viewed in this study as well as others, such as by Simons, R., Sonawane, N., Verbruggen, M., & Chaudary, J., might been separated and disentangled from the other observational data. A longer time period might allow the observed placebo effect to become minimized if there is actually a correlation between the therapeutic treatment of lignin extracts and Begnin Prostatic Hyperplasia. If there is not a therapeutic effect then the reductive data seen in the short term studies in the treatment groups, would potentially decease back towards the baseline.
A significant internal confounding variable, in which the authors of the study acknowledged, was that the SDG intervention supplement contained other substances besides the SDG component such as 15.1% coumaric acid glycoside, 8.1% of Ferulic acid glycoside, 15.6% hydroxylmethylglutaric acid and the rest constituents were water and other components 11. The increase in the quality of life, the reduction in BPH and LUTS symptoms and the reduction seen in the prostatic volume could have been due to these other components or possibly be a result of a synergistic effect between all of these components. The coumaric acid glycoside component, has anti-inflammatory and anti-oxidant properties, which could have resulted in the reduction in IPSS scores, an increase in quality of life, and the reduction of prostatic volume found by this target study. The authors did not mention how the flaxseed lignan extract tablets or the placebo tablets were administered to the study participants, possibly reducing the control of potential assessment bias that the study tried to implement with its double blinded nature. It was only stated by the researchers that the lignan extract tablets and placebo tablets were administered twice a day, once at breakfast and once at dinner 11. The subjects were also instructed to not change their regular daily routines, however it was not stated how the lignan extract or placebo tablets were physically administered to the subjects, what environment they were in nor the emotional state they were in at the time of each administration. Observed in this study the placebo comparative group did display some significant therapeutic effect, in which could have accounted for some of the beneficial effects even within the intervention groups because of the same administration process and similar perceivable tablets. This placebo effect limitation comes into fruition when the researchers found a significant reduction in prostatic volume across all three groups including the comparative 0mg SDG group, compared to the baseline. For the prostatic volume three was no significant between group prostatic volume reductions, with the comparison between the placebo and treatment groups. Lastly, another possibly internal validity confounding variable could be due to the fact that the authors of this study only monitored the triglyceride and total cholesterol levels in the subjects blood serum in all three of the groups, where as another study performed by Demark-Wahnefried et al, added another variable group which had a low-fat intake diet of the participants 2. The reason for the decision to add another comparative variable group which isolates and separates the lignan extract supplementation factor from a supplementation or diet containing low fat which eliminates this possible internal validity confounding variable. The reason for low fat intake being a possible confounding variable is that it has been found by previous research is that diets with low fatty intake also have been observed to have beneficial reductive effects on begnin prostatic hyperplasia 2. Therefore it would have benefited Zhang et al to also create another variable group separating the low fat diet variable and the lignan extract variable to have a better determination as to which variable correlates with the reductive effects on the enlarged prostate.
CAP: Part Three
Possible Reductive Effects of Lignan Extracts from Flaxseed husks in Regards to Benign Prostatic Hyperplasia
Students need to perform future research into possible correlations between lignan extracts and prostate hyperplasia with a greater longitudinal time frame. The longest human subject study which tried to observe this correlation to date only had a temporal frame of four months, which was not enough time to tease the potential placebo effect from the possible therapeutic effects of the lignan extracts. A greater amount research needs to be done to compare the efficacy of the pharmaceutical BPH reductive agents mentioned previously in Part two and the efficacy of the reductive effects lignan extract supplementation, such as the comparison that was done in this study by Zhang et al. Lastly, studies need to be done to analyze possible synergistic effects of lignans with isoflavones in Soy or lycopene’s in tomatoes etc.
In the study above, it was displayed in the results that there was a significant increase in the QOL score, and therefore the quality of life, of the participants of the study 11. It was also shown that there was a reduction in the IPSS score, which includes urinary symptoms associated with prostate enlargement, as well as a reduction in the prostatic volume itself 11. The time frame in which the Zhang et al study was performed was as long as four months, and resulted in the observable reductive data within this time frame 11. In order to have appropriate patient application to obtain and reproduce the reductive effects on begnin prostatic hyperplasia found in the Zhang et al, study, patients must be instructed by their practitioner to ingest roughly 300 or 600mg four times a day of supplemental lignan extract 11. The patients can utilize options of ingestion such as, if they are able to find the supplementation in the form of tablets and capsules, or they can sprinkle ground up flaxseed onto their breakfast cereal and add it to their dinner meals or they can sprinkle supplemental lignan extract powder onto their meals. A good meal to mix the ground flax or the powdered lignan extract with is with beans or vegetables, such as with cooked vegetables because of their soft texture and easier to chew and swallow which could prevent potential lodging or partial blockages that might occur.
