Life Cycle Analysis of AA (Alkaline) Batteries
Info: 6873 words (27 pages) Dissertation
Published: 30th Nov 2021
Tagged: EngineeringElectronics
Abstract
At their base, alkaline batteries are nothing more than a chemical reaction taking place between two opposing poles, the anode and the cathode. Despite the fact that most manufacturers today will boast that their batteries do not contain the likes of mercury and that it is therefore acceptable to dispose of spent cells in your household trash bin, the reality of the situation is in fact quite the opposite. Many alkaline batteries do in fact still contain trace amounts of mercury, among other substances that, although deemed inconsequential to the environment, can indeed pose a significant ecological threat if deposited in sufficient quantities. Of course, this is specifically the case with landfills.
So despite their supposed eco-friendly properties, provided enough spent cases accumulate in a landfill, the cumulative effects of some of the toxins and leftover metals in question may ultimately poison the soil and even seep its way into local waterways after many years of sitting in the earth. It is for this reason that a proper Life Cycle Analysis is more than necessary for these types of devices.
One must be well aware of the potential dangers related to the improper disposal of alkaline batteries and the necessity of having easy access to proper disposal systems. This type of information is not only pertinent to the end user, but also to the manufacturer, whose duty it is to strive to make their products as eco-friendly as possible, all the while ensuring that proper channels through which their customers may dispose of the spent products in a safe and responsible manner remain open and easily accessible to the general public.
Table of Contents
Click to expand Table of Contents
Introduction
Inventory Analysis
Manufacturing Process
Cathodes
Separator
Anodes
Brass Nail
Casing
Plastic Seal
Labels
Manufacturing Flow Chart
Input & output
Raw material
Production Process
Waste Management
Packaging
Transportation Phase
Battery Usage
Impact Analysis
Water
Land
Air
Toxicity
Overall Impact
Improvement Analysis
Improvements in Design
Manufacturing and Disposal Life-Time Improvement
Shipping
Conclusion
Work Cited/References
Introduction
Energy was at first difficult to transport from one place to another. The invention of batteries allowed objects to be transported from one place to another where the user can choose when to use its power or not. This energy was possible by the chemical potential energy. This chemical potential energy thus was stored into a container called a battery. The components of most batteries are the top, bottom, the shell, the anode, cathode, and separator.
The typical dimensions of a double AA battery are 5 cm in length and 1.4 cm in diameter. Inside the battery is found two terminals, one of which is an anode and the other is the cathode. In between the anode and the cathode lies the electrolyte also known as the separator. Electrolyte separates the anode and cathode, which allows for chemical charge to flow between them.
The battery can be made of many different combinations of acids and metals [1]. Batteries have energy stored in them from the chemicals. For the battery to release energy the chemicals inside the battery release electrons from the anode to the electrolyte through an oxidation reaction. After it reaches the electrolyte, the electrons are accepted by the cathode on the positive side. This allows for the stored chemical energy in a battery to become electrical energy [1].
The outside of a battery is called the can and that can be made of different kinds of metals that help define the type battery. The can is a cylindrical shape. Inside the can is the acid inside the battery which also varies on pairs decided for best effectiveness. The top of the battery is for the positive charge and the bottom is for the negative charge. The acid metal pair types for the double AA battery have different characteristics. Characteristics can vary based on the length of the battery life, based on the device used for, the effective temperature, the shelf life, materials used, and if the battery is rechargeable [3].
AA batteries can be divided into two general categories describing whether it is renewable or not; Primary batteries being non-rechargeable and secondary batteries being rechargeable [2]. Primary batteries have their chemical energy only can be transferred one way. Secondary can have energy reaction reversed [1]. Batteries can be made with alkaline, Alkaline Manganese, Lithium/Iron Disulfide, Nickel Metal Hydride (NiMH), Zinc Manganese Dioxide [3].
Inventory Analysis
All batteries are manufactured differently since they consist of different chemical components. However, all batteries do go through a similar process. All battery consists of three main parts- cathode, anodes and electrolytes. Cathodes are positively charged whereas the anode are negatively charged. A battery needs a chemical reaction to function therefore, it needs both electrons and protons. A majority of the batteries use these two types of chemicals to form batteries which are zinc as anode (-) and magnesium dioxide as cathode (+) or lithium as anode (-) and copper oxide as cathode (+) but there are many other elements that can be used to manufacture batteries. The one that we will be discussing is zinc and manganese dioxide.
Manufacturing Process
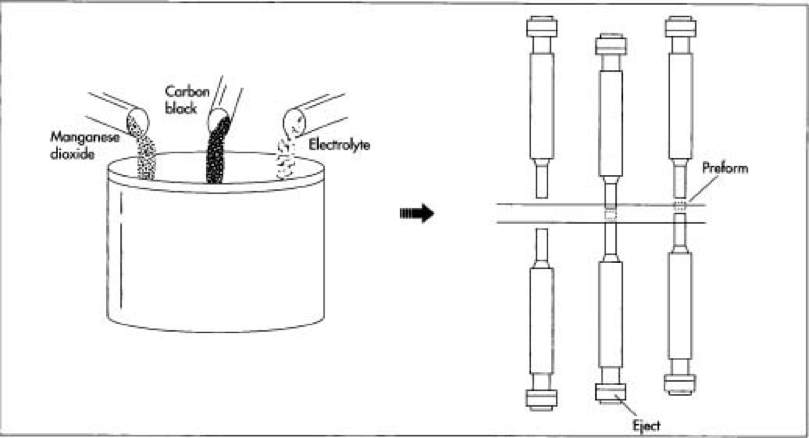
Figure 1- Mixing of Elements (1)
Cathodes
To manufacture alkaline batteries, the process begins by mixing a large amount of manganese dioxide, black carbon and potassium hydroxide (electrolyte) at the production site. These raw materials are obtained by mining and mineral extraction and then used for ideal purposes. These 3 elements are mixed in a large barrel in large quantities. Once the mixture is ready, it is granulated and compressed into preforms which are hollow cylinders. The process requires these preforms to descend into the barrel of the mixture and then compacting each preform. Depending on the battery size being manufactured, many of preforms can be stacked in a battery. Once these preforms are individually packed, these preforms are then inserted into nickel –plated steel cylinder which is what cathode is made from. The cylinder that contains the cathode is made from nickel-plated steel by the process of cutting. Asphalt or epoxy is placed on top of the can to prevent leakage [6].
Separator
The cathode and anode are blocked from being in contact with one another by a separator which can be made from layered paper or porous synthetic material. The layered paper is manufactured from wood pulp. Wood pulp is known as the liquid made from cellulose wood fibers, lignin, water and many other chemicals. The wood pulp is obtained by a process known as CTMP where it involves soaking the wood with sodium sulfate before the grinding of the wood to make the paper stronger [7]. The paper is laid cross grain to each other inside the cylinder. The porous synthetic is the smallest visible unit of a fabric which can be used instead of layered paper [8]. They are made from extruding fiber forming materials by spinnerets (a device used to melt polymers to form fibers) into air and water which forms thread [9]. Layered paper or porous synthetic material can be used as a separator for anode and cathode.
Anodes
The anode which is a thick zinc powder gel is then inserted in the cylinder along with potassium hydroxide electrolytes. The gel isn’t filled to the top since efficient space needs to be left for chemical reactions that occur within the battery when used. The extraction of zinc powder is from electrolysis or smelting. Electrolysis is a process which involves the electrical current to pass through a zinc ore and result in zinc powder. This occurs inside an electrolytic cell where it is covered in a solution containing positive and negative charged ions. This process is used to get fine powdered and pure zinc. Smelting, on the other hand, is a process used to heat zinc ore to obtain powder zinc [10].
Brass Nail
Once the anode is added to the cylinder, a brass nail is inserted two thirds within the centre of the cylinder through the zinc gel. The nail is known as the “current collector” and it aids in transporting electrons to and from the electrodes into the electrical circuit [11]. The nail can be made of Brass or Bronze. Brass is an alloy of copper and zinc. The amount of copper varies between 55% and 95% by weight and zinc varies between 5% and 40% depending on the type of its intended use. In a battery, there must be an equal concentration of both zinc and copper to facilitate the necessary reactions. Copper and zinc are extracted through the process of mining or by leaching and used to facilitate the production of materials. An appropriate amount of copper is melted after the quantity needed. Once the copper is melted, an appropriate amount of zinc is added. The melting operation culminates with the formation of slabs called “cakes.” Once the cakes are cool enough, they are moved to the rolling area for storage. From the stored element, the nails are fabricated at the production site or ordered from manufacturing companies and then inserted to the batteries [12].
Casing
The casing of the battery is the next step of manufacturing a functional battery. The casing of the battery as an important aspect to ensure that it sufficiently converts its chemical energy to electrical energy which leads to powering all electrical devices. The casting consists of plastic seal, metal cover and metal cap [11].
Plastic Seal
The plastic seal in a very thin sheeting that is placed right over the nail. The purpose of the seal is to protect the cathode anode and the nail. It exists to ensure that the potential of the battery doesn’t run out after a certain time, in unison with two other protective layers- the nail and the Metal end cap. The plastic seal can be made from possible layers of various raw materials such as polyethylene terephthalate layers, a polymer layer and a polypropylene layer. The plastic seal can sometimes cause a lot of gas to accumulate leading to the rupture of the battery, but engineers have made the plastic seal layer thinner so that the plastic seal will rupture of a part rather than the entire battery. By adding wax-filled holes in the plastic so that the excess heat may escape through the wax is a solution to prevent rupture. The top and the end of the battery cylinders are then closed with a steel plate which is welded or glued with an epoxy cement [4,12].
