Drug Use and Misuse in Adolescents: Neural and Psychological Mechanisms
Info: 10313 words (41 pages) Dissertation
Published: 15th Feb 2022
Tagged: PsychologyYoung PeopleNeurology
Summary
It has long been established that adolescence is a time of ‘storm and stress’, tarnished by high rates of crime, high-risk behaviour and drug use and misuse. Less clear, however, is whether the heightened vulnerability to initiate drug use and development of addiction during adolescence is a direct result of this neurobiological developmental period. Changes in dopaminergic auto-receptor density and the protracted development of the prefrontal cortex are two examples of neural mechanisms that occur during adolescence, giving rise to sensation seeking behaviour and increased impulsivity, respectively. These enhanced behavioural traits are not only a consequence of the neurodevelopmental period in question, but genetics also have an impact.
Likewise, sensation seeking and impulsivity are not the sole contributing factors leading to drug use and abuse during adolescence. Psychological mechanisms are also prominent. These can be at a social level, encompassing the impact of peers, parents, and socioeconomic state.
Furthermore, there are also biological psychological mechanisms. Adolescence is a stressful time regarding romantic relationships, academic success, and body image for example. Initiation of drug use and development of addiction can result from habitual coping mechanisms established to deal with uncontrollable stress. Although innate neurobiological factors unique to adolescence increase vulnerability to drug use and misuse, psychological mechanisms are required for the transition of this vulnerability into a reality.
Introduction
Adolescence is “defined as the period of physical, psychological, and social transition between childhood and adulthood (Blakemore, 2008, p267). Furthermore, it is a period tarnished by stereotypes (Devlin & Ireland. , 2007) related to risk taking behaviour, drug abuse, and crime. ‘Drug Misuse: findings from the 2015/16 Crime Survey for England and Wales 2nd edition’ (Wingfield, Office for National Statistics, 2016)(manually reference) revealed 1:12 (8.4%) adults (aged 16-59) had taken illicit drugs in the previous year of the study, compared to 1:5 (18%) adolescents (aged 16-24). Additionally, the chance of developing a drug addiction (including that of alcohol) is considerably higher if initiation of drug use commenced before the age of 18 (1 in 4), in comparison to those initiating drug use after the age of 21 (1 in 25) (National Center on Addiction and Substance Abuse, 2011).
“Drug addiction is increasingly viewed as the endpoint of a series of transitions from initial drug use—when a drug is voluntarily taken because it has reinforcing, often hedonic, effects—through loss of control over this behaviour, such that it becomes habitual and ultimately compulsive” (Everitt & Robbins, 2005). I intend to focus specifically on the use of, and addiction to, drugs of abuse. These are substances taken non-medicinally, for example cocaine, nicotine, alcohol, and amphetamine, having a vast array of psychological and physiological effects. Despite this, a common neurobiological effect is the enhancement of dopaminergic signalling in the mesolimbic system (Di Chiara & Imperato, 1988).
The causes of enhanced likelihood of drug use and addiction during adolescence are vast. I will focus on sensation seeking and impulsive traits- both of which are prominent in adolescence, as well as being highly associated with initiation of drug use and transition to addiction (Belin, Mar, Dalley, Robbins, & Everitt, 2008). I will explore the neural underpinnings of sensation seeking traits and impulsivity, as well as potential impacts genetics can have on such. In terms of psychological factors, I will primarily explore the impact that social psychology such as peer interactions and socioeconomic status has on individuals. I will also look at the neurobiological changes associated with stress and how coping mechanisms through habit formation can be maladaptive. My intention is to decipher the significance that both nature and nurture, and the interaction between the two, have on adolescents’ impulsion to take drugs, and vulnerability to develop an addiction.
Impulsivity and sensation seeking traits - neurobiological factors
Sensation seeking
Sensation or novelty seeking is essentially the attraction towards and arousal over novel stimuli (Adriani, Chiarotti, and Laviola, 1998). This trait is typically assessed through observation of animal reactivity to novel environments. An experimental set up used by Adriani, Chiarotti, and Laviola (1998) involved placing mice in a white compartment with a wide meshed floor (‘familiar compartment’) for twenty minutes. After this time, the partition was removed and the mice had the freedom to remain in the ‘familiar compartment’ or move into a black compartment, with a narrow meshed floor (‘novel compartment’). Observations of periadolescent mice (33-43 days old) and adult mice (61-71 days old) were compared. Periadolescent mice spent more time and showed greater activity in the novel compartment compared to the adults, demonstrating novelty-seeking behaviour. This demonstrated the greater arousal and desire of adolescents to be exposed to novel stimuli.
Locomotor activity is a measure of arousal in novel environments. Rats with high sensitivity to novel environments are termed ‘high responders’ (Piazza, Deminière, Le Moal, & Simon, 1989). An early hypothesis of reward deficiency was used to explain the high sensation seeking traits of adolescents (Steinberg, 2008). This was thought to arise from an increase in the density of dopamine receptors in the prefrontal cortex relative to the mesolimbic system (Steinberg, 2008). Furthermore there is differential development of dopaminergic systems during adolescence. During adolescence, there is a dramatic reduction in glutamatergic inputs to the PFC (Spear, 2000). Consequentially, the pruning of some synapses frees up the potential for increases in other inputs to the PFC; this is demonstrated through a transient rise in dopaminergic and serotoninergic inputs (Spear, 2000). This increase in dopaminergic activity is evident in the PFC, but not the mesolimbic system. Dopaminergic concentration and fibre density in the PFC reaches a maximum during adolescence, before pruning to adult levels (Spear, 2000) (inverted U shape development). In contrast, the dopaminergic synthesis, turnover rates, and ultimately activity in the mesolimbic system is reduced in adolescents, compared to adults (Spear, 2000). This low level of dopaminergic activity in the mesolimbic system is thought to lead to high sensation seeking traits. One hypothesis is that sensation seeking is an adaptive measure to low levels of dopamine in the mesolimbic system (Spear, 2000). Adolescents “actively seek out not only addicting drugs, but also environmental novelty and sensation as a type of behavioural remediation of reward deficiency” (Gardner, 1999, p82, cited in Steinberg, 2008,).
An alternative hypothesis is that sensation seeking is a result of enhanced reward salience during adolescence (Steinberg, 2008), rather than a compensatory mechanism for reward deficiency. Enhanced reward salience is likely to stem from the low dopaminergic auto-receptor density during adolescence (Steinberg, 2008). Auto-receptors provide negative feedback, a regulatory control over dopaminergic release in response to rewards (Spear & Brake, 1983). A deficit in such a system would lead to accentuated dopamine release in response to a reward presented in adolescence, compared to in childhood and adulthood. Dopamine encodes prediction error (Schultz, 1998). Therefore, the greater rise in dopamine release in response to a rewarding stimulus would result in positive prediction error. The increase in salience of rewarding stimuli would be likely to lead to the enhanced sensation-seeking traits of adolescents (Steinberg, 2008).
Impulsivity
“In the broadest terms, impulsivity describes poor self- control, characterised by making decisions quickly, without forethought or regard for potential consequences” (Dalley & Roiser, 2012, p42) Impulsivity is typically coupled with risk taking ability; these however have distinct neurobiological underpinnings. Both traits come under the more general construct of cognitive control (Dalley & Robbins, 2017). This encompasses the ability to suppress maladaptive thoughts or behaviours, in preference of goal directed processes (Casey, Jones, & Hare, 2008).
Different areas of the brain have different profiles of development. The PFC provides top down control and has a protracted profile of development (Huttenlocher, 1979). The deficit in prefrontal functioning in adolescence, and consequential deficit in behavioural inhibition, is likely to give rise to increased impulsive behaviour (Casey et al., 2008). In comparison, the subcortical limbic system provides bottom up control and matures earlier than the PFC (Casey et al., 2008). Rather than having a linear profile of development, responses of the limbic system (e.g. nucleus accumbens, NAc) are particularly enhanced during adolescence (Steinberg, 2008). This observation is prominent during observation of or participation in high-risk activities. This is supported by the accentuated activity of the nucleus accumbens (NAc) when observing or partaking in high- risk activities in adolescence, in comparison to childhood and adulthood (Galvan et al., 2006). When taken together, immature cognitive control, as seen in adolescence, is likely to be the product of misbalance in the maturity and the functioning of the PFC and the limbic system (Casey et al., 2008).
Another aspect of neurobiological development influencing impulsive behaviour is the change in grey matter volume over time. This developmental profile has an inverted U shape that is shifted with respect to time for different areas of the brain (Gogtay et al., 2004). The PFC grey matter volume rises to a maximum around the time of puberty onset, 11.0 years for female and 12.1 years for males, before declining to adult levels (Giedd et al., 1999). There are connections between the limbic system and the PFC involved in cognitive control. Such connections are refined and strengthened through synaptic pruning (Casey et al., 2008). This involves the elimination of synapses that are no longer frequently active(Blakemore, 2008). The PFC is involved in inhibiting inappropriate behaviours (Arnsten, 2009). High grey matter volume of the PFC during early adolescence therefore reflects upon immature functioning of the PFC and highly impulsive behaviour. Functional imaging MRI (fMRI) supports this. PFC activation is more focussed in adulthood whereby pruning has taken place and cognitive control has improved (Casey et al., 2008).
In addition to the contribution that structural developmental changes in the brain make to high impulsivity in adolescence, changes in levels of particular neurotransmitters are also involved. Association between dopaminergic input and cognitive ability of the PFC is thought to follow an inverted U shape function (Cools & D’Esposito, 2011). The enhancement of dopaminergic input during adolescence would therefore result in diminished cognitive control.
Extensive studies have been carried out on individuals with Parkinson’s Disease (PD) and ADHD, both of which are characterised by dopaminergic dysfunction (Dalley & Roiser, 2012). Drugs that potentiate dopaminergic activity have opposing effects on the two groups of individuals. Where dopaminergic D2/3 agonists give rise to an increase in impulsivity in individuals with PD (Dalley & Roiser, 2012); methylphenidate (dopamine reuptake inhibitor) depresses such symptoms typical of ADHD (Dalley & Roiser, 2012). These contradictions allude to the complexity of this literature. It is likely that impulsivity is not due to dysregulation of the dopaminergic system alone, but rather a product of interactions between different neurotransmitters and other neurobiological factors (Dalley & Roiser, 2012).
In addition to the increase in dopaminergic activity, there is also a marked increase in serotonergic input into the PFC during adolescence (Romer, 2010). Cherek, Lane, Pietras, & Steinberg (2002) found that chronic administration of Paroxetine (selective serotonin reuptake inhibitor, SSRI) over 21 days lead to a shift in preference of a test group, from a small reward after a short delay to a larger reward after a longer delay. SSRIs increase [5-HT] in synapses (Dalley & Roiser, 2012), correlating with a decrease in impulsive response. The outcomes of this study must be treat with caution due to a small sample size. Despite this, such results are supported through tryptophan depletion experiments. Tryptophan is the precursor of 5-HT (Dalley & Roiser, 2012), thus tryptophan depletion reduces synthesis of such. Tryptophan depletion (reducing [5-HT]) is found, through numerous observations including that of diminished ability in the delay discounting task and impaired conditioned suppression, to increase impulsivity (Dalley & Roiser, 2012).
As discussed previously, both dopaminergic and serotonergic activity increases in adolescence. Since dopamine increases impulsivity, and serotonin decreases impulsivity, it would be predicted that effects would cancel out. This is not the case as the activity of both systems increase disproportionately and at different times during adolescence (Andrew Chambers, Taylor, & Potenza, 2003). The ratio of dopamine metabolite to serotonin metabolite is high during this developmental period, thus dopamine turnover must exceed serotonin turnover (Takeuchi et al., 2000). The misbalance in excitatory and inhibitory systems reflects upon the impulsive behaviour of adolescents that could render them vulnerable to addiction (Chambers et al., 2003).
Belin et al. (2008) investigated the significance of both sensation seeking and impulsivity in relation to addiction. To do so, they identified groups of high responder (HR) and low responder rats (LR) from investigation of locomotor reactivity of rats in a novel compartment. Groups of rats with high impulsivity (HI) and low impulsivity (LI) were also identified, through means of a 5- choice serial reaction time test (5-CSRTT). HR and LR rats showed no significant difference in impulsivity; likewise, HI and LI rats showed no significant difference in sensation seeking. This aids comparison to be made between the impacts of impulsivity and sensation seeking on the development of addiction. Three traits of addiction were considered: motivation to take drugs, inability to restrict drug usage, and compulsion to take drugs. Rats were exposed to 40 days of cocaine self-administration and the number of addictive traits identified. Where the majority of LI, HR and LR rats only showed 0-1 addictive traits; the proportion of HI rats showing all three outnumbered those with no addictive traits. Additionally, analysis of addicted individuals showed no significant difference between HR and LR groups in predicting vulnerability to drug addiction. This evidence suggests that initial experimentation with drugs may be a sensation seeking behaviour. Yet it is high impulsivity that is prominent in predicting vulnerability to addiction. Adolescents have high impulsivity and sensation seeking traits, thus have a double hit vulnerability.
Evidently, adolescence is a unique and complex neurobiological developmental period that all individuals must pass through. Despite this, not all individuals experiment with drugs, and among those that do, not all develop an addiction. Neurobiological changes giving rise to increased sensation seeking and impulsivity could act as catalysts. Although they increase the vulnerability to the initiation of drug use and transition to addiction, additional risk factors must also be necessary for both.
Genetics
Sensation seeking and impulsivity influence initiation of drug use and transition to addiction respectively (Belin et al., 2008). There will be additional contributions from risk-taking, environmental factors and stress responsivity (Kreek, Nielsen, Butelman, & LaForge, 2005). As well as having neurodevelopmental underpinnings, contributing factors will also have their own extensive genetic profile. This gives rise to the complexity regarding genetic contribution to initiation of drug use and addiction itself (Kreek et al., 2005). The following table outlines associations of candidate genes and addiction to particular drug(s). The mechanisms through which these are facilitated, at the level of impulsivity, risk- taking, the environment, stress responsivity and/or addiction are also provided.
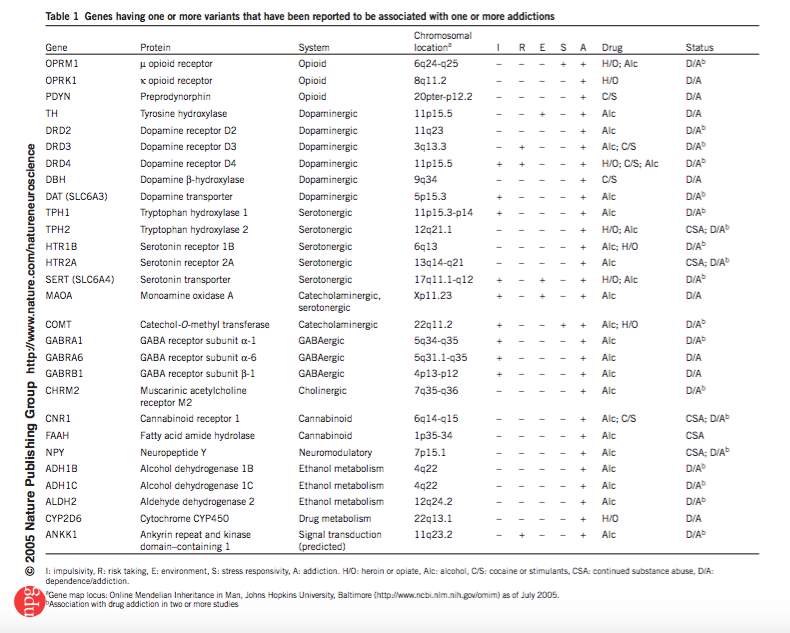
Figure 1: Kreek et al. (2005)
The above represents a number of disorder specific genes. In addition to this, studies also suggest that genes influencing externalising disorders in general are also associated with drug use and abuse (Dick & Agrawal, 2008). Although not unique to adolescence, genetics undoubtedly contributes to increased risk to drug use or abuse. The neurobiological susceptibility, alongside adverse external influences characteristic of adolescence could translate this increased risk into reality. Some individuals however do not succumb to drug use or addiction even in the presence of internal and external risk factors (Cadet, 2016). Such individuals are termed resilient (Cadet, 2016). Cadet outlines numerous genetic and epigenetic underpinnings to resilience. Epigenetic underpinning mechanisms included the maintenance of normal levels of G9a histone methyl-transferase in the NAc and H3K27 methylation in the presence of chronic stress (Cadet, 2016). In comparison, in susceptible animals, levels of both would be altered. These genetic and epigenetic changes offer protection against transition to drug use and misuse even in the vulnerable period of adolescence.
Social influences on adolescents - peers and parents
Some contributing factors to drug use and addiction in adolescence is beyond nature’s control. Puberty is not solely a period of hormonal and neurobiological changes, but there is also a shift in social interactions. Adolescents spend more time with, and are more heavily influenced by peers rather than parents. (Bradford Brown, & Larson, 2009). Additionally, the salience of peer interactions in adolescence is also increased (Gardner & Steinberg, 2005). Parental influence is not lost though, and still has an impact on modulating the risk-taking behaviours of adolescents (Steinberg, Fletcher, & Darling, 1994).
Peer influence on adolescent risk-taking behaviours/actions
Adolescents place a large emphasis on status and prestige (Brown et al., 2009). Popularity is a complex subject, particularly during adolescence. Popularity is not necessarily defined by the extent to which an individual is liked by peers (sociometric popularity), but may be determined by status or prestige assigned to an individual (perceived popularity) (Brown et al., 2009). Adolescents’ perception of peers is however often inaccurate. They frequently overestimate the actions of their peers, including their “involvement in antisocial behaviour, unhealthy, or maladaptive behaviour such as drug use, sexual activity, or inattentiveness to schoolwork” (Prinstein & Wang, 2005, cited in Brown et al., 2009, p78). They may be covertly influenced by the true or misperceived actions or behaviours of peers (Brown et al., 2009). Overt or covert peer pressure could lead to initiation of drug use or abuse.
The efforts of adolescents to impress peers and to fit into a particular social group can involve carrying out risk taking behaviour, for example drug use (Brown et al., 2009). Peer influence on risk taking behaviour during adolescence is supported by studies by Gardner and Steinberg (2005), and Chein, Albert, O’Brien, Uckert, & Steinberg (2011). Both experiments involved the ‘chicken’ or ‘stop light’ game. This game required the subject to watch a car move across a screen. When the traffic lights turn from green to amber, the subject can choose to stop the car or to continue driving- with the known risk of crashing if it does not cross the junction in time. The aim being to get as far as possible without crashing, in order to receive points (reward). The study by Gardner and Steinberg (2005) involved 106 adolescents (aged 13-16), 105 youths (aged 18-22), and 95 adults (aged 24+). The subjects each had to choose two friends to join the study. In one condition, all three subjects were in one room completing the game and were allowed to interact, and in the other condition the subject was alone. Results are shown below:
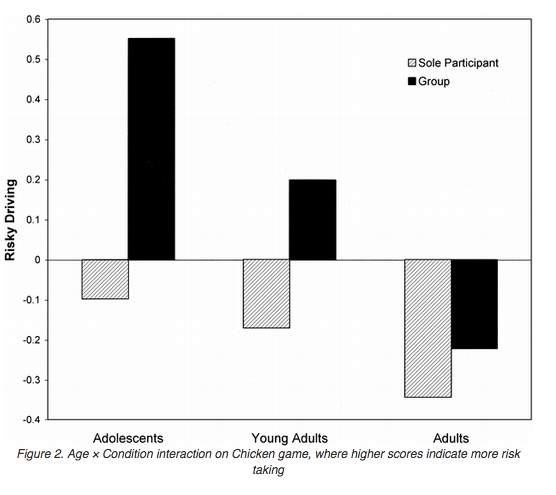
Fig. 2: Gardner and Steinberg (2005)
Measures including the number of car restarts per round and the percentage of time the car was in motion for were collated to assess risk-taking behaviour. Results showed that adolescents had the riskiest driving, in both conditions. Furthermore, the risk taking behaviour was accentuated in a group environment.
Chein et al. (2011) further investigated the influence of peers on risk taking behaviour of adolescents. The same experimental set up as Gardner and Steinberg was used in the study. It did however differ in that subjects in the peer condition were in the room alone but were told that peers were in the other room observing them. Unlike in the previous study, conclusions drawn were not the result of overt peer pressure on subjects but simply their observation and perceived judgement. The same conclusion was made from their results, that peer relationships become more salient in adolescence (Brown et al., 2009).
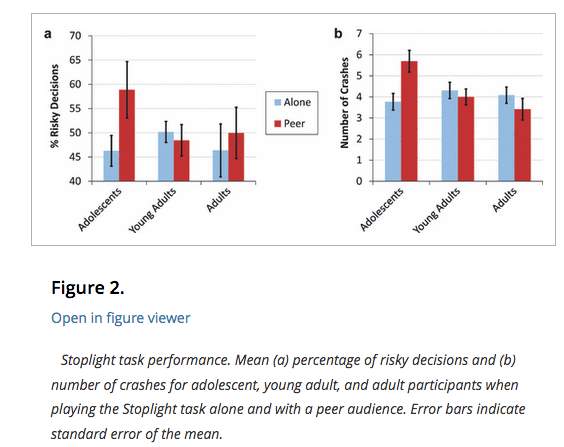
Fig. 3: Chein et al. (2011)
Results suggest that in the alone condition, the proportion of risky decisions is not significantly higher in adolescents, compared to young adults/adults. The peer condition does not have a significant effect on risky decision- making for young adults/adults, but does for adolescents.
Alongside the experiment, they carried out fMRI. They recorded the activity in the ventral striatum and the orbitofrontal cortex (OFC), both associated with incentive processing (Chein et al., 2011). Additionally, they analysed the activity in the lateral PFC (lPFC), associated with cognitive processing of decision-making (Chein et al., 2011). Not only did adolescents take more risks than adults, they also showed significantly greater activity in the ventral striatum and orbitofrontal cortex when doing so. The enhanced activation in these areas was only evident in adolescents (not adults) and solely in the peer condition. This suggests that the presence of peers sensitises adolescents to, and enhances the reward value of risk- taking behaviours. In comparison, the activation of the ventral striatum and the orbitofrontal cortex in adults remained at baseline throughout the experiment. Despite this, adults had increased activation (multiple sites of activation) of the lPFC when making decisions. The lPFC is mature in adulthood and insensitive to changes in the presence of peers (Chein et al., 2011). This could explain the enhanced ability of adults to override high- risk decisions, in favour of strategic ones.
The influence peers have on the risk taking behaviours of adolescents is bidirectional. Steinberg et al. (1994) carried out a longitudinal study across three school years, involving 6494 adolescents. He investigated the effect of peer influence and parental monitoring on substance use. Not all peer influence is negative. Females were found to be equally likely to increase or decrease drug use, depending on the nature of influence. Males, however, were more likely to be affected by negative peer interactions leading to drug addiction, compared to positive influences. A meta-analysis by Cross, Copping, & Campbell (2011) investigated sex differences in impulsivity. Findings were not highly conclusive, nor concordant between different types and measures of impulsivity. Despite this, it was found through questionnaires that males had higher sensation seeking and risk taking behaviour scores than females. This could bias males towards transition to drug use, regardless of the nature of peer and parental interactions.
Furthermore, the mentalising ability during adolescence is suboptimal and could also contribute to increased peer salience (Blakemore & Robbins, 2012). By the age of eleven, the understanding that others have distinct thoughts and feelings from themselves has been established, as well as ‘formal operational thought’. This allows for adolescents to carry out hypothetical and deductive reasoning (Elkind, 1967). Despite this, their ability to distinguish between hypothetical and actual thoughts of others is lacking. This could lead to the egocentric belief of adolescents that others are thinking about or judging their appearance or actions(Elkind, 1967). This concept of an ‘imaginary audience’ is associated with increased self consciousness. Additionally, this deficit in mentalising ability could contribute to a highly salient, but inaccurate influence of peers on decision making (Blakemore & Robbins, 2012).
Parenting influence on adolescent risk-taking behaviours/actions
Steinberg et al. (1994) found similar trends with respect to parental monitoring, decreasing drug use of female adolescents, with no significant effect on males. This is further supported by DiClemente et al. (2001) who found a negative correlation between parental monitoring and marijuana use and alcohol consumption. The study involved 512 black adolescents between the ages of 14-18, in a low- income environment. The niche selection criteria decrease the ability to generalise conclusions made. As well as the effects of parental monitoring, quality of parental attachment (Bahr, Hoffmann, & Yang, 2005), and other qualities of parenting styles (Baumrind, 1991) such as commitment, demandingness, and responsiveness can also influence adolescent substance use.
A study by Borawski, Ievers-Landis, Lovegreen, & Trapl (2011) found that parental monitoring, when considered in terms of a controlling social factor, only reduced a small number of high risk behaviours. Results showed that mutual respect and trust between parent and adolescent had a greater impact on their participation in health risk behaviours such as drug taking and unprotected sex. This trust could be demonstrated through negotiated unsupervised time between the parent and adolescent; this is when independence is given to the adolescent with some agreement between the parent and child on boundaries. Surprisingly, the likelihood of them experimenting with potentially high-risk activities under these circumstances was increased, but these activities were executed in a more responsible way.
Neurobiological development consists of a definite set of changes that occur during adolescence. In comparison, although the shift in salience of social interactions is well established, it is not fixed. Individuals’ social experiences will vary dramatically, in terms of quality, nature of interactions, and time spent with peers/parents. Any conclusions should therefore be taken with caution, and will not apply to all.
Environmental influences
As with peer and parental influences, environmental factors are not fixed for individuals but vary greatly. The scope for environmental factors that could influence the vulnerability of adolescents to develop addiction is vast. I will focus on effects of socioeconomic differences and stress on vulnerability to drug use and abuse.
Socioeconomic risk
As a general principle, a low socioeconomic status is characterised by low levels of education and employment, correlating with high levels of substance use and misuse (Mennis, Stahler, & Mason, 2016). Potential causes can be direct or indirect. At a simple level, the proximity of alcohol stockists to residential areas correlates with greater consumption and alcohol related deaths (Mennis et al., 2016). Disadvantaged areas have an excessive number of alcohol (Mennis et al., 2016) and tobacco (Mennis et al., 2016) stockists, compared to more affluent neighbourhoods. Not only does the ease of access to alcohol and tobacco stockists have an effect on consumption, the amount of advertisement for these in the area also has a negative impact (Mennis et al., 2016).
At a more fundamental level, the association between low socioeconomic status and substance use or abuse can be due to stress. Stress can be a very personal manifestation of unemployment for example and the consequential financial and social impact it may have (Redonnet, Chollet, Fombonne, Bowes, & Melchior, 2012). On a broader scale, disadvantaged areas tend to lack in quality of surroundings due to vandalism, noise, abandoned infrastructure and lack of green space for example (Mennis et al., 2016). This can have a negative impact on the mental wellbeing of a wider population (Mennis et al., 2016). Furthermore, the quality of services provided in disadvantaged areas can also be lacking; there are typically less recreation centres, libraries, medical and social services (Mennis et al., 2016). Social interactions and support are usually facilitated by such services, thus social cohesion in low-income areas is poor (Mennis et al., 2016). Social interactions are pivotal to coping with and relieving stress, thus the lack of such leads to a downwards spiral in mental wellbeing. Individuals could initiate drug use as a coping mechanism (Sinha, 2008).
There are however some contradictory findings within the literature. Alcohol consumption and marijuana use have been found to go against the trend. High level and frequency of consumption by adolescents is associated with high socioeconomic status (high parental education and income) (Humensky, 2010). There are many plausible explanations for this trend. It could simply be due to more disposable income being available to spend on alcohol and drugs (Humensky, 2010). Certain environments, such as universities, traditionally more accessible to individuals of higher socioeconomic status, can have a higher prevalence of drug use (Schulenberg & Maggs, 2002). This could be due to university culture, increased independence, and peer pressure within a novel community. Additionally, quality of interaction with parents and parenting techniques could also underlie such an association. It has been found that parents of high socioeconomic status were more likely to work longer hours and have less contact with or supervision of their children (Patrick et al., 2012). As a result perhaps, these parents are more lenient and inconsistent in parenting tachniques and punishment these parents are more permissibleless consistent with parenting techniques and punishment, thus are more permissible are also more likely to practice more permissible and inconsistent parenting techniques and methods of punishment(Patrick et al., 2012). All of which could facilitate drug use without parental consequences or controls.
Socioeconomic factors can have a negative impact on all age groups. When considering adolescents, these factors are simply one of many with the potential to initiate drug use within this particularly vulnerable group.
Stress during adolescence and coping mechanisms
Arnett reconsidered Hall’s (1904) view of adolescence being a time of ‘storm and stress’ (Arnett, 1999). The difficult nature of adolescence can be expressed in three key ways: conflict with parents, mood disruptions, and risk behaviour (Arnett, 1999). Concordant with all age groups, stress may arise from a major, or traumatic life event (Compas, Davis, Forsythe, & Wagner, 1987). Additional, prevalent origins of stress during adolescence include peer interactions and relationships (Bakker, Ormel, Verhulst, & Oldehinkel, 2010), perceived importance of body image (Siegel, Yancey, Aneshensel, & Schuler, 1999), and academic stress (Kaplan, Liu, & Kaplan, 2005). The origins of stress vary across countries and cultures (Arnett 1999), depending on which aspects of life are valued the most.
The coping mechanisms to deal with stress are determined by whether the stressor is controllable or uncontrollable. Coping with controllable stressors is an active, problem-based process, regulated at the level of the ventral- medial PFC (mPFCv), and the dorsal raphe nucleus is also inhibited (Amat et al., 2005). On the other hand, coping with uncontrollable stressors is a more passive, emotionally focused procedure at the level of the amygdala (Arnsten, 2009). Uncontrollable stressful stimuli induce deficits in PFC working memory (Murphy, Arnstent, Goldman-Rakict, & Roth, 1996) (Arnsten, 2009). The underlying mechanism behind this PFC function deficit is at the level of neurotransmitters.
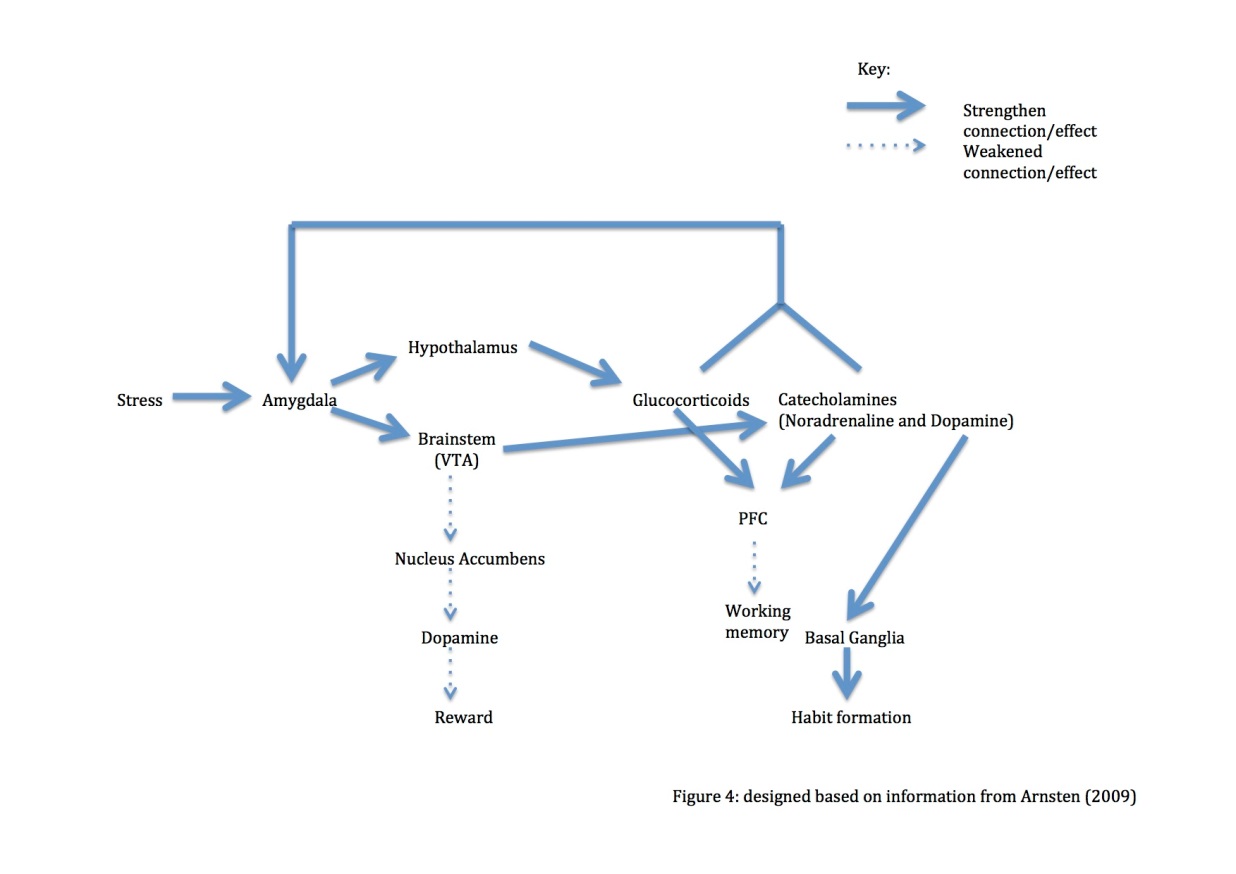
Fig. 4: diagram based on information derived from Arnsten (2009)
Uncontrollable stress up-regulates the activity of the amygdala. This results in increased levels of glucocorticoids and catecholamines. These act back upon and further strengthen amygdala functioning. High catecholamine input also depresses PFC function, manifesting as a deficit in working memory. Additionally, high levels of dopamine strengthen habit formation by the basal ganglia.
Use of habitual behaviours is a passive mechanism to cope with uncontrollable stress. Habits do not require much cognitive effort, thus cognitive efforts (Arnsten, 2009) can be focused on how to remove, avoid or reduce the stressor itself. Despite this, if coinciding with stress and high dopamine levels, maladaptive behaviours practiced could develop into habits (Arnsten, 2009). This could put individuals at risk of developing or relapsing into habitual behaviours such as drug addiction. Additionally, exposure to an uncontrolled stressor reactivates the salience of drug related cues; interestingly, stress may also promote craving in the absence of such cues (Sinha, 2008).
Opposite to the effects in the PFC, exposure to an uncontrollable stressor decreases dopamine in the NAc (Shirayama & Chaki, 2006). This would manifest as reward deficiency. The increase in dopamine in the mesolimbic system due to acute exposure to drugs will greatly exceed the dopaminergic response due to natural rewards (Di Chiara & Imperato, 1988). The positive prediction error produced by drugs will be enhanced. This would increase the incentive to take drugs in order to compensate for the reward deficit. The use of drugs as a coping mechanism can be maladaptive. Associating drugs as a mechanism to cope with stress can be a high- risk strategy, especially in the presence of chronic or reoccurring stress (Arnsten, 2009). This is likely to lead to increased drug use and contribute to increased vulnerability of adolescents to develop an addiction. In the presence of stress, the transition to addiction would also be aided by the increase in dopamine in the PFC (Arnsten, 2009), facilitating habit formation.
Uncontrollable stress facilitates a shift from goal directed behaviours to habitual behaviour for all ages. The PFC is not completely developed during adolescence (Huttenlocher, 1979), thus they would be more likely to cope with stress through habitual behaviours, rather than goal directed behaviours. As discussed, habits can be maladaptive, such as addiction.
Epigenetics
Epigenetics bridges the gap between environmental factors and the impact these have on the neurobiology of individuals and consequential vulnerability to initiate drug use or develop an addiction. It is the study of changes in gene function or activity through mechanisms independent to changing the sequence of DNA itself. Such modifications are transmissible through mitosis and/or meiosis (Jaenisch & Bird, 2003).“Epigenetic processes are essential for development and differentiation, but can also arise in mature humans and mice, either by random change or under influence of the environment” (Issa, 2000, cited in Jaenisch & Bird, 2003, p245).
Maternal and perinatal influences
Epigenetic modification is a broad term. The most stable form of epigenetic modification is DNA methylation. This modification has high specificity, involving addition of a methyl group at position 5 of the cytosine pyrimidine ring. This typically occurs at a position whereby cytosine and guanine are adjacent to one another (Wong, Mill, & Fernandes, 2011).
Cecil et al. (2016) identified 65 loci in which epigenetic variations (DNA methylation) underlie the increased vulnerability for individuals to develop addiction in adolescence. Five of these loci identified were associated with methylation quantitative trait loci (mQTLs), involved in regulation of methylation. Not all of the loci identified were linked to mQTLs, but also candidate genes PACSIN1, NEUROD4 and NTRK2, involved in neurodevelopment (Cecil et al., 2016). Interestingly, when mQTLs were excluded from data analysis, the risk value for association remained the same. This implies that DNA methylation patterns are not solely genetically influenced, but the environment can also lead to aberrant DNA methylation patterns.
Environmental factors that are associated with changes in DNA methylation at these loci involve maternal tobacco smoking (1st trimester), maternal risks (e.g. stress, psychopathology such as depression), and contextual risks (e.g. factors associated with low socioeconomic status)(Cecil et al., 2016). Exposure to stressors beyond utero can also cause epigenetic changes that could also have an impact on susceptibility to initiation of drug use and development of addiction in adolescence. These stressors include those of maternal separation, childhood abuse, or an early traumatic life event, such as a parental death (Cadet, 2016). Maternal tobacco smoking during pregnancy appeared to have the most significant impact on DNA methylation of the offspring epigenome and consequential increased chance of substance use in adolescence. Not only did this increase the likelihood of tobacco use in adolescence, but it also had an impact at a broader level, increasing the likelihood of using other drugs such as cocaine.
Participants for this study were selected from the ‘Avon Longitudinal Study of Parents and Children’, whereby measures of DNA methylation were taken at various ages. Information about substance use was also obtained through a questionnaire. A correlation between DNA methylation and drug use risk was only found with DNA methylation measures were taken in neonates, but not at age 7. This could reveal an experimental flaw whereby this difference could be due to the different tissue sources the DNA is obtained from; umbilical cord for neonates, in contrast to a sample of blood at age 7 (Cecil et al., 2016). It could alternatively demonstrate a critical period in early life in which epigenetic modifications due to maternal smoking for example must occur in order to have an impact on future drug use. Epigenetic modifications may not need to be maintained over time in order to have an effect, and by age 7 the critical period is likely to be exceeded (Cecil et al., 2016).
Evidentially, risk factors for development of addiction during adolescence do not have to occur during this developmental period. Epigenetic variations can be a result of exposure to external stressors or risk factors prenatally or perinatally. This programming is likely to increase vulnerability to develop an addiction, but may still require presence of external risk factors to facilitate this.
Influence of acute and chronic drug exposure on transcriptional and epigenetic changes
Epigenetic modifications increasing vulnerability to addiction can occur beyond utero.
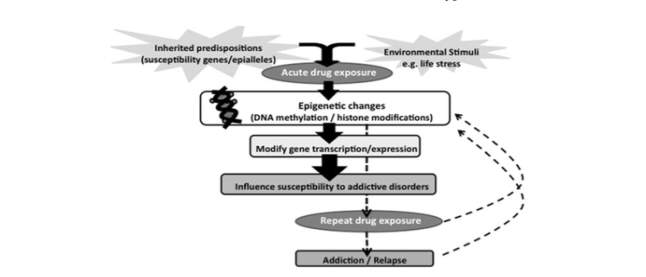
Figure 5: Wong et al. (2011)
Psychological and genetic predispositions increase susceptibility to initiate drug use. Acute drug exposure, through epigenetic changes, renders individuals at increased vulnerability to develop an addiction. Repeated acute drug use as a result cause further epigenetic changes. This perpetual cycle (dashed arrows) increases the likelihood to develop an addiction.
Genetic and epigenetic susceptibility, in conjunction with adverse environmental factors can increase the likelihood of acute drug use. I would extend this model further by adding that developmental vulnerability of high impulsivity and sensation seeking traits during adolescence also make important contributions. Furthermore, acute drug use leads to a more general alteration of gene expression. This can be facilitated through epigenetic mechanisms or through up-regulation or repression of transcription factors. I will outline these associations, alongside the effects that could increase vulnerability to further drug use and addiction.
**INSERT EPIGENETIC TABLE**- see other doc (excellent table!)
Figure 6:
Table adapted from Wong et al. (2011), using additional information from Robison and Nestler (2011), and Bowers (2010)
Future directions - treatments for addiction
Research shows that measures of high impulsivity correlate with poor response outcomes to treatment for addiction (Stevens et al., 2014). The studies included in the meta-analysis tested impulsivity of participants through tests, such as go/no go, stoop test, and immediate/ delayed memory task. They assessed the correlation between levels of impulsivity and treatment retention and drug abstinence. The treatments used in the studies were conventional pharmacological interventions and Cognitive Behavioural Therapy (CBT), and Contingency Management. High impulsivity correlated with short retention and low abstinence levels (Stevens et al., 2014). Adolescents typically have high levels of impulsivity, thus treatment success in this developmental period is unlikely to be successful. A different approach must be found, as one method of treatment evidently does not fit all (Stevens et al., 2014).
Due to the protracted development of the PFC (when does it fully mature?), impulse control is deficient compared to intellectual ability in adolescence. “Adolescents may be capable of making informed choices about their future…but do not yet have full capability to override impulses in emotionally charged situations that require decisions in the heat of the moment” (Casey et al., 2008, p.112). Treatment should therefore be focussed at reducing impulsive behaviours that could render adolescents vulnerable to drug use and misuse (Stevens et al., 2014). Such treatment options include Goal Management Training, and Mindfulness- based meditation (Stevens et al., 2014).
Intervention should be focussed at the level of prevention and protection. Stress is one psychological factor that could manifest in initiation of drug use as a coping mechanism (Sinha, 2008). Efforts should be made to mentor and promote more alternative, active methods of coping (Cadet, 2016). Successful mechanisms of coping could be gained from looking at resilient individuals (Cadet, 2016). Resilience does have a genetic and epigenetic basis, but could also be developed at a behavioural level. Identifying behavioural and cognitive factors contributing to resilience could allude to more potential ways to cope with stress and reduce the chance of initiating drug use or developing addiction to start with.
Conclusion
Statistics show disproportionately high prevalence of drug use and transition to addiction during adolescence. This is a product of neural and psychological risk factors, as well as interactions between the two. Adolescents have a distinct and unique neurobiological profile, giving rise to increased impulsivity and sensation seeking traits. Where sensation seeking is associated with initiation of drug use, impulsivity is involved in the transition to addiction. These traits are not sufficient in isolation to lead to drug use and abuse. Socio-psychological interactions must also be considered at the level of the individual and interactions made between them and their immediate surroundings (micro level). Furthermore, interactions between the microsystems (meso level) and, on a greater scale, the influence of culture and socioeconomic status (macro level) also have an impact (Bronfenbrenner, 1994)- manually in bibliography. The epigenetic modifications resulting from these potential stressors and influences are another contributing factor to the potential of drug use and addiction in adolescents.
Acknowledgments
Adriani, W., Chiarotti, F., & Laviola, G. (1998). Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience, 112(5), 1152–66. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9829793
Amat, J., Baratta, M. V, Paul, E., Bland, S. T., Watkins, L. R., & Maier, S. F. (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience, 8(3), 365–371. Retrieved from https://doi.org/10.1038/nn1399
Andrew Chambers, R., Taylor, J. R., & Potenza, M. N. (2003). Developmental Neurocircuitry of Motivation in Adolescence: A Critical Period of Addiction Vulnerability. Am J PsychiatryAm J Psychiatry, 1606(160), 1041–1052. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12777258
Arnett, J. J. (1999). Adolescent storm and stress, reconsidered. The American Psychologist, 54(5), 317–26. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10354802
Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–22. Retrieved from https://doi.org/10.1038/nrn2648
Bahr, S. J., Hoffmann, J. P., & Yang, X. (2005). Parental and Peer Influences on the Risk of Adolescent Drug Use. The Journal of Primary Prevention, 26(6), 529–551. Retrieved from https://doi.org/10.1007/s10935-005-0014-8
Bakker, M. P., Ormel, J., Verhulst, F. C., & Oldehinkel, A. J. (2010). Peer Stressors and Gender Differences in Adolescents’ Mental Health: The TRAILS Study. Journal of Adolescent Health, 46(5), 444–450. Retrieved from https://doi.org/10.1016/j.jadohealth.2009.10.002
Baumrind, D. (1991). The Influence of Parenting Style on Adolescent Competence and Substance Use. The Journal of Early Adolescence, 11(1), 56–95. Retrieved from https://doi.org/10.1177/0272431691111004
Belin, D., Mar, A. C., Dalley, J. W., Robbins, T. W., & Everitt, B. J. (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science (New York, N.Y.), 320(5881), 1352–5. Retrieved from https://doi.org/10.1126/science.1158136
Blakemore, S.-J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. Retrieved from https://doi.org/10.1038/nrn2353
Blakemore, S.-J., & Robbins, T. W. (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15(9), 1184–1191. Retrieved from https://doi.org/10.1038/nn.3177
Borawski, E. A., Ievers-Landis, C. E., Lovegreen, L. D., & Trapl, E. S. (2011). Parental Monitoring, Negotiated Unsupervised Time, and Parental Trust: The Role of Perceived Parenting Practices in Adolescent Health Risk Behaviors. Journal of Adolescent Health, 33(2), 60-70 https://doi.org/10.1016/S1054-139X(03)00100-9
Bowers, M. S. (2010). Activators of G-protein signaling 3: a drug addiction molecular gateway. Behavioural Pharmacology, 21(5–6), 500–13. Retrieved from https://doi.org/10.1097/FBP.0b013e32833dcfa5
Brown, B. B., Larson, J., Brown, B. B., & Larson, J. (2009). Peer Relationships in Adolescence. In Handbook of Adolescent Psychology. Hoboken, NJ, USA: John Wiley & Sons, Inc. Retrieved from https://doi.org/10.1002/9780470479193.adlpsy002004
Cadet, J. L. (2016). Epigenetics of Stress, Addiction, and Resilience: Therapeutic Implications. Molecular Neurobiology, 53(1), 545–60. Retrieved from https://doi.org/10.1007/s12035-014-9040-y
Casey, B. J., Jones, R. M., & Hare, T. A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–26. Retrieved from https://doi.org/10.1196/annals.1440.010
Cecil, C. A. M., Walton, E., Smith, R. G., Viding, E., McCrory, E. J., Relton, C. L., … Barker, E. D. (2016). DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Translational Psychiatry, 6(12), e976. Retrieved from https://doi.org/10.1038/tp.2016.247
Chein, J., Albert, D., O’Brien, L., Uckert, K., & Steinberg, L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1-10. Retrieved from https://doi.org/10.1111/j.1467-7687.2010.01035.x
Cherek, D. R., Lane, S. D., Pietras, C. J., & Steinberg, J. L. (2002). Effects of chronic paroxetine administration on measures of aggressive and impulsive responses of adult males with a history of conduct disorder. Psychopharmacology, 159(3), 266–274. Retrieved from https://doi.org/10.1007/s002130100915
Compas, B. E., Davis, G. E., Forsythe, C. J., & Wagner, B. M. (1987). Assessment of major and daily stressful events during adolescence: the Adolescent Perceived Events Scale. Journal of Consulting and Clinical Psychology, 55(4), 534–41. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3624609
Cools, R., & D’Esposito, M. (2011). Inverted-U–Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biological Psychiatry, 69(12), e113–e125. Retrieved from https://doi.org/10.1016/j.biopsych.2011.03.028
Cross, C. P., Copping, L. T., & Campbell, A. (2011). Sex differences in impulsivity: A meta-analysis. Psychological Bulletin, 137(1), 97–130. Retrieved from https://doi.org/10.1037/a0021591
Dalley, J. W., & Robbins, T. W. (2017). Fractionating impulsivity: neuropsychiatric implications. Nature Reviews Neuroscience, 18(3), 158–171. Retrieved from https://doi.org/10.1038/nrn.2017.8
Dalley, J. W., & Roiser, J. P. (2012). Dopamine, serotonin and impulsivity. Neuroscience, 215, 42–58. Retrieved from https://doi.org/10.1016/j.neuroscience.2012.03.065
Devlin, M., & Ireland. Equality Authority. (2007). Inequality and the stereotyping of young people. Equality Authority.
Di Chiara, G., & Imperato, A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America, 85(14), 5274–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2899326
Dick, D. M., & Agrawal, A. (2008). The Genetics of Alcohol and Other Drug Dependence Genetic Epidemiology of AOD Dependence. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3860452/pdf/arh-31-2-111.pdf
DiClemente, R. J., Wingood, G. M., Crosby, R., Sionean, C., Cobb, B. K., Harrington, K., … Oh, M. K. (2001). Parental monitoring: association with adolescents’ risk behaviors. Pediatrics, 107(6), 1363–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11389258
Elkind, D. (1967). Egocentrism in Adolescence. Child Development, 38(4), 1025. Retrieved from https://doi.org/10.2307/1127100
Everitt, B. J., & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–1489. Retrieved from https://doi.org/10.1038/nn1579
Galvan, A., Hare, T. A., Parra, C. E., Penn, J., Voss, H., Glover, G., & Casey, B. J. (2006). Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. Journal of Neuroscience, 26(25). Retrieved from http://www.jneurosci.org/content/26/25/6885
Gardner, M., & Steinberg, L. (2005). Peer Influence on Risk Taking, Risk Preference, and Risky Decision Making in Adolescence and Adulthood: An Experimental Study. Developmental Psychology, 41(4), 625–635. Retrieved from https://doi.org/10.1037/0012-1649.41.4.625
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Castellanos, F. X., Liu, H., Zijdenbos, A., … Rapoport, J. L. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. Retrieved from https://doi.org/10.1038/13158
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. Retrieved from https://doi.org/10.1073/pnas.0402680101
Huan, V. S., See, Y. L., Ang, R. P., & Har, C. W. (2008). The impact of adolescent concerns on their academic stress. Educational Review, 60(2), 169–178. Retrieved from https://doi.org/10.1080/00131910801934045
Humensky, J. L. (2010). Are adolescents with high socioeconomic status more likely to engage in alcohol and illicit drug use in early adulthood? Substance Abuse Treatment, Prevention, and Policy, 5, 19. Retrieved from https://doi.org/10.1186/1747-597X-5-19
Huttenlocher, P. R. (1979). Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Research, 163(2), 195–205. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/427544
Jaenisch, R., & Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics, 33(3s), 245–254. Retrieved from https://doi.org/10.1038/ng1089
Kaplan, D. S., Liu, R. X., & Kaplan, H. B. (2005). School related stress in early adolescence and academic performance three years later: the conditional influence of self expectations. Social Psychology of Education, 8(1), 3–17. Retrieved from https://doi.org/10.1007/s11218-004-3129-5
Kreek, M. J., Nielsen, D. A., Butelman, E. R., & LaForge, K. S. (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience, 8(11), 1450–1457. Retrieved from https://doi.org/10.1038/nn1583
Lader, D. (2016). Drug Misuse: Findings from the 2015/16 Crime Survey for England and Wales.
Mennis, J., Stahler, G. J., & Mason, M. J. (2016). Risky Substance Use Environments and Addiction: A New Frontier for Environmental Justice Research. International Journal of Environmental Research and Public Health, 13(6). Retrieved from https://doi.org/10.3390/ijerph13060607
Murphy, B. L., Arnstent, A. F. T., Goldman-Rakict, P. S., & Roth, R. H. (1996). Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Neurobiology, 93, 1325–1329. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8577763
National Center on Addiction and Substance Abuse at, C. U. (2011). Adolescent Substance Use: America’s #1 Public Health Problem. National Center on Addiction and Substance Abuse at Columbia University. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=eric&AN=ED521379&lang=ja&site=ehost-live
Patrick, M. E., Wightman, P., Schoeni, R. F., & Schulenberg, J. E. (2012). Socioeconomic status and substance use among young adults: a comparison across constructs and drugs. Journal of Studies on Alcohol and Drugs, 73(5), 772–82. Retrieved from https://doi.org/10.15288/JSAD.2012.73.772
Piazza, P. V, Deminière, J. M., Le Moal, M., & Simon, H. (1989). Factors that predict individual vulnerability to amphetamine self-administration. Science (New York, N.Y.), 245(4925), 1511–3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2781295
Redonnet, B., Chollet, A., Fombonne, E., Bowes, L., & Melchior, M. (2012). Tobacco, alcohol, cannabis and other illegal drug use among young adults: The socioeconomic context. Drug and Alcohol Dependence, 121(3), 231–239. Retrieved from https://doi.org/10.1016/j.drugalcdep.2011.09.002
Robison, A. J., & Nestler, E. J. (2011). Transcriptional and epigenetic mechanisms of addiction. Nature Reviews Neuroscience, 12(11), 623–637. Retrieved from https://doi.org/10.1038/nrn3111
Romer, D. (2010). Adolescent risk taking, impulsivity, and brain development: implications for prevention. Developmental Psychobiology, 52(3), 263–76. Retrieved from https://doi.org/10.1002/dev.20442
Schulenberg, J. E., & Maggs, J. L. (2002). A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. Journal of Studies on Alcohol. Supplement, (14), 54–70. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12022730
Schultz, W. (1998). Predictive reward signal of dopamine neurons. Journal of Neurophysiology, 80(1), 1–27. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9658025
Shirayama, Y., & Chaki, S. (2006). Neurochemistry of the Nucleus Accumbens and its Relevance to Depression and Antidepressant Action in Rodents. Current Neuropharmacology, 4(4), 277–291. Retrieved from https://doi.org/10.2174/157015906778520773
Siegel, J. M., Yancey, A. K., Aneshensel, C. S., & Schuler, R. (1999). Body image, perceived pubertal timing, and adolescent mental health. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 25(2), 155–65. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10447043
Sinha, R. (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141, 105–30. Retrieved from https://doi.org/10.1196/annals.1441.030
Spear, L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 24(4), 417–463. Retrieved from https://doi.org/10.1016/S0149-7634(00)00014-2
Spear, L. P., & Brake, S. C. (1983). Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology, 16(2), 83–109. Retrieved from https://doi.org/10.1002/dev.420160203
Steinberg, L. (2008). A Social Neuroscience Perspective on Adolescent Risk-Taking. Developmental Review : DR, 28(1), 78–106. Retrieved from https://doi.org/10.1016/j.dr.2007.08.002
Steinberg, L., Fletcher, A., & Darling, N. (1994). Parental monitoring and peer influences on adolescent substance use. Pediatrics, 93(6 Pt 2), 1060–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8197008
Stevens, L., Verdejo-García, A., Goudriaan, A. E., Roeyers, H., Dom, G., & Vanderplasschen, W. (2014). Impulsivity as a vulnerability factor for poor addiction treatment outcomes: A review of neurocognitive findings among individuals with substance use disorders. Journal of Substance Abuse Treatment, 47(1), 58–72. Retrieved from https://doi.org/10.1016/j.jsat.2014.01.008
Takeuchi, Y., Matsushita, H., Sakai, H., Kawano, H., Yoshimoto, K., & Sawada, T. (2000). Developmental Changes in Cerebrospinal Fluid Concentrations of Monoamine-Related Substances Revealed With a Coulochem Electrode Array System. Journal of Child Neurology, 15(4), 267–270. Retrieved from https://doi.org/10.1177/088307380001500415
Wong, C. C. Y., Mill, J., & Fernandes, C. (2011). Drugs and addiction: an introduction to epigenetics. Addiction, 106(3), 480–489. Retrieved from https://doi.org/10.1111/j.1360-0443.2010.03321.x
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Neurology"
Neurology is the specialist branch of medicine that deals with the treatment of disorders of the nervous system. This means that neurologists concern themselves with issues affecting the brain, the nerves, and the spinal cord.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




