The Role of vitamin D in the Development of Asthma and Allergies
Info: 7484 words (30 pages) Dissertation
Published: 14th Feb 2022
Tagged: BiologyMedicinePharmacology
Lay abstract
Every year worldwide more and more people are told to have either asthma or food allergy, which both conditions become very common. Asthma is a lung condition causing problems in breathing, whereas food allergy happens when the immune system reacts wrongly to specific foods producing mouth itching. Both conditions have no cure, which need to be in good management. There are many causes of asthma and food allergy, and several studies have found a positive link between the lacks of vitamin D in the blood with having asthma or food allergy. Thus, assuming that a low vitamin D level could be a risk factor. However, other studies have seen the opposite. Vitamin D can be taken from the sun or throughout eating food containing vitamin D. A large number of people will have lack of vitamin D because they don’t spend enough time outdoors under the sun. Also, some people do not eat food include vitamin D like oily fish and eggs. This report looks at what makes the level of vitamin D associated with asthma and food allergy, including its actions on some immune system cells that play a role in such conditions.
Scientific abstract
In the last decades, the incidence of asthma and food allergy has been increasing and become the most common chronic diseases worldwide. Asthma is an inflammatory disorder that is characterised by airways hyper responsiveness and airflow obstruction causing breathlessness. Whereas, food allergy is an abnormal response to a food triggered by the immune system causing itching in the mouth. There is no cure for both conditions, which require proper administration. Many factors are implicated in developing asthma and food allergy, and some studies suggested Vitamin D as a risk factor. It was found that an individual with a deficiency in vitamin D has a higher risk of developing asthma and food allergy. In contrast, some investigations stated the reverse. Vitamin D can be synthesised in the skin after the exposure of sunlight or throughout the dietary intake. A large population are with low vitamin D serum because of less sun exposure and inadequate nutritional intake. This report analyses the role of vitamin D in developing asthma and food allergy highlighting the importance of vitamin D in the modulation of the immune system.
1.0 Introduction
Asthma and allergies are examples of the most common chronic inflammatory diseases affecting millions of population worldwide, and their prevalence in the last few decades has risen, particularly in low and middle-income countries. For asthma, the World Health Organization has estimated that 300 million people have asthma and by 2025 it is expected to reach 400 million because of the rising trends (1)(2). According to Asthma UK, the United Kingdom has a high rate of asthma in the world, which is an about 5.4 million individual as the prevalence is higher among females than males (3). Likewise, other allergies disorders such as eczema, food allergies and rhinitis have shown increases in incidence. A study published in British Medical Journals reviews a substantial increase of allergic diseases over recent decades in the United Kingdom (4).
The pattern of increased allergic diseases in developed countries could be a result of numerous possible reasons. Some nutrients particularly vitamin D has been suggested to be one of the risk factors that could demonstrate an essential role of the raises in asthma and allergic diseases. In addition to that Vitamin D is also related to bone mineral metabolism and other extra-skeletal conditions such as cancer, inflammatory bowl disease and type 2 diabetes mellitus (5)(6). The objective of this report is to assess the evidence that vitamin D play a significant role in both asthma and allergic diseases.
2.0 Vitamin D
Vitamins are vital substances that consist of organic compounds that are involved in normal growth and health maintenance. Vitamins cannot be synthesised in adequate concentrations by the body, therefore vitamins must be accomplished through the dietary intake in small quantities (6). In the early 20th-century, vitamin D, also known as calciferol, first classified as a vitamin and now it is defined as a pro-hormone. The changing of this classification is due to the unique aspect of vitamin D differing from other vitamins, since the human body can produce it through the action of sunlight, therefore, makes it challenging to develop dietary reference intake values (7). Vitamin D has several types; the two major forms are vitamin D2 or ‘ergocalciferol’ and vitamin D3 or ‘cholecalciferol’. Vitamin D2 is synthesised via the UV irradiation of the ergosterol outside the human body in plants and fungi, mainly in mushrooms. Vitamin D3 is generated via the UV irradiation of the 7-dehydrocholesterol (7-DHC) in the skin. In this process, which is considered as a non-enzymic process, cholecalciferol is created through a two-step process, in which the B ring of the 7-DHC is destroyed by UV light radiation from the sun (280–320 ultraviolet B), forming pre-D3. Pre-D3 then goes through a thermo-sensitive process that isomerises it to D3. The effectiveness of cutaneous synthesis of vitamin D3 could be limited by some factors. For instance, clothing and the use of sunscreen on the skin blocks UVB from reaching 7-DHC, thus reducing D3 production [Figure 1].
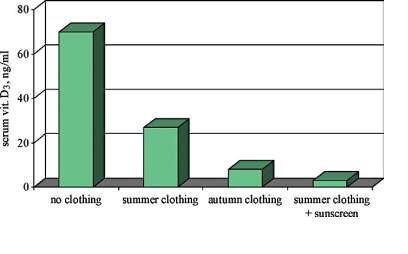
In addition to that age affects D3 the synthesis, because of the inverse relationship between age and the amount of 7-DHC in the epidermis as this is shown in [Figure 2].
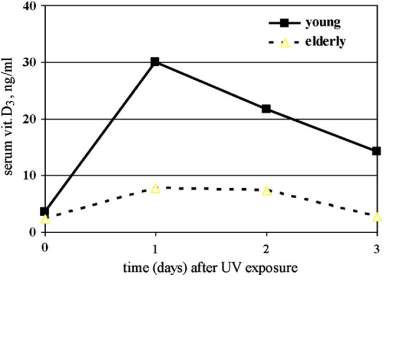
Vitamin D can be obtained from most sorts of food expect from fatty fish because of containing little vitamin D unless fortified. Vitamin D found in fish with the use of fortification is often ergocalciferol (D2), whereas cholecalciferol (D3) can be found in fish alone. D2 differs from D3 in having a methyl group at C24 and a double bond between C23 and C22 in the side chain. These differences limit D3 conversion to 25 hydroxyvitamin D (25OHD) and make its clearance from the circulation faster. Therefore, D3 supplementation when given daily does not result in as low a blood level of 25OHD as comparable amounts of D2. (8)(9)(10)(11)(12).
2.1 Vitamin D metabolism
After vitamin D has entered into the body by the synthesis of vitamin D3 in the skin or throughout the ingestion of food containing either ergocalciferol (D2) or cholecalciferol (D3) or sometimes by taking dietary supplements of these substances, vitamin D and its metabolites are bound to a carrier molecule called the vitamin D binding protein (DBP), for systemic transport (14). Regardless of the source of vitamin D and how our body is receiving it, it must be hydroxylated twice to become biologically active. The first hydroxylation of vitamin D occurs in the liver by 25-hydroxylase at carbon 25-position. To achieve this hydroxylation step, some cytochrome P450 (CYP) isoforms have been proposed; including the microsomal CYP3A4, CYP2J3 and CYP2R1 and the mitochondrial CYP27A1. The CYP2R1 cytochrome is considered to be the high-affinity 25-hydroxylase (15)(16). The regulation of 25-hydroxylases is poorly known but since the reflection of vitamin D nutritional status is reflected by 25- hydroxyvitamin D (25(OH) D3), the 25-hydroxylase is believed to be poorly regulated enzyme. The primary indicator of vitamin D status is 25(OH) D3, as it is the primary circulating form of vitamin D with approximately two weeks half-life. 25- hydroxyvitamin D concentrations in the blood vary between individuals as this is shown in the following table [Table 1].
[Table 1] Recommended criteria of vitamin D status – Adapted from (13)
| 25-OH-D3 level | Status | ||
| nmol/l | ng/ml | ||
| Deficient | |||
| 50-75 | 20-29 | Insufficient | |
| 75-375 | >30 | Adequate | |
| >250 | >100 | Excess | |
However, 25-hydroxyvitamin D requires the second hydroxylation, which mainly occurring in the kidneys by the 1-a-hydroxylase (CYP27B1) to form the biologically active form of vitamin D known as 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) or calcitriol. CYP27B1-activity in the kidneys could be either positively or negatively regulated. Phosphate and calcium and some regulating hormones such as insulin-like growth factor (IGF), growth hormone, calcitonin and parathyroid hormone (PTH) are positively regulating 1-a-hydroxylase. Whereas 1,25(OH)2D3 itself, klotho and fibroblast growth factor (FGF) are the negative regulators. For example, when there is a low calcium level in the serum the parathyroid glands release PTH that excites the activity of renal CYP27B1 therefore 1,25(OH)2D3 production. Whereas when calcium is in normal levels, PTH is released and consequently the CYP27B1 action is switched off. Moreover, 1,25(OH)2D3 prevents excessive vitamin D signaling and defines its own activity via producing 24- hydroxylase (CYP24) that executes the first step of vitamin D catabolism (16)(17)(18)(19). Vitamin D metabolism hydroxylation processes are summarized in the diagram [Figure 3].
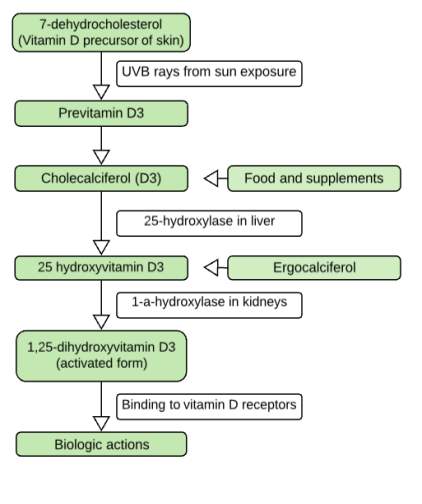
The mechanism of action is explained when 1,25(OH)2D3 binds to vitamin D receptor (VDR), a member of the nuclear receptor superfamily. VDR consists of two discrete domains the C-terminal ligand-binding domain (LDB) and N-terminal DNA-binding domains (DBD). When 1,25(OH)2D3 binds to the LBD it undergoes conformational changes; allowing the hetero-dimerization of the receptor with the retinoid X receptor (RXR) within the nucleus. Afterwards, VDR-RXR heterodimer binds to selective or promoter site of the target cell DNA. The new complex then recruits various co-activators and co-repressors that influence gene expression and alter cellular activity; this includes protein synthesis and secretion and cellular proliferation. Cellular response is determined on the cell type, location, number of VDR and the affinity of active VDRs. VDRs cannot be found only in tissues like kidneys, bone and skin but also can be found in non-classical organs such as b-cells, immune cells, muscles, heart, eyes and thyroid and parathyroid glands (20)(21).
2.2 Vitamin D and immune system
The discovery of Vitamin D receptors (VDRs) in nearly all immune cells including B cells, T cells, antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages, has emphasised the critical role of Vitamin D in the regulation of immune system responses. Importantly, in some immune cells, the expression of VDRs is regulated by immune signals. Whereas VDRs are abundantly present against the activation of T cells, therefore at a high VDR level T cells proliferation is reduced which make T cells only present at low VDR levels. By contrast, VDR-expression enhances the differentiation of monocytes either into DCs or macrophages, hence, plays a critical role in innate immune responses against pathogens. Collectively, Vitamin D has a role in modulating both innate and adaptive immune responses are supported throughout the high abundance of its receptors found on immune cells and their regulation by immune signals (22).
2.2.0 Modulation of innate immunity
The first-line defence against severing microbial invaders is provided by essential members of the innate immune compartment, macrophages and monocytes, which use pattern-recognition receptors, such as Toll-like receptors (TLRs) that give the ability to sense pathogen-associated molecular patterns (PAMPs) of numerous infectious agents. In this context, since the mid-19th century, it has been recognised that 1,25(OH)2D3 is a significant mediator of innate immune responses, which magnify the antimicrobial attributes of human immune cells like monocytes and macrophages (23). Accordingly, 1,25(OH)2D3 promotes the differentiation of monocytes into macrophages, which down-regulate granulocyte-macrophage-colony-stimulating factor (GM-CSF) expression and produce the immunosuppressant prostaglandin (E2). In contrast, 1,25(OH)2D3 inhibits macrophages from producing pro-inflammatory chemokines and cytokines. If Vitamin D is inadequate, macrophages maturation is impaired as well as the production of macrophage-specific membrane antigens needed for antimicrobial activity such as hydrogen peroxide and lysosomal acid phosphatase. So when adding 1,25(OH)2D3, free oxygen radicals, enzymes and the expression of membrane markers increases, enhancing phagocytosis and chemotaxis (24). When macrophages become activated, in response to interferon γ (IFNγ) and the toll-like receptors (TLRs) activation, they produce 1,25(OH)2D3. Hence, supplementing 100 nM of 1,25(OH)2 D3 to a human monocyte alters the TLR9-dependent production of IL-6 and represses the innate-immunity receptors TLR4, TLR9, and TLR2 expression (25). Additional different effect of 1,25(OH)2D3 is down-regulating class 2 major histocompatibility complex (MHC2) antigens expression at the cell surface and inducing the cathelicidin production, a peptide associated with the antimicrobial response. Moreover, the macrophages antigen-presenting function is reduced by 1,25(OH)2D3 through the down-regulation of (MHC2) membrane expression (26).
2.2.1 Modulation of adaptive immunity
Effects on dendritic cells
Dendritic cells (Dcs), the antigen-presenting cells, are the fundamental elements of the adaptive immune system that present antigens to T cells. These DCs consider as the prime targets for 1,25-dihydroxyvitamin D3 (27). 1,25(OH)2D3 diminishes the processes in which DCs proliferate and differentiate, which further inhibits the differentiation of monocytes into DCs. Another effect of 1,25(OH)2D3 is the inhibition of Dcs lifespan and maturation, thus reducing T-cell stimulation by DCs. Also, 1,25(OH)2D3 has an indirect inhibitory effect on Th1 cells response through inhibition of IL-12 secreted by DCs, as IL-12 is essential in the activation VDR/NF-kappaB pathways. As a result, the activation of Th1 cells is down-regulated. Beside inhibiting IL-12, 1,25(OH)2D3 raises the releasing of IL-10 by dendritic cells, hence decreasing the response of Th1 and the induction of type 1 regulatory cells (Tr1). As 1,25(OH)2D3 acts on co-stimulation molecules, the T-cell/Dc interaction is affected via decreasing the expression of CD40, CD80, and CD86 molecules on (MHC2) T cells membrane. These molecules activate T-cells and increase the releasing of pro-inflammatory cytokines such as TNF-alpha and IL-1. Thus by decreasing their expression, their function is reduced. This will result producing more IL-10 and TGF-beta and producing less IL-2 and IFN-gamma (22)(28).
