Isolating Listeria Monocytogenes in Food Sample
Info: 2953 words (12 pages) Dissertation
Published: 9th Dec 2019
Tagged: Biology
Report 4# Listeria monocytogenes
Introduction
Listeria monocytogenes is a gram positive, facultative anaerobe and a non-spore forming bacterium that is extensively spread in several environments and has been mostly detected in many RTE (ready to eat) foods. The safety limit for Listeria monocytogenes in foods according to the European Union is set to 100 CFU/g(1). It can survive in a wide range of pH (4.0 to 9.5), temperature (1-45C), salt (12%) and water activity of 0.92 (2). Different parts of factories like floors, drains, stagnant water, food manufacturing and processing equipment like freezers, Listeria are frequently isolated and detected(3). These are biofilm formers and hence more ubiquitous in the processing environment(4). Since most of the decontamination process in factories are alkaline based it doesn’t affect Listeria as it has the capability to survive under alkaline conditions. Inside a processing plant, the most vulnerable sources of contamination include conveyor belts, slicing equipment’s, brining and packaging machines (in the case of cheese).
Listeria monocytogenes is an adaptable foodborne pathogen which causes two types of disease namely enteric and systemic which can cause abortion and still births for a pregnant women and meningitis in the case of newborn infants and immunocompromised adults (5). The traditional methods used for the detection of Listeria monocytogenes involves enrichments, selective and differential plating to obtain colonies followed by confirmatory testing using different biochemical characteristics exhibited by Listeria(6). All these methods take extended time for isolation and detection. Hence newer methods like rapid testing methods which can give results in hours are being widely used(6).The dairy products have been mostly associated with Listeria outbreak(7) particularly fresh cheese. Soft cheese provides excellent growth conditions for Listeria monocytogenes (8).
Listeria contamination isgenerally through post processing (environment)as it cannot survive the high temperature short time pasteurization treatment or it can also originate from the raw milk in the case of soft cheese(1). In the dairy industry, predominantly soft cheeses are posing a major risk to the health as it is one of the leading source for the outbreak of L.monocytogenes. The foodborne disease affects all the vulnerable age groups of population (immunocompromised, neonatal, pregnant women, elderly and children). 20-30% is the mortality rate for this foodborne infection. The contamination occurs primarily due to the conditions prevailing in cheese processing which favors the growth of this pathogen. Almost every dairy processing plant are likely to be contaminated with the Listeria monocytogenes. Due to the specific abilities of Listeria to with stand all the processing parameters and the conditions it is creating a potential threat to the control its growth in food products(2).
The conditions of cheese manufacturing, ripening process and storage environments influences the growth and the survival of Listeria in cheese products. The soft cheese generally has a pH 4.5-6.5, NaCl 2.3% to 3.5% and these conditions are considered to be a part of hurdle technology for the food preservation but it does not help to prevent the growth of Listeria as it is resistant to these conditions. During the ripening process the brine solutions used may be contaminated with Listeria monocytogenes.(9).As a result these normal traditional preservation methods fail in preventing the growth of Listeria monocytogenes.
Objective
The objective of this experiment was to detect and isolate Listeria monocytogenes in food sample (Queso Fresco) using different enrichment methods and detect Listeria monocytogenes using selective and differential media followed by performing confirmatory tests for biochemical reactions for the isolated colonies of Listeria.
Methods

Samples of Fresco was brought from a grocery store. The fresco was weighed to 25g and 225 ml of UVM1 broth was added to it in a stomacher bag. Another 25g of Queso Fresco was weighed and 225 ml of water was added to it along with LESS powder into another stomacher bag. Both the samples were homogenized using a stomacher for 2 minutes. The bags were sealed and incubated for 24 hours at 30°C.1ml of the UVM1 sample ( after incubation) is transferred into 9 ml of Fraser broth in duplicated and incubated at 35°C for 24 hours. Environmental sampling was done by swabbing the freezer and 3 ml of the swab was transferred into the Listeria Petri film and incubated at 35°C for 30 hours. Three phase streaking was done with 4 Modified Oxford Agar (MOA) plates and 4 PALCAM agar plates from the incubated Fraser tubes. 2 of plates of MOX and PALCAM for each test tube (Fraser) and were incubated at 35°C for 24 hours. 1 ml of the incubated sample from LESS was transferred to an empty test tube and boiled for 15 minutes. 200μl of the sample was transferred into a Reveal test well and the test strip was inserted into the well and left for about 20 minutes and the number of bands on the strip was noted and examined. The MOX and PALCAM plated were checked for the growth of colonies. Stabbing was done in Tryptic Soy Agar with blood (TSA+Blood) agar at 4 different spots and with 4 different colonies. 4 quadrant streaking was done with the Tryptic Soy Agar with Yeast Extract (TSAYE) and Rapid L.monocytogenes agar. Both the plates were incubated at 35°C for 24 hours. The plates were then observed, examined and compared with the controls. 2 selected colonies from the TSAYE agar plates were selected for Gram staining. Hydrogen peroxide was added to Rapid L.monocytogenes agar plated and the production of gas (Catalase test) was observed and recorded. The complete procedure for streak plating, aseptic techniques, colony counting rules, Gram staining were all done according to the method stated in the laboratory manual [13].
Results
Table 1: Isolation of presumption L.monocytogenes colonies on selective agar media and biochemical and morphology of isolates.
| Source: Queso Fresco | Observation |
| MOX Agar | White colonies were observed and no black precipitate was observed |
| PALCAM agar | No growth was observed in the PALCAM agar |
| TSAYE agar | White colonies were observed on the plates |
| Gram staining | Gram positive cocci |
| Catalase test | Positive |
| Hemolysis- TSA+ Blood agar | β-hemolysis |
* MOX – Modified Oxford agar, TSAYE-Tryptic Soy Agar with Yeast Extract, TSA+Blood – Tryptic Soy Agar with blood agar.
Table 2: Colony morphology of the isolated colonies with Gram staining
| Colony Number | Color | Form | Elevation | Margin | Gram stain color | Gram positive /Gram negative | Shape |
| 1 | White | Circular | Raised | Entire | Violet | Gram positive | Cocci |
| 2 | White | Circular | Raised | Entire | Violet | Gram positive | Cocci |
Discussion
The individual colonies isolated from Quesco Fresco were found to be negative for Listeria monocytogenes. UVM 1 broth is the primary enrichment step which is done to enhance only the growth of Gram positive Listeria as it contains nalidixic acid, acriflavin which inhibits the growth of Gram negative bacteria and Gram positive bacteria other than Listeria. Fraser broth is also enrichment media but it is selective as it contains lithium chloride in addition to acriflavin and nalidixic acid which inhibits the growth of enterococci. The media also contains esculin, ferric acid and ammonium citrate which acts as differential agents. The Fraser tubes had a dark precipitate at the bottom which may be due to contamination of the broth. The broth appeared turbid which indicates the growth and presence of Listeria spp. There was no growth of Listeria in the petri film which was incubated for environmental sample. The place( freezer) which was swabbed was found to be clean and negative for Listeria monocytogenes. For the Rapid LESS test there was appearance of only a single band which indicates that a negative test for the presence of Listeria monocytogenes.
The MOX (Modified oxford Agar) is a selective differential medium used for the isolation of Listeria monocytogenes as it can grow in the presence of antimicrobials and the medium contains lithium chloride, colistrin sulphate and moxalactam. The Listeria can produce an enzyme which can hydrolyze esculin and this product in turn reacts with iron (ferric ions) of ferric ammonium citrate to produce a characteristic black precipitate. All the 4 incubated MOX agar plates were positive for the growth of the colonies and were white in color but no black precipitate was observed. PALCAM agar is another selective differential medium which is used for the isolation of Listeria spp. The medium contains lithium chloride, polymyxin, acriflavin, ceftazidime as selective agents and esculin, ferric ammonium citrate and mannitol- phenol red as differential agents. The hydrolysis of the esculin leads to medium blackening and also the growth of mannitol fermenting bacteria will increase the acidity of the medium and changes the color of the medium from red to gray to yellow. All the 4 incubated plated for the PALCAM agar did not have any growth in them and the reason may be due to the agar. The medium was soft and the agar was not properly prepared as even the positive and negative controls did not grow in the medium.
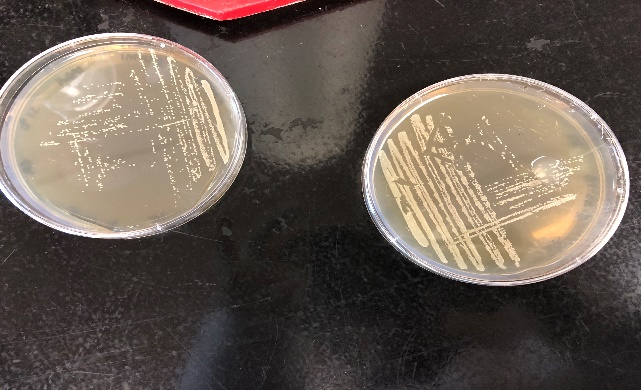
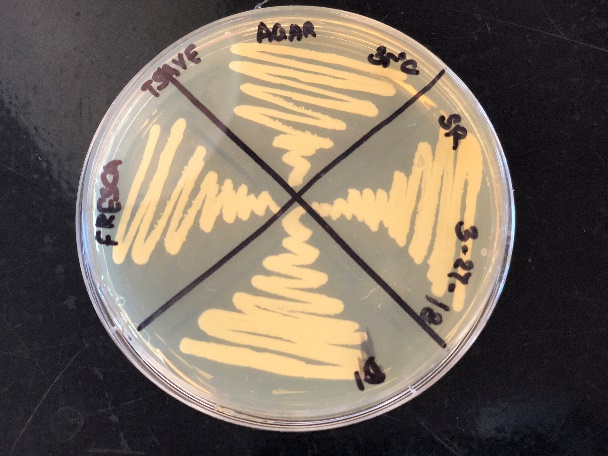
Figure 2: Growth of Listeria monocytogenes in MOX agar (left) and TSAYE agar (right)
The tryptic soy agar with blood (TSA+Blood) agar is a medium used for fastidious microorganisms to grow and also to differentiate those microorganisms which can cause blood hemolysis. Listeria monocytogenes produces distinct narrow zones of β-hemolysis around the stabs. There are 3 different types of hemolysis: α-hemolysis (produces greenish discoloration), β-hemolysis (clear zones around the stabs), γ-hemolysis (no change in color no hemolysis). The blood agar resulted in the formation of distinct narrow zones of β-hemolysis. The microorganism may be suspected to be Listeria monocytogenes as it can also produce a distinct narrow zone with β-hemolysis on the TSA blood agar which was observed in the control sample.
The TSAYE (tryptic Soy Agar with yeast extract) is a nutrient rich, non-selective medium which contains both TSA and 2% yeast extract and is generally used for the colony morphology as it provides a clear background. The colonies in the TSAYE agar were observed to be white and there was no appearance of any bluish grey taint of Listeria colonies. The Rapid Biorad Listeria monocytogenes agar is also a nutrient rich medium and helps in the identification of the L.monocytogenes. The color of the agar changes from orange to purple in the presence of Listeria monocytogenes. There was no significant changes in color in the agar plates except for the growth of colonies.
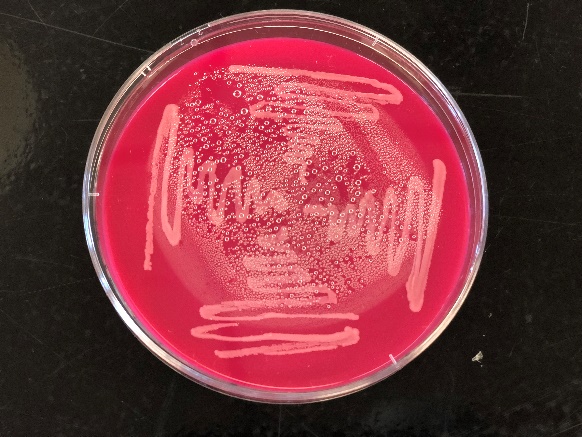
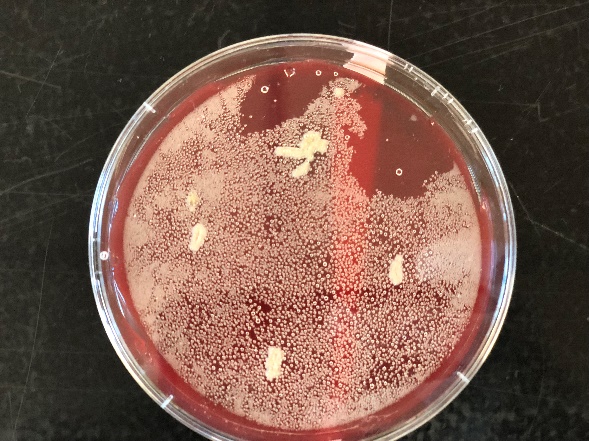
Figure 3: Growth of colonies in Rapid L.monocytogenes agar (left) and β-hemolysis on TSA Blood agar (right)
The 2 colonies that were tested for the Gram staining were found to be Gram positive bacteria but the cells where cocci shaped and were stained violet which indicates that the colonies were Gram positive. But Listeria is also identified as Gram positive but a rod shaped bacteria and hence the colonies that were identified were suspected to be other bacteria used in the starter cultures like lactic acid bacteria since the product tested was cheese.
The catalase test is specific for Listeria monocytogenes as it is catalase positive. The Hydrogen peroxide is hydrolyzed to water and oxygen by the catalase enzyme which is produced by Listeria monocytogenes and produces instant effervescence. The catalase test was positive due to the effervescence which was observed. This proves the presence of catalase enzyme and the organism may be suspected to be Listeria monocytogenes. Since most of the test show a negative result to the presence of Listeria monocytogenes the food sample tested is found to be clear without any Listeria monocytogenes.
Listeria monocytogenes can grow between to -0.4 to 50˚C. It is catalase positive and oxidase negative and also produces an enzyme for β hemolysis (10). Queso Fresco is a Hispanic soft cheese which has a shelf life of 30 to 60 days if they are kept refrigerated. The production of Fresco is only allowed with pasteurized milk in the United States. The cheese curds are only processed at 40˚C. Fresco can be contaminated during the cheese manufacturing or during post process packaging. Due to the high moisture, low salt content and neutral pH, it is an excellent substrate for the growth of L.monocytogenes as well as other spoilage bacteria(11). Queso Fresco (is one of the popular fresh soft Mexican style cheeses) has been classified as FDAs cluster 1 to ready to eat food products with the highest risk of Listeria monocytogenes contamination. The conditions of the cheese manufacturing and processing enhances the ability of L.monocytogenes cells to multiply under refrigeration storage(12).
References
1. Rosshaug PS, Detmer A, Ingmer H, Larsen MH. 2012. Modeling the growth of Listeria monocytogenes in soft blue-white cheese. Appl Environ Microbiol 78:8508–8514.
2. Melo J, Andrew PW, Faleiro ML. 2015. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res Int 67:75–90.
3. Mendoza‐Yepes MJ, Abellan‐Lopez O, Carrion‐Ortega J, Marin‐Iniesta F. 1999. Inhibition of Listeria monocytogenes and other bacteria in Spanish soft cheese made with Lactococcus lactis subsp. diacetylactis. J Food Saf 19:161–170.
4. Lin C-M, Zhang L, Doyle MP, Swaminathan B. 2006. Comparison of Media and Sampling Locations for Isolation of Listeria monocytogenes in Queso Fresco Cheese. J Food Prot 69:2151–2156.
5. Ryser ET, Marth EH. 1987. Behaviour of Listeria monocytogenes during the manufacture and ripening of cheddar cheese. J Food Prot 50:7–13.
6. Fluit AC, Torensma R, Visser MJ, Aarsman CJ, Poppelier MJ, Keller BH, Klapwijk P, Verhoef J. 1993. Detection of Listeria monocytogenes in cheese with the magnetic immuno-polymerase chain reaction assay. Appl Environ Microbiol 59:1289–1293.
7. Barancelli G V, Camargo TM, Reis CMF, Porto E, Hofer E, Oliveira C a F. 2011. Incidence of Listeria monocytogenes in cheese manufacturing plants from the northeast region of São Paulo, Brazil. J Food Prot 74:816–819.
8. Rudolf M, Scherer S. 2001. High incidence of Listeria monocytogenes in European red smear cheese. Int J Food Microbiol 63:91–98.
9. McLauchlin J, Greenwood MH, Pini PN. 1990. The occurrence of Listeria monocytogenes in cheese from a manufacturer associated with a case of listeriosis. Int J Food Microbiol 10:255–262.
10. Farber JM, Peterkin PI. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev 55:476–511.
11. Soni KA, Nannapaneni R, Schilling MW, Jackson V. 2010. Bactericidal activity of lauric arginate in milk and Queso Fresco cheese against Listeria monocytogenes cold growth1. J Dairy Sci 93:4518–4525.
12. Soni KA, Desai M, Oladunjoye A, Skrobot F, Nannapaneni R. 2012. Reduction of Listeria monocytogenes in queso fresco cheese by a combination of listericidal and listeriostatic GRAS antimicrobials. Int J Food Microbiol 155:82–88.
13. Yousef, A. (2003). Food Microbiology – a laboratory manual. New York: John Wiley & Sons, pp.69-73.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




