Development of Lead Smelting, Refining and Recycling Processes
Info: 6725 words (27 pages) Dissertation
Published: 10th Jun 2021
Tagged: Chemistry
ABSTRACT
Phase equilibria of the Pb-Fe-Si-O system have been investigated at 958-1913 K (685-1640°C) for oxide liquid in equilibrium with air and solid oxide phases: (a) quartz, tridymite or cristobalite SiO2; (b) hematite Fe2O3; (c) spinel Fe3O4+x; (d) complex lead-iron silicates (melanotekite PFS = PbO·FeO1.5·SiO2, barysilite P9-xFxS6 = (9-x)PbO·xFeO1+y·6SiO2, “P5FS” = 5PbO·FeO1.5·SiO2, and “P6FS” = 6PbO·FeO1.5·SiO2); (e) lead silicates (alamosite PS = PbO·SiO2, P2S = 2PbO·SiO2, P11S3 = 11PbO·3SiO2, P5S = 5PbO·SiO2); (f) lead ferrites (magnetoplumbite PbO·12FeO1.5, plumboferrite PbO·(5+x)FeO1.5, 1:1 lead ferrite PbO·(1±x)FeO1.5); and (g) lead oxide (PbO, massicot). High-temperature equilibration on primary phase or inert metal (platinum, gold) substrates, followed by quenching and direct measurement of Pb, Fe and Si concentrations in the phases with the electron probe X-ray microanalysis (EPMA) has been used to accurately characterize the system in equilibrium with air. All results are projected onto the PbO-“FeO1.5”-SiO2 plane for presentation purposes. The present study is the first systematic characterization of liquidus over a wide range of compositions in this system in equilibrium with air.
I. INTRODUCTION
The chemical compositions of slags in typical lead smelting operations can be represented by the Pb-Zn-Fe-Cu-Ca-Si-Al-Mg-O system. Accurate data on slag-solid-metal phase equilibria, activities of lead species in the slag, and partitioning of elements between gas, slag, metal and solid phases are essential to support the further optimization and development of complex lead smelting, refining and recycling processes.
The present experimental studies of gas-slag-solid oxide phase equilibria in the Pb-Fe-Si-O “low-order” system is an important part of an integrated thermodynamic modeling and experimental research program for the above multicomponent system. The earlier studies of the lead-containing slag systems [2-12] were focused on the multicomponent Pb-Zn-Fe-Ca-Si-O equilibria in air. Glasser [13] examined a subsolidus isothermal section of the PbO-“Fe2O3”-SiO2 system in air at 923 K (650°C). Some information on the liquidus surface in air can also be derived from the work by Nitta (1989) [14]. Some preliminary studies on the solid-liquid equilibria in the PbO-“Fe2O3”-SiO2 system in air have been performed using the equilibration/quenching/microanalysis technique [15]. The information available at that time was used for the development of the thermodynamic database [16] for the system Pb-Zn-Fe-Ca-Si-O phase equilibria based on the results at different conditions (including liquidus in air [15] and in equilibrium with metallic iron [17]), using FactSage [16, 18-20]. Accurate thermodynamic predictions require experimental data on all low-order systems (binary and ternary) and all phases that can be formed. Significant gaps in experimental data in the Pb-Zn-Fe-Cu-Si-Ca-Al-Mg-O system have been identified to be essential for revision of the current thermodynamic database [16, 20]. Several issues have been identified in the preliminary dataset [15], that required further experimental work. The study on the liquidus of the PbO-“FeO”-SiO2 system in equilibrium with metallic Pb has been completed recently [1]. To evaluate the effect of oxygen potential on phase equilibria at oxidizing conditions, the current study of the liquidus in the PbO-“FeO1.5”-SiO2 system in air is conducted.
According to the current public FactSage 6.2 database [16, 18-20], most of the liquidus surface in the PbO-“FeO1.5”-SiO2 system in equilibrium with air belongs to the quartz, tridymite, cristobalite (SiO2), hematite (Fe2O3), spinel (Fe3O4+x), and magnetoplumbite (“PbFe10O16”) primary phase fields. Liquid immiscibility was expected at very high temperatures (>1873 K (1600°C)) over the cristobalite liquidus field. Other predicted primary phase fields were melanotekite (PbO·FeO1.5·SiO2), 5PbO·FeO1.5·SiO2, lead silicates (alamosite PbSiO3, lead orthosilicate Pb2SiO4, and “4-lead silicate Pb4SiO6”), lead ferrites (1:1 lead ferrite PbO·FeO1.5, plumboferrite “PbO·4FeO1.5”), and lead oxide (massicot, PbO). However, no experimental studies reporting the exact extents of these phase fields were found in literature. The current study focuses on the equilibria of the slag-(solid oxide)-air phases over wide range of temperatures (938-1743 K (685-1470°C)) and compositions.
