Dissertation on Pharmaceutical Industry Patent Reforms
Info: 18878 words (76 pages) Dissertation
Published: 15th Nov 2021
Tagged: International StudiesPatent LawIntellectual Property Law
ABSTRACT
China’s State Intellectual Property Office in 2015 became the first office to receive over a million patent applications in a single year. The office received almost as many applications as the leaders in IP area combined (Japan, the Republic of Korea and U.S.). On the other hand, China`s pharmaceutical market became the world 2nd (in terms of industry value), coming from the 9th place, in the period between 2005 to 2016, according to a IMS Health report. Based on a patent search and literature research, this study carefully analyzes the impact of recent reforms in the patent applications from pharmaceutical industry in China, identifies the principal fields of technology developed, main inventors and companies patenting products in the country, and a trend in the applications after two major reforms in the patent Law. Moreover, based on a questionnaire and interviews to 18 companies with operations in China, the study identifies the factors that companies in pharmaceutical industry considered the most important for their IP strategy and patent application; it has been found that (i) partnership (ii) exclusivity and (iii) licensing opportunities are the factors that influence those decisions in the organization. Additionally, it has been found that companies are satisfied with the IPR system and found it user-friendly, as well as helpful to the industry growth.
Keywords: Intellectual Property, Patent Law, IP strategy, Pharmaceutical Industry.
LIST OF ABBREVIATIONS
IP – Intellectual Property
IPR – Intellectual Property Rights
WIPO – World Intellectual Property Organization
IPC – International Patent Classification
SFDA – State Food and Drug Administration
SIPO – State Intellectual Property Office
USPTO – United States Patent and Trademark Office
Click to expand contents
TABLE OF CONTENTS
TITLE 1
ABSTRACT 2
LIST OF ABBREVIATIONS 3
TABLE OF CONTENTS 4
LIST OF TABLES & FIGURES 6
Chapter 1. INTRODUCTION 7
- Research Background 7
- Research Objectives 9
- Research Question 9
Chapter 2. LITERATURE REVIEW 10
- Chinese Patent Law 10
- Intellectual Property and Pharmaceutical Industry 16
- Intellectual Property Strategy 17
Chapter 3. ACTUAL PATENT LAW AND PHARMACEUTICAL INDUSTRY 19
3.1. The actual Chinese patent Law 19
3.1.1 Intellectual Property Reforms 24
3.2 PHARMACEUTICAL INDUSTRY 32
3.2.1 Pharmaceutical Industry in China 33
3.2.2 Patented drug market 37
3.2.3 Diseases profile 38
3.2.4 Domestic market and competitive environment 39
3.2.5 The SFDA 40
Chapter 4. HYPOTHESIS AND METHODOLOGY 42
4.1 Hypothesis 42
4.2 Methodology 44
4.2.1 Research Design 44
4.2.2 Research Area 44
4.2.3 Research Instrument 45
4.2.3.1 Patent Search 45
4.2.3.2 Questionnaire 46
4.2.3 4 Sampling 47
4.3 Data analysis and findings 48
Chapter 5. CONCLUSIONS and DISCUSSION 61
5.1 Research Finding Discussion 61
5.2 Conclusions 62
5.3 Significance of the study 63
5.4 Study Limitation 64
5.5 Direction for future research 64
REFERENCES 66
APPENDIX 1 69
APPENDIX 2 70
APPENDIX 3 76
ACKNOWLEDGEMENTS 84
LIST OF TABLES & FIGURES
Table 1. Patent applications by top fields of technology (2001 – 2015)
Table 2. Determinant factors in company decision to apply for a patent in China
LIST OF FIGURES
Figure 1. Patent granting procedure in China
Figure 2. Top 10 Total pharmaceutical Markets in the World, 2005-2016
Figure 3. Revenue of Major pharmaceutical circulation Enterprises in China,2013
Figure 4. Diagram of China`s approval procedures for new drugs
Figure 5. Patented drug sales in China, 2007- 2015
Figure 6. Patent application in pharmaceutical Industry 2004 – 2014 in China
Figure 7. Top Assignee in pharmaceutical Industry patent application period 2004-2014
Figure 8. Top IPC marks in pharmaceutical Industry patent application period 2004-2014
Figure 9. Chinese IPR system to pharmaceutical companies.
