Disinfection and Sterilisation and their Main Applications on Clinical Microbiology Laboratories
Info: 3339 words (13 pages) Dissertation
Published: 11th Dec 2019
Tagged: Health and SafetyMicrobiology
Clinical laboratories, regardless of their specialty, deal with clinical samples that are potentially infective on a daily basis as part of their work routine. In particular, clinical microbiology laboratories will more likely be exposed to infectious clinical samples in order to carry out the identification of pathogenic organisms. In order to handle these samples, the laboratory personnel not only need to be trained for this purpose but also the laboratory must have clear policies that ensure that the staff can work in a safe environment and perform their tasks adequately. These policies generally address protocols that aim at inhibiting microbial growth and making surfaces, items and equipments safe to handle, and most procedures can be grouped under the umbrella term “decontamination” (Madigan et al., 2015). They aim to care for the health of the workers and prevent contamination at the workplace and adjacent laboratories. Two of the most common decontamination procedures to reduce the exposure to infectious agents are disinfection and sterilisation. These are not new concepts in laboratories; historically, they have been used for over a century, being originated by Joseph Lister who introduced aseptic practices in the field of surgery (Mahon et al., 2015). In fact, disinfection is present in our daily lives in simple activities such as using chemicals to clean the house to prevent infections, whereas sterilisation is more restrict to laboratory environments. However, the terms disinfection and sterilisation are often used interchangeably, and even erroneously, despite having specific definitions that distinguish them. It is, therefore, fundamental to highlight the differences between these two concepts as well as their applications in clinical microbiology laboratories, as it will be covered next.
According to Ford (2014), the definition for disinfection states that it is “the process of destroying harmful micro-organisms at point of transmission” whereas the process of sterilisation “destroys all forms of microbial life”. These definitions imply that disinfection will render an item free of pathogenic microorganisms, although more resistant microbial forms may remain viable e.g. endospores, mycobacteria, viruses and fungi (Murray et al., 2016). Endospores are extremely resilient due to their spore coats which prevents the penetration of most chemical disinfectants (Madigan et al., 2015). Conversely, sterilisation is a categorical concept, that is, an object is either sterile or is not, given that this process can destroy all microorganisms, including sporicidal activity (Favero and Arduino, 2006). This can be proved by the “sterility assurance level”, which defines that the survival rate for microorganisms after an object has been subjected to sterilisation is less that one in one million (Favero, 1998, Favero, 2001, cited in: Favero and Arduino, 2006). The reason, however, that disinfection and sterilisation are sometimes interchanged derives from the fact that there can be different levels of disinfection: high, intermediate and low level disinfection – it was defined by the traditional microbial hierarchy by Spaulding (1957, cited in: McDonnell and Burke, 2011) and is still in use today (Figure 1). Spaulding determined the levels of disinfection and sterilisation and the infection risk posed when a medical device was used with a patient (McDonnell and Burke, 2011). These levels will differ in the killing activity spectrum by each disinfectant, in which high-level disinfection can generally meet sterilisation standards in terms of efficiency, whilst intermediate-level disinfection is not sporicidal, and a wider range of microorganisms can survive low-level disinfection (Murray et al., 2016).
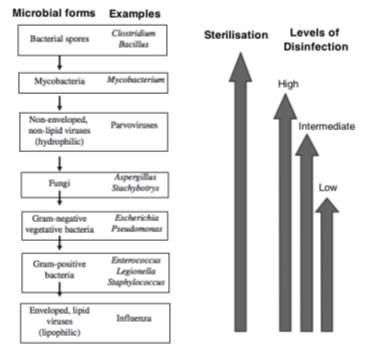
Figure 1. Sterilisation and levels of disinfection across the different microbial forms based on the Spaulding classification, with the decreasing microbial resistance hierarchy shown on the left (adapted from McDonnell and Burke (2011)).
The extent to which disinfection or sterilisation will be used mainly depends on the purpose of the decontamination; usually disinfection is used when sterilisation is deemed unnecessary, not attainable or impractical such as an inadequately sized object or if it cannot withstand sterilisation. However, sterilisation cannot be replaced for disinfection in all situations as it is a superior process which can have its effectiveness checked (HSE, 2013). It is also important to notice that prior to either process you need to clean any soiled items to maximise surface contact with the disinfectant/sterilant so as to avoid having microorganisms trapped within debris that will not be in contact with the disinfectant/sterilant, thus having the ability to keep multiplying and causing infection (Ford, 2014). Additionally, when choosing a disinfectant it must supersede the resistance to disinfection imposed by the pathogenic organisms (as shown above in Figure 1), as well as meeting other equally important factors: concentration, pH in which liquid disinfectant are most active, contact and exposure duration, bacterial load, presence of organic matter, the type of item to be disinfected and temperature (CDC, 2017, HSE, 2013, Ford, 2014).
There are several ways to accomplish disinfection; they range from physical to chemical methods. By far, chemical disinfection in the form of liquids is more common in laboratories (Mahon et al., 2015); the most common chemical agents being alcohols (ethyl, isopropyl), halogen disinfectants (chlorine compounds) and aldehydes (glutaraldehyde) (Ford, 2014, Murray et al., 2016). Alcohols wipes in high concentrations (e.g. 70-80%) are generally used in healthcare facilities, but they present major disadvantages: they do not possess sporicidal activity and they have a poor penetration on organic debris. As such, they are mostly used in surfaces that are already clean so as to maximise the disinfectant action and circumvent its quick evaporation (Ford, 2014, HSE, 2013). Chlorine compounds are still widely used today, usually in the form of liquid sodium hypochlorite found in household bleach, an inexpensive disinfectant in its dilutable form. Its microbicidal activity is due to the oxidative effects of hypochlorous acid which is formed when dissolved chloride ions are in water solution, thus conferring a broad microbicidal spectrum (Mahon et al., 2015). Other advantages of diluted sodium hypochlorite include its fast-acting activity, it is non-flammable, it is unaffected by hard water, reduces bacterial biofilms on surfaces and it is a relatively stable compound (Rutala and Weber, 2016). Despite all of these characteristics, it cannot be used as a sterilant due to a major drawback: it would require a long exposure time for it to be sporicidal and it can be inactivated by organic matter. Additionally, sodium hypochlorite poses a reaction hazard when in contact with acids and ammonias as well as production of trihalomethane, it leaves salt residues, is corrosive to metals, it causes discolouration and staining of fabrics and its odours may cause irritation at high concentrations (Rutala and Weber, 2016). As for glutaraldehyde, while it is an inexpensive and effective disinfectant, its use in the laboratory must be restricted due to its irritating effects on the respiratory tract and pungent odour, especially considering that there are other lower hazard alternatives available (HSE, 2013, Rutala and Weber, 2016). It is worth noting that glutaraldehyde (as well as other aldehydes, e.g. formaldehyde), can be used both as sterilants or high-level disinfectants, varying only on their aqueous concentration (Favero and Arduino, 2006, Murray et al., 2016).
The chemical agents mentioned above exert their bactericidal actions through different mechanisms, sometimes a combination of them, such as damaging the integrity of the cytoplasmic membrane, denaturation of cellular proteins, reaction with groups of enzymes containing a thiol (-SH) group and damage to RNA and DNA thus inhibiting the replication of microorganisms. And as a general rule, a disinfectant must be effective against the expected spectrum of microorganisms (Mahon et al., 2015). Whilst less common than chemical methods that employ these mechanisms, physical disinfection may be used when other methods are unavailable or not applicable. Moist heat disinfection can be achieved through boiling (to a 100oC temperature) and pasteurisation, the latter being more common in the food industry rather than clinical laboratories. These methods do not possess a sporicidal activity but they are valid alternatives for a minimum disinfection process (WHO, 2015, Mahon et al., 2015). At last, the simplest form of disinfection available in all laboratories must also be highlighted here: hand-washing. Even when handling biohazardous materials requires the use of appropriate gloves, this does not replace the thorough washing of hands by laboratory workers. Be it ordinary soap and water, germicidal soap or an alcohol hand rub, hand hygiene cannot be overemphasised (WHO, 2015). It must be noted, though, that the use of chemical agents for the disinfection of skin or other living tissues is classified under the concept of “antisepsis” (Murray et al., 2016). Such processes are worth mentioning in order to highlight that occasionally minimum disinfection processes can be sufficient for an effective prevention and control of infection, therefore they must not be discarded.
As with disinfection, sterilisation can also be achieved through a variety of different means; sterilants can be physical, gas vapours or chemicals. Heat (moist and dry heat) constitutes a physical method and is the most commonly used in clinical microbiology laboratories. In order to ensure the complete killing of microorganisms and particularly bacterial and fungal spores, it requires temperatures above 120oC over a set period of time given that any temperature drops can be critical to its efficiency (Murray et al., 2016). This can be exemplified by the use autoclaves (saturated steam under pressure), which are commonly found in most laboratories, as they employ moist heat dispersed through all of its contents thus penetrating all areas (including medical packing) for a complete microbicidal effect (Ford, 2014, Rutala and Weber, 2016). An added benefit is the fact that the autoclaving procedure can have its effectiveness easily monitored e.g. the use of commercial preparations of Bacillus stearothermophilus spores – if the procedure is successful, these spores are killed and fail to grow when incubated at 37oC (Murray et al., 2016). Therefore it is considered the most effective and reliable method for the sterilisation of laboratory materials; its main applications are the disposal of laboratory waste (e.g. clinical samples, culture plates) and to sterilise instruments and culture media (WHO, 2015, Ford, 2014). It is worth adding that any item that has been sterilised must be handled and stored in such manner as to keep them sterile until used. It has, however, a few disadvantages such as the potential for burns, it is inappropriate for heat-sensitive instruments as well as leaving instruments wet, which could lead to rusting (Rutala and Weber, 2016). Dry heat can also act as an sterilant, however it requires prolonged exposure times and higher temperatures than moist heat which could damage many instruments (Murray et al., 2016). Despite its drawbacks, it is still used in clinical microbiology laboratories for tasks such as sterilising glassware and inoculation loops, although the latter ones are being replaced by sterile plastic disposable loops in many laboratories (Ford, 2014, Mahon et al., 2015).
Examples of other physical sterilants include filtration, ultraviolet (UV) radiation and ionising radiation. Filtration is especially useful when your material cannot withstand heat; a pertinent example of that in a clinical microbiology laboratory is the production of selective media that requires the addition of antibiotics, which need to be filtrated so that it is not destroyed with the heat (Ford, 2014). Moreover, it is possible to filtrate both liquids and air; that can be attained through the use of membrane filters where the liquid can be pulled by either vacuum or pushed by pressure against the filter matrix and collected into a sterile vessel, whereas air filtration is accomplished with high efficiency particulate air (HEPA) filters (e.g. removal of microorganisms in biological safety cabinets for the manipulation of cell and microbial cultures) (Mahon et al., 2015, Madigan et al., 2015). Among the radiation methods, UV radiation has its applications mostly limited to the sterilisation of work surfaces and as “germicidal” UV lights on laminar flow hoods; that is due to the poor penetration properties of UV radiation (Madigan et al., 2015). Ionising radiation methods, conversely, are not so widely used in clinical laboratories due to the nature of the ionising source which is lethal to all cellular types (Ford, 2014).
Furthermore, gas vapours sterilants (e.g. ethylene oxide, hydrogen peroxide vapour, plasma gas) and chemical sterilants (e.g. peracetic acid and glutaraldehyde at different concentrations than that of disinfectants) are alternative sterilisation methods that are particularly important for heat and/or pressure-sensitive items. Gas vapours such as ethylene oxide, though, despite its high efficiency has its use strictly regulated due to the toxic gases generated during the sterilisation process as well as it being flammable and explosive. Similarly, chemical sterilants also have a limited use for sterilisation because of their toxicity to viable tissues thus making them less interesting methods for laboratories (Murray et al., 2016). Conversely, some extreme sterilisation methods like incineration may be necessary in clinical microbiology laboratories that work with prions, for example. These infective proteins cannot be eliminated using other conventional procedures, thus requiring burning them at temperatures ranging from 870oC to 980oC for the treatment of infectious waste. This method also involves the emission of toxic gases, however, limiting the use of incineration in some countries (Tille, 2014).
Each of the disinfection and sterilisation methods that have been discussed have their own particularities. The extent to which they will be used will vary in different clinical microbiology laboratories solely for the fact that laboratories will adopt methods that will suit their needs in terms of costs, practicality, type of microorganisms being handled and the level of exposure to them (Siani and Maillard, 2015). The expectations associated with each of these methods are based on the understanding of microbiology, in which disinfection and sterilisation can be claimed under the basis of passing established efficiency methods (e.g. the killing of Bacillus stearothermophilus spores after an autoclaving process) (McDonnell and Burke, 2011). However, it is worth noting that bacterial resistance mechanisms to disinfection have been documented (McDonnell, 2007, cited by: McDonnell and Burke, 2011). But these observations require closer examination, given that they might not necessarily have a relevant biohazard impact in the current disinfection practices, but it is worth emphasising that caution must be taken when choosing disinfectants as well as using them prudently to avoid further resistance with actual clinical implications (Meyer and Cookson, 2010, cited by: McDonnell and Burke, 2011).
In light of what has been discussed, understanding the principles of disinfection and sterilisation is of paramount importance for the implementation of appropriate biosafety procedures in clinical microbiology laboratories. These processes aimed at antimicrobial reduction have been proven useful for a wide array of applications, ranging from the disinfection of surfaces, air filtration on laminar flow hoods, sterilisation of loops and glassware, to the autoclaving of laboratory material and clinical waste. The specific methods which will be selected for each of these tasks will primarily depend on the activities that are being performed and the level of pathogenicity associated with the microorganisms being handled in the daily routine. Regardless of the methods used, the compliance to the decontamination protocols should be sufficient to ensure the safe conduct of work with biohazardous agents. In turn, these measures are beneficial not only for reducing the possibility of infections at the workplace, the general public and the environment but also by preventing cross-contamination in the laboratory.
REFERENCES
CDC 2017. Biosafety in Microbiological and Biomedical Laboratories. In: SERVICES, U. S. D. O. H. A. H. (ed.) Fifth ed.: Centers for Disease Control and Prevention.
FAVERO, M. 1998. Developing indicators for sterilization. In: RUTALA, W. A. (ed.) Disinfection, Sterilization and Antisepsis in Health Care. Champlain, N.Y.: Association for Professionals in Infection Control and
Epidemiology, Inc.
FAVERO, M. 2001. Sterility assurance: concepts for patient safety. In: RUTALA, W. A. (ed.) Disinfection, Sterilization and Antisepsis: Principles and Practices in Healthcare Facilities. Washington, D.C.: Association
for Professionals in Infection Control and Epidemiology, Inc.
FAVERO, M. S. & ARDUINO, M. J. 2006. Decontamination and Disinfection. In: FLEMING, D. & HUNT, D. (eds.) Biological Safety. Washington DC: American Society for Microbiology Press.
FORD, M. 2014. Medical microbiology, Oxford University Press.
HSE 2013. Safe working and the prevention of infection in clinical laboratories and similar facilities. In: EXECUTIVE, U. K. H. A. S. (ed.).
MADIGAN, M., MARTINKO, J., BENDER, K., BUCKLEY, D. & STAHL, D. 2015. Brock Biology of Microorganisms, Pearson Education.
MAHON, C. R., LEHMAN, D. C. & MANUSELIS, G. 2015. Control of Microorganisms. Textbook of Diagnostic Microbiology. Fifth ed.: Elsevier.
MCDONNELL, G. & BURKE, P. 2011. Disinfection: is it time to reconsider Spaulding? Journal of Hospital Infection, 78, 163-170.
MCDONNELL, G. E. 2007. Antisepsis, disinfection and sterilization: types, action and resistance, Washington, DC.
MEYER, B. & COOKSON, B. 2010. Does microbial resistance or adaptation to biocides create a hazard in infection prevention and control? J Hosp Infect, 76, 200-5.
MURRAY, P. R., ROSENTHAL, K. S. & PFALLER, M. A. 2016. Sterilization, disinfection and antisepsis. Medical Microbiology. Eight ed. Philadelphia, PA: Elsevier, Elsevier Health Science.
RUTALA, W. A. & WEBER, D. J. 2016. Disinfection, sterilization, and antisepsis: An overview. American Journal of Infection Control, 44, e1-e6.
SIANI, H. & MAILLARD, J.-Y. 2015. Best practice in healthcare environment decontamination. European Journal of Clinical Microbiology & Infectious Diseases, 34, 1-11.
SPAULDING, E. H. 1957. Chemical disinfection and antisepsis in the hospital. Journal of Hospital Research, 9, 5-31.
TILLE, P. M. 2014. Bailey & Scott’s Diagnostic Microbiology. Thirteenth ed. St. Louis: Mosby, Inc.
WHO 2015. Laboratory Biosafety Manual. Third ed.: World Health Organization.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Microbiology"
Microbiology is the study of microscopic organisms, or microorganisms. Microorganisms are simple life forms that include bacteria, fungi, algae, and viruses.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




