Mechanisms of Bird Migrations
Info: 7993 words (32 pages) Dissertation
Published: 9th Dec 2019
Tagged: Environmental Studies
Bird Migrations
DEFINITION
Seasonal outward and return movement of populations between regions where conditions are alternately favorable and unfavorable.
INTRODUCTION
The word migration recalls images of heroic movements of large populations over vast distances. Blackpoll warblers (Setophaga striata) during fall migration converge on the Eastern coast of the United States and Canada before embarking on remarkable overwater flights across the Atlantic Ocean to the Caribbean or South America, a nonstop journey of up to 2,400 kilometers or more over the course of 3 days, before reaching wintering grounds in Venezuela or Colombia. Migration here is defined as a seasonal to-and-fro movement of populations between regions where conditions are alternately favorable and unfavorable. Migrating birds can fly great distances, visiting many unique landscapes, and thus face many ecological problems which can be solved either through natural selection shaping innate behavioral programs or through cognitive processes. Although some aspects of migratory behavior are genetically predetermined such as initial departure time, direction and distance, every migrating bird embarks on a journey that is unique, and therefore exploration, experience and learning necessarily play a fundamental role in overcoming the challenges of migration. Migration is a complex behavior that offers a rich study system for evolutionary biologists, as it involves countless morphological and physiological adaptations for efficient movement, behavioral adaptations for optimal use of environmental factors such as winds and sensory cues for orientation, and cognitive adaptations for map learning, long-term memory and social interaction (Ǻkesson & Hedenström, 2007).
PERFORMANCE (SPEED AND DISTANCE)
The highly physical and energetically costly nature of movement lends itself to optimality analyses. The use of optimality perspectives in analyzing behavioral adaptations and strategies has become an essential aspect of understanding bird migration (Alerstam, 2011). If migration is viewed as a conflict between (re-)fueling and movement towards a goal location, then the overall speed of migration can be determined by three variables: rate of energy accumulation, flight speed and rate of energy consumption during flight (Ǻkesson & Hedenström, 2007). Fueling between migratory events is an important determinant of migration performance. The rate of fuel accumulation partially determines the overall migration speed. One might therefore expect adaptations which maximize both food intake and its conversion into fuel stores, such as fat, which contains the most energy per unit of mass compared to alternatives. Migratory birds are required to locate and ingest food, break it down, absorb it, and create and transport fat bodies to specialized fat deposits. These processes require special adaptations, which among others include flexible organs that grow and shrink in relation to the requirements of fueling and movement. During fueling, migratory birds have been shown to ingest and process food close to their metabolic capacity. To do so they grow a larger intestine, gizzard, and liver to boost their food processing capacity, and grow larger flight muscles to lift and transport increasing fuel loads (McWilliams, Guglielmo, Pierce, & Klaassen, 2004).
Quickly reaching a migratory goal naturally also depends upon flight speed. The sum of the different power components (parasitic, induced, and profile power, along with the basal metabolic rate), which constrain powered flight, result in a U-shaped curve which relates power (energy consumed per unit time) and speed (Pennycuick, 1975). From the power performance curve, two flight speeds of special interest can be calculated: (1) the optimal flight speed for minimizing energy costs (Vmp or minimum power) per unit of time (i.e., to keep flying for as much time as possible on a given energy reserve, irrespective of the distance covered) and (2) The optimal flight speed for minimizing the energy costs per unit of distance covered (i.e. the speed associated with the minimum ratio of power to velocity, Vmr or maximum range) found by drawing a tangent from the origin to the power curve. Vmr allows birds to fly the maximum distance on a given fuel reserve (Pennycuick, 1975).
Temperature, air density and oxygen concentration all decline with increasing altitude, which can have dramatic effects on a bird’s flight aerodynamics, respiratory efficiency and water economy (risk of dehydration). Ascending-descending flight behavior appears to be used for sampling the most favorable winds throughout the air column, and the horizontal wind component is thought to be the most important factor predicting cruising altitude (Bruderer, Underhill, & Liechti, 1995). Migrating birds have been regularly observed flying at altitudes up to 6000 m above grounds level (agl) and some migrants such as the bar-headed goose (Anser indicus) regularly reach altitudes of 8000 m or more when crossing the Himalayas. When confronted with barriers such as mountain ranges, birds must either make a detour around the barrier or ascend high enough to cross the mountain peaks. Birds which rely on thermal updrafts for migration have been observed at great altitudes, such as the Ruppell’s griffon vulture (Gyps rueppellii) observed at an altitude of 11,300 m above the Ivory Coast (Braunitzer & Hiebl, 1988). High altitude migratory species are capable of such feats because they possess several types of hemoglobin which operate side by side providing a greater respiratory capacity (oxygen affinity) at high altitudes than closely related lower altitude species. For example, four different forms of hemoglobin have been discovered in the Ruppell’s griffon vulture (Braunitzer & Hiebl, 1988).
SPECIALIZATION VS PLASTICITY
In nearly all bird species there exists tension between ecological specialization, selecting habitats, foraging sites and food for which they are best adapted, and the ability to respond to unpredictable and novel resources. Ecological plasticity is described as the ability to adaptively respond behaviorally to variable resources in the environment. Plasticity may vary among species, individuals of the same species and developmentally in an individual. We can assume that the transient period of migration during the annual cycle could provide the greatest challenge for a behavioral profile of dedicated ecological specialization. Stochastic events during migration may play a large role in determining the habitats that migrating birds come to occupy during stopover. Prevailing wind patterns over the Gulf of Mexico can influence the geographic position of arrival following trans-Gulf flight (Gauthreaux, Belser, & Welch, 2006). Upon arrival decisions about stopover habitat are more influenced by physiological condition than resource cues (Buler, Moore, & Woltmann, 2007). Habitats and food become more and more dissimilar the further a bird may migrate from their natal area. Stopover sites become critical during migration; the decision where to land is vital for whether sufficient resources can be found and fat reserves restored. In low-quality habitats, birds may have to leave the area completely in search of better stopover sites. Many migrating birds show consistently different habitat use and foraging preferences during the breeding and wintering periods. For example, Worm-eating warblers (Helmitheros vermivorum) during the breeding season forage for insects in live foliage. However, during the overwinteringperiod throughout their Central American and Caribbean range worm-eating warblers forage on insects found in dead foliage trapped in the vegetation of the forest understory (Greenberg, 1987). Greenberg’s (1987) detailed observations and experiments with juveniles have demonstrated that a complex interaction between innate habitat preference, patterns of substrate exploration and learning play an important role in developing this seasonal foraging specialization. Similarly, foraging opportunism among Setophaga warblers is greatest among conifer-breeding species that winter in tropical areas. In winter, these species occupy broad-leaf habitats, and hence, necessarily employ opportunistic strategies for locating, catching and handling a diverse array of food types. Exploring novel habitats and monitoring many diverse resources instead of a specialized few would seem likely to require unique cognitive capabilities and/or personality traits. In addition, several studies have found that migrants may use social information to gather information about the quality of habitats. For example, migrants may initially join flocks at stopover sites before foraging on their own, and may use resident species as an indicator of high-quality breeding habitats. Such social interactions may support information gathering (Németh & Moore, 2007).
IN FLIGHT DECISION-MAKING: WHEN TO DEVIATE FROM A DIRECT PATH TOWARD A MIGRATORY GOAL
Wind and Drift
Decision-making involves determining an action given the available information about relevant environmental features integrated with past experience. One environmental feature, wind, has a very profound influence on the migratory movements of birds. Depending on the direction and strength of the wind, migrants may greatly benefit if the wind flows in the same general direction as the intended course or may be hindered during flight if the wind flows in an opposing direction (Ǻkesson & Hedenström, 2007). In cross winds, a bird may become drifted off its intended course unless it can accurately detect and compensate for displacement. Therefore, selecting favorable winds during migration is important for optimal migration performance if “optimality” is to be achieved (Liechti & Bruderer, 1998). The relationship between a migrant’s track, heading and wind direction is shown in figure 1. The track (movement with respect to the ground) vector is the additive sum of the heading and wind vectors. An in-flight decision of full or at least partial compensation could be achieved if a bird could perceive lateral displacement from a preferred direction. One way this could be accomplished is by monitoring fixed visual landmarks relative to a bird’s heading (Alerstam & Pettersson, 1977; see also Bingman, Able, & Kerlinger, 1982).
Recent studies have demonstrated that migratory birds can choose when to fly to avoid adverse wind conditions and thus improve travel speeds (McLaren, Shamoun-Baranes, Dokter, Klaassen, & Bouten, 2014). Successful arrival at breeding or wintering grounds depends at least in part on the ability of migrants to make time-sensitive decisions on where to orient to exploit beneficial wind patterns thus maximizing energetic efficiency. However, to expedite travel to their intended goal, migrating birds may initiate migration under non-optimal wind conditions. During autumn migration in eastern North America, migrants in flight are often drifted laterally by crosswinds, but are observed compensating for drift when near the Atlantic coast. The tendency to compensate for wind drift along the Atlantic coast also increased through the night, while no temporal differences were observed inland. The observed behavior suggests that birds migrate in an adaptive way to conserve energy by actively assessing in flight the degree to which they should optimally compensate for wind drift (Horton et al., 2016). Upgrades to the United States national weather radar network to dual-polarization allows a more direct determination of migrant heading (body axis direction) and track (the resultant direction of bird movement, see Figure 1). However, the effects of drift are difficult to quantify as differential departures of migrants with different preferred directions can occur and lead to a phenomenon known as “pseudodrift” (Evans, 1966).
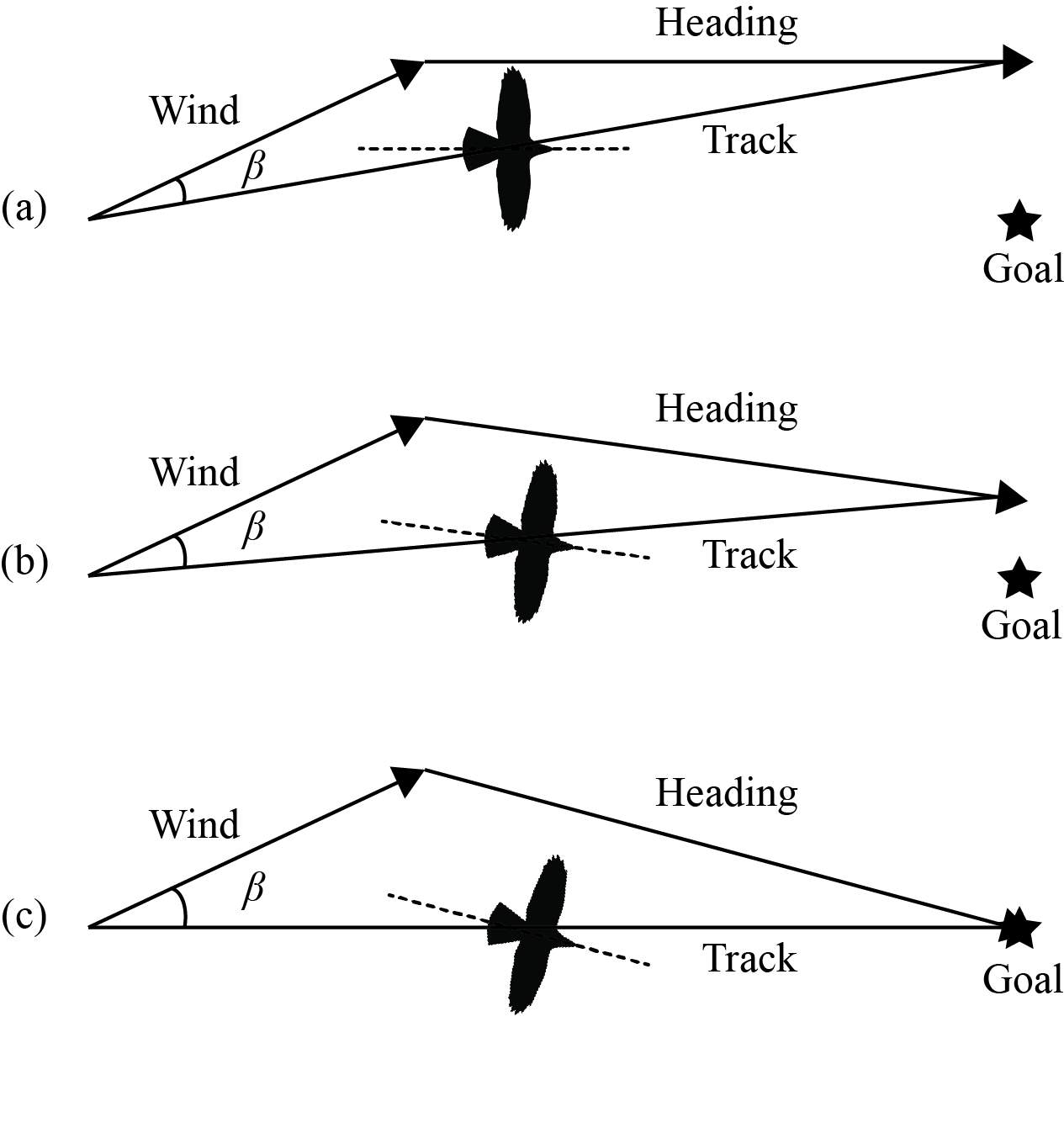
Fig. 1. The resultant velocities for three different situations (heading can also be considered air velocity and track can also be considered ground velocity): (a) full wind drift, (b) partial drift/partial compensation, and (c) full compensation. β = angle between track and wind direction. Note that the body axis of the bird in the figure is aligned with the heading vector.
Barriers
Migrating birds can store large fuel reserves and make nonstop flights of thousands of kilometers. Yet there are many cases where birds refrain from crossing barriers (like open water, deserts or ice), which are clearly within their flight range capabilities. Why then do birds not always migrate along the most direct and shortest routes to their destinations? Many factors may influence the costs and benefits of alternative migration routes such as drift, disorientation, predation, stopover conditions and risks of starvation, and weather, most prominently wind. Detours are found among both soaring and flapping flight birds. Large increases in the fuel stores of a bird contribute to higher total body mass, which consequently increases both parasitic and induced drag. Therefore, the relationship between fuel and flight range has a concave function, which means that birds could ultimately save energy if they migrate by short flight steps and low fuel loads rather than departing on longer flights with heavy fuel loads. This optimal fuel load configuration will promote avoidance of barriers and preference for detours if they increase the number of short flight steps travelled (Alerstam, 2001). When confronted with migratory barriers or obstacles, a migrant faces a decision regarding seeking out available fuel to cross the barrier in one go, or take an alternative but longer detour around the barrier. The occurrence of directional shifts towards a coastline during the night suggests that the disposition of migrants to cross large barriers depends in part on physical condition (Bruderer & Liechti, 1998). In autumn, migrants approaching the Mediterranean Sea later in the night show a decline in motivation to cross, possibly due to lowering of fuel stores through the night and/or increasing hesitation to cross barriers of unknown extents (Fortin, Liechti, & Bruderer, 1999).
Long-Term Memory
How much are decisions about suitable stopover sites influenced by prior experience? Memory of suitable stopover sites would be a great advantage for optimizing migratory routes and potentially increasing travel speeds. Banding data reveals that experienced adults typically spend a shorter period of time at a particular stopover site than inexperienced juveniles. Many migrants utilize the same stopover or wintering areas year after year (Nolan & Ketterson, 1991). Such observations raise the question of whether long-distance migrants have specialized neural capabilities related to long-term memory compared to residents? In one study, migratory Garden warblers (Sylvia borin) and resident Sardinian warblers (Sylvia melanocephala) were hand-raised and subsequently allowed to explore two unfamiliar rooms during the autumn migratory period. One room contained food while the other did not. The Garden warblers remembered the room with food for at least 12 months, whereas the resident Sardinian warblers demonstrated considerably shorter long-term memory ability (Mettke-Hofmann & Gwinner, 2003). Memory and experience may also influence the establishment of new migration patterns. Teitelbaum et al. (2016) demonstrated that experienced migratory whooping cranes (Grus Americana) can innovate new migratory routes, which once established, eventually spread to younger individuals via social learning. The presence of older individuals in a group increased the likelihood of occupying new overwintering sites, suggesting that innovative behavior often increases with age because those individuals are more experienced and thus better suited to infer adaptive solutions to novel problems.
NAVIGATION: MAPS AND COMPASSES
An important distinction must be made between three types navigational/homing ability as described by Griffin (1955):
- Type I or piloting. To locate a goal by referring to familiar landmarks or landscape features, but may also entail random or systematic searches until familiar landmarks are found. For birds, landmarks are generally thought to be visual, but there is no reason why they would be limited to visual cues. Landmarks in themselves cannot provide compass or directional information, but used in conjunction with compass cues can be used to maintain directionality during oriented flight. Landmarks can also be substituted for compass orientation systems in familiar areas.
- Type 2 or compass orientation. To orient in a specified compass direction without reference to landmarks when released in unfamiliar territory. This may only result in successful navigation if the compass direction leads to a goal or familiar territory. Vector navigation carried out by young birds embarking on their first migration possess inherited information about the direction and distance to population-specific wintering ranges (Berthold & Helbig, 1992).
- Type 3 or true navigation. True navigation allows for the determination of a specific direction toward a goal when in unfamiliar territory and in the absence of familiar landmarks or any form of sensory contact with the goal. This is often considered the most complex form of navigational ability, and it is presumed that until goal areas (e.g., breeding or wintering grounds) are established through experience no true navigation can occur.
Compass Mechanisms
The most remarkable aspect of migration is how birds can routinely return to previous breeding and overwintering sites following migration of often thousands of kilometers. They do so using many sophisticated sensory adaptations and navigation capabilities (Alerstam, Hedenström, & Åkesson, 2003). The term orient means to determine, relative to some external cue, and keep a compass bearing, while navigation refers to moving from a starting location to a specific goal. To accomplish true navigation, birds are thought to use one or several compasses in conjunction with a map (Able, 2001). Migratory birds have access to several compass mechanisms for orientation, using information from the sun (both the position and the pattern of skylight polarization), stars and Earth’s magnetic field.
The use of a sun compass by birds was first demonstrated by Kramer (1950). Kramer used mirrors to alter the apparent position of the sun and found that European Starlings (Sturnus vulgaris) altered their directional preference accordingly. The avian sun compass is based on the sun’s azimuth (the angle of the sun’s position projected on the horizon relative to north), with the sun’s altitude being irrelevant. Because the sun appears to move across the sky during the day, the use of a sun compass requires calibration by an internal clock (circadian clock) because it is the sun’s azimuth at a particular time of day that provides the directional information. By experimentally shifting the internal clock of a bird, used in so-called clock-shift experiments, and recording directional preferences in homing pigeon or migrant orientation cage experiments, the internal clock has been shown to be important for correctly implementing the sun compass. Inexperienced birds must learn to use the sun compass; in homing pigeons learning appears to take place after the bird is confronted with the need to home, likely spontaneously during extended exercise flights around the home loft. Moore (1987) demonstrated that the position of the setting sun functions as a source of directional information for night-migrating birds. Experimental evidence indicates that several species of migratory birds (but not all; see Wiltschko, Munro, Ford, Stapput, & Wiltschko, 2008) are apparently able to perceive polarized light and use it as a cue for orientation. There is also evidence that information from different compass cues can be integrated. For example, researchers have provided experimental evidence that suggests that migratory Savannah Sparrows (Passerculus sandwichensis), Swainson’s (Catharus ustulatus) and Gray-cheeked (C. minimus) thrushes, and White-throated Sparrows (Zonotrichia albicollis) use polarized light cues at sunrise or sunset to calibrate their magnetic compass. The avian visual Wulst of the anterior forebrain and hippocampus may be involved in learning processes supported by the sun-compass in homing pigeons (Bingman & Jones, 1994), though the sun compass itself is still operational after Wulst and hippocampal lesions (Bingman, Bagnoli, Ioalè, & Casini, 1984).
During the night, when the sun compass is unavailable, migrating birds may rely on information provided by other cues for orientation. In a series of classic experiments, Emlen (1967, 1969) demonstrated that indigo buntings (Passerina cyanea) use the stars for orientation during migration. By experimentally manipulating the location and movements of stars and constellations in a planetarium, Emlen (1967) found that buntings derived directional information from the pattern of constellations relative to each other and to the celestial pole, the point around which stars appear to rotate during the night. Inexperienced juvenile buntings learned the location of north by observing the rotation of constellations around the celestial pole. Star-compass orientation has been demonstrated in several species of nocturnal migrating songbirds, including Bobolinks (Dolichonyx oryzivorus), Savannah Sparrows (P. sandwichensis), European Robins (Erithacus rubecula), and Garden Warblers (Sylvia borin). Little is known about specific neural structures involved in stellar orientation other than the obvious assumption that elements of the visual system are involved. Mouritsen, Heyers, & Güntürkün, (2016) proposed a hypothetical framework for the forebrain organization of both the sun and star celestial compasses.
The Earth’s magnetic field also provides compass orientation information for migratory birds. The geomagnetic compass provides migratory birds directional information based on inclination angle and not the polarity of the field (an inclination compass; Wiltschko & Wiltschko, 1995). Inclination angle or magnetic dip is the angle of Earth’s geomagnetic field relative to gravity or any vertical reference. Along the magnetic equator the Earth’s magnetic field runs parallel to the earth’s surface and towards the poles the angle becomes increasingly perpendicular. Observing changes in the orientation preference of European robins (E. rubecula) by altering the magnetic field around orientation cages by using Helmholtz coils provided the first evidence of a magnetic compass (Wiltschko, 1968). To date, no studies in birds have demonstrated compass orientation derived from either total intensity or polarity of the Earth’s magnetic field. Currently, the peripheral transduction mechanism used by migratory birds to gather and process information from Earth’s magnetic field is under debate. Magnetic information may be detected by magnetoreceptors in the upper beak and transmitted through the ophthalmic branch of the trigeminal nerve to the brain (Mora, Davison, Wild, & Walker, 2004). More likely, however, magnetic field direction would be sensed by special photopigments in the eyes (Ritz, Adem, & Schulten, 2000) and this geomagnetic signal would then be processed in cluster N, a specialized, night-time active forebrain region found in nocturnal migrants. European robins (E. rubecula) with lesions of cluster N are unable to orient to magnetic cues but can perform sun compass and star compass orientation behavior.
Vector Navigation
Juvenile European Starlings have been shown to migrate by orienting toward a preferred direction, but not to a goal. In a classic experiment, Perdeck (1958) demonstrated that juvenile starlings displaced during fall migration from Holland to Switzerland continued southwest along their normal migratory direction ending to reach southern France. Adults, however, could compensate for their geographic displacement and reoriented northwest towards their typical wintering grounds in northern France. Migrating white-crowned sparrows (Zonotrichia leucophrys) were similarly displacement longitudinally across North America and only adults, but not juveniles, showed a clear goal (typical wintering area)-directed shift in orientation after displacement (Thorup et al., 2007). The presumably innate direction and distance program of first-time migrants is termed vector navigation, which is not a true navigational mechanism as no cognitive representation of a goal is defined. Only predetermined genetic information on direction and distance is used. To gauge the distance to fly, many inexperienced migrants have been experimentally demonstrated to rely on an inherited temporal program, which specifies how long they should travel (Berthold, 2003) and direction (Helbig, 1991). The behavioral mechanisms which guide inexperienced migratory birds is a typical example of vector navigation because it is based on a simple determination of direction and distance from a starting location.
Map-based Navigation
Map-based navigation denotes a situation in which a bird determines its spatial position relative to a goal based solely on information available at some distant location. Based principally on homing pigeon research, fundamental differences between two theoretical representational types of map are distinguished: a mosaic map and gradient map. The mosaic map introduced by Papi (1976) is formed from the learned spatial relationships among landscape features (for Papi, those landscape features would be odor patches) and their relative arrangement with respect to a goal. These relationships are presumed to be learned as a bird explores increasingly larger areas of its home range. Although this type of map may be useful over distances ranging perhaps a few hundred kms, for example, some large visual landmarks may be seen at great distance or remote olfactory patches can be coded as being a certain direction from a goal by winds, a mosaic map would be of little use after a displacement far removed from an animal’s local experience. Mosaic maps are often considered equivalent to so-called cognitive maps, but it is unclear whether this is correct. O’Keefe and Nadel (1978) refer to cognitive maps as a mental representation in which spatial relationships among spaces are coded with respect to not only position and direction but also distance. Thus, an animal possessing a cognitive map should be capable of inferring novel shortcuts between any two points on the map. But there is evidence suggesting that homing pigeons are capable of navigating between two such points within their range. Blaser et al. (2013) released food deprived and satiated pigeons from an unfamiliar site, equidistant between a known food site and home loft. The pigeons knew their geographic position relative to the familiar targets and chose a flight direction reflective of their motivational state.
More discussed as a hypothetical model of map-based navigation relevant at migratory-bird spatial scales are so-called gradient maps (Wallraff, 2005), which are thought to be based on at least two gradients of any physical substrate, e.g., atmospheric odors or the earth’s magnetic field, which may vary systematically over large spatial scales. Utilizing a bi-coordinate gradient map, birds would learn how navigational cues change in some parameter across their familiar, experienced space. If a gradient parameter varies predictably beyond the familiar experienced space, a displaced bird could obtain an estimate of its position relative to a goal by implementing a neural algorithm comparing remembered values of stimulus at home with the measured values at the displacement site/current location.
Kramer’s Map and Compass Model
Kramer (1953) outlined what came to be called the “map and compass” model of homing pigeon navigation, which in principle would generalize to migratory birds. Kramer’s conceptual framework for understanding bird navigation remains the central explanatory paradigm in the field. He described navigation as a two-step process. In the first step, a displaced individual estimates its position in space relative to a goal, such as “I am west of the goal”. This step yields the direction to the goal as a bearing. In the second step, a compass mechanism is employed to identify the actual geographic direction of the goal-directed bearing, e.g., “That way is east.” The map and compass model has three defining characteristics: (1) a two-component process dependent upon a position-fixing system (map) and an independent compass mechanism; (2) the positioning system computes a bearing towards a goal; and (3) a biological compass is used to identify the direction of this goal-oriented bearing. For homing pigeons, the sun compass identifies the goal direction, and countless clock-shift experiments have been performed as demonstration. Importantly, those same clock-shift experiments also revealed that the map component remained intact, providing evidence that the position-finding component is independent of the compass component.
Bingman and Cheng (2005) described a hypothetical sequential model of migratory bird navigation which consisted of three phases each supported by three separate, hypothetical map mechanisms. Using a Le Conte’s sparrow (Ammodramus leconteii) as an example of a nocturnal songbird migrant, a hypothetical spring migration from wintering grounds in Florida, USA to breeding grounds in Saskatchewan, Canada could be described (Fig. 2). At a global scale, an experienced nocturnal migrant may first use a low-resolution global gradient map, perhaps based on the Earth’s magnetic field, to roughly determine its position relative to its goal location in Saskatchewan. A higher spatial resolution but shorter range olfactory gradient map of atmospheric odors could then guide the migrant in areas within a few hundred kilometers from the goal similar to the navigation of homing pigeons. Finally, an even higher spatial resolution but shorter range visual landmark or landscape map, operating within tens of kilometers from the intended goal, may help guide the final approach. The model is designed such that each “map” need only serve to provide enough spatial information to bring the migrant to within the ‘catchment’ area of the next phase map.
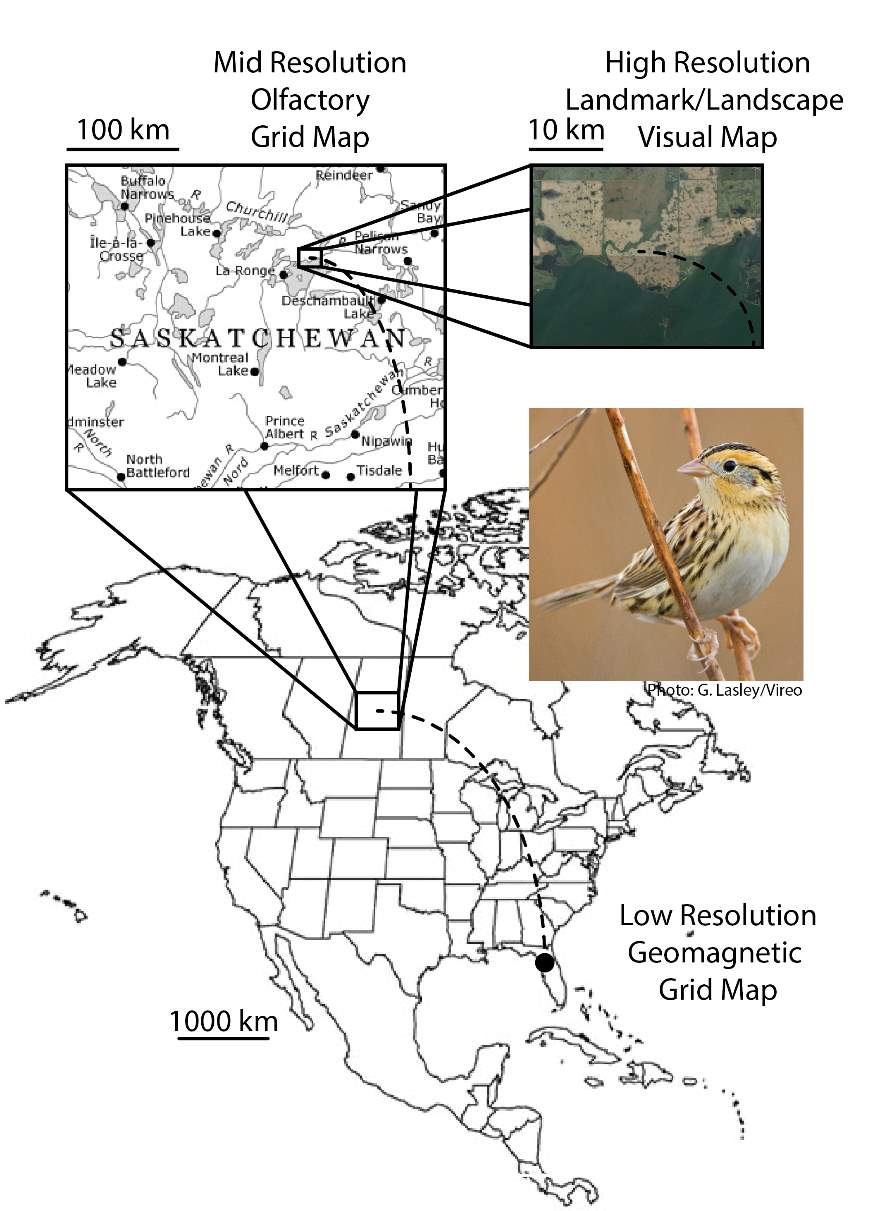
Fig. 2. Hypothetical migration map of a Le Conte’s sparrow (Ammodramus leconteii) using a phase model of global-navigational mechanisms operating at different spatial scales and resolutions. For illustrative purposes, an experienced nocturnal migrant begins its spring migration at wintering grounds in Florida, USA, and journeys to breeding grounds in Saskatchewan, Canada. See Bingman and Cheng (2005).
BRAIN ARCHITECTURE
Evolution of a sedentary or migratory phenotype, and the associated evolutionary adaptations for coping with or escaping the challenges of highly seasonal environments, are known to correlate with changes in brain organization (Fuchs, Winkler, Bingman, Ross, & Bernroider, 2014). Species which migrate longer distances typically have smaller brains than closely related sedentary species. Brain size may be energetically constrained by low food availability, developmentally constrained by short breeding seasons or restricted by bill adaptations, which can limit braincase volume. Small brain size may lead to migratory behavior to compensate for low behavioral flexibility or vice versa. Although generally larger brain volumes are observed in sedentary compared to long-distance migratory birds, long-distance migrants are observed to possibly have relatively larger dorsal pallial regions (hyperpallium) and hippocampus (Fuchs et al. 2014). The avian pallium tends to be relatively large, comprising ~75% of the telencephalic volume. The question arises as to whether the observed differences in brain organization can be explained by differences in developmental constraints or differences in behavioral adaptations. One possible explanation for smaller brains in long-distance migrants may be a consequence of a restricted developmental window available to juveniles. Comparisons of hatching date and onset of migratory restlessness in stone-chats (Saxicola sp.) clearly shows shorter time available for maturation in the longer-distance migrant populations (Helm, Gwinner, & Trost, 2005). These observations may account for differences in overall brain size, but cannot explain the sparing of the hyperpallium and hippocampus from volume reduction in migratory species. One functional explanation is that the hyperpallium is characterized by a visual area, which receives a strong input from the thalamus. A larger hyperpallium may correlate with high visual acuity as observed in mammalian visual cortex. Possible specialized functions such as visual control of the sun-compass and retinal-mediated, geomagnetic sensitivity hosted by the avian hyperpallium could be advantageous in migratory behavior (Bingman & Cheng, 2005).
The homology between the avian hippocampal formation and the mammalian hippocampus encourages the hypothesis that the avian hippocampus plays a fundamental role in navigation by migratory birds. The hippocampal formation of migratory species tends to be relatively larger compared to closely related sedentary species (Fuchs et al., 2014). For example, a larger and more microglial dense hippocampal formation was observed in a shorebird species that migrates over a visually complex landscape compared to another shorebird species that migrates over water (Diniz et al., 2016). The growing body of evidence which correlates hippocampus morphology to migratory behavior supports the hypothesis that the hippocampal formation houses some specialized spatial representational mechanism(s), which supports navigation during migration. Notable however, there is little evidence that the hippocampus is necessary for the operation of the sun compass in both homing pigeons and migratory savannah sparrows (P. sandwichensis;Bingman, Able, & Siegel, 1999). Lesions of the hippocampal formation in homing pigeons results in an impaired ability to use familiar landmarks and landscape for navigation including the carrying out of corrective re-orientations following displacement (Gagliardo et al., 2001). Based on the homing pigeon data it is reasonable to assume that migratory birds similarly rely on the hippocampal formation when navigating a familiar, local space (See Figure 2). The sparing (or even relatively larger) of hippocampus size reduction in migratory birds may be at least partially explained by having to hold multiple representations of local spaces (breeding, stop-over and overwintering sites).
CONCLUSIONS
Migratory birds are confronted with a variety of challenges, each requiring unique physiological, behavioral and cognitive adaptations. Even mundane problems such as selection of flight speed are affected by several factors operating simultaneously. The seemingly routine migration of birds belies its complexity. Migratory birds successfully carry out goal-directed flight of often thousands of kilometers or more over often diverse and unique landscapes. By employing a complex multisensory navigational system, migratory birds overcome challenges encountered such as inclement weather, winds, and migratory obstacles to regularly return year after year to within a few meters of the previous year’s breeding or wintering site. Migratory birds exhibit low neophobia, being less hesitant than resident species to enter novel environments, representing a personality trait that may be beneficial when encountering unfamiliar areas during migration. But migrants typically do not invest a lot of time, and consequently energy, exploring unfamiliar environments. Therefore, it is possible that migrants, which also rely heavily on long-term memory, may be at a disadvantage responding to rapidly changing environments, either climate or human-induced, within a single generation. Social interactions may aid many migratory species, which rely on learning and experience to perhaps rapidly alter their migration patterns when confronted with environmental change. Maintaining a diverse age structure within populations, which includes older and experienced migrants, may be critical in adapting to global change (Teitelbaum et al., 2016). To dampen the negative impact of environmental change on migrants, maintaining suitable habitats across the migratory journey is essential. Bird migration remains perhaps one of the most spectacular and mysterious wildlife events.
CROSS REFERENCES
Aves (Birds)
Cognitive Map
Compass Orientation
Homing
Migration
Passerine Cognition
Passerine Navigation
REFERENCES
Able, K. P. (2001). The concepts and terminology of bird navigation. Journal of Avian Biology, 32(2), 174-183.
Åkesson, S., & Hedenström, A. (2007). How migrants get there: migratory performance and orientation. BioScience, 57(2), 123-133.
Alerstam, T., & Pettersson, S. G. (1977). Why do migrating birds fly along coastlines?. Journal of Theoretical Biology, 65(4), 699-712.
Alerstam, T. (2001). Detours in bird migration. Journal of Theoretical Biology, 209(3), 319-331.
Alerstam, T., Hedenström, A., & Åkesson, S. (2003). Long‐distance migration: evolution and determinants. Oikos, 103(2), 247-260.
Alerstam, T. (2011). Optimal bird migration revisited. Journal of Ornithology, 152(1), 5-23.
Berthold, P., & Helbig, A. J. (1992). The genetics of bird migration: stimulus, timing, and direction. Ibis, 134(1), 35-40.
Berthold, P. (2003). Genetic basis and evolutionary aspects of bird migration. Advances in the Study of Behavior, 33, 175-229.
Bingman, V. P., Able, K. P., & Kerlinger, P. (1982). Wind drift, compensation, and the use of landmarks by nocturnal bird migrants. Animal Behaviour, 30(1), 49-53.
Bingman, V. P., Bagnoli, P., Ioalè, P., & Casini, G. (1984). Homing behavior of pigeons after telencephalic ablations. Brain, Behavior and Evolution, 24(2-3), 94-108.
Bingman, V. P., & Jones, T. J. (1994). Sun compass-based spatial learning impaired in homing pigeons with hippocampal lesions. Journal of Neuroscience, 14(11), 6687-6694.
Bingman, V. P., Able, K. P., & Siegel, J. J. (1999). Hippocampal lesions do not impair the geomagnetic orientation of migratory Savannah sparrows. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 185(6), 577-581.
Bingman, V. P., & Cheng, K. (2005). Mechanisms of animal global navigation: comparative perspectives and enduring challenges. Ethology Ecology & Evolution, 17(4), 295-318.
Blaser, N., Dell’Omo, G., Dell’Ariccia, G., Wolfer, D. P., & Lipp, H. P. (2013). Testing cognitive navigation in unknown territories: homing pigeons choose different targets. Journal of Experimental Biology, 216(16), 3123-3131.
Braunitzer, G., & Hiebl, I. (1988). Molecular aspects of high altitude respiration of birds. Hemoglobins of the striped goose (Anser indicus), the Andean goose, (Chloephaga melanoptera) and vulture (Gyps rueppellii). Die Naturwissenschaften, 75(6), 280-287.
Bruderer, B., Underhill, L. G., & Liechti, F. (1995). Altitude choice by night migrants in a desert area predicted by meteorological factors. Ibis, 137(1), 44-55.
Bruderer, B., & Liechti, F. (1998). Flight behaviour of nocturnally migrating birds in coastal areas: crossing or coasting. Journal of Avian Biology, 29, 499-507.
Buler, J. J., Moore, F. R., & Woltmann, S. (2007). A multi-scale examination of stopover habitat use by birds. Ecology, 88(7), 1789-1802.
Diniz, C. G., Magalhães, N. G. M., Sousa, A. A., Santos Filho, C., Diniz, D. G., Lima, C. M., Oliveira, M. A., Paulo, D. C., Pereira, P. D. C., Sherry, D. F., & Picanço-Diniz, C. W. (2016). Microglia and neurons in the hippocampus of migratory sandpipers. Brazilian Journal of Medical and Biological Research, 49(1). doi: 10.1590/1414-431X20155005
Emlen, S. T. (1967). Migratory orientation in the Indigo Bunting, Passerina cyanea. Part II: Mechanism of celestial orientation. The Auk, 84(4), 463-489.
Emlen, S. T. (1969). The development of migratory orientation in young indigo buntings. Living Bird, 8, 113-126.
Evans, P. R. (1966). Migration and orientation of passerine night migrants in northeast England. Journal of Zoology, 150(3), 319-348.
Fortin, D., Liechti, F., & Bruderer, B. (1999). Variation in the nocturnal flight behaviour of migratory birds along the northwest coast of the Mediterranean Sea. Ibis, 141(3), 480-488.
Fuchs, R., Winkler, H., Bingman, V. P., Ross, J. D., & Bernroider, G. (2014). Brain geometry and its relation to migratory behavior in birds. Journal of Advanced Neuroscience Research, 1, 1-9.
Gagliardo, A., Ioalè, P., Odetti, F., Bingman, V. P., Siegel, J. J., & Vallortigara, G. (2001). Hippocampus and homing in pigeons: left and right hemispheric differences in navigational map learning. European Journal of Neuroscience, 13(8), 1617-1624.
Gauthreaux Jr, S. A., Belser, C. G., & Welch, C. M. (2006). Atmospheric trajectories and spring bird migration across the Gulf of Mexico. Journal of Ornithology, 147(2), 317-325.
Greenberg, R. (1987). Seasonal foraging specialization in the Worm-eating Warbler. Condor, 89, 158-168.
Griffin, D. R. (1955). Bird Navigation. InA. Wolfson (Ed.), Recent Studies in Avian Biology (pp. 157-197). Urbana, IL: University of Illinois Press.
Helbig, A. J. (1991). Inheritance of migratory direction in a bird species: a cross-breeding experiment with SE-and SW-migrating blackcaps (Sylvia atricapilla). Behavioral Ecology and Sociobiology, 28(1), 9-12.
Horton, K. G., Van Doren, B. M., Stepanian, P. M., Hochachka, W. M., Farnsworth, A., & Kelly, J. F. (2016). Nocturnally migrating songbirds drift when they can and compensate when they must. Scientific reports, 6. doi: 10.1038/srep21249
Kramer, G. (1950). Weitere Analyse der Faktoren, welche die Zugaktivität des gekäfigten Vogels orientieren. Naturwissenschaften, 37, 377-378.
Kramer, G. (1953). Die Sonnenorientierung der Vogel. Verhandlungen der Deutschen Zoologischen Gesellschaft,1953, 72–84.
Liechti, F., & Bruderer, B. (1998). The relevance of wind for optimal migration theory. Journal of Avian Biology, 29, 561-568.
McWilliams, S. R., Guglielmo, C., Pierce, B., & Klaassen, M. (2004). Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. Journal of Avian Biology, 35(5), 377-393.
Mettke-Hofmann, C., & Gwinner, E. (2003). Long-term memory for a life on the move. Proceedings of the National Academy of Sciences, 100(10), 5863-5866.
Moore, F. R. (1987). Sunset and the orientation behaviour of migrating birds. Biological Reviews, 62(1), 65-86.
Mora, C. V., Davison, M., Wild, J. M., & Walker, M. M. (2004). Magnetoreception and its trigeminal mediation in the homing pigeon. Nature, 432, 508-511.
Mouritsen, H., Heyers, D., & Güntürkün, O. (2016). The neural basis of long-distance navigation in birds. Annual review of physiology, 78, 133-154.
Nolan, V., & Ketterson, E. D. (1991). Experiments on Winter‐site Attachment in Young Dark‐eyed Juncos. Ethology, 87(1‐2), 123-133.
O’Keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford, England, UK: Clarendon Press.
Papi, F. (1976). Olfactory navigation system of the homing pigeon. Verhandlungen der Deutschen Zoologischen Gesellschaft, 69, 184-205.
Papi, F., 1990. Olfactory navigation in birds. Experientia, 46(4), 352-363.
Pennycuick, C. J. (1975). Mechanics of flight. Avian biology, 5, 1-75.
Perdeck, A. C. (1958). Two Types of Orientation in Migrating Starlings, Sturnus yulgaris L., and Chaffinches, Fringilla coelebs L., as Revealed by Displacement Experiments. Ardea, 46(1–2), 1-2.
Ritz, T., Adem, S., & Schulten, K. (2000). A model for photoreceptor-based magnetoreception in birds. Biophysical journal, 78(2), 707-718.
Teitelbaum, C. S., Converse, S. J., Fagan, W. F., Böhning-Gaese, K., O’Hara, R. B., Lacy, A. E., & Mueller, T. (2016). Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nature Communications, 7. doi: 10.1038/ncomms12793
Thorup, K., Bisson, I. A., Bowlin, M. S., Holland, R. A., Wingfield, J. C., Ramenofsky, M., & Wikelski, M. (2007). Evidence for a navigational map stretching across the continental US in a migratory songbird. Proceedings of the National Academy of Sciences, 104(46), 18115-18119.
Wallraff, H. G. (2005). Avian navigation: pigeon homing as a paradigm. Berlin, Germany:Springer Science & Business Media.
Wiltschko, W. (1968). Über den Einfluß statischer Magnetfelder auf die Zugorientierung der Rotkehlchen (Erithacus rubecula). Zeitschrift für Tierpsychologie, 25, 537-558.
Wiltschko, W., & Wiltschko, R. (1995). Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. Journal of Comparative Physiology A, 177(3), 363-369.
Wiltschko, R., Munro, U., Ford, H., Stapput, K., & Wiltschko, W. (2008). Light-dependent magnetoreception: orientation behaviour of migratory birds under dim red light. Journal of Experimental Biology, 211(20), 3344-3350.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Environmental Studies"
Environmental studies is a broad field of study that combines scientific principles, economics, humanities and social science in the study of human interactions with the environment with the aim of addressing complex environmental issues.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