Figure 10. The Chinese IPR system and its help in growth to pharmaceutical Companies.
Figure 11. The Chinese IPR system is user-friendly
Figure 12. The process of filling patent application in China is user-friendly
Figure 13. The period required to register a patent in China is adequate.
Figure 14. Preparation of patent application in the companies
Figure 15. The company met the goal/target for patent applications in the last year?
Figure 16. Are all patent application functions centralized in the IP department?
Figure 17. Expenditure in IP services or operation during reforms timeline
Figure 18. IP strategy in the reforms timeline
Figure 19. Determinant factors in IP strategy
Chapter 1. Introduction
Research Background
China is worldwide recognized as one of the countries with highest rates of Intellectual property infringement, one example is the reported software piracy, which it can be over 77% according to the report: Shadow Market; the report shows a total pirated value of $8.902 ($M USD) in 2011. (BSA Business Software Alliance 2011). China`s tend to patent or copyright infringement has been studied from different perspectives, one of the most recognized scholars is William P. Alford from Harvard University who dedicated most of his work to explain how Chinese culture differs from other western countries in the application of Intellectual Property Law and its history.
Books as “To steal a book is an elegant offense: Intellectual Property Law in Chinese civilization” (Stanford University Press, 1995), explain how during the changing era the application of new legislations in China, were more focused in agriculture than in commerce. Basically, the author refers to the influence of legislation to transform industries. Moreover, part of his work recognizes how China it has a historical inconvenient way to follow western legal values. (Alford 1995) Alford discussion maintains the theory that some of the issues that move China back in the time, in comparison to other countries legislations, and it creates difficulties to face the developing of the intellectual property law system, it is the Confucian ideology, that highly influenced the people’s behavior. (Paul S. Ropp 1990)
According to the scholar, the imitation and copying can be considerate as flattery in many cases for Chinese people, even a way to show respect and admiration. In comparison with western societies where copying it can be seeing as an insult. Managing intellectual property related processes it becomes a challenge if you added to the formula a cultural factor that affects the understanding of the law and an international legislation based on many agreements and treaties.
The pharmaceutical industry in today`s economy is one of the most controversial in IP legislation, since has a high R&D intensity and it is also a high-tech industry, also it has an increasing demand for innovation. (Andrew Ward 2015) China`s estimate population reach 1.38 billion, with a population over 65 years old of 131 million (9.6%). At the time that population is increasing, it is also the income levels and chronic diseases burden. The changes in diets, lifestyles, air and water pollution, are contributing to an increased incidence of cancer, heart disease, diabetes and other chronic conditions. China has 110 million diabetics, with much more undiagnosed, what opens the door to a discussion of opportunities for the industry in the country. (Sjolin 2016)
The costs in product development in the pharmaceutical industry are enormous, the estimated cost of discovering, developing, and launching a new drug depending on the therapy or the developing firm it can be around $500 million to more than $2,000 million. (Christopher P. Adams 2006) Besides the high costs of product innovation in the industry, is also important to highlight the protection of the safety of drugs that enter a country, since the benefits of intellectual property it helps to introduce products more rapidly into the market, encouraging pioneers. It is a fundamental part of market segmentation in the market for the industry. (Richard 2011)
This study shows an overview of the Chinese IP law, the started point of the patent law and its actual stage, the pharmaceutical industry in China. The connection between the patent law and pharmaceutical industry focusing in the Chinese market, and the effects of the last reforms in the patent application for the pharmaceutical industry. The study relays in the opinion of 18 companies that are applying for patents in China, with specific characteristics of time in the market and product development. Additionally, considered public information of patent applications from 2004 to 2014, filtered with a patent search formula, containing moderators of IPC marks especially used in the protection of new products for the industry, State of application and designated states.