II. EXPERIMENTAL TECHNIQUE AND PROCEDURE
A. Experimental Procedure and Sample Examination
The experimental procedure and apparatus have been described in detail in previous publications by PYROSEARCH [12, 21]. Initial mixtures were made by mixing high-purity powders of Fe2O3 (99.945 wt.% purity), PbO (99.9 wt.% purity), SiO2 (99.9 wt.% purity), supplied by Alfa Aesar, MA, USA. The initial compositions of the mixtures were selected so that following the experiments one or more crystalline phases would be present in equilibrium with liquid slag. The volume fraction of solids in the final phase assemblage in the range of relatively fluid liquid was targeted to be below 50%, and preferably about 10%, to achieve acceptable quenching (to avoid crystallization during quenching). An iterative procedure involving preliminary experiments was often needed to achieve the targeted proportion of the phases for given final temperature and composition, since the exact liquidus coordinates were not initially known. Less than 0.5 g of mixture was used in each equilibration experiment.
Four types of substrates were used for equilibration, depending on the conditions:
1. Silica open crucibles or ampoules with drilled holes (to ensure contact with air but avoid excessive evaporation of PbO).
2. Hematite (Fe2O3) baskets, prepared by heating a folded iron foil (99.5 wt.% purity Fe, 0.1 mm thickness, supplied by Sigma Aldrich, MO, USA) in air at 1473 K (1200°C) for 2-3 hours (the stoichiometry of Fe2O3 was checked gravimetrically), were used for the experiments where hematite was one of the primary phases.
3. Platinum foil envelopes (>99.9% purity, 0.05 mm thickness, provided by Johnson Matthew, Australia) were used for equilibration of slags with melanotekite (PbO·FeO1.5·SiO2), magnetoplumbite (PbO·12FeO1.5), and sometimes hematite (Fe2O3) and spinel (Fe3O4+x) at temperatures above 1073 K (800°C).
4. Gold foil baskets (99.99% purity, 0.127 mm thickness, provided by Sigma Aldrich, Australia) were used for equilibration of PbO-rich slags with various primary solid phases at or below 1073 K (800°C), because platinum reacts at such conditions forming lead platinates PbPt2O4 and Pb2PtO4 [22].
All equilibration experiments were carried out in a vertical reaction tube (impervious recrystallized alumina, 30-mm inner diameter) within an electrical resistance heated silicon carbide (SiC) furnace, bottom open to ensure natural air flow. A working thermocouple in a recrystallized alumina sheath was placed immediately next to the sample to monitor the actual sample temperature. The working thermocouple was periodically calibrated against a standard thermocouple (supplied by the National Measurement Institute of Australia, NSW, Australia). The overall absolute temperature accuracy of the experiments was estimated to be ±3 K.
The sample was suspended in the hot zone of the furnace by Kanthal support wire (0.7 or 1-mm diameter) at temperatures below ~1753 K (1480°C); at higher temperatures, Pt or Pt-Rh wires were used. Samples were pre-melted for various times (0.5 to 19 h, see Table 2) at 20-200 K above the equilibration temperature, to form a homogeneous slag. This was followed by equilibration at the final target temperature for the required time (from 0.5-1 hour in tridymite and spinel primary phase fields at high temperatures to 3-5 days for quartz primary phase field at low temperature, see Table 2), to allow solid phases to crystallize from the melts. For some experiments where the crystallization from the viscous slag was slow, in particular, for the lead and lead-iron silicates liquidus below 1073 K (800°C), an extra pre-crystallization stage was introduced between the premelt and the final equilibration. To avoid undercooling, the sample was kept below the final temperature for several hours or days, up to 4 months for the PbSiO3 primary phase field.