Labels
The final step of manufacturing batteries are the placing the labels before they are sent to packing, Labels are generally made from very thin plastic or paper and then glued to the outer part of the battery. Since batteries vary in sizes therefore labels are fabricated based on the dimensions and proportion of the battery. The reason why labels are wrapped around the battery is to ensure the type, size, function and other information to the consumers. These labels must be added before packing and supplying [4].
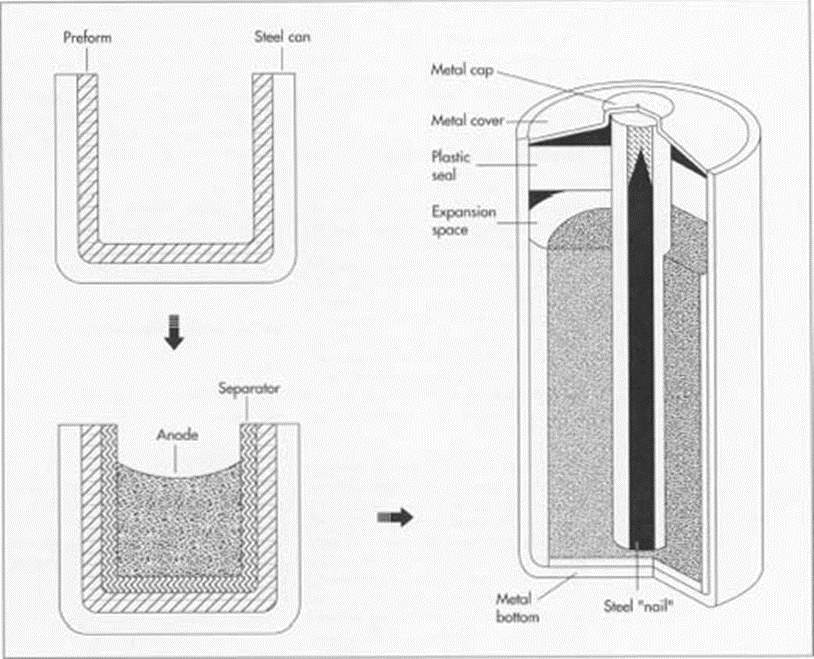
Figure 2- Inside of a battery (4)
Manufacturing Flow Chart
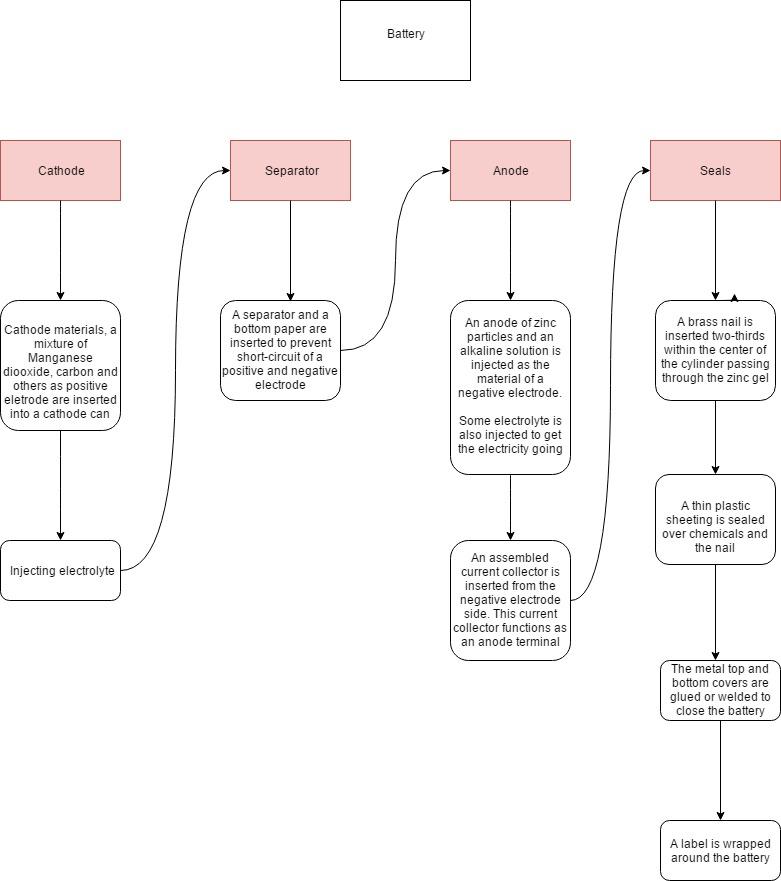
Input & output
Raw material
The standard AA alkaline battery is typically cylindrically shaped, formed with a nickel-plated steel which makes up 25% of the battery [14]. Natural elements are also part of the composition of the battery such as: manganese dioxide, potassium hydroxide solution and graphite. The rest of the battery is composed of plastic and metal used for labels, sealing and other small details to make a fully functioning battery.
Given that all these materials are from natural resources and recyclable, 100% of its product can be repurposed. Cathode is made up by the following elements shown: manganese dioxide, carbon black (graphite), and an electrolyte (potassium hydroxide in solution). Most of these organic matters can be obtained by mining methods [15]. Mining for these materials contributes to the greenhouse gases generated in the mining industry.
The battery also requires a separator that is usually made of paper or a man-made material that is porous. Paper is made with wood chips and a chemical- water mixture, which is then pressured cooked to make a pulp. This pulp can be customized in several ways by different colour dyes and various types of coating/finishes [16]. The use of paper requires gathering material from the lands and forestry sector.
The anode is in the form of gel, which has a thick viscosity with a pasty texture. This substance is produced by zinc powder, potassium hydroxide electrolyte and other compounds.
To seal the battery, it has three components involved: a brass nail is used, a plastic cap and a metal cap. Brass is a metal alloy that consists of copper and zinc, where the cooper is proportionally bigger than zinc. Zinc and Copper can be physically extracted from mines [18]. Plastic caps are produced through processing several organic matters such as cellulose, coal, natural gas, salt and, of course, crude oil [17]. Epoxy glue is also used to adhere the metal cap to the cylindrical body.
For labelling, there are two types of possible material: paper or plastic shrink wrap. The paper label would require glue to be used and is considered to have a loose fit. Comparative to the plastic shrink wrap, it is very heated sensitive and will take shape of an object when exposed to heat, giving a “sealed” tight fit. This is made possible due to a specific class of polymer called polyolefin, a common type of polyolefin is polypropylene and polyethylene which are considered as thermoplastics [19].
Production Process
In large quantity, the cathode is made by mixing manganese dioxide, carbon black (graphite), and an electrolyte (potassium hydroxide in solution). After the mixing thoroughly, the mixture is then granulated and pressed into moulds shaped into cylinders called performs. All performs may vary due to different uses and type of battery. Once moulded, the content is then placed inside the nickel-plated shell and is then sealed off will either epoxy or asphalt. The sealant is present to prevent any type of leakage happening. The separator is applied into the cylindrical shell, where it would cover the cylinder’s walls. This will act as a barrier between the cathode and anode, thus will not interfere in each other’s activities.
The description above is shown by figure 2. The anode is then the last mixture to be placed into the shell. The anode will not fill the entire shell, but rather have enough space for the reaction to occur once the battery is at use. Then begins the process of sealing the battery, a brass nail is placed in the centre of the cylindrical shaped battery from the top. This will pierce through all the content approximately two third of the battery, the nail will aid the current to pass through. A plastic cap is then placed to cover the battery’s opening and followed by a metal cap, at which the nail will go through the plastic cap and welded to the metal end. The other opening of the battery is sealed with a metal cap using epoxy glue [15].
Waste Management
In today’s modern age, it is safe to place batteries into the regular trash bin. Unless the batteries contained mercury, it must be recycled for the well-being of the public’s health. However, not recycling regular alkaline batteries would not be environmentally friendly because it would simply generate more waste for society. The main solution to deal with such waste is to recycle alkaline batteries. By recycling, it will generate less waste, reduce the amount of pollution, energy is saved and reduce the need to import from outside sources.
Packaging
In order, to sell separate batteries in packages. Those packages are composed of plastic and paperboard. Some companies prefer to package them in bulk. According to the regulations, the manufacturers need to make proper packaging to deliver their products. For instance, each package must be labelled with flammable icon, in order for the workers to know that they are handling hazardous material. Furthermore, the batteries need to be isolated with plastic in order to avoid any short-circuit and fire hazard. The packaging must also be strong enough to resist a fall from up to 1.2 m [20]. According to Duracell, the normal packaging of a battery consists of 2 cardboard and clear plastic covering [21]. For the packaging, they use 55% of recycled material, reducing the carbon footprint caused by the packaging of batteries [22]. This packaging can be recycled as it is designed for consumers. The use of plastic and paperboard have an impact on the emission level as that material is gathered from natural resources. This contributes to the annual C02 emission already existent in the market.
Transportation Phase
Most of the double AA batteries manufactures are located in China (i.e around 2000 manufacturers) [23], therefore, the transportation plays a role in the life-cycle analysis. For instance, using the distance the battery travel as a parameter an estimation of greenhouse gases can be done. In one research, it is shown that the transportation accounts for only 4% of the emission related to the production of batteries [24]. Transportation, carbon footprint is important as it accounts for 26% of the total US greenhouse emission and has been rising every year [25]. Therefore, imports of batteries is an important matter in the greenhouse gases related to the production of batteries; however, it is only a small part of the production of a battery.
Battery Usage
Double AA batteries are commonly used for household objects to power small electronic devices. In general, if the batteries are not used and properly stored they can last between 3 and 10 years. If the battery is primarily composed of lithium, it would store for at least 10 years, whereas if it is composed of nickel it may last from 3 to 5 years [26]. The battery composition can be recycled. Material such as steel, zinc, manganese and potassium can be recovered from the battery [27]. Nevertheless, “each year, Americans throw away more than 3 billion batteries.” [28]. This contributes to the carbon footprint related to waste of batteries. It is also important to note that 98 to 99% of lead acid batteries are being recycled, hence the emphasis on recycling the batteries once they no longer work [27]. By recycling batteries, material can be re-used as an amount of the minerals can be extracted, hence reducing greenhouse gases due to the production of double AA batteries.
Impact Analysis
Water
When the casing of alkaline batteries corrodes, the chemicals within may leak into the soil and make its way into water streams such like rivers and oceans. As mentioned before the main components of alkaline batteries; zinc and manganese oxide are not toxic. However, a higher concentration of these minerals can change the PH level of water and modify its quality. For example, a high concentration of zinc is known to be toxic to some aquatic life, such as fishes and invertebrates [29] Thus, the presence of potassium hydroxide (KOH) as an electrolyte in alkaline batteries could be corrosive not only toxic. A high concentration in water will result in toxic effects on aquatic organisms. Also, it will raise the PH of water when present in high concentration. However, a small quantity of KOH will be neutralized by other substances present in water.
Land
According to EPA (AA) batteries represent up to 20% of the household hazardous materials in America's landfills. As the battery casing corrodes, chemicals leak into the soil and make their way into our water supply [31]. The major components in alkaline batteries are zinc and manganese dioxide. Both components do not pose a direct threat to the environment. Definitely zinc and manganese are essential for the growth of many crops but an excess concentration in soil may be toxic. Another component in alkaline batteries is nickel-plated steel cylinders. Once released into the environment after batteries degradation, nickel ends up in soil and attaches particles containing iron or manganese. Under acidic conditions, it may seep into groundwater. Also, it is important to mention that the chances of mercury leakage are very small and almost negligible [30].
Air
Alkaline battery is composed of harmful metal even though it is not considered a hazardous material due to its small doses of quantity, it can still have a huge impact to the environment [41]. More specifically, an impact on the Air we breathe. When alkaline are thrown away with the household wastes, they are transported to the landfill. At the landfill, they go through photochemical reactions. This creates gas emissions of various types including ozone and many more harmful gases. Even though battery is not the only thing contributing to the greenhouse effect, it clearly still does contribute to the global warming issue [42]. It is almost important to note that battery have toxic leakage. Chemicals that are leaked from these batteries could be exposed and/or could react to other chemicals at the landfill. Hydrogen gas is generated. These, as we know, are extremely flammable. Thus, creating an explosive atmosphere and releasing more toxic chemicals in the atmosphere [43].
Toxicity
In May 13, 1996, Congress passed the Mercury-Containing and Rechargeable Battery Act [33]. Due to this, alkaline batteries now contain little to no traces of mercury in them. Because of the Battery Act, many individuals believe that it is safe to throw away their Alkaline Batteries with their household wastes. Many popular companies such as Duracell and Energizer also promote and clearly state on their website that alkaline batteries are not required to be recycled and that they can safely be disposed with household wastes [38]. However, it is important to note that theses household wastes are transported to landfills [34].
Unfortunately, despite having little to no mercury in alkaline batteries, these batteries still contain other chemicals and metals that are not only harmful to the environment but to us humans as well [36]. For example, alkaline batteries are still toxic because their leakage contains potassium hydroxide [35]. Potassium hydroxide is harmful to humans and are hazardous/exposed to skin, eyes, ingestion and inhalation [37]. It is important to note that alkaline batteries also contain metals such as zinc and manganese which are hazardous [40].
Yes, these chemicals and metals are discarded because they are marked as safe (non-hazardous waste) by the Environmental Protection Agency (EPA) due to their doses of impact in small quantity [41]. But when these chemicals and metals accumulate through time they become a huge quantity. According to EPA, on average, more than 86,000 tons of alkaline batteries are thrown away every year. In terms of size, if one was to line these 86,000 tons of these batteries. It could potentially go around the world about six times [39].
Clearly, the metals in these alkaline batteries accumulated over a year are huge and exceed the “doses” that are marked safe by the EPA. These batteries are actually still required to be recycled in order to be disposed properly because these chemicals and metals are accumulating and piling up at the same landfill which is used to store wastes. It is always good to remember that these chemicals and metals are leaching into the soil which is causing damages to our ecosystem and they are also going into our water supply [34].
Overall Impact
Although alkaline batteries no longer contain traces of mercury in them, it can still not be disposed into any landfill. Removing mercury had a net positive outcome but there has been a resulting downside to the revelation.
These batteries still contain certain chemicals and metals that are harmful to the environment. These components were labelled as safe by the EPA in small doses which is a great issue since it allows these batteries to be thrown into the environment. These chemicals will slowly add up and become a large amount and will harm the environment [24]. Each battery contains only a small amount of non-safe elements which is why they are considered safe to be thrown away whereas if the same elements were thrown away in a larger scale, it wouldn’t be safe anymore [46].
These chemicals endanger the air, ground, waterways, and the environment. This is why most battery types should not be categorized as safe to throw away. The most dangerous elements for the environment that come from batteries are lead, mercury, and cadmium. The effect they have on the ecosystem is harmful towards the animal and plant life. They can cause illnesses and even prevent them from reproducing.
Improvement Analysis
Improvements in Design
The City of Richland's Environmental Education Programme claims that about two-button batteries, and ten disposable alkaline batteries are owned by an average resident of the United States. Additionally, there are about eight household batteries per year, in average, that are being thrown away, resulting in water, soil, groundwater, and surface water pollution [49]. Thus, we can conclude that the disposal issue regarding the alkaline batteries occupies the centre stage due to its deteriorating effects on well-being of humans and the environment surrounding them. However, the improvement of design and manufacturing processes of alkaline batteries could lead to a significant reduction of these impacts [50].
The improvement of design can be done by reducing the mass of steel in the battery casing and by improving the performance and the useful life of the battery.
Firstly, the reduction of the thickness of steel in alkaline batteries could be accomplished by engineers with the aim of achieving the same performance and reliability as that of a typical alkaline battery. Since steel use generates a big environmental footprint, this opportunity would help in making the environment less polluted. In 2011, a study team from the University of California claimed that many battery manufacturers have already considered reducing the steel composition of their batteries due to reasons of cost and competitiveness among the world’s major battery companies. However, an additional five percent reduction in steel mass might be achievable and more environmentally friendly, taking into consideration the reduction of greenhouse gas emissions this opportunity could result in [50].
Secondly, battery lifetime extension is another way to reduce the impact of alkaline batteries. According to a case study from the California Department of Resources Recycling and Recovery, if engineers expand the lifetime of batteries by 30%, the annual purchases of new batteries would be reduced by a factor of 1/1.3. This results in the demand of fewer batteries over the same period to meet residents’ needs. The table below shows the analysis details of these two opportunities [50].
| Opportunity Area | Analysis Details | Analysis Case | |
| Reduced steel mass in casing | Key assumption(s) | Partial savings | Maximum savings |
| GHG emissions savings compared to base case (Mg/yr) | Steel mass in casing reduced by 2.5% | Steel mass in casing reduced by 5% | |
| Extended lifetime | Key assumption(s) | Battery lifetime extended by 15% | Battery lifetime extended by 30% |
| GHG emissions savings compared to base case (Mg/yr) | 16,900 | 33,600 | |
Table: Summary of analysis assumptions for life-cycle improvement opportunities. (Contractor’s Report to CalRecycle, page 17) [50]
Manufacturing and Disposal Life-Time Improvement
Several ideas were pitched decades ago to find a way to improve the efficiency of alkaline batteries. For example, reusable batteries were introduced in 1992. The advantages of these batteries included inexpensiveness (compared to disposable batteries), multiple recharges and low self-discharge [51]. However, it suffered from a low life-cycle and could only be used for flashlights and other light-duty implementations.
Today, the market continues to look for ways to increase energy consumption and the life-cycle for AA batteries. Microelectronics is a perfect example of a technology that has steadily grown over the 20th century to the point that you could shrink a heavy-duty car battery to the size of a small coin if batteries have been blessed with such advancements [52]. Yet, batteries work through an electro-chemical process which has its own limitations.
Research addressing the problem can only gain 6% in energy capacity for these batteries per year. The logic here is that if you increase the lifetime of the battery, consumers wouldn’t need to buy more batteries therefore aiding the environment in the process since you’re also reducing waste. But a case study conducted by the State of California and its Department of Resources Recycling and Recovery found out how the supply chain could be improved to reach its maximum efficiency through a peculiar method called: eStep Modelling Methodology [53].
The methods goal is to reduce the greenhouse gas emissions coming from every sector (manufacturing, commercial, agricultural, mining, etc.). The method assumes every component of the chain respects and practices the method whether it’s a primary sector or a really distant supplier [53].
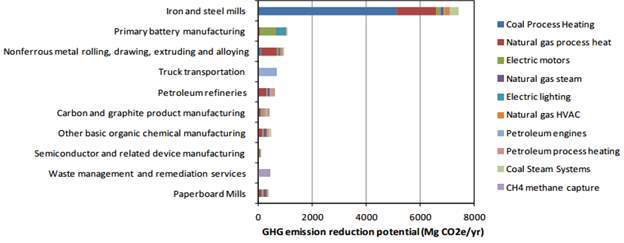
From this chart, the model indicates that iron and steel mills are a number one sector that could be drastically improved when 288 million dollars’ worth of batteries were manufactured in California in 2010. Even though we are looking at a lifetime away from groundbreaking alternatives to alkaline batteries like superior electro-chemical batteries, plenty of businesses around the world are looking into their research and development departments to find ways to recycle the batteries.
To expand on my point, the EU put out regulations to force manufacturers to recycle 45% of waste batteries by 2016 which equals to about 120 000 metric tons of batteries. RecAlkaline Oy(Ltd) is a Finnish company that boasts how it can exceed the standards put out by the EU. The recycling process will yield manganese and zinc which are necessary for the growth of plants. Plus, the minerals could also increase the efficiency of fertilizers. This would bring the recycling rate to about 90% compared to the other recycling lines who do 50%.
AkkuSer owns 49% of RecAlkaline and has now invested greatly in the technology that has been praised to be safe since it operates at room temperature [54]. This goes to show that there is a market to be filled in the recycling of batteries that can further help save the environment and diminishes waste in landfills.
Shipping
Batteries that are not sealed appropriately can cause leakage, thus may cause the battery to implode due to the numerous chemicals involved. As a result of these chemicals, disposal of batteries can be damaging to the environment [56]. The shipment of batteries is dependent of the state or province, if they are deemed as a hazard. Most states will classify alkaline batteries, as a public health risk thus requiring large amount of paperwork. In consequence, a specific way of packaging is mandatory where, typically a strong wooden box or a fiberboard is used [58].
Conclusion
Batteries and with improvements made upon them have made them more versatile. From the initial creation of a primary battery allowing us to keep a charge while on the move. To the creation of secondary rechargeable batteries where we waste fewer resources. Then with the inclusion of different battery types, we can use our resources more efficiently thus be more efficient in resource use. Over time the development more environmentally friendly methods for the production and disposal of batteries helps will greatly help our global community.
Work Cited/References
"How Does a Battery Work?" MIT School of Engineering. N.p., 01 May 2012. Web. 20 Mar. 2017.
Crompton, T. R. (2000). Battery reference book. Oxford : Newnes: Reed Education and Professional Publishing. (page ix) Retrieved March 21, 2017
"Battery Comparison Chart." Energizer. N.p., n.d. Web. 21 Mar. 2017.
"Battery." How Products Are Made. N.p., n.d. Web.
"How Do Batteries Work?" NorthWestern. N.p., n.d. Web.
"Alkaline Battery." Wikiwand. N.p., n.d. Web.
"Types of Pulping Processes." Paper Online - Types of Pulping Processes. N.p., n.d. Web.
"Synthetic Fibers and Fabrics Information." GlobalSpec. N.p., n.d. Web.
"Synthetic Fiber." Wikipedia. Wikimedia Foundation, 29 Mar. 2017. Web.
"Zinc Processing." Encyclopædia Britannica. Encyclopædia Britannica, Inc., n.d. Web.
Howard, Rick. "The Role of Battery Materials and Electrode Fabrication in Cell Performance." Blog.flexelbattery.com. N.p., n.d. Web.
"Brass." How Products Are Made. N.p., n.d. Web.
BatteryEducation, Author. "What Materials Are Used To Make A Battery?" Battery Education. N.p., n.d. Web.
"What's Inside A Battery." What's Inside A Battery. Raw Materials Company Inc., n.d. Web. 05 Apr. 2017.
"Battery." How Products Are Made. N.p., n.d. Web. 05 Apr. 2017. CHECK #4
Smith, Vern. "How Paper Is Made." WI Paper Council. N.p., n.d. Web. 05 Apr. 2017.
"How Do You Make Paper From a Tree?" Wonderopolis. N.p., n.d. Web. 05 Apr. 2017.
"Copper Extraction." Wikipedia. Wikimedia Foundation, 29 Mar. 2017. Web. 05 Apr. 2017.
"Thermoplastic." Wikipedia. Wikimedia Foundation, 04 Apr. 2017. Web. 05 Apr. 2017.
IATA. Lithium Batteries - Significant Changes on the Way. N.p.: International Air Transport Association (IATA), n.d. PDF.
"Battery Packaging Design." Behance. Behance.net, n.d. Web. 05 Apr. 2017.
"Battery Care, Use and Disposal." Duracell Batteries. N.p., n.d. Web. 05 Apr. 2017.
"Alkaline Battery Market." Frost.com. N.p., n.d. Web. 05 Apr. 2017.
Masanet, Eric, and Arpad Horvath. Single-Use Alkaline Battery Case Study. Berkeley, California: CalRecycle, May 2012. PDF.
"Sources of Greenhouse Gas Emissions." EPA. Environmental Protection Agency, 14 Feb. 2017. Web. 05 Apr. 2017.
"How to Store Batteries." Battery University. N.p., n.d. Web. 05 Apr. 2017.
"What's Inside a Battery?" What's Inside A Battery. N.p., n.d. Web. 05 Apr. 2017.
"Battery Statistics." Everyday-Green.com. N.p., n.d. Web. 05 Apr. 2017.
Sound Environmental Management of Spent Primary Batteries. N.p.: The Association of Electrical Equipment and Medical Imaging Manufacturers, Nov. 2001. PDF.
"Toxic Substances Portal - Nickel." Agency for Toxic Substances and Disease Registry. Centers for Disease Control and Prevention, 21 Jan. 2015. Web. 01 Apr. 2017.
"How Do I Recycle?: Common Recyclables." EPA. Environmental Protection Agency, 05 Jan. 2017. Web. 01 Apr. 2017.
"Environmental Effects Associated with Battery Disposal." Frost & Sullivan. N.p., n.d. Web. 01 Apr. 2017.
PUBLIC LAW 104–142. N.p.: 104th Congress, 13 May 1996. PDF.
"What Do Batteries Do to the Environment If Not Properly Recycled?" What Do Batteries Do to the Environment If Not Properly Recycled? | Education - Seattle PI. Seattle PI, 13 Nov. 2013. Web. 02 Apr. 2017.
"Clean Up After an Alkaline Battery Leak." Alkaline Batteries. N.p., 01 Jan. 1970. Web. 02 Apr. 2017.
"Disposal of Batteries." University of California, San Diego. University of California, San Diego, n.d. Web. 02 Apr. 2017.
"Potassium Hydroxide." The National Institute for Occupational Safety and Health (NIOSH). Centers for Disease Control and Prevention, 01 July 2014. Web. 02 Apr. 2017.
Murphy, Mike. "FYI: It’s Totally Fine to Throw Away Most Batteries." Quartz. Quartz, 23 Jan. 2015. Web. 02 Apr. 2017.
"Battery Statistics." Everyday-Green.com. N.p., n.d. Web. 02 Apr. 2017.
"Alkaline Batteries." Electrical4u. N.p., n.d. Web. 02 Apr. 2017.
"BATTERY DISPOSAL." InfoHouse. N.p., n.d. Web. 02 Apr. 2017. http://infohouse.p2ric.org/ref/07/06033.htm
Merry, Micheal. "Environmental Problems That Batteries Cause." Sciencing. N.p., n.d. Web. 02 Apr. 2017. .
"Energizer Design Considerations - Hydrogen Generation." Energizer. N.p., n.d. Web. 02 Apr. 2017.
Mercury in Batteries." EPA. Environmental Protection Agency, n.d. Web. 30 Nov. 2016.
http://www.calrecycle.ca.gov/publications/Documents/1433%5C20121433.pdf
http://www.epbaeurope.net/090607_2006_Oct.pdf
Batteries for IT Systems: Environmental Issues." Greenit. GreenIt, 2005. Web. 30 Nov. 2016.
3Battery Waste Management Life Cycle Assessment." Epbaeurope. ERM, 18 Oct. 2006. Web. 30 Nov. 2016.
"Environmental Effects Associated with Battery Disposal." Frost & Sullivan. N.p., n.d. Web.
Masanet, Eric, and Arpad Horvath. Single-Use Alkaline Battery Case Study. Berkeley, California: CalRecycle, May 2012. PDF.
"Will the Reusable Alkaline Battery Have a Future?" Battery University. N.p., n.d. Web. 02 Apr. 2017.
"The Future Battery." Battery University. N.p., n.d. Web. 02 Apr. 2017.
Masanet, Eric, and Arpad Horvath. Single-Use Alkaline Battery Case Study. Berkeley, California: CalRecycle, May 2012. PDF.
http://www.recalkaline.fi/docs/rec_alkaline_recycling_alkaline_batteries.pdf
"Recycle Your Batteries." Call2Recycle®. N.p., n.d. Web. 03 Apr. 2017.
Asde. "Did You Know That Lithium Batteries Are Dangerous Goods?" ASDE. N.p., 11 May 2016. Web. 03 Apr. 2017.
"Wastesaver Technologies LLC." Metal Finishing 93.6 (1995): 74. Web. 03 Apr. 2017.
"Shipping Batteries or Devices with Batteries." UPS. N.p., n.d. Web. 03 Apr. 2017. https://www.ups.com/content/ca/en/resources/ship/packaging/guidelines/batteries.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Electronics"
Electronics regards the science and technology involved in the development of electrical circuits and electronic devices and equipment that use them.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




