Dissertation on Prescription of Exercise for Breast Cancer Survivors
Info: 6578 words (26 pages) Dissertation
Published: 12th Nov 2021
Tagged: Cancer
ABSTRACT
Purpose: Clinical guidelines recommend that breast cancer (BrCa) survivors be prescribed exercise. Healthcare providers often do not make these prescriptions citing multiple health conditions often found among cancer survivors which may impede survivors from safely completing home/community based exercise. This study evaluated the proportion and characteristics of breast cancer survivors who may need further evaluation prior to being prescribed home/community based exercise.
Methods: Participants included BrCa survivors treated in a university health care system between 2009-2014. We applied previously identified published guidelines for health conditions that may impede BrCa survivors from completing a home/community based exercise program. Logistic regression models were used to quantify the magnitude of association between demographic and clinical characteristics and the ability to perform home/community based exercise.
Results: Among 667 BrCa survivors, 65% to 75% were classified as able to complete home/community based exercise as recommended by the clinical guidelines. Older age, black race, treatment with chemotherapy, and treatment with radiation were associated with the potential need for further medical evaluation prior to starting exercise.
Conclusions: A large proportion of BrCa survivors can be prescribed home/community based exercise. Future research will need to determine how to identify the subset of BrCa survivors that may benefit from medical evaluation prior to starting exercise in a manner that doesn’t interrupt clinical oncology workflow. Approximately 35% of BrCa survivors may benefit from medical evaluation prior to starting home/community based exercise.
INTRODUCTION
Research that has emerged over the past 20-years demonstrates that exercise may improve multiple physiologic and psychological sequelae of breast cancer (BrCa) treatment [1-3], including cancer-related fatigue, cardiovascular fitness, muscular strength, physical function, and quality of life [3-7]. In addition, regular participation in physical activity may be associated with a lower risk of BrCa recurrence and death [8-12]. The American College of Sports Medicine (ACSM), American Cancer Society (ACS), and National Comprehensive Cancer Network (NCCN) clinical practice guidelines recommend all cancer survivors to perform: 1) 150-minutes of moderate-intensity aerobic activity or 75-minutes of vigorous-intensity aerobic activity per week; 2) 2–3 weightlifting or muscle strengthening sessions per week; and 3) neuromuscular and flexibility activities on days of exercise [1, 13, 14]. Exercise possesses pharmacological properties like a medicine, and is most efficacious when prescribed with the appropriate volume, intensity, and modality to improve a specific health outcome [3, 15].
Despite clinical recommendations that encourage all BrCa survivors to engage in regular exercise [1, 13, 14], healthcare providers may have reservations about prescribing exercise to their patients due, in part, to competing health conditions often found among cancer survivors, such as lymphedema, cardiovascular disease, and cancer-related fatigue [16]. Consequently, only one-in-five of BrCa survivors report that their healthcare provider has offered recommendations about engaging in healthy lifestyle behaviors, such as participating in regular physical activity [17]. Similar exercise prescribing patterns are observed among colorectal and endometrial cancer survivors.
The current infrastructure in oncology often results in cancer survivors participating in exercise with minimal guidance and limited or no supervision [18]. BrCa survivors with multiple health conditions may require different volumes, intensities, and modalities of exercise to safely and effectively improve health outcomes. Healthcare providers may benefit from a standardized approach to identify BrCa survivors for whom it may be appropriate to safely prescribe a community/home-based exercise program that is consistent with the ACSM/ACS/NCCN clinical guidelines. Prior studies have estimated that as few as 20% of colorectal cancer survivors and 15% of endometrial cancer survivors could be prescribed a community/home based exercise program without the potential need for further medical screening [19, 20]. However, no study has quantified the proportion of BrCa survivors that can be prescribed a community/home based exercise program.
METHODS
Study Sample
Participants were eligible if they were: aged ≥21 years; diagnosed with BrCa (International Classification of Disease, 9th Revision [ICD-9]: 174.*); and received surgery for BrCa in the University of Pennsylvania Health System (UPHS) between the years of 2009 and 2014. Participants were also required to have a subsequent visit approximately six-months (± three-months) after completion of their cancer-directed therapies (with exception of hormonal or targeted treatment). We excluded women who were aged ≥90 years; with metastatic disease; or without the requisite health information. Data were abstracted from UPHS electronic medical records. This study was approved by University of Pennsylvania Institutional Review Board.
Primary Dependent Variable
Cancer survivors may be most likely to adopt recommendations about healthy lifestyle behaviors six-months after completing their primary cancer-directed therapies [21]. Six-months is suggested because it is the shortest interval of time that allows the acute symptoms of cancer treatment to subside while survivors are still receptive to learning, and adopting new healthy lifestyle practices [19, 20].
In order to develop a detailed list to define whether survivors would be able to perform unsupervised exercise after a cancer diagnosis (Appendix Table 1, [19]), a review of previously published clinical recommendations for exercise was conducted that identified potential health conditions that may suggest the need for further medical evaluation [cite]. This list has been applied in colorectal and endometrial cancer survivors previously [19, 20]. The health conditions were classified into ten system-specific categories (Table S1). The identification of one or more relevant health conditions signified the potential need for further medical evaluation. For this analysis, if no relevant health conditions were identified, the ability to perform community/home based unsupervised exercise was assumed.
Data abstraction
Data abstracted from electronic medical records included demographic information, including age and race. Clinical information included stage (American Joint Committee on Cancer Staging Manual, Seventh Edition), and cancer-directed therapies. Measures for cancer care were collected at six-months after completing cancer-directed therapies (with exception of hormonal or targeted treatment) using ICD-9 or procedure codes listed in the electronic medical record.
Covariates
Age was calculated at the time of cancer diagnosis. Race was categorized as white, black, or other. Cancer-related characteristics included cancer stage (Ductal carcinoma in situ (DCIS), I, II, III) [22], type of cancer-directed therapy (radiation and chemotherapy), type of surgery (lumpectomy vs mastectomy), reconstructive surgery, and the Charlson comorbidity index [23].
Statistical Analysis
Using the abstracted electronic medical record data, a composite outcome defined as the sum of all health conditions was generated and then categorized into BrCa survivors that had zero versus one or more health conditions(s). Values of zero signified the ability to perform community/home based exercise. Values of one or more signified the potential need for further medical evaluation. Means and standard deviations, and t-tests were used to describe and compare continuous variables. Frequencies and proportions, and chi-square tests were used to describe and compare categorical variables. Univariate logistic regression and multivariable logistic regression models were used to quantify odds ratios (OR) and 95% Confidence Intervals (CIs). For all covariates, we had ≥80% statistical power to detect odds ratios 1.6. We also conducted sensitivity analyses that excluded common health conditions among BrCa survivors including hypertension, diabetes, arthritis, morbid obesity (Body Mass Index ≥40 kg/m2), and hyperlipidemia [24, 25]. P
RESULTS
A total of 1,520 BrCa survivors were identified with having the requisite six-month follow up data. Among those ineligible: 93 had stage IV (metastatic) BrCa; 449 had missing BMI measures; three were ≥90 years; and 308 were excluded due to missing baseline treatment-related characteristics. Overall, 667 BrCa survivors met all inclusion criteria. BrCa survivors included in the analytic sample were younger (55.7±12.3 vs 58.0±12.3; P
Among the 667 survivors who met all inclusion criteria, the mean age at diagnosis was 55.0±12.3 years. Age ranged from 24 to 89 (Table 1). Most women were white (74%); 19% had ductal carcinoma in situ (DCIS); 47.1% had stage I disease; 25% had stage II disease; and 9% stage III disease. Forty-three percent were treated with breast conserving therapy (lumpectomy); 44% were treated with chemotherapy; 39% were treated with radiation; and approximately 30% completed reconstructive surgery.
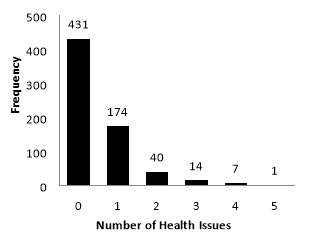
The prevalence of individual and system-specific health conditions, which may suggest the potential need for further medical evaluation, varied widely (Table 2). Health conditions with the highest prevalence included hypertension (10.6%), morbid obesity (7.5%), thyroid disease (6.7%), anemia (6.0%), and diabetes (4.2%). The median number of health conditions was zero and ranged from 0 to 5 (Figure 1 (a)). Sixty-five percent of the study population had no identified health conditions, 26.1% had one, 6% had two, and 3% had three or more of the selected health conditions.
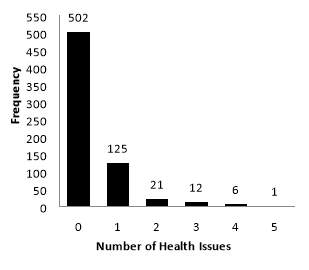
In sensitivity analysis, we excluded health conditions common among BrCa survivors including hypertension, diabetes, arthritis, morbid obesity (BMI ≥40 kg/m2), and hyperlipidemia. In this sensitivity analysis, the median number of health conditions was zero and ranged from 0 to 5 (Figure 1 (b)). Seventy-five percent of the study population had no identified health conditions, 18.7% had one, 3.1% had two, and 2.8% had three or more of the selected health issues.
Older age, black race, treatment with chemotherapy, and treatment with radiation were variables associated with being less likely to be able to participate community/home-based exercise in the univariate logistic regression model (Table 3). Conversely, having a mastectomy or having reconstructive surgery was independently associated with being more likely to be able to perform community/home-based unsupervised exercise. In multivariate-adjusted logistic regression, older age (OR: 0.98, 95% CI: 0.96-0.99; P =0.015), black race (versus white) (OR: 0.44, 95% CI: 0.30-0.64; P
DISCUSSION
The principal finding of this study is that approximately 65% of BrCa survivors may be able to participate community/home-based exercise at the dose suggested by the ACSM/ACS/NCCN clinical guidelines, six-months after completion of their cancer-directed therapies. A second important finding is that BrCa survivors who were older, of black race, were treated with chemotherapy or radiation were more likely to potentially need further medical evaluation prior to engaging in community/home-based exercise. These results in BrCa complement the previously-described relationship examining the ability to prescribe community/home-based exercise among endometrial and colorectal survivors [19, 20]. The discussion herein will compare and contrast our results with these prior studies [19, 20], and review the clinical implications of our new findings.
Our finding that 65% of BrCa survivors could be able to participate in community/home-based exercise is in contrast to prior studies that suggest as few as 14% of endometrial and 21% of colorectal cancer survivors could be able to participate community/home-based exercise [19, 20]. Potential explanations for these differences might include differences in patient age and BMI. The median age at the diagnosis of colorectal cancer is 71 years [26], which is 15 years older than the median age in our sample. Older age is often accompanied by an increased burden of comorbid health conditions among cancer survivors [27]. Therefore, contrasts in age of cancer diagnosis may explain, in part, the differences in estimates between BrCa and colorectal cancer survivors. A higher BMI is associated with an increased risk for endometrial cancer development; consequently, up to 38% of endometrial cancer survivors are morbidly obese (BMI≥35 kg/m2) [28]. This contrasts with our data, where 7.5% of BrCa survivors were morbidly obese. In our sample, older age was associated with a lower likelihood of being able to participate in community/home-based exercise. This is consistent with previous findings in colorectal and endometrial cancer [19, 20]. However, an unanticipated finding that was not observed in colorectal and endometrial cancer was that black BrCa survivors were 56% less likely to be able to participate community/home-based exercise program compared to white BrCa survivors. This observation is consistent with prior reports that suggest black BrCa survivors may have more comorbid health conditions at the time of their BrCa diagnosis compared to white BrCa survivors [29].
As outlined in the introduction, there are multiple established benefits of exercise among BrCa survivors. Observational studies suggest that increased volumes of post-diagnosis physical activity may be associated with a lower risk of BrCa recurrence and BrCa specific mortality [4, 8]. In addition, randomized clinical trials have demonstrated that exercise improves bone health, cardiovascular fitness, and preserves physical functioning [30-33]. Randomized trials have also demonstrated that exercise reduces cancer-specific side effects such as musculoskeletal symptoms from hormonal breast cancer therapies, lymphedema, and cancer-related fatigue [6, 30, 34].
Given the above-described benefits of exercise for BrCa survivors, it is important that healthcare providers encourage their patients to participate in regular exercise. The provider recommendation is reported by patients as one of the biggest catalysts to promote behavior change [35]; however only 25% of providers recommend exercise to their patients [17]. Healthcare providers often cite the lack of an infrastructure to refer patients [36], and patients often cite uncertainty about what types of exercise are safe, and how to commence or sustain an exercise program [35]. Although 65% of BrCa survivors may be able to participate community/home-based exercise, it is noteworthy that 35% of BrCa may require additional resources to enable their safe participation in an exercise program. Thirty-five percent of the approximate three-million BrCa survivors currently living in the United States translates to approximately one-million women for whom there may be value for further medical evaluation prior to engaging in community/home-based exercise [37, 38]. Given the large number of BrCa survivors (and other types of cancer survivors) who may benefit from referral, there exists an urgent need to train health and fitness professionals with the knowledge, skills, abilities, and sources that are necessary to safely individualize and implement exercise programs that are appropriate for the unique needs of cancer survivors. Our data can also be leveraged to guide healthcare providers in the identification of the subset of patients who may benefit from further medical evaluation prior to engaging in community/home-based exercise.
There are several limitations that should be acknowledged to add context to our findings. One major limitation is the potential lack of generalizability. This is a cross-sectional study in one university healthcare system. Estimates for the prevalence of common health issues such as hypertension and diabetes were lower in our sample population than estimates found in prior studies using national data matched for age and race [39]. These discrepancies may be attributed to the observation that the included hospitals are large tertiary care centers, and the characteristics of BrCa survivors that are treated in this health system may not reflect characteristics of BrCa survivors treated in the community setting across the United States. Additionally, although all necessary information required for the ACSM/ACS/NCCN guidelines for exercise prescription was available in this population, our analyses were limited to what was found in the electronic medical record. It is therefore possible that common, but non-malignant health issues were not systematically recorded in the electronic medical record. Our exploratory logistic regression models had sufficient statistical power to detect effects as small as 1.6. It is plausible that smaller, yet still clinically important effects may have been missed. Another limitation is that over 50% of our sample size were lost to exclusion criteria. The women in the analytic sample were significantly younger. The younger age of our analytic sample may make our estimates conservative. Based on these limitations, it is plausible that our results may overestimate the percentage of BrCa survivors for whom oncologists could safely prescribe community/home based exercise.
Conversely, there are several strengths to this study such as a large sample size, which allowed us to have sufficient statistical power to examine demographic and clinical correlates. Our sample included 28% non-white BrCa survivors, which improves the demographic generalizability of our sample to the broader population of BrCa survivors in the United States. Our data were abstracted from the electronic medical record following a systematic search process that has been validated [19, 20].
In conclusion, our findings suggest that 65% to 75% of BrCa survivors may be able to participate community/home-based exercise at the dose suggested by the ACSM/ACS/NCCN clinical guidelines, six-months after completing cancer-directed therapies. BrCa survivors who were older, of the black race, received chemotherapy, and received radiation, may benefit from further medical evaluation prior to engaging in community/home-based exercise. Although the prescription of exercise to cancer survivors is complex and multifaceted, beyond that of the presence or absence of comorbid health conditions, these data are the first steps in providing support for continued research to empirically determine the efforts necessary to safely prescribe exercise to the three-million BrCa survivors living in the United States.
REFERENCES
1. Ligibel, J.A. and C.S. Denlinger, New NCCN guidelines® for survivorship care. Journal of the National Comprehensive Cancer Network, 2013. 11(5S): p. 640-644.
2. Courneya, K.S., et al., Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Journal of Clinical Oncology, 2007. 25(28): p. 4396-4404.
3. Irwin, M. and A.C.o.S. Medicine, ACSM’s guide to exercise and cancer survivorship. 2012: Human Kinetics.
4. Holmes, M.D., et al., PHysical activity and survival after breast cancer diagnosis. JAMA, 2005. 293(20): p. 2479-2486.
5. Schmitz, K.H., et al., Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. Jama, 2010. 304(24): p. 2699-705.
6. Schmitz , K.H., et al., Weight Lifting in Women with Breast-Cancer–Related Lymphedema. New England Journal of Medicine, 2009. 361(7): p. 664-673.
7. Denlinger, C.S. and P.F. Engstrom, Colorectal Cancer Survivorship: Movement Matters. Cancer Prev Res (Phila), 2011. 4(4): p. 502-11.
8. McTiernan, A., et al., Recreational physical activity and the risk of breast cancer in postmenopausal women: The women's health initiative cohort study. JAMA, 2003. 290(10): p. 1331-1336.
9. Moradi, T., et al., Physical activity and risk for breast cancer a prospective cohort study among Swedish twins. International journal of cancer, 2002. 100(1): p. 76-81.
10. Colditz, G., et al., Physical activity and risk of breast cancer in premenopausal women. British journal of cancer, 2003. 89(5): p. 847-851.
11. Luoto, R., et al., The effect of physical activity on breast cancer risk: a cohort study of 30,548 women. European journal of epidemiology, 2000. 16(10): p. 973-980.
12. Lee, I.-M., et al., Physical activity and breast cancer risk: the Women’s Health Study (United States). Cancer Causes & Control, 2001. 12(2): p. 137-145.
13. Schmitz, K.H., et al., American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc, 2010. 42(7): p. 1409-26.
14. Rock, C.L., et al., Nutrition and physical activity guidelines for cancer survivors. CA: a cancer journal for clinicians, 2012. 62(4): p. 242-274.
15. Naci, H. and J.P.A. Ioannidis, Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ-BRITISH MEDICAL JOURNAL, 2013. 347: p. f5577.
16. Bluethmann, S.M., A.B. Mariotto, and J.H. Rowland, Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiology Biomarkers & Prevention, 2016. 25(7): p. 1029-1036.
17. Sabatino, S.A., et al., Provider Counseling about health behaviors among cancer survivors in the United States. JOURNAL OF CLINICAL ONCOLOGY, 2007. 25(15): p. 2100-2106.
18. Brown, J.C., E.M. Ko, and K.H. Schmitz, Development of a risk-screening tool for cancer survivors to participate in unsupervised moderate-to vigorous-intensity exercise: Results from a survey study. PM&R, 2015. 7(2): p. 113-122.
19. Brown, J.C. and K.H. Schmitz, The prescription or proscription of exercise in colorectal cancer care. Medicine and science in sports and exercise, 2014. 46(12): p. 2202.
20. Zhang, X., et al., The prescription or proscription of exercise in endometrial cancer care. Gynecologic oncology, 2015. 139(1): p. 155-159.
21. Demark-Wahnefried, W., et al., Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol, 2005. 23(24): p. 5814-30.
22. Edge, S.B. and C.C. Compton, The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology, 2010. 17(6): p. 1471-1474.
23. Charlson, M.E., et al., A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases, 1987. 40(5): p. 373-383.
24. Patnaik, J.L., et al., The Influence of Comorbidities on Overall Survival Among Older Women Diagnosed With Breast Cancer. Journal of the National Cancer Institute, 2011. 103(14): p. 1101-1111.
25. McCaskill-Stevens, W. and J.S. Abrams, Comorbidities in the Aging Breast Cancer Population: Are Current Assessments Leading to Improved Outcomes? Journal of the National Cancer Institute, 2011.
26. United States cancer statistics: 1999–2012 incidence and mortality web-based report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute, 2015.
27. Hewitt, M., J.H. Rowland, and R. Yancik, Cancer survivors in the United States: age, health, and disability. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2003. 58(1): p. M82-M91.
28. Santoso, J.T., et al., Obesity and perioperative outcomes in endometrial cancer surgery. Archives of gynecology and obstetrics, 2012. 285(4): p. 1139-1144.
29. Schmitz, K.H., et al., Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. Journal of the National Cancer Institute, 2013: p. djt223.
30. Winters-Stone, K.M., et al., Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat, 2011. 127(2): p. 447-56.
31. Dobek, J., et al., Musculoskeletal changes after 1 year of exercise in older breast cancer survivors. Journal of Cancer Survivorship, 2014. 8(2): p. 304-311.
32. Rogers, L.Q., et al., Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat, 2015. 149(1): p. 109-19.
33. Brown, J.C. and K.H. Schmitz, Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. Journal of Clinical Oncology, 2015. 33(19): p. 2184-2189.
34. Irwin, M.L., et al., Randomized exercise trial of aromatase inhibitor–induced arthralgia in breast cancer survivors. Journal of Clinical Oncology, 2015. 33(10): p. 1104-1111.
35. Jones, L.W. and K.S. Courneya, Exercise counseling and programming preferences of cancer survivors. Cancer practice, 2002. 10(4): p. 208-215.
36. Karvinen, K.H., L.J. Carr, and C. Stevinson, Resources for physical activity in cancer centers in the United States. Clin J Oncol Nurs, 2013. 17(6): p. E71-E76.
37. DeSantis, C.E., et al., Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians, 2014. 64(4): p. 252-271.
38. Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics, 2016. CA: a cancer journal for clinicians, 2016. 66(1): p. 7-30.
39. Piccirillo, J.F., et al., The Changing Prevalence of Comorbidity Across the Age Spectrum. Crit Rev Oncol Hematol, 2008. 67(2): p. 124-32.
| Table 1. Demographic and clinical variables | ||
|
|
Overall (n=667) | |
| Age at Diagnosis a | 55.0±12.3 | |
| N | % | |
| Race | ||
| White | 481 | 72.1 |
| Black | 154 | 23.1 |
| Other | 32 | 4.8 |
| Stage | ||
| DCIS | 126 | 18.9 |
| I | 314 | 47.1 |
| II | 167 | 25.0 |
| III | 60 | 9.0 |
| Surgery | ||
| Lumpectomy | 284 | 42.6 |
| Mastectomy | 383 | 57.4 |
| Chemotherapy | ||
| No | 407 | 61.0 |
| Yes | 260 | 39.0 |
| Radiation | ||
| No | 371 | 55.6 |
| Yes | 296 | 44.4 |
| Reconstructive Surgery | ||
| No | 468 | 70.2 |
| Yes | 199 | 29.8 |
a Variables are mean ± standard deviation
| Table 2. Health-issues that preclude to participate in unsupervised exercise program (N=667) | ||
| Health Issues | N | % |
| Hematologic – any of the following | 46 | 6.90 |
| White Blood Cells | 18 | 2.70 |
| Low Hemoglobin ( | 40 | 6.00 |
| Musculoskeletal – any of the following | 3 | 0.45 |
| Fracture of Hip/Back/Legs | 3 | 0.45 |
| Systemic – any of the following | 18 | 2.70 |
| Fever >100°F | 5 | 0.75 |
| Malaise | 13 | 1.95 |
| Gastrointestinal – any of the following | 12 | 1.80 |
| Severe Nausea | 10 | 1.50 |
| Fecal or Urinary Incontinence | 2 | 0.30 |
| Cardiovascular – any of the following | 40 | 6.00 |
| Chest Pain | 9 | 1.35 |
| Pulse >100 or | 12 | 1.80 |
| Irregular Pulse | 7 | 1.05 |
| Ankle Edema | 10 | 1.50 |
| Congestive Heart Failure | 3 | 0.45 |
| Heart Valve Disease | 10 | 1.50 |
| Aortic Stenosis | 3 | 0.45 |
| Ventricular Ectopy | 6 | 0.90 |
| Coronary Angioplasty | 2 | 0.30 |
| Pulmonary – any of the following | 25 | 3.75 |
| Severe Dyspnea | 10 | 1.50 |
| Coughing or Wheezing | 14 | 2.10 |
| Chest Pain with Deep Breath | 6 | 0.90 |
| Neurologic – any of the following | 4 | 0.60 |
| Blurred Vision | 2 | 0.30 |
| Neuropathy | 2 | 0.30 |
| Comorbidities – any of the following | 164 | 24.59 |
| Hypertension | 71 | 10.64 |
| Heart Murmur | 2 | 0.30 |
| Diabetes | 28 | 4.20 |
| Arthritis | 2 | 0.30 |
| Osteoporosis | 17 | 2.55 |
| COPD | 4 | 0.60 |
| Depression | 24 | 3.60 |
| Morbid Obesity | 50 | 7.50 |
| Lymphedema | 8 | 1.20 |
| Hyperlipidemia | 18 | 2.70 |
| Thyroid Disease | 41 | 6.15 |
| Liver Disease | 4 | 0.60 |
| Table 3. Association between demographic and clinical variables and who can be safely prescribed community/home-based unsupervised exercise program | ||||
| Variable | Univariate Model | Multivariate Model | ||
| OR (95% CI)a | P | OR (95% CI)a | P | |
| Age – Continuous | 0.98 (0.97-0.99) | 0.020 | 0.98 (0.96-0.99) | 0.015 |
| Race | ||||
| White | 1.00 (Ref) | 1.00 (Ref) | ||
| Black | 0.41 (0.28-.59) | 0.44 (0.30-0.64) | ||
| Other | 1.13 (0.51-2.49) | 0.772 | 0.90 (0.40-2.03) | 0.804 |
| Pathology Stage | ||||
| DCIS | 1.19 (0.76-1.87) | 0.430 | — | — |
| I | 1.00 (Ref) | — | — | |
| II | 0.79 (0.54-1.17) | 0.237 | — | — |
| III | 0.72 (0.41-1.27) | 0.261 | — | — |
| Chemotherapy | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 0.61 (0.44-0.85) | 0.003 | 0.61 (0.43-0.87) | 0.007 |
| Radiation | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 0.58 (0.42-0.81) | 0.69 (0.49-0.98) | 0.039 | |
| Surgery (%) | ||||
| Lumpectomy | 1.00 (Ref) | — | — | |
| Mastectomy | 1.59 (1.12-2.12) | 0.008 | — | — |
| Reconstructive Surgery | ||||
| No | 1.00 (Ref) | — | — | |
| Yes | 1.39 (0.97-1.99) | 0.066 | — | — |
a Odds Ratio (OR) from Logistic Regression and 95% Confidence Interval (95% CI).
Figure 1a. Distribution of health-issues that preclude unsupervised exercise in (a) the primary outcome analysis, and (b) the sensitivity analysis that excluded hypertension, diabetes, arthritis, morbid obesity (BMI ≥40 kg/m2), and hyperlipidemia.
(b).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Cancer"
Cancer is a disease in which cells grow or reproduce abnormally or uncontrollably. Cancerous cells have the potential to spread to other areas of the body in a process called metastasis.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