Research Objectives
The aim of the research can be divided into the following points:
- To study the Chinese IP Law, its reforms and development, focused in the articles/legislation that would likely affect the pharmaceutical industry.
- To study the impact of Chinese IP Law, recent reforms (2006 -2016), on pharmaceutical company’s patent application.
- To study the pharmaceutical companies’ perception and understanding of IP system in China.
- To highlight the determinant factors in the patent application procedure and companies IP strategy for the pharmaceutical Industry in China.
Research questions
The main research questions related to the study are as follows:
- What is the effect of the new legislation in the decision of pharmaceutical companies to apply for a patent in China?
- What is the effect of the new legislation in the IP strategy for pharmaceutical companies?
- What have been the main factors that affect the patent application process for the pharmaceutical companies?
Chapter 2. Literature Review
2.1 Chinese Patent Law
Some scholars like Awokuse, Titus O.; Yin, Hong; 2010 and Pang, Laikwan, 2008, studied the Chinese Intellectual Property system, its legislation and the issues associated with it and stated that: China is well known around the world because of its weak intellectual property law, most of the studies around this area are related to the lack of protection for trademark, commercial names, and inventors. (Keupp, Beckenbauer y Gassmann. 2009) The researchers are mainly focused on the factors that impact the patent application procedure from a procedural perspective.
The first stage in the literature review showed a meaningful base of patent value studies, conclusions as “patent value from Chinese owners is much lower than that of overseas owners”. (Zhang Gupeng, Chen Xiandong, 2012) and “China`s R&D intensification is not the main force of patent explosion” (Albert Guangzhou Hu, Gary H Jefferson, 2009), allowed me to infer that the procedures in patent application could be influenced by different factors in comparison with other countries.
The Chinese Law has also been studied from scholars who analyze and describe the evolution of different aspects of the Law, M Sidel 1985; Hill, Evans 1994 and Yueh 2009. They explore whether the IPR system has resulted in innovation and how factors, as international pressure, culture, industries, and inventors, have transformed and improved the system in the different periods.
The paper “The value of invention patents in China: Country origin and technology field differences” (Zhang Gupeng, Chen Xiangong), relies on a model applied to patent data in different technical fields to discuss the patent value from Chinese owners, concluding that it is much lower the one from overseas owners. The Chinese IP environment it was deeply studied and referenced in the paper “conducting R&D in Countries with weak IPR protection” by Minyuan Zhao, which presents a close understanding of firm strategy and external environments. Additionally, it proposes to firms to combine and integrate the capabilities to efficiently perform globally. In terms of innovation and performance referring to the Patent law, Linda Yueh published “Patent Laws and Innovation in China”, where through an economic analysis concludes that the impact patent laws over innovation will likely depend on the context of the region.
Indian scholars had widely studied the implications of patent law and intellectual property rights on pharmaceutical Industry; Cheri Grace discuss the competitive advantages of Indian pharmaceutical companies and their strategy, the scholar considered the size of the market, the quality of chemist, engineers and the services offered for re-engineering. On the other hand, Peter F. Drucker, “Innovation is not the same as invention”, discusses the importance of innovation in the actual pharmaceutical firms, not focusing in the conception of new products, but valuing the positioning in the market.
The starting point of Chinese patent Law
The origin of the Intellectual Property practices in China take place in the 19th century. Most of the activities that generate a base for the actual law, took place in Qing Dynasty, during this time, new technologies developed in foreign countries were introduced to China. In order to achieve higher results in the industrialization era, Chinese started to use the inverse engineer to transform the imports in a national production. Copying products and imitating capabilities, the country it was able to reproduce many products that were imported at this time.
During the late 1890`s there was a regulation on the development of technology, the government focused the strategies to develop an industrial machinery for the country, in this terms, the main focus for Chinese it was more in producing rather than inventing. (Liu 1996) Since China is a country driven by collectivism, the efficiency of the law and protection of rights related to inventions was low, even after the creation of the first legislation, there was no definition for the inventions and no processes for examination in order to guarantee a proper statement for inventors. (Zheng, Intellectual Property 1999)
During the Qing Dynasty in 1910, China makes a big step in terms of Intellectual Property Law, introducing the Copyright Law. In comparison with England, where the first copyright statute occurred with the Statute of Anne in 1709, China it can be seen behind for more than 200 years. “The Hang Wu publishing house” it was back in time the first recorded copyright, with 20-year copyright for a famous author, Yan Fu. (Zheng, Textbook for Intellectual Property Law 1997)
The first formal Law
The major source of legal advice to support activities that required a support to protect their novelty until 1944 it was the “Temporary statute of technology reward” announced during 1912. Among this time, the rights were not named as patent but as a reward for new technologies. The first formal Law, named as patent law appeared in 1944. (D. Bosworth 2002) One of the provisions in this legislation showed how close it was China at the time, prohibiting foreigners to apply for patents in the country. Trademark regulations were introduced in 1923, there was a notorious predominance of foreign registration with over 50.000 trademark registration by 1948.
The reward system
The reward system, started during the establishment of Mao Zedong as leader of the communist regime and the Peoples republic of China (1949 to 1957). The system basically worked as a figure to promote the industrialization and innovation trough Intellectual Property practices. The reward system allowed the inventors to get medals, bonuses or certifications in reward for their new creations, the period of protection for the new inventions it was limited and it can’t be compared with what they have nowadays, but it was followed by the beliefs of that time. (Liu 1996)
Changes in the IP system
The reforms in Chinese legislation related to Intellectual Property Protection, that created an environment capable of being managed as a system, started back to 1979 during the negotiation of the Sino-US Trade Agreement. During this time the US government argued over the importance to develop an agreement considering the importance of Intellectual Property Protection, as an integral part of the trade in terms of science and technology. These negotiations with the US, were the beginning of many research from Chinese in the area, principally to understand and get experience in some clauses of the agreement. (Yang 2003)
The biggest transformation in Chinese Intellectual Property legislation it was in 1980 when China became a member of the World Intellectual Property Organization (WIPO). Since that time, the government continued active in the area, ratifying a series of World Intellectual Property conventions and treaties. In 1985 China ratified the Paris Convention, moving to ratify the Madrid agreement in 1989, and 1989 became a signatory country for the integrated Circuits Treaty. Also, the State Intellectual Property Office (SIPO) it was established and the Trademark Office. The judicial organs for Intellectual Property Protection it was restructured and the biggest step it was made by the promulgation of a wide range of IP laws. By the middle of 1990`s with new reforms and legislation in the area, China got enough framework in terms of Intellectual Property to develop a proper system.
Improvements in the system
Following a strategy to form an IP system in China, and to generate an appropriated IP environment, the country has intensified the policies, by ratifying more IP conventions and treaties. The most recent internationally improvements include the following in a chronological order: Berne Convention, Universal Copyrights Convention, Geneva Convention, Patent Cooperation Treaty.
China becoming a World Trade Organization (WTO) member in November 2001, it gave a significant step forward, not only in terms of Intellectual Property Protection but also joining in world discussions in innovation related movements. The country participated in the negotiations and signed the last document of Uruguay Round for the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement in 1995, even when it was not a member of the WTO.
In terms of local efforts, there are many reforms that affected the system in the country: In 1991, it was announced the Statute on Computer Software Protection; in 1992, it was promulgated the Regulations on the Enforcement of Universal Copyrights Convention; in 1993, China announced the Law of the People`s Republic of China for Countering Unfair Competition (Anti-Unfair Competition Law), and by 1994, China enacted the Decision on Infringement Punishment.
Influence from developed countries
The international pressure is one of the factors that influence China`s decision to involve the IP protection in its agenda. Also, after the WTO, it is possible to identify a major number of reforms or assets related to this legislation in the country. The USA is one of the countries that have campaigned for an increased protection of the inventions and products in developing countries. The commercial treaties and agreements are usually the first stage in order to get a fairer trade. (D. Yang 2000)
In addition, the US also uses “special mention”, which refers to a list of countries that should further enhance their IPP because of existing or emerging problems. Following a decision of the US International Trade Commission (USITC), Section 337 can be used to authorize the US Customs to detain all the imported products associated with IPR infringement. The US using Sections 301 and 337 appears to have exerted a strong influence on IPP in China, especially in the 1990s.
Influence from International Organizations
The World Intellectual Property Organization (WIPO) have played a significant role in the development of China`s IP Law, while the World Trade Organization (WTO) have been an influence in terms of improvements to the Intellectual Property system. (Kwang 1999) China`s Patent Law, Trademark Law, and Copyright Law were based on the Paris Convention, Madrid Convention, and the Universal Copyright Convention respectively, these Laws were specially marked after 1980 when China became a contracting country of WIPO. Nowadays, China is an active member of the Organization and also maintains in line with the different conventions.
China`s actual development plan, it has as an important point in the agenda to ensure that Intellectual Property Protection is sufficiently strong to attract foreign investors, capital, know-how, technology and also to protect the consumers, ensuring product quality and safeguard fair competition. The actual plan also highlights the importance of motivating inventors and creators.
2.2 Intellectual Property and Pharmaceutical Industry
Keith E. Maskus in “Intellectual Property rights in the global economy” offers a brief resume of the “complexity of patents” in pharmaceuticals, biotechnology, and plant varieties; the document highlights the difficulties for the industry, principally with substitute products. It includes important data related to expenses in R&D for the American drug companies, costs, and sector distribution. This document takes into consideration only the challenges of the sector in terms of legal scope.
Robert Weissman in “A long, strange TRIPS: the pharmaceutical industry drive to harmonize global intellectual property rules, and the remaining WTO legal alternatives available to third world countries”, the document focus in legal effects for international trade agreements, taking as a example the “North America and Uruguay” trade; also offer a review of the changes proposed and made during the negotiation process.
Anna Lanoszka in “The global politics of intellectual property rights and pharmaceutical drug policies in developing countries”, the paper focus on the legal framework, effects, and negotiation of the Trade-Related aspects of intellectual property rights (trips). Also, remind the challenges for developing countries in terms of patents for pharmaceutical products. Widener L. Symp. J. in “TRIPS, pharmaceutical patents, and the HIV/AIDS crisis: Finding the proper balance between intellectual property rights and compassion”, refers to the dispute between the World Trade Organization WTO members in the compulsory licensing provision and other tools that allow the production of products that have been patented and maintain the rights in a territory, taking in consideration a legal scope.
Lara J Glasgow in “stretching the limits of Intellectual Property Rights: Has the pharmaceutical industry gone too far?”, provides insights into the pharmaceutical industry strategies to extend the protection of their name drugs, through actions like suing generic manufacturers, using loopholes to apply for extensions, merging with competitors and recombining drugs. The paper focus in the U.S. pharmaceutical industry and the different tools offered by the I.P. to protect the inventions. The paper main conclusions are related to legal inabilities and strategies.
2.3 Intellectual Property strategy
Intellectual Property Strategies have been widely studied over the world, scholars in the IP and innovation field approach the topic from different perspectives. Xiang, Tang 2016 and Wang Zh 2013; refers to China in their research to explain how companies can succeed in China`s innovation environment, considering the China`s IP system. Additionally, the documents discuss areas as antitrust enforcement, IP securitization and IP value involved in the IP strategies.
Guide to Intellectual Property: What it is, how to protect it, how to exploit it by the Economist and Stephen Johnson, 2015. It offers a clear guide to companies on the operation of strategies in IP; this book focuses on the challenges that companies have to face because of the large number of infringements that are present in this area. One of the most common cases and discussed by the authors is the generic drugs in pharmaceutical industry. Because of these cases, companies must rethink their protection strategies through the tools in the IP system, such as licenses, trademarks, copyrights, designs and trade secrets.
As for patents, the authors determine activities such as: internationalization, partnership and marketing; which focus on a particular process which it must be done before and after the patent is granted, and it can provide a complete strategy in those areas. For example, early publications on inventions related to parts of the drug-making process, may help other companies not to approach the invention generated, but also to market the product in an early stage, or it can help to find partners or investors to finish the invention process.
