Role of Imaging in Early Diagnosis of Acute Ischemic Stroke
Info: 7544 words (30 pages) Example Literature Review
Published: 23rd Dec 2021
Tagged: Medical Technology
Authors: Mohammad Amin Akbarzadeh1, Sarvin Sanaie2,3, Mahshid Kuchaki Rafsanjani3 and Mohammad‑Salar Hosseini1,2,4*
Abstract
Stroke is a serious health condition that is responsible for more than 5% of total deaths. Near 20% of patients experiencing stroke die every year, resulting in the stroke being at the top of the list of preventable causes of death. Once an acute stroke is suspected, a golden hour of less than an hour is available to prevent the undesirable consequences. Since neuroimaging is mandatory in the diagnosis of stroke, the proper use of neuroimaging could help saving time and planning the right treatment for the patient. Some of the available imaging methods help us with rapid results, while others benefit us from a more accurate diagnosis. Hereby, we aim to provide a clinical review of the advantages and disadvantages of different available neuroimaging methods in approaching acute stroke to help clinicians choose the best method according to the settings.
Introduction
Cerebrovascular diseases are serious health conditions that can affect the quality of life by leading to disabling consequences, such as dysarthria, paralysis, and amnesia [1, 2]. A stroke happens when the brain’s blood-supplying vessels are ruptured or blocked, usually along with thrombus formation, displaced embolism, stenosis in cerebral arteries, and hemorrhage in the brain parenchyma [3]. These events can lead to a significant decrease in blood flow and oxygen to the brain, resulting in a stroke. Stroke is responsible for more than 10% of deaths worldwide [4], and one in five stroke patients die each year, putting stroke at the top of the list of preventable causes of death [5]. Risk factors attributable to stroke are mainly the ones that are also common in other non-communicable diseases, such as diabetes, blood pressure, being obesity, smoking, and alcohol consumption; although, the prevalence of them may vary between different countries [6, 7].
In the management of acute stroke, a “golden time” of less than an hour is available from the onset of the symptoms, where making prudent diagnostic and therapeutic choices may result in fewer complications [8, 9]. Sense of weakness or numbness, visual loss or blurred vision, sensory disturbance, impaired consciousness, dizziness and loss of balance, dysphagia, headache, and speech problems are the most common findings in favor of stroke [10]. Like other life-threatening emergencies, an accurate and rapid diagnosis is crucial. Delay in diagnosis could result in irreversible damages, since the brain tissue is lost for each minute delay. Several stroke assessment scales [such as Face Arm Speech Time (FAST), Cincinnati Prehospital Stroke Scale (CPSS), Los Angeles Prehospital Stroke Scale (LAPSS), and Melbourne Ambulance Stroke Scale (MASS)], and severity grading scores [such as Los Angeles Motor Scale (LAMS), Kurashiki Prehospital Stroke Scale (KPSS), and National Institutes of Health Stroke Scale (NIHSS)] have been developed, trying to facilitate the rapid diagnosis of stroke through different criteria. Simpler scales such as CPSS and FAST have been shown to have enough sensitivity for clinical purposes, while the more complex scales might have lower sensitivity and, therefore, miss more cases [11]. Although some important clinical manifestations, such as face drooping, arm weakness, and speech difficulty, may suggest stroke in the initial assessment, the definitive diagnosis is primarily determined based on imaging [12]. Therefore, an intelligent choice of imaging technique could result in an early, lifesaving diagnosis of acute stroke. This article aims to review the role of different imaging methods in the diagnosis of acute ischemic stroke and determine the advantages and disadvantages of each.
Keywords: Brain infarction, Cerebrovascular disorders, Diagnostic imaging, Intravenous thrombolysis, Stroke
Etiology and pathophysiology of ischemic stroke
The main pathology of ischemic stroke is loss or abruption of blood circulation in specific parts of the brain, which could be due to the thrombotic or embolic occlusion [13]. The thrombotic ischemic strokes are generally associated with atherosclerotic accidents and are classified based on the size of the involved vessels [14]. Depending on the speed of the plaque rupture, it can be presented with sudden devastating damages or subtle pathologic changes that manifest slowly [15]. In the latter situation, collateral circulation would partly compensate the circulation loss. The small-vessel ischemic stroke could occur as a consequence of systemic and chronic diseases, such as diabetes [16]. Rarely, vasculitis can predispose the vessel walls to narrowing and obstruction [17]. On the other hand, the embolic ischemic stroke is mainly caused by atrial fibrillation [18]. In addition, several monogenic disorders (such as Ehlers–Danlos, Fabry’s disease, and Marfan syndrome) are associated with ischemic stroke [19, 20].
Regardless of the etiology, the abruption in blood circulation, a cascade of ischemia-related events initiate in both cellular level and macroscopic scale [13, 21]. In the first place, the energy supply by mitochondria gets interrupted—even in the absence of complete blood occlusion—leading to loss of function of the membranous proteins and an impaired gradient between intracellular and extracellular space, which eventually results in swelled neurons and glial cells (also referred to as cytotoxic edema) [22, 23]. Besides, the release of excitatory neurotransmitters adds extra stress in terms of energy supply by the cells, ectopic activation of destructive enzymes, such as lipases and proteases, destruction of vital organelles in neurons, and production of oxygen free radicals, eventually leading to necrotic death of the neurons in the core of the ischemic region, and triggering the cascade of apoptotic events in the peripheral neurons [24–26].
Importance of imaging in approach to stroke
For the following reasons, the suspicion of ischemic stroke should be followed by early neuroimaging:
Exclude the presence of hemorrhage
Apart from stabilizing the patient, the initial management of a patient with stroke depends on the type and etiology of stroke [27]. While thrombolytic therapy benefits ischemic strokes, it is contraindicated in hemorrhagic ones [28]. This process should be done fast, as there is a golden time of 4.5 h available from the symptom onset to safely use Tissue Plasminogen Activators (tPA), such as Alteplase [29, 30].
Confirmation of the stroke
Although a stroke is generally accompanied by clinical signs and symptoms, such as face drooping on one side, arm weakness, and difficulty in speech, this diagnosis should be confirmed by detecting signs of infarction and early signs of ischemia (such as presence of hyperdense area in a vessel—mainly in the middle cerebral artery—which is a common sign of intravascular thrombus or embolus) through imaging [31, 32]. In addition, although the collateral flow tends to compensate the lack of blood flow in the affected area, early signs such as hypoattenuation and loss of grey–white matter differentiation would be evident [33].
Determining the status of brain‑supplying arteries
The status of brain-supplying arteries should be assessed in the work-up to specify the possible etiologies, indicate the damage severity, and determine the recurrence risk in some cases [34].
Rule out the stroke mimics
Tumors are stroke-mimics that should be ruled out. Migraines and seizures are other diseases that could present with stroke-like symptoms and require imaging for ruling out the stroke [27, 35–37].
Common imaging modalities in healthcare settings
Ranging from Magnetic Resonance Imaging (MRI) to Computed Tomography Perfusion (CTP), there are various imaging techniques available [38, 39]. Different modalities of Computed Tomography (CT) include CT Angiography of head and neck (CTA), CTP, and noncontrast CT. As for MRI modalities, various measures can be useful, such as Apparent Diffusion Coefficient (ADC) Diffusion-Weighted Imaging (DWI), Gradient Recalled Echo (GRE), Fluid-Attenuated Inversion Recovery (FLAIR), and MR Angiography of head and neck (MRA) [40–42]. Although CT is still the preferred imaging modality in the early management of acute stroke, MRI with DW measure is more sensitive than noncontrast CT for the very early detection of acute ischemia and is shown to be more efficient than the first CT, second CT and MRI in the evaluation of patients who are presented within the 24 h [43]. The main reason for this accuracy is the capability of the DW imaging to show the slight differences in diffusion of the water molecules across the membranes during the cytotoxic edema; therefore, more visually available contrast will be presented in contrast to CT imaging which requires significant amount water molecule activity and retention and more time to exhibit detectable contrasts [44, 45].
However, not all medical centers have convenient access to MRI, and due to patient contraindications and intolerance to MRI scans, the amount of time it takes, and despite providing more accurate insight in tissue and vascular pathology, CT is considered the first-line imaging study at most centers [46]. Widespread availability, rapid scanning features, and ease of detecting intracranial hemorrhage make noncontract CT of the head an appealing choice in early acute stroke evaluation [47]. Although, approaches including multimodal CT/MRI protocols seem to be valuable tools in prevention, diagnosis and tracking the pathologic course of stroke and therapeutic response of interventions [48]. In addition, after the initial treatment is given and the acute emergency phase has passed, carotid ultrasound should be performed to evaluate the vessels’ status in terms of artery occlusion, decreased blood flow, and potential sources of embolism. The characteristics of the brain infarct (including the size and the location) are key findings to determine the subtype of stroke (Intracerebral hemorrhage, subarachnoid hemorrhage, and brain ischemia) [49].
Early signs of ischemic stroke in CT are chiefly described as hypoattenuation, loss of differentiation between the grey and the white matter, parenchymal swelling and edema, hyperattenuation of the MCA (middle cerebral artery), infarction in the territory of the brain-supplying arteries, and cortical sulcal effacement on the affected side [50–52]. The Alberta Stroke Program Early CT Score (ASPECTS) is a quantitative scoring system that evaluates early changes of MCA stroke, based on the topographic involvement of the MCA-supplying territories of the brain [53]. For each area involved, one point is deducted from the total of ten points. Some clinical variations of ASPECTS have also been introduced, such as pc-ASPECTS which evaluates the stroke of the posterior circulation [54]. On the other hand, speaking of MRI, an increase in DWI signals is evident from the first hours of the ischemic stroke [55]. While the ADC mapping shows reduced signals, T1- and T2-weighted sequences do not show significant changes in the early hyperacute phase of the ischemic stroke (0–6 h after stroke) [56]. T1-weighted MRI usually shows low signal intensity after 16 h, and T2-weighted MRI is usually high-signal after 8 h [56]. Therefore, the presentations of different MRI sequences could determine the age of the infarct.
Angiography modalities
Non-invasive methods such as CT Angiography (CTA) or Magnetic Resonance Angiography (MRA) are accessible and typically used for screening [57, 58]. Although noninvasive imaging modalities prevent a significant number of stroke cases, choosing the right strategy may differ based on the time that has been passed from the transient ischemic attack [59]. With intravenous administration of contrast, CTA can illustrate a precise anatomical picture of the vascular system with sufficient details [60]. On the other hand, MRA is an appropriate modality to evaluate the dynamic changes, since the technique used in this imaging is based on changes in the vasculature flow [61]. Four-dimensional CTA and MRA are also less invasive alternatives to determine the clot burden and grading the collateral blood flow in large vessel occlusions [62]. CTA could be considered as the most appropriate approach to diagnose stenosis and occlusions [63]. However, as the injection of contrast material can cause kidney failure, it requires a thorough history, which is not always possible and time permitting in an acute stroke situation [64, 65].
Role of ultrasonography in early identification of stroke
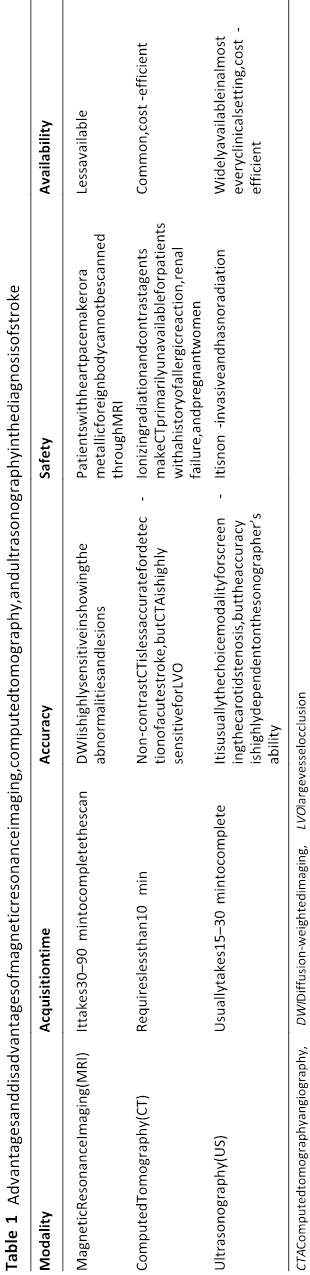
Ultrasound methods are also available as non-invasive measures, though they are practically used only for the evaluation of non-acute cerebrovascular accidents [66]. In addition, strokes of large arteries and patients with transient ischemic attack (TIA) can possibly benefit from Carotid Duplex Ultrasound (mostly extracranial vessels) and Transcranial Doppler (mostly intracranial vessels), where both of them are non-invasive [43, 67]. Transcranial Doppler (TCD) relies on the physical phenomenon known as the Doppler effect [68]. In this case, changes in sound wave frequencies secondary to the motion of the blood in the arteries are measured and evaluated usually by calculating the Doppler shift. In addition, The peak systolic, diastolic, and mean flow velocities, along with the Gosling pulsatility index are also usually calculated, providing vital information about blood flow and whether it is altered or absent, or there is a change in pulsatility of the concerned vessel [69, 70]. Detecting these changes helps the clinicians to find out about the presence of occlusion and determine if the vessel has been recanalized after the initiation of treatment or not. The previous studies have demonstrated that prehospital diagnosis of middle cerebral artery occlusion with or without microbubble contrast agents in stroke patients has a significant amount of sensitivity and specificity, and therefore, prehospital assessment with ultrasonography may provide therapeutic benefits for stroke patients [71]. Especially utilizing imaging techniques that describe the morphologic characteristics of lesions that lead to embolic events is useful in the early stages of stroke pathology, with ultrasound being the leading modality for monitoring the plaque rupture and dynamic changes in the vessels [72]. Doppler ultrasound may provide prognostic value that may guide the therapeutic approach in stroke patients and better risk scoring for stroke patients; As an example, previous studies reported that asymptomatic embolic signals, detected in patients with symptomatic carotid artery stenosis, may be useful in developing better risk score system for prognostic purposes and efficient antithrombotic therapy [73–75]. Table 1 provides a summary of the benefits and limitations of MRI, CT, and ultrasound.
Telemedicine and neuroimaging
Since the health burden of stroke has led to the development of specialized centers and units for providing stroke-related care, an increasing need for expanding a constant and reliable communication between these centers and the primary healthcare units is tangible. Therefore, new concepts such as telehealth and telemedicine have become bold, especially in emergencies, introducing even more specific terms, such as teleneurology and telestroke [76–78]. Telestroke aims to virtually examine the suspected stroke patient by evaluating the imaging results and providing clinical recommendations [79]. Neuroimaging is the fundamental element of telediagnosis for stroke, and appropriate imaging would indicate the best treatment approach for each patient.
Not all stroke patients need a transfer to a comprehensive stroke center (CSC). Several indications have been defined for the patients in need of CSC transfer, including qualification for tPA administration (ischemic stroke who would remain in the golden time after the transfer), endovascular thrombectomy, largevessel occlusion, and massive stroke [80, 81]. Apart from making the final decision for which patients should be transferred, the patients’ transfer time will also decrease significantly by implementing the telestroke strategies. From the several available modalities, CT is an essential for the primary units and local hospitals, since it benefits from availability in most emergency departments and rapid results. Based on the patient’s presentations, results of CT imaging, and the facilities of the primary unit, the stroke specialists might request the local team to perform vascular imaging, such as CTA, MRA, or Digital Subtraction Angiography (DSA) [82].
Providing a standard and detailed protocol for the application of telestroke could help the patients with a rapid and more successful management, and the healthcare system by reducing the patient burden.
Conclusions
According to the clinical importance of stroke and the advantages of early management, imaging plays a vital role in the patients’ survival. MRI is more accurate in ruling out the intracranial hemorrhage and MRI with DWI is more precise in the detection of acute ischemic stroke. However, due to the availability and lower acquisition time, CT is preferred in most healthcare settings. Being cognizant of the early warning signs of a stroke, alongside developing rapid, simple, and handy imaging techniques could help us improve the outcomes and overcome the burden of stroke.
Abbreviations
ADC: Apparent Diffusion Coefficient; ASPECTS: Alberta Stroke Program Early CT Score; CPSS: Cincinnati Prehospital Stroke Scale; CSC: Comprehensive stroke center; CT: Computed tomography; CTA : Computed tomography angiography; CTP: Computed Tomography Perfusion Imaging; DSA: Digital Subtraction Angiography; DWI: Diffusion‑Weighted Imaging; FAST: Face Arm
Speech Time; FLAIR: Fluid‑Attenuated Inversion Recovery; GRE: Gradient
Recalled Echo; KPSS: Kurashiki Prehospital Stroke Scale; LAMS: Los Angeles
Motor Scale; LAPSS: Los Angeles Prehospital Stroke Scale; MASS: Melbourne Ambulance Stroke Scale; MCA: Middle cerebral artery; MRA: Magnetic resonance angiography; MRI: Magnetic Resonance Imaging; NIHSS: National Institutes of Health Stroke Scale; TIA: Transient ischemic attack; TCD: Transcranial Doppler; tPA: Tissue Plasminogen Activators.
Acknowledgements None.
Authors’ contributions
SS and MSH developed the idea, MAA, MSH, and MKR carried out the data gathering, MAA and MSH prepared the manuscript, and SS and MKR revised the manuscript critically. All authors read and approved the final manuscript.
Funding None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate Not applicable.
Consent for publication Not applicable.
Competing interests
The authors declare that they have no competing interests.
Author details
1 Student Research Committee, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran. 2 Neurosciences Research Center, Tabriz University of Medical Sciences, Golgasht Street, 5166/15731 Tabriz, EA, Iran. 3 Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran. 4 Emergency Medicine Research Team, Tabriz University of Medical Sciences, Tabriz, Iran.
Open Access: This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
References
1. Geisler F, Ali SF, Ebinger M, Kunz A, Rozanski M, Waldschmidt C, et al. Evaluation of a score for the prehospital distinction between cerebrovascular disease and stroke mimic patients. Int J Stroke. 2019;14(4):400–8. https:// doi. org/ 10. 1177/ 17474 930188 06194.
2. Chiaramonte R, Pavone P, Vecchio M. Speech rehabilitation in dysarthria after stroke: a systematic review of the studies. Eur J Phys Rehabil Med. 2020;56(5):547–62. https:// doi. org/ 10. 23736/ s1973‑ 9087. 20. 06185‑7.
3. Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. https:// doi.org/ 10. 1038/ s41572‑ 019‑ 0118‑8.
4. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res.
2017;120(3):439–48. https:// doi. org/ 10. 1161/ CIRCR ESAHA. 116. 308413.
5. Yang Q, Tong X, Schieb L, Vaughan A, Gillespie C, Wiltz JL, et al. Vital signs: recent trends in stroke death rates—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;66(35):933.
6. Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8(4):345–54.
7. Almobarak A, Badi S, Elmadhoun W, Tahir H, Ahmed M. The prevalence and risk factors of stroke among Sudanese individuals with diabetes: cross‑sectional survey. Brain Circ. 2020;6(1):26–30. https://doi.org/10.4103/bc.bc_15_19.
8. Advani R, Naess H, Kurz MW. The golden hour of acute ischemic stroke. Scand J Trauma Resusc Emerg Med. 2017;25(1):54.
9. Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41(7):1431–9. https://doi.org/10.1161/strokeaha.110.583815.
10. Di Stefano V, De Angelis MV, Montemitro C, Russo M, Carrarini C, di Giannantonio M, et al. Clinical presentation of strokes confined to the insula: a systematic review of literature. Neurol Sci. 2021. https://doi.org/10.1007/s10072‑021‑05109‑1.
11. Purrucker JC, Hametner C, Engelbrecht A, Bruckner T, Popp E, Poli S. Comparison of stroke recognition and stroke severity scores for stroke detection in a single cohort. J Neurol Neurosurg Psychiatry. 2015;86(9):1021–8.
12. Brunser AM, Hoppe A, Illanes S, Díaz V, Muñoz P, Cárcamo D, et al. Accuracy of diffusion‑weighted imaging in the diagnosis of stroke in patients with suspected cerebral infarct. Stroke. 2013;44(4):1169–71.
13. Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7(5):378–85.
14. Alqahtani SA, Stemer AB, McCullough MF, Bell RS, Mai J, Liu A‑H, et al. Endovascular management of stroke patients with large vessel occlusion and minor stroke symptoms. Cureus. 2017;9(6):e1355.
15. Tziotzios C, Dawson J, Walters M. Stroke in practice: from diagnosis to evidence‑based management. Boca Raton: CRC Press; 2017.
16. Umemura T, Kawamura T, Hotta N. Pathogenesis and neuroimaging of cerebral large and small vessel disease in type 2 diabetes: a possible link between cerebral and retinal microvascular abnormalities. J Diabetes Investig. 2017;8(2):134–48.
17. Younger DS. Stroke due to vasculitis in children and adults. Neurol Clin. 2019;37(2):279–302.
18. Perera KS, Vanassche T, Bosch J, Swaminathan B, Mundl H, Giruparajah M, et al. Global survey of the frequency of atrial fibrillation‑associated stroke: embolic stroke of undetermined source Global Registry. Stroke. 2016;47(9):2197–202.
19. Majersik JJ, Skalabrin EJ. Single‑gene stroke disorders. Seminars in neurology. New York: Thieme Medical Publishers Inc.; 2006.
20. Rosenberg RN, Pascual JM. Rosenberg’s molecular and genetic basis of neurological and psychiatric disease, vol. 2. Cambridge: Academic press; 2020.
21. Zhao X‑J, Larkin TM, Lauver MA, Ahmad S, Ducruet AF. Tissue plasminogen activator mediates deleterious complement cascade activation in stroke. PLoS ONE. 2017;12(7):e0180822.
22. Gerriets T, Walberer M, Ritschel N, Tschernatsch M, Mueller C, Bachmann G, et al. Edema formation in the hyperacute phase of ischemic stroke. J Neurosurg. 2009;111(5):1036–42.
23. Wang P, Shao B‑Z, Deng Z, Chen S, Yue Z, Miao C‑Y. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163:98–117.
24. Endres M, Dirnagl U, Moskowitz MA. The ischemic cascade and mediators of ischemic injury. Handb Clin Neurol. 2008;92:31–41.
25. Rodrigo R, Fernández‑Gajardo R, Gutiérrez R, Manuel Matamala J, Carrasco R, Miranda‑Merchak A, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord‑Drug Targets (Formerly Curr Drug Targets‑CNS Neurol Disord). 2013;12(5):698–714.
26. Guo Y, Li P, Guo Q, Shang K, Yan D, Du S, et al. Pathophysiology and biomarkers in acute ischemic stroke—a review. Trop J Pharm Res. 2013;12(6):1097–105.
27. Kamalian S, Lev MH. Stroke imaging. Radiol Clin N Am. 2019;57(4):717–32. https:// doi. org/ 10. 1016/j. rcl. 2019. 02. 001.
28. Chugh C. Acute ischemic stroke: management approach. Indian J Crit Care Med Peer‑rev. 2019;23(Suppl 2):S140.
29. Alonso de Leciñana M, Egido JA, Casado I, Ribó M, Dávalos A, Masjuan J, et al. Guidelines for the treatment of acute ischaemic stroke. Neurologia (Barcelona, Spain). 2014;29(2):102–22. https:// doi. org/ 10. 1016/j. nrl. 2011.09. 012.
30. Liao XL, Wang CX, Wang YL, Wang CJ, Zhao XQ, Zhang LQ, et al. Implementation and outcome of thrombolysis with alteplase 3 to 4.5 h after acute stroke in Chinese patients. CNS Neurosci Ther. 2013;19(1):43–7. https:// doi. org/ 10. 1111/ cns. 12031.
31. Merino JG, Warach S. Imaging of acute stroke. Nat Rev Neurol. 2010;6(10):560.
32. Chrzan R, Gleń A, Urbanik A. Hyperdense middle cerebral artery sign as the only radiological manifestation of hyperacute ischemic stroke in computed tomography. Neurol Neurochir Pol. 2017;51(1):33–7.
33. Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Correlation of early CT signs in the deep middle cerebral artery territories with angiographically confirmed site of arterial occlusion. AJNR Am J Neuroradiol. 2001;22(4):654–9.
34. Weimar C. Stroke of undetermined cause: workup and secondary prevention. Curr Opin Neurol. 2016;29(1):4–8. https:// doi. org/ 10. 1097/ wco. 00000 00000 000280.
35. Ifergan H, Amelot A, Ismail M, Gaudron M, Cottier JP, Narata AP. Strokemimics in stroke‑units. Evaluation after changes imposed by randomized trials. Arq Neuropsiquiatr. 2020;78(2):88–95. https:// doi. org/ 10. 1590/ 0004‑ 282x2 01901 54.
36. Quenardelle V, Lauer‑Ober V, Zinchenko I, Bataillard M, Rouyer O, Beaujeux R, et al. Stroke mimics in a stroke care pathway based on MRI screening. Cerebrovasc Dis (Basel, Switzerland). 2016;42(3–4):205–12. https:// doi. org/ 10. 1159/ 00044 5956.
37. Psychogios K, Kargiotis O, Safouris A, Magoufis G, Gelagoti M, Bonakis A, et al. Perfusion imaging averting intravenous thrombolysis in stroke mimics. Neurol Sci. 2021;42(6):2591–4. https:// doi. org/ 10. 1007/ s10072‑0 21‑0 5090‑9.
38. Provost C, Soudant M, Legrand L, Ben Hassen W, Xie Y, Soize S, et al. Magnetic resonance imaging or computed tomography before treatment in acute ischemic stroke. Stroke. 2019;50(3):659–64. https:// doi. org/ 10. 1161/ strok eaha. 118. 023882.
39. Zhang W, Cheng J, Zhang Y, Wang K, Jin H. Analysis of CT and MRI combined examination for the diagnosis of acute cerebral infarction. J Coll Physicians Surg Pak. 2019;29(9):898–9. https:// doi. org/ 10. 29271/ jcpsp. 2019. 09. 898.
40. Malhotra K, Liebeskind D. Overview of neuroimaging of stroke. In: Welch KM, Caplan LR, Siesjo BK, Weir B, Reis DJ, editors. Primer on cerebrovascular diseases. Amsterdam: Elsevier; 2017. p. 676–85.
41. Wang Y, Zhou Z, Ding S. FLAIR vascular hyperintensity‑DWI mismatch most likely to benefit from recanalization and good outcome after stroke. Medicine. 2020;99(2):e18665. https:// doi. org/ 10. 1097/ md. 00000 00000 018665.
42. Boss SM, Moustafa RR, Moustafa MA, El Sadek A, Mostafa MM, Aref HM. Lesion homogeneity on diffusion‑weighted imaging is a marker of outcome in acute ischemic stroke. Egypt J Neurol Psychiatry Neurosurg. 2019;55(1):59. https:// doi. org/ 10. 1186/ s41983‑ 019‑ 0101‑z.
43. Oliveira‑Filho J, WJ K. Initial assessment and management of acute stroke. UpToDate. Waltham: UpToDate Retrieved January. 2010.
44. Mullins ME, Schaefer PW, Sorensen AG, Halpern EF, Ay H, He J, et al. CT and conventional and diffusion‑weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology. 2002;224(2):353–60. https:// doi. org/ 10. 1148/ radiol. 22420 10873.
45. van Gelderen P, de Vleeschouwer MHM, DesPres D, Pekar J, van Zijl PCM, Moonen CTW. Water diffusion and acute stroke. Magn Reson Med. 1994;31(2):154–63. https:// doi. org/ 10. 1002/ mrm. 19103 10209.
46. Almandoz JED, Romero JM. Advanced CT imaging in the evaluation of hemorrhagic stroke. Neuroimaging Clin. 2011;21(2):197–213.
47. Zerna C, Thomalla G, Campbell BC, Rha J‑H, Hill MD. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet. 2018;392(10154):1247–56.
48. Liebeskind DS, Alexandrov AV. Advanced multimodal CT/MRI approaches to hyperacute stroke diagnosis, treatment, and monitoring. Ann N Y Acad Sci. 2012;1268:1.
49. Kang DW, Jeong HG, Kim DY, Yang W, Lee SH. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibilityweighted magnetic resonance imaging. Stroke. 2017;48(6):1554–9. https:// doi. org/ 10. 1161/ strok eaha. 116. 016217.
50. Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment—systematic review. Radiology. 2005;235(2):444–53. https:// doi. org/ 10. 1148/ radiol. 23520 40262.
51. Lövblad KO, Altrichter S, Mendes Pereira V, Vargas M, Marcos Gonzalez A, Haller S, et al. Imaging of acute stroke: CT and/or MRI. J Neuroradiol. 2015;42(1):55–64. https:// doi. org/ 10. 1016/j. neurad. 2014. 10. 005.
52. Guberina N, Dietrich U, Radbruch A, Goebel J, Deuschl C, Ringelstein A, et al. Detection of early infarction signs with machine learning‑based diagnosis by means of the Alberta Stroke Program Early CT score (ASPECTS) in the clinical routine. Neuroradiology. 2018;60(9):889–901. https:// doi. org/ 10. 1007/ s00234‑ 018‑ 2066‑5.
53. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet (Lond, Engl). 2000;355(9216):1670–4. https:// doi. org/ 10. 1016/ s0140‑ 6736(00) 02237‑6.
54. Pallesen LP, Gerber J, Dzialowski I, van der Hoeven EJ, Michel P, Pfefferkorn T, et al. Diagnostic and prognostic impact of pc‑ASPECTS applied to perfusion CT in the Basilar Artery International Cooperation Study. J Neuroimaging. 2015;25(3):384–9. https:// doi. org/ 10. 1111/ jon. 12130.
55. Lansberg MG, Albers GW, Beaulieu C, Marks MP. Comparison of diffusionweighted MRI and CT in acute stroke. Neurology. 2000;54(8):1557–61. https:// doi. org/ 10. 1212/ wnl. 54.8. 1557.
56. Allen LM, Hasso AN, Handwerker J, Farid H. Sequence‑specific MR imaging findings that are useful in dating ischemic stroke. Radiographics. 2012;32(5):1285–97. https:// doi. org/ 10. 1148/ rg. 32511 5760 (discussion 97-9).
57. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275(2):510–20.
58. Eastman AL, Muraliraj V, Sperry JL, Minei JP. CTA‑based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma. 2009;67(3):551–6. https:// doi. org/ 10. 1097/ TA. 0b013 e3181 b84408 (discussion 5-6).
59. Wardlaw JM, Stevenson MD, Chappell F, Rothwell PM, Gillard J, Young G, et al. Carotid artery imaging for secondary stroke prevention. Stroke. 2009;40(11):3511–7. https:// doi. org/ 10. 1161/ STROK EAHA. 109. 557017.
60. Liebeskind DS, Kosinski AS, Saver JL, Feldmann E, Investigators S. Computed tomography angiography in the stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) study. Interv Neurol. 2013;2(4):153–9.
61. Liebeskind DS, Kosinski AS, Lynn MJ, Scalzo F, Fong AK, Fariborz P, et al. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis. J Neuroimaging. 2015;25(1):87–91. https:// doi. org/ 10. 1111/ jon. 12101.
62. Kilburg C, McNally JS, de Havenon A, Taussky P, Kalani MYS, Park MS. Advanced imaging in acute ischemic stroke. Neurosurg Focus. 2017;42(4):E10.
63. Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40(11):3646–78.
64. Myung JW, Kim JH, Cho J, Park I, Kim HY, Beom JH. Contrast‑induced acute kidney injury in radiologic management of acute ischemic stroke in the emergency setting. AJNR Am J Neuroradiol. 2020;41(4):632–6. https:// doi. org/ 10. 3174/ ajnr. A6472.
65. Hopyan JJ, Gladstone DJ, Mallia G, Schiff J, Fox AJ, Symons SP, et al. Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am J Neuroradiol. 2008;29(10):1826–30. https:// doi. org/ 10. 3174/ ajnr. A1257.
66. Connolly F, Röhl JE, Guthke C, Wengert O, Valdueza JM, Schreiber SJ. Emergency room use of “fast‑track” ultrasound in acute stroke: an observational study. Ultrasound Med Biol. 2019;45(5):1103–11. https:// doi. org/ 10. 1016/j. ultra smedb io. 2019. 01. 001.
67. Buskens E, Nederkoorn PJ, Buijs‑Van Der Woude T, Mali WP, Kappelle LJ, Eikelboom BC, et al. Imaging of carotid arteries in symptomatic patients: cost‑effectiveness of diagnostic strategies. Radiology. 2004;233(1):101–12. https:// doi. org/ 10. 1148/ radiol. 23310 30863.
68. Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Semin Neurol. 2012;32(4):411–20. https:// doi. org/ 10. 1055/s‑ 0032‑1 331812.
69. de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, et al. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66. https:// doi. org/ 10. 1007/ s12028‑0 12‑9 672‑6.
70. Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial Doppler: a stethoscope for the brain‑neurocritical care use. J Neurosci Res. 2018;96(4):720–30. https:// doi. org/ 10. 1002/ jnr. 24148.
71. Schlachetzki F, Herzberg M, Hölscher T, Ertl M, Zimmermann M, Ittner KP, et al. Transcranial ultrasound from diagnosis to early stroke treatment— part 2: prehospital neurosonography in patients with acute stroke—The Regensburg Stroke Mobile Project. Cerebrovasc Dis. 2012;33(3):262–71.
72. Giannoni MF, Vicenzini E, Sbarigia E, Di Piero V, Lenzi GL, Speziale F. Early ultrasound imaging of carotid arteries in the acute ischemic cerebrovascular patients. Perspect Med. 2012;1(1–12):108–11.
73. Markus HS, MacKinnon A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. 2005;36(5):971–5. https:// doi. org/ 10. 1161/ 01. STR. 00001 62717. 62684. 40.
74. Prabhakaran S. Imaging markers of stroke risk in asymptomatic carotid artery stenosis. Brain Circ. 2015;1(1):38–46. https:// doi. org/ 10. 4103/ 2394‑ 8108. 166373.
75. Tang Y, Wang M‑Y, Wu T‑T, Zhang J‑Y, Yang R, Zhang B, et al. The role of carotid stenosis ultrasound scale in the prediction of ischemic stroke. Neurol Sci. 2020;41(5):1193–9. https:// doi. org/ 10. 1007/ s10072‑0 19‑0 4204‑8.
76. Mahmoodpoor A, Akbarzadeh MA, Sanaie S, Hosseini MS. Role of telehealth in outbreaks—where the classical healthcare systems fail. Infect Control Hosp Epidemiol. 2020;41(8):992–4. https:// doi. org/ 10. 1017/ ice. 2020. 120.
77. Birns J, Bhalla A, Rudd A. Telestroke: a concept in practice. Age Ageing. 2010;39(6):666–7. https:// doi. org/ 10. 1093/ ageing/ afq125.
78. Hubert GJ, Corea F, Schlachetzki F. The role of telemedicine in acute stroke treatment in times of pandemic. Curr Opin Neurol. 2021;34(1):22– 6. https:// doi. org/ 10. 1097/ wco. 00000 00000 000887.
79. Agarwal S, Warburton EA. Teleneurology: is it really at a distance? J Neurol. 2011;258(6):971–81. https:// doi. org/ 10. 1007/ s00415‑0 11‑5 920‑5.
80. Laghari FJ, Hammer MD. Telestroke imaging: a review. J Neuroimaging. 2017;27(1):16–22. https:// doi. org/ 10. 1111/ jon. 12402.
81. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large‑vessel ischaemic stroke: a meta‑analysis of individual patient data from five randomised trials. Lancet (Lond, Engl). 2016;387(10029):1723–31. https:// doi. org/ 10. 1016/ s0140‑ 6736(16) 00163‑x.
82. Pedragosa A, Alvarez‑Sabín J, Rubiera M, Rodriguez‑Luna D, Maisterra O, Molina C, et al. Impact of telemedicine on acute management of stroke patients undergoing endovascular procedures. Cerebrovasc Dis (Basel, Switzerland). 2012;34(5–6):436–42. https:// doi. org/ 10. 1159/ 00034 5088.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical Technology"
Medical Technology is used to enhance the medical care and treatment that patients are given in healthcare settings. Medical Technology can be used to identify, diagnose and treat medical conditions and illnesses.
Related Articles
DMCA / Removal Request
If you are the original writer of this literature review and no longer wish to have your work published on the UKDiss.com website then please: