Medical Device Technology Industry Operational Excellence Models
Info: 8191 words (33 pages) Dissertation
Published: 16th Dec 2019
Tagged: Medical Technology
Learning Requirement:
Based on my research of the Med Tech industry and the range of Operational Excellence models that exist, the aim was to develop a new conceptual Operational Excellence Assessment model that identifies the critical parameters (e.g. product, process, culture, etc.) that are essential for current and future success in any Med Tech business and can be utilised to assess and drive improvement across the Design Cycle of a medical device.
- Introduction; Overview of a new Business Model, ‘The approach to Operational Excellence called-Hexygon’ P. 1
- Targeted Disease or Disorder P.1
- The Medical device P. 1
- The new OE model P. 1
- Key Principles of the Model P. 2
- Part 1 of the OE Model:: p. 5
- Part 2 of the OE model p. 9
- How to use new OE model would be used in an organisation p. 21
- Framework assessment tool and how an organisation would use a framework to asses a new OE model p. 24
- Two level new improvement plan for continuous improvement p.25
6.0. References p.26
- Introduction; Overview of a new Business Model, ‘The approach to Operational Excellence called-Hexygon’
Before discussing this new operational excellence model the author of this work wishes to briefly introduce the medical device and targeted disease, disorder or ailment, which this new OE model could be applied to.
- Targeted Disease or Disorder
Severe chronic pain is a wide and complex spectrum therefore the area of focus for this document is a medical device that targets severe chronic pain, its treatment, prevention and or management of Chronic Severe Migraine (CSM) leading into Cluster Headaches (CH). Both usually last a minimum of between 4 to 72 hours and can even continue into several days and weeks. This pain can be accompanied by numbness, or pins and needles compounded by a hot burning sensation of the skin (paresthesia) paticullary the face, temple and ears. The suffer may experience thought and or speech difficulties, vision problems and confusion. In early diagnoised and for long term sufferers as well, this experience can be a frightening one as these symptoms are similar to those of a stroke. This weakness may last from one hour to several days, but usually it goes within 24 hours refer to (MigraineTrust 2017).
(Fischell et al 2008) described such types of headaches as a “human affliction” and stated that migraine headaches occur in approximately 11% of the population in the U.S.A. and Western Europe each year with more than 2.5 million people in the U.S.A. The number of migraine sufferers in Ireland according to (Tompkins 2016) is approximately 0.5 million or about 15 % of the total population. While the (Migraineresearchfoundation 2017) affects 39 million men, women and children in the U.S. and approcimately1 billion worldwide. Prof. Peter Goadsby considered by peers to be the world’s leading researcher on migraine and cluster headaches was quoted by (choosenatural. 2017) paraphrased here and said, “Women with cluster headache will say such an attack is worse than giving birth without anaesthetic, imagine such cranial pain once or twice a day, for between six to ten weeks at a time”. In closing, this affliction is particularly close to the author of this paper whom is a chronic migraine sufferer.
- The Medical device
The device is called the gammaCore and is a neuro-modulation or electro stimulus handheld placement device available by prescription only. It contacts the skin and delivers electrical stimulation to the vagus nerve. It positioned in neck region as shown in figure 1. It is new device to this field medicine and has shown promise in treating different types of migraine and primary headache aliments. Suffers hold the device against their neck and the device sends a mild electrical signal via metal electrodes and conductive gel through the skin to the vagus nerve. On April 18, 2017 the FDA approved the device for use and according to the manufacturer (electrocore. 2017) who evaluated 85 patients with episodic attacks found that 34% of patients experienced a reduction in pain from episodic attacks about 15 minutes after use. The device according to the manufactures is about the size or slightly larger than a mobile phone and a conductive gel is required and applied on the metal stimulation electrode surfaces and it’s then placed on the neck. An early version of the device had a thumbwheel which is used to vary the stimulation strength until the patient feels a mild sensation underneath the skin. Each dose takes approximately 90 seconds with each complete treatment just under two minutes. The image in figure 1 is taken from (gammaCore 2017).
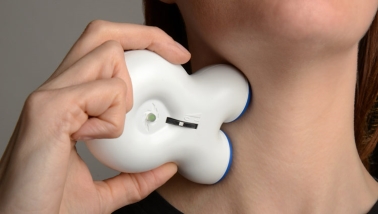
(Image taken from http://gammacore.co.uk/about-gammacore)
Figure 1. The non-invasive gammaCore® device being applied for vagus nerve stimulation
- The new OE model
In order to formulate a new OE model the author of this work looked to nature which is the greatest teacher of all. Nature in many way is a very complex system with infinite integral parts which all seem (at least with current understanding) to work in complete harmony. Within this infinite system, the author looked in particular to the extremely busy but efficient environment of bees and their life around the honeycomb structure. Such a structure serves as a sturdy home against the elements, food store for the winter months, nursery for the next generation, family and community space for development and living while providing a working environment in order to plan for the future and inevitable change. The author of this paper comes from an engineering background and looked at the honeycomb hexagonal structure and conceptualised a new OE model entitled “Hexygon; A New Operational Excellence Model” for Medical Devices. This new model is based on a theme of efficiency of design, functional use, strength, integration, seamless connectivity, lean development, future planning, operational excellence, continuous improvement, living and working cultural.
In line with the core principle or teaching of (Sheedy 2017) the author thought that such a structure is optimal in design and therefore fitting to the “Sheedy Principle” which states that a system which has no waste but has no value or value add is almost pointless and not beneficial to either individual or company. In other words, it is important to have a system with value over no waste, because a system which has no waste but bring no value will not sustain in a business context.
On a scientific and engineering note this new OE model has beauty, form function and efficiency. Such geometric shapes were not fully understood until (Hales 1999) a mathematician proved that hexagons divide a plane into shapes of equal area with the least perimeter or land mass. Hexagons also have the densest circle packing of the plane, where every circle is tangent to 6 other circles which fill just over 90% of the area of the plane in question, therefore this is a pretty efficient context overall. No other shape provides such an optimal use of space within bounded environments, efficient use of materials, functionality, and strength to weather time and the changes it brings. The hexagonal structure is one of the most stable and efficient structures known in the modern world.
Such structures allow the minimization of the material, efficient use of space with minimal weight and because of its formation; it has maximum strength for load bearing when times are tough. When one thinks about this in an OE sense, design, compactness, functionality efficiency, lean use of man and material combined with strength for functional longevity what could be a better basis for a new lean OE model. Figure 2 shows a graphical representation of the model.
 Figure 2. Graphical representation of Hexagon; The new OE model for medical devices
Figure 2. Graphical representation of Hexagon; The new OE model for medical devices
- Key Principles of the Model
In the context of medical devices, the model has two very distinct parts named parts 1 &2. In total, both parts make-up 14 Hexagonal integrated cells. The left hand side (part1) of the model addresses the primary high-level requirements that all medical device companies need to address with before ever a device is even conceptualised. This consists of six distinct parts or cells, as follows;
- Compliance with regulation and directives
- Compliance with standards (eg ISO, ASTM, BS)
- Specifics: As in what sector of medical device is it, i.e. wearables, implantables, diagnostic’s
- Sales and operational planning
- Understanding the Cost of Quality from Taguchi loss function
- Culture, is the company’s culture right in order to succeed?
The right hand side (part 2) of the model deals more with specific methods and tools of operational excellence and lean initiatives. Methods such as these are used daily by a growing number of companies to carry out and ensure the sustainability of operational excellence, lean initiatives and performance which if done well, will ensure longevity and growth in an ever volatile market, the eight distinct parts or cells, are as follows;
- Vision/Idea; The medical device itself, what is it?
- Goal; Demographic; Disease Targeted for Treatment
- Strategy
- Process Planning
- Creativity
- Tracking, Measurement, Documentation
- CAPA
- Success and Continuous Improvement
It can be seen in figure 2 the gap between the left and right parts of the model (cell 1.6) there is an integration piece. For explanatory purposes only; this connectivity is depicted by two workers with colour coded red and blue cables. The key thing to note about this integration is that right hand side of the model should never come before the left hand side. That is to say that is pointless and dangerous in terms of medical devices to try and apply lean tools and operational excellence models to any medical device if the company has not first understood that the regulatory requirements, standards, specifics of device sector. Secondly, from a profit share and functional business perspective sales/operational planning, cost of quality and culture need to be just right and understood in order for a business to work.
In other words if one doesn’t know what value add you want to bring to the market, who you want to help, know that you need to plan, know where growth for shareholder is versus the cost of quality and finally above all know that the regulations and standards must be followed and adhered too, then you won’t have a business of any kind in the medical device space to apply lean and operational excellence initiatives to.
Linking this train of thought to Business Excellence Framework (BEF) (Saiglobal 2017) states that (BEF) is an integrated leadership and management system that describes the elements essential to sustainable organisational and operational excellence. The framework has proven relevance to all organisations specifically medical devices companies. The (BEF) can be used to assess and improve any aspect of an organisation/division including strategy, leadership, planning, people, information, product quality, knowledge, safety, service, delivery, and most of all results. The key benefits of (BEF) is to engage teams (i.e. right culture) in the process of CI, increase staff satisfaction, improve decision-making capabilities, increase capacity to manage change, demonstrate to key stakeholders that the organisation and department has a structured and systematic approach to improving and achieving best practice and excellence. In summary, the (BEF) is based on enduring principles of continuous improvement organisational improvement and organisational excellence. With this in mind this introduction section explains the key elements that should be considered first before constructing a new OE model.
- Part 1 of the OE Model:
2.1.1. Cells: 1.1,1.2 &1,3.
In order to design a new operational excellence model (OE) it’s important to approach this model with high degree of “regulatory and standards” inclusiveness. The three key pillars required for this approach in a medical device context is shown in figure 3. This figure shows the triangular method proposed by (Blunnie, 2017). The figure’ sets out graphically “the three pillars” that have to be present and used when talking about OE models in a medical device context. Starting at the top of the triangle is (pillar 1) which is what everyone must do as set out by the regulation and directives, (pillar 2) is how and what to do as set out by the standards and (pillar 3) is what specifics is one addressing (i.e. is it wearable, implantable, drug, device or a combination of both). Note for this triangular tool to work pillars 1 & 2 should to be considered first and then fed into pillar 3. In summary, the discussion presented in this introductory section describes where a new OE model should FIRST BEGIN from in the context of QSR, standards and cost of quality for both the EU and US medical device markets.
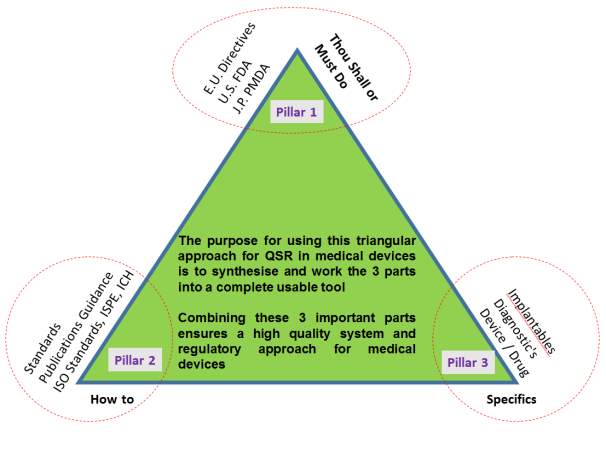
Figure 2. Triangular approach /tool to understanding and discussing the basis of OE
Note (see reference section) but the QSR and International Standards considered in this triangular tool are, see reference section for full references of these
- European Directives (EU) 90/385/EEC-AIMD, 93/42/EEC-MDD superseded by
(EU) 2017/745 MDR
- European Directives (EU) 98/79/EEC-IVD superseded by (EU) 2017/746 IVD
- USA Directive; (US) FDA 21 CFR Part 820 for medical devices
- Standards: (EN ISO 13485-2016),
- (EN ISO 14971-2012),
- EN ISO 9001 and cGMP
2.1.2. Cell: 1.4
According to (Dodd 2011) modern operational excellence (last 6 years) is 75% Lean principles/methods tools, 25% sales, and operational planning (S&OP) in terms of effort, focus and direction. The author states that S&OP comes first because it sets the overall direction of the organisation and this is why it features in part 1 of the “Hexagon OE model”. Figure 3 depicts this belief as a waterfall model (left image) for medical devices similar to the waterfall diagram used in design controls refer to (EN ISO 13485:2016–3, 2017) and figure 4. Also shown are the key industry dynamics (right image of figure 3) comparing medical devices to other industries like consumer-packaged goods (CGP).
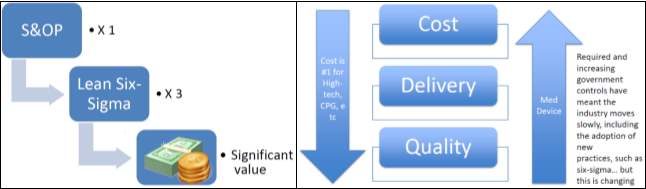
Figure 3. Steps of S&OP, and Lean leading to significant values for medical device companies and key industry dynamics
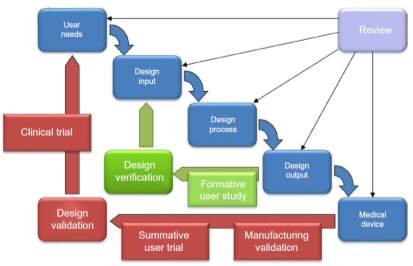
Figure 4. Medical devices waterfall diagram used in design controls refer to (EN ISO 13485:2016-3, 2017
2.1.3. Cell 1.5: The Cost of Quality
According to (Blunnie 2017) the Taguchi loss function is the standard model used to understand the Cost of Quality as shown in figure 5. The image shows that loss in value progressively increases as variation increases from the intended mean condition labelled T. This graph describes quality and cost of quality but also helps understand Continuous Improvement (CI) with the greater goal of implementing O.E.
The Taguchi loss function explains that from the customer’s point of view the drop in quality is not apparent or sudden. The model says that the customers experience a loss of quality the moment product specification deviates from the ‘target value’ T. This ‘loss’ is depicted by a quality loss function that follows a parabolic curve. The loss is given by L(y) = k(y–T)2, where T is the theoretical quality characteristic (i.e. target value or mean value) and y is the actual size of the product, with k being the constant and L the loss. This means that if the difference between ‘actual size’ and ‘target value’ i.e. (y–T) is large, loss would be more, irrespective of tolerance specifications.
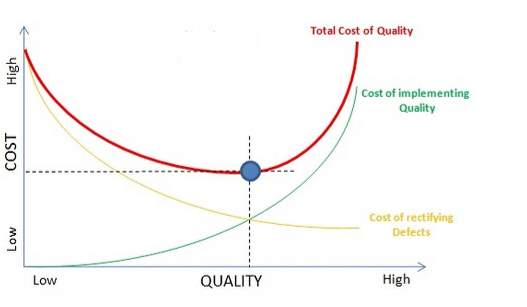

Figure 5 Cost of Quality using Taguchi Loss function
2.1.4. Cell 1.6: Culture
The author looked to the regulations on this to try and understand culture in the medical device setting. The FDA particularly the (FDASIA 2012) have started thinking ahead plus doing more company audits based on the FDA Safety and Innovation Act (FDASIA). Its focus is on understanding, measuring, rewarding the development and growth of a quality based culture within business and across all levels. This new scrutiny from the FDA means more and more companies are driving a principal of quality based staff culture.
Nypro_Jabil Bray Ireland (where the author of this paper works) expects all staff to behave in a professional manner based on a quality based culture and value system. (Creaner 2014) who founded GetReskilled Inc is a practitioner (claiming over 30 years of experience) in the field of cultural values within business particularly in the life sciences and claims to have trained over 3,000 pharmaceutical and medical device operators since 2010. (Creaner 2014) also stated that at International Society for Pharmaceutical Engineering 2013 (ISPE) annual meeting a key statement from FDA representative was to “Develop a quality culture from the ground up, all the way to the executives.”(Creaner 2014) also states that quality issues in a cultural context are often two things:
- Human error and the tedium of repetitive work
- Error reporting; brining problems to the attention of superiors can sometimes be difficult in certain environments (because it could be deemed that one is questioning authority
To offset this (Creaner 2014) also states the following ideologies as positive ways to incentivise a quality-based culture and the author of this work uses some of these here as a basis of culture for the new OE model.
- Making it personal connecting what you do to real life symptoms and health
Creaner’s ideas tie into what Nypro Ireland do. Nypro have a saying that “all staff members should be mindful of what they do as the next patient might be you”. This is a simple statement, which connects the process and device to actual diseases or symptoms while clearly connecting what staff do to curing or alleviating symptoms and enabling better quality of life and the struggle of illness.
- Facts and data (The foundation of engineers)
Managers specifically engineers now recognize that using data-based training can help staff to ask questions about the “engineering and science,” rather than be in a position of questioning upper management about a quality issue.
Where possible, management should measure for levels and increased levels of capability, which then feed training records for annual performance reviews.
- Psychometric testing can aid data and measurement
Nypro Ireland carry out such testing on all managers in order to identify conscientiousness which has been found to relate to being alert to possible errors /contamination issues. Testing is usually conducted with different scenario-based questions to identify key underlying traits that staff may not be aware of and indicate how staff might behave under certain circumstances.
Such studies can demonstrated a high correlation and similarity between the behaviour / characteristics of staff such as (analytical, imaginative, conceptual etc). The belief that once a type of cultural profile is identified a behavioural-based positioning system can then be employed to ensure a better natural quality and cultural fit for the staff and the whole the organisation.
- Saying something important if the situation require it
Personnel with strong cultural values have a variety of different traits such as
- Conscientious; pays attention to quality issues; detail-oriented; follows procedures, rules, and regulations
- Planners; systematic, organized, and plans ahead
- Thinkers; are more likely to be distracted due to inattentiveness
- Employee training and upskilling a form of appreciation
Taking time to offer regular, consistent quality training shows a company wiliness to invest in its staff and this can be morale boost for employees
- Part 2 of the OE model
Note: Please refer to the accompanying excel document entitled “BE Excel Model Vincent Lawlor Part 2 OE Assignment” which is linked to part 2 of the OE model. The eight cells (2.1 to 2.8) are shown in Figure 6 and 7, also refer to the sheet BE MODEL (IDEA APP MED DEVICE) tab of the excel sheet.
Figure 6 is a screen grab of the excel model itself and associated tab/sheets.
There are seven sheets in the excel model in total and listed as follows;
- BE MODEL (IDEA APP MED DEVICE)
Is an overview of the IDEA and BE Model applied to the Medical Device, the Demographic of suffers and Disease/Disorder
- Introduction
Is a high Level Introduction to Part 2 of the new OE model in line with the material covered by Sheedy F., (2017),
- Overview to Execute Program
Presents a high Level Introduction to how the model would be executed using a analysis visual and a SIPOC tool that summarizes the inputs and outputs of one or more process in tabular form.
- Goal and Strategy
Purpose is to understand the overall goal / Strategy and what is the trajectory, considering the following three key points and underlining questions
Strategic opportunity – the trajectory our organisation could pursue if it faced no constraints or limits
Strategic reality Trajectory – the trajectory our organisation is currently taking, the path we are currently on and is it the right one?
Strategic intent Trajectory – the trajectory our organisation has chosen to take based upon an understanding of the trajectory of strategic reality and trajectory of strategic possibility by asking a series of questions
- Planner and Tracker
Assessing ourselves; as in how do we plan, execute and track what we do?.
- Creativity
This sheet show an example Visual Spider Diagram used to track and see amount of problem solving and creative solutions that are being made in order for product success and growth. The diagram is a monthly review of new problem solving opportunities and creative solutions that are being generated in order to solve or advance the current product pipeline.
Creative Questions that we should be asking ourselves in order to make technological and business advances. How good are we at it?
- CAPA
This sheet show three part to doing a CAPA
CAPA Process Flow Diagram
CAPA Complaints Log and Tracker
Tracking visual
- Continuous Improvement
This sheet show continuous improvement measure is to have more visuals and rapid learning cycles for example here is an A3 for the test, assembly and measurement of the battery of the gammaCore. Such checks should ensure continued success
It also shows an eight-step guide for problem solving for continuous improvement
Figure 7. Show the Part 2 of the new OE model with its eight sub-parts or cells
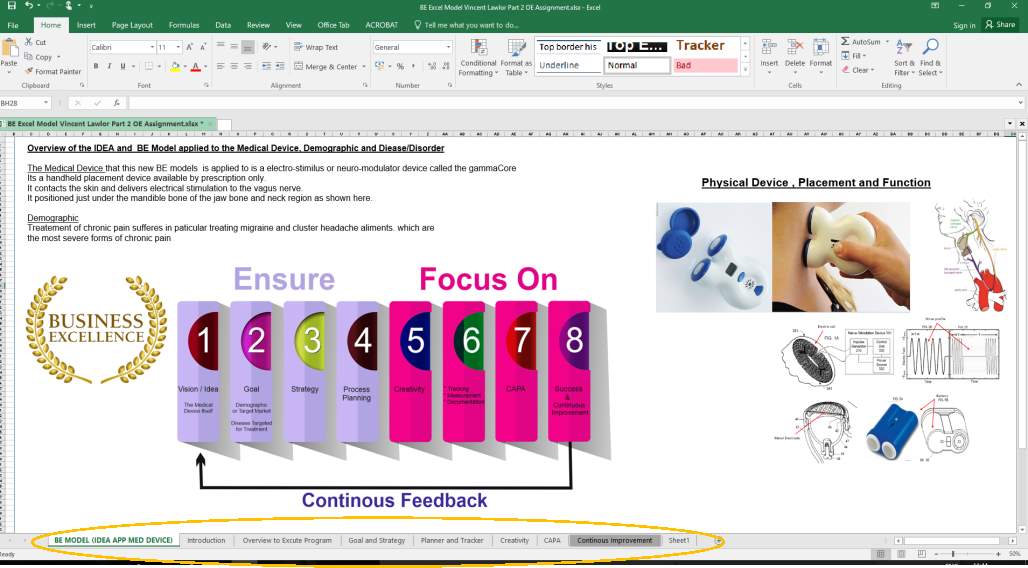
Figure 6. The complete BE excel model
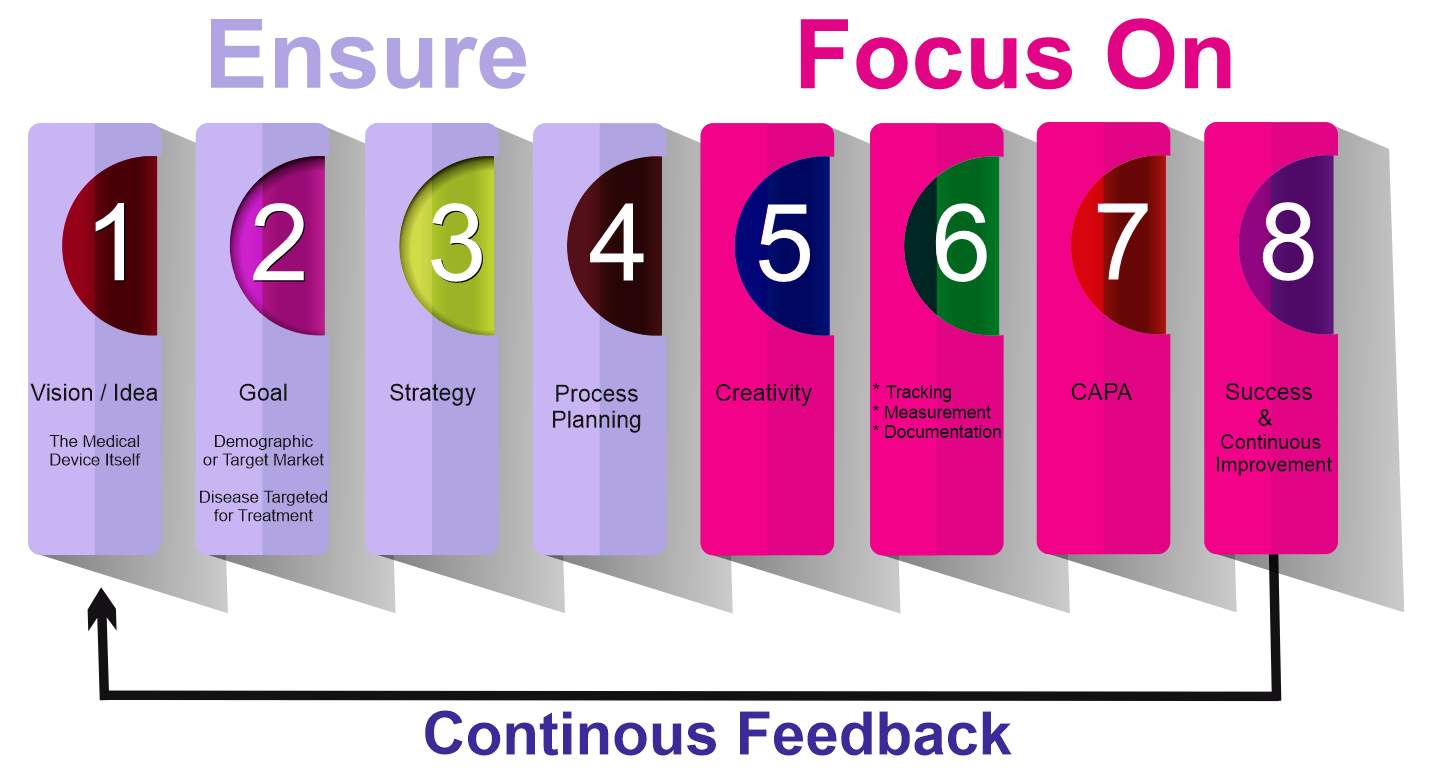
Figure 7. Part 2 of the new OE model with its 8 sub-parts of cells
2.2.1 Cell 2.1.Vision / Idea
The vision/ idea of this operational excellence model is to;
- Guide a company or individual in making the best possible (neuromodulatory or electo stimulus) device that will help reduce, prevent, abort and manage the intensity, frequency and duration of debilitating chronic migraine or cluster headaches and to enable a better quality of life by reducing cost and the dependency of complex and expensive medication programs.
- Design and OE model to be a game changer in what we do
- Ensure efficacy and safety of this device that when it reaches the market in accordance with the FDA general wellness draft guidance document for wellness products refer to (Bain 2015).
2.2.2. Cell 2.2 Goal
The high-level questions are presented here with more detailed questions outlined in the Business Excellence excel model called which accompanies this document.
The goal of this operational excellence model is to guide companies or individuals in making a device that will be;
- Cost effective financial alternative to incumbent specialised strong medication by supplementing treatment with such a device
- Physically sized and weigh comparable or less the volume medication sufferers carry on a daily basis
- Simple, self-administered hand-held treatment device that can be used at any time unlike medication programs, which limits max dosage per 24 hrs
- Effective treatment; less than 2 minutes to administer versus the slow absorption rates of the body with incumbent drugs and blood brain barrier
- An improvement in the quality of life and overall wellness by supplementing such a device which will therefore reduce medication and the possibility or susceptibility of liver disease due to a reduction or removal of medication overuse.
2.2.3. Cell 2.3. Strategy
The high-level questions are presented here with more detailed questions outlined in the Business Excellence excel model called which accompanies this document.
Objective of this part is to ask the right Strategic questions for our business and product to succeed and be a world leader in chronic pain management and alleviation in suffering
- Starting Point; How do we assess our current Strategy
- Assessing our Competition: What are they doing that we are not?
- Assessing the Future; How do we do it?
- Distribution: Assessing our connections; How do we do it?
- Assessing our Outlets Overall; How do we do it?
- Product Offering Assessing our Market; How do we do it?
- Assessing the Value to our Customers; How do we do it?
- Assessing the Future; How do we do it?
- Pricing & Promotion: Assessing our Pricing; How do we do it?
- Assessing our Promotions; How do we do it?
- Assessing our Sales; How do we do it?
2.2.4. Cell 2.4 Planning Process
The high-level questions are presented here with more detailed questions outlined in the Business Excellence excel model which accompanies this document.
Objective of this part is to ensure effective planning and tracking and to make sure every voice is heard and give due consideration
- Project Conception and Initiation
- Project Definition and Planning
- Project Launch and Execution
- Project Performance / Monitoring
2.2.5. Cell 2.5 Creativity
The objective of this part is to understand creativity. The creativity part of the OE model has two parts again the high-level questions are presented here with more detailed questions outlined in the Business Excellence excel model which accompanies this document.
Part 1 is a 12 by 12 question and rating format which feeds into a Visual Spider Diagram used to track and see amount of problem solving and creative solutions that are being made in order for product success and growth.
The spider diagram is a quick visual tracker used for monthly review of new problem solving opportunities and creative solutions that are being generated in order to solve or advance the current product pipeline. High-level creative questions are asked in order to drive technological and business advances and presented in following
.
Such creative items to address are;
- Addressing Product Cost
- Addressing Device Power Consumption
- Portability
- Weight
- Battery life
- Quality and Cost of quality
- Functionality
- Learning environment
- Developing partnerships
- Next logical engineering steps
- Reliability
- Human Factors
While for each question there is a key ratings of importance as shown in Table 1.
Table 1 How creative questions are ranked
| Ratings Level | Definition |
| 1 | Very poor |
| 2 | Poor |
| 3 | significantly below average |
| 4 | Below average |
| 5 | Average |
| 6 | Above Average |
| 7 | significantly above average |
| 8 | Good |
| 9 | Very good |
| 10 | Great |
| 11 | Excellent |
| 12 | Most Significant; Class of its own |
Parts 2 is a table of creative questions that we should be asking ourselves in order to make technological and business advances. How good are we at it?. Again, these questions have six rating levels ranging from excellent to non-relevant and cover a process of questioning and understanding thus making a excellent idea or business solution as opposed to a good idea or non-relevant one;
- What’s turning customers on and off?
- How do we discover opportunities especially missed ones?
- How do we decide what to build and why?
- How would we improve the targeted company’s product/site
- Could we give an example of a well-executed product? How would or could we improve it?
- What makes a product the differentiator in the market its competing in?
- How would we or could we explain what we do especially the product to a 5 year old?. Because if we can explain it simply then we don’t understand it well enough or the market, vision , goal
- If we had only key one input to this pain treatment device what would it be and why?
- If we had to remove one design feature, form this pain treatment device what would it be and why?
2.2.6. Cell 2.6 Tracking, measurement and documentation
The objective of this part is to ensure effective tracking and to make sure every voice is heard and given due consideration again the high-level questions are presented here with more detailed questions outlined in the Business Excellence excel model which accompanies this document.
Using the design principle of (Mascitelli 2011) his work entitled “Mastering Lean Product Development” a GANTT chart with active swim lanes is presented as one of the ways for effective tracking. The chart has activities set out on a weekly basis with assigned tasks, owners and due dates for completion. The key visual on this chart is the column which presents the percentage of the task complete or near completion and if one looks horizontally it can be seen which the percentage is tracked too.
The four high level tasks presented here but under each task are more refined task titles with more detailed questions outlined in the Business Excellence excel model which accompanies this document.
- Project Conception and Initiation
- Project Definition and Planning
- Project Launch and Execution
- Project Performance / Monitoring
Documentation control is in accordance with (EN ISO 13485:2016-3, 2016) and (EN ISO 14971 2007-09) refer to reference section.
2.2.7. Cell 2.7 CAPA
Objective here is to be in line with the (FDA. 2017) Corrective and Preventive Actions (CAPA). Presented in this section is method or a process flow diagram and the steps to be taken if a customer complaint is logged right through the CAPA process to final resolution. Also presented is a visual bar chart tracker and CAPA complaints log and tracker, which tries to understand the questions or source of complaints with a view to understanding the demographics and percentages of complaints handled. Again the high-level questions are presented here with more detailed questions outlined in the Business Excellence excel model which accompanies this document.
- Number of telephone complaints received
- Number of written complaints received (including letters, emails, texts)
- Total complaints received
- Number of complaints resolved by the end of the first working day after which the complaint was received (day+1)
- Percentage of complaints outstanding after day+1
- Number of complaints resolved between day+2 and 31 calendar days
- Percentage of complaints outstanding after 31 calendar days
- Number of repeated complaints
- Percentage of repeated complaints
- Total number unresolved issues
- Number of complaints taken up within Ombudsman terms of reference
- Number of complaints taken up outside Ombudsman terms of reference
- Total number of complaints taken up by the Ombudsman for resolution (i.e. number of forms completed as reported by TOSL inside and outside of the terms of reference)
- Number of final decisions issued by the Ombudsman
- Total number of awards made by the Ombudsman (financial, non-financial and both) to the complainant
- % of final decisions in favour of complainant
2.2.8. Cell 2.8 Success with Continuous Improvement
Objective is to present a guide or 8 step problem solving process for for continuous improvement , again the high-level steps/questions are presented here with more detailed questions outlined in the Business Excellence excel model which accompanies this document.
- Step 1: Always clarify the Problem
- Step 2: Continually breakdown the Problem
- Step 3: Always set Targets
- Step 4: The Analyse the Root Cause of each
- Step 5: Followed by Developed Countermeasures
- Step 6: Rank and Pick a Countermeasure in order of importance and implement
- Step 7: Continually Monitor Results & Process
- Step 8: Always Standardise & Share Success once resolved
Finally step 8 states, document the new process and set it as a new standard. Share the new standard through horizontal deployment. With this success then reflect and celebrate the wins, but most importantly DON’T STOP there, start the next Improvement and repeat.
Figure 7 8 and 9 following shows how a new OE model would be used by an organisation or group, how the organisation would assess its performance while using such a model. Finally presented is a developed improvement plan to use on a fully time basis to address continuous improvement which is the core foundation of operational excellence?
Figure 7. Depicts a nine-step flow diagram on how the new OE model would be used starting from step 1 mission and vison top left of the image to step 9 which is maintain goals. The flow diagram also has a feedback loop from step 9 back to step 3 which means that once a organisation has a process flow diagram to implement the new OE model its important to always feedback learnings and continually keep cycling the process.
- How to use new OE model would be used in an organisation
Figure eight show how to use the new OE model by taking a nine-step approach as shown in the following process flow diagram
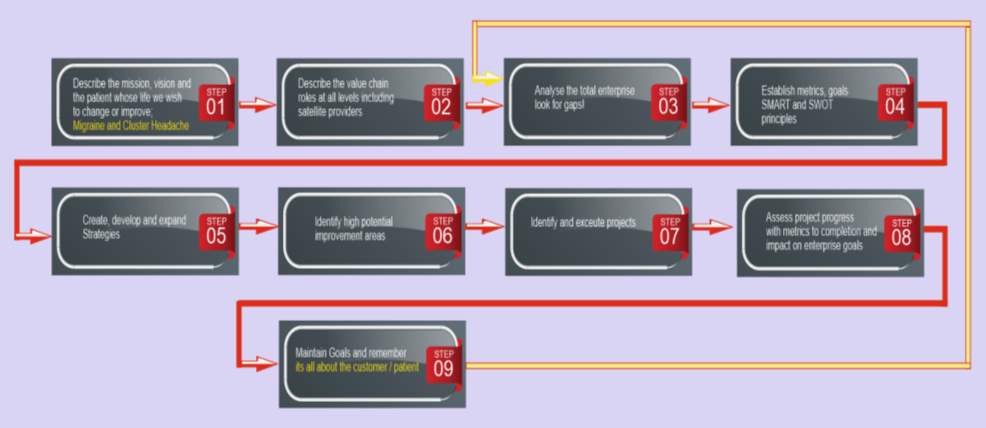
Figure 7. Nine step flow diagram or guide on how the new OE model would be used in an organisation
In order to assess the performance of using the such a model a review paper by (Rusev and Salonitis 2016) looked at the principles of assessment which Rusev and Salonitis stated could be classified under four dimensions; Culture, Continuous Process Improvement, Enterprise Alignment and Results and is summarise in table 2 taken directly from the paper.
Table 2. Categorisation of components of existing assessment tools versus Shingo dimension and principles (% weight of each category) (Table taken from Rusev and Salonitis 2016)
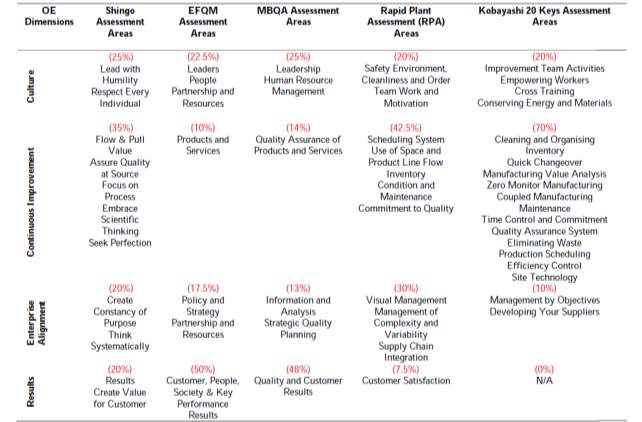
A thing to note is that out of the five assessment tools tabulated only the RPA comes close to the Shingo tool in terms of addressing the four dimensions. However, this four-dimensional approach could be used by companies to implement and achieve OE, however they would have to attain a high maturity level and measured success in these four areas by external assessment.
This is a costly exercise so a good way the organisation can prepare is to develop a matrix to critically evaluate and compare in areas such as dimensions, assessed them develop a scoring criteria and usability. The matrix could be validated by managers and the results of the assessment could be used to form the basis onto which a roadmap of OE would then be developed. Figure 8 is redrawn and adapted from the work of Rusev and Salonitis but the author of this paper believe that their work is very comprehensive and detailed show how this framework could be used by organisation a new OE model
- Framework assessment tool and how an organisation would use a framework to asses a new OE model
Figure 8 shows a framework assessment tool and how an organisation would use a framework like this to asses a new OE model
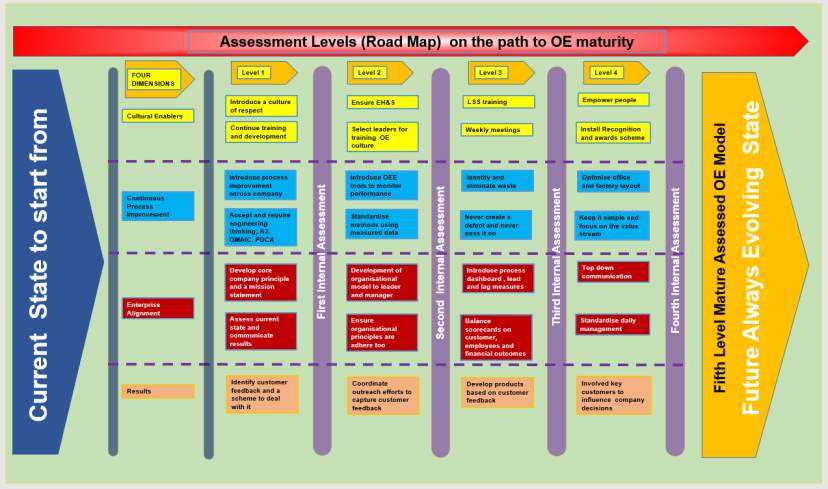
Figure 8 Framework Assessment tool; How an organisation would use a framework to asses a new OE model
- Two level new improvement plan for continuous improvement
Figure 9 shows atwo level new improvement plan developed by the author of this paper to continually improve the new OE model
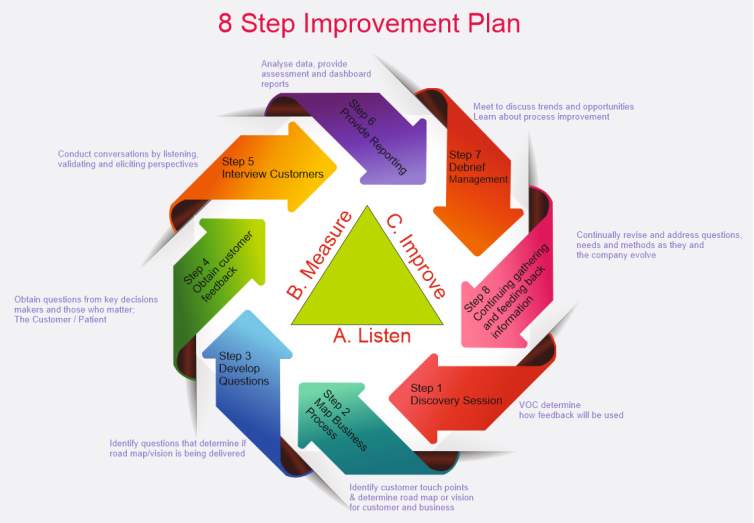
Figure 9 Two level New improvement plan (Developed by the author of this paper)
Figure 9 shows a two level new improvement plan. Level one is the ABC level which is (A) always listen to what is going on, (B) then measure this feedback and (C) don’t settle aim for improvement. Level 2 is the 8 step approach to support level 1 ranging for the VOC all the way through to continued success by continually gather information and feedback and use it and start all over again.
- References
Note reference in this section are lists as they appear in the paper.
- Migrainetrust (2017), Hemiplegic migraine A rare condition involving temporary weakness on one side of the body. [ONLINE] Available at: https://www.migrainetrust.org/about-migraine/types-of-migraine/hemiplegic-migraine/, [Accessed 14 December 2017].
- Fischell et al. (2008), Means and methods for treating headaches. [ONLINE] Available at: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=20080045776&OS=20080045776&RS=20080045776, [Accessed 14 December 2017].
- Tomkins, E., (2016), Migraine: the facts. [ONLINE] Available at: https://www.irishtimes.com/life-and-style/health-family/migraine-the-facts-1.2575275, [Accessed 14 December 2017]
- Migraineresearchfoundation (2017), Raising Money for Migraine Research. [ONLINE] Available at: http://migraineresearchfoundation.org/about-migraine/migraine-facts/, [Accessed 14 December 2017].
- Choosenatural, (2017), Cluster Headaches. [ONLINE] Available at: http://www.choosenatural.com/headaches/cluster-headaches/, [Accessed 14 December 2017].
- Electrocore (2017), FDA Releases gammaCore®, the First Non-Invasive Vagus Nerve Stimulation Therapy Applied at the Neck for Acute Treatment of Pain Associated with Episodic Cluster Headache in Adult Patients, [ONLINE] Available at:http://www.electrocore.com/fda-releases-gammacore-the-first-non-invasive-vagus-nerve-stimulation-therapy-applied-at-the-neck-for-acute-treatment-of-pain-associated-with-episodic-cluster-headache-in-adult-patients, [Accessed 14 December 2017]
- gammacore (2017), About gammaCore. [ONLINE] Available at: http://gammacore.co.uk/about-gammacore, [Accessed 14 December 2017].
- Sheedy F., (2017), Lecture Series & Notes, Module in Operational Excellence and the Science of Innovation, Semester 2, PGDip in Medical Device Technology and Business, Innopharma Dublin and Griffith College Dublin
- Hales (1999) The Honeycomb Conjectur, Cornell university Library. [ONLINE] Available at: https://arxiv.org/abs/math/9906042, [Accessed 26 November 2017].
- Saiglobal, (2017), Business Excellence Framework (BEF). [ONLINE] Available at: https://www.saiglobal.com/business-improvement/process/framework/excellence.htm, [Accessed 25 November 2017].
- Blunnie P., (2017 )Lecture Series & Notes, Module in Quality Systems and Regulation (QSR), Semester 1, PGDip in Medical Device Technology and Business, Innopharma Dublin and Griffith College Dublin
- ec.europa.eu, (2017). Active implantable medical devices, EU)90/385/EEC-AIMD, and 93/42/EEC-MDD [ONLINE] Available at: https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/implantable-medical-devices_en. [Accessed 12 December 2017].
- ec.europa.eu, (2017). Regulatory framework, The new Regulations on medical devices, (EU) 2017/746 IVD. & (EU) 2017/745 MDR [ONLINE] Available at: https://ec.europa.eu/growth/sectors/medical-devices/regulatory-framework_en. [Accessed 12 December 2017].
- ec.europa.eu, (2017), In vitro diagnostic medical devices Directive 98/79/EC. [ONLINE] Available at: https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/iv-diagnostic-medical-devices_en. [Accessed 12 December 2017].
- ISO Standards. (2017), International Organization for Standardization, ISO 9000 – Quality management. [ONLINE] Available at: https://www.iso.org/iso-9001-quality-management.html. [Accessed 12 December 2017]
16. ISO Standards, (2017), International Organization for Standardization, ISO 14971:2012, Medical devices — Application of risk management to medical device, [ONLINE] Available at: https://www.iso.org/standard/38193.html, [Accessed 12 December 2017]
17. ISO Standards (2017), International Organization for Standardization, ISO 13485:2016, Medical devices — Quality management systems — Requirements for regulatory purposes, [ONLINE] Available at: https://www.iso.org/standard/59752.html, [Accessed 12 December 2017]
- FDA Medical Devices (2017), Quality System (QS) Regulation/Medical Device Good Manufacturing Practices, [ONLINE] Available at: https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/QualitySystemsRegulations/, [Accessed 12 December 2017].
19. (EN ISO 13485:2016-3, (2016) Medical devices — Quality management systems — Requirements for regulatory purposes, International Organization for Standardization, pp. 1-36
20. (EN ISO 14971 2007-09, (2009), Medical devices – Application of risk management to medical devices, pp. 1-82
- FDA, (2017), Corrective and Preventive Actions (CAPA), [ONLINE] Available at:https://www.fda.gov/iceci/inspections/inspectionguides/ucm170612.htm#page2 [Accessed 12 December 2017].
- Dodd D., (2011), Sales & Operations Planning Summit, The Orthopaedic Implant Company, Sales & Operations Planning Summit, Boston, pp. 1-12
- FDASIA (2012), Food and Drug Administration Safety and Innovation Act (FDASIA). [ONLINE] Available at: https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDASIA/default.htm, [Accessed 19 December 2017].
- Creaner (2014), How To Develop A Quality Culture In Your Medical Device Manufacturing Operation. [ONLINE] Available at: https://www.meddeviceonline.com/doc/how-to-develop-a-quality-culture-in-your-medical-device-manufacturing-operation-0001, [Accessed 12 December 2017].
- Rusev S.J., and Salonitis K., (2016), Operational excellence assessment framework for manufacturing companies, 5th CIRP Global Web Conference Research and Innovation for Future Production, Procedia CIRP, Vol.55, pp. 272 – 277
- Bain et al (2015), Non-Invasive Neuromodulation of the Central Nervous System: Opportunities and Challenges: Workshop Summary, The national academies of science, engineering, medicine, pp.1-119
- Mascitelli, R., (2011), Mastering Lean Product Development: A Practical, Event-Driven Process for Maximizing Speed, Profits, and Quality, pp. 1-323
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical Technology"
Medical Technology is used to enhance the medical care and treatment that patients are given in healthcare settings. Medical Technology can be used to identify, diagnose and treat medical conditions and illnesses.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: