Comparison of Soil Samples for Zinc Solubilizing Bacteria
Info: 10139 words (41 pages) Dissertation
Published: 10th Dec 2019
Tagged: BiologyMicrobiology
Content
| Chapter | Content | Page No. |
| 1 | Introduction | 14-17 |
| 2 | Review of Literature | 19-25 |
| 3 | Materials and Methods | 26 |
| 3.1 | COLLECTION OF SOIL SAMPLE AND SOIL ANALYSIS | 26 |
| 3.2 | PRIMARY SCREENING AND ISOLATION ZINC SOLUBILZI NG BACTERIA | 26-27 |
| 3.2.1 | MORPHOLOGY AND CULTURE CHARACTERISTICS OF 23 ISOLATES | 27 |
| 3.2.2 | IDENTIFICATION OF COLIFORM AND NON COLIFORMS | 27 |
| 3.2.3 | PHOSPHATE AND POTASSIUM SOLUBILIZATION | 27 |
| 3.3 | QUANTITATIVRE ESTIMATION OF ZINC SOUBILIZATION | 27-29 |
| 3.3.1 | DETERMINATION OF SOLUBLE ZINC BY AAS METHOD | 28-29 |
| 3.4 | ROOT COLONIZATION AND PLANT GROWTH PROMOTING ACTIVITY OF BACTERIAL ISOLATES | 29-30 |
| 3.5 | GENETIC IDENTIFICATION OF BACTERIAL ISOLATES | 30 |
| 4 | Results and Discussion | 31 |
| 4.1 | COLLECTION OF SOIL SAMPLE AND SOIL ANALYSIS | 31-32 |
| 4.2 | PRIMARY SCREENING AND ISOLATION ZINC SOLUBILZI NG BACTERIA | 32-37 |
| 4.2.1 | IDENTIFICATION OF COLIFORM AND NON COLIFORMS | 38 |
| 4.2.2 | PHOSPHATE AND POTASSIUM SOLUBILIZATION | 38-41 |
| 4.3 | QUANTITATIVRE ESTIMATION OF ZINC SOUBILIZATION | 42-47 |
| 4.4 | ROOT COLONIZATION AND PLANT GROWTH PROMOTING ACTIVITY OF BACTERIAL ISOLATES | 48-54 |
| 4.5 | GENETIC IDENTIFICATION OF BACTERIAL ISOLATES | 55 |
| 5 | Conclusion | 56 |
| 6 | Appendix | 57-60 |
| 7 | Bibliography |
Abstract
The soil sample from 4 states of India namely Maharashtra, Rajasthan, Karnataka and Andhra Pradesh exhibiting low levels of Zn and high pH were used for isolation of zinc solubilizing bacteria (ZSB) on ZnSB medium containing ZnO as source of insoluble Zn. Sixty bacterial cultures were isolated from 4 soil samples. The qualitative screening on the basis of diameters of zone of Zn solubilization revealed that 23 isolates exhibited measurable zones of Zn solubilization while only 4 isolates showed zone of solubilization higher than 10 mm, minimum value for zone of Zn solubilization suggested by FCO guideline. The quantitative estimation of Zn solubilization by selected isolates was done by growing them in ZnSB broth containing ZnO, ZnCO3 or ZnPO4 as source of insoluble Zn. MHSS 1 was found to be the most efficient strain solubilizing 446.23 ppm, 378 ppm and 252 ppm from ZnO, ZnCO3 and ZnPO4, respectively. The solubilization by all ZSBs was correlated to reduction in pH of broth suggesting secretion of organic acids as mechanism for solubilized Zn from various Zn compounds. Corn and Wheat root colonization by MHSS 1 and RJSS 6 was studied by seed germination assay. Both the isolates could colonize corn and wheat roots (1 ± 0.5*108 cfu/g root) and exhibited plant growth promoting effects on corn and wheat. There was significant increase in vigour indices of both seedlings treated with selected isolates as compared to untreated control.
The present study thus revealed that isolate MHSS 1 exhibiting highest Zn solubilization, effective root colonization and higher plant growth promoting effects on corn and wheat is potential candidate for development of ZSB biofertilizer. Isolate MHSS 1 was identified as Acinetobacter calcoaceticus based on 16’s rDNA sequence analysis.
List of Figures
Figure 1: Atomic Absorption spectrophotometer (AAS)
Figure 2: Twenty three Zn solubilizing isolates from 4 different soil samples
Figure 5: Time dependent correlation of pH and Zn concentration in RJSS 6
Figure 6: Time dependent correlation of pH and Zn concentration in MHSS 1
Figure 7: Time dependent correlation of pH and Zn concentration in KTSS 8
Figure 8: Time dependent correlation of pH and Zn concentration in RJSS 5
Figure 9: % seed germination of wheat seeds in presence of 4 isolates
Figure 10: Vigour index of wheat seeds in presence of 4 isolates
Figure 11: Germination of wheat seeds in presence of 4 isolates
Figure 12 : Germination of wheat seeds in presence of 4 isolates
Figure 13: % seed germination of corn seeds in presence of 4 isolates
Figure 14: Vigour index of corn seeds in presence of 4 isolates
Figure 15: Plant growth promoting activity of 4 isolates on corn seeds with respect to control
Figure 16: Germination of corn seeds in presence of 4 isolates
List of Tables
Table 1: pH and Zn content of soil samples collected from different states………18
Table 2: Qualitative estimation of Zn solubilizing activity of 60 isolates……….20
Table 3 : Colony characteristics of 23 isolates………………………22
Table 4: Potassium mobilization and phosphate solubilization activity of 4 isolates…26
Table 5: Solubilization of Zn in presence of different source of insoluble Zn…….33
Table 6: Estimation of plant growth promoting activity of 4 isolates using wheat seeds 34
Table 7:Estimation of plant promoting activity of 4 isolates using corn seed……………………38
Introduction
The growth of plant several macro and micro nutrients are required. Nutrients are absorbed through inorganic or organic forms by the plant roots along with water. For optimum plant growth Zinc (Zn) is one of the essential micronutrient and plays a vital role in metabolism (Hughes & Poole, 1989). Zinc is essential for crop yield and required optimal size of fruit, photosynthetic tissues which consist of all carbonic enzyme in that the zinc is present, and also required for biosynthesis chlorophyll (Ali, Riaz, Mairaj, Arif, Fida, & Bibi, 2008; Graham, Welch, & Bouis, 2000). Zinc plays vital role in the healthy growth plants. In plants, zinc acts a key role many important biochemical pathways which are mainly concerned with: carbohydrate metabolism, both in photosynthesis and in the conversion of sugars to starch, protein metabolism, growth regulator metabolism, the maintenance of the integrity of biological membranes, the resistance to infection by certain pathogens (Brian J. Alloway, 2008).
When the supply of zinc to the plant is inadequate, one or more important physiological functions of zinc are unable to operate normally and plant growth is adversely affected. The changes in plant physiological mechanisms brought about by a deficiency of zinc can result in the plant developing visible symptoms of stress which might include one or more of the following: stunting (reduced height), interveinal chlorosis (yellowing of the leaves between the veins), bronzing of chlorotic leaves, small and abnormally shaped leaves and/or stunting and rosetting of leaves (where the leaves form a whorl on shortened stems). These different types of symptoms vary with plant species and are usually only clearly displayed in severely deficient plants. In cases of marginal deficiency, plant yields can often be reduced by 20% or more without obvious visible symptoms (Brian J. Alloway, 2008).
In soil, Zn undergoes a complex dynamic equilibrium of solubilisation and precipitation that is greatly influenced by pH and microflora (Di Simine, Sayer, & Gadd, 1998; Martino & Gadd, 2003) and that ultimately affects their accessibility to roots for absorption. Phosphorus is the important element that interferes on zinc uptake, as zinc uptake by plants reduces by increasing phosphorus in soil (Salimpour, Khavazi, Nadian, & Besharati, 2010; Das, 2005; Marschner, Oberle, Cakmak, & Romheld, 1990; Sharma, Krants, Brown, & Quick, 1986). The total value of zinc in soil depends on chemical composition of raw materials in the soil. Zinc value of earth’s crust is 80 mg/kg approximately, and its value in soil is usually 10 to 300 mg/kg with an average of 50 mg/kg. Zinc is in different forms in the earth’s crust, such as sulphate, silica and carbonate minerals. Also zinc is in different forms in the soil as water-soluble, exchange, bound to organic matter and stabilized by the secondary clay minerals (Alloway, 2008). Zinc deficiency is related to soil pH and its value is very low in calcareous soils which has high pH (Alam, Abedin, & Azad, 2010; Alloway, 2008). Concentration of zinc in soil solution ranged from less than 0.5 ppm to more than 1.0 ppm (low – 0.5 ppm, marginal – 0.6 to 1.0 ppm and high – 1.0 ppm).
Zinc mobility and uptake in soil has also been shown to be dependent on many other factors such as soil acidity, available zinc content in the soil, organic matter and soil type. Total available zinc may be very low in highly acidic soils due to the intense soil leaching. Its usability decreases by increasing soil pH, because the minerals solubility reduced and zinc uptake increases by soil colloidal particles such as clay minerals, iron and aluminium oxides, organic matter and calcium carbonate. Its usability decreases by decreasing temperature and light intensity due to limited root development. Its ability by plants decreases by high levels of phosphorus in the soil. Zinc uptake by plants inhibited by some metal cations such as Cu2+ and Fe2+, due to the same carriers for these elements in the plant roots (Chang, 2007; Alloway, 2008; Sillanpaa, 1990).
The distribution of micro nutrient deficiencies across agro-ecological zones indicates zinc deficiency to be about 40 %. Zn deficiency is wide spread in the calcareous soils of Bihar, Vertisols and Inceptisols of Andhra Pradesh, Tamil Nadu, Alfisols of Karnataka, swell- shrink soils of Maharashtra and Madhya Pradesh and Aridisols of Haryana resulting in low crop yield (SHRUTHI.P., 2013). Zinc deficiency is a serious constraint to rice production in many parts of the world (Anonymous, 1993) and this could only be compensated by the application of costly chemical fertilizers, either as foliar or soil applications (Reyes, 1989). Alternatively, numerous microorganisms, especially those associated with roots, have the ability to increase plant growth and productivity (Okon, 1985; Kloepper, 1998; Yanni, 2001; Rodriguiz, 2004) by increasing the supply of mineral nutrients of low mobility in the soil like P, Zn and Cu (Cunningham, 1990; Tarafdar, 1994; Thompson, 1996; Goldstein, 1995). Among these microorganisms, a group of bacteria referred to as plant growth promoting rhizobacteria (PGPR) are involved in nutrient cycling (Weller, 1983; Glick, 1995).
Many plant species are affected by zinc deficiency on a wide range of soil types in most agricultural regions of the world. The major staple cereal crops: rice, wheat and maize are all affected by zinc deficiency, together with many different fruit, vegetable and other types of crops including cauliflower, cotton and flax. Maize is the crop species which is most susceptible to zinc deficiency and, in many countries, it receives the highest proportion of zinc fertiliser applications. Rice is also highly susceptible to zinc deficiency, especially that grown in lowland (paddy) production systems, because the chemical conditions in the waterlogged soils are conducive to zinc deficiency (Brian J. Alloway, 2008) (SHRUTHI.P., 2013).
Zinc occurs in soil as sphalerite, olivine, hornblende, augite and biotite. However, availability of zinc from these sources is guided by many factors among which biochemical actions of rhizosphere microorganisms play an important role in converting such unavailable sources into available ones (Bhupinder S., 2005).
Microorganisms play a key role in zinc solubilization. Some species of rhizobacteria are capable of mobilizing zinc in accessible form in soils. Mineral zinc solubilization by microbes which enhances crop growth and yield. Zinc solubilizing bacteria are capable of solubilizing ZnO, ZnCO3 and zinc phosphate through production and excretion of organic acids. The special focus on Zn solubilizer was due to the fact that zinc is one of the essential micronutrient required by all crops. It is a key element in many physiological and biochemical processes. Currently, very little information is available on zinc solubilization by bacteria, their mechanisms of solubilization and their effect on growth, Zn uptake and yield of several crops (Brian J. Alloway, 2008).
Objectives
1. Isolation and screening highly efficient strains of zinc solubilizing bacteria from soil samples obtained from Maharashtra, Rajasthan, Karnataka and AndhraPradhesh.
2. Assessment of Zn solubilization efficiency of isolates using quantitative assay.
3. Assessment of root colonization and plant growth promoting effects of selected ZSB isolates on corn and wheat using seed germination assay.
Review of Literature
Zinc is a trace element found in varying concentrations in all soils, plants and animals and it is essential for the normal healthy growth of higher plants, animals and humans. Zinc is needed in small but critical concentrations and if the amount available is not adequate, plants and/or animals will suffer from physiological stress brought about by the dysfunction of several enzyme systems and other metabolic functions in which zinc plays a part. When the supply of zinc to the plant is inadequate, one or more of the many important physiological functions of zinc are unable to operate normally and plant growth is adversely affected. Availability of Zinc in majority of the soils of the world is too low for the production of good yields. Certain groups of micro-organisms including bacteria, fungi and actinomycetes are known to solubilize zinc mineral into soluble form which can be utilized by the plants. The literature pertaining to solubilization of zinc minerals by bacteria is very scanty. The available literature on zinc solubilization by bacteria and their mechanisms of solubilization, other beneficial traits and their agronomic importance is reviewed in this chapter (SHRUTHI.P., 2013).
OCCURRENCE AND ISOLATION OF ZINC SOLUBILISING BACTERIA (ZSB)
Saravanan V. S., 2004, reported zinc solubilizing bacterial culture from soil and ore sources, both by direct plating and by enrichment technique in the medium with 0.1% ZnO. Three cultures were isolated by direct plating and one by enrichment technique. Among those two strains were characterized as Bacillus spp. and Pseudomonas spp. Further the potential to correct the Zn deficiency was assessed using soybean plant. The result revealed that Pseudomonas spp. was able to correct Zn deficiency used along with 1% ZnO.
Xinxia, 2011, investigated zinc solubilizing bacteria from roots, stems and leaves of Zn/Cd hyper accumulator Sedum alfredii. A total of fourteen bacterial endophytes were isolated and are closely related phylogenetically to Pseudomonas, Bacillus, Stenotrophomonas, Acinetobacte by16S rRNA sequence analysis. Both plate and broth assay proved that strain VI8L1, VI8L2, II8L4 andVI8R2 were able to effectively solubilize ZnCO3 and Zn3 (PO4)2.
Silva, 2010, obtained 3 bacterial strains from leaves and roots of sugarcane. Subsequently, these isolates were evaluated for their ability to solubilize insoluble nutrients in LGI medium containing calcium tertiary phosphate and zinc oxide. Isolate SCB4789F-1 was the most efficient for the solubilization of phosphorus, with a halo zone of solubilization diameter on average0.75 cm. The solubility of zinc isolates SCB4789F-1 and SCB4789F-2 were the most efficient, with a halo zone whose diameter was on average 1.34 and 1.29 cm, respectively.
Li, 2012, reported several strains of bacteria from the rhizosphere of S.alfredii. Among the different strains isolated, Burkholderia cepacia showed the highest ability in mobilizing Cd and Zn as well as resisting high concentrations of soluble Zn (500 mg L−1). The soluble Zn concentration in the medium increased from 13 to 72 and 99% (p<0.001) after bacterial inoculation in the medium supplemented with insoluble zinc oxide and zinc carbonate. The present results showed that certain bacteria associated with metal hyper accumulators could contribute significantly in mobilizing heavy metals, which will enhance the phyto-extraction process.
Materials and Methods
3.1 COLLECTION SOIL SAMPLE AND SOIL ANALYSIS
Soil sample were obtained from soil testing department of Kanbiosys Pvt. Ltd. Soil samples were from 4 different states namely Rajasthan, Maharashtra, Karnataka, and Andhra Pradesh were collected and analyzed.
These soil samples were analyzed for pH using pH meter and Zn content using by atomic absorption spectrophotometer (AAS) in soil testing department of Kanbiosys Pvt. Ltd.
3.2.1 ISOLATION OF ZINC SOLUBILISING BACTERIA
Heat shock treatment at 450C was given to each soil sample for continuous 24 hrs to reduce the vegetative load of gram negative bacteria such coliforms and non coliforms or non bacilli. Isolation, Each soil sample 2gm was inoculated in 500 ml nutrient broth. They were incubated at room temperature on shaker at 160 rpm 24 hr. The tenfold serial dilution (10-1 to 10-8) of 24 hr old nutrient broth samples were prepared using sterile saline (0.85% NaCl w/v) as diluents. The pour plate technique was used to isolate zinc solubilizing bacteria. Media used for isolation was Zinc solubilizing agar (ZnSB) (0.1% zinc oxide insoluble source of zinc) as per mentioned in (Biofertilizer and Organic Fertilizers in Fertilizer (Control) Order, 1985)(FCO). Nutrient agar plates were incubated at 280C for 48 hrs while ZnSB agar plate incubated at 280C for 7 days.
3.2.2 Qualitative assay for Zn solubilization
Saline suspension of each isolate was used for cell count and total viable count. Total 108 cfu/ml suspension of each isolate was used to check zinc solubilization on ZnSB medium containing 0.1% Zinc oxide insoluble source of zinc (pH 7.5). Plates were incubated at 280C for 7 days.
The diameter of halo zone and diameter of colony was determined for each isolate in order to calculate zinc solubilization index. The formula for Zn solubilization index is given below:
Zn Solubilization Index = (Colony Diameter + Halo zone Diameter) / Colony Diameter
The bacterial isolates showing maximum zones of clearance in the range of 12-15 mm were selected (Biofertilizer and Organic Fertilizers in Fertilizer (Control) Order, 1985)and further analyzed.
3.2.3 MORPHOLOGICAL & CULTURAL CHARACTERISTICS OF 23 ISOLATES
Morphological and cultural characteristics (Size, Shape, Color, Margin, Elevation, Opacity, Consistency and gram character) of the isolates were determined on Nutrient Agar after 24 hrs of incubation at 280C.
3.2.4 IDENTIFICATION OF COLIFORMS AND NON COLIFORMS
The Gram negative (MHSS 1, RJSS 5, KTSS 8) isolates selected from above 23 isolates were streaked on MacConkey’s Agar plate and incubated at 370C for 24 hr. the plates were observed for lactose fermentation resulting in pink coloured colonies, if any.
3.2.5 PLATE ASSAY FOR PHOSPHATE AND POTASSIUM SOLUBILISATION
Phosphate and potassium solubilization activity for 4 selected isolates was estimated. Tenfold serial dilution in saline of selected isolated were pour plated on Pikovaskaya agar medium (0.4 % of tricalcium phosphate was added as insoluble phosphate) and Aleksandrov agar medium (0.1 % of mica was added as insoluble potassium), respectively. In both the medium Bromothymol blue dye was added as a pH indicator. The plates were incubated at 280C for 4 days (Biofertilizer and Organic Fertilizers in Fertilizer (Control) Order, 1985). The yellow zones showing acid secretion and P and K solubilization around isolated colonies of selected isolates were measured and recorded.
3.3 QUANTITATIVE ESTIMATION OF ZINC SOLUBILIZATION
Twenty four hr old Nutrient agar slant were used to prepare saline suspensions of the 4 selected isolate. Erlenmeyer flasks (250 ml capacity) containing 125 ml of ZnSB broth. The broth contains 0.1 % of Zinc Oxide (ZnO) as a source of insoluble Zinc. The flasks were sterilized by autoclaving at 15 lbs for 15 minutes. Saline suspensions 2 ml of 4 isolates (≥1*108) were inoculated in ZnSB. Then the flasks were incubated on rotary shaker (160 rpm) at room temperature (280C) for 7days. The experiment was done in triplicates.
During incubation period, at the interval of 24 hrs, sample 5 ml were drown from each of the test flasks. The samples were assayed for cell count, gram character and pH. 10 ml broth was centrifuged at 4000 rpm for 30 minutes and (soluble) Zinc content in the supernatant was estimated suing atomic absorption spectrophotometer.
Microscopic observations were performed to confirm purity and morphological stages of bacterial isolates. Cell count was performed to check the viability of cultures. pH was measured in order to check the acid production.
3.3.1 DETERMINATION OF SOLUBLE ZINC BY AAS METHOD
- Preparation of working standard and standard curve:
Take 1ml of standard zinc solution in 100 ml volumetric flask & make up the volume 100 ml with acidified water. This 10 ppm zinc solution is ready. Take following volume of 10 ppm zinc solution in 100 ml volumetric flasks & make the volume with acidified water.
Dilution scheme for standard graph ranging from 0.4 to 1.6 ppm
| Flask No. | Volume of 10 ppm standard Zinc Solution
(ml) |
Concentration of Zinc after making volume of 100ml
(ppm) |
| 1 | 4 | 0.4 |
| 2 | 10 | 1 |
| 3 | 16 | 1.6 |
- Flaming of solution:
Flaming the standard and filtered sample by AAS at wavelength of 213.8 µ (µ line of the instrument)
- Calculation:
Prepare standard curve of known concentration of Zinc solution by plotting the absorption value on Y axis against their respective Zinc Concentration values on X axis. Determine the concentration of Zinc in the sample using standard graph.
- Estimation of Zn (soluble) in test sample:
Take 1ml of sample supernatant in 100 ml volume flask and make up the volume with acidified water. The sample was further diluted to 1:250 and 1:500 times in order to get the reading within the range of standard graph (Biofertilizer and Organic Fertilizers in Fertilizer (Control) Order, 1985).

Figure 1: Atomic Absorption spectrophotometer (AAS)
3.4 ROOT COLONIZATION AND PLANT GROWTH PROMOTING ACTIVITY OF BACTERIAL ISOLATES
The ability of selected organisms to colonies roots and exerts plant growth promoting effects if any was assessed using seed germination assay. The study was done using corn and wheat seeds. Seeds were surface sterilized by using 0.4% of sodium hypochloride solution for 30 seconds and then washed 3 times with sterile distilled water.
The seeds were soaked in suspension (109cfu/ml) of each isolate and then placed on sterile petri plates containing tissue paper (soaked with sterile distilled water). Seeds were then incubated at room temperature for 9-12 days. Seeds soaked in sterile distilled water were kept as a control.
After 12 days, ability of selected isolates to colonize roots of the corn and wheat seedlings was assessed. The roots of wheat and corn plant were cut aseptically and 1 gm of roots were dipped in 100 ml sterile saline and shaked for 30 minutes on shaker at 160 rpm, at room temperature. Total viable count of ZSB isolates on 1 gm/ml root was estimated by pour plating tenfold serial dilution prepared in saline. Colony characteristics of selected isolates were confirmed on nutrient agar and Zn solubilizing activity was confirmed on ZnSB agar medium.
Plant growth promoting effect of selected isolates on corn and wheat were estimated by measuring % seed germination, root length, shoot length and vigour index.
Formulae for % seed germination and vigour index are as follows:
% Seed Germination = (No. of Germinated seeds / No. of total seeds) * 100
Vigour index= (mean root length + mean shoot length) * % Seed Germination
3.5 GENETIC IDENTIFICATION OF BACTERIAL ISOLATES
Two isolates were selected on the basis of their maximum zinc solubilizing capacity and plant growth promoting activity. Genetic identification of these two organism was done by 16s rRNA sequence analysis by Bioresource Laboratories, Pune.
Results & Discussions
4.1 COLLECTION SOIL SAMPLES AND SOIL ANALYSIS
Worldwide scenario revealed that the India is highly zinc deficient country (Singh, 2006). Soil samples from 4 states namely Maharashtra, Rajasthan, Karnataka and Andhra Pradesh of India were collected and analyzed for Zn content and alkaline pH. This because there are maximum chances of highly efficient strains of Zn different soils having alkaline pH. The results of said analysis are giving in table no. 1
| States | pH | Zn (ppm) | |
| 1 | Maharashtra | 8.2 | 0.62 |
| 2 | Karnataka | 7.9 | 1.16 |
| 3 | Rajasthan | 8.27 | 0.58 |
| 4 | Andhra Pradesh | 7.6 | 1.76 |
Table 1: pH and Zn content of soil samples collected from different states
Table 1 reveals that pH of all 4 samples was in the range of 7.6 to 8.3. Soil samples from Maharashtra, Rajasthan were more alkaline than that of Karnataka and Andhra Pradesh.
The normal quantity of zinc that is available in the soil is 0.65 ppm according to government standards (Biofertilizer and Organic Fertilizers in Fertilizer (Control) Order, 1985). Table 1 reveals that soil samples of Maharashtra, Rajasthan is Zn deficient with Zn content of 0.62 and 0.58 ppm, respectively. However, Zn content of soil samples from Karnataka and Andhra Pradesh was slightly higher than the normal quantity. These observations are similar to reported by (Brian J. Alloway, 2008).
4.2 ISOLATION OF ZINC SOLUBILISING BACTERIA
The soil samples were processed for isolation of zinc solubilizing bacteria. Before isolation of zinc solubilizing bacteria, heat shock treatment at 450C was given to each soil sample for 24 hrs. The purpose behind it was to isolate Gram positive spore forming bacillus species that can withstand high temperature as well as to reduce the load of gram negative bacteria coliforms and non coliforms that cannot survive at this temperature.
This because high temperature tolerant spore forming Zn solubilizing bacilli were thought to be a better alternative for developing shelf stable biofertilizer formulation as compared to gram negative bacteria. Furthermore coliforms are kwon human pathogen and need to be excluded from present study. After heat shock treatment soil sample (2 gm) were inoculated in nutrient broth and were incubated for 28-300C for 24 hrs on rotary shaker (160 rpm). The 24 hrs old nutrient broths cultures were serially diluted and plated on ZnSB medium. After 7 days of incubation 60 bacterial cultures were isolated from 4 soil sample. Each bacterial culture purified in nutrient and ZnSB plates colony characteristics and zones of Zn solubilization for each isolates were recorded.
Table 2 reveals that there are 23 isolates form 4 soil samples that show growth and Zn solubilization zone on ZnSB medium after 7 days incubation period. Whereas 37 isolates from these soil samples showed only growth on ZnSB medium. Though, Zn solubilization zone were not detected for these cultures after 7 days of incubation.
Table 2: Qualitative estimation of Zn solubilizing activity of 60 isolates
| Source | Isolates | Zone of clearance (mm) |
| Maharashtra | 7 | Only growth |
| 1 | 1 | |
| 1 | 2 | |
| 1 | 3 | |
| 1 | 4 | |
| 1 | 5 | |
| 1 | 8 | |
| 1 | 15 | |
| Rajasthan | 13 | Only growth |
| 1 | 1 | |
| 1 | 2 | |
| 1 | 4 | |
| 1 | 7 | |
| 1 | 12 | |
| 1 | 14 | |
| Karnataka | 9 | Only growth |
| 1 | 1 | |
| 1 | 3 | |
| 1 | 5 | |
| 1 | 7 | |
| 1 | 14 | |
| AndhraPradhesh | 8 | Only growth |
| 1 | 1 | |
| 1 | 2 | |
| 1 | 3 | |
| 1 | 4 | |
| 1 | 12 |
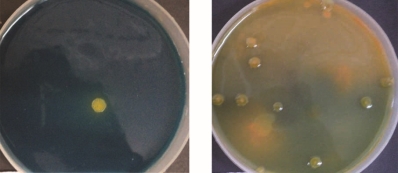
The FCO guidelines suggest that the organism used as ZSB biofertilizer should show zinc solubilization zone of minimum 10 mm. Figure 2 reveals that only 5 isolates shows zinc solubilization zone higher than 10 mm. (Ranging from 10- 15 mm).
Figure 2: Twenty three Zn solubilizing isolates from 4 different soil samples
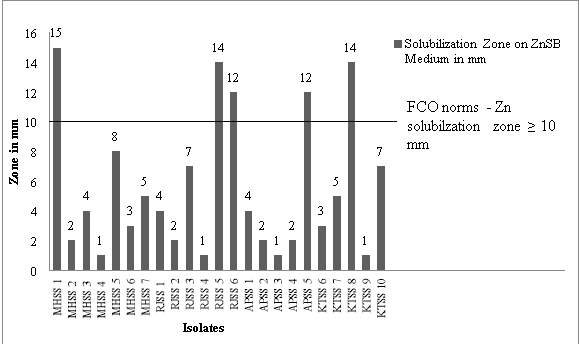
Growth conditions – ZnSB agar medium, incubated at 280C for 7 days
The highest zinc solubilization zones (14 to 15 mm) were showed by isolates MHSS 1 > RJSS 5 > KTSS 8. These isolates were selected for further study. RJSS 6 and APSS 5 showed slightly laser Zn solubilization zone (12 mm) than the above three mentioned isolates. Though RJSS 6 have exhibited ZN solubilization zone above FCO norm and also the only Zn solubilizing bacillus (Gram positive endospore forming) species isolated from present study and hence selected for further study.
COLONY MOROPHOLOGY OF 23 ISOLATES A3
Table 3 : Colony characteristics of 23 isolates
The colony characteristics of 23 isolates given in table 3.
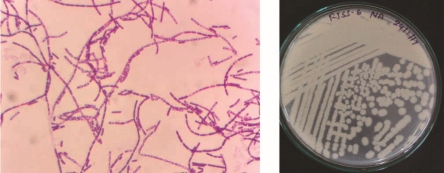
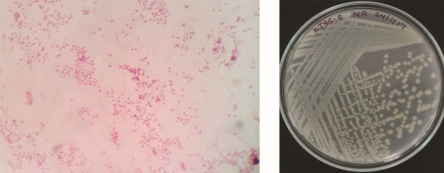
The colony morphology of 4 selected bacterial isolates is as follows: MHSS1 isolate was gram negative very short rods. It appeared on nutrient agar as off white, circular, raised pin point colonies.
RJSS 6 isolate was gram positive very short rods. It appeared on nutrient agar as 2 to 3mm, white, circular, raised spreading colonies.
KTSS 8 isolate was gram negative very short rods. It appeared on nutrient agar as 2 mm, circular, raised, white colored slimy colonies.
RJSS 5 isolate was gram negative very short rods. It appeared on nutrient agar as 1 to 2 mm, circular, raised, white colored slimy colonies.
Bright field images of gram staining and a photograph of isolated colonies of 4 potential Zn solubilizing isolates depicted in figure 4.
Figure 3: Bright field image of gram staining and a photograph of isolated colonies of 4 potential Zn solubilizing isolates.

MHSS 1
RJSS 6
KTSS 8
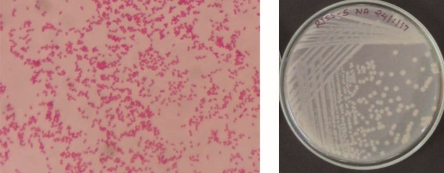
RJSS 5
4.2.1 IDENTIFICATION OF COLIFORM AND NON COLIFORM
The aim of the study was to isolates highly effective strains of zinc solubilizing bacteria which can be used to developed biofertilizer formulation. The three selected isolates were gram negative short rods and it was necessary to confirm that these are non coliform before further study. This because coliforms are known human pathogens and unsafe for handling as well as developing a biofertilizer.
The three gram negative isolate were streaked on MacConkey’s agar plates. The MacConkey’s agar is selective media for differencing between coliform and non coliform on the basis of lactose fermentation. The coliform grows on macConkey’s agar giving pink color colonies. Coliform ferment lactose by acid production and these acid production changes colour of pH indicator neutral red used in medium impacting pink colour of colonies. Non coliform does not ferment lactose and colonies are colourless in appearance. The 3 gram negative isolates are identified as non coliform because they are colourless on MacConkey’s agar plate. This result thus concludes that the gram negative isolates were non coliform and safe for handling for further study of development of ZBS biofertilizer.
4.2.2 CHARACTERIZATION OF POTASSIUM MOBILIZING & PHOSPHATE SOLUBILIZING ACTIVITY
Solubilization shows a common mode of action or mechanism i.e. solubilization of Zn as well as major plant nutrients P and K in alkaline soil is secretion of organic acid by various bacteria. The selected isolates have shown that organic acid secretion or lowering of pH is important factor in solubilizing Zn and so their potential to solubilize major crop nutrients P and K was also assessed.
For examination of these allied activities of phosphate solubilization and potassium mobilization the pour plate technique was used. For this experiment Pikovaskaya’s agar (0.1 % Tricalcium phosphate insoluble source of phosphate) and Aleksandrov’s agar (0.4 % mica insoluble source of potassium) were used for assessment of phosphate solubilization and potassium mobilization activities respectively. These media contain Bromothymol blue pH indicator which shows colour change from blue to yellow in pH range of alkaline to acidic.
Table 4: Potassium mobilization and phosphate solubilization activity of 4 isolates
| Isolates | K Mobilization zone in mm | P Solubilization zone in mm |
| MHSS 1 | 2 | 21 |
| RJSS 6 | 1 | 16 |
| RJSS 5 | 4 | 3 |
| KTSS 8 | 5 | 12 |
Growth conditions – Aleksandrov agar medium and Pikovaskaya agar medium for K and P mobilization and solubilization, respectively, incubated at 280C, for 7days
Table no. 4 reveals that 4 isolates showed solubilization of insoluble source phosphate and potassium while figure 4 shows growth and P and K solubilization zones of said isolates on Pikovaskaya’s and Aleksandrov’s media plates.
MHSS 1, RJSS 6 and KTSS 8 showed P solubilization activity significantly higher than FCO norms for PSB biofertilizer. Whereas all the isolates showed KMB activity but solubilization zones were below the FCO norms for KMB biofertilizer.
.
Figure 4: Photograph of 4 isolates showing P solubilization and K mobilization activity in terms of zone of clearance
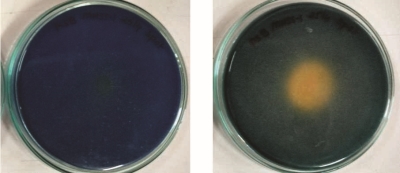
MHSS 1
RJSS 6
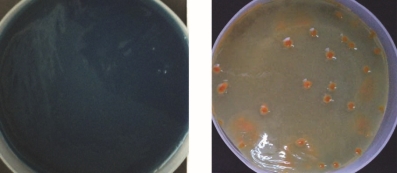
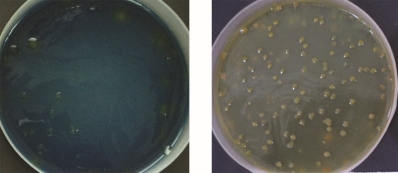
KTSS 8
RJSS 5
4.3 QUANTITATIVE ESTIMATION OF ZINC SOLUBILIZATION
For quantitative estimation of Zn solubilization selected isolates were grown in ZnSB broth (containing 0.1% ZnO i.e. 901.6 ppm Zn) at 280C on rotary shaker (160 rpm) for 7 days.
Samples were drawn from test flasks at an interval of 24 hrs during incubation period. Soil samples were processed for estimation of cell count and morphology of bacteria, pH and Zn content.
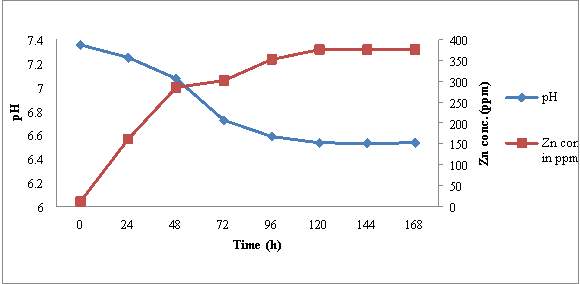
4.5.1 RJSS 6
The initial Zn content of ZnSB broth was estimated to be 11.39 ppm and pH of medium was 7.35. After inoculation of RJSS 6, the pH of medium start decreasing where as Zn concentration in culture broth started increasing during the incubation period of 7 days (fig 5). Within 24 hrs pH decreased slightly from 7.35 ± 0.08 to 7.25 ± 0.05. While Zn concentration in broth increased significantly from 11.39 ppm to 161.43 ± 0.5 ppm. Decrease in pH (7.35 to 6.5) and increase in soluble Zn content (11.39 to 376.16 ppm) in broth continued till 120 hrs of incubation and then no significant change in pH and Zn content was recorded up to 168 hrs incubation.
Microscopic observation of these sample revealed that there was increase in cell no. / OEL field from ≥ 10cfu/microscopic field to ≥ 200 cell/ OEL field during incubation period upto 96 hrs. After this morphological transition set, in sporangia were observed at 96 hrs and endospores become predominant morphological from after 120 hrs of incubation. From these observations it may be concluded that increase in Zn concentration and decrease in pH of medium also shows correlation with growth and morphological transition of RJSS 6. During growth phase of RJSS 6, there was significant increase in Zn solubilization associated with decrease in pH. Once morphological transition endospore formation set in no change in pH and Zn content of broth was noted.
Figure 5: Time dependent correlation of pH and Zn concentration in RJSS 6
 Growth condition- ZnSB broth medium, incubated at room temperature for 7 days on shaker at 163 rpm.
Growth condition- ZnSB broth medium, incubated at room temperature for 7 days on shaker at 163 rpm.
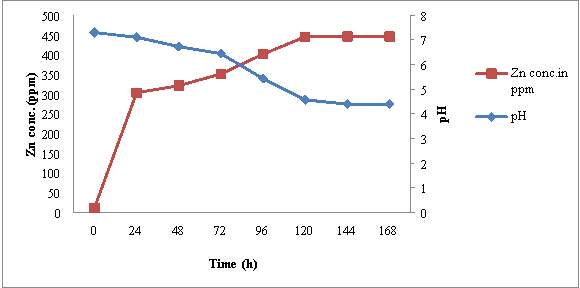
4.5.2 MHSS 1
The initial Zn content of ZnSB broth was estimated to be 11.39 ppm and pH of medium was 7.3. After inoculation of MHSS 1, the pH of medium start decreasing where as Zn concentration in culture broth started increasing during the incubation period of 7 days (fig 6). Within 24 hrs pH decreased slightly from 7.3 ± 0.1 to 7.10 ± 0.1. While Zn concentration in broth increased significantly from 11.39 ppm to 303.40 ± 0.5 ppm. Decrease in pH (7.35 to 4.39) and increase in soluble Zn content (11.39 to 446.23 ppm) in broth continued till 120 hrs of incubation and then no significant change in pH and Zn content was recorded up to 168 hrs incubation.
Microscopic observation of these samples revealed that there was in cells no. / OEL microscopic field from ≥10 cfu/ OEL field to ≥ 250 cfu/ OEL field. During incubation period upto 120 hrs to further incubation of broth culture did not show significant increase in cell no. from these observation it may be concluded that increase in Zn concentration and decrease in pH of medium also show correlation with active growth phase of MHSS 1. No change in pH and Zn concentration was recorded after 120 hrs of incubation.
Figure 6: Time dependent correlation of pH and Zn concentration in MHSS 1
Growth condition- ZnSB broth medium, incubated at room temperature for 7 days on shaker at 163 rpm.
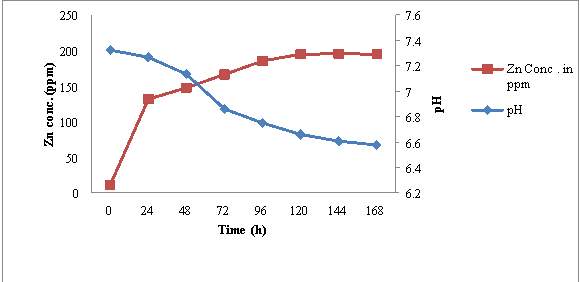
4.5.3 KTSS 8
The initial Zn content of ZnSB broth was estimated to be 11.39 ppm and pH of medium was 7.32. After inoculation of KTSS 8, the pH of medium start decreasing where as Zn concentration in culture broth started increasing during the incubation period of 7 days (fig 7). Within 24 hrs pH decreased slightly from 7.32 ± 0.75 to 7.26 ± 0.06. While Zn concentration in broth increased significantly from 11.39 ppm to 131.64 ± 0.6 ppm. Decrease in pH (7.32 to 6.60) and increase in soluble Zn content (11.39 to 195.44 ppm) in broth continued till 144 hrs of incubation and then no significant change in pH and Zn content was recorded up to 168 hrs incubation.
Microscopic observation of these samples revealed that there was in cells no. / OEL microscopic field from ≥10 cfu/ OEL field to ≥ 250 cfu/ OEL field. During incubation period upto 144 hrs to further incubation of broth culture did not show significant increase in cell no. from these observation it may be concluded that increase in Zn concentration and decrease in pH of medium also show correlation with active growth phase of MHSS 1. No change in pH and Zn concentration was recorded after 120 hrs of incubation.
Figure 7: Time dependent correlation of pH and Zn concentration in KTSS 8
Growth condition- ZnSB broth medium, incubated at room temperature for 7 days on shaker at 163 rpm.
4.5.4 RJSS 5
The initial Zn content of ZnSB broth was estimated to be 11.39 ppm and pH of medium was 7.32. After inoculation of RJSS 6, the pH of medium start decreasing where as Zn concentration in culture broth started increasing during the incubation period of 7 days (fig 8.). Within 24 hrs pH decreased slightly from 7.32 ± 0.08 to 7.26 ± 0.05. While Zn concentration in broth increased significantly from 11.39 ppm to 139.97 ± 0.5 ppm. Decrease in pH (7.32 to 6.58) and increase in soluble Zn content (11.39 to 195.21 ppm) in broth continued till 144 hrs of incubation and then no significant change in pH and Zn content was recorded up to 168 hrs incubation.
Microscopic observation of these samples revealed that there was in cells no. / OEL microscopic field from ≥10 cfu/ OEL field to ≥ 250 cfu/ OEL field. During incubation period upto 120 hrs to further incubation of broth culture did not show significant increase in cell no. from these observation it may be concluded that increase in Zn concentration and decrease in pH of medium also show correlation with active growth phase of MHSS 1. No change in pH and Zn concentration was recorded after 120 hrs of incubation.
Figure 8: Time dependent correlation of pH and Zn concentration in RJSS 5
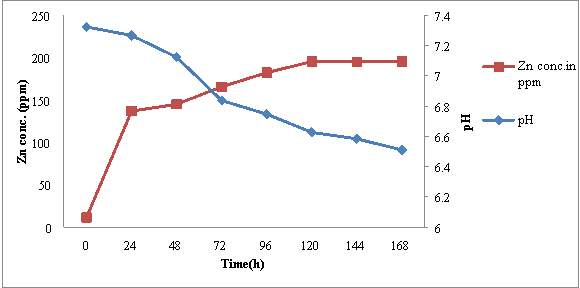
Growth condition- ZnSB broth medium, incubated at room temperature for 7 days on shaker at 163 rpm.
All the selected isolates named RJSS 6, MHSS 1, KTSS 8 and RJSS 5 showed Zn solubilization upto 120 hrs. The Zn solubilization was associated with decrease in pH of medium suggesting the organic acid secretion is the probable mechanism for solubilization of Zn by these bacterial isolates. The Zn solubilization by 4 selected isolates was MHSS 1 (Zn 446.23 ppm) > RJSS 6 (Zn 376.16 ppm) > KTSS 8 (Zn 195.44 ppm) > RJSS 5 (Zn 195.21 ppm)
Beside ZnO, Zn may be present in ZnCO3 and ZnPO4 in soil and Zn in these compounds are also unavailable for plants.
MHSS 1 and RJSS 6 isolates exhibiting very high efficiency of solubilization of Zn from ZnO, were selected for further study, on solubilization of Zn from ZnCO3 and ZnPO4.
Thus, these two isolates were grown in ZnSB broth containing either ZnCO3 or ZnPO4 as source of insoluble Zn. These flasks were incubated at 28 – 300C on rotary shaker (160 rpm) for 168 hrs. The initial and final pH and Zn content were recorded. Table no 5. shows the solubilization of Zn by RJSS 6 and MHSS 1 on different source of Zn.
Table 5: Solubilization of Zn in presence of different source of insoluble Zn
| Insoluble Zn source | Isolates | Zn conc. (ppm) after 0 hrs. | Zn conc. (ppm) after 168 hrs. | Initial pH | Final pH |
| ZnO | MHSS 1 | 11.39 | 446.23 | 7.32 | 4.39 |
| RJSS 6 | 11.39 | 376.16 | 7.32 | 6.53 | |
| ZnCO3 | MHSS 1 | 12.34 | 378.65 | 7.45 | 4.84 |
| RJSS 6 | 12.36 | 352.96 | 7.48 | 6.34 | |
| ZnPO4 | MHSS 1 | 14.15 | 252.36 | 7.39 | 4.59 |
| RJSS 6 | 14.21 | 204.56 | 7.36 | 6.45 |
Table no 5 reveals that MHSS 1 best Zn solubilization with ability to solubilize Zn from different source of insoluble Zn as compared to RJSS 6. The decrease in pH was correlated to solubilization of Zn irrespective of source of insoluble Zn used. This suggest the secretion of organic acids is playing important role in Zn solubilization from various insoluble Zn compounds.
4.4 ROOT COLONIZATION AND PLANT GROWTH PROMOTING ACTIVITY OF SELECTED ISOLATES
Zinc solubilizing bacteria are known to grow along root surface or in rhizospheric soil, solubilize Zn and secrets other plant growth promoting substance like auxins, cytokines etc. Thus ZSB’s improve plant growth by improving PGPRs.
The seed germination assays were conducted using corn
and wheat seeds. The total viable count of selected isolates on roots of 12days old corn and wheat seedlings were estimated. Study revealed that all the selected isolates can colonize roots of wheat and corn seedling.
TVC of selected isolates on wheat roots were MHSS 1 (2*108) > RJSS 6 (1*108) > KTSS 8 (1*107) > RJSS 5 (1*106) and on wheat seeds MHSS 1 (1*108) > RJSS 6 (1.2*108) > KTSS 8 (1*107) > RJSS 5 (1*106). Which is best root colonizes MHSS 1. Highest count of MHSS 1 organism was detected on wheat and corn.
Figure 9: % seed germination of wheat seeds in presence of 4 isolates
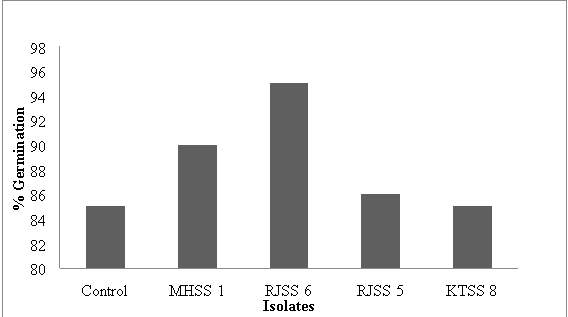
Figure 10: Vigour index of wheat seeds in presence of 4 isolates

Figure 11: Germination of wheat seeds in presence of 4 isolates

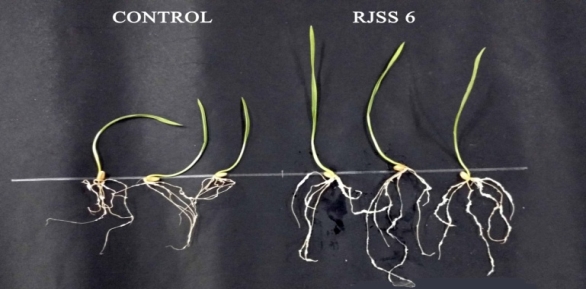
Figure 12 : Germination of wheat seeds in presence of 4 isolates


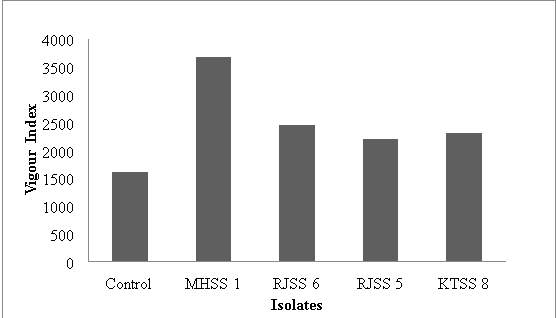
Table 7: Estimation of plant promoting activity of 4 isolates using corn seeds
| Isolates | Mean root length (cm) | Mean shoot length (cm) | % Germination | Vigour Index |
| Control | 13.45 | 5.48 | 85 | 1325.1 |
| MHSS 1 | 21.69 | 19.14 | 90 | 3674.7 |
| RJSS 6 | 18.69 | 9.52 | 86.66 | 2444.6786 |
| RJSS 5 | 15.98 | 9.32 | 86.66 | 2192.498 |
| KTSS 8 | 15.1 | 10.46 | 90 | 2300.4 |
Figure 13: % seed germination of corn seeds in presence of 4 isolates
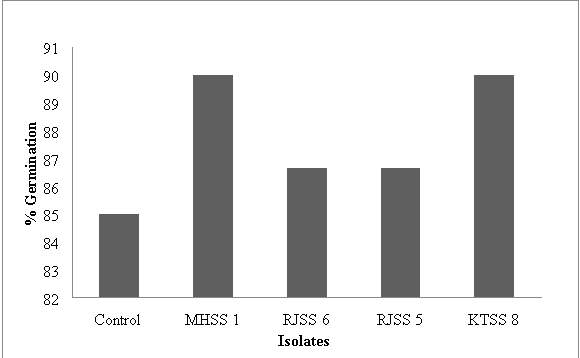
Figure 14: Vigour index of corn seeds in presence of 4 isolates
Figure 15: Plant growth promoting activity of 4 isolates on corn seeds with respect to control
CONTROL

MHSS 1

RJSS 6
Figure 16: Germination of corn seeds in presence of 4 isolates
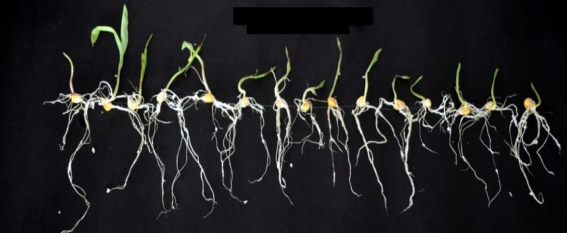
KTSS 8

RJSS 5
4.5 GENETIC IDENTIFICATION
MHSS 1 and RJSS 6 exhibiting highest Zn solubilization activities (446.23 ppm and 376.16 ppm) respectively. They were found to exhibit good root colonization for wheat and corn seedlings. And they exhibited highest plant growth promoting effects of wheat (vigour index 1508.4 and 1494.3 respectively) and corn (vigour 3674.7 and 2444.6 respectively). Thus, these two isolates exhibited highest potential for development of ZBS biofertilizer. The identification of these isolates becomes essential before further studies with respect to optimization of conditions for biomass products of these isolates. Hence, isolates were identified using 16’s rDNA sequences analysis of Bioresource Laboratories, Pune.
The results conclude that the isolate MHSS 1 was identified as Acinetobacter calcoaceticus and isolate RJSS 6 was identified as Bacillus thurengensis.
Conclusion
The soil samples from 4 states in India namely Maharashtra, Rajasthan, Karnataka and Andhra Pradesh were screened for isolation of highly efficient strains of zinc solubilizing bacteria. Quantitative screening was done using ZnSB medium containing ZnO as source of insoluble Zn. The said medium is used in present study as it is the medium assessment of Zn solubilization suggested for efficiency of ZnSB in laboratory as per the guidelines of FCO. The FCO norms suggests that ZnSB used in biofertilizer should give Zn solubilization zone of 10 mm diameter (minimum) on ZnSB medium within 7- 8 days.
From 4 soil samples, 60 isolates were obtained that could grow and / or solubilized Zn on ZnSB medium. Out of these 23 isolates show measurable zone of solubilization of Zn (12 to 15 mm). Bacterial isolates (4 NO.s) exhibiting Zn solubilization zones higher than 10 mm diameter were selected for further study. RJSS 6 Gram positive rod and MHSS 1, RJSS 5 and KTSS 8 are gram negative short rods. BY checking the growth on MacConkey’s agar, the all non coliform nature of gram negative rods was confirmed. The isolates namely, MHSS 1, RJSS 6, KTSS 8 and RJSS 5 were grown in ZnSB broth for 7 days on rotary shaker. The Zn solubilization efficiency was quantified by soluble Zn present in culture broth at interval of 24 hrs, during incubation period. The Zn solubilization by selected isolates was MHSS 1 (446.23 ppm) > RJSS 6 (376.16 ppm) > KTSS 8 (195.44 ppm) > RJSS 5 (195.21 ppm). The isolates of MHSS 1 and RJSS 6 were also grown in ZnSB medium containing either ZnCO3 or ZnPO4 as source of insoluble zinc. The highest Zn solubilization of all the three Zn compounds was observed in presence of MHSS 1 compared to RJSS 6 solubilization of Zn in ZnSB broth was found to correlate with decrease in pH of culture broth, This suggest the secretion of organic acids by ZnSB cultures as mechanism for solubilizing Zn. Organic acid secretion by various bacteria also plays important role in solubilizing insoluble phosphate as well as potassium salts. Hence efficiency of P and K solubilization of selected isolates was studied using Pikovaskaya’s and Aleksandrov’s media. The P solubilization assay results showed MHSS 1 (21 mm) > RJSS 6 (16 mm) > KTSS 8 (12 mm) > RJSS 5 (3 mm) and K solubilization assay results showed MHSS 1 (2 mm) > RJSS 6 (1 mm) > KTSS 8 (5 mm) > RJSS 5 (4 mm).
The selected isolates were then assessed for their ability to colonize roots of corn and wheat as well as for their effects on plant growth by conducting a seed germination assay. All the isolates were able to colonize roots of corn and wheat seeds. The total viable counts of selected isolates on corn roots MHSS 1 (2*108) > RJSS 6 (1*108) > KTSS 8 (1*107) > RJSS 5 (1*106) and on wheat seeds MHSS 1 (1*108) > RJSS 6 (1.2*108) > KTSS 8 (1*107) > RJSS 5 (1*106). A significant increase in % seed germination and vigour index of seedlings was observed in presence of all the selected isolates. The % seed germination for corn seeds was MHSS 1 (90) > RJSS 5 (90) > KTSS 8 (86.66) > RJSS 6 (86.66) and that for wheat seeds for MHSS 1 (90) > RJSS 6 (95) > KTSS 8 (86) > RJSS 5 (85). The plant growth promoting effects of selected isolates can be summarized as their vigour indices. For corn seedlings vigour indices in presence of selected isolates were MHSS 1 (3674.7) > RJSS 6 (24404.6) > RJSS 5 (2300.4) > KTSS 8 (2192.4). For wheat seedling vigour indices were MHSS 1 (1508.4) > RJSS 6 (1494.3) > RJSS 5 (1352.8) > KTSS 8 (1152.6). The present study thus revealed that MHSS 1 and RJSS 6 isolates exhibiting highest Zn solubilization efficiencies on 3 different compounds of Zn, good root colonization ability and highest plant growth promoting effects on corn and wheat are potential candidates for development of biofertilizer (ZSB). Before further studies on optimization of conditions for mass productions of bacterial cultures was important. Hence, MHSS 1 and RJSS 6 were identified by 16’s rDNA sequence analysis at Bioresource Laboratories, Pune. MHSS 1 was genetically identified as Acinetobacter calcoaceticus and RJSS 6 was identified as Bacillus thurengensis.
MHSS 1: Acinetobacter calcoaceticus was found to be the best isolates for further studies on development of ZSB Biofertilizer.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Microbiology"
Microbiology is the study of microscopic organisms, or microorganisms. Microorganisms are simple life forms that include bacteria, fungi, algae, and viruses.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: