Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa) for Bacterial Infection Treatment
Info: 21971 words (88 pages) Dissertation
Published: 10th Dec 2019
Tagged: Microbiology
ABSTRACT
Aquaculture has expanded enormously on the world, yet there are different issues related with aquaculture establishment one of which is the utilization of antimicrobial agents bringing about safer bacterial strains which antagonistically influence human wellbeing and the common habitat. Plants are rich in a wide assortment of auxiliary metabolites, for example, tannins, alkaloids, and flavonoids, of the phytochemical constitutes, found in vitro to have antimicrobial properties. A considerable lot of the flavors and herbs utilized today have been esteemed for their antimicrobial impacts and therapeutic forces. Medications of bacterial infections with different herbs have been securely utilized broadly in natural agribusiness, veterinary and human solution. Since old circumstances, therapeutic plants have been utilized for the treatment of regular irresistible illnesses and medications with plants having antibacterial action are a possibly valuable option in aquaculture. Moreover, plant-inferred phytomedicines give a less expensive source of treatment and more prominent precision than chemotherapeutic operators in this field. The present review was expected to purify and characterize the active compounds present in Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa) for the treatment of bacterial infection in Tilapia Fish (Oreochromis mossumbicus). This work was led to assess the effect of Tulsi and Turmeric by epitomizing the joining separate in caseinate beads on their growth performance, feed utilization, structural changes, enzymatic parameters. The present review uncovered that the consolidated plant concentrate can be utilized for treating the disease. This can be utilized as a sustain for treating the disease in aquaculture, which can be utilized as the other option to antibiotics.
Keywords: – Medicinal Plants (Tulsi & Turmeric), Aquaculture, Tilapia.
CHAPTER 1
1. INTRODUCTION
1.1 HISTORY OF AQUACULTURE
Aquaculture, also named as called aqua farming, is the cultivation of aquatic organisms, for example, crustaceans, mollusks, and aquatic plants. As per the FAO, aquaculture “is comprehended to mean the cultivation of aquatic life forms including fish, mollusks, crustaceans and aquatic plants”. Cultivation suggests some type of mediation in the raising procedure upgrade generation, for example, standard stocking, feeding, assurance from predators, and so on. Cultivation likewise suggests an individual or corporate responsibility for the stock being developed. It is developing more quickly than other section of the animal culture industry. Specific strategies incorporate aquaponics and coordinated multi-tropic aquaculture, both of which coordinate fish cultivation and plant cultivation. Aquaculture in India has a long history and there are references to fish culture in Kautilya’s Arthashastra and ruler Someswara’s Manasoltara. The customary routine of fish culture in little lakes in eastern India is known to have existed for a long time and noteworthy advances were made in the condition of West Bengal in the mid-nineteenth century with the controlled rearing of carp in bandhs (tanks or impoundments where stream conditions are reproduced). Fish culture got remarkable consideration in Tamil Nadu as right on time as 1911, therefore, states as Bengal, Punjab, Uttar Pradesh, Baroda, Mysore, and Hyderabad started fish culture through the foundation of fisheries departments. Aquaculture includes the development of freshwater and saltwater populace under controlled conditions and can be appeared differently in relation to business fishing, which is the harvesting of wild fish.
Aquaculture likewise knew as fish or shellfish cultivation, it suggests to the reproducing, raising and collecting of plants and animals in a wide range of water situations including lakes, waterways, lakes. Aquaculture is generally perceived as a viable approach to meet the fish requests of a developing populace. All around, marine and inland catch fisheries give 66% of the aggregate nourishment fish supply with the staying 33% being gotten from aquaculture.
Around 430 (97%) of the species cultivated at 2007 were farm during the 21st century, of which a likely 106 came in the decade to 2007. Given the long haul significance of farming, it is exciting to note that to date just 0.08% of known land plant species and 0.0002% of known land species have been accomplished, compared with 0.17% of known marine plant species and 0.3% of known marine animals.
Controlling aquatic species includes fewer dangers to people than land animals, which took a huge toll on human lives. Many human diseases found in aquatic animals for example, smallpox and diphtheria.
Secure stagnation in wild fisheries and overexploitation of prominent marine species, consolidated with a developing interest for fantastic protein, urge water culturists to control other marine species.
“The detailed worldwide generation of sustenance from aquaculture, including fishes, shellfish, mollusks and other aquatic animals for human utilization, achieved an amazing stature of 52.5 million tons in 2008. The commitment of aquaculture to the aggregate generation of fisheries and aquaculture kept on developing, ascending from 34.5% in 2006 to 36.9% in 2008. In the period 1970-2008, the generation of sustenance fish from aquaculture expanded at a normal yearly rate of 8.3% as indicated by The Condition of World Fisheries and Aquaculture 2010”.
Marine aquaculture alludes to bringing animal groups that live up in the sea, including shellfish, mollusks, mussels, shrimp, and salmon. Freshwater aquaculture produces species that are local to waterways, lakes, and streams, for example, trout, catfish, and tilapia.
Aquaculture in India can be categorised into 3 groups (i) Freshwater aquaculture (ii) Brackish water aquaculture and (iii) Mari culture.
1.2 IMPACT OF AQUACULTURE
Sustainble marine aquaculture has many advantages. For instance, aquaculture makes work and business openings in littoral groups, gives safe and sustainable seafood, and backings marine fish populace and living spaces. Like any human action, aquaculture can affect the earth and sustenance afety directions. when it is maintained responsibily, aquaculture’s effect on wild fish, shellfish populaces, marine environments, and water quality are minimum.
1.3 OVERVIEW OF MEDICINAL PLANTS
Plants have been utilized for restorative purposes some time before recorded history. Primitive men watched and valued the colossal differing qualities of plants accessible to them. Plants give sustenance, clothing, lodging, and medication. A significant part of the therapeutic utilization of plants is by all accounts created through perceptions of wild animals, and by experimentation. As time went on, every population included the therapeutic vitality of herbs in their area to its knowledge base. They systematically gathered data on herbs and formed easily characterized pharmacopoeias.
Many medications recorded as customary drugs were initially gotten from plants. Salicylic acid, a precursor of aspirin, which was initially gotten from white willow bark and the meadowsweet plant. Cinchona bark is the origin of quinine, which is active against malarial disease. Another remedial territory where regular items have majorly affected life span and personal satisfaction is in the treatment of the tumor. A vast majority of anticancer medications are derived from plants or micro-organisms. Some examples of anticancer medications are Doxorubicin, Bleomycin, Vinblastine, Vincristine, and now the current expansion of Ironotecan, Paclitaxel (Taxol), Tenoposide and Etoposide. Probably the most energizing natural items found in the current years are the cholesterol-lowering agents from fungi.
India has a rich culture of therapeutic herbs and flavors, which incorporates more than 2000 species and has an inconceivable topographical region with high potential capacities for Ayurvedic, Unani, Siddha customary drugs. However just not very many have been considered synthetically and pharmacologically for their potential medicinal value.
People have utilized plants for the treatment of various illnesses for a large number of years. As per the World Health Organization, most populaces still depend on natural plant solutions for their mental and physical health requirements, since they can’t bear the cost of the results of Western pharmaceutical enterprises, together with their symptoms and absence of healthcare facilities. Regional zones of many developing nations still depend on conventional pharmaceutical for their essential human services needs and have found a place in everyday life. These medications are moderately more secure and less expensive than engineered or present day drug. Individuals living in local arears from their own experience realize that these customary cures are significant source of common items to keep up human wellbeing, however they may not comprehend the science behind these meds, but rather realized that some medicinal plants are very successful just when utilized at helpful dosages.
1.4 OVERVIEW OF THE STUDY
The worldwide use of antibiotics in aquaculture for prophylactic and medicinal purposes has caused an increase in bacterial resistance in exposed microbial environments, affecting both animal and public health. Microbial infection and diseases cause a comMercial loss in aquaculture on a large scale, and the use of these synthetic antibiotics for disease treatment produces various undesirable side effects and biological hazards
Now a day, many medicinal plants have grown an effective defence capability against various pathogenic bacteria, though there is a growing interest in these plants as sources for natural antibacterial agents
In this context, this study was undertaken to Purify and characterise the bio-active compounds present in Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa) extracts, and also to evaluate the combined effect of these extracts on the performance, growth and innate immunity of common Tilapia (Oreochromis mossambicus) and its challenge against Aeromonas hydrophila infection
1.5 TULSI (Ocimum tenuiflorum)

Fig no.1 (Ocimum tenuiflorum)
SCIENTIFIC CLASSIFICATION
| Kingdom | Plantae |
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Order | Lamiales |
| Family | Lamiaceae |
| Genus | Ocimum |
| Species | tenuiflorum |
Table no.1 (Scientific classification of Ocimum tenuiflorum)
1.5.1 IMPORTANCE OF TULSI
The tulsi plant or Indian basil involves a vital place in the Hindu religion. The name “tulsi” denotes “the exceptional one” which is a sweet-smelling plant. It is local to Indian
Subcontinent and it is widely spread throughout the Southeast Asian tropics. Tulsi or Ocimum tenuiflorum is an aromatic plant belonging to the family Lamiaceae. It is widely used for religious and medicinal purposes, and for its important oil. A large number of the component are present in tulsi leaves, the main constituents of Tulsi are Oleanolic acid, Ursolic acid, Eugenol, Rosmarinic acid, Linalool, Carvacrol, and β caryophyllene, have been used widely for many years in food products, perfumery, dental and other oral products. Phytochemical analysis of the tulsi leaves reveals the presence of saponins, alkaloids, flavonoids, cardiac glycosides, steroids, phenols and tannins in it. It is broadly referred to over the Indian Subcontinent as a therapeutic plant and regularly utilized as a part of Ayurveda, where it is considered as the destroyer of all the doshas. It has an essential part inside the “Vaishnavite tradition of Hinduism”, in which followers do devotion including Tulsi plants.
1.6 TURMERIC (Curcuma longa)

Fig no.1 (Curcuma longa)
SCIENTIFIC CLASSIFICATION
| Kingdom | Plantae |
| Phylum | Magnoliophyta |
| Class | Liliopsida |
| Order | Zingiberales |
| Family | Zingiberaceae |
| Genus | Curcuma |
| Species | longa |
Table no. 2 (Scientific classification of Curcuma longa)
1.6.1 IMPORTANCE OF TURMERIC
Turmeric (Curcuma longa) is a medicinal plant broadly utilized as a part of Ayurveda, Unani and Siddha medicine as a primary treatment for different diseases. It belongs to the family named Zingiberaceae and is one of the essential medicinal plants around the world. Turmeric is used as a food improver (spice), preservative and colouring agent in Asian countries, including China and South East Asia. It is also measured as a promising part of all religious festivals. It is broadly utilized for the treatment of sprains and swelling brought about by injury. Now a day, regular Indian medication uses turmeric powder for the treatment of the biliary issue, anorexia, coryza, cough, diabetic injuries, hepatic issue, and sinusitis. In China, C. longa is utilized for diseases related to stomach pain. The coloring standard of turmeric is the main fundamental property of this plant. It contains many bio active components for example, curcumin, turmerone, curcuminoids, turmerone, arturmerone, and zingiberene, that have antioxidant activties. Although recent studies have revealed that turmeric displays an extensive variety of pharmacological impacts, for example, antioxic, antitumor, hepatoprotective, antimutagenic, antiangiogenic, immunomodulatory, antimicrobial, anticancer, and wound healing
1.7 ANTIOXIDANTS
An antioxidant is nothing but a molecule that prevents the oxidation of other molecules. Oxidation is a chemical response that exchanges electrons or hydrogens from a substance to an oxidizing agent. The oxidation reaction can produce free radicals. These radical in addition can start chain reactions. When the chain reaction started in a cell, it can cause damage or death to the cells. Antioxidant agents can end this chain response by expelling free radical’s intermediates, and suppress other oxidation responses. They do this by being oxidized themselves, so antioxidants are often reducing agents, for example, thiols, ascorbic corrosive, or polyphenols.
In spite of the fact that oxidation responses are important for life, they can likewise be harming, plants and animals keep complex systems of numerous types of antioxidants, for example, glutathione, vitamin C, vitamin A, vitamin E, and catalysts, for example, catalase, superoxide dismutase, and different peroxidases. Deficient levels of antioxidants, or prevention of the antioxidant enzymes, cause oxidative anxiety and may harm or execute cells. Oxidative anxiety is harm to cell structure and cell function by excessively reactive oxygen-containing particles and chronic excessive inflammation. Oxidative anxiety appears to assume a huge part in many human diseases, including cancer. The utilization of antioxidants in pharmacology is seriously studied, especially as medications for stroke and neurodegenerative diseases. Hence, oxidative anxiety can be thought to be both the cause and the outcome of a few diseases.
Antioxidants are generally utilized as a part of dietary supplements and have been explored for the prevention of diseases, for example, tumour, coronary heart disease. Though initial studies proposed that antioxidant supplements might stimulate health issues, later large clinical trials have been carried out with a limited number of antioxidants, which had detected no benefit and even proposed that additional supplementation with certainly recognized antioxidants may be harmful. Antioxidants also have many industrial uses, such as preservatives in food and cosmetics and to prevent the degradation of rubber and gasoline.
1.7.1 MECHANISM OF ANTIOXIDANT
Oxidative anxiety, defined as the difference between oxidants and antioxidants in favor of the former, leads to many biochemical alterations and is a significant contributing element in several human chronic diseases, such as atherosclerosis and cardiovascular diseases, mutagenesis and cancer, some neurodegenerative disorders, and also likely the aging process. The antioxidant enzymes are accompanied by small-molecule antioxidants, some of which are derived fully from the diet and are vitamins. These small molecule antioxidants are present extracellularly and intracellularly and include ascorbic acid (vitamin C), glutathione (GSH), and tocopherols (primarily α-tocopherol; vitamin E). Intracellular concentrations of these compounds can be substantial, i.e., in the (milimolar) range for both ascorbate and GSH. α-Tocopherol is the richest lipid-soluble antioxidant in humans, exists in cellular and subcellular membranes and lipoproteins. The mechanisms by which these antioxidants act on the molecular and cellular level includes a role in gene expression and regulation, apoptosis, and signal transduction. Therefore, antioxidants are involved in fundamental metabolic and homeostatic mechanisms. However, there are still many gaps in our knowledge of the basic mechanisms of oxidative damage and antioxidant defenses. Filling of these gaps would allow us to more precisely target treatments and derive best benefits from the antioxidants for health issues and disease control.
1.8 ANTIBACTERIAL ACTIVITY
Bacterial infection causes a high rate of mortality in the human population as well as on aquaculture organism/animals. The organism chosen for this study is Aeromonas huydrophila. A. hydrophila is a Gram-negative, facultatively anaerobic, motile, rod-shaped bacterium, which is belonging to the family Aeromonadaceae. It can produce several diseases like hemorrhagic septicemia, fin, and tail rot disease, and furunculosis in several fish species, causing mass damage and mortalities in several fish species. This bacterium can infect warm-water fish, causing red-sore disease in comMon Tilapia (Oreochromis mossambicus). Thus, disease control in comMercial fish farming is very crusial. Bacterial diseases are controlled by chemical drugs and veterinary medicines, like antibiotics, pesticides, and disinfectants. The excessive use of these chemical agents in aquaculture has led to an increase in antibiotic-resistant bacteria that have the potential to infect the nearby ecosystem. Moreover, the cost of the chemicals is too high and also they cause a negative effect on the hosts. Though, due to decrease efficiency and resistance of pathogens to the antibiotics has necessitated for the development of several alternatives to antimicrobial treatment. In addition, one of the best alternatives of antimicrobials is the use of medicinal plants. Many bioactive and pharmacologically important have been obtained from medicinal plants and widely used in medicine production. These constituents have growth and imMunostimulating activities for fishes. They also are used as feed supplements and chemotherapeutics.
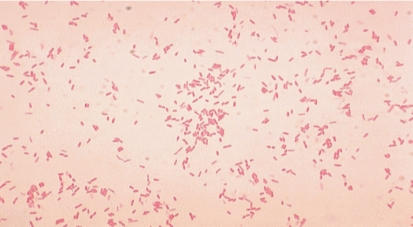
Fig no 3. Aeromonas hydrophila
SCIENTIFIC CLASSIFICATION
Aeromonas hydrophila
| Domain | Bacteria |
| Kingdom | Proteobacteria |
| Phylum | GamMaproteobacteria |
| Class | Aeromonadales |
| Genus | Aeromonas |
| Species | hydrophila |
Table no. 3 (Scientific classification of Aeromonas hydrophila)
1.9 AIM AND OBJECTIVES
- To purify the bio active compounds, present in the plant extracts.
- To determine and characterise the active compounds Tulsi and Turmeric by GC-MS, FT-IR, HPLC, FT-RAMAN.
- To estimate the Antibacterial activity of the purified plant extracts individually and by combining the two plant extracts (Ocimum tenuiflorum and Curcuma longa)following in vitro, in vivo and oral method against the Tilapia pathogen Aeromonas hydrophila.
- To estimate the Antioxidant activity (Sequential and maceration process) of both the plant extracts.
- To estimate the rate of the permanence of the Tilapia Fishes after treatment with the plant extracts.
- To determine different Enzymatic and Biochemical parameters after and before treatment.
- SEM analysis for sodium caseinate beads.
CHAPTER 2
2. REVIEW OF LITERATURE
2.1 MEDICINAL PLANTS
India has 2.4% of world’s region with 8% of worldwide bio diversity. It is one of the great diversity regions of the world. The other diverse countries are Colombia, USA, Brazil, Mexico, China, Venezuela, South Africa, Ecuador, Indonesia, Peru, and Bolivia. The forests of India are assessed to harbor 90% of India’s therapeutic plants assorted qualities. Just around 10% of the known medicinal plants of India are limited to non-forest natural surroundings. The assessed quantities of plant species and those utilized for medicinal reason vary. As indicated by, one fifth of the considerable number of plants found in India are utilized for a medicinal and treatment purpose. The world normal stands at 12.5% while India has 20% plant types of medicinal values
Yet, India has around 44% of plant life, which is utilized remedially. Despite the fact that it is hard to appraise the number of medicinal plants introduces around the world, the reality says that India stands first with its rich diversity of therapeutic plants. The presence of the conventional drug relies on upon plant species, differing qualities and the related information of their utilization as natural medicine. Both plants and traditional knowledge are imperative to the herbal solution exchange and the pharmaceutical industry where plants give crude materials and the conventional essential information
Medicines made up from plants are in extraordinary demand in both made and making nations as a wellspring of essential human services inferable from their qualities having wide natural and therapeutic actions, good safety margins, and lesser expenses. Plant extract molecules are protected and would defeat the resistance delivered by the pathogens as they exist in a consolidated shape or in a pooled type of more than one particle in the protoplasm of the plant cell. Indeed, even with the appearance of present day or allopathic pharmaceutical, Balick and Cox (1996) have noticed that various critical current medications have been gotten from plants, utilized by indigenous individuals.
Traditional utilization of medicine is perceived as an approach to find out about potential future medications. Specialists have recognized a number of components utilized as a part of standard pharmaceutical medicacy, which were developed from “ethnomedical” plant sources. Plants are utilized therapeutically in various nations and are a wellspring of numerous intense and strong medications
2.2 ANTIBIOTIC PROPERTIES OF NATURAL PLANTS
The plant chemicals are categorized as primary or auxiliary metabolites. Primary metabolites are generally dispersed in nature, happening in some frame in for all intents and all life forms. In greater plants, such compounds are regularly moved in seeds and vegetative storing organs and are required for physiological advancement due to their involvement in essential cell metabolism. Primary metabolites acquired from higher plants for business uses, which are high in volume-low esteem mass chemicals (e.g. vegetable oils, unsaturated fats, sugars and so on). Generally, plants produce numerous auxiliary metabolites, which are biosynthetically derived from Primary metabolites and constitute a critical origin of pesticides, microbicides and numerous pharmaceutical medications. From a long time, medicinal herbs and plants or their auxiliary metabolites have been clearly playing an essential part in the human life to battle against illnesses
Auxiliary metabolites have no use in a plant’s essential metabolism, however regularly have a biological part, as some pollinator attractants, represents chemical compounds adjustments to ecological anxieties or fill in as concoction protection against small animals, microorganisms, Flies, insects and higher predators and even different plants (allelochemics). Auxiliary metabolites are present in a lesser amount in the plants than the primary metabolites
As opposed to primary metabolites, they are produced in particular cell categories and at mistakable growing stages, making their extraction and filtration hard. Subsequently, secondary metabolites that are utilized business-wise as a naturally active compound, are for high esteem low volume items than the essential metabolites (e.g. steroids, terpenoids, alkaloids, quinines, and flavonoids), which are utilized as a part of medication formulate by the pharmaceutical businesses. These are largely acquired from plant materials by steam refining or by extraction with natural or fluid solvents. Some naturally active plant mixes have some application as medication elements or as model mixes for medication combination and semi-amalgamation.
An overview of current pharmaceutical utilize uncovered that of the aggregate physician endorsed drugs administered, 25% are plant inferred. Plant mixes profoundly fluctuate in structure; many are fragrant substances, the majority of which are phenols or their oxygen-substituted subsidiaries. In any case, there is an expanded consideration on concentrates and organically dynamic mixes separated from plant species utilized as a part of home grown solution, because of the symptoms and the resistance that pathogenic miniaturized scale life forms work against the anti-infection agents. New mixes repressing microorganisms, for example, benzoin and emetine have been separated from plants. Of the different pharmaceuticals utilized as a part of present day medication, ibuprofen, atropine, ephedrine, digoxin, morphine, quinine, reserpine, and tubocurarine fill in as cases of medications found through perceptions of indigenous therapeutic practices. Eloff (1999) expressed that the antimicrobial mixes from plants may restrain microscopic organisms by an alternate system than the directly utilized anti-microbials and may have a clinical incentive in the treatment of safe microbial strains.
Plant constituents might be separated and utilized specifically as medication specialists or as beginning materials for medication amalgamation or they may fill in as models for pharmacologically dynamic mixes in medication union. The general research strategies incorporate a legitimate choice of therapeutic plants, planning of rough concentrates, organic screening, itemized chemo pharmacological examinations, toxicological and clinical reviews, institutionalization and utilization of dynamic moiety as the lead molecule for medication configuration
2.3 SCOPE OF MEDICINAL THERAPY
The treatment of several diseases with pure plant pharmaceutical agents is generally a better phenomenon. Be that as it may, as European travellers and traders spread out toward the Western and Eastern parts of the world, a part of the advantages is that they would bring back were newfound pharmaceutical planning of comMon birthplace. One of the most punctual examples of overcoming adversity in building up a medication from a characteristic item was headache medicine. Today we are worried about the diseases like depression, cancer, TB and heart problems brought about by flawed sustenance and stress. The need of option treatment is to cover great happiness for all. Natural treatment is one of the accepted procedures to conquer sickness. Customary Indian practice held that specific medications ought to be planned through the expansion have picked a substance that upgrades bioavailability of the medication. Pepper has affirmed bioavailability enhancer property and indicates the dynamic segment as the particle piperine. An anti TB medicate Rifampicin must be given at a higher dose than required with a specific point to adjust for misfortunes while in transit to the objective site. Detailing of Piperine with Rifampicin will have counter effects
2.4 ANTIOXIDANTACTIVITYOFMEDICINALPLANTS
In living organisms, oxidation is an essential portion of the comMon metabolic process. In which ROS or reactive oxygen species (hydrogen peroxide and hypochlorous corrosive) and other free radicals (hydroxyl radical (OH) and superoxide anion) are created. Quick generation of free radicals may bring about adjustment in the membrane structure and capacity of cell constituents and can brings about human neurologic problem, and many other diseases for example, diabetes, cancer, asthma, Tuberculosis, heart diseases and neurodegenerative disorders. Hence, the counteractive action of the above mentioned conditions requires the antioxidant presence or the free radical scavenging particles in the body.
There are a lot of anti-oxidants elements exist in plants (vegetables, fruits, medicinal plants) and the free radical rumMaging elements in them represents as phenolic mixes (e.g. phenolic acids, tannins, coumarins, flavonoids quinones, lignans,), nitrogen mixes (amines, alkaloids), terpenoids, vitamins (as well as carotenoids), and some different endogenous metabolites, So to keep up a solid body, one ought to dependably increase the consumption of foods rich in various antioxidants properties that lower the problem of certain long lasting diseases .
Anti-oxidants naturally can be utilized as a part of daily diet and furthermore for the inhibition of certain pathogens. Free radical-related diseases also cured by using antioxidants, present in plants, which can likewise be supplanted by financially accessible, artificial anti-oxidants, for example, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), which are very dangerous to utilize and is limited because of their cancer-causing impacts. Nitric oxide (NO) is an intense pleiotropic inhibitor of physiological procedures, for example, smooth muscle loosening, neuronal flagging, restraint of platelet total and control of cell mediated toxicity. This is a diffusible free radical, which has a various effect on an effectors particle in a differing organic system including vasodilatation and antitumor and antimicrobial exercises.
The most usually utilized strategies for measuring anti-oxidant movement are those which include the era of free radicals which are then killed by cancer anti-oxidants mixes. DPPH is an outstanding radical and a dupe (“scavenger”) for different radicals. Hence, rate drop of a chemical response upon addition of DPPH is utilised as a sign of the radical nature of that reaction. On account of a solid assimilation band focused at around 520 nanometer, the DPPH radical has formed a dark violet colour in the soluton, and when it is neutral, it turns into a colour lees solution or light yellow. This property permits visual checking of the response, and the number of primary radicals can be included from the change of the optical absorption at 520 nanometer. DPPH measures the efficacy of electron donating activity of different compounds present in the solution and subsequently gives an assessment of anti-oxidant action because of the free radical scavenging. Any particle that can give an electron or hydrogen will respond with DPPH, hence by neutralising its shading from a dark purple to a light yellow by electrons from the oxidant mixes. The total concentration and structure of different compound being scavenged at the end of the reaction indicates the concentration of DPPH
2.5 ANTIMICROBIAL PROPERTIESOFPLANTS
Plants with medicinal properties have always dependably been considered as the origin for hale and healthy life for individuals. Medicinal properties of therapeutic plants are exceptionally valuable in mending different infection and diseases and the benefit of these therapeutic plants are normal. All around the world, these plants have been utilized for its antibacterial, antifungal and antiviral exercises for a long time.
Scientists are progressively turning their concern for comMon natural items and searching for new options to treat diseases like cancer, and also for certain fungal, microbial and viral infections. A few artificial antibiotics were utilized in the treatment of severe infection and transmittable diseases. The hazardous microorganisms can be controlled with medications and this has brought about the development of different medication resistant microscopic organisms and it has made disturbing clinical circumstances in the treatment of contagions. Microorganisms have the hereditary capacity to transmit and gain resistance to certain synthetic medication, which is used as beneficial agents. Hence, moves must be made to diminish this kind of problems, for example, to bound the utilization of antibiotics. And we should develop an alternative to inhibit the resistance microorganism and to proceed with studies to grow new anti-microbial, antifungal compounds with differing chemical structures and novel activity against the microorganism
The antimicrobial study has revealed that Gram-positive microscopic organisms show a lesser resistance to plant extracts than the gram negative microbes. This might be because of the variety of the cell wall structures of Gram-negative and Gram-positive microorganisms. Basically, Gram-negative microbes contain an external membrane that is made out of high thickness lipopolysaccharides that serves as a boundary to numerous ecological substances with antibiotics. Though many plant species have been tried for antimicrobial properties, most by far of have not been enough assessed.
The Indian vegetation offers awesome conceivable outcomes for the revelation of novel compounds with better remedial activity against diseases and also support the imMune system. These compounds found in plants may inhibit the bacterial contaminations by various mechanism than the comMercially used drugs. The unpredictable usage of antimicrobial agents has brought about numerous bacterial pathogens quickly getting resistant to various originally found antimicrobial agents.
Hence, there was a constant research going on for the identification of new antibiotics from the plants, which may offer another origin of antibacterial agents. This is to be sure essential since Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli are a part of main human pathogens, as they are able resistant to antibiotics.
2.6 MEDICINAL PROPERTIES OF PLANTS
The therapeutic usages of S. roxburghiana incorporate treatment for stomach irritations, diarrhoea ear infection, and haemorrhoids. Generally, in treating haemorrhoids and ear infections, the leaf of S. roxburghiana plant is warmed and then squeezed all over the infected zone. The leaves of S. roxburghiana were roasted and then utilized as an emollient. Root stocks are utilized as a drug for treating cough. Juice of delicate shoot was given to kids for the treatment of sore throats. In India, expectorant and in bone setting problems delicate roots and rhizome are utilized. For certain viral diseases somewhat warmed leaf juice is utilized as nasal drops.
The therapeutic properties of Sansevieria species are very much recorded. A few animal categories have an ethnopharmacological basis, specifically, S. trifasciata which was found in South Africa and tropical America is utilized for the treatment of provocative conditions and sold as a raw medication in the market to treat casualties of snakebite. S. liberica plant leaves and roots which are widely found in Nigeria are utilized as a part of conventional remedial method for treating stomach pains, piles, snakebite, hypertension, asthma, menorrhagia, colic, dermatitis, haemorrhoids, gonorrhoea, and injuries of the foot.
2.7 MEDICINAL PLANTS IN AQUACULTURE
Asian nations have seen the development of aquaculture in recent years. A definitive objective is to create the best conceivable weight per culture unit in most aquaculture operations for culture fish, crustaceans or mollusc. As aquaculture industry turns out to be more concentrated, the occurrence of disease including different irresistible infections has expanded subsequently. Illnesses are a significant component which supresses the development of aquaculture. Different chemotherapeutants have been utilized for the treatment of various diseases. Although, the use of antimicrobial agents in aquaculture has brought about many antibiotic resistant bacterial strains. These resistant bacterial strains could affect the fish during their treatment or can produce human diseases. Hence, Plants and Herbs have been generally utilized as a part of the veterinary and human solution. They are normal items that are safe for consumers as well as broadly accessible throughout Asia.
Satavari (Asparagus racemous) is broadly utilized in India as an ayurvedic pharmaceutical for advancing human health and progress. It delivered a comparable outcome in Labeo rohita infections. Shagnliang et al. revealed the antimicrobial action of 5 Chinese medicinal plant extracts (Impatiens biflora, Stellaria aquatica, Oenothera biennis, Lonicera japonica and Artemisia vulgaris) was affecting, against 2 viral and 13 bacterial fish pathogens. Edwardsiella ictaluri and Aeromonas salmonicida were the most susceptible to these concentrates. S. aquatica has the capacity to inhibit various kind of fish pathogen. L. japonica revealed some inhibitory activity against both IPN and IHN infections. while S. aquatica and A. vulgaris can olny inhibit the IHN infection. In Vietnam, the Institute of Ecology and Bioresources has embraced connected research on some medicinal plants for the treatment of fish and shrimp maladies, for example, ulceration, white mouth and head, red skin, intestinal sickness, and red spot in fishes. In 1983, during the Thailand outbreak of epizootic ulcerative disorder (EUS) farmers has utilized the bark of plug wood tree (Sesbania grandiflora) for the treatment of the snake-head fishes in Uthaitanee. The vast majority of the fish retrieved after treatment. Since 1990 numerous species of medicinal plants have been used to treat several infectious diseases in fish farms. A study has revealed that the antimicrobial activity of guava (Psidium guajava) against the pathogenic bacteria for fishes was started.
2.8 FISH PATHOGENICITY
Aeromonas hydrophila causes infection in fishes named as “Motile Aeromonas Septicemia” (MAS), “Hemorrhagic Septicemia,” “Ulcer Disease,” or “Red-Sore Disease”. The numerous equivalent words of this illness identify with the injuries brought about by this bacterium which incorporates septicemia where the microbes or bacterial poisons are available inside various organs of the fish. Aeromonas hydrophila is a gram-negative, facultative anaerobe, rod shaped bacterium, which is regularly confined from freshwater lakes and which is an ordinary occupant of the gastrointestinal tract. “Septicaemia” brought on by this bacterium basically influences freshwater fish, for example, Tilapia, catfish, and numerous types of tropical or decorative fish.
Aeromonas hydrophila was considered as an opportunistic pathogen. This appears like an inconsistency in wording, since most microscopic organisms which are named “opportunistic” more often that they don’t bring about infection unless different factors are included, and those microorganisms which are considered as a “pathogen” can cause diseases without any changes in intrinsic or extrinsic factors. Though, Aeromonas hydrophila was considered as an “opportunistic pathogen” so it can cause any diseases whenever favourable condition was present. As previously mentioned, that Aeromonas hydrophila was persistent in nature and is even present in the intestinal tract of the fish. In regular circumstances, fishes infected with Aeromonas hydrophila are most likely a minor issue. However, with concentrated fish-fanning frameworks, regardless of whether these frames are open air lakes or tanks, different elements must be considered. The basic condition of this infection identifies with stress conditions or variables of the fish. Fish specialists revealed that fishes are naturally stressed when water quality is not good. Many trials demonstrate that the fishes which are in poor conditions because of inadmissible water quality, for example, low levels of oxygen(DO), high nitrite levels or elevated amounts of carbon dioxide (CO2) are more subject to disease by Aeromonas hydrophila. Furthermore, an occasional frequency of a higher number of rep-ted fish death in the spring is related to diminished water temperatures.
CHAPTER 3
3. MATERIALS AND METHODS
3.1 REAGENTS AND CHEMICALS
Ethanol, Quercetin, Dichloromethane, Chloroform, potassium hydroxide, AmMonium Molybdate, Bovine serum albumin, Phosphate Buffer Saline (PHOSPHATE BUFFER SALINE), anhydrous amMonium acetate, Hexane, Folin-Ciocalteu Reagent, Gallic Acid, EDTA, , Sodium Carbonate, ABTS, Potassium Acetate, Hydrogen Peroxide, Aluminium Chloride, DPPH, Sodium Phosphate, Copper sulphate, Sulphuric Acid, TCA, Ascorbic Acid, Ferric Chloride, Potassium Ferricyanide, Ferric Chloride, Ferrozine, Hydrogen Peroxide, Potassium Dihydrogen Phosphate, Ferrous Sulphate, Sodium Salicylate, APS, Xanthum Gum, Sodium potassium tartarate, Glycerol, tripalmitin, Sodium Caseinate, Orthophosphoric Acid, Acetonitrile, Acetone, Disodium Phosphate, Trichloroacetic acid, Zinc Hydroxide, Potassium Chloride, HCL, Trichloroacetic Acid, Calcium Chloride, Sodium Chloride, Acetyl acetone, Muller-Hinton Agar, Were Purchased From Sigma Aldrrich Co. (St. Louis, MO, USA). All chemicals used were of analytical grade.
3.2 GLASSWARES AND PLASTIC WARES
Conical flasks of 100, 250, 500milliliter Petriplates, Test tubes, Falcon tubes, 2milliliter centrifuge tubes, Microtitre plate, Glass beakers were purchased from Tarson and Borosil glass work limited.
3.3 COLLECTION OF MEDICINAL PLANTS AND PROCESSING
Fresh Tulsi (Ocimum tenuiflorum) leaves and Turmeric (Curcuma longa) roots were collected from Vellore district, Tamilnadu, India. They were transported to the laboratory in polythene bags and washed with the help of fresh water to remove dust and soil particles from the outer surfaces. The cleaned sample was then dried by keeping the samples at 50-60 °C for 72 hr. These dry samples were then kept in sealed plastic bags at a dry and cool place to avoid deterioration. Then the dried samples were ground into fine powder by using a grinder. The samples were sieved to get uniform particle size, then kept in an air-tight container and stored in a freezer (4⁰C) for further analysis.
3.4 COLLECTION OF EXPERIMENTAL ANIMALS
Healthy fresh water Tilapia (Oreochromis mossambicus) fishes were collected from a farm located in Walajah Road, Chennai. They were transported in a live condition in twenty Litres tanks with freshwater and aeration. In the laboratory, they were maintained in thousand litres fibre glass tanks filled with fresh water under constant aeration. The animals were fed twice a day with pellet feed.
3.5 PREPARATION OF BACTERIAL INOCULUM
Aeromonas hydrophila waspurchased from Microbial type culture collection (MTCC). The revival of the cultures was done as per the instruction is given by the MTCC catalogue. After revival, the organisms were grown on nutrient agar plates. After incubation at 37°C for 24 hours, the culture was picked by sweeping it into sterile nutrient broth with a folded bent inoculation needle. The tube containing the culture stored in the freezer for future use.
3.6 PREPARATION OF EXTRACT BY DIFFERENT METHOD
The dried plant sample weight was measured and transferred into a glass beaker. The samples were then extracted in hundred millilitre of the following solvents ethanol, water, dichloromethane in a soxhlet extractor. The extraction process was repeated thrice to get a required quantity of extract for both the plant samples. The extracts were kept in a rotary evaporator to reduce the sample concentration.
3.6.1 maceration process
5gram of dried fine plants powder (Ocimum tenuiflorum and Curcuma longa) was soaked in 100 millilitre of five different solvents like Dichloromethane, Ethanol, and water. Then they kept in shaking incubator at 30⁰C for 48 hours. After incubation, the suspension was filtered through Whatman No.1 filter paper. The filtrate was then concentrated to dryness and dried extract was dissolved in respective solvents like Dichloromethane, Ethanol, and Water. All the extracts were stored at 4⁰C for further use.
3.6.2 Sequential process
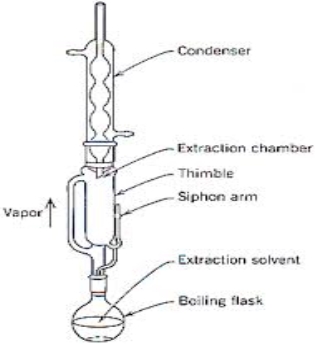
Fig no. 4 (Soxhlet Extractor)
The samples were kept inside a thimble made from thick filter paper, which is loaded into a main chamber of the soxhlet extractor. The extractor is placed on to a flask containing the extraction solvent. The soxhlet has an attached condenser. The solvents were heated to reflux. The vapour of the solvent travels through a distillation arm and floods into the chamber containing the sample. The condense ensure that each solvent vapour should cool and drips back down into chamber holding the sample. The sample fills with the warm solvent and some of the desired compounds will then dissolve in the warm solvent. When the soxhlet is about to full, the chamber automatically lets down by a side arm, with the solvent running back down to the distillation flask. This cycle was repeated for many times, during each cycle, a portion of the non-volatile compound dissolves in the solvent. The solvent is then dried and again dissolved in the desired solvent. The final product was stored for future studies at 4◦C.
3.7 DETERMINATION OF MOISTURE CONTENT
To find the moisture content, 1gram of plant samples was taken in two vials, an empty vial and the vial with the sample was weighed, then the vial with the sample was kept in hot air oven at 92ºc for around 3hours and the vial weight was measured continuously every 1 hour.
The moisture content was determined by using the following formula:
 %W = x –y × 100
%W = x –y × 100
X
Where:
%W = percentage of moisture in the sample
x = initial bottle weight with sample
y = final bottle weight with sample
X = sample weight
3.8 SEPARATION OF COMPOUNDS BY COLUMN CHROMATOGRAPHY
8g of sephadex G50 was weighed and added in n-hexane to obtain a slurry suspension. The slurry was then poured into the column using a glass rod until it was full. After a few minutes, the gel bed in the column started to settle, leaving 2-4 cm clear solution above it. The screw of the column was opened slightly to allow the n-hexane to drip slowly from the column into a waste beaker.
Using an automatic pipette, very slowly pipetted twenty millilitre of solvent (ethanol) down the wall of the column on the sephadex, ensuring the top of the bed remained even. The process was continued until the column was full. At any time, the column bed was not allowed to run dry. Always kept it topped up with solvent. Now adjust the screw clip so that the solvent drips from the column at a rate of 0.8millilitre per minute. Placed a few clean test tubes under the outlet tube. The test samples (Tulsi and Turmeric extracts) was very slowly added to the top of the column. As the solvent flows down the different compounds are moved down the column according to their different sizes. The separated fraction collected in a test tube and kept for further analysis.

Fig no. 5 (column chromatography)
3.9 PHYTOCHEMICAL ANALYSIS
The prepared plant extracts were subjected to perform following preliminary phytochemical screening test
.3.9.1 Test for alkaloid
Alkaloid identification was done by the revised quantitative method of one milllitre of plant extract, one millitre of concentrated hydrochloric acid was added in a test tube. Then few drops of Mayer’s reagent were added to the mixture. The presence of green color or white precipitate shows the presence of alkaloids.
3.9.2 Test for flavonoid
For flavonoid identification, two millilitre of plant extract and one millilitre of 2N sodium hydroxide was added in a tube and mix well. The presence of yellow color specifies the presence of flavonoids.
3.9.3 Test for saponin
For Saponin identification, frothing test was used in which two millilitre extract and two milllitre of distilled water were added to a clean test tube and the mixture was kept in the shaker for around 1 hour. Development of foam confirms the presence of saponin.
3.9.4 Test for tannin
The presence of tannin in the plant extracts was detected by adding few drops of 0.1% FeCl3 to 1 millilitre of extract. Development of bluish black colour indicates tannin.
3.9.5 Test for steroid
The Steroid identification was done by adding 1 milliter of acetic anhydride into 0.5 milliliter of plant extract, followed by 2 drops of concentrated H2S04. Development of a violet/brown ring at the junction indicates the presence of steroid
.
3.9.6 Test for carbohydrates
In a test tube, 2 milliliter of plant extract, 1 milliliter of Molisch’s reagent and few drops of concentrated sulphuric acid were added. Purple or reddish colour indicates the presence of carbohydrates.
3.9.7 Test for glycosides
For glycoside identification, 2 milliliter of plant extract, 3 milliliter of chloroform and 10% amMonia solution was added in a tube. Development of pink color shows the presence of glycosides.
3.9.8 Test for terpenoids
For terpenoid identification, 0.5 milliliter of extract and 2 milliliter of chloroform was added along with concentrated sulphuric acid. Red brown colour at the interface indicates the presence of terpenoids.
3.9.9 Test for phenols
For phenol identification, 1 milliliter of the extract and 2 milliliter of distilled water was added in a tube followed by few drops of 10% ferric chloride. Development of blue or green colour indicates the presence of phenols.
3.9.10 Test for proteins and amino acids
For this, identification for Ninhydrin test was used, in which 2 milliliter of plant extract, few drops of 0.2% Ninhydrin was added and heated for 5 min. Formation of a blue colour indicates the presence of proteins.
3.9.11 Test for lactones
Treat the extract with sodium picrate solution. The appearance of yellow to orange colour indicates the presence of lactone.
3.9.12 Test for fixed oils and fatty acid
Prepared spot on a filter paper with the plant extract and add oil staining on the filter paper which indicates the presence of fixed oil & fats.
3.10 ANTIOXIDANT ACTIVITY
The following antioxidant assays were done for both the plant extracts, of both maceration and sequential method.
3.10.1 Determination Total Phenolic Content
“The total concentration of polyphenols content was measured by Folin- Ciocalteau method. In this method, 10 microliter of Tulsi and Turmeric extracts (Maceration and Sequential) were taken into separate test tubes and mixed with 20microliter of Folin-Ciocalteu reagent and they allowed it to stand for 2 minutes. After this 50 microliter of sodium carbonate and 920 microliter of distilled water was added to the mixture and incubated at dark for 1 hour. The sample absorbance was deliberate at 725 nanometer using an UV Bio photometer. Gallic acid was used as the standard (milligram of Gallic acid/g)”.
3.10.2 Determination of Total Flavonoid Content
“This method was used for the determination of total flavonoid content, which was slightly modified from Yafang et al., (2011). Briefly, 10microliter of the plant samples were added with 365microliter of 95% ethanol, in this 25microliter of Aluminium chloride, 25microliter of potassium acetate and 700microliter distilled water was mixed. The reaction mixture was kept under the dark condition for around 30 minutes. Then absorbance was checked at 415 nanometer against a blank. Quercetin was used as standard”.
3.10.3 Determination of Total Antioxidant Activity
“The total antioxidant activity of Tulsi and Turmeric was calculated by Phospho molybdenum according to the procedure of Prieto et al., (1999). The antioxidant can reduce from Mo (IV) to Mo (V) and the green phosphate / Mo (V) compounds which have an absorption peak at 695 nanometer, were made consequently”
“10 microliter of samples was mixed with 90microliter of ethanol, in which 1milliliter of reagent solution (1:1:1 ratio mixture of sulphuric acid (1.66g/50milliliter water), sodium phosphate (0.532g/50milliliter water) and milimolar onium molybdate (0.247g/50milliliter water). This mixture was incubated in water bath at 98⁰C for 90 minutes. Then the sample was cooled at room temperature for 10 minutes. The absorbance was read at 695 nanometer by using an UV Bio photometer. Ascorbic acid was used as a standard. Total Antioxidant activity is stated as the number of equivalents of ascorbic acid in milligrams per grams of the extracts”.
3.10.4 Determination of free radicals scavenging by DPPH method
“This assay was done according to the modified protocol described by Brand-Williams, Cuvelier, and Berset (1995). Free radical scavenging activity pf the plant extracts was determined by using,1-diphenyl-2-picryl hydrazyl (DPPH). DPPH was made by adding 0.78 milligram in 10 milliliter of ethanol. 10microliter plant extract was mixed with 495 microliter of ethanol and 500 microliter of 0.1 millimolar DPPH. The mixture was incubated for around for 30 min. The absorbance of the sample was checked at 515 nanometer using a UV-spectrophotometer. The blank was made without adding plant extract samples and Quercetin was used as a standard. The percentage of scavenged DPPH was calculated using the following equation”:
 DPPH Scavenged (%) = A control – A test × 100
DPPH Scavenged (%) = A control – A test × 100
Acontrol
Where Acontrol stands for the absorbance of DPPH radicals without samples and Asample stands for the absorbance of DPPH with samples.
3.10.5 Hydrogen Peroxide Scavenging Capacity
“The ability of the plants to scavenge H2O2 was determined by following the method of Ruch et al., 1989 with the slight modification of Gulcin et al., 2003. 10 milliliter solution of H₂O₂ was prepared by combining 9950 microliter of phosphate buffer and 49.3 microliter of H₂O₂. 10microliter of the plant extract was added with 2799 microliter of phosphate buffer and 600 microliter of H₂O₂. The absorbance was measured using UV visible spectrophotometer at 240 nanometer against a blank solution containing phosphate buffer without H₂O₂. Quercetin was used as a standard. The scavenging effect of H₂O₂ was calculated by using following formula”:
% Scavenged [H2O2] = [(AC – AS)/AC] x 100
Where AC denotes the absorbance of H₂O₂ radical without adding sample and AS denotes the absorbance of H₂O₂ radicals with samples.
3.10.6 Hydroxyl radical scavenging assay
“The scavenging activity of Tulsi and Turmeric extract was performed by using the slightly modified method of Costa et al., (2009). In this process, 10microliter of the sample was added with 500μl FeSO4, 350μl H2O2, and 150μl sodium salicylate. In the case of control, the sample was exchanged to respective solvents. In A2, sodium salicylate was changed to H2O. The reaction solution was incubated at 37°C for 1 hour. Absorbance was measured at 562nanometer using an UV Biophotometer. Distilled water used as a blank. Quercetin was used as the standard. The scavenging effect was calculated in % by using the below mentioned formula”:
Scavenging activity (%) = [(1-(A1 – A2)) / A0] ×100
3.10.7 ABTS radical scavenging assay
“The ABTS (2, 2’-azino-bis3-ethylbenzthiazoline-6-sulfonic) radical scavenging assay was done by the method of Re et al. (1999) with some specific modifications. The ABTS reagent mixture was made by adding 10 milligram of ABTS in 5 milliliter of distilled water. 2.2milligram of APS (AmMonium Per sulphate) was dissolved in 5 milliliter of distilled water. Both the mixture was mixed well and made up to 50 milliliter by adding distilled water. Then the ABTS solution was incubated in dark for 6 h. After incubation, 10microliter from the sample was added with 1 milliliter of ABTS solution and 90 microliter of ethanol. The absorbance was measured at 734 nanometer using UV- spectrophotometer”. Trolox was used as a standard. The percentage of scavenging of the sample was calculated by the following equation:
% of scavenging = ((Ao-A1) / Ao) × 100
Where Ao indicates the absorbance of control and A1 indicates the absorbance of the sample.
3.11 ANTIBACTERIAL ACTIVITY
The antibacterial activity of Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa) against Aeromonas hydrophila was performed. It was done by preparing Muller Hinton agar and poured into the sterilised Petri plates, which was allowed to solidify. After this, the petri plates used for the well diffusion and disc diffusion method.
3.11.1 Well diffusion method
For agar well diffusion the method of Perez et al, (2006) was followed. In this, a sterile cotton swab stick was dipped into the broth containing the bacterial inoculum. The cotton swab was then pressed against the tube wall to remove excess inoculum. Then the surface of the agar plates was inoculated by swabbing three times. The lid of the petri plate was replaced and the was kept at room temperature for 5-10 minutes to settle or to dry the inoculum. After this wells were punched on the agar plates by using a cork screw borer. The wells are then filled with 10microliter, 20 microliters, 30microliter and 40microliter of the purified plant extract respectively. This method was followed for both the plant extracts individually, and also by combining the two plant extracts. The extract was then allowed to diffuse for certain time. The plates were then kept for incubation at 37ºc for 24-48 hours. After incubation, the antibacterial activity was evaluated by measuring the zone of inhibition (millimeter) formed around the wells. The size of the zones was also measured.
3.11.2 Disc diffusion method
Muller Hinton agar was sterilised in an autoclave and after sterilisation, they poured onto the sterile petri plates. After the agar get solidified a cotton swab was taken and dipped into the broth culture suspension. The cotton swap was then pressed against the test tube wall to remove excess inoculum. The surface of the solidified agar was then swabbed three times to form a lawn of bacterial culture. The lid was replaced to its original position and the plate was kept at room temperature for 10 minutes. The discs loaded with plant extracts was then placed in the petri plates. Then the plates were incubated at 37ºc for 24-48 hours. Evidence of antibacterial activity was expressed when the zone of inhibition (millimiter) was observed around the discs.
3.11.3 Minimum Inhibitory concentration (MIC)
MIC of the plant extracts against Aeromonas hydrophila was determined by the tube dilution technique. The nutrient broth was prepared and kept in an autoclave for sterilisation. The broth was then allowed to cool. 4milliliter of nutrient broth was then transferred into 12 different tubes. Varying concentrations of the plant extracts (10, 20, 30, 40, 50, 60, 70, 80, 90, 100g/milliliter) were added to the test tubes containing nutrient broth. The bacterial suspension was then inoculated into the tubes and the tubes were incubated at 37oC for 24 hrs. After 24 hrs the tubes were examined for bacterial growth. The growth of the organism in the broth was measured by a spectrophotometer.
3.11.4 Growth Kinetic Study
Growth kinetic study was nothing but the effect of crude and purified plant extracts against the fish pathogen (Aeromonas hydrophila). For growth kinetic study 500milliliter of sterile nutrient broth was prepared and then 75milliliter of this were added to 6 different side arm flasks aseptically. After this, 100microliter of bacterial suspension was added to the 5 side arms flasks containing 75milliliter of nutrient broth, one flask without bacterial culture was kept as blank. In the other 4 flasks, 50microliter of crude and purified plant extracts were added. The remaining flask which contains bacterial culture but no plant extracts were kept as control. The reading of all the flask was taken against the blank, by using a spectrophotometer at 570nanometer in every 2 hours gap between incubation. The obtained results are then calculated by plotting a standard graph.
3.12 Identification and characterisation of active compounds from plants extracts
3.12.1GC-MS analysis
The ethanolic extract was prepared by drying the plant extracts. 2g of this dried biomass was saturated in absolute ethanol and then kept in a shaker for about 24 hours. After incubation, the extract was filtered through whattman no 1 filter paper. The filtrate was then evaporated and the powered form was given for GC-MS analysis. The identification of the chemical constituents of the plant extracts (Ocimum tenuiflorum and Curcuma longa) was performed by using a GC-MS spectrograph (Agilent 6890) fixed with an electron impact mode. The plant extracts were injected with the help a Hamilton syringe to the GC-MS manually for total ionchromato-125 graphic analysis in split mode. In quantitative analysis, selected ion monitoring (SIM) mode was active during the GC–MS analysis. SIM plot of the ion current resulting from very small mass range with only compounds of the selected mass was detected and plotted.
3.12.2 FT-IR analysis
FT-IR is a valuable tool for the identification and characterisation of chemical components and the functional groups (chemical bonds) present in the unidentified mixture of crude and purified plant extracts. Basically, the FT-IR spectra of pure components are like a molecular “fingerprint” as they are so unique in nature. The identification of the unknown compounds was done by comparing the comMon plant compounds stored in the system library. FTIR samples were prepared in various ways. For liquid samples, a drop of samples was placed between the 2 plates of sodium bromide. The sample then forms a thin film between the plates. The obtained results were then interpreted by comparing the crude and purified samples.
3.12.3 FT-RAMAN analysis
“Fourier-transform Raman spectroscopy was done by using a “Bruker IFS 66 instrument with a FRA 106 Raman module connection and a Nd3+ /YAG laser effective at 1064nanometer” in the near infrared. The liquid samples of plant extracts (Crude and purified for both Tulsi and Turmeric) were studied in aluminium cups with a spectral “footprint” of 100m and the spectra were noted at a 4cm−1spectral resolution over the wavenumber range 50–3500cm−1. To increase the spectral signal-to-noise ratio, 2000 scans were gathered over a period of about 30min in replicate. All spectra were got more than twice from each position and no changes in band positions and intensities were observed. Comparison of the bands in the specimens was done by the spectra noted before for a project. The obtained results are compared with the control samples”.
3.12.4 HPLC analysis
“HPLC is an adaptable, powerful, and broadly utilized method for the segregation of comMon natural compounds. At present, this system is picking up fame among different expository methods as the primary decision for fingerprinting study for the quality control of herbal and medicinal plants. Purification of the compound of interest utilizing HPLC is the way toward isolating or separating the objective compound from other (structurally related) mixes or contaminants. Each compound ought to have a unique peak under certain chromatographic conditions. Depending upon what should be isolated and how firmillilitery related the samples are, the chromatographer may pick the conditions, for example, the mobile phase, run time, flow rate, comprehensive detectors and column to get the best separation. Identification of compounds is a crucial part of any HPLC assay. To identify certain compounds by HPLC, we have to select the best detector for the study. Once the detector is chosen and is set to ideal detection settings. The parameters of this assay ought to be with the end goal that a perfect peak of the known sample is seen from the chromatograph. The distinguishing peak ought to have a reasonable retention time and ought to be very much isolated from extraneous peaks at the recognition levels which the assay will be performed. The samples initially treated in such a way that the components of interest are efficiently liberated into the solution”.
The samples were injected in the column by using a syringe. The mobile phase, run time, the flow rate was fixed for each sample. From the obtained result we can easily identify the major components present in the plant extract samples.
3.13 Interaction of protein with bacterial culture by SEM method
Different concentration of purified plant extract was used to control the bacterial infection.in this study, the concentration was ranging from 10-1000microliter. For this experiment, 6 clean test tubes have been taken and sterilized properly. To this tubes 1milliliter of bacterial culture was added, into which different concentration of purified plant extracts was poured. The tubes were kept for incubation for 24 hours at 37ºc. After incubation, one loop full of the liquid suspension was taken from each tube and added to clean slides to form a thin smear. These slides are then given for SEM analysis to observe the interaction between purified plant extract and the bacterial pathogen.
3.14 Experimental pathogenicity of Tilapia (Aeromonas hydrophila)
The pathogenicity of Aeromonas hydrophila in Oreochromis mossambicus was studied by imMersion and intramuscular injection according to the standard method.
3.14.1 Infection via intramuscular method
For infection via intramuscular injection, the method of was followed. The experiment was performed in triplicates. A 30 microliter of serially diluted bacterial suspension (Neat, 10 ˉ1,10 ˉ2, 10ˉ3,10ˉ4, 10 ˉ5 and 10ˉ6 CFU/animal) was injected intramuscularly into each Tilapia fishes. Tilapia (Oreochromis mossambicus) fishes were conserved in fibre glass tanks holding freshwater at 28 ºC. Sterile PHOSPHATE BUFFER SALINE buffer was injected to the control fishes.The fishes were checked twice a day to check if there are any clinical signs of diseases and mortality. The infected fishes were observed for around 120 hours.
3.14.2 Infection via imMersion method
For this study, 3 fishes were taken in each fibre glass tank filled with 2litre of fresh water and oxygen. 100microliter of diluted bacterial culture was added from the different dilution (Neat, 10 ˉ1,10 ˉ2, 10ˉ3,10ˉ4, 10 ˉ5 and 10ˉ6) in that 7 different tanks containing tilapia fishes. The fishes were then checked twice a day for any clinical changes and mortality. The fishes were checked for 5 days.
3.15 Encapsulation of Plant Extracts
Encapsulation is a process in which different active compounds presents within a matrix or membrane in a particulate form to achieve one or more desired effects. Sodium caseinate and Xanthum gum have added in a proportion of 7:1 to 100 milliliter of double distilled water. To this mixture, 1% of glycerol was added. The mixture was then mixed with the aid of a hot plate with a magnetic stirrer. 1-2milliliter of plant extract was added to the mixture and kept for overnight shaking. After this, the mixture was kept for centrifugation at a 1000rpm for 2 min, to break the air pockets. 1N HCl was prepared and to this, the above mixture was added drop by drop utilizing a 5milliliter syringe. The dots acquired were washed thrice with distilled water, and then dried. The dried beads were kept for further investigation.
3.16 Characterization of Beads
SEM study of the beads was done by using a HIGH RESOLUTION SCANING ELECTRON MICROSCOPE Jeol JSM-6360 (Japan). The sodium caseinate beads were attached to the stubs using a two-sided adhesive tape to fix them at a particular position. The beads were then examined by using an acceleration voltage of 10 kilovolts.
3.17 In vivo treatment with combined plant extracts
Aeromonas hydrophila infection in tilapia fishes was treated by combining the two plant extract (Tulsi and Turmeric). This is done by three methods; they are (1) Intramuscular Injection (2) Immersion (3) Oral (Feed scale).
3.17.1 Intramuscular injection
Oreochromis mossambicus was experimentally infected by the bacterium Aeromonas hydrophila using the intramuscular method as defined previously. The infected fishes with clinical signs and symptoms were collected and nurtured separately in sterile freshwater. Then they are treated by injecting 40microliter of plant extracts. The fishes were checked daily if there is any progress in their survival. The fishes surviving at the end of 20 and 30 days were calculated to define the optimum exposure time of plant extracts required for the tilapia fishes to recover from the infection caused by Aeromonas hydrophila.
3.17.2 Immersion method
In this method, 100microliter of bacterial suspension was inoculated into 2litres of fresh water containing 3 tilapia fishes in fibre glass tank. To this, 50microliter of combined plant extracts was added. The fishes were checked for any kind of sign and symptoms of bacterial infection. The fishes survived at the end of 15-20 days were deliberate to explain the optimum exposure time of plant extracts requisite for the tilapia fishes to recover from the infection.
3.17.3 Oral Encapsulated Feed Treatment
This treatment process was done by infecting the tilapia fishes through intramuscular injection. The fishes were then maintained in fibre glass tank which contains fresh water and oxygen supply. To these fishes, the encapsulated beads which contain the plant extracts were given as their regular diet instead of that comMercial feed. The fishes were checked daily and the survival rate of the fishes has been also noted.
3.18 HISTOPATHOLOGICAL INVESTIGATION
For the histopathological study, different organs such as gill, tissue, Intestine, Liver, and brain were dissected out from the control fish, infected fish and the treated with plant extracts. All the dissected organs of the fishes were stored in screw cap bottles containing the formalin. Organs go through 4 washing steps before staining. After washing with different concentration of alcohol, the organs were left in a mixture of chloroform and paraffin wax at room temperature for 24 hours. Before implanting, the organs were saturated in three changes of paraffin wax with ceresin. The organs were sliced in between 5 to 7 µm thickness using a manual rotatory microtome. These sliced organs were then stained with Harris alum haematoxylin and counter stained with 1% Eosin. The histopathological sections were studied by the help of Carl Zeiss compound microscope. Photographs were taken by the camera fixed with the microscope.
3.19 Ultra SEM analysis of fish organ
For ultra SEM analysis, the organs (gills and liver) are dissected from the Control, Infected and Treated fishes. These organs firstly washed with absolute ethanol for 2-5 seconds, these organs were then dipped into isopropanol for 3-5 minutes. After the washing procedure was over, moisture present in the organs was soaked by using a paper towel or tissue paper. These dried samples then kept in a hot air oven for 10 minutes at 30ºc. Then the samples are given for the SEM analysis, to check whether there are any damages occurred in the cells due to infection by the bacterial culture. The results were compared with the treated sample results to find out the effectiveness of the plant extracts against the fish pathogen.
3.20 Enzymatic Analysis
3.20.1 Assay of Superoxide Dismutase (SOD)
SOD activity was done by following the method of Beauchamp and Fridovich (1995) using Nitro blue tetrazolium (NBT) in the presence of riboflavin. For this experiment, 0.1milliliter of cell lysate supernatant was taken in a test tube. To this tube 0.2milliliter of 0.1M EDTA (Prepared by dissolving in PHOSPHATE BUFFER SALINE, PH 7.8) was added. 0.1milliliter of 1.5mM NBT (dissolved in DMF) was added to the reaction mixture. Lastly, 50microliter of riboflavin (10µm) was added and kept for incubation for about 30 minutes. After the incubation period, the absorbance of the samples was taken at 560nanometer. The obtained data was analysed to plot a standard graph.
3.20.2 Assay of Catalase
Catalase protects the cell from oxidative damages by ROS, a comMon enzyme found in all organ of fishes. The catalase activity was determined according to the protocols of Sinha, (1972). For this, 33microliter of samples was mixed with 666microliter of phosphate buffer (50mM). To this 333microliter of hydrogen peroxide were added. The absorbance was taken at 240nanometer for 3 minutes at every 30 seconds.
3.20.3 Estimation of Lipid peroxidation
The lipid peroxide content was determined by thiobarbaturic acid reaction defined by. For this assay, 0.1milliliter of the sample was mixed with 1.5milliliter acetic acid, 0.2 milliliter SDS, 1.5milliliter TBA reagent in a test tube and to this distilled water was added to make the volume 5milliliter. Blank and standards were also prepared by following the same procedure. These tubes were then kept in a water bath for 1 hour. The tube suspension was then centrifuged at 6000 rpm for 10 minutes. A supernatant was pinkish in colour and the absorbance of this suspension was taken at 530-540nanometer.
3.20.4 Determination of Total Reduced Glutathione (GSH)
GSH was estimated by following the method of. Homogenates of gill, liver, and tissue (0.5milliliter) were precipitated with 0.1milliliter of 5% TCA and 0.4milliliter of distilled water. The substances were assorted well for complete precipitation of proteins and then centrifuged. 2.5milliliter of 0.2 M phosphate buffer and 50 microliter of DTNB were added to 0.5milliliter of the clear supernatant. The absorbance was taken at 412 nanometer compared to a blank comprising TCA in addition to the sample. The amount of glutathione is expressed as µg glutathione/milligram protein.
3.21 TOTAL ERYTHROCYTE AND LEUCOCYTE COUNTS
Red blood cells (RBC) and white blood cell (WBC) diluting fluids were utilised for determining total erythrocyte and leukocyte counts respectively. It was done by mixing 20microliter of the blood sample with 3980microliter of corresponding diluting fluid in a clean tube. Cell counts were done by using a Neubauer’s counting chamber. Diluting fluids were prepared as follows.
WBC diluting fluid
For this 200microliter of glacial acetic acid was mixed with10milliliter of distilled water.
RBC diluting fluid
For this 3g of sodium citrate was mixed with 1milliliter of formalin. The final content was made up to 100milliliter.
3.22 CYTOTOXICITY STUDY BY COMET ASSAY
The assay was performed to estimate the DNA to the cells (control, infected and treated). 3 buffers were prepared i.e. lysis buffer, electrophoresis buffer and neutralising buffer. 25microliter of sample was sandwiched between alternating layers of HMA, LMA, HMA. They were then immersed in lysis buffer for 2 hours in the dark. They were then subjected into electrophoresis buffer for 20 minutes and also run in the same buffer for 15 minutes. After electrophoresis they were soaked in neutralising buffer. Ethidium bromide was added and the samples were observed under fluorescent microscope.
CHAPTER 4
4. RESULTS
4.1 MOISTURE CONTENT
The moisture content of Tulsi (Ocimum tenuiflorum) and turmeric (Curcuma longa) sample was calculated. Moisture content present in Ocimum tenuiflorum was 40% and Curcuma longa also contains 20% moisture content.
| Calculation | Ocimum tenuiflorum | Curcuma longa |
| % sample free from moisture | 60% | 80% |
| Moisture content present in sample | 40% | 20% |
Table No. 4 (moisture content analysis)
4.2 PHYTOCHEMICAL ANALYSIS OF THE PLANT EXTRACTS
| Phytochemicals | Test | Appearance | Inference (Tulsi) | Inference (Turmeric) |
| Alkaloids | Wagner’s test | Red precipitate | + | + |
| Tannins and Phenolic compounds | Lead test | Bluish Green colour | – | – |
| Terpenoids | Salkowaski’s test | Reddish-brown colour | + | – |
| Saponins | Foam test | Presence of emulsion | + | – |
| Flavonoids | Ferric chloride test | White precipitate | + | + |
| Glycosides | Brown ring | + | – | |
| Carbohydrates | Fehling’s test | Brown ring | + | + |
| Lactones | Baljet’s test | – | – | – |
| Proteins | Biuret’s test | – | – | + |
| Fixed oils and fatty acid | Spot test | Brown ring | + | + |
Presence (+), Absence (-)
Table no.5 (Phytochemical analysis of Tulsi and Turmeric)
4.3 ANTIOXIDANT ACTIVITY
4.3.1 TOTAL POLYPHENOL ACTIVITY
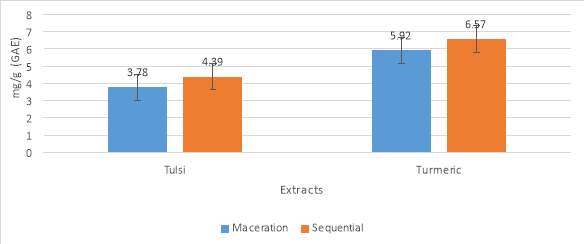
The analysis of total polyphenol content of Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa) was performed by two different processes (Maceration and Sequential). The results were shown in table no. 1 and graph no 1. The ethanolic extracts of these two plants showed a concentration of 3.78milligram/g, 4.39milligram/g for tulsi and 5.92milligram/g, 6.57milligram/g for turmeric of total polyphenol content in both the methods. In both cases, the sequential method showed a higher activity than the maceration. The results were stated as Gallic acid equivalents.
4.3.2 TOTAL FLAVANOID CONTENT
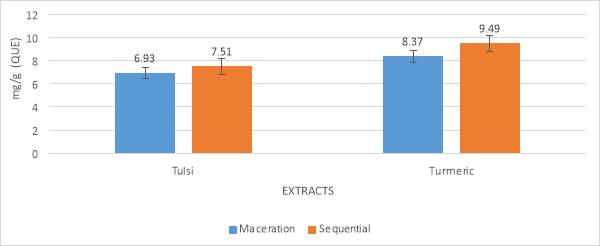
Total flavonoid content analysis was also done by two processes, maceration and sequential. The results were shown in table no. 2 and graph no. 2. The ethanolic extract of Tulsi and Turmeric showed the concentration of 6.93milligram/g, 7.51milligram/g for tulsi and 8.37milligram/g, 9.49milligram/g for turmeric. When comparison was done between the two process for both the cases, the sequential process shows a higher impact than the maceration process. Here the total flavonoid content is described as the number of equivalent quercetin.
Graph No.1 Total polyphenol content
| Methods | Tulsi | Turmeric |
| Maceration (milligram/g) | 3.78 | 5.92 |
| Sequential (milligram/g) | 4.39 | 6.57 |
Table No. 6 Total polyphenol content
Graph No. 2 Total flavonoid content
| Methods | Tulsi | Turmeric |
| Maceration (milligram/g) | 6.93 | 8.37 |
| Sequential (milligram/g) | 7.51 | 9.49 |
Table No. 7 Total flavonoid content
4.3.3 TOTAL ANTIOXIDANT ACTIVITY
Total antioxidant activity analysis of Ocimum tenuiflorum (Tulsi) and Curcuma longa (Turmeric) was determined by method. The antioxidant activity was performed by using extracts of both maceration and sequential process. The results showed by Tulsi extracts are 27.23 milligram/g, 34.94 milligram/g and in Turmeric extract 31.75milligram/g. 38.17 milligram/g. In both the cases, the activity of sequential process was higher than the maceration. The results were shown in table no 3 and graph no 3. The results were expressed as the number pf equivalents of ascorbic acid in milligram per gram of extract.
4.3.4 DPPH RADICAL SCAVENGING ASSAY
Total DPPH radical scavenging analysis of Ocimum tenuiflorum (Tulsi) and Curcuma longa (Turmeric) was performed by using the ethanolic extracts of both maceration and sequential process. The scavenging showed by Tulsi extracts are 22.41%, 31.52% and in Turmeric extract 25.12%, 43.39%. In both the cases, the activity of sequential process was higher than the maceration. The results were shown in table no 4 and graph no 4.
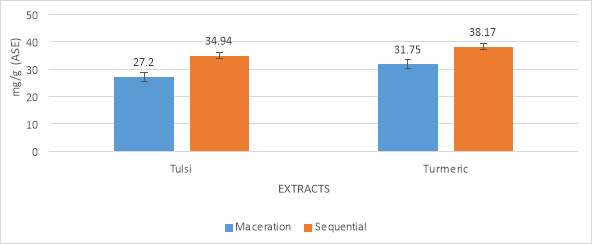
Graph No. 3 Total antioxidant assay
| Methods | Tulsi | Turmeric |
| Maceration (milligram/g) | 27.20 | 31.75 |
| Sequential (milligram/g) | 34.94 | 38.17 |
Table No. 8 Total antioxidant assay
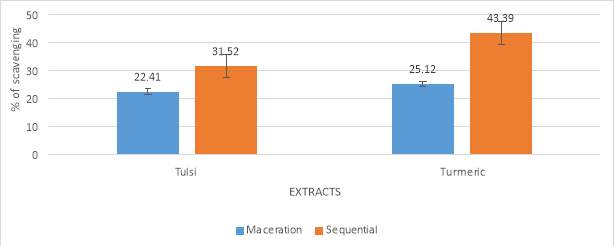
Graph No. 4 DPPH (2,2-diphenyl-1-picrylhydrazyl)
| Methods | Tulsi | Turmeric |
| Maceration (% scavenging) | 22.41 | 25.12 |
| Sequential (% scavenging) | 31.52 | 43.39 |
Table No. 9 DPPH (2,2-diphenyl-1-picrylhydrazyl)
4.3.5 HYDROGEN PEROXIDE SCAVENGING ASSAY
Many plant extracts have the scavenging capability of H2O2. Which are able to cross the membrane barrier and can slowly oxidize a certain number of components. H2O2 is not highly reactive but can show some toxic effects to the cells because of the increasing amount of free radicals in the cells. This process was also carried out by using the ethanolic plant extracts (Tulsi and Turmeric). Scavenging activity of Tulsi is 47.85% and 59.2% (Maceration and Sequential). For Turmeric it showed 52.97% and 67.18% scavenging. In this case, also, the sequential method showed a higher scavenging activity than the maceration. The results are described in table 5 and graph 5.
4.3.6 HYDROXYL RADICAL SCAVENGING ASSAY
This assay was performed by following the modified method of. The two processes (Maceration and Sequential) were compared for both Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa). The inhibition was higher in the sequential process than the maceration. Tulsi and Turmeric extracts showed 37.92%, 53.34% and 46.28%, 61.53% (Maceration and Sequential) of inhibition. The results were shown in table no 6 and graph no 6.
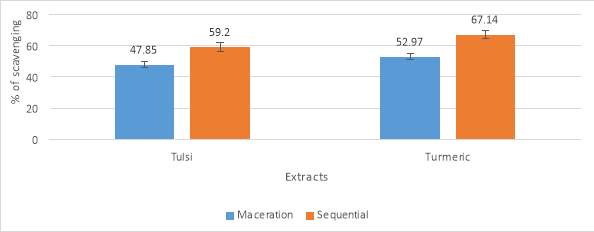
Graph No. 5 Hydrogen Peroxide scavenging assay
| Method | Tulsi | Turmeric |
| Maceration | 47.85 | 52.97 |
| Sequential | 59.2 | 67.14 |
Table No. 10 Hydrogen Peroxide scavenging assay
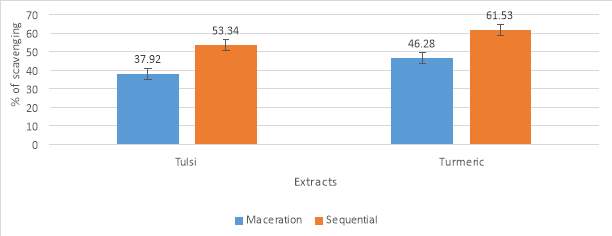
Graph No. 6 Hydroxyl radical assay
| Method | Tulsi | Turmeric |
| Maceration | 37.92 | 46.28 |
| Sequential | 53.34 | 61.53 |
Table No. 11 Hydroxyl radical assay
4.3.7 ABTS RADICAL SCAVENGING ASSAY
After performing the ABTS radical scavenging assay, it showed that the anti-oxidant activity increases as the concentration increases. The scavenging activity was shown by the ethanolic extract of Tulsi (27.48% and 32.19%) and in Turmeric (34.77% and 43.31%). The squentil process showing a higher activity than the maceration process in both extracts.
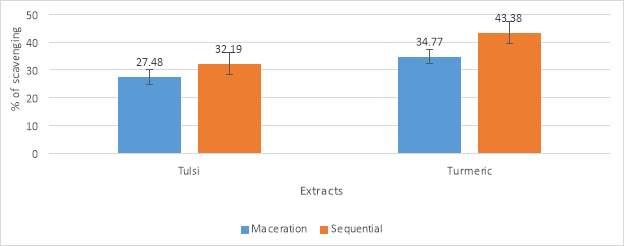
Graph No. 7 ABTS2,2´-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)
| Method | Tulsi | Turmeric |
| Maceration | 27.48 | 34.77 |
| Sequential | 32.19 | 43.38 |
Table No. 12 ABTS radical scavenging assay
4.4 ANTIBACTERIAL ACTIVITY
In the present study, the extracts (Ethanol solvent) of Tulsi (Ocimum tenuiflorum) and Turmeric (Curcuma longa) were tested against Aeromonas hydrophila by well diffusion method and disc diffusion method. The combination study of the plant extracts was also done. The results show that the extracts can inhibit the growth of the bacteria. It was also observed that the combined extract was more potent than individual once. The well diffusion results showed that the ethanol extract of Tulsi, Turmeric shows a zone of inhibition of 5millimeter, 6millimeter. Whereas the combined extract shows a zone of inhibition of 8millimeter. In disc diffusion study showed the zone of inhibition of Tulsi, Turmeric, and the combined one was as follows 3millimeter, 4millimeter, 6millimeter.

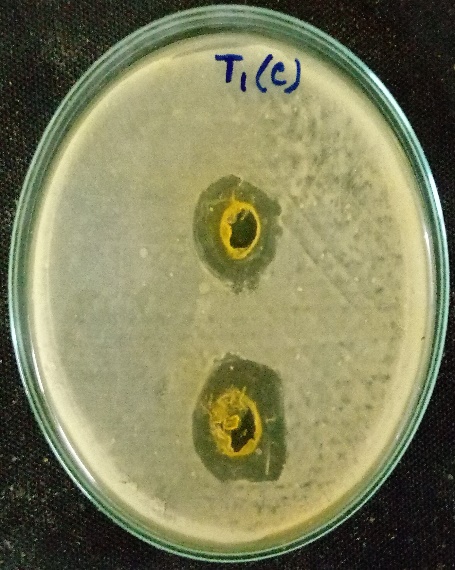
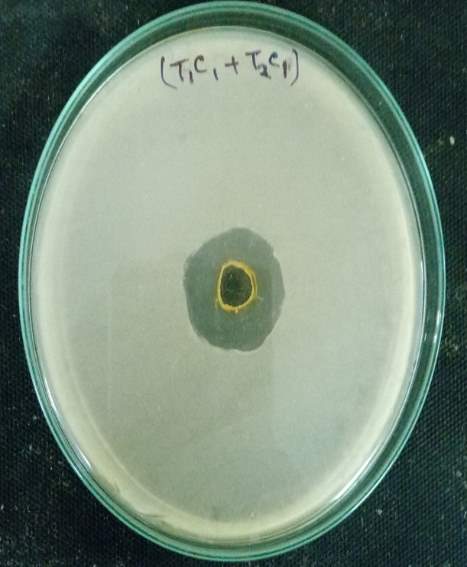 (a) Tulsi Extract (b) Turmeric Extract (c) Combined Extract
(a) Tulsi Extract (b) Turmeric Extract (c) Combined Extract
Fig No.6 The antibacterial activity by well diffusion method of (a) Tulsi (Ocimum tenuiflorum), (b) Turmeric (Curcuma longa) and the (c) combined extract against Aeromonas hydrophila.
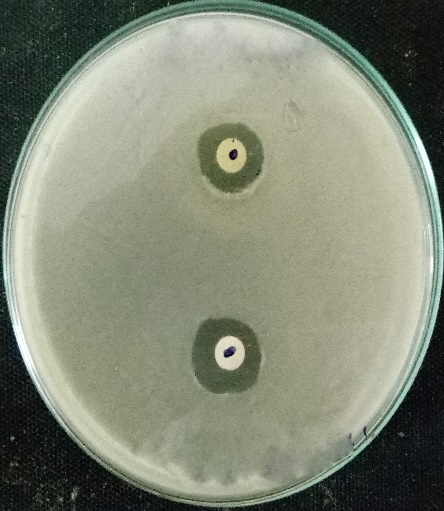
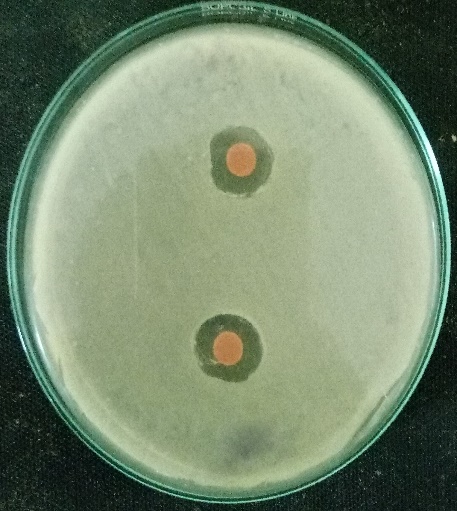
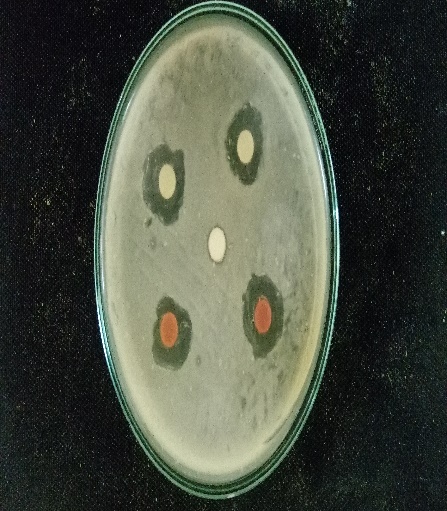
(a) Tulsi Extract (b) Turmeric Extract (c) Combined Extract
Fig No.7 The antibacterial activity by disc diffusion method of (a) Tulsi (Ocimum tenuiflorum), (b) Turmeric (Curcuma longa) and the (c) combined extract against Aeromonas hydrophila.
4.4.1 MINIMUM INHIBITORY CONCENTRATION (MIC)
| Extracts | 10µl | 20µl | 30µl | 40µl | 50µl | 60µl | 70µl | 80µl | 90µl | 100µl | culture |
| Tulsi (c) | + | + | + | + | [+] | – | – | – | – | – | + |
| Turmeric(c) | + | + | + | [+] | – | – | – | – | – | – | + |
| Tulsi crude | + | + | + | + | [+] | – | – | – | – | – | + |
| Turmeric crude | + | + | + | [+] | – | – | – | – | – | – | + |
The MIC of Ocimum tenuiflorum and Curcuma longa (crude and purified)against Aeromonas hydrophila was described by the tube dilution method. Ocimum tenuiflorum and Curcuma longa extracts were effective in controlling Aeromonas hydrophila. Hence the minimum inhibitory concentration was found to be 50microliter in Tulsi and 40microliter in turmeric for both crude and purified extracts. The results are presented in the following table
The growth of Aeromonas hydrophila- Good Growth (+), No growth (-), Poor growth [+].
Table No. 13 (Minimum inhibitory concentration)
4.4.2 GROWTH KINETIC STUDY
The growth kinetic study was performed by using the crude and purified extracts of both the plants against the bacterial pathogen. The optical density measured at 570 nanometre. The results were shown in the below mentioned graph and table.
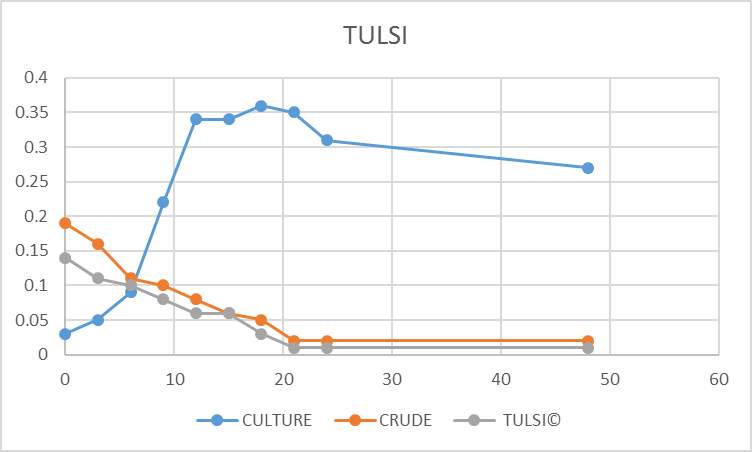
Graph No. 8 (Growth kinetic study of Tulsi)
| TIME | CULTURE | CRUDE | TULSI(C) |
| 0th | 0.03 | 0.19 | 0.14 |
| 3rd. | 0.05 | 0.16 | 0.11 |
| 6th | 0.09 | 0.11 | 0.1 |
| 9th | 0.22 | 0.1 | 0.08 |
| 12th | 0.34 | 0.08 | 0.06 |
| 15th | 0.34 | 0.06 | 0.06 |
| 18th | 0.36 | 0.05 | 0.03 |
| 21st | 0.35 | 0.02 | 0.01 |
| 24th | 0.31 | 0.02 | 0.01 |
| 48th | 0.27 | 0.02 | 0.01 |
Table No. 14 (Growth kinetic study of Tulsi)
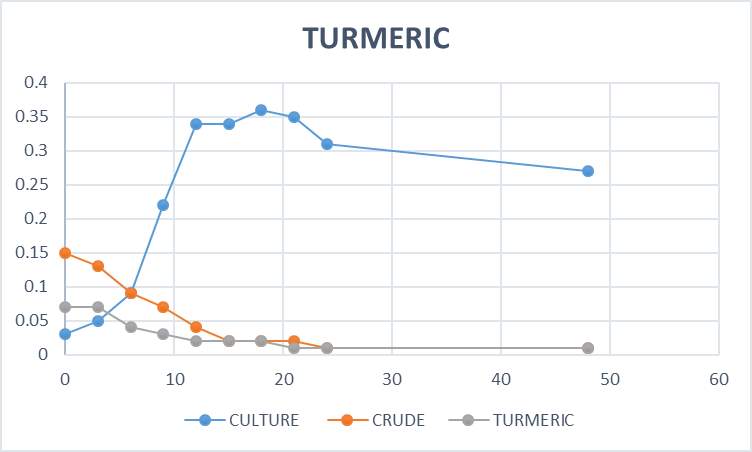
Graph No. 9 (Growth kinetic study of Turmeric)
| TIME | CULTURE | CRUDE | TURMERIC(C) |
| 0 | 0.03 | 0.15 | 0.07 |
| 3 | 0.05 | 0.13 | 0.07 |
| 6 | 0.09 | 0.09 | 0.04 |
| 9 | 0.22 | 0.07 | 0.03 |
| 12 | 0.34 | 0.04 | 0.02 |
| 15 | 0.34 | 0.02 | 0.02 |
| 18 | 0.36 | 0.02 | 0.02 |
| 21 | 0.35 | 0.02 | 0.01 |
| 24 | 0.31 | 0.01 | 0.01 |
| 48 | 0.27 | 0.01 | 0.01 |
Table No. 15 (Growth kinetic study of Turmeric)
4.5 IDENTIFICATION OF COMPOUNDS
4.5.1 GC-MS analysis
This identification was made by comparing the mass spectra on both columns with phytochemical and ethnobotanical databases libraries or with mass spectra from the literature. The phytocomponents present in Tulsi and Turmeric were observed in GC –MS and some of them compounds possess antibacterial activity. The results are expressed in the following table.
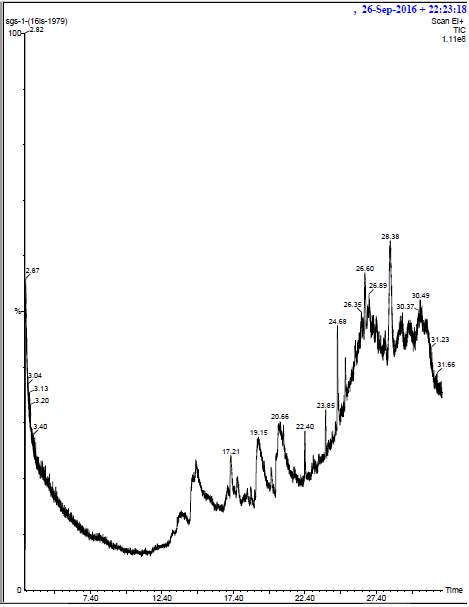
(Chromatogram of Tulsi Extract)
| No | RT | Name of Compound | Molecular Formula | MW | ** Activity |
| 1 | 17.2 | Phytol | C20H40O | 296 | antibacterial |
| 2 | 19.12 | Eicosanoic acid | C20H40O2 | 312 | No activity |
| 3 | 22.40 | SULFUROUS ACID, OCTADECYL 2-PROPYL ESTER | C21H44O3S | 376 | Antibacterial |
| 4 | 20.62 | PENTADECANAL | C15H30O | 226 | Antibacterial, antifungal. |
| 5 | 20.90 | Oleic acid | C18H34O2 | 282 | No activity |
Table No. 16 (GC-MS analysis of Tulsi extract)
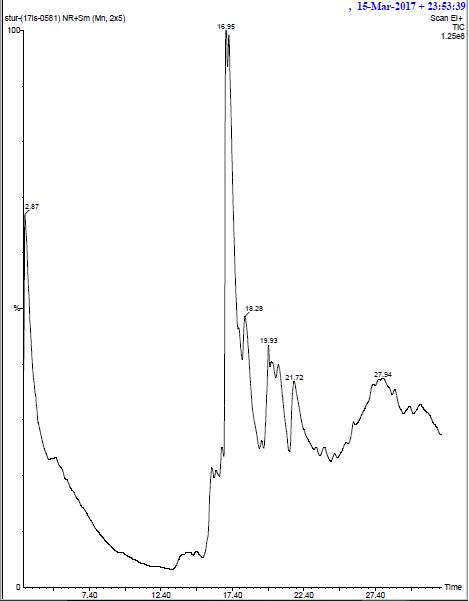
| No | RT | Name of Compound | Molecular Formula | MW | ** Activity |
| 1 | 2.87 | METHYLENE CHLORIDE | CH2Cl2 | 84 | No Activity |
| 2 | 16.95 | AR-TUMERONE | C15H20O | 216 | Antibacterial, antifungal, |
| 3 | 19.93 | CYCLOHEXYLPHENYLACETIC ACID | C14H18O2 | 218 | Antibacterial Activity |
| 4 | 21.72 | PROPANE, 1,2-DIBROMO-2-METHYL | C4H8Br2 | 214 | Antibacterial Activity |
| 5 | 27.94 | HEXESTROL DI-TMS | C24H38O2Si2 | 414 | Antibacterial, antifungal, antiprotozoans |
(Chromatogram of Turmeric Extract)
Table No. 17 (GC-MS analysis of Turmeric extract)
4.5.2 FT-IR analysis
FT-IR was done for both the tulsi and turmeric extract. The crude extract was taken as a control to compare with the purified one. The functional groups present the Tulsi crude extract were identified alcohol, alkane, carbonyl, amine, aromatic, ester, ether, and alkene, where in purified extract alcohol, alkane, Primary amine, Aromatic phosphates and disulphides bond were found. The resultant peak frequencies were recognized from the standard peaks.
Tulsi (Ocimum tenuiflorum)
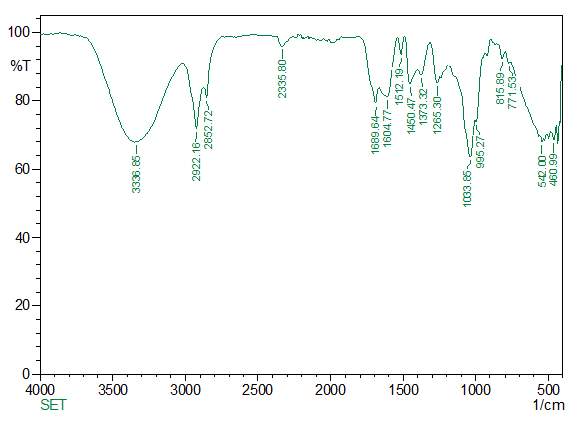
(FT-IR analysis of Tulsi Crude extract)
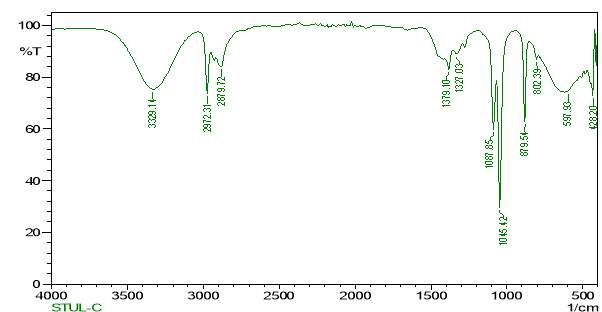
(FT-IR analysis of Tulsi purified extract)
Turmeric (Curcuma longa)
The functional groups present the Tulsi crude extract were identified alcohol, carboxylic acid, alkane, amine, ether, and alkene, where in purified extract alcohol, alkane, alkene, alcohol, ether, carboxylic acid bond were found. The resultant peak frequencies were recognized from the standard peaks.
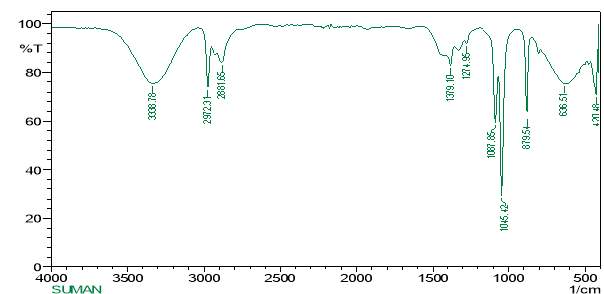
(FT-IR analysis of Turmeric crude extract)
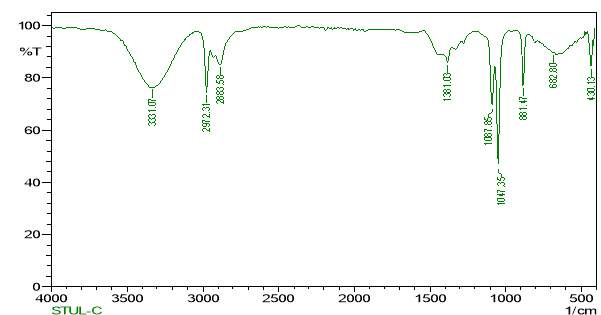
(FT-IR analysis of Turmeric purified extract)
4.5.3 FT-RAMAN analysis
FT-RAMAN analysis was also done for the identification of compounds for both tulsi and turmeric extract. The crude extract was taken as a control to compare with the purified one. The functional groups present in the Tulsi crude extract were identified alkane, carboxylate, aryl sulphides, a carbonyl group, where in the purified extract were bond were a carboxylic acid, alkane found. The resultant peak frequencies were recognized from the standard peaks
Tulsi (Ocimum tenuiflorum)
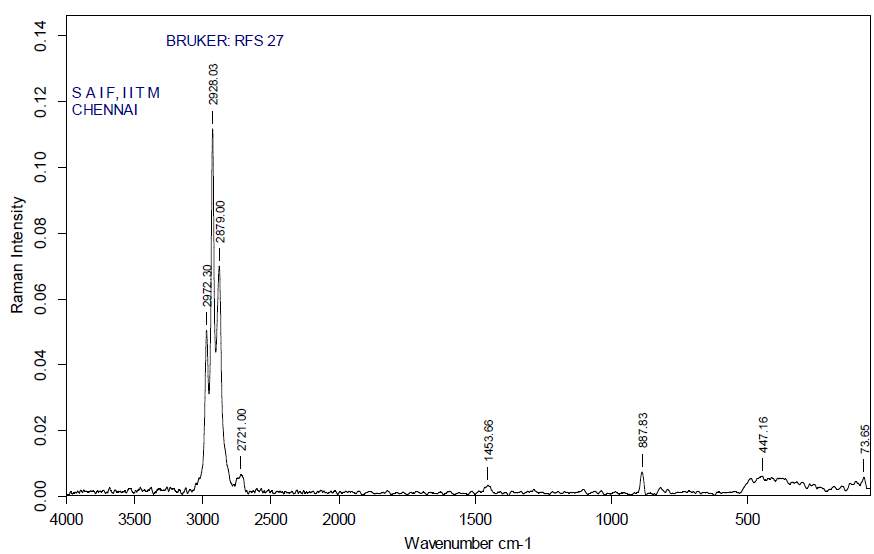 (FT-RAMAN analysis of tulsi crude Extract)
(FT-RAMAN analysis of tulsi crude Extract)
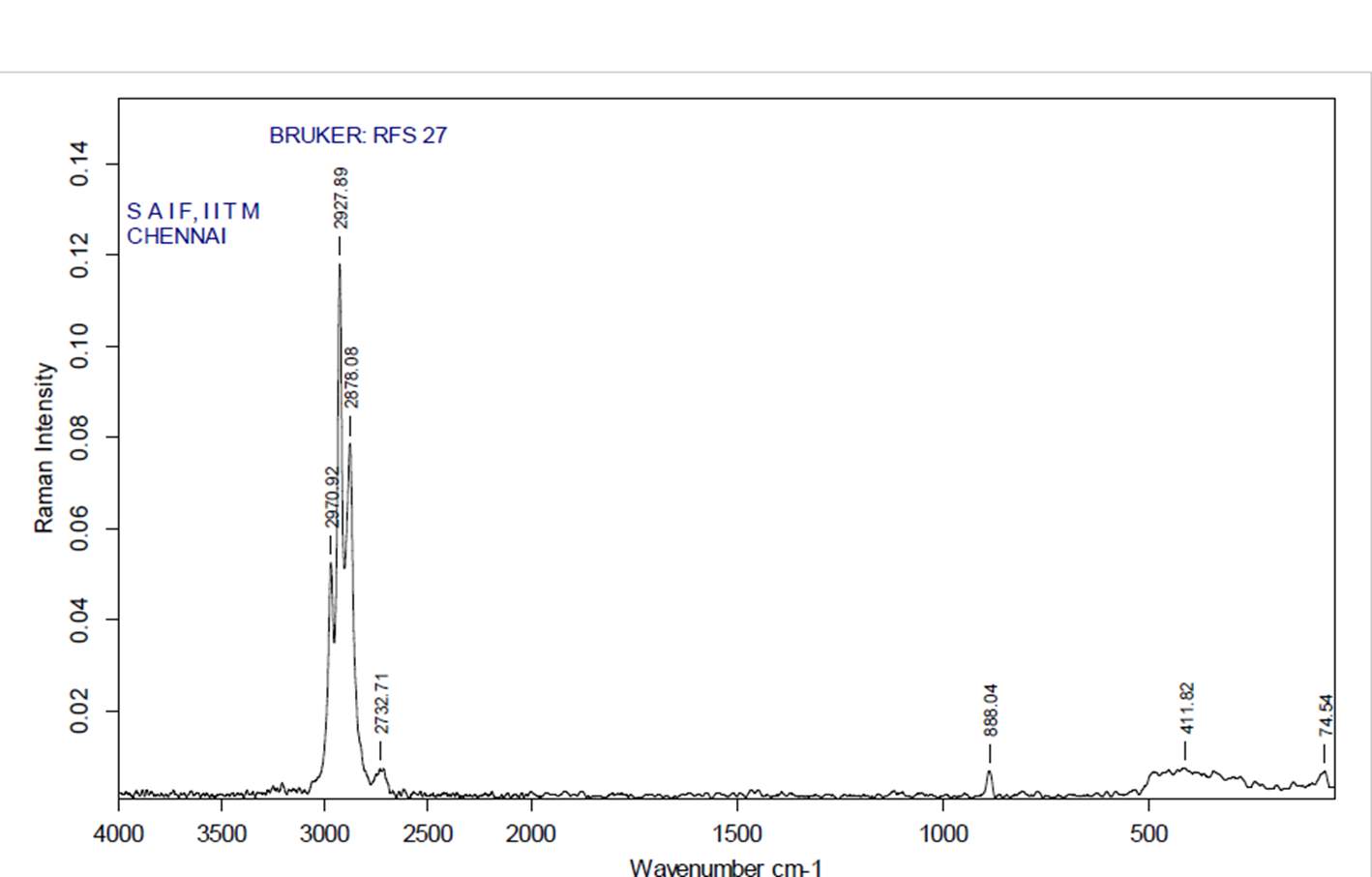
(FT-RAMAN analysis of Tulsi purified extract)
Turmeric (Curcuma longa)
The functional groups found in the crude turmeric extracts are hydroxyl, amine, alkane, alkyne, thiol, aryl sulphides. In the purified extract the identified functional groups were alkyne, thiol, alkanes, aryl sulphides, carboxylate.
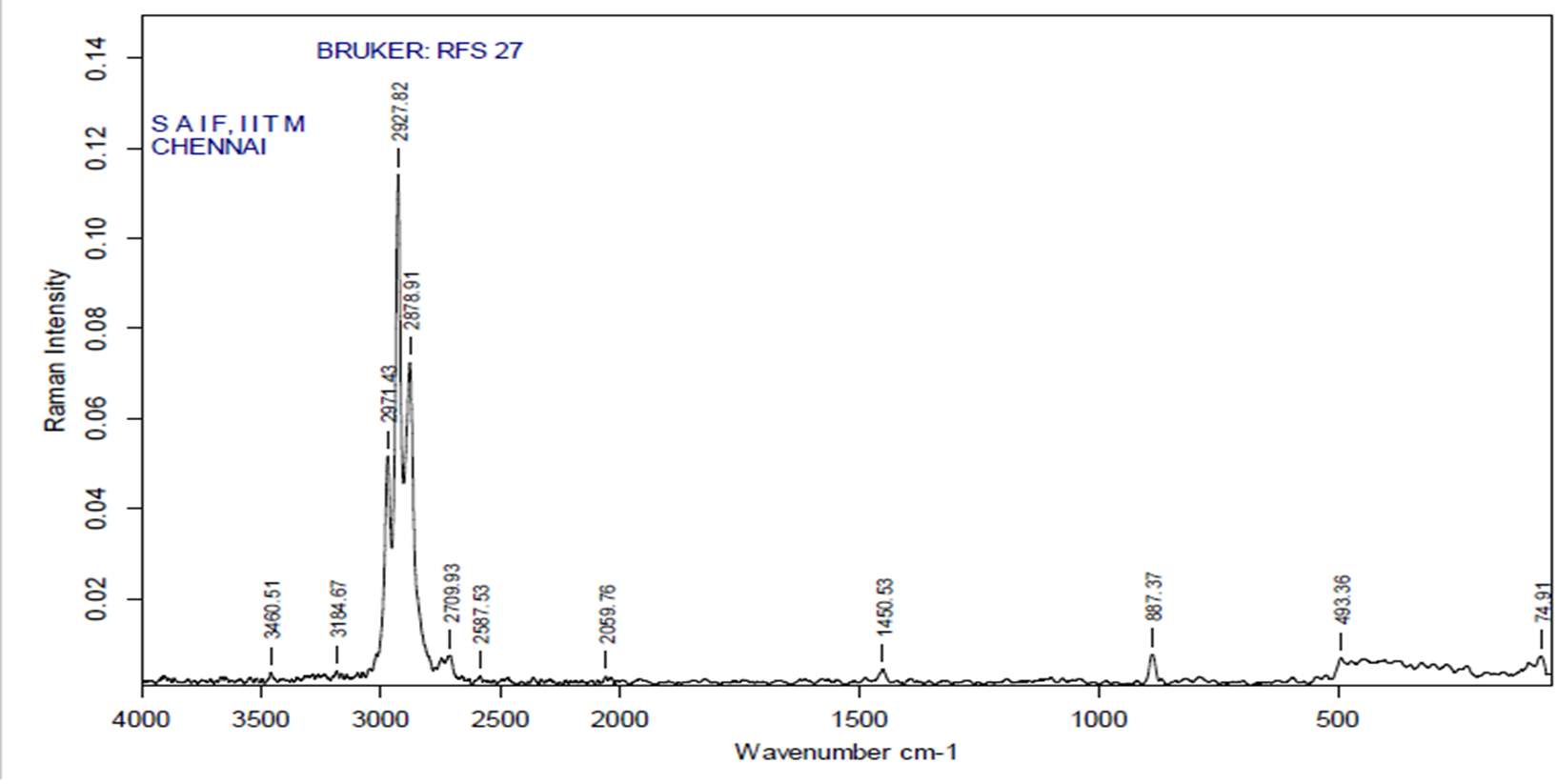
(FT-RAMAN analysis of Turmeric Crude Extract)
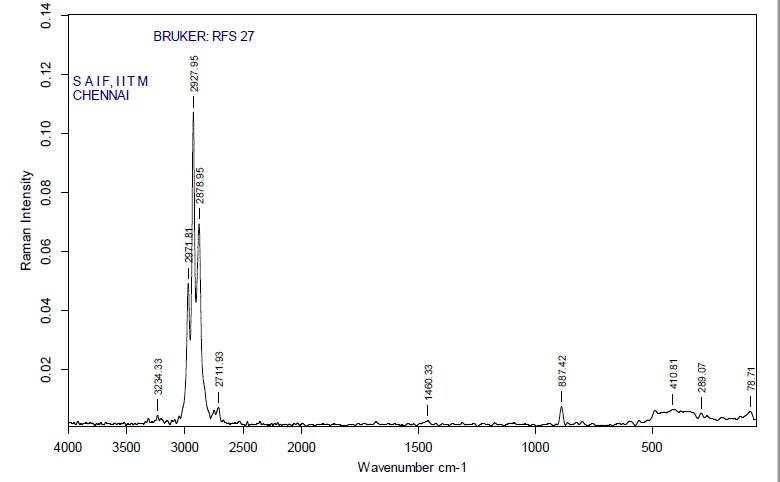
(FT-RAMAN analysis of Turmeric purified Extract)
4.5.4 HPLC analysis
High performance liquid chromatography was performed for both the purified samples (Tulsi and Turmeric) to identify the components present in the purified solution. Suitable parameters were optimized like, run time, mobile phase, flow rate within acceptable limits. Both the samples were run for 20 minutes. After completion of the run, the retention time for tulsi showed only one peak i.e. 1.958, which indicates the presence of urosilic acid and for turmeric also a single peak was found i.e. 9.130, which indicates the presence of palmitic acid. The results are shown below:
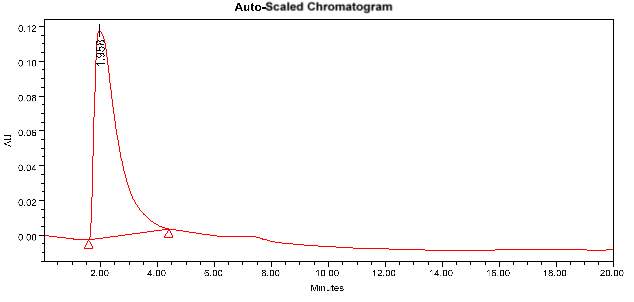 (Chromatogram of purified tulsi extract)
(Chromatogram of purified tulsi extract)
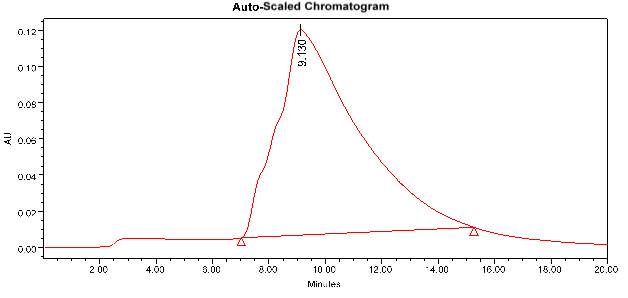
(Chromatogram of purified Turmeric extract)
4.6 EXPERIMENTAL PATHOGENICITY
The pathogenicity of Tilapia (Oreochromis mossambicus) to Aeromonas hydrophila was confirmed by the intramuscular injection and immersion method. The clinical symptoms on the fishes are ulceration, tumour formation at the site of injection, fin and tail damage, external bleeding and redness all over the body surface. The highest concentration of Aeromonas hydrophila showing 100% mortality within 96 hours, when injected intramuscularly. And in immersion method the mortality rate was same but it takes around 120 hours. However, the lower dilution of Aeromonas hydrophila caused 60% and 50% mortality, after 120 and 96 hours of post immersion and injection respectively. The LD50 value of Aeromonas hydrophila for intramuscular injection was got to be 2.82 × 10-3 at 96 hours. The LC50 value for the immersion method was found to be 2.12 × 10-3 at 120 hours. The results are shown in the below mentioned graphs and tables:

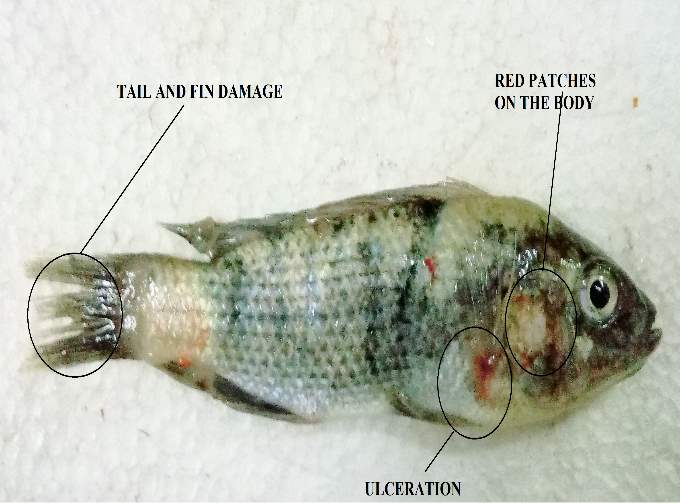
(A) Control (B) Infected
Fig No. 8 (Experimental pathogenicity)
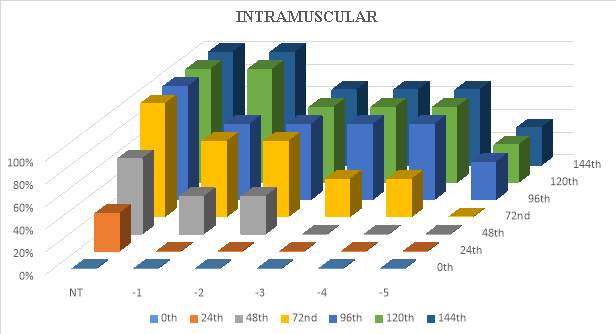
Graph No. 10 (Pathogenicity by Intramuscular Injection)
| TIME(hr) | NT | 10-1 | 10-2 | 10-3 | 10-4 | 10-5 |
| 0th | 0% | 0% | 0% | 0% | 0% | 0% |
| 24th | 33% | 0% | 0% | 0% | 0% | 0% |
| 48th | 33% | 0% | 0% | 0% | 33% | 0% |
| 72nd | 100% | 33% | 33% | 33% | 33% | 0% |
| 96th | 100% | 100% | 33% | 33% | 33% | 0% |
| 120th | 100% | 100% | 66% | 66% | 66% | 33% |
| 144th | 100% | 100% | 66% | 66% | 100% | 66% |
Table No. 18 (Pathogenicity by Intramuscular Injection)
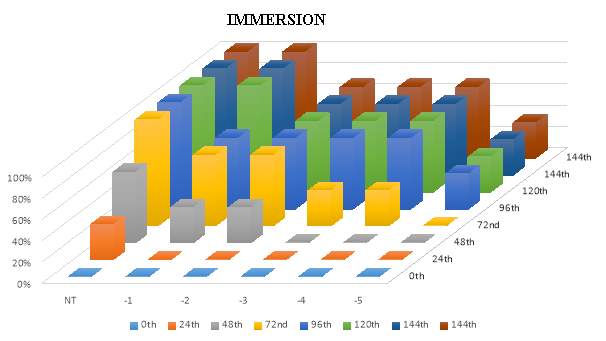
Graph No. 11 (Pathogenicity by Immersion method)
| TIME(hr) | NT | 10-1 | 10-2 | 10-3 | 10-4 | 10-5 |
| 0th | 0% | 0% | 0% | 0% | 0% | 0% |
| 24th | 33% | 0% | 0% | 0% | 0% | 0% |
| 48th | 66% | 33% | 33% | 0% | 0% | 0% |
| 72nd | 100% | 66% | 66% | 33% | 33% | 0% |
| 96th | 100% | 66% | 66% | 66% | 66% | 33% |
| 120th | 100% | 100% | 66% | 66% | 66% | 33% |
| 144th | 100% | 100% | 66% | 66% | 66% | 33% |
Table No. 19 (Pathogenicity by ImMersion method)
4.7 ENCAPSULATION
Encapsulation of the purified plant extract by using casein and xanthum gum is a biologically significant process to capture the proteins and active other biomolecules. In this study, the purified compounds of Tulsi and Turmeric extracts were encapsulated in the form of spherical beads. These spherical beads further used for feed scale treatment. Some pictures of bead formation are mentioned below:



- (b)


(c)
Fig No. 9 (Method of Bead formation)
4.8 CHARACTERISATION OF BEADS BY SEM
The SEM micrograph of the beads shown the circular shape morphology of the encapsulated beads with a substantial mean diameter of approx. 100micrometre. No visible pores, breakage or cracks were observed on the outer area of the beads. The results are shown in the following pictures:
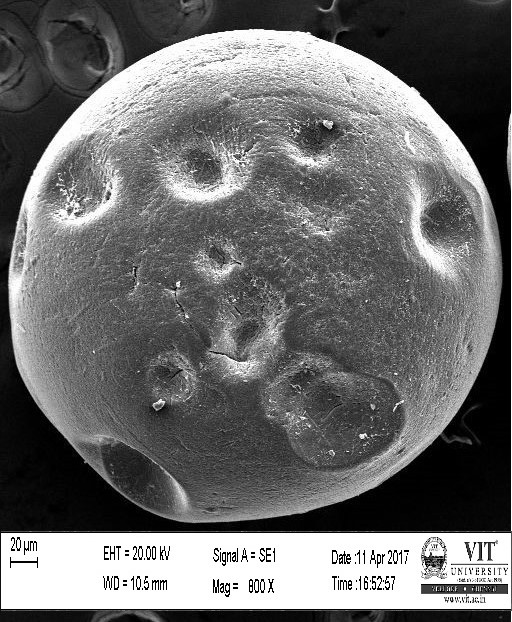

Fig No. 10 Fig No. 11
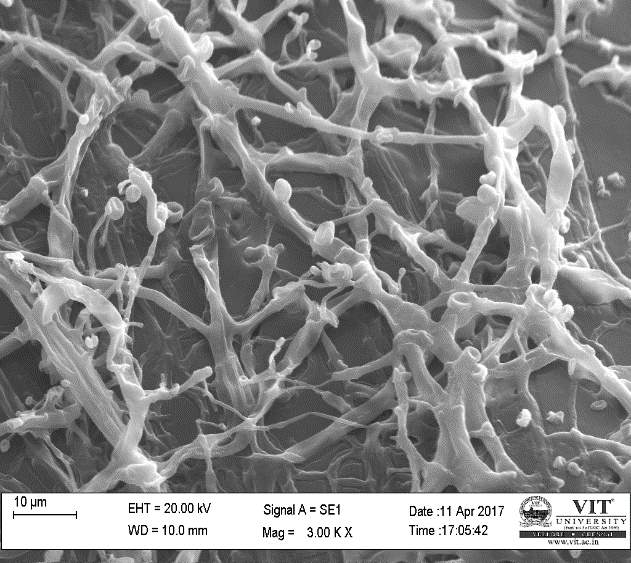

Fig No. 12 Fig No. 13
Fig No. 10 and 11 showed that at the outer surface of beads there were no cracks, where as in Fig No. 12 and 13 the outer surface of the beads was observed. In the outer surface, we can see how the plant extract molecules bind to it.
4.9 IN VIVO TREATMENT
This study revealed that, when the combined plant extract injected intramuscularly into Tilapia, the mortality rate has decreased. It showed an antibacterial activity on the fish pathogen and able to kill or inhibit the bacterial (Aeromonas hydrophila) infection. which is responsible for causing diseases and infections in Tilapia (Oreochromis mossambicus). The fishes were healthy and no signs of symptoms were seen after the treatment. In general, the combined plant extract can use a remedy for treating Aeromonas hydrophila infection. ImMersion and feed scale treatment was also performed for treating the fishes. In imMersion method, 2milliliter of combined extract was added into the water containing infected fishes. After 15 days the no clinical symptoms or infections were observed on the fishes. For the feed scale treatment, the combined extracts were encapsulated by using sodium caseinate. These beads were given as a normal diet for the infected fish for 15-20 days. The results showing a positive effect on the bacterial infection. Because fishes having no symptoms of infection on their body.


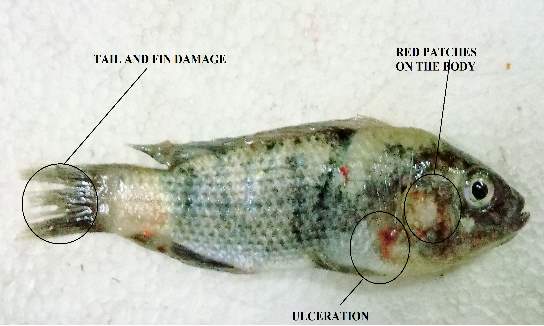
Fig No. 14 (Control Fish) Fig No. 15 (Infected Fish)


Fig No. 16 (Treated Fish)
4.10 HISTOPATHOLOGICAL ANALYSIS
Histopathological analysis has been done by dissecting the Control, Infected, and Treated fishes. Organs like gill, liver, intestine, Tissue were given for the analysis. In the infected fishes, the organs are damaged due to the bacterial infection. But after treatment, the damages of the organ were very less when compared with the control fishes. The changes observed in the gills of infected fishes were a loss of epithelium integrity and epithelium detachment leading to disagreement and consequent haemorrhage. Where as in the liver the number of kuffler cells has decreased and lots of vacuoles are observed. In skin and tissue sample the epidermis and dermis layer has been damaged. The changes occurred in the intestine of the infected animal were an alteration in connective tissues of lamina propria, critical damage in CEC, critical mucus excretion, and separation of epithelial layer from lamina propria. All these signs and symptoms were cured after treatment by using the plant’s extract. The pictures of some dissected organ were given below:

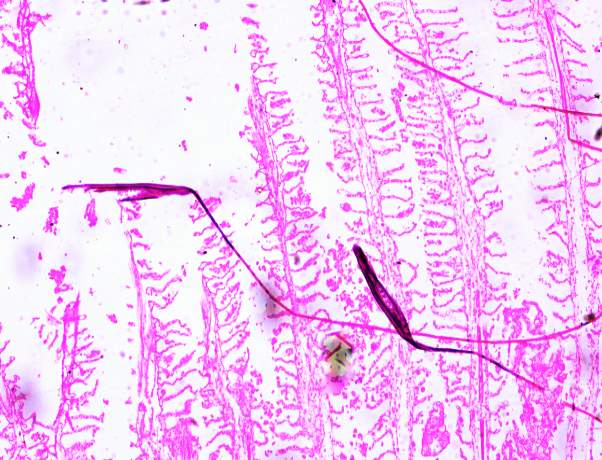
Fig No. 17 (Control gills) Fig No. 18 (Infected gills)

Fig No. 19 (Treated gills)
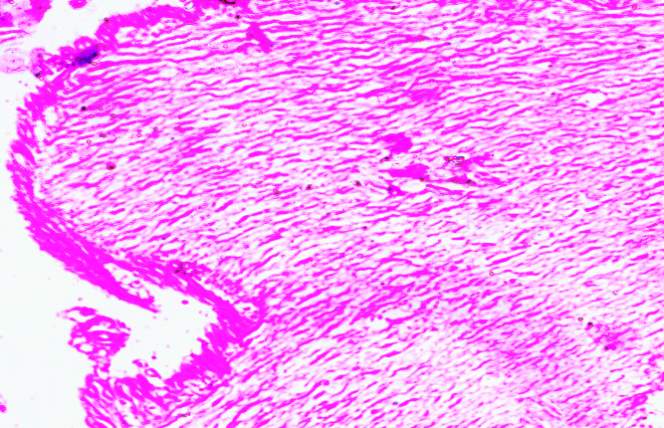
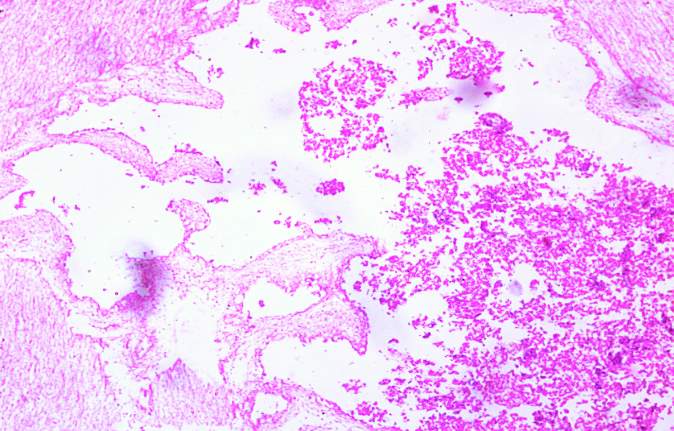
Fig No. 20 (Control Intestine) Fig No. 21 (Infected Intestine)
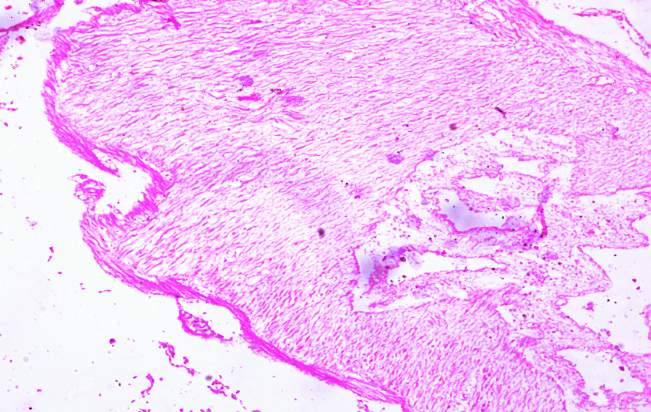
Fig No. 22 (Treated Intestine)
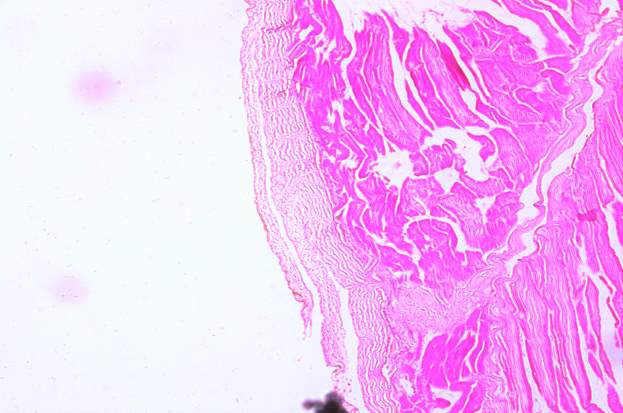
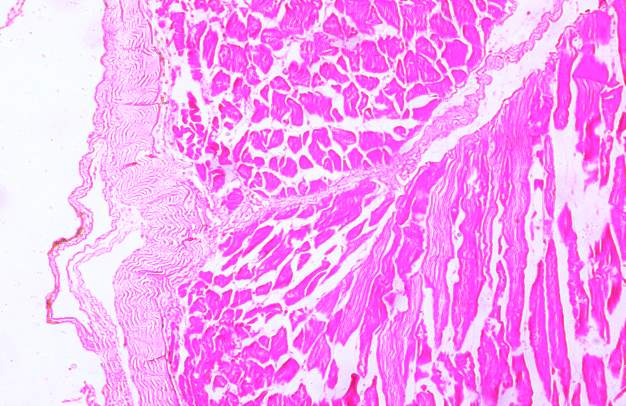
Fig No. 23 (Control skin and muscle) Fig No. 24 (Infected skin and muscle)
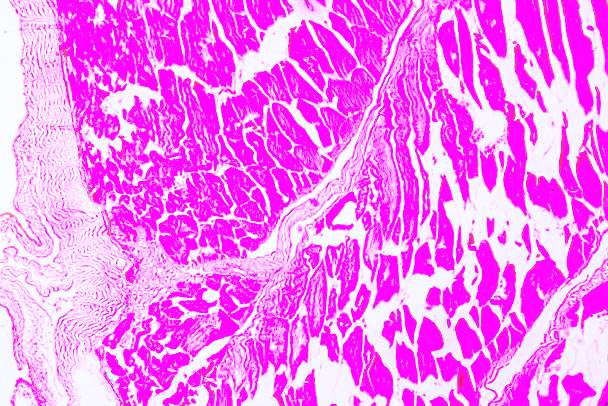
Fig No. 25 (Treated skin and muscle)
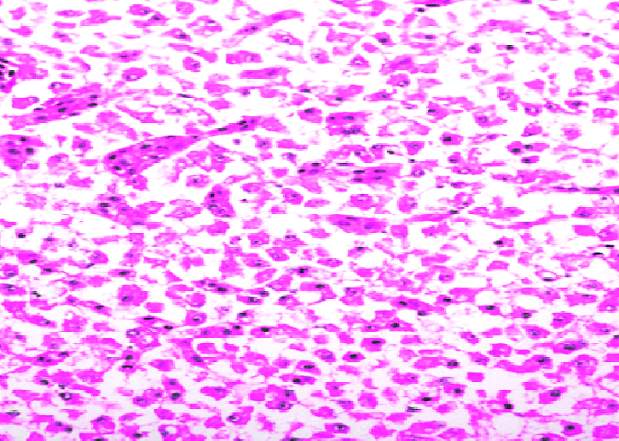
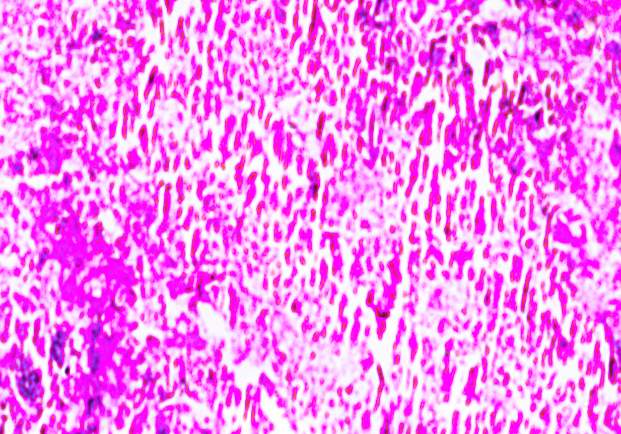
Fig No. 26 (Control liver) Fig No. 27 (Infected liver)
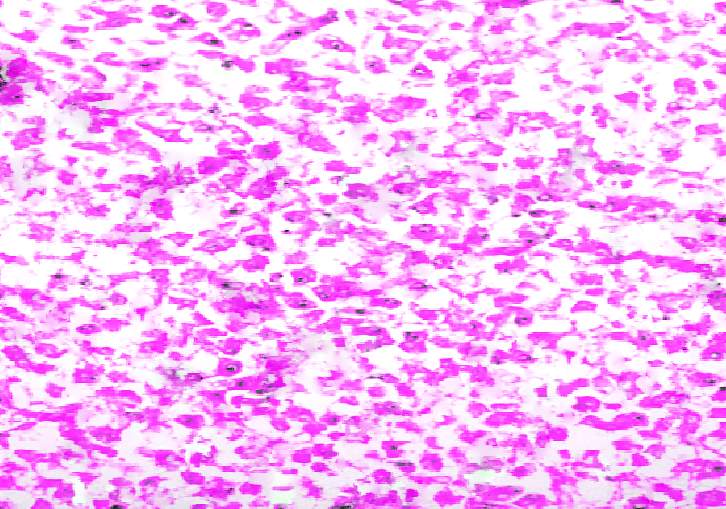
Fig No. 28 (Treated liver)
4.11 PROTEIN INTERACTION STUDY BY SEM METHOD
In this study, the ability of both the plant extracts to inhibit or kill the bacterial pathogen was observed on a glass slide by using Scanning electron microscope. The pictures show that how the active molecules bind to the bacteria.
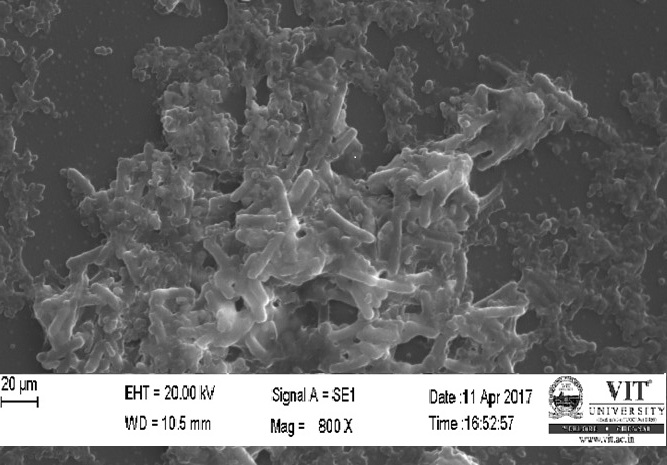


Fig No. 29 (control) Fig No. 30 (Tulsi Extract) Fig No. 31(Turmeric)
4.12 ULTRA SEM ANALYSIS OF FISH ORGANS
The infected and control gill and liver were given for the ultra SEM analysis. This study reveals the that how much damage happened to the cells. In the case of gill, it was observed that the primary lamellae and secondary lamellae possess some damages in the infected animal, where as it was absent in the control. In the liver, the kuffler cells and vacuoles were observed. The ultra SEM pictures are given below:
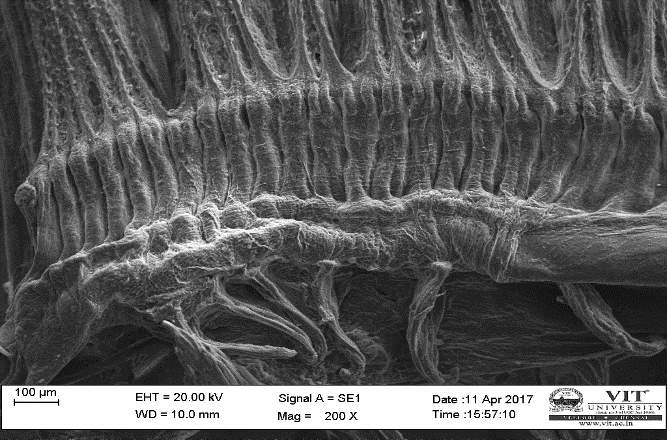
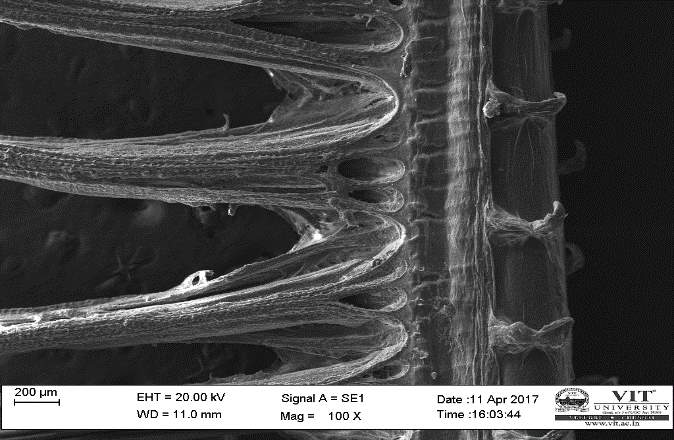
Fig No. 32 (control gill) Fig No. 33 (Infected gill)
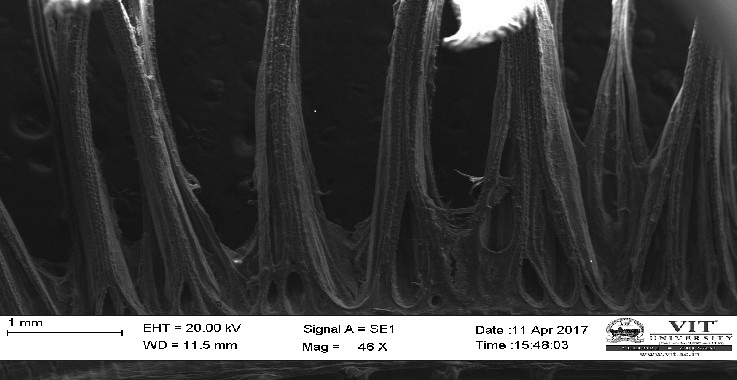

Fig No. 34 (Treated gill) Fig No. 35 (Treated gill)
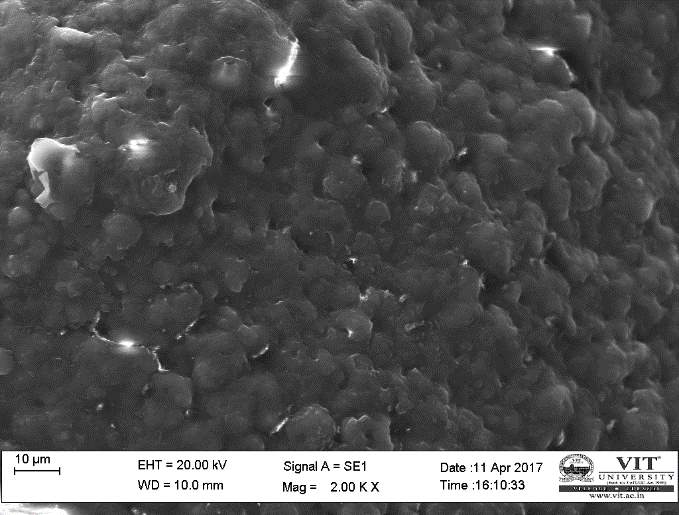
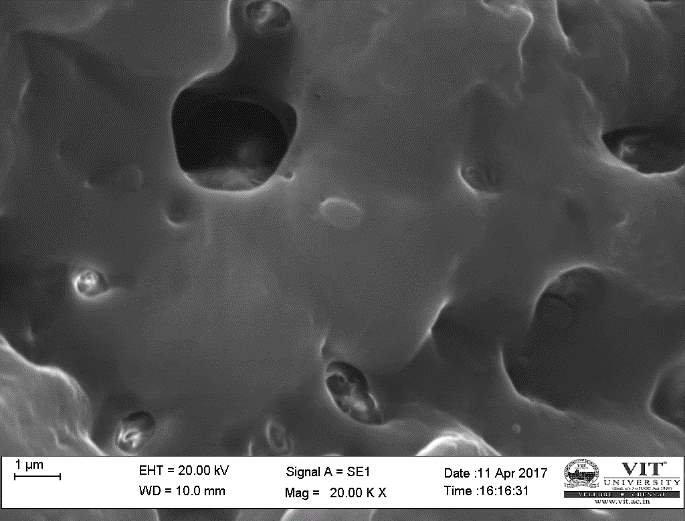
Fig No. 36 (Control Liver) Fig No. 37 (Infected Liver)
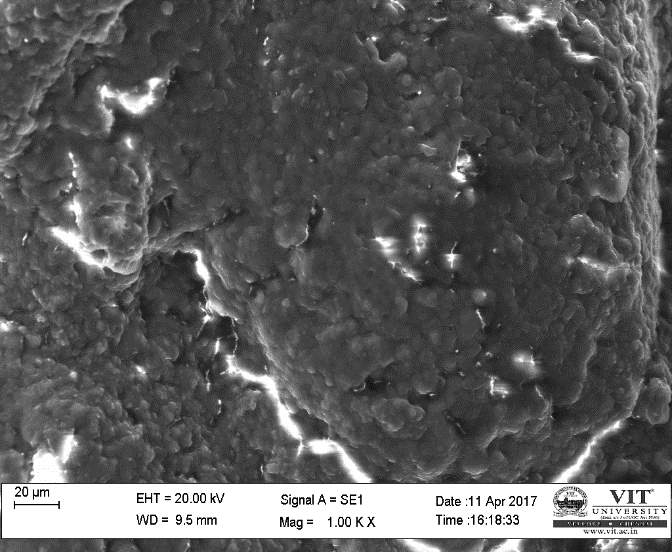
Fig No. 38 (Treated Liver)
TOTAL RBC AND WBC COUNT
Total RBC and WBC count were done for control, infected and treated fish blood samples. In control fish blood sample, the amount of WBC was 1.8×103/microliter and RBC was 0.62×106/microliter. In the infected fish blood sample, the amount of WBC was 0.7×103/microliter and RBC was 0.080×106/microliter. Where as in the treated sample, the amount of WBC was 1.2×103/microliter, and RBC was 0.23×106/microliter. The results indicate that the plant extracts were active against the bacterial pathogen. And it can actively kill the pathogen present in the blood cells.
COMET ASSAY
After performing this assay, it was observed that the comet tail was present in case of infected samples, the length of the tail was 1.94micrometre. which indicates the destruction of DNA within the cells. Where as in the control and treated sample there was no sign of comet. Some pictures are mentioned below

Fig No. 39 (Control cells) Fig No. 40 (Infected cells)

Fig No. 41(Treated cells)
ENZYMATIC ANALYSIS
Different enzymatic assays have been performed. SOD, catalase, LPO and GSH activity was increased in the treated samples when compared the infected samples. The mechanism of action was catalysed by the addition of natural antioxidant and radical scavenging molecules from the plant extract for the control of intracellular reactive oxygen species formation.
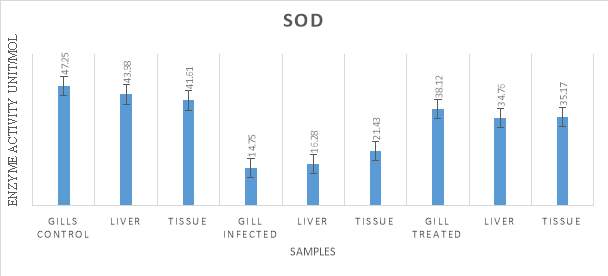
Graph No. 12 (Super Oxide dismutase)
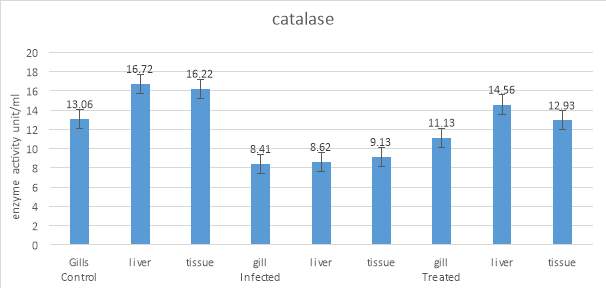
Graph No. 13 (Catalase Activity)
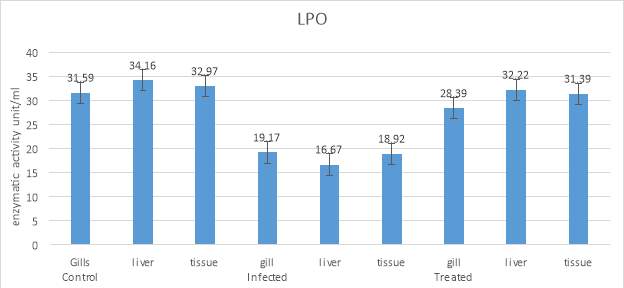
Graph No. 14 (Lipid peroxidation assay)
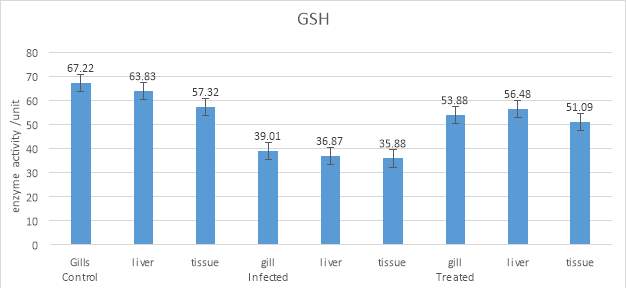
Graph No. 15 (Total Reduced Glutathione)
5. DISCUSSION
This study relies on the use of medicinal plants, which are used from ancient times. People have utilized plants for the treatment of various illnesses for a large number of years. As per the World Health Organization, most populaces still depend on natural plant solutions for their mental and physical health requirements. These medications are moderately more secure and less expensive than engineered or present day drug. In this study, I have used Tulsi and Turmeric plant extract, as they are well known for treating various diseases found in humans. Now a day, various microorganisms were resistance against many antibiotics. Hence, these medicinal plants are the best alternative for comMercial antibiotics.
The powdered plant substance from Tulsi and Turmeric undergoes through Maceration and Sequential process to remove all the impurities from the solution. The filtrate solution was given for identification and characterisation of bio active compounds by GC-MS, FT-IR, FT-RAMAN, and HPLC. The prepared plant extracts were subjected to perform following preliminary phytochemical screening test. The results showed some interesting active compounds which have antibacterial, antifungal, antiviral activity.
Anti-oxidants are successful in ensuring living beings against oxidative harms brought about by ROS. Consequently, antioxidants agents which are found comMercially have been sought after, and the majority of them are synthesized, including butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and propyl gallate (PG). These manufactured antioxidants have been accounted for to be dangerous and cancer-causing in animals (Safer 1999). Therefore, the wellbeing issues identified with manufactured antioxidant agents have prompted an expanding enthusiasm for the advancement of protected and reasonable supplements of normal source.
Plant extracts have been received a significant attention for their potential as natural antioxidants. Most of the compounds of medicinal plants show anti-bacterial activities. In this study Ocimum tenuiflorum and Curcuma longa were used to investigate the antioxidant and antibacterial activity.
Solvents such as methanol and ethanol are believed to be more efficient than water for extracting antioxidant compounds from plant material because of the break down cell walls and other structures in plants more effectively.
These purified plant extracts were used for in vitro study against Aeromonas hydrophila by well diffusion, disc diffusion, MIC, and growth kinetic study methods. Thus the purified extracts were used for treating Aeromonas hydrophila infection in Oreochromis mossambicus.
The in vivo study was performed by intramuscular, and immersion method. The results showed a high mortality rate as previously observed. The treatment has been done by injecting the plant extracts with th bacterial pathogen on the fishes. Treatment also is done by immersion and oral method using encapsulated beads. Encapsulated beads were prepared by following the methods of, in which all the bioactive molecules are trapped inside a spherical sodium caseinate bead.
Different organs of control, infected and treated fishes were given for histopathological analysis and SEM analysis. The damages found on the infected fishes were compared with the treated and control samples. The ultra SEM analysis of the organs also shown the damages present in the organs on the infected fishes. The protein interaction study by SEM reveals how the plants extract molecules to inhibit the bacterial pathogen.
From the above reports its concluded that Aeromonas hydrophila were more susceptible to both the plant extracts (Tulsi and Turmeric). The bacterial infection found in Tilapia fish was effectively inhibited by using the combined plant extract and it can be used as an alternative to several antibiotics.
6.CONCLUSION
In this study, the ethanolic extract of both the sample shown an antibacterial activity against the bacterial pathogen in all of the in vitro techniques. Ocimum tenuiflorum and Curcuma longa showed as a Total polyphenol, Total flavonoid, Total antioxidant, DPPH, Hydrogen Peroxide, Hydroxyl radical and ABTS assay. From these study, we can conclude that the Tulsi and Turmeric exhibit a good antioxidant activity. Both the Plants extracts were purified and characterised by different methods and all the active compounds are encapsulated in a single bead. These liquid extracts and beads were used as a medication for treating the infection found in Tilapia. Histopathological, SEM and ultra SEM analysis has revealed the morphological changes after and before the treatment when compared with the control fishes. The active compounds present in Tulsi and Turmeric extract has the potential to inhibit certain bacterial infection. But when the two extracts were combined the efficacy of that product was much better than the individual once. Hence, it was concluded that the combined plant extract could be a good alternative to the antibiotics.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Microbiology"
Microbiology is the study of microscopic organisms, or microorganisms. Microorganisms are simple life forms that include bacteria, fungi, algae, and viruses.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