The strategies for the protection and use of inventions vary according to the type of product and the purpose of the company. The book “Essentials of Licensing Intellectual Property” by Alexander I. Poltorak and Paul J. Lenner, offers a group of practices, techniques and tips, for companies in all industries that seek to license their inventions. The authors refer to all types of licensing, focusing on the contracts that can be generated and discussing the processes related to licensing, as well as the strategies that companies should use. For example, in the case of the pharmaceutical industry, companies that seek to license their inventions must know in depth the production capacity of the licensee and his activities in research and development, since the licensee it can become a future competitor in the invention field.
Chapter 3. ACTUAL PATENT LAW AND PHARMACEUTICAL INDUSTRY
3.1 The actual Chinese patent Law
The Chinese patent law, it was adopted at the 4th Meeting of the Standing Committee of the Sixth National People`s Congress on March 12, 1984, is administered by the State Intellectual Property Office (SIPO). Patent Law of the People`s Republic of China (PRC) has been amended on September 4, 1992, amended for the second time on August 25, 2000, and amended for the third time on December 27, 2008. (Patent Law of PRC 2008) (Preface)
The law recognized three types of patents, invention-creations that mean inventions as a new technical solution proposed for a product, process or the improvement thereof; utility models, meaning new technical solutions proposed for the shape and structure of a product, or the combination thereof, which fit for practical use and designs, as new designs of the shape, pattern, or the combination thereof, that are rich in an aesthetic appeal and are fit for industrial application. (Patent Law of PRC 2008) (Article 2)
Scope of Patents
Patents are national, not international, in scope. What it means is that patentee`s right to exclude others is extinguished at the geographic borders of the granting country. For example, the owner of a Chinese patent can not rely on the patent to stop an unauthorized third party from copying and selling the invention in Italy. Rather, the owner should obtain an Italian patent and enforce that patent against the infringer in Italy.
Consequently, there is not such an “international patent” or patent that provides rights to the owner in more that one country, the owner of the invention should apply for the patent in the countries where he considers it is necessary to protect it. There are, however, international treaties, agreements, and conventions that make it much easier to obtain protection in multiple countries. The Paris Convention for the Protection of Industrial Property (Paris Convention) (WIPO 1979). , was the first truly international agreement concerning industrial property. Before the Paris Convention an application even one day before in a different country, it was considered as a reason to invalidate the novelty of the invention calling it “prior art”, this kind of difficulties forced the patentees to apply for an invention in multiple countries the same day.
Conditions for Granting Patent Rights
The patent law in China defines three conditions for granting patent rights to inventions patents that shall be novel, creative and of practical use. (Patent Law of PRC 2008) (Article 22)
Novelty; The invention that is claimed shall be novel, meaning that is not an existing technology; also haven’t been exposed to the general public (published, used, made) before the date of filing of the application. For this, are counted both within the country and abroad. (Patent Law of PRC 2008) (Article 22)
Creativity; Creativity means, as compared with existing technologies, the new technology shall possess prominent substantive features and remarkable advancements, For assessing this requirement It is compared with the closest prior art and technical problem solved. It shouldn’t be obvious to a person skilled in the art. (Patent Law of PRC 2008) (Article 22)
Practical Use; Practical use means the claimed technology or utility model can be used for production or be utilized and may produce positive results. (Patent Law of PRC 2008) (Article 22)
Not patentable
The Chinese patent law excludes from patentability invention-creations that violate the law or social ethics; rules and methods for mental activities; methods of nuclear transformation and substances obtained thereof; human embryonic stem cell and methods of producing the same; animal or plant varieties; designs that are mainly used for making the pattern, color or the combination of the two of prints; human body or various stages of its formation and inventions based on genetic resources obtained in violation of the prevailing laws. (Patent Law of PRC 2008) (Article 5-25)
Filing the patent application
For Chinese citizens, a patent can be filed by hand or by post at SIPO or representative’s offices at all state capitals and districts headquarters. Chinese Intellectual Property system also offers the option for an electronic application. (Lei 2012) In the case of foreign applicants without a regular residence or business site in China, must entrust a legally established patent agency with the application and such matters. (Patent Law of PRC 2008) (Article 19)
The Chinese government in order to encourage patent filing by domestic applicants, provide subsidy in the patent filing and examination fee to them. This strategy has achieved good results. (Lei, Sunt y Wright s.f.)