Effects on T and B cells
Vitamin D is either directly or indirectly affecting both T and B cells, critical cell types in the adaptive immunity, and alters their activation response. For T cells, the activation of CD4+ T cells is associated with an increase by five-fold in the VDR expression level on its membrane. Thus at a low level of VDR expression CD4+ T cells are inactive. T cells are affected directly by 1,25(OH)2D3 through the inhibition of T-cell proliferation and indirectly via the dendritic cells as mentioned above. In relating to this, 1,25(OH)2D3 is targeting 102 genes in the CD4+ T cells (29). Moreover, 1,25(OH)2D3 inhibits T-cells differentiation indirectly by inhibiting the Th1 response. The Th1 response is limited by 1,25(OH)2D3 via reducing cell proliferation and decreasing in genes transcription encoding IL-2 and IFN-gamma as well as reducing protein expression of these genes. In contrast, 1,25(OH)2D3 stimulates the response of Th2 indirectly via lowering the production of IFN-gamma and directly by enhancing the production of IL-10 and IL-5 (30). In addition, 1,25(OH)2D3 suppresses the immune response when combining with glucocorticoids by inducing type 1 regulatory cells (Tr1) that produce IL-10. Another indirect effect of 1,25(OH)2D3 is blocking the production of IL-6 and IL-23 that inhibits Th17 differentiation and circulation (31). Finally, 1,25(OH)2D3 decreases the cytotoxicity of CD8+ T cells (32). Overall 1,25(OH)2D3 down-regulates the effect of T-cells in the immune response through promoting the Th2 and Tr1 immune modulatory responses and by reducing Th1 and Th17 pro-inflammatory responses.
For the B cells, 1,25(OH)2D3 inhibits their proliferation and differentiation into plasma cells, thus reducing the production of immunoglobulins by the B cells. Since reported in several studies, lower serum 25(OH)D levels have been recorded in patients with autoimmune diseases comparing them with healthy individuals. In order to prevent or decrease the severity of most of the autoimmune diseases, vitamin D supplementation has been suggested, considering vitamin d deficiency is linked to many autoimmune conditions (32)(33).
3.0 Asthma
3.1 Asthma overview
Asthma is a chronic inflammatory disorder, that is characterised by airways hyper responsiveness and variable airflow obstruction that makes airways challenging to breathe through. Consequently, leading to clinical manifestation including dyspnoea (difficulty breathing), chest tightness, wheezing and coughing mainly early in the morning or at night (34). Although the specific causes of asthma are ultimately unknown and thought to be caused by a combination of genetic and environmental factors. Considering particular genes have been identified that increase the risk of developing asthma as well as having a family history of asthma. Environmental factors include outdoor/Indoor pollutants, tobacco smoke, infections and diet. People with asthma can have asthma exacerbation or asthma attacks, which are usually initiated by environmental triggers that cause immune cells to generate inflammation in the lungs, thus making airways even narrower and potentially be life threatening.
In asthma, there is often an excessive reaction from Th2 against specific allergens. Th2 cells are an immune cell subtype distinguished to be involved in asthma as well as atopic dermatitis and allergic rhinitis making up the atopic triad. Accordingly, when allergens such as tobacco smoke enter the airways, they are picked up by dendritic cells and presented to Th2 cells. Thus Th2 cells produce cytokines like interleukins (IL-4 and IL-5) leading to some features of asthma. For example, IL-4 leads to the production of immunoglobulin E (IgE) antibodies that coat mast cells and stimulate them to release granules containing histamines, leukotrienes and prostaglandins (type 1 hypersensitivity reaction). On the other hand, IL-5 activates eosinophils, the disease-fighting white blood cells, which promote an immune response by releasing more cytokines and leukotrienes. In consequence of the interaction between inflammatory mediators and cells, besides the impairment of immunogenic tolerance, airways endothelium become injured. This process is known as airway “remodelling”. The process involves epithelial goblet cell hyperplasia that increases the mucus secretion, smooth muscle hypertrophy and protein deposition in the airway extracellular matrix [Figure 4]. These actions drive an obstruction of the airflow causing the respiratory symptoms of asthma (34)(35)(36).
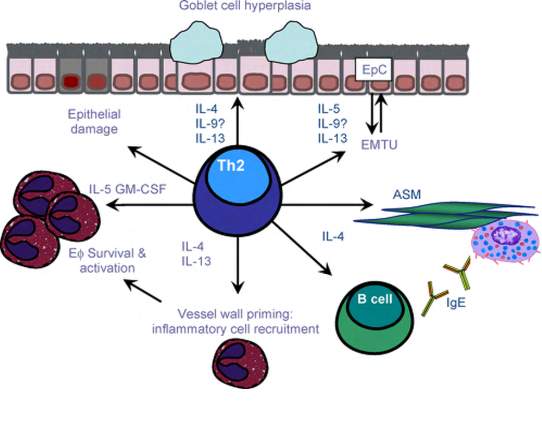
Asthma can be classified according to the frequency of symptoms, in particular, nighttime or early morning symptoms, forced expiratory volume in one second (FEV1) or peak expiratory flow rates (PEFR). Both FEV1 and PEFR measure the amount of obstruction in the airways and how often a person is using asthma medication to help with symptoms. While there is no cure for asthma, there are treatments available that can help manage symptoms and prevent the development of an asthma attack. First people with asthma should minimise or avoid contact with triggering substances by vacuuming and removing rugs. Also, some medications can reduce asthma symptoms such as bronchodilators which are short-acting beta-adrenoreceptor agonists and anticholinergic, that often administered through emergency inhalers. These fast-acting types of drugs cause the smooth muscles in the lungs to relax and therefore dilate the airways and make breathing easier. For severe asthma corticosteroids, the long-acting beta-adrenoreceptor agonist or leukotriene antagonist is recommended (37)(38).
3.2 Asthma and Vitamin D
Recently, some studies suggested that the status of vitamin D is presumably related to the risk of developing asthma. Vitamin D appears to have effects on lung and immune system development and function during in-utero and post-natal periods. In-utero period, the process in which lung mature is taken place continues through the first few years of life. At a late stage of foetal pulmonary maturation, the alveolar epithelium is differentiated making lungs after birth suitable for the gas (oxygen and carbon dioxide) exchange (39). The alveolar epithelium comprises two main cell types, type I and type II alveolar cells. Type I alveolar cells are flat and cover approximately 95% of the alveolar surface area, thus enabling them to play an essential role in gas exchange. Whereas, type II alveolar cells comprise a small fraction of the alveolar surface area and involve in the synthesis of pulmonary surfactants (40). Surfactants, a mixture of phospholipids within the alveoli, that reduce the surface tension at the air/liquid interface in the lungs. Therefore, surfactants dysfunction causes breathing problems (41). Through a series of studies, it has shown that vitamin d (1,25(OH)2D3) effects type II alveolar cells and the production of surfactants (42)(43). Another effect of vitamin D in utero is the development of the immune system and exert its influence in early in life. As mentioned above, vitamin D has an impact on the immune cells development specifically the development of dendritic and T-regulatory cells. The consequence of this effect is to suppress Th17 responses, ultimately a decrease in sensitisation to allergens and balance between Th1 and Th2 responses (39). Different aspect shows the relationship between vitamin D and lung function impairment is a study reported that children with insufficient vitamin D levels have a slightly lower mean FEV1 compared with children with sufficient vitamin levels (5)(44). Moreover, it has been proved the vital correlation between vitamin D serum level and predicted FVC and asthma symptoms is more controllable by children with higher serum levels of vitamin D (5)(45).
Vitamin D has different mechanisms for decreasing the risk of asthma exacerbations [Figure 5].
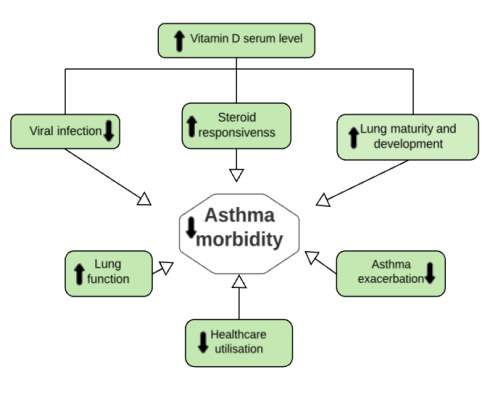
Vitamin D is associated with innate immune responses and in the handling of respiratory infections in early life. This involves down regulating of inflammatory mediators associated with viral infections and up regulating of cathelicidin, beta-defensins and other antimicrobial compounds. Consequently, improving lung function, decreasing airway responsiveness and preventing airway smooth muscle over proliferation. Moreover, vitamin D levels affect the effectiveness of anti-asthma therapy like glucocorticoids. For example, when vitamin D level is low, it contributes to a more severe asthmatic condition. Thus, achieving a therapeutic effect in low vitamin D serum requires administering higher doses of glucocorticoids. Vitamin D enhances asthma response to steroids by reversing the steroid-resistant vis up-regulating of IL-10 production (39)(46).
4.0 Food allergies
4.1 Food allergies overview
Food allergy (FA) is a medical condition refers to an immune system reaction against food component most commonly proteins. The sponsored guidelines of the US National Institutes of Allergy and Infectious Diseases (NIAID) have defined FA as ‘‘an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food’’ (47)(48). In the last several decades, food allergy prevalence seems to be rising throughout westernised countries, affecting 2% of adults and 8% of young children (49). A systematic review and meta-analysis of the epidemiology of FA in Europe show that the prevalence of FA is highest among north-western Europe while in Southern Europe appears to have the lowest prevalence (50). Different types of response could cause food allergies; either IgE mediated reactions or non-IgE mediated reactions, or a combination of both (51). IgE mediated reactions are the most common type of food allergy and involve the gastrointestinal tract, respiratory tract and skin. During an allergic reaction, the immune system responds to an ordinarily harmless substance as if it were a threat. 170 foods have been stated to cause such reaction, and the majority include soy, milk, peanut, tree nuts, eggs, wheat, and crustacean shellfish (47)(48).
In FA, allergens bind to the antigen receptor called immunoglobulin expressed on the surface of B-cells. Thus, B-cells bind allergen and interact with a CD4+ T cell, which has specificity for the same allergen. When interaction occurs, T-cell helper sends signals to the B-cells to undergo the production of antibody. In the circumstance of allergen presentation, Th2 cell produces IL-4, IL-5 and IL-13 and interacts with a CD40 ligand on its surface with CD40 on the B-cell surface causing B-cells to undergo class switch recombination and begin making IgE antibodies. This class of antibody binds to immune cells called mast cells and basophils that circulate throughout the body. When the body is exposed to the food allergen, it attaches to the IgE antibodies (52)(53)(54). IgE antibodies/food allergen binding complex signals the immune cells to release histamine and other chemicals like and leukotrienes, causing allergy symptoms such as hives, swelling of the lips and shortness of breath that typically occurs within 2 hours (47)(55).
The most severe kind of reaction is called anaphylaxis, causing a sudden drop in blood pressure, trouble breathing, dizziness and possibly death. An anaphylactic episode must be treated with a hormone called epinephrine, which maintains blood pressure and opens up the airways. To deal with accidental exposure, people diagnosed with FA are prescribed a medical device called an autoinjector that delivers a single dose of epinephrine into the thigh muscle (56)(57). FA could be diagnosed using blood tests for specific IgE antibodies or/and skin prick test (47). There is no cure for food allergy. Therefore, the logical way to manage FA is to avoid allergenic food and read food labels carefully. There are some factors correlated with the increased risk of FA, include genetics (HLA-gene), sex (male sex in children) and vitamin D insufficiency (54)(58).
4.2 Food allergies and Vitamin D
Vitamin D hypothesis is one of the numerous suggestions on food allergy development. As discussed above FA is mainly Th2-biased and discriminated by interleukins (IL-4, IL-5 and IL-13) and allergen-specific IgE antibody secretion. As explained previously, vitamin D in the modulation of the adaptive immunity decreases B-cells proliferation, differentiation and the immunoglobulin production. That happens when there is an adequate serum level of 1,25-dihydroxyvitamin D3, which enhances the expression of IL-10 by T and dendritic cells. Thus inhibiting antibodies expression by B-cells (plasma cells). However, IL-10 production is reduced in anti-CD40/IL-4-activated B cells either indirectly by calcium modulation or directly via VDR/IL-10 binding. In brief, by increasing the secretion of IL-10, consequently decreasing IgE secretion. Thus the allergic reaction is diminished (59)(60)(61)(62).
In contrast, other studies found that excess and insufficiency of vitamin D serum levels in infants contribute the subsequent food allergy. A study shows that insufficient vitamin D in the infantile blood rises the IgE-mediated food allergy risk (63). Another research found that during pregnancy low vitamin D levels in the maternal blood increases the infantile risk of developing atopic dermatitis (64). On the other hand, children born to mothers with low levels of vitamin D (30 ng/ml) were in higher risk of developing eczema (65). Moreover, other suggests an increased risk of food allergies in children and adults when taking vitamin D supplementation excessively in early childhood (66).
The limitations of studies discussing vitamin D status of the prenatal and its impact on the immune status of the newborn require further searches and more precise data. This includes the following infantile atopic outcomes in early life. Especially the investigation associated with the recommendation of a vitamin D supplementation during pregnancy or in early childhood to lessen children risk of developing asthma and allergies. Therefore, proving whether the use of vitamin D supplements reduce and prevent allergies disorders or not.
5.0 Conclusion
In conclusion, the prevalence of asthma and allergic conditions including food allergies is on increasing. This raising in incidence can be attributed to a variety of factors including genetic and environmental factors. Recently, many studies indicate, that vitamin D performs an essential role in the modulation of both innate and adaptive immunity, therefore suggested vitamin D to be one of the risk factors. Vitamin D is a pro-hormone that can be produced either by its synthesis in the skin after the exposure of sunlight or throughout the dietary supplements. The primary circulation form of vitamin D is 25-hydroxycholecalciferol-D3, known as calcidiol, which consider as an indicator of vitamin D in the serum. Vitamin D receptors (VDRs) have been discovered nearly in all immune cells, which indicate its function in reducing inflammation. It’s, in turn, diminishes the proliferation of immune cells including T-cells, B-cells and dendritic cells. Also, vitamin D. limits B-cells differentiation into plasma cells that produced antibodies against allergens as wells as reduces the secretion of pro-inflammatory mediators. Since asthma and food allergy development is characterised by most of these cells, adequate vitamin D serum level can reduce their development. However, other studies found that excess and insufficient vitamin D levels can raise the risk of developing such conditions.
Concerning this, it is necessary to carry out further studies in greater depth investigating the role of vitamin D in the development of both asthma and allergies. Also to determine whether vitamin D supplementation for individuals with deficient vitamin D level is beneficial in limiting asthma and allergies development or not.
References
1. Pawankar R, Canonica G, Holgate S, Lockey R. Allergic diseases and asthma. Current Opinion in Allergy and Clinical Immunology. 2012;12(1):39-41.
2. Pawankar R, Canonica G, Holgate S, Lockey R. World Allergy Organization (WAO) white book on allergy. United Kingdom: WAO; 2011.
3. Asthma Data Visualisations | Asthma UK [Internet]. Asthma UK. 2018 [cited 27 March 2018]. Available from: https://www.asthma.org.uk/get-involved/campaigns/data-visualisations/
4. Gupta R, Sheikh A, Strachan D, Anderson H. Time trends in allergic disorders in the UK. Thorax. 2007;62(1):91-96.
5. Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. 2011;67(1):10-17.
6. Varsavsky M, Alonso G, García-Martín A. Vitamin D: Present and future. Revista Clínica Española (English Edition). 2014;214(7):396-402.
7. Holick M. Vitamin D. [Totowa]: Humana; 2010.
8. DRI – Dietary Reference Intakes – Calcium and Vitamin D20122DRI – Dietary Reference Intakes – Calcium and Vitamin D. Institute of Medicine of the National Academies, , ISBN: 13‐978‐0‐309‐16394‐1. Nutrition & Food Science. 2012;42(2):75-79.
9. Tripkovic L, Lambert H, Hart K, Smith C, Bucca G, Penson S et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2012;95(6):1357-1364.
10. Holick M, Biancuzzo R, Chen T, Klein E, Young A, Bibuld D et al. Vitamin D2Is as Effective as Vitamin D3in Maintaining Circulating Concentrations of 25-Hydroxyvitamin D. The Journal of Clinical Endocrinology & Metabolism. 2008;93(3):677-681.
11. Bikle D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chemistry & Biology. 2014;21(3):319-329.
12. Lehmann B, Meurer M. Vitamin D metabolism. Dermatologic Therapy. 2010;23(1):2-12.
13. Combs G. Vitamin D. The Vitamins. 2012;:139-180.
14. Zhang J, Habiel D, Ramadass M, Kew R. Identification of two distinct cell binding sequences in the vitamin D binding protein. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 2010;1803(5):623-629.
15. Cheng J, Levine M, Bell N, Mangelsdorf D, Russell D. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proceedings of the National Academy of Sciences. 2004;101(20):7711-7715.
16. Dusso A, Brown A, Slatopolsky E. Vitamin D. American Journal of Physiology-Renal Physiology. 2005;289(1):F8-F28.
17. Meyer M, Goetsch P, Pike J. A Downstream Intergenic Cluster of Regulatory Enhancers Contributes to the Induction ofCYP24A1Expression by 1α,25-Dihydroxyvitamin D3. Journal of Biological Chemistry. 2010;285(20):15599-15610.
18. Bosworth C, Levin G, Robinson-Cohen C, Hoofnagle A, Ruzinski J, Young B et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney International. 2012;82(6):693-700.
19. Henry H. Regulation of vitamin D metabolism. Best Practice & Research Clinical Endocrinology & Metabolism. 2011;25(4):531-541.
20. Bikle D. Nonclassic Actions of Vitamin D. The Journal of Clinical Endocrinology & Metabolism. 2009;94(1):26-34.
21. DeLuca H. Overview of general physiologic features and functions of vitamin D. The American Journal of Clinical Nutrition. 2004;80(6):1689S-1696S.
22. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Current Opinion in Pharmacology. 2010;10(4):482-496.
23. Gombart A. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiology. 2009;4(9):1151-1165.
24. Helming L. 1 ,25-dihydroxyvitamin D3 is a potent suppressor of interferon -mediated macrophage activation. Blood. 2005;106(13):4351-4358.
25. Dickie L, Church L, Coulthard L, Mathews R, Emery P, McDermott M. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology. 2010;49(8):1466-1471.
26. Liu P. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science. 2006;311(5768):1770-1773.
27. Baeke F, Etten E, Overbergh L, Mathieu C. Vitamin D3 and the immune system: maintaining the balance in health and disease. Nutrition Research Reviews. 2007;20(01):106.
28. Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1α,25-Dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in Human Monocytes. Cytokine. 2009;45(3):190-197.
29. Mahon B, Wittke A, Weaver V, Cantorna M. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. Journal of Cellular Biochemistry. 2003;89(5):922-932.
30. Boonstra A, Barrat F, Crain C, Heath V, Savelkoul H, O’Garra A. 1 ,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. The Journal of Immunology. 2001;167(9):4974-4980.
31. Daniel C, Sartory N, Zahn N, Radeke H, Stein J. Immune Modulatory Treatment of Trinitrobenzene Sulfonic Acid Colitis with Calcitriol Is Associated with a Change of a T Helper (Th) 1/Th17 to a Th2 and Regulatory T Cell Profile. Journal of Pharmacology and Experimental Therapeutics. 2007;324(1):23-33.
32. Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier M. Vitamin D and inflammation. Joint Bone Spine. 2010;77(6):552-557.
33. Holick M. Vitamin D Deficiency. New England Journal of Medicine. 2007;357(3):266-281.
34. Murdoch J, Lloyd C. Chronic inflammation and asthma. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2010;690(1-2):24-39.
35. Interactions between innate and adaptive immunity in asthma pathogenesis: New perspectives from studies on acute exacerbations. Journal of Allergy and Clinical Immunology. 2010;125(5):973-974.
36. Shifren A, Witt C, Christie C, Castro M. Mechanisms of Remodeling in Asthmatic Airways. Journal of Allergy. 2012;2012:1-12.
37. James D, Lyttle M. British guideline on the management of asthma: SIGN Clinical Guideline 141, 2014. Archives of disease in childhood – Education & practice edition. 2016;101(6):319-322.
38. Kumar P, Clark M. Kumar and Clark’s Clinical Medicine E-Book. 8th ed. London: Elsevier Health Sciences; 2012.
39. Litonjua A. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Current Opinion in Allergy and Clinical Immunology. 2012;12(2):179-185.
40. MASON R. Biology of alveolar type II cells. Respirology. 2006;11(s1):S12-S15.
41. Perez-Gil J, Weaver T. Pulmonary Surfactant Pathophysiology: Current Models and Open Questions. Physiology. 2010;25(3):132-141.
42. Zosky G, Berry L, Elliot J, James A, Gorman S, Hart P. Vitamin D Deficiency Causes Deficits in Lung Function and Alters Lung Structure. American Journal of Respiratory and Critical Care Medicine. 2011;183(10):1336-1343.
43. Lykkedegn S, Sorensen G, Beck-Nielsen S, Christesen H. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;308(7):L587-L602.
44. Brehm J, Schuemann B, Fuhlbrigge A, Hollis B, Strunk R, Zeiger R et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. Journal of Allergy and Clinical Immunology. 2010;126(1):52-58.e5.
45. Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner A. Vitamin D Serum Levels and Markers of Asthma Control in Italian Children. The Journal of Pediatrics. 2011;158(3):437-441.
46. Paul G, Brehm J, Alcorn J, Holguín F, Aujla S, Celedón J. Vitamin D and Asthma. American Journal of Respiratory and Critical Care Medicine. 2012;185(2):124-132.
47. Burks A, Tang M, Sicherer S, Muraro A, Eigenmann P, Ebisawa M et al. ICON: Food allergy. Journal of Allergy and Clinical Immunology. 2012;129(4):906-920.
48. Boyce J, Assa’a A, Burks A, Jones S, Sampson H, Wood R et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. Nutrition. 2011;27(2):253-267.
49. Sackeyfio A, Senthinathan A, Kandaswamy P, Barry P, Shaw B, Baker M. Diagnosis and assessment of food allergy in children and young people: summary of NICE guidance. BMJ. 2011;342(feb23 2):d747-d747.
50. Nwaru B, Hickstein L, Panesar S, Muraro A, Werfel T, Cardona V et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2013;69(1):62-75.
51. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C et al. EAACI Food Allergy and Anaphylaxis Guidelines: diagnosis and management of food allergy. Allergy. 2014;69(8):1008-1025.
52. Geha R, Jabara H, Brodeur S. The regulation of immunoglobulin E class-switch recombination. Nature Reviews Immunology. 2003;3(9):721-732.
53. Vickery B, Chin S, Burks A. Pathophysiology of Food Allergy. Pediatric Clinics of North America. 2011;58(2):363-376.
54. Sicherer S, Sampson H. Food allergy. Journal of Allergy and Clinical Immunology. 2010;125(2):S116-S125.
55. Food allergy – Symptoms and causes [Internet]. Mayo Clinic. 2018 [cited 10 April 2018]. Available from: https://www.mayoclinic.org/diseases-conditions/food-allergy/symptoms-causes/syc-20355095
56. Jacobsen R, Millin M. The Use of Epinephrine for Out-of-Hospital Treatment of Anaphylaxis: Resource Document for the National Association of EMS Physicians Position Statement. Prehospital Emergency Care. 2011;15(4):570-576.
57. Anaphylaxis | Would you know what to do? | Allergy UK [Internet]. Allergyuk.org. 2018 [cited 10 April 2018]. Available from: https://www.allergyuk.org/information-and-advice/conditions-and-symptoms/33-anaphylaxis-and-severe-allergic-reaction
58. Sicherer S, Sampson H. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. Journal of Allergy and Clinical Immunology. 2014;133(2):291-307.e5.
59. Jones A, Tulic M, Rueter K, Prescott S. Vitamin D and Allergic Disease: Sunlight at the End of the Tunnel?. Nutrients. 2011;4(1):13-28.
60. Heine G, Niesner U, Chang H, Steinmeyer A, Zügel U, Zuberbier T et al. 1,25-dihydroxyvitamin D3promotes IL-10 production in human B cells. European Journal of Immunology. 2008;38(8):2210-2218.
61. Suaini N, Zhang Y, Vuillermin P, Allen K, Harrison L. Immune Modulation by Vitamin D and Its Relevance to Food Allergy. Nutrients. 2015;7(8):6088-6108.
62. Vassallo M, Camargo C. Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. Journal of Allergy and Clinical Immunology. 2010;126(2):217-222.
63. Feuille E, Nowak-Wegrzyn A. Vitamin D Insufficiency Is Associated With Challenge-Proven Food Allergy in Infants. PEDIATRICS. 2013;132(Supplement):S7-S8.
64. Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. European Respiratory Journal. 2009;35(6):1228-1234.
65. Gale C, Robinson S, Harvey N, Javaid M, Jiang B, Martyn C et al. Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition. 2007;62(1):68-77.
66. KULL I, BERGSTROM A, MELEN E, LILJA G, VANHAGE M, PERSHAGEN G et al. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. Journal of Allergy and Clinical Immunology. 2006;118(6):1299-1304.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Pharmacology"
Pharmacology involves the study of drugs and how they affect the body. A pharmacologist contributes to drug development by researching and testing how the body reacts to medication, and whether the medication can have a positive impact on the body in terms of fighting illness and disease.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