The question of whether or not there is a therapeutic effect of phytoestrogens, containing hydroxylated bicyclobenzylbutane diol, such as lignans, on begnin prostatic hyperplasia; has been somewhat answered by the study provided by Zhang et al, as well as a few other studies. In multiple human subject studies including the study analyzed by Zhang et al, it has been found and replicated that the overall quality of life and lower urinary tract symptoms of individuals containing BPH, was altered significantly by supplementary lignan extracts with varying dosages 11. The significant increase in quality of life and the significant decrease in lower urinary tract symptoms were also observed temporally over many months in some of the studies 11. It was also seen in the Zhang et al study, that there was also a reduction in the prostate volume compared to the baseline 11. However, the quality of life and lower urinary tract symptoms were obtained through subjective measures via survey answers from the participants rather than objective measures. The reduction in the prostate volume of participants found in this study was only significantly different from the baseline and not between groups compared to the placebo group and was only found in this study 11. A lot of the significant values found in this study and in others must be taken also with caution due to a larger confidence interval existing as a result of the studies small population size. Lastly more studies need to be done with greater temporal frames, in order to see the long term effects on BPH. It seems as though there is a consensus that lignan extract supplementation does help individuals with quality of life and reducing BPH urinary symptoms, and therefore in a sense answers the question initially proposed.
As mentioned earlier there has only been a few studies performed that have tried to uncover if whether or not phytoestrogenic lignan extracts have a therapeutic effect on BPH within human individuals. Two prominent studies performed by Zhang et al and Simons, R., Sonawane, N., Verbruggen, M., & Chaudary, J. found an increase the quality of life and decrease in enlarged prostatic symptoms through measures such as QOL, IPSS and AUASI (American Urological Association Index) 11 8. The authors, Simons, R., Sonawane, N., Verbruggen, M., & Chaudary, J. found a significant reduction in urinary obstructive symptoms, and an increased management of irritable BPH symptoms compared to the baseline for the placebo, high dose and low dose groups reflected by the AUASI questionnaire 8. Therefore there was no significant between group differences 8. The high dose group received one 100mg SDG capsule and one maltodextrin placebo capsule, whereas the high does group received two capsules each containing 100mg of SDG 8. This study reflects and supports the study of interest performed by Zhang et al, where there was an overall increase in the quality of life and decrease in BPH symptoms of the study participants and the existence of a significant placebo affect compared to baseline in both studies. It was also found that DHT (dihydrotestosterone) levels were significantly reduced in both the low and high dose treatment groups significantly 8. The only difference between the two studies was that Zhang et al found a significant reduction in the prostate volume whereas Simons, R and company did not.
There has been a significant amount of research done however in regards to the therapeutic effects of the lignan extracts on prostatic hyperplasia in its cancerous form. It has been displayed in a study performed by Azrad, M.et al that an increase in the SDG metabolites, enteroiol (ED) and enterolactone (EL) lead to an inversely proportional decrease in the prostate specific antigen’s (PSA) Ki67 and VEG1 (indication of vasculature growth) 1. However, there was only an significant inverse relationship found between the pre-mentioned SDG metabolizes and Ki67, indicating that the administered flaxseed supplementation delivered to the patients at a dosage of 30g/day for thirty days, had a therapeutic effect on the enlarged cancerous prostate 1. This reduction in the PSA levels of Ki67 in response to increased levels of enteroiol (ED) and enterolactone (EL) was also found by Demark-Wahnefried, W. et al, except their study also incorporated and extra variable group of just a low-fat diet and not flaxseed supplementation 2. Flaxseed supplementation dosage was also 30g/day for thirty days 2. A lot fat diet can also have therapeutic reductive effects on an enlarged prostate and the authors therefore wanted to separate the possible therapeutic effects of flaxseed supplementation and a low-fat diet. These results again support the idea that lignan extracts from flaxseeds do have a therapeutic effect on begnin prostatic hyperplasia, as well as with cancerous prostatic hyperplasia. Lastly there was a study performed by Hong SJ, Kim SI, Kwon SM, Lee JR, Chung BC. which yielded conflicting results, where they found that there was no significant difference between levels of lignan metabolites, ED and EL, in the blood plasma and extracted prostatic tissue of Asian men subjects who had bladder cancer when compared to middle aged Asian men who had BPH 6. However they did find a significant difference between the two groups in regards to isoflavone levels, genistein, due to a high soy diet, with the BPH comparative group having significantly lower levels of isoflavones in their blood serum and prostatic tissue 6. The authors as a result concluded that reduction in BPH symptoms and prostate size was due to the influence of isoflavones rather than lignan sources 6.
