Glutamine Supplementation in Athletic Performance, Body Composition and Immune Function
Info: 19073 words (76 pages) Dissertation
Published: 24th Feb 2022
Tagged: HealthSportsBiomedical Science
INTRODUCTION
Maintaining athletic performance is one of the issues that has constantly been considered by trainers and athletes (1). Nowadays, athletes employ several approaches for success in competitions and the use of pharmaceuticals or nutritional products are the most common among them. Some of the reasons cited by athletes to intake these products are increasing performance, accelerating recovery, and reducing muscle damage.(2) It has been frequently suggested by some studies that nutritional supplements increase athletic performance (3). Physical performance is highly related to muscle function and muscle protein synthesis, and its degradation is important in determining the muscle strength (4). Amino acids are the construction blocks of muscle and employed as a source of energy for skeletal muscle (5). There is a little evidence to suggest that nonessential amino acids stimulate muscle protein synthesis. However, glutamine (GLN), which is a nonessential amino acid is particular (6). Supporting the immune system, increasing production of glycogen, anticatabolic effect and increasing the absorption of water and electrolytes caused the GLN supplementation receive support from some well-controlled scientific studies (7). It was shown a direct relationship between free GLN levels in muscle and the rate of muscle protein synthesis in laboratory animals (6). Sustaining positive protein balance and anabolic effects of GLN supplementation may potentially improve athletic performance (namely power, vertical jump performance, or overall muscle strength) that is the direct result of increase in muscle mass (3) .
GLN is the most abundant amino acid in plasma and skeletal muscles. It makes up 60% of the total free amino acid in skeletal muscle (8, 9) and about 20% of plasma amino acids (10). GLN could be used as a precursor for the synthesis of amino acids, proteins, nucleotides and many other biological molecules (11). As well, it is essential for homeostasis (including fluid balance, PH, regulate body temperature and heart rate), and optimal function of some body tissues, especially the immune system and gastrointestinal tract (10). This amino acid is the most important fuel source for some immune cells and may has a special effect on immune stimulation .(12) For years it had been proved that immune cells use glucose as a fuel (13), but in the early ’80s, it was found that these cells use GLN equally and in the same way as glucose (14). Recently the role of GLN in the immunosuppression has become a live topic. A decrease in plasma GLN is associated with immune suppression after intensive exercise and overtraining syndrome (15). However, there is no direct evidence for the association between low plasma GLN (after exercise or overtraining syndrome) with impaired immune function (16). Based on limited studies GLN increases the number of circulating lymphocytes and macrophages and it has been found a relationship between plasma GLN concentration and viral diseases in nonathletes (17, 18) .
Decrease in plasma GLN levels after prolonged exercise may be ascribable to an increase in body demand and uptake of GLN by the tissues more than normal. The plasma GLN levels can decline due to reduced production or decrease in release of GLN by muscle .(19) Despite of existing claims about the effect of GLN on enhancing athletic performance and improving immune system still results of the studies are conflicting. This study was conducted to evaluate the effect of GLN supplement on athletic performance, body composition and immune function.
Methods
This research conducted by following the guidelines and the PRISMA statement for reporting systematic reviews and meta-analyses of studies. Because of the study type (meta-analysis and systematic review) ethical approval was not necessary according to local legislation.
Literature Search
Two authors (AR and ER) independently performed a Pubmed, Scopus, ISI Web of Science, SID and Cochrane Central Register of Controlled Trials and Cochrane Library database extended literature search of all studies published as original full-text article until April 2016. There was no restriction on publication year and all studies published in English or Persian included. The following medical subject heading terms and words was used for search, in all possible combination: “glutamine” or “dipeptide”, “L-glutamine”, ” L-Alanyl-L-Glutamine”, “sustamine”, “oral glutamine”, “supplement of glutamine”, “glutamine supplementation”, AND “athletes”, or “exercise”, “sport”, “training”, “athletics” AND “body composition”, “muscle mass”, “lean mass”, “lean body mass”, “fat mass”, “body mass”, “weight”, “immune function”, “immune”, “immune response”, “immunity”, “white blood cell”, “lymphocyte”, “leukocyte”, “neutrophil”, “cytokine”, “performance”, “aerobic performance”, “anaerobic performance”, “power”, “strength”, “endurance”, “resistance”, “Vo2”, “growth hormone”, “GH”, “glucose”, “creatine kinase”, “CK”, ” creatine phosphokinase”, “phosphor-creatine kinase”, “CPK”. The “related article” function was used to expand the search and the reference lists of articles selected for full-text review were searched for extre articles.
Study Selection
The term “glutamine supplementation” was defined as any treatment containing GLN or GLN dipeptide (as L-Alanyl-L-Glutamine or sustamine) in combination or not with any form of artificial nutrition as reported in the articles reviewed.
We included trials with the following eligibility criteria: a) Studies enrolling patients with age >18 years, b) Athletes with regular exercise, c) controlled trial, d) GLN or L-Alanyl-L-Glutamine supplementation, e) Trials reporting at least 1 of the outcomes considered in the systematic review and meta-analysis, f) English or Persian language.
We considered all studies irrespectively if GLN was given in powder forms, pills or as sport drinks. Trials with the following criteria were excluded: a) Observational studies (cohort study, case-control study, ecological studies, case reports, case series), b) GLN combined with other nutrients with potential metabolic activity (for example amino acids, nucleotides, Creatine and omega-3 fatty acids), c) No full-text available articles, opinion pieces, review articles and editorials.
Data Extraction
An electronic database was created to collect all relevant trials data. The data were extracted independently by 2 investigators (AR and ER) and in case of disagreement superpartes referent (AM) cross-examined doubtful data and the decision was made after consensus meeting. Information extracted from the trials include: first author, country of origin, year of publication, study type (parallel or cross-over), gender, double blinding, GLN dosage, administration method, and period of supplementation, the regimen of the control groups, sport type, and the different outcome measures.
The primary purpose of this systematic review and meta-analysis was to evaluate if GLN supplementation could affect athletics performance, immune function and body composition. Most frequent outcomes used for conducting meta-analysis. Data related to body mass, lean body mass and body fat percent before and after supplementation extracted for evaluating effects of GLN supplementation on body composition. For athletic aerobic performance Vo2 max data were extracted. We used data of lymphocyte, leukocyte and neutrophil counts for determining the effect of GLN on immune function. As a secondary endpoint of the analysis, we considered the effect of GLN on concentration of GH, CK and blood glucose that indirectly related to sport performance and body composition. Studies quality was assessed by 2 independent reviewers (AR and ER) according to the Jadad score (20).
Statistical Analysis
The effect size that estimated by the mean difference (MD) was used to performing the fixed method meta-analysis. We performed a random-effects meta-analysis for each outcome that there was a significant heterogeneity between studies (21). Heterogeneity was assessed using I2 index and testing the null hypothesis that all studies share a common effect size. We used I2 more than 25% considered as a threshold for the presence of moderate heterogeneity and more than 50% as high heterogeneity. For identifying potential source of heterogeneity, some stratified analyses were performed according to the following indicators: GLN dosage (≥0.2 g/kg/day or <0.2 g/kg/day), duration of GLN supplement (Acute or chronic) and study quality (low or high). Acute supplementation embraces interventions that have been conducted in a single day. For all the analyses, we tested the presence of a different effect between subgroups.
We used funnel plots to visual inspection for presence of publication bias. Besides, for further investigation on publication bias Begg’s rank correlation and Egger’s linear regression tests were used. All the analyses were carried out using Stata, version 12 SE (Stata Crop, College Station, TX, USA). P-values <0.05 were considered statistically significant.
Results
Study Characteristic
As shown in figure 1, early electronic search resulted in 1064 studies, after duplicate removal. Following a title and abstract screen, 996 studies excluded because of reporting unrelated data, animal subjects, review articles, and language was not English or Persian. Overall, 68 studies evaluated for eligibility and 21 studies excluded for the following reasons: a) used a combination preparation with other ergogenic aids or amino acids (5, 15, 22-30), b) did not report the outcomes related to the aims of this study (31-39), and c) was not performed on human subjects (40). From 45 studies included in systematic review, 25 trials fit the inclusion criteria for meta-analysis. Table 1 summarized information on all trials included in the systematic review. A total of 27 studies (60%) employed a parallel study design, whereas 18 studies (40%) used a cross-over design. Most studies (n=35) conducted in male subjects, although 8 trials involved both male and females, and 2 recruited only females. Majority of the trials tested the effects of GLN in acute supplementation (n=22), while intervention duration in 15 studies were fewer than 4 weeks and in 7 trials were longer than 4 weeks. Dosage of GLN supplements are not directly comparable, as in some studies authors considered subjects weight for prescribing supplement, while in others there was no difference in receiving supplement according to weight. Based on JADAD score, in more than half of the trials (n=25), study quality was high, although 20 trials received a poor score due to lack of randomization or double-blinding.
Glutamine supplementation and immune function
Qualitative synthesis
Overall 20 studies considering the effect of GLN supplementation on immune function. One study showed that 7 days GLN supplementation significantly reduced incidence of infection in athletes (18).
One study showed a significant reduction in the ratio of CD4+ helper/CD8+ suppressor cells in the placebo group than in the GLN group (41). On the other hand, in one study, CD8+ reduction due to GLN supplementation caused a significant increase in the ratio of CD4+/CD8+ after training, while this ratio was unchanged in the control group (42). However, in 4 studies the numbers of CD4+ and CD8+ was not different between the GLN group and the placebo group (14, 43-45). The percentage of T-cells was significantly higher in the GLN group compared to the placebo only 16 hours after exercise, in one study (14). Also, a study showed a significant elevation in post-training Natural Killer (NK) activity in athletes who received GLN supplement (42). However, three studies found no effect of GLN supplementation on NK cells activity (14, 44, 46).
One study that evaluated the effect of GLN supplementation on B cell counts found no difference between experimental and control group (14). Effect of GLN supplementation on the change of salivary immunoglobulin A (s-IgA) was not significant in 3 studies (1, 47, 48). Also, some studies found no difference between the GLN group and placebo in plasma levels of Ig M (42, 49), IgA (42, 49, 50) and IgG (42, 49, 50). However, one study reported that nasal IgA was greater in athletes receiving GLN compared to placebo (48).
In 10 studies that considering the effect of GLN on total leukocyte counts, 8 studies found there is no significant difference in total leukocyte numbers after GLN supplementation compared to placebo (14, 16, 41, 44, 45, 50-52). Two of the studies showed that leukocyte numbers significantly increased in the GLN group compared to the placebo group (49, 53). All nine studies that examined lymphocyte numbers reported no difference between the GLN and the placebo group (14, 16, 41, 43-46, 51, 52). In 6 studies, GLN supplementation did not abolish the change in neutrophil numbers (16, 43-45, 51, 52). One study showed that neutrophil numbers less enhanced in the GLN group compared to placebo (46). Sasaki and colleagues found that in one week (but not 2 weeks) following GLN supplementation neutrophil numbers increased significantly after exercise, although in the placebo group there was a little reduction in neutrophil numbers compared to pre-exercise (50). Also, another study reported more increasing in the neutrophil numbers in the GLN group (49). Three studies reported that GLN supplementation has no effect on monocyte numbers (44, 45, 52).
Also, according the studies that evaluating the effects of GLN supplementation on athletes immune function there was no any difference in the plasma concentration of complements (C3, C4 (49, 50) and C5a (14)), CRP (14), neopterine (14), IL-6 (14), IFɣ (14), ConA stimulated proliferative response (44), Lymphokine activated killer (LAK) (43, 44, 46), phytohaemagglutinin stimulated lymphocyte (PHA) proliferative response (43, 44), and phagocyte activity (49, 50) between the GLN and placebo. However, one study showed that immediately after exercise, plasma IL-6 levels were significantly greater in the GLN group than in the placebo group (54). Moreover, two studies showed that peripheral blood mononuclear cells (PBMC) level of IκBα increased in response to exercise in the GLN group (55, 56). These two studies also found that HSP70 expression was higher in GLN group when compared to placebo (55, 56). Also, Zuhl and colleagues reported that plasma levels of TNF-α were significantly lower in the GLN group compared to placebo (56).
Quantitative synthesis
Nine trials, including 173 subjects (n = 89 treated and 84 controls) provided data on Leukocyte numbers for meta-analysis (14, 16, 41, 45, 46, 49, 51-53). As shown in figure 2 L- GLN supplementation did not reveal a significant leukocyte increasing effect compared to placebo (mean difference=198.07 [95% CI: -749.1, 1145.3] n/µl; P =0.68). There was moderate heterogeneity among studies (I2=64.5, P = 0.004). The subgroup analysis of study duration (acute or chronic supplementation), quality (high or low) and supplement dose (<200 mg/kg body weight/day or ≥200 mg/kg body weight/day) marks that heterogeneity was significant in trials with chronic intervention (I2=79.6, P = 0.001), supplement dose more than 200 mg/kg BW (I2=83.4, P < 0.001), and low quality studies (I2=81.6, P = 0.004). Also, in low quality studies, leukocyte counts significantly increased after GLN supplementation compared to placebo group (mean difference=1794.90 [95% CI: 542.7, 3047.1] n/µl; P =0.005). Sensitivity analysis suggesting no difference in the results following excluding any of the trials. Funnel plot (figure 3) marked no publication bias of trials to investigate the effect of GLN supplementation on leukocyte numbers (Egger’s test P=0.95; Begg’s test P=0.53).
Overall, 8 studies provided enough data for the effect of GLN supplementation on neutrophils numbers (n=84 treated and 81 controls). According to the meta-analysis, GLN supplementation has no significant effect on neutrophils numbers compared to the placebo group (mean difference=-112.70 [95% CI: -389.7, 164.3] n/µl; P =0.42; figure 4). There was low heterogeneity between studies (I2=24.6, P = 0.23). Subgroup analysis of study duration (acute or chronic) showed that there is no difference between acute or chronic supplementation of GLN on neutrophil counts. It seems that chronic supplementation of GLN is a moderate source of heterogeneity (I2=45.6, P = 0.11). Supplement dose more than 0.2 gr/kg BW GLN resulted in a significant decline in neutrophil numbers (mean difference=-605.77 [95% CI: -1200.0, 52.1] n/µl; P =0.03). Sensitivity analysis did not provide further information. Funnel plot did not show any publication bias between trials (Egger’s test P=0.36; Begg’s test P=0.80).
Eight studies reported the effect of GLN supplementation on lymphocyte numbers. The meta-analysis on mean change of lymphocyte numbers after GLN or placebo supplementation in plasma was conducted on 141 subjects (73 treatments and 67 placebo). We found that GLN supplementation did not significantly change lymphocyte numbers compared to placebo (mean difference=-65.71 [95% CI: -275.7, 144.2] n/µl; P =0.54). The heterogeneity was not obvious among studies (I2=0.0, P = 0.71). The effect sizes were constant in the sensitivity analysis. There was no significant effect of GLN on lymphocyte numbers in the subgroups of studies supplemented low dose of GLN (<0.2 gr/kg/day) or high doses (≥200 mg/kg/day). Similarly, the lymphocyte numbers did not significantly alter by GLN in either subgroups of study duration (acute or chronic supplementation) and study quality (high or low). For trials considering lymphocyte numbers, neither Begg’s (P=0.57) and Egger’s test (P=0.64) nor visual inspection of funnel plot showed any publication bias.
Glutamine supplementation and body composition
Qualitative synthesis
Overall 6 studies (3, 49, 57-60) examined the effects of GLN supplementation on body composition. One of these studies examined the effect of a mixture of GLN and creatine on body composition that in both creatine monohydrate and creatine monohydrate+GLN groups, body mass and LBM (measured by skinfolds) increased more than the placebo group (p=0.016). Fat mass and percentage of body fat presented no significant changes overtime (3). Nomura and colleagues found body weight decreased significantly at post-practice of pre- and post-intervention for both the GLN and the placebo groups( p<0.01 for all) (49). Candow and colleagues found GLN supplementation during 6 weeks of resistance training has no significant effect on body composition (60). Also, another study showed both treatment and placebo groups lost significant amounts of body mass (p < 0.001), lean mass (p< 0.001), and fat mass (p< 0.001) over the 12-day period but there were no significant differences between groups (57). Two studies showed that 8 weeks GLN supplementation leads to a significantly increase in total body mass and lean body mass and decrease in body fat percentage (58, 59).
Quantitative synthesis
Totally, 6 studies provided enough data to evaluate the effect of GLN supplementation on body composition. Five studies include 122 subjects (62 treated and 60 controls) investigated the effect of GLN supplementation on fat mass and body weight. Also, 5 studies include 69 subjects in the group treated by GLN and 64 controls, examined the effect of GLN on lean mass. The results of these studies pointed to that GLN consumption inversely associated with body weight (mean difference=-1.36 [95% CI: -2.55, -0.16] kg, p = 0.02). There was no heterogeneity among the studies (I2 = 0.0%, p = 0.93). subgroup analysis showed that l- GLN has a significant effect on weight in studies with low quality (mean difference=-1.47 [95% CI: -2.69, -0.24] kg, p = 0.02) and in dose lower than 0.2 gr/kg BW (mean difference=-1.45 [95% CI: -2.67, -0.23] kg, p = 0.02). Based on funnel plot and Egger’s test, there was a publication bias between trials (p = 0.01), while Begg’s Test marked no publication bias.
No association was observed between GLN consumption and fat or lean mass (mean difference=1.01 [95% CI: -0.19, 2.22] kg, p = 0.09 and mean difference=0.38 [95% CI: -2.94, 3.71] kg, p = 0.81 respectively), and there was no heterogeneity between studies (I2 = 0.0%, p = 0.58 and I2 = 0.0%, p = 0.99 respectively). According to subgroup analysis, a significant increase in body fat mass was observed in low quality studies (mean difference=1.64 [95% CI: 0.11, 3.17] kg, p = 0.03). Also, similar results were found in studies supplemented GLN in doses lower than 0.2 gr/kg BW (mean difference=1.61 [95% CI: 0.09, 3.12] kg, p = 0.04). Although it appears that only one study caused this results (59), sensitivity analysis did not confirm that. There were not significant differences in effect of GLN intake on lean body mass after divided by study quality and supplement dose to different groups. Funnel plot and Egger’s test showed a bias in published articles related to lean mass (p = 0.04), however Begg’s test did not show a significant publication bias between studies (p=0.14). No bias noted in published articles related to fat mass (Begg’s Test p=0.99, and Egger’s p= 0.23). Sensitivity analysis did not show any change in the results for fat mass and lean body mass.
Glutamine supplementation and athlete’s performance
Qualitative synthesis
According to our systematic review, 16 studies evaluated the effects of GLN supplementation on different aspects of athletic performance.
Two studies reported that GLN supplementation has no effect on aerobic capacity evaluated by VO2max compared to the placebo group (10, 61). One study concluded that GLN supplementation did not affect oxygen consumption and energy expenditure during exercise. Also, GLN had a trivial effect on exogenous glucose oxidation rate relative to control group (62). In addition, two studies found that pulmonary oxygen consumption (VO2), mean response time of %Hb (as an indicative of muscle deoxygenation kinetics), respiratory exchange ratio, and expired ventilation was not different between the GLN and placebo groups (63, 64). However, in one of these studies the mean response time of VO2 was faster in the GLN group. Moreover, taking GLN during the early phase of exercise increased muscle oxygen consumption, %Hb and oxidative metabolism (63). One study, also, showed that GLN supplementation has a significant enhancement effect on aerobic power (VO2max) (59). In three studies there was no significant difference between GLN and placebo groups in heart rate (48, 64, 65).
Three studies found a significant increase in peak, minimum and mean anaerobic power (Rast or Wingate test) following GLN supplementation compared to placebo (59, 66, 67). On the other hand, in one study the authors did not find any significant relationship between GLN supplementation and improvement in anaerobic performance (measured by shuttle run test), muscle strength of the upper limbs (evaluated by pushups), muscle strength of lower limbs (evaluated by horizontal jump), flexibility (determined by the sit and reach test), and abdominal muscle endurance (determined by sit-up test) (61). According to one study lactate threshold and lactate tolerance did not change following GLN supplementation in comparison with placebo (10).
A study in basketball players showed that GLN supplementation resulted in a significant improvement in shooting drill, shooting performance and visual reaction time, although, there was no difference between trials in lower body reaction, motor response, vertical jump power and player loads. In this study low and high dose of GLN had a similar effect (65). In contrast, another study that compared rehydrating with two doses of L-Alanyl-L-Glutamine (low dose and high dose) and a simple electrolyte drink, indicated that visual, physical and motor reaction time was likely faster for a low dose of L-Alanyl-L-Glutamine than other trials. Also, this study showed a possible advantage for high doses of supplements in the number of successful hits. In both low and high doses of supplementation an improve in lower body response time was observed. There was no difference in cognitive performance between trials (68).
One study suggested a significant greater peak torque (as an indicator for strength) over 96 hours after exercise, in the GLN group than placebo (69). Another study found that GLN supplementation resulted in higher peak torque post-exercise only in the men, but not in the women (70). Two studies showed that rating of perceived exertion (RPE) tend to be higher in the GLN group compared to the placebo (16, 62).
One study showed that GLN supplementation causes an improvement in total distance covered and duration of tolerance (71). Two other studies showed that subjects who received GLN supplements experienced less fatigue than placebo group and mean time elapsed to fatigue or exhaustion was longer for athletes in the GLN group (67, 71). In contrast, one study reported a small increase in leg muscle tiredness with GLN supplement compared to glucose or placebo (62). Moreover, two studies showed that there was no significant difference in fatigue perception, between GLN and placebo trials (66, 72).
One study considering the effect of GLN supplementation on surface electromyography (sEMG; as the indicator of muscle fatigue and muscle damage) concluded that there is no difference between GLN and placebo and sEMG decreased significantly after exercise in both groups (73).
In one study, GLN supplementation did not affect the timing and magnitude of the soreness, although it diminished soreness more rapidly than the control group (69). Also, Rahmani-Nia and colleagues Reported that GLN supplementation has no effect on muscle soreness compared to placebo (73). In return, in another study a lower rate of knee extensor and muscle soreness in GLN group than placebo, was noted (70).
Quantitative synthesis
Overall, only 3 studies (56 subjects; 27 in treatment and 29 in the placebo group) remained to be included in meta-analysis according to the inclusion criteria. We found that GLN supplementation has no significant effect on Vo2max compared to placebo (mean difference=-0.96 [95% CI: -5.1, 3.2] ml/kg/min; P =0.65). There was moderate heterogeneity among studies (I2=68.7, P = 0.04). Sensitivity analysis did not show further information. Egger’s (P=0.26) and Begg’s (P=0.11) did not indicate any publication bias in studies.
Glutamine supplementation and other outcome
Qualitative synthesis
Six trials studied the effect of GLN supplementation on blood glucose and none of them found significant associations (16, 46, 74-77).
We found 4 studies about the effect of GLN supplementation on blood growth hormone. Three of these studies found no effect of GLN supplementation on growth hormone (46, 75, 78) but Hakimi and colleagues (58) showed 0/35 gr/kg/day GLN during 8 week caused significantly greater increases in blood GH in GL group compared to the PL group.
Among 6 studies that investigated the effect of GLN supplementation on blood CK, one study showed Serum CK decreased 1 week after the intervention, compared to pre-intervention (50). Another study indicated that 6 g/day GLN supplementation during 1 week caused a lower level of CK in the GLN supplementation group at recovery stage than at the end of exercise (p<0.05) (79). 4 studies found no significant relationship between GLN supplementation and blood CK (49, 69, 73, 75).
Quantitative synthesis
Data from 6 studies (16, 46, 74-77), including 56 treatments and 56 controls was used to explore the effect of GLN on blood glucose immediately after exercise. Two studies tested two different doses of GLN on blood glucose levels (75, 77), therefore, each dose entered to analysis separately. Overall, 8 effect size entered for comparing the relationship between GLN supplementation and blood glucose post-exercise. There was no differences between low and high dose of GLN in affecting blood glucose (77). According to these studies GLN intake in athletes did not correlate with blood glucose (mean difference=0.27 [95% CI: -0.24, 0.78] mmol/l; P =0.29). There was a high heterogeneity between these studies (I2 = 88.5%, p<0.001). Potential source of variation evaluated by subgroup analysis and we found that supplement dose more than 0.2 gr/kg BW may be a source of heterogeneity (I2 = 87.3%, p<0.001). Subgroup analysis of study quality did not provide further information in detecting source of heterogeneity. However, in high quality studies, plasma glucose level was significantly lower in the GLN group compared to placebo group (mean difference=-0.46 [95% CI: -0.55, -0.36] mmol/l; P <0.001). Based on Egger’s test and Funnel plot there is a significant bias in publishing articles (P = 0.001), but Begg’s Test indicated no publication bias (P=0.32). Sensitivity analysis did not provide further information.
Study on 66 subjects from 3 studies (49, 50, 79) (36 treatments and 35 control) did not show a significant effect of GLN on blood CK after exercise (mean difference=-20.29 [95% CI: -86.55, -45.97] UI/l; P =0.54). One study indicated no effect of dose on blood Ck changes (75). There was no significant heterogeneity between articles (I2 = 0.0%, P= 0.67) and publication bias was not evident (Begg’s Test p=0.60, and Egger’s p= 0.91). Sensitivity analysis did not show any change in results.
Meta-analysis on three studies (46, 58, 78), including 30 treated and 30 control did not found significant effect of GLN on blood GH immediately post-exercise (mean difference=0.17 [95% CI: -1.50, 1.85] ng/l; P =0.83). Articles were statistically heterogeneous (I2 = 64.2%, p = 0.06) and there was no publication bias in articles related to GH (Begg’s Test p=0.60, and Egger’s p= 0.72). Subgroup analysis did not found source of heterogeneity.
Discussion
In the present meta-analysis l-glutamine supplementation was not associated with a significant change in athlete’s immune function (Leukocyte, lymphocyte and neutrophil counts), aerobic capacity (Vo2max), body composition (fat mass and lean body mass), and plasma levels of glucose, CK and GH after exercise. However, GLN supplementation resulted in a significant weight reduction in athletics.
Immune function
Early studies found that heavy-load exercise can lead to a decrease in athletes immune function and proposed that may be GLN have some effects to prevent this condition (80). According to meta-analysis GLN does not affect leukocyte, lymphocyte, and neutrophil numbers. However, GLN supplementation in doses more than 0.2 gr/kg BW significantly reduced neutrophil numbers compared to placebo. Further, despite the high quality studies, leukocyte counts were significantly higher in low quality studies.
At the other extreme, some studies reported a significant alteration in immune function following GLN ingestion. Decline in the incidence of infection in athletes (18), change in the ratio of CD4+/CD8+ (41, 42), increase in the percentage of T-cells (14), elevation in natural killer activity (42), increase in nasal Ig-A (48), enhancement of leukocyte numbers (49, 53), change in neutrophil counts (46, 49, 50), increase in IL-6 (54), elevation in IκBα (55, 56), increase in HSP70 expression (55, 56), and lower plasma levels of TNF-α (56) reported after GLN supplementation compared to placebo. It is likely long-term stress due to overtraining leads to a reducing in GLN synthesis and at the same time increasing body’s GLN demand (42). It has been showed that the immune system needs GLN as an energy source and also it is needed for nucleic acid synthesis, proliferation and differentiation of lymphocytes and macrophages (81). Moreover, GLN supplementation can ameliorate stress induced intestinal permeability that causes an increase in pro-inflammatory plasma proteins (i.e., TNF-α, IL-6, IL-7), activation of the NF-κB pathways, endotoxin leakage, so GLN can play a protective role by stabilization of the intestinal wall (56).
Nevertheless, most of the studies failed to show a significant effect of GLN intake on the numbers of CD4+ and CD8+ (14, 43-45), NK cells activity (12, 14, 44), B cell counts (14), s-IgA (1, 12, 48), plasma levels of Ig A, M and G (42, 49, 50), leukocyte numbers (14, 16, 41, 44, 45, 50-52), lymphocyte counts (14, 16, 41, 43-46, 51, 52), neutrophil numbers (16, 43-45, 51, 52), monocyte numbers (44, 45, 52), plasma concentration of complements (14, 49, 50), CRP (14), neopterine (14), IL-6 (14), IFɣ (14), ConA stimulated proliferative response (44), LAK (43, 44, 46), PHA proliferative response (43, 44), and phagocyte activity (49, 50). Based on these findings it is more likely that GLN do not affects immunosuppression that remarked in athletes. Although the duration of study and dose of supplement are important factors to earn the desired effect, this meta-analysis found that there is no difference in acute or chronic intervention duration. However, in some studies change in immune function was varied in different time (14, 50). Also, Supplement dose made some difference as described earlier for neutrophil numbers.
Body composition
According to our research, GLN supplementation has no significant relationship with lean body mass and fat mass, but there was an inverse correlation with body weight. Heterogeneity between studies related to body composition was not obvious, but there was a publication bias in articles related to body weight and lean body mass that may cause observing unrealistic results. Consistent with meta-analysis, several studies have found no correlation between consuming GLN and body composition (49, 57, 60), But some studies have shown opposite results. Hakimi (58) and Ghanbarzadeh (59) showed that GLN supplementation increases body weight and lean mass and significantly reduces fat mass. Lehmkuhl and colleagues suggested that both body mass and LBM increased, while Fat mass did not show any significant change after the 8-week GLN supplementation (3). A reason for this variation could be the differences in the amount of GLN intake. As Lehmkuhl showed that adding 4 grams of GLN to creatine monohydrate resulted no significant increase in body mass and LBM compared to single creatine monohydrate, that this may be due to inadequate dose of GLN (3). For the effect of GLN on increasing muscle GLN synthetase (59) and the subsequent increasing muscle protein synthesis and maintaining muscle mass (6) the amount of GLN must be enough to increase plasma GLN levels (82).
Our results demonstrated an inverse association between the intake of GLN supplements and body weight. Weight loss may be affected by severe training program (59) or the effect of GLN in reducing body fat. GLN can mediate lipid metabolism and thereby have an impact on the amount of fat tissue (59). Weight loss may also be due to the effect of exercise on body fluid balance (49). Another reason for the differences in the results of studies on the effect of GLN on body composition can be difference in mixture of supplements (59).
Athletic performance
This meta-analysis concluded that GLN supplementation has no effect on aerobic performance of athletes. Also, there was no effect of GLN on oxygen consumption, oxygen kinetics in muscles, energy cost and aerobic capacity (10, 61-64). Also, GLN had no effect on heart rate and thus not affect supplying blood to muscles (48, 64, 65). In contrast to these findings, some researchers found a significant relationship between GLN supplementation and faster mean response time of Vo2, increase in muscle oxygen consumption, muscle deoxygenation kinetics, oxidative metabolism, and aerobic power (59, 63). It might GLN helps to preserve phosphocreatine and glycogen in muscle oxidative fibers as a tricarboxylic acid cycle intermediate metabolite (59). Overall, it appears that GLN have a minor role in improving athletic aerobic ability.
Researches for effect of GLN on anaerobic performance are more conflicting. Several studies reported a significant improvement in anaerobic power (59, 66, 67), strength (69, 70), perceived exertion (16, 62), reaction time and shooting performance (65, 68). At the other extreme, some studies failed to find a significant relationship between GLN ingestion and anaerobic performance, muscle strength, flexibility, lactate threshold, motor response, vertical jump and lower body reaction (10, 61, 65). In general, it seems that GLN has a positive effect on some aspects of anaerobic power and strength. Additionally, based on previous studies, it may be efficient for enhancing athletes tolerance and time to fatigue (67, 71). Furthermore, GLN helped to diminish muscle soreness more quickly (69, 70). However, in some trials GLN did not affect athletic endurance (61, 66, 72, 73), and time and intensity of muscle soreness (69, 73) or even an unfavorable consequence was observed (62).
GLN has several effects on the immune system, and maintaining body protein balance and may play an important role in cell regulatory system (83). Possibly GLN improves muscle function by alleviating inflammatory response due to exercise (84). Also, reducing protein degradation by GLN leads to an increase in the size of fast twitch fibers and enhancing buffering capacity (85). In addition, more functions such as increasing muscle cells hydration state that reduces the release of CK, inflammatory process, and cell lesions (86, 87), reducing sensitivity to dehydration by contributing to a more efficient fluid and electrolyte uptake (65), promoting muscle glycogen re-synthesis during recovery period (38), and reduction in plasma lactate concentration (62), suggested for GLN in improving muscle strength and anaerobic power. Discrepancies that observed in different studies may be due to the form and dosage of supplements, study duration and quality, difference in the type and intensity of exercise, and gender of participants. For example, some derivatives of GLN such as sustamine be more stable than GLN itself (88). Further, accompanying GLN with maltodextrin has been shown to be more effective than each one separately (66). Also, different results were obtained in a study that compared the effect of GLN on strength between male and female participants (70).
Blood factors (CK, GH, Glucose)
The results of the current meta-analysis indicated that GLN supplementation did not significantly affect blood glucose, growth hormone and creatine kinase levels.
Since there was significant heterogeneity between studies related to blood glucose and growth hormone, the results did not reflect the factual findings while studies of creatine kinase did not have any heterogeneity. Heterogeneity between studies related to glucose not abolished by performing subgroup analysis, but dose of supplements was found as a source of heterogeneity. In line with meta-analysis, none of the studies found significant correlation between the consumption of GLN and blood glucose (16, 46, 74-77). Among these studies, one study showed higher levels of blood glucose in the placebo group compared to the supplementation group (74) it may be due to administration of maltodextrin in the placebo group, while the intervention group was given only GLN. Thus, the differences in the placebo type and intervention time can lead to different results.
Among the studies in relation to GH, one study showed increased levels of this hormone in response to GLN (58) . Also, other studies indicated that GLN can increase the resting levels of GH (89, 90) but not the post-exercise levels (90). It also has been suggested that the stimulatory role of GLN in growth hormone release is during prolonged critical illness when GLN levels drop below normal range(91) . The type of exercise also affects the growth hormone response to GLN. Some studies have shown that GLN supplementation during prolonged heavy endurance training has no effect on increasing serum GH (78).
Creatine kinase is a marker of muscle damage that increases in blood after exercise and is closely linked to energy metabolism(79) . Consistent with other studies(49, 50, 69, 73) meta-analysis found no effect of GLN supplementation on blood levels of CK but Koo et al. have shown that GLN reduces CK levels (79). Reduced levels of serum CK after GLN supplementation may be due to cellular hydration state. Transportation of GLN into the cell is sodium dependent. The subsequent entry of water to the cell and potassium release from the cells (92, 93), influences the cell’s volume and hydration status which results in resistance of cells against lesions, and decreasing the release of intracellular enzymes like CK (86, 93).
indicated that both body mass and LBM increased, while Fat mass did not show any significant change after the 8-week GLN supplementation (3). One of the reasons for this variation could be the differences in the amount of GLN ingested. As Lehmkuhl showed that adding 4 grams of GLN to supplement creatine monohydrate resulted no significant increase in body mass and LBM compared to creatine monohydrate, that this may be due to insufficient dose is GLN (3). For the effect of GLN on increasing muscle GLN synthetase (59) and the subsequent increasing muscle protein synthesis and maintaining muscle mass (6) the amount of GLN must be sufficient to increase plasma GLN levels (82).
Our results demonstrated an inverse association between the intake of GLN supplements and body weight. Weight loss may be affected by severe training program (59) or the effect of GLN in reducing body fat. GLN can mediate lipid metabolism and thereby have an impact on the amount of fat tissue (59). Weight loss may also be due to the effect of exercise on body fluid balance (49). Another reason for the differences in the results of studies on the effect of GLN on body composition can be difference in mixture of supplements (59).
Athletic performance
This meta-analysis concluded that GLN supplementation has no effect on aerobic performance of athletes. Also, there was no effect of GLN on oxygen consumption, oxygen kinetics in muscles, energy expenditure and aerobic capacity (10, 61-64). Also, GLN had no effect on heart rate and thus not affect supplying blood to muscles (48, 64, 65). In contrast to these findings, some researchers found a significant relationship between GLN supplementation and faster mean response time of Vo2, increase in muscle oxygen consumption, muscle deoxygenation kinetics, oxidative metabolism, and aerobic power (59, 63). It is possible that GLN helps to maintain phosphocreatine and glycogen in muscle oxidative fibers as a tricarboxylic acid cycle intermediate metabolite (59). Overall, it appears that GLN have a minor role in enhancing athletic aerobic capacity.
Researches for effect of GLN on anaerobic performance are more conflicting. Several studies reported a significant improvement in anaerobic power (59, 66, 67), strength (69, 70), perceived exertion (16, 62), reaction time and shooting performance (65, 68). At the other extreme, a number of studies failed to find a significant relationship between GLN ingestion and anaerobic performance, muscle strength, flexibility, lactate threshold, motor response, vertical jump and lower body reaction (10, 61, 65). In general, it seems that GLN has a positive effect on some aspects of anaerobic power and strength. Additionally, based on previous studies, it may be efficient for enhancing athletes tolerance and time to fatigue (67, 71). Furthermore, GLN helped to diminish muscle soreness more quickly (69, 70). However, in some trials GLN did not affect athletic endurance (61, 66, 72, 73), and time and intensity of muscle soreness (69, 73) or even an unfavorable consequence was observed (62). GLN has several effects on the immune system, and maintaining body protein balance and may play an important role in cell regulatory system (83). It is possible that GLN improves muscle function by alleviating inflammatory response due to exercise (84). Also, reducing protein degradation by GLN leads to an increase in the size of fast twitch fibers and enhancing buffering capacity (85). In addition, more functions such as increasing muscle cells hydration state that reduces the release of CK, inflammatory process, and cell lesions (86, 87), reducing sensitivity to dehydration by contributing to a more efficient fluid and electrolyte uptake (65), promoting muscle glycogen resynthesis during recovery period (38), and decrease in plasma lactate concentration (62), suggested for GLN in improving muscle strength and anaerobic power. Discrepancies that observed in different studies may be due to the form and dosage of supplements, study duration and quality, difference in the type and intensity of exercise, and gender of participants. For example, some derivatives of GLN such as sustamine be more stable than GLN itself (88). Further, accompanying GLN with maltodextrin has been shown to be more effective than each one separately (66). Also, different results were obtained in a study that compared the effect of GLN on strength between male and female participants (70).
Blood factors (CK, GH, Glucose)
The results of the current meta-analysis indicated that GLN supplementation did not significantly affect blood glucose, growth hormone and creatine kinase levels.
Since there was significant heterogeneity between studies related to blood glucose and growth hormone, the results did not reflect the factual findings while studies of creatine kinase did not have any heterogeneity. Heterogeneity between studies related to glucose not abolished by performing subgroup analysis, but dose of supplements was found as a source of heterogeneity. In line with meta-analysis, none of the studies found significant correlation between the consumption of GLN and blood glucose (16, 46, 74-77). Among these studies, one study showed higher levels of blood glucose in the placebo group compared to the supplementation group (74) it may be due to administration of maltodextrin 8% in the placebo group, while the intervention group was given only GLN. Thus, the differences in the placebo type and intervention time can lead to different results.
Among the studies in relation to GH, one study showed increased levels of this hormone in response to GLN (58) . Also, other studies indicated that GLN can increase the resting levels of GH (89, 90) but not the post-exercise levels (90). It also has been suggested that the stimulatory role of GLN in growth hormone release is during prolonged critical illness when GLN levels drop below normal range(91) . The type of exercise also affects the growth hormone response to GLN. Some studies have shown that GLN supplementation during prolonged heavy endurance training has no effect on increasing serum GH (78).
Creatine kinase is a marker of muscle damage that increases in blood after exercise and is closely linked to energy metabolism(79) . Consistent with other studies(49, 50, 69, 73) meta-analysis found no effect of GLN supplementation on blood levels of CK but Koo et al. have shown that GLN reduces CK levels (79). Reduced levels of serum CK after GLN supplementation may be due to cellular hydration state. Transport of GLN into the cell is sodium dependent. The subsequent entry of water to the cell and potassium release from the cells (92, 93), influences the cell’s volume and hydration status which results in resistance of cells against lesions, and decreasing the release of intracellular enzymes like CK (86, 93).
References
1. Dabidi Roshan V, Fallah Mohammadi Z, Barzegar Zadeh H. the effect of short-term supplementation of glutamine on A im- munoglobulin in saliva of active males following an exhaustive activity. Olympic Journal. 2007;2(38):7-15.
2. Sobal J, Marquart LF. Vitamin/mineral supplement use among athletes: a review of the literature. International journal of sport nutrition. 1994;4(4):320-34.
3. Lehmkuhl M, Malone M, Justice B, Trone G, Pistilli E, Vinci D, et al. The effects of 8 weeks of creatine monohydrate and glutamine supplementation on body composition and performance measures. Journal of strength and conditioning research / National Strength & Conditioning Association [Internet]. 2003; 17(3):[425-38 pp.].
4. Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72(2 Suppl):551s-7s.
5. Ohtani M, Maruyama K, Sugita M, Kobayashi K. Amino acid supplementation affects hematological and biochemical parameters in elite rugby players. Bioscience, biotechnology, and biochemistry. 2001;65(9):1970-6.
6. Jepson M, Bates P, Broadbent P, Pell J, Millward D. Relationship between glutamine concentration and protein synthesis in rat skeletal muscle. American Journal of Physiology-Endocrinology And Metabolism. 1988;255(2):E166-E72.
7. Piattoly T, Welsch MA. L-Glutamine supplementation: Effects on recovery from exercise. Medicine and science in sports and exercise. 2004;36(5):S127-S.
8. Antonio J, Street C. Glutamine: a potentially useful supplement for athletes. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee. 1999;24(1):1-14.
9. Kreider RB. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports medicine (Auckland, NZ). 1999;27(2):97-110.
10. Akbarnezhad A., Ravasi A.A., Aminian Razavi T.D., I. N. The effect of creatine and glutamine supplements on athletic performance in elite wrestlers after one acute period of weight losing. Harakat. 2006(27):173-88.
11. Smith RJ. Glutamine metabolism and its physiologic importance. JPEN Journal of parenteral and enteral nutrition. 1990;14(4 Suppl):40s-4s.
12. Krzywkowski K, Petersen EW, Ostrowski K, Link-Amster H, Boza J, Halkjaer-Kristensen J, et al. Effect of glutamine and protein supplementation on exercise-induced decreases in salivary IgA. Journal of applied physiology (Bethesda, Md : 1985). 2001;91(2):832-8.
13. Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, et al. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN Journal of parenteral and enteral nutrition. 1994;18(2):128-33.
14. Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. European journal of applied physiology. 1997;75(1):47-53.
15. Sawaki K, Takaoka I, Sakuraba K, Suzuki Y. Effects of distance running and subsequent intake of glutamine rich peptide on biomedical parameters of male Japanese athletes. Nutrition Research. 2004;24(1):59-71.
16. Walsh NP, Blannin AK, Bishop NC, Robson PJ, Gleeson M. Effect of oral glutamine supplementation on human neutrophil lipopolysaccharide-stimulated degranulation following prolonged exercise. International journal of sport nutrition and exercise metabolism [Internet]. 2000; 10(1):[39-50 pp.].
17. Rowbottom DG, Keast D, Morton AR. The emerging role of glutamine as an indicator of exercise stress and overtraining. Sports medicine (Auckland, NZ). 1996;21(2):80-97.
18. Castell LM. Does glutamine have a role in reducing infections in athletes? European journal of applied physiology and occupational physiology. 1996;73(5):488-90.
19. Walsh NP, Blannin AK, Robson PJ, Gleeson M. Glutamine, exercise and immune function – Links and possible mechanisms. Sports Medicine. 1998;26(3):177-91.
20. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996;17(1):1-12.
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177-88.
22. Antonio J, Sanders MS, Kalman D, Woodgate D, Street C. The effects of high-dose glutamine ingestion on weightlifting performance. Journal of strength and conditioning research / National Strength & Conditioning Association [Internet]. 2002; 16(1):[157-60 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/754/CN-00377754/frame.html.
23. Falk DJ, Heelan KA, Thyfault JP, Koch AJ. Effects of effervescent creatine, ribose, and glutamine supplementation on muscular strength, muscular endurance, and body composition. Journal of strength and conditioning research / National Strength & Conditioning Association [Internet]. 2003; 17(4):[810-6 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/726/CN-00472726/frame.html.
24. Cury-Boaventura MF, Levada-Pires AC, Folador A, Gorjão R, Alba-Loureiro TC, Hirabara SM, et al. Effects of exercise on leukocyte death: Prevention by hydrolyzed whey protein enriched with glutamine dipeptide. European journal of applied physiology. 2008;103(3):289-94.
25. Naclerio F, Larumbe-Zabala E, Cooper R, Allgrove J, Earnest CP. A multi-ingredient containing carbohydrate, proteins L-glutamine and L-carnitine attenuates fatigue perception with no effect on performance, muscle damage or immunity in soccer players. PloS one [Internet]. 2015; 10(4). Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/190/CN-01088190/frame.html
http://journals.plos.org/plosone/article/asset?id=10.1371/journal.pone.0125188.PDF.
26. Ionescu AM, Vasilescu MM, Carmoci A, Nica AS, Ionescu S. Long-term glutamine supplementation in elite gymnasts. Farmacia [Internet]. 2014; 62(4):[761-6 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/482/CN-00998482/frame.html.
27. Lowery RP, Joy JM, Dudeck JE, Oliveira de Souza E, McCleary SA, Wells S, et al. Effects of 8 weeks of Xpand® 2X pre workout supplementation on skeletal muscle hypertrophy, lean body mass, and strength in resistance trained males. Journal of the International Society of Sports Nutrition. 2013;10.
28. Willems MET, Sallis CW, Haskell JA. Effects of multi-ingredient supplementation on resistance training in young males. Journal of human kinetics. 2012;33:91-101.
29. Zachwieja JJ, Witt TL, Yarasheski KE. Intravenous glutamine does not stimulate mixed muscle protein synthesis in healthy young men and women. Metabolism-Clinical and Experimental. 2000;49(12):1555-60.
30. Little JP, Forbes SC, Candow DG, Cornish SM, Chilibeck PD. Creatine, arginine α-ketoglutarate, amino acids, and medium-chain triglycerides and endurance and performance. International journal of sport nutrition and exercise metabolism. 2008;18(5):493-508.
31. Bassini-Cameron A, Monteiro A, Gomes A, Werneck-de-Castro JPS, Cameron L. Glutamine protects against increases in blood ammonia in football players in an exercise intensity-dependent way. British journal of sports medicine. 2008;42(4):260-6.
32. Carvalho-Peixoto J, Alves RC, Cameron LC. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme [Internet]. 2007; 32(6):[1186-90 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/674/CN-00628674/frame.html
33. Hall G, Saris WH, Schoor PA, Wagenmakers AJ. The effect of free glutamine and peptide ingestion on the rate of muscle glycogen resynthesis in man. International journal of sports medicine [Internet]. 2000; 21(1):[25-30 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/855/CN-00371855/frame.html
https://www.thieme-connect.com/DOI/DOI?10.1055/s-2000-10688.
34. Wilkinson SB, Kim PL, Armstrong D, Phillips SM. Addition of glutamine to essential amino acids and carbohydrate does not enhance anabolism in young human males following exercise. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme [Internet]. 2006; 31(5):[518-29 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/952/CN-00576952/frame.html
35. Lambert GP, Mason B, Broussard L, Xia T, Summers RW, Gisolfi CV. Intestinal absorption and permeability during exercise with aspirin: Effects of glutamine in replacement beverages. Faseb Journal. 1998;12(4):A370-A.
36. Bowtell JL, Marwood S, De Vito M, Uusi-Hakala H, Zaidell L. No effect of acute oral glutamine on glutathione status and lipid peroxidation during and in recovery from exercise. Journal of Physiology-London. 2002;543:88P-9P.
37. Kim PL, Wilkinson SB, Armstrong D, Phillips SM. No effect of glutamine (Gln) on whole body protein turnover after exercise. Faseb Journal. 2002;16(5):A786-A7.
38. Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. Journal of applied physiology (Bethesda, Md : 1985) [Internet]. 1999; 86(6):[1770-7 pp.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/772/CN-00304772/frame.html.
39. Iwashita S, Mikus C, Baier S, Flakoll PJ. Glutamine supplementation increases postprandial energy expenditure and fat oxidation in humans. Journal of Parenteral and Enteral Nutrition. 2006;30(2):76-80.
40. Curi R, Lagranha CJ, Lima TM, Pithon-Curi TC. Prevention Of Exercise-induced Neutrophil Apoptosis By Glutamine Supplementation. Medicine and science in sports and exercise. 2005;37:S37-S.
41. Castell LM, Newsholme EA. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition (Burbank, Los Angeles County, Calif) [Internet]. 1997; 13(7-8):[738-42 pp.].
42. Song Q-H, Xu R-M, Zhang Q-H, Shen G-Q, Ma M, Zhao X-P, et al. Glutamine supplementation and immune function during heavy load training. International journal of clinical pharmacology and therapeutics. 2015;53(5):372-6.
43. Rohde T, Asp S, MacLean DA, Pedersen BK. Competitive sustained exercise in humans, lymphokine activated killer cell activity, and glutamine–an intervention study. European journal of applied physiology and occupational physiology [Internet]. 1998; 78(5):[448-53 pp.].
44. Rohde T, MacLean DA, Pedersen BK. Effect of glutamine supplementation on changes in the immune system induced by repeated exercise. Medicine and science in sports and exercise [Internet]. 1998; 30(6):[856-62 pp.].
45. Alijani E, Hosseini Z. The effect of glutamine supplementation on immune system in female athlete students of shahid chamran university. Harakat. 2008;37:155-69.
46. Krzywkowski K, Petersen EW, Ostrowski K, Kristensen JH, Boza J, Pedersen BK. Effect of glutamine supplementation on exercise-induced changes in lymphocyte function. American journal of physiology Cell physiology [Internet]. 2001; 281(4):[C1259-65 pp.].
47. Krzywkowski K, Petersen EW, Ostrowski K, Link-Amster H, Boza J, Halkjaer-Kristensen J, et al. Effect of glutamine and protein supplementation on exercise-induced decreases in salivary IgA. Journal of applied physiology [Internet]. 2001; 91(2):[832-8 pp.].
48. Krieger JW, Crowe M, Blank SE. Chronic glutamine supplementation increases nasal but not salivary IgA during 9 days of interval training. Journal of applied physiology (Bethesda, Md : 1985) [Internet]. 2004; 97(2):[585-91 pp.].
49. Nomura T, Umeda T, Takahashi I, Iwane K, Okubo N, Chiba Y, et al. Effects of L-glutamine intake on muscle fatigue and neutrophil functions during a judo training camp. Hirosaki Medical Journal. 2014;64(2-4):144-57.
50. Sasaki E, Umeda T, Takahashi I, Arata K, Yamamoto Y, Tanabe M, et al. Effect of glutamine supplementation on neutrophil function in male judoists. Luminescence : the journal of biological and chemical luminescence [Internet]. 2013; 28(4):[442-9 pp.].
51. Banaeifar A. NHA, Javadi E., Namazi Zadeh M. The effect of glutamine supplement on changes in levels of plasma glutamine and immune factors in wrestling. Harakat. 2003(17):123-36.
52. V Ziaei , A Akbarnejad, R Kordi, Z Ahmadinejad, AA Ravasi, Mansournia M. The Effects of Weight Loss and Glutamine-Creatine Supplementation on Peripheral White Blood Cells in Elite Athletes ZJRMS. 2008;10(3):191-9.
53. Abbasalipour M, Parsay S, Melkumyan K, Minasyan S. Effects of creatine and glutamine supplements in comparison with proper nutrition on performance factors of wrestlers. Advances in Environmental Biology. 2014;6(10):2726-30.
54. Hiscock N, Petersen EW, Krzywkowski K, Boza J, Halkjaer-Kristensen J, Pedersen BK. Glutamine supplementation further enhances exercise-induced plasma IL-6. Journal of applied physiology (Bethesda, Md : 1985) [Internet]. 2003; 95(1):[145-8 pp.].
55. Zuhl MN, Lanphere KR, Kravitz L, Mermier CM, Schneider S, Dokladny K, et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. Journal of applied physiology (Bethesda, Md : 1985). 2014;116(2):183-91.
56. Zuhl M, Dokladny K, Mermier C, Schneider S, Salgado R, Moseley P. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell stress & chaperones. 2015;20(1):85-93.
57. Finn KJ, Lund R, Rosene-Treadwell M. Glutamine Supplementation did not Benefit Athletes During Short-Term Weight Reduction. Journal of sports science & medicine. 2003;2(4):163-8.
58. Hakimi M, Mohamadi MA, Ghaderi Z. The effects of glutamine supplementation on performance and hormonal responses in non- athlete male students during eight week resistance training. Journal of Human Sport and Exercise. 2012;7(4):770-82.
59. Ghanbarzadeh M, Sedaghatpour M. Effect consumption of glutamine supplement on aerobic power, anaerobic power and body composition of soccer players. Journal of Physical Education and Sport. 2011;11(3):313-6.
60. Candow DG, Chilibeck PD, Burke DG, Davison KS, Smith-Palmer T. Effect of glutamine supplementation combined with resistance training in young adults. European journal of applied physiology [Internet]. 2001; 86(2):[142-9 pp.].
61. da Silveira CL, Paiva de Souza TS, Batista GR, de Araujo AT, Gomes da Silva JC, Cirilo de Sousa MdS, et al. Is Long Term Creatine and Glutamine Supplementation Effective in Enhancing Physical Performance of Military Police Officers? Journal of human kinetics. 2014;43(1):131-8.
62. Rowlands DS, Clarke J, Green JG, Shi X. L-Arginine but not L-glutamine likely increases exogenous carbohydrate oxidation during endurance exercise. European journal of applied physiology [Internet]. 2012; 112(7):[2443-53 pp.].
63. Marwood S, Bowtell JL. Effects of glutamine and hyperoxia on pulmonary oxygen uptake and muscle deoxygenation kinetics. European journal of applied physiology [Internet]. 2007; 99(2):[149-61 pp.].
64. Bruce M, Constantin-Teodosiu D, Greenhaff PL, Boobis LH, Williams C, Bowtell JL. Glutamine supplementation promotes anaplerosis but not oxidative energy delivery in human skeletal muscle. American Journal of Physiology-Endocrinology And Metabolism. 2001;280(4):E669-E75.
65. Hoffman JR, Williams DR, Emerson NS, Hoffman MW, Wells AJ, McVeigh DM, et al. L-alanyl-L-glutamine ingestion maintains performance during a competitive basketball game. Journal of the International Society of Sports Nutrition. 2012;9.
66. Khorshidi-Hosseini M, Nakhostin-Roohi B. Effect of glutamine and maltodextrin acute supplementation on anaerobic power. Asian journal of sports medicine. 2013;4(2):131-6.
67. Piattoly T, Parish TR, Welsch MA. L-glutamine supplementation: Effects on endurance, power and recovery. Current Topics in Nutraceutical Research. 2013;11(1-2):55-62.
68. Pruna GJ, Hoffman JR, McCormack WP, Jajtner AR, Townsend JR, Bohner JD, et al. Effect of acute L-Alanyl-L-Glutamine and electrolyte ingestion on cognitive function and reaction time following endurance exercise. European journal of sport science. 2014;16(1):72-9.
69. Street B, Byrne C, Eston R. Glutamine supplementation in recovery from eccentric exercise attenuates strength loss and muscle soreness. Journal of Exercise Science & Fitness. 2011;9(2):116-22.
70. Legault Z, Bagnall N, Kimmerly DS. The influence of oral L-glutamine supplementation on muscle strength recovery and soreness following unilateral knee extension eccentric exercise. International journal of sport nutrition and exercise metabolism [Internet]. 2014; 25(5):[417-26 pp.].
71. Favano A, Santos-Silva PR, Nakano EY, Pedrinelli A, Hernandez AJ, Greve JMD. Peptide glutamine supplementation for tolerance of intermittent exercise in soccer players. Clinics. 2008;63(1):27-32.
72. Haub MD, Potteiger JA, Nau KL, Webster MJ, Zebas CJ. Acute L-glutamine ingestion does not improve maximal effort exercise. The Journal of sports medicine and physical fitness [Internet]. 1998; 38(3):[240-4 pp.].
73. Rahmani-Nia F, Farzaneh E, Damirchi A, Majlan AS, Tadibi V. Surface Electromyography Assessments of the Vastus medialis and Rectus femoris Muscles and Creatine Kinase after Eccentric Contraction Following Glutamine Supplementation. Asian journal of sports medicine. 2014;5(1):54-62.
74. Caris AV, Lira FS, Mello MT, Oyama LM, dos Santos RV. Carbohydrate and glutamine supplementation modulates the Th1/Th2 balance after exercise performed at a simulated altitude of 4500 m. Nutrition (Burbank, Los Angeles County, Calif) [Internet]. 2014; 30(11-12):[1331-6 pp.].
75. Hoffman JR, Ratamess NA, Kang J, Rashti SL, Kelly N, Gonzalez A, et al. Effect Of Acute L-alanyl-l-glutamine Ingestion And Dehydration On Immune, Inflammatory And Oxidative Stress Responses During Anaerobic Exercise. Medicine and science in sports and exercise. 2010;42(5):790-.
76. Karami S, Kashef M, Gaeini A, Rajabi H, Amani M. The effect of glutamine supplement on changes in hsp72, cortisol and plasma glucose after exercise. Iranian Journal of Endocrinology and Metabolism. 2013;15(2):166-73.
77. McCormack WP, Hoffman JR, Pruna GJ, Jajtner AR, Townsend JR, Stout JR, et al. Effects of l-Alanyl-l-Glutamine Ingestion on One-Hour Run Performance. Journal of the American College of Nutrition. 2015;34(6):488-96.
78. Ghasemi A, Haeri M, Kazemi A, A.A. R. Effect of glucose and glutamine supplementation during 4 weeks exhaustive periodic-endurance training on serum growth factors in nonathlete mens. journal of Qom university of medical science. 2012;5(4).
79. Koo GH, Woo J, Shin KO, Kang S. Effects of supplementation with BCAA and L-glutamine on blood fatigue factors and cytokines in juvenile athletes submitted to maximal intensity rowing performance. Journal of physical therapy science. 2014;26(8):1241-6.
80. Castell L, Redgrave N. 23 Role of Glutamine in Exercise-Induced Immunodepression in Man. Glutamine: CRC Press; 2017. p. 333-44.
81. Tung JN, Lee WY, Pai MH, Chen WJ, Yeh CL, Yeh SL. Glutamine modulates CD8alphaalpha(+) TCRalphabeta(+) intestinal intraepithelial lymphocyte expression in mice with polymicrobial sepsis. Nutrition (Burbank, Los Angeles County, Calif). 2013;29(6):911-7.
82. Boelens PG, Nijveldt RJ, Houdijk AP, Meijer S, van Leeuwen PA. Glutamine alimentation in catabolic state. The Journal of nutrition. 2001;131(9 Suppl):2569S-77S; discussion 90S.
83. Wernerman J. Clinical use of glutamine supplementation. The Journal of nutrition. 2008;138(10):2040S-4S.
84. Paulsen G, Crameri R, Benestad HB, Fjeld JG, Morkrid L, Hallen J, et al. Time course of leukocyte accumulation in human muscle after eccentric exercise. Medicine and science in sports and exercise. 2010;42(1):75-85.
85. Phillips GC. Glutamine: the nonessential amino acid for performance enhancement. Current sports medicine reports. 2007;6(4):265-8.
86. Blomstrand E, Essén-Gustavsson B. Changes in amino acid concentration in plasma and type I and type II fibres during resistance exercise and recovery in human subjects. Amino acids. 2009;37(4):629.
87. Miles MP, Naukam R, Hackney AC, Clarkson P. Blood leukocyte and glutamine fluctuations after eccentric exercise. International journal of sports medicine. 1999;20(05):322-7.
88. Fürst P. New developments in glutamine delivery. The Journal of nutrition. 2001;131(9):2562S-8S.
89. Welbourne TC. Increased plasma bicarbonate and growth hormone after an oral glutamine load. Am J Clin Nutr. 1995;61(5):1058-61.
90. Suminski RR, Robertson RJ, Goss FL, Arslanian S, Kang J, DaSilva S, et al. Acute effect of amino acid ingestion and resistance exercise on plasma growth hormone concentration in young men. International journal of sport nutrition. 1997;7(1):48-60.
91. Duska F, Fric M, Pazout J, Waldauf P, Tuma P, Pachl J. Frequent intravenous pulses of growth hormone together with alanylglutamine supplementation in prolonged critical illness after multiple trauma: effects on glucose control, plasma IGF-I and glutamine. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2008;18(1):82-7.
92. Gleeson M, Walsh NP, Blannin AK, Robson PJ, Cook L, Donnelly AE, et al. The effect of severe eccentric exercise-induced muscle damage on plasma elastase, glutamine and zinc concentrations. European journal of applied physiology and occupational physiology. 1998;77(6):543-6.
93. Miles MP, Naukam RJ, Hackney AC, Clarkson PM. Blood leukocyte and glutamine fluctuations after eccentric exercise. International journal of sports medicine. 1999;20(5):322-7.
94. Rahmani-Nia F, Farzaneh E, Damirchi A, Majlan AS. Effect of L-Glutamine Supplementation on Electromyographic Activity of the Quadriceps Muscle Injured By Eccentric Exercise. Iranian journal of basic medical sciences. 2013;16(6):808-12.
Figure 1- flow diagram of literature search according to the PRISMA statement

Identification
Screening
Records identified through database searching
(n = 1829)
Records after duplicates removed
(n = 1064)
Records excluded
(n = 998)
Records screened for title and abstract (n = 1064)
 44
44
Full-text articles assessed for eligibility
(n = 66)
Full-text articles excluded, with reasons
(n = 21)
Eligibility
Studies included in qualitative synthesis
(n = 45)
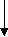
Studies included in quantitative synthesis (meta-analysis)
(n = 25)
Included
Table 1- Characteristics of the Included Trials
| Author (year) | Country | Study design | Gender | Blindness | Quality | No. Of population | No. Of intervention /placebo | Intervention duration | Supplement dose | Outcomes | Sport type |
| Castell, L. M. et al. (1997) (18) | Belgium | Parallel | Male and female | Double | High | 151 | 72/79 | Acute | 5 gr | Immune function | ultra-marathon or marathon runners, Rowers |
| Castell, L. M. et al. (1997) (14) | Belgium | Parallel | Male and female | Double | High | 18 | 10/8 | Acute | 100 mg/kg | Immune function | Ultra-marathon or marathon runners, rowers |
| Castell, L. M. et al. (1997) (41) | Belgium | Parallel | Male | Double | High | 18 | 10/8 | Acute | 5 gr | Immune function | Marathon runners |
| Rohde, T. et al. (1998) (43) | Denmark | Parallel | Male | Single | Low | 16 | 9/7 | Acute | 400 mg/kg | Immune function | Marathon runners |
| Rohde, T. et al. (1998) (44) | Denmark | Cross-over | Male | Single | Low | 8 | 8/8 | Acute | 900 mg/kg | Immune function | – |
| Haub, M. D. et al. (1998) (72) | USA | Cross-over | Male | Double | High | 10 | 10/10 | Acute | 30 mg/kg/day | Performance | physically active subjects |
| Walsh, N. P. et al. (2000) (16) | England | Cross-over | Male | Single | Low | 7 | 7/7 | Acute | 42 gr | Plasma glucose,
Immune function, performance |
Cycle |
| Akbarnejad, A. et al. (2001) (10) | Iran | Parallel | Male | Single | Low | 21 | 7/7 | 1 week | 300 mg/kg/day | Performance | Wrestling |
| Candow, D. G. et al. (2001) (60) | Canada | Parallel | Male and female | Double | High | 31 | 17/14 | 6 weeks | 900 mg/kg of lean body mass/ day | Body composition | Resistance training 2-4 times a week |
| Krzywkowski, K. et al. (2001) (46) | Denmark | Cross-over | Male | Double | High | 10 | 10/10 | Acute | 17.5 gr | Immune function,
Plasma glucose Growth hormone |
Elite athletes |
| Krzywkowski, K. et al. (2001) (47) | Denmark | Cross-over | Male | Double | High | 11 | 11/11 | Acute | 17.5 gr | Immune function | endurance trained sportsmen |
| Bruce, M. et al. (2001) (64) | England | Cross-over | Male | Double | Low | 7 | 7/7 | Acute | 125 mg/kg | Performance,
Glucose |
Cyclist |
| Banaeifar, A. (2003) (51) | Iran | Parallel | Male | Single | Low | 20 | 10/10 | 1 month | 50 mg/kg/day | Immune function | Wrestler |
| Lehmkuhl, M. et al. (2003) (3) | USA | Parallel | Male and female | Double | High | 29 | 10/9 | 8 weeks | 4 gr/day | Body composition | Track & field |
| Finn, K. J. et al. (2003) (57) | USA | Parallel | Male | Double | High | 18 | 9/ 9 | 12 days | 350 mg/kg/day | Body composition | Wrestler |
| Hiscock, N. et al. (2003) (54) | Denmark | Cross-over | Male | Double | Low | 8 | 8/8 | Acute | 3.5 gr | Immune function,
Plasma glucose |
Healthy trained |
| Krieger, J. W. et al. (2004) (48) | USA | Parallel | Male and female | Double | High | 13 | 6/7 | 2 weeks | 100 mg/kg/day | Immune function, performance | Runner |
| Marwood, S. et al. (2007) (63) | England | cross-over | Male | Single | Low | 8 | 8/8 | 2 days | 125 mg/kg/day | performance | Cyclist |
| Dabidi Roshan, V. et al. (1) | Iran | Parallel | Male | Double | High | 23 | 12/11 | Acute | 100 mg/kg | Immune function | Students |
| Alijani, E. et al. (2008) (45) | Iran | Parallel | Female | Double | High | 30 | 10/10 | Acute | 14 gr | Immune function | Athletics |
| Ziaee, V. et al. (2008) (52) | Iran | Parallel | Male | Single | Low | 21 | 7/7 | 1 week | 300 mg/kg/day | Immune function | Wrestler |
| Favano, A. et al. (2008) (71) | Brazil | Parallel | Male | Double | High | 16 | 9/7 | Acute | 3.5 gr | Performance | soccer |
| Hoffman J. R. et al. (2010) (75) | USA | Cross-over | Male | Single | Low | 10 | 10/10 | Acute | 200 mg/kg
50 mg/kg |
Blood factors | Physically active subjects |
| Ghanbarzadeh, M. et al. (2011) (59) | Iran | Parallel | Male | Single | Low | 20 | 10/10 | 8 weeks | 100 mg/kg/day | Performance,
Body composition |
Soccer |
| Ghasemi, A. et al. (2011) (78) | Iran | Parallel | Male | Single | Low | 10 | 5/5 | 4 weeks | 28 gr. Twice/week | Blood factors | Student athletes |
| Street, B. (2011) (69) | England | Parallel | Male | Single | Low | 15 | 7/8 | 4 days | 300 mg/kg/day | Performance,
Blood factors |
drop jumps (eccentric exercise) |
| Hoffman J. R. et al. (2012) (65) | USA | Cross-over | Female | Double | Low | 10 | 10/10 | Acute | 1 gr/day
2 gr/day |
Performance | Basketball |
| hakimi, M. et al. (2012) (58) | Iran | Parallel | Male | Double | High | 30 | 15/15 | 8 weeks | 350 mg/kg/day | Blood factors, Body composition | Non athlete healthy young male students |
| Rowlands, D. S. et al. (2012) (62) | New Zealand | Cross-over | Male | Double | High | 8 | 8/8 | Acute | 9.9 gr | Performance,
Blood factors |
cycle & triathlete |
| Karami, S. et al. (2013) (76) | Iran | Parallel | Male | Double | High | 14 | 7/7 | Acute | 500 mg/kg | Blood factors | Soccer |
| Sasaki, E. et al. (2013) (50) | Japan | Parallel | Male | Single | Low | 26 | 13/13 | 2 weeks | 3 gr/day | Blood factors, Immune function | Judo |
| khorshidi-hosseini, m. et al. (2013) (66) | Iran | Parallel | Male | Double | High | 14 | 7/7 | Acute | 250 mg/kg/day | Performance | physical education students |
| Piattoly, T. et al. (2013) (67) | USA | Parallel | Male | Double | High | 12 | 6/6 | 6 days | 300 mg/kg/day | Performance | Cyclist |
| Abbasalipour, M. et al. (2014) (53) | Iran | Parallel | Male | Double | High | 14 | 7/7 | 15 days | 300 mg/kg/day | Immune function | Elite wresteler |
| Nomura, T. et al. (2014) (49) | Japan | Parallel | Male | Single | Low | 35 | 18/17 | 9 days | 6 gr/day | Immune function,
Body composition, CK, |
Judo |
| da Silveira, C. L. et al. (2014) (61) | Brazil | Parallel | Male | Double | High | 32 | 10/12 | 12 weeks | 300 mg/kg/day | Performance | Military police officers |
| Caris, A.V. et al. (2014) (74) | Brazil | Cross-over | Male | Double | High | 9 | 9/9 | 3 weeks | 20 gr/day | Blood factors | Colorado Altitude Training |
| Koo, G. H. et al. (2014) (79) | Korea | Cross-over | Male | Single | Low | 5 | 5/5 | 1 weeks | 6 gr/day | Blood factors | Elite rowing athletes |
| Rahmani-Nia, F. et al. (2014) (73, 94) | Iran | Parallel | Male | Double | High | 17 | 9/8 | 2 days | 100 mg/kg/day | Blood factors,
performance |
Untrained healthy |
| Pruna, G. J. et al. (2014) | USA | Cross-over | Male | Double | High | 12 | 12/12 | Acute | 0.6 gr
1 gr |
Performance | Endurance runners |
| Zuhl, M. et al. (2014) (55) | USA | Cross-over | Male and female | Double | Low | 8 | 8/8 | 2 weeks | 900 mg/kg of fat-free mass | Immune function | University student |
| Legault, Z. et al. (2014) (70) | Canada | Cross-over | Male and female | Double | High | 16 | 16/16 | 3 days | 300 mg/kg/day | Performance | Eccentric exercise |
| Zuhl, M. et al. (2015) (56) | USA | Cross-over | Male and female | Double | Low | 7 | 7/7 | Acute | 900 mg/kg of fat-free mass | Immune function | Trained endurance athletes |
| Mccormack, W. P. et al. (2015) (77) | USA | Cross-over | Male | Double | High | 12 | 12/12 | Acute | 300 mg/500 ml
1 g/500 ml (250 ml every 15 minutes) |
Blood factors | Endurance runners |
| Song, Q. H. et al. (2015) (42) | China | Parallel | Male | Single | Low | 24 | 12/12 | 6 weeks | 10 gr/day | Immune function | Swimmers |
Table 2- overall estimates of meta-analysis on the effect of glutamine on study outcomes
| Outcomes | Subgroups | No. of trials | References | WMD (95% CI) | P-value | I2 (%) | P-value for heterogeneity |
| Immune function | |||||||
| Leukocyte (n/µl) | 9 | (14, 16, 41, 45, 46, 49, 51-53) | 198.07 (-749.1, 1145.3) | 0.68 | 64.5 | 0.004 | |
| study duration | acute | 4 | (14, 16, 41, 46) | -486.94 (-2300.1, 1375.2) | 0.60 | 0.0 | 0.48 |
| chronic | 5 | (45, 49, 51-53) | 181.33 (-194.6, 557.3) | 0.34 | 79.6 | 0.001 | |
| supplement dose | <200 mg/kg BW | 5 | (14, 16, 41, 49, 51) | 59.25 (-336.5, 455.1) | 0.76 | 0.0 | 0.60 |
| ≥200 mg/kg BW | 4 | (45, 46, 52, 53) | 780.32 (-230.2, 1790.9) | 0.13 | 83.4 | <0.001 | |
| quality | low | 3 | (41, 46, 53) | 1794.90 (542.7, 3047.1) | 0.005 | 81.6 | 0.004 |
| high | 6 | (14, 16, 45, 49, 51, 52) | -0.37 (-386.0, 385.26) | 0.99 | 0.0 | 048 | |
| Neutrophil (n/µl) | 8 | (16, 43, 45, 46, 49-52) | -112.70 (-389.7, 164.3) | 0.42 | 24.6 | 0.23 | |
| study duration | acute | 3 | (16, 43, 46) | -430.79 (-2000.0, 1111.8) | 0.58 | 0.0 | 0.41 |
| chronic | 5 | (45, 49-52) | -102.10 (-383.6, 179.4) | 0.47 | 45.6 | 0.11 | |
| supplement dose | <200 mg/kg BW | 5 | (16, 43, 49-51) | 51.91 (-268.0, 371.8) | 0.75 | 3.2 | 0.38 |
| ≥200 mg/kg BW | 3 | (45, 46, 52) | -605.77 (-1200.0,-52.1) | 0.03 | 0.0 | 0.57 | |
| Lymphocyte (n/µl) | 8 | (14, 16, 41, 43, 45, 46, 51, 52) | -65.71 (-275.7, 144.2) | 0.54 | 0.0 | 0.71 | |
| study duration | acute | 5 | (14, 16, 41, 43, 46) | -3.31 (-358.5, 351.8) | 0.98 | 0.0 | 0.43 |
| chronic | 3 | (45, 51, 52) | -99.22 (-359.5, 161.1) | 0.45 | 0.0 | 0.73 | |
| supplement dose | <200 mg/kg BW | 5 | (14, 16, 41, 43, 51) | -21.11 (-253.7, 211.5) | 0.85 | 0.0 | 0.44 |
| ≥200 mg/kg BW | 3 | (45, 46, 52) | -262.2 (-750.4, 225.9) | 0.29 | 0.0 | 0.94 | |
| quality | low | 2 | (41, 46) | -260.06 (-811.5, 291.35) | 0.35 | 0.0 | 0.88 |
| high | 6 | (14, 16, 43, 45, 51, 52) | -32.75 (-259.8, 194.3) | 0.77 | 0.0 | 0.54 | |
| Body composition | |||||||
| Body mass | 5 | (3, 49, 57-59) | -1.36 (-2.55, -0.17) | 0.02 | 0.0 | 0.93 | |
| supplement dose | <200 mg/kg BW | 3 | (3, 49, 59) | -1.45 (-2.67, -0.23) | 0.02 | 0.0 | 0.89 |
| ≥200 mg/kg BW | 2 | (57, 58) | 0.95 (-5.06, 6.72) | 0.78 | 0.0 | 0.82 | |
| quality | low | 2 | (49, 59) | -1.47 (-2.69, -0.24) | 0.02 | 0.0 | 0.78 |
| high | 3 | (3, 57, 58) | 0.95 (-4.63, 6.54) | 0.73 | 0.0 | 0.96 | |
| Fat mass | 5 | (3, 49, 57-59) | 1.01 (-0.19, 2.22) | 0.09 | 0.0 | 0.58 | |
| supplement dose | <200 mg/kg BW | 3 | (3, 49, 59) | 1.61 (0.09, 3.12) | 0.03 | 0.0 | 0.55 |
| ≥200 mg/kg BW | 2 | (57, 58) | -0.01 (-1.99, 1.97) | 0.99 | 0.0 | 0.81 | |
| quality | low | 2 | (49, 59) | 1.64 (0.11, 3.17) | 0.03 | 7.3 | 0.29 |
| high | 3 | (3, 57, 58) | -0.02 (-1.98, 1.94) | 0.98 | 0.0 | 0.97 | |
| Lean body mass | 5 | (3, 49, 57, 58, 60) | 0.38 (-2.94, 3.71) | 0.81 | 0.0 | 0.99 | |
| supplement dose | <200 mg/kg BW | 2 | (3, 49) | -0.002 (-4.76, 4.76) | 0.99 | 0.0 | 0.77 |
| ≥200 mg/kg BW | 3 | (57, 58, 60) | 0.77 (-3.75, 5.29) | 0.73 | 0.0 | 0.94 | |
| Aerobic capacity | |||||||
| Vo2max (ml/kg/min) | 3 | (10, 59, 61) | 0.96 (-5.1, 3.2) | 0.65 | 68.7 | 0.04 | |
| Blood factors | |||||||
| Glucose (mmol/l) | 8 | (16, 46, 74-77) | 0.27 (-0.24, 0.78) | 0.29 | 88.5 | <0.001 | |
| quality | low | 3 | (16, 75) | 0.43 (-0.10, 0.96) | 0.11 | 31.0 | 0.23 |
| high | 5 | (46, 74, 76, 77) | -0.46 (-0.55, -0.36) | <0.001 | 91.6 | <0.001 | |
| supplement dose | <200 mg/kg BW | 3 | (75, 77) | 0.45 (0.11, 0.78) | 0.008 | 0.0 | 0.95 |
| ≥200 mg/kg BW | 5 | (16, 46, 74-76) | -0.51 (-0.61, -0.41) | <0.001 | 87.3 | <0.001 | |
| CK (UI/l) | 3 | (49, 50, 79) | -20.29 (-86.55, 45.97) | 0.54 | 0.0 | 0.67 | |
| GH (ng/l) | 3 | (46, 58, 78) | 0.17 (-1.50, 1.85) | 0.83 | 64.2 | 0.06 | |
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biomedical Science"
Biomedical Science focuses on how cells, organs and systems function in the human body and underpins much of modern medicine. Biomedical Science applies parts of natural and/or formal sciences to help develop advances in healthcare.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