At the end of the equilibration process, the sample was rapidly quenched in iced calcium chloride brine (
The samples were examined by optical microscopy, carbon-coated, and the phase compositions were measured using an electron probe X-ray microanalysis technique with wavelength dispersive detectors (JEOL 8200L EPMA; Japan Electron Optics Ltd., Tokyo, Japan). The EPMA was operated with 15-kV accelerating voltage and 20 nA probe current using the Duncumb–Philibert atomic number, absorption, and fluorescence correction (ZAF correction). Hematite (Fe2O3), quartz (SiO2), wollastonite (CaSiO3) (supplied by Charles M. Taylor Co., Stanford, CA, USA), and K-456 lead silicate glass (71 wt. pct. PbO, supplied by NIST, Wash. D.C., USA) standards were used for calibration of EPMA. Based on the previous work [1], where fayalite (Fe2SiO4) and lead silicates (PbSiO3 and Pb2SiO4) served as additional stoichiometric standards in the Fe-Si-O and Pb-Si-O systems, subsequent correction [23] was applied to the compositions obtained after the JEOL ZAF correction:
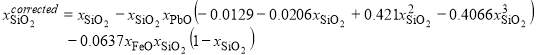 ; (1)
; (1)
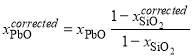 ; (2)
; (2)
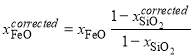 . (3)
. (3)
Only concentrations of metal cations were measured with EPMA. The ratios of the different oxidation states of iron cations were not measured with EPMA, and the iron oxide concentrations were recalculated as FeO1.5 for presentation purposes. The molar ratios were used to avoid any ambiguity.
According to FactSage predictions, the ferrous (FeO) content in slags are very low across the whole range of concentrations except very high temperatures (spinel primary phase field and two liquid miscibility gap over the cristobalite primary phase field).
The ability to produce samples containing the primary phase solids and glassy or amorphous phase on quenching depends on the composition of the slag and the equilibration temperature. The most significant quenching difficulties were observed for the high-iron oxide slags in the spinel and hematite primary phase fields, where the areas of glassy homogeneous slag phase were limited to locations at the surfaces, directly contacting the quenching medium, and the upper limit of temperatures could not exceed 1673 K (1400°C). The approach used to obtain accurate, repeatable, and objective measurements by EPMA-line analysis was similar to the one described by Nikolic et al [24], in which the average composition of the liquid phase is calculated from 20 points measured in a well-quenched area within 15 microns from the surface, with the allowed limit of standard deviation 1.5”-SiO2 slags in air has been observed to be much better than the PbO-“FeO”-SiO2 slags in equilibrium with Pb metal, possibly due to the fact that Fe3+ ion acts as a moderate glass-former rather than Fe2+ that promotes highly fluid slags.
The absence of evaporation loss of Pb species during EPMA measurement of a single point up to 1 minute was confirmed in the present study. The accuracy of the EPMA measurements was estimated to be within 1 wt. %, and the typical detection limit of minor components was estimated to be about 0.01 wt. % [11]. The major uncertainty sources of the results include: deviation from targeted temperature and other experimental conditions; phase inhomogeneity due to incomplete achievement of equilibrium and segregation on quenching; sample contamination due to initial reagent impurities, surface roughness; EPMA standard analysis uncertainties; electron beam stability; EPMA ZAF correction inaccuracies. Special attention was given in this study to test and minimize all potential sources of uncertainties.
B. Confirmation of Achievement of Equilibrium
To ensure the achievement of equilibrium in the samples, the four-point test approach [21, 25] was used including: (1) variation of equilibration time; (2) assessment of the compositional homogeneity of phases by EPMA; (3) approaching the final equilibrium point from different starting conditions; and, importantly, (4) consideration of reactions specific to this system that may affect the achievement of equilibrium or reduce the accuracy. Several dedicated series of experiments were carried out for these purposes, resulted in the experimental design features described below.
Lead silicate master-slag (the eutectic at 40 mol.% PbO melting 2 + Fe2O3 + master-slag) or (Pb3O4 + Fe2O3 + master-slag) powders. Pb3O4 powder, prepared from PbO heated in air at 723 K (450°C) for 24 h, was used instead of PbO to protect Pt and Au substrates from alloy formation with Pb metal and destruction that could happen locally due to reducing conditions.
Fe2O3 powder particles have been found to stay in finely dispersed state in the samples at hematite, melanotekite, and magnetoplumbite primary phases at T2O3 in the initial mixture was carefully avoided, so that the initial premelt was able to convert the sample to fully liquid state, from which larger crystals of the primary phase would precipitate at final equilibration temperature.
A major source of incomplete achievement of equilibrium in the present study was found to be a failure of some phases to nucleate from viscous slag at low temperatures. In particular, the melanotekite (PbFeSiO4.5)-hematite (Fe2O3), melanotekite (PbFeSiO4.5)-quartz (SiO2), and melanotekite (PbFeSiO4.5)-alamosite (PbSiO3) boundaries required long experimental time and careful selection of preheat and precrystallisation heat treatments.
IV. RESULTS AND DISCUSSION
Examples of micrographs of quenched PbO-“FeO1.5”-SiO2 samples are given in Figure 1(a-p). All phases observed in the system in the present study are listed in Table 1. Note that the abbreviations accepted in the present study are P = PbO, S = SiO2, F = FeO or FeO1.5 (not Fe2O3), i.e. the iron atoms are counted regardless of their oxidation state. This facilitates the representation of the EPMA results that do not give accurate information on the oxygen concentration, as well as unambiguously reporting the concentrations in terms of the metal cation ratios and projecting the concentrations onto the PbO-“FeO1.5”-SiO2 plane.
Figure 1. Backscattered scanning electron micrographs of quenched slag in the PbO-“FeO1.5”-SiO2 system in equilibrium with one or two crystalline phases and air, p(O2) = 0.21 atm.
In the previous study [1], some phases not included into the FactSage database [16, 18-20] were discovered: barysilite “P8FS6”=8PbO·FeO·6SiO2 and “P6FS”=6PbO·FeO1.5·SiO2. Along the PbO-SiO2 join, Pb11Si3O17 and Pb5SiO7 [26, 27] were found to be stable instead of Pb4SiO6 [16]. In the Pb-Fe-O system, Pb1+xFe12‑xO19‑x (magnetoplumbite [28]) and PbFe5+xO8.5+1.5x (plumboferrite) were found to be stable instead of PbFe10O16 and PbFe4O7 [16]. In a number of samples in air, barysilite (P9-xFxS6) phase was also observed; unlike 8PbO·FeO·6SiO2 that exists at reducing conditions, it is shifted towards the Pb9Si6O21 (Pb3Si2O7) end-member that can be stable only at subsolidus conditions [26]. The oxidation state of iron in the observed Pb9‑xFexSi6O21+y barysilite was not determined, with possible variation of y from 0 (Fe2+) to x/2 (Fe3+); 0
The results of experiments after correction according to Eqs. 1-3 are reported in Table 2. The phase diagram constructed based on the present results is given in Fig. 2(a-b). The pseudobinary diagram PbO-“FeO1.5”, updated at high temperature part after the previous study [28], is shown in Fig. 2c. The diagram in wt.% of components (without the experimental points) is given in Fig. 3(a-b). The estimated liquidus invariant points involving the observed phases are listed in Table 3.
Figure 2. Liquidus surface of the PbO-“FeO1.5”-SiO2 system in air, p(O2)=0.21 atm. (a) – complete diagram; (b) detail for Pb-rich area; (c) updated PbO-“FeO1.5” pseudobinary diagram. All temperatures are in °C. PbO-SiO2 and PbO-“FeO1.5” binaries are based on [26] and [28], respectively. Selected points [15] given for comparison.
Figure 3. Liquidus surface of the PbO-“FeO1.5”-SiO2 system in air, p(O2)=0.21 atm. (a) – complete diagram; (b) detail for Pb-rich area. All temperatures are in °C and compositions in weight %.
The liquidus in quartz, tridymite and cristobalite (SiO2) primary phase fields has been determined over a wide range of temperatures, 993-1743 K (720-1470°C) and Fe/(Fe+Pb) molar ratios in slag (from 0 at the binary PbO-SiO2 system [26] to ~0.8). The quartz-tridymite and tridymite-cristobalite transitions were not determined directly and accepted from FactSage database [16, 18-20] as 1144 K (867°C) and 1742 K (1465°C), respectively. A range of liquid immiscibility is known to exist in the “FeO1.5”-SiO2 system [29, 30] and it is expected to extend over a part of the ternary PbO-“FeO1.5”-SiO2 system but not reach the binary PbO-SiO2 system [26]. This area involves extreme experimental difficulties due to poor quenching from high temperatures; only a few successful experiments have been obtained in the present study. The area of two immiscible liquids does not extend more than 7 mol.% PbO, which is much less than predicted by previous FactSage model [16, 18-20]. This may indicate existence of charge compensation effect that involves stabilization of tetrahedral Fe3+ by the large Pb2+ ions.
All area of hematite (Fe2O3) has been covered by the current investigation. Its boundary with spinel (Fe3O4+x), where x~0.11 at the transition temperature 1664 K (1391°C) in air, p(O2) = 0.21 atm, according to public FactSage database [16, 18-20]. Spinel was experimentally observed at 1673 K (1400°C) and above, and hematite – at 1663 K (1390°C) and below, in agreement with the predictions. Several experiments have been dedicated to determine the slag-hematite (Fe2O3)-magnetoplumbite (PF12 = PbFe12O19) invariant reaction in the silica-free PbO-“Fe2O3” system, which has been bracketed between 1593 K (1320°C) and 1613 K (1340°C).
The melanotekite (PFS = PbO·FeO1.5·SiO2) has been found to form through the peritectic reaction Liquid + hematite (Fe2O3) → melanotekite (PFS) estimated to occur at 1203±5 K (930±5°C). The melanotekite primary phase field is assumed to be limited by seven ternary invariant points at fixed p(O2) = 0.21 atm:
Liquid → melanotekite (PFS) + quartz (SiO2) + alamosite (PbSiO3) at 993±5 K (720±5°C),
Liquid + melanotekite (PFS) → barysilite (P9-xFxS6) + alamosite (PbSiO3) at 993±5 K (720±5°C),
Liquid → melanotekite (PFS) + barysilite (P9-xFxS6) + Pb2SiO4 at 988±5 K (715±5°C),
Liquid → melanotekite (PFS) + Pb2SiO4 (P2S) + P5FS at 953±5 K (680±5°C),
Liquid + magnetoplumbite (PF12) → melanotekite (PFS) + P5FS at 963±10 K (690±10°C),
Liquid + hematite (Fe2O3) → melanotekite (PFS) + magnetoplumbite (PF12) at 1163±10 K (890±10°C), and
Liquid + hematite (Fe2O3) → melanotekite (PFS) + quartz (SiO2) at 1173±10 K (900±10°C).
It also has two saddle (maximum) points on the boundary lines with alamosite (PbSiO3) and Pb2SiO4:
Liquid → melanotekite (PFS) + alamosite (PbSiO3) at 1018±10 K (745±10°C),
Liquid → melanotekite (PFS) + Pb2SiO4 at 998±5 K (725±5°C)
The barysilite (P9-xFxS6) primary phase field has been determined less accurately compared to other primary phase fields in the present study due to experimental difficulties related to the sluggishness of achievement of equilibrium in the corresponding range. As it was observed in equilibrium with alamosite (PbSiO3) and Pb2SiO4 below liquidus, barysilite is expected to be involved at least in three invariant points: Liquid + melanotekite (PFS) → barysilite (P9-xFxS6) + alamosite (PbSiO3), Liquid → PFS + barysilite (P9-xFxS6) + Pb2SiO4 (see above), and Liquid → barysilite (P9-xFxS6) + alamosite (PbSiO3) + Pb2SiO4.
The phases P5FS and P6FS melt incongruently at 978±5 K (705±5°C) and 998±5 K (730±10°C), respectively. Their primary phase fields are surrounded by the Pb2Fe2O5, plumboferrite (PF5+x), magnetoplumbite (P1+xF12-x), melanotekite (PFS), Pb2SiO4 (P2S), Pb11Si3O17 (P11S3), Pb5SiO7 (P5S), and massicot (PbO) primary phase fields, with the following invariant reactions being suggested:
Liquid + magnetoplumbite (PF12) → P5FS + melanotekite (PFS) at 963±10 K (690±10°C),
Liquid → P2S + P5FS + melanotekite (PFS) at 956±10 K (683±10°C),
Liquid → P5FS + P2S + P11S3 at 958±5 K (685±5°C),
Liquid + P6FS → P5FS + P11S3 at 960±5 K (687±5°C),
Liquid + P5S → P6FS + P11S3 at 978±5 K (705±5°C),
Liquid + massicot (PbO) → P5S + P6FS at 980±5 K (707±5°C),
Liquid + massicot (PbO) → PF1±x + P6FS at 1003±10 K (730±10°C),
Liquid + P6FS → P5FS + PF1±x at 978±5 K (705±5°C),
Liquid + PF1±x → P5FS + plumboferrite (PF5+x) at 973±5 K (700±5°C),
Liquid + plumboferrite (PF5+x) → P5FS + magnetoplumbite (PF12) at 968±5 K (695±5°C).
The solidus surface of the PbO-“FeO1.5”-SiO2 system in air is presented in Fig. 4. Note that the phase relations in high-PbO corner, particularly involving the barysilite, PF1±x, P6F1+xS1-x, and P11FxS3-x solid solutions, are not well-established in the present work.
Figure 4. Solidus surface of the PbO-“FeO1.5”-SiO2 system in air, p(O2)=0.21 atm.
Solid solution ranges in the PbO-“FeO1.5”-SiO2 system in air
The ranges of compositions of the observed solid oxide phases in the PbO-“FeO1.5”-SiO2 system in air are listed in Table 1. Cristobalite and tridymite show limited solubility of iron oxide (Fig. 5), which reached 0.75 mol.% “FeO1.5” for equilibrium with the slags of the highest iron concentration close to the Liquid + tridymite + spinel boundary. Solubility of iron in quartz has not been measured, since all corresponding samples contained too small quartz crystals that gave inaccurate values. None of the silica polymorphs (including the points in the PbO-SiO2 system [26]) showed any significant solubility of PbO, 2+ and Fe3+ in all silica polymorphs, as well as the differences between tridymite and cristobalite and the effects of temperature, are below the accuracy of the present measurements. The principal parameter that determines the solubility of “FeO1.5” in silica polymorphs is the Fe/(Fe+Pb) molar ratio in slag.
Figure 5. Iron oxide concentrations in solid quartz, tridymite and cristobalite crystals in equilibrium with slags of the PbO-“FeOx”-SiO2 system and air, as a function of molar ratio Fe/(Fe+Pb) in the slag. Labels indicate the experimental temperatures (°C). Data at reducing conditions [1] are given for comparison.
The solubility of silica and PbO in hematite Fe2O3 and spinel Fe3O4+x (Fig. 6) is 2 and 2 and PbO in spinel in this system at reducing conditions [1]. The solubility of SiO2 shows clear trend against Si/(Pb+Si) molar ratio in slag, describing both hematite and spinel. However, there is no such trend for PbO solubility, indicating that the results may represent the noise from slag inclusions in hematite crystals or secondary fluorescence of PbO in slag surrounding those crystals.
Figure 6. SiO2 (a) and PbO (b) concentrations in hematite and spinel crystals in equilibrium with slags of the PbO-“FeO1.5”-SiO2 system in air, as a function of molar ratio Si/(Pb+Si) in the slag. Labels indicate the experimental temperatures (°C).
Melanotekite (PbFeSiO4.5) (Fig. 7) was found to have systematic excess of iron oxide, with the average “FeOx” concentration of 34.0 mol.% instead of theoretical 33.3%. This is consistent with the results at reducing conditions [1]. In air, the primary phase field of melanotekite includes much larger range of slag compositions, so it is possible to analyze the compositions of solid in more detail. As found, they can be approximated with substitution of SiO2 with FeO1.5, keeping the Pb/(Pb+Fe+Si) ratio close to constant. The extent of substitution of SiO2 with excess FeO1.5 in the ideal composition PbFeSiO4.5 is the smallest (1-1.5 mol.%) when melanotekite is in equilibrium with slags close to the ratio Pb:Si = 1, i.e. near the PbFeSiO4.5-PbSiO3 join (Fig. 7b). The largest concentration of excess iron in melanotekite (3 mol.%) is found in samples with low-silica slags, which are far from the PbFeSiO4.5-PbSiO3 join.
Figure 7. Melanotekite (PFS = PbFeSiO4.5) compositions measured on crystals in equilibrium with slags of the PbO-“FeO1.5”-SiO2 system in air. Labels indicate the experimental temperatures (°C).
Lead metasilicate (alamosite, PS = PbSiO3) (Fig. 8a) and orthosilicate P2S = Pb2SiO4 (Fig. 8b) are both close to stoichiometric composition, with limited solubility of FeOx (9-xFxS6 (Fig. 8c) was not studied well enough to give conclusions about its behavior: there is only one point in equilibrium with liquid, and some subsolidus points in equilibrium with PS and P2S. The observed compositions agree with the formula Pb9-xFexSi6O21+y, x~0.7-0.8.
Figure 8. PS (a), P2S (b) and barysilite P9-xFxS6 (c) compositions measured on crystals in equilibrium with slags (or at subsolidus conditions) of the PbO-“FeO1.5”-SiO2 system in air. Labels indicate the experimental temperatures (°C).
Only two points have been obtained for the Pb11Si3O17 (P11S3) compound, and one (in Fe-free sample) for P5S; so, their deviations from stoichiometry cannot be currently analyzed in detail. Up to 0.8 mol.% “FeOx” were observed in P11S3. The Pb5SiO7 (P5S) compound is unstable below 975 K (702°C) [27].
The compositions of P5FS (5PbO·FeO1.5·SiO2) and P6FS (P6F1+xS1-x, 6PbO·(1+x)FeO1.5·(1-x)SiO2) measured in equilibrium with slags or at subsolidus conditions in the PbO-“FeO1.5”-SiO2 system in air (Fig. 9), are consistent with the data from previous work at reducing conditions [1].
Figure 9. P5FS and P6F1+xS1-x compositions measured on crystals in equilibrium with slags (or at subsolidus conditions) of the PbO-“FeO1.5”-SiO2 system in air, compared to the results in equilibrium with Pb metal [1] (small font labels). Labels indicate the experimental temperatures (°C).
Among the three lead ferrites, magnetoplumbite (PF12) and 1:1 lead ferrite (PF1±x) have been studied extensively in the present work. Only two points have been obtained for plumboferrite (PF5+x), since its primary phase field is very narrow. Most magnetoplumbite compositions correspond to the Fe:Pb molar ratio between 10 and 12 (i.e., 7.7 to 9.1 mol.% Pb). The points outside of this range could be contaminated with inclusions of plumboferrite or slag in magnetoplumbite crystals. The mechanism of solubility of (PbO+FeO) replacing (Fe2O3) in the magnetoplumbite structure described in [1, 28, 31] cannot operate at oxidizing conditions due to low stability of (FeO), so the formula of magnetoplumbite can be represented as PbO·(12-x)FeO1.5, 0
The 1:1 lead ferrite (PF1±x) has a range of solid solutions, so the points measured within the present work have 51 to 56 mol.% Pb/(Pb+Fe). Typically, a composition of 1:1 lead ferrite is variable even within a single particle, seen as a pattern in SEM microstructures (Fig. 1f). This suggests that part of observed PF1±x was of non-equilibrium composition, formed at initial higher temperatures during the premelt, and unable to reach the equilibrium at final temperature due to slow solid-state diffusion. All lead ferrites also showed limited solubility of SiO2 (
Figure 10. Magnetoplumbite PF12 (a) and 1:1 lead ferrite PF1±x (b) compositions in equilibrium with liquid (or at subsolidus conditions) in the PbO-“FeO1.5”-SiO2 system in air. Labels indicate the experimental temperatures (°C).
In summary, present experimental study has produced the first comprehensive experimental dataset for the Pb-Fe-Si-O system in air that is essential for the lead smelting and recycling industry, and provides valuable new data that can be used to optimize thermodynamic databases of these systems.
IV. CONCLUSIONS
New phase equilibria information in the PbO-“FeO1.5”-SiO2 system in air has been obtained. The studied range of temperatures covered 923-1913 K (670-1640°C), and included the equilibria of slag with one or two crystalline phases: quartz, tridymite, cristobalite (SiO2), hematite Fe2O3, spinel Fe3O4+x, melanotekite PFS = PbO·FeO1.5·SiO2, barysilite P9-xFxS6 = (9-x)PbO·xFeO1+y·6SiO2, “P5FS” = 5PbO·FeO1.5·SiO2 and “P6FS” = 6PbO·(1+x)FeO1.5·(1-x)SiO2 ternary compounds, lead silicates (alamosite PbSiO3, Pb2SiO4, Pb11Si3O17, Pb5SiO7), lead ferrites (magnetoplumbite PF12 = PbO·12FeO1.5, plumboferrite PbO·(5+x)FeO1.5, 1:1 lead ferrite PbO·(1±x)FeO1.5), and lead oxide (PbO, massicot). This is the first systematic study of the Pb-Fe-Si-O system in air, as a part of the multicomponent system Pb-Zn-Fe-Cu-Si-Ca-Al-Mg-O, essential for the lead smelting and recycling industries.
LIST OF FIGURES CAPTIONS
Figure 1. Backscattered scanning electron micrographs of quenched slag in the PbO-“FeO1.5”-SiO2 system in equilibrium with one or two crystalline phases and air, p(O2) = 0.21 atm:
a) Liquid + PF (PbFeO2.5) + P6FS (Pb6FeSiO9.5);
b) Liquid + P2S (Pb2SiO4) + PFS (melanotekite, PbFeSiO4.5);
c) Liquid + PFS (melanotekite, PbFeSiO4.5);
d) Liquid + PbO (massicot) + PF (PbFeO2.5);
e) Liquid + P2S (Pb2SiO4) + P11S3 (Pb11Si3O17)
f) Liquid+ tridymite (SiO2) + hematite (Fe2O3)
g) Liquid + PFS (melanotekite, PbFeSiO4.5)
h) Liquid + hematite (Fe2O3) + magnetoplumbite (PF12)
i) Liquid + PbO (massicot) + P6FS (Pb6FeSiO9.5)
j) Liquid + spinel (Fe3O4+x) + tridymite (SiO2)
k) PbSiO3 + Pb2SiO4 + barysilite (Pb9-xFexSi6O21+y)
l) Liquid + PbSiO3 + quartz (SiO2) + PFS (melanotekite, PbFeSiO4.5)
m) Liquid + cristobalite (SiO2)
n) Liquid + P5FS (Pb5FeSiO8.5)
o) Liquid + Pb5SiO7
p) Liquid + plumboferrite (PF5) + magnetoplumbite (PF12)
q) 2 liquids + cristobalite (SiO2)
Figure 2. Liquidus surface of the PbO-“FeO1.5”-SiO2 system in air, p(O2)=0.21 atm. (a) – complete diagram; (b) detail for Pb-rich area; (c) updated PbO-“FeO1.5” pseudobinary diagram. All temperatures are in °C. PbO-SiO2 and PbO-“FeO1.5” binaries are based on [26] and [28], respectively. Selected points [15] given for comparison.
Figure 3. Liquidus surface of the PbO-“FeO1.5”-SiO2 system in air, p(O2)=0.21 atm. (a) – complete diagram; (b) detail for Pb-rich area. All temperatures are in °C and compositions in weight %.
Figure 4. Solidus surface of the PbO-“FeO1.5”-SiO2 system in air, p(O2)=0.21 atm.
Figure 5. Iron oxide concentrations in solid quartz, tridymite and cristobalite crystals in equilibrium with slags of the PbO-“FeOx”-SiO2 system and air, as a function of “FeOx” concentration (a) and molar ratio Fe/(Fe+Pb) (b) in the slag. Labels indicate the experimental temperatures (°C).
Figure 6. SiO2 (a) and PbO (b) concentrations in hematite and spinel crystals in equilibrium with slags of the PbO-“FeO1.5”-SiO2 system in air, as a function of molar ratio Si/(Pb+Si) in the slag. Labels indicate the experimental temperatures (°C).
Figure 7. Melanotekite (PFS = PbFeSiO4.5) compositions measured on crystals in equilibrium with slags of the PbO-“FeO1.5”-SiO2 system in air. Labels indicate the experimental temperatures (°C).
Figure 8. PS (a), P2S (b) and barysilite P9-xFxS6 (c) compositions measured on crystals in equilibrium with slags (or at subsolidus conditions) of the PbO-“FeO1.5”-SiO2 system in air. Labels indicate the experimental temperatures (°C).
Figure 9. P5FS and P6F1+xS1-x compositions measured on crystals in equilibrium with slags (or at subsolidus conditions) of the PbO-“FeO1.5”-SiO2 system in air, compared to the results in equilibrium with Pb metal [1]. Labels indicate the experimental temperatures (°C).
Figure 10. Magnetoplumbite and 1:1 lead ferrite compositions in equilibrium with liquid (or at subsolidus conditions) in the PbO-“FeO1.5”-SiO2 system in air. Labels indicate the experimental temperatures (°C).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Chemistry"
Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