The information gathered from parts one through three; indicate that there is a therapeutic effect to be found in regards to lignan extracts from flaxseed supplementation and begnin prostatic hyperplasia. Numerous of studies support this idea, including the target study in which they found an overall increase in the individual’s quality of life and a reduction in prostatic hyperplasia symptoms. The study performed by Zhang et al, also found lignan extract supplementation to be just as effective as other botanicals and pharmaceutical 5 alpha reductase inhibitors and AR blockers 11. However Zhang, et al was the only study to find a significant reduction in prostate volume compared to baseline for all groups, but not when compared to the placebo comparative group, and was the longest study temporally compared to the others which did not find a significant reduction compared to the baseline 11. Therefore more studies need to be performed before it can be stated that lignan extracts have a strong correlation with reducing prostate volume. However there was conflicting results by Hong SJ, Kim SI, Kwon SM, Lee JR, Chung BC, who found no significant difference between the two case report groups in blood serum and prostatic tissue concentrations of ED and EL but a significant difference between isoflavone, genistein levels 6. This could indicant that it is not the SDG metabolites alone that have a therapeutic effect in lignan extracts but also other synergistic components. Lastly it was found that this therapeutic treatment would be cost effective compared to the available pharmaceutical inhibitors and blockers on the market presently, however a practioner should be careful when administering this treatment and should not use this treatment plan with a thought to reduce the prostate volume, but rather to have the mindset that it will reduce BPH symptoms and increase the overall quality of life of the patient.
References:
- Azrad, M., Vollmer, R. T., Madden, J., Dewhirst, M., Polascik, T. J., Snyder, D. C., . . . Demark-Wahnefried, W. (2013). Flaxseed-Derived Enterolactone Is Inversely Associated with Tumor Cell Proliferation in Men with Localized Prostate Cancer. Journal of Medicinal Food, 16(4), 357-360. doi:10.1089/jmf.2012.0159
- Demark-Wahnefried, W., Polascik, T. J., George, S. L., Switzer, B. R., Madden, J. F., Ruffin, M. T., . . . Vollmer, R. T. (2008). Flaxseed Supplementation (Not Dietary Fat Restriction) Reduces Prostate Cancer Proliferation Rates in Men Presurgery. Cancer Epidemiology Biomarkers & Prevention, 17(12), 3577-3587. doi:10.1158/1055-9965.epi-08-0008
- Finasteride Dosage Guide with Precautions. (n.d.). Retrieved April 07, 2018, from https://www.drugs.com/dosage/finasteride.html#Usual_Adult_Dose_for_Androgenetic_Alopecia
- Finasteride 5 mg Price Comparisons – Discounts, Cost & Coupons. (n.d.). Retrieved April 07, 2018, from https://www.pharmacychecker.com/generic/price-comparison/finasteride/5 mg/
- Flax naturally concentrated lignans – 5.3oz powder. (n.d.). Retrieved April 07, 2018, from http://store.heartlandnatural.com/product_p/flaxnatlignanpowder.htm
- Hong, S. J., Kim, S. I., Kwon, S. M., Lee, J. R., & Chung, B. C. (2002). Comparative Study of Concentration of Isoflavones and Lignans in Plasma and Prostatic Tissues of Normal Control and Benign Prostatic Hyperplasia. Yonsei Medical Journal, 43(2), 236. doi:10.3349/ymj.2002.43.2.236
- Product review submission form. (n.d.). Retrieved April 07, 2018, from https://www.qualityprescriptiondrugs.com/pricedetail.aspx?drugname=finasteride&affiliate=1157
- Simons, R., Sonawane, N., Verbruggen, M., & Chaudhary, J. (2015). Efficacy and Safety of a Flaxseed Hull Extract in the Symptomatic Management of Benign Prostatic Hyperplasia: A Parallel, Randomized, Double-Blind, Placebo-Controlled, Pilot Study. Journal of Medicinal Food, 18(2), 233-240. doi:10.1089/jmf.2013.3129
- Roehrborn, G, C. (2005). Benign Prostatic Hyperplasia: An Overview. Reviews in Urology, 7(Suppl 9): S3–S14
- Trost, L., Saitz, T. R., & Hellstrom, W. J. (2013). Side Effects of 5‐Alpha Reductase Inhibitors: A Comprehensive Review. Sexual Medicine Reviews, 111, 24-41. doi:10.1002/smrj.3
- Zhang, W., Wang, X., Liu, Y., Tian, H., Flickinger, B., Empie, M. W., & Sun, S. Z. (2008). Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. Journal of Medicinal Food, 11(2), 207-214. doi:10.1089/jmf.2007.602
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Food and Nutrition"
Food and Nutrition studies deal with the food necessary for health and growth, the different components of food, and interpreting how nutrients and other food substances affect health and wellbeing.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