Patent granting procedure in China
As it can be seen in Figure 1, the patent granting procedure in China, according to the European Patent Office, starts with the application, moving to formality check, classification of the technology, following laid-open publication; after this period the applicant should request for examination, where it can be notified of a refusal or it will be granted. Finally, after the patent is granted, there is a publication of the granted patent.
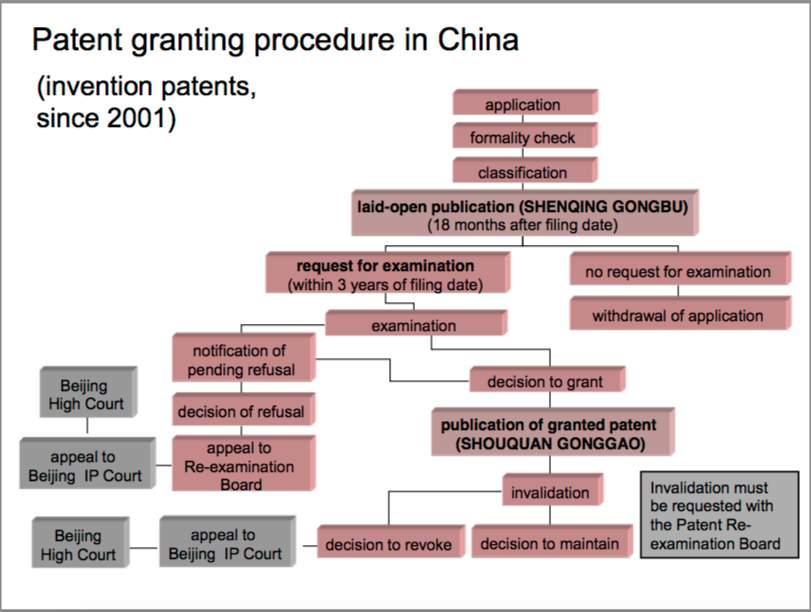
Source: European Patent Office
Figure 1. Patent granting procedure in China
Compulsory license for exploitation of a patent
The provisions related to compulsory licensing in the Chinese patent law are described in chapter VI, from the article 48 to 58. Moreover, the China State Intellectual Property Office (SIPO) issued “Measures for compulsory licensing of patent implementation” in march, 2012. The main objective of these measures it was to standardize the procedures of compulsory licenses of patents, focusing in granting, fees adjudication and termination.
In terms of compulsory licenses, the provision more important for this study are listed:
- According to the article 48, the patent administration department may grant a compulsory license for exploitation of an invention patent or utility model patent under the conditions: (i) have been three years since the patent right is granted and four years since the application is submitted, since the patentee, fails to have the patent exploited or fully exploited, without legitimate reasons. (Patent Law of PRC 2008) (Article 48)
- The article 49 provides SIPO the authority to grant a compulsory license, where a national emergency or any extraordinary state of affairs occurs, also public interests so require. Also for the benefit of public health, SIPO may grant a compulsory license for manufacture of the drug, patented, and for its export to the countries or regions that conform to the provisions of relevant international treaties. (Patent Law of PRC 2008) (Article 49-50)
Patent linkage
According to the Drug Registration Regulation, approved by SFDA, the applicants shall submit the necessary documentation explaining the China patent status and ownership rights. Moreover, it shall submit a letter of guarantee stating that the drug will not infringe patent rights of others. In case there is an infringement dispute after completion of registration, the parties shall resolve the matter according to relevant laws, regulations, rules through judicial organs, patent administration institutions or negotiate a resolution. The article 13, focus on drugs that have obtained patent protection in China, applicants may apply for registration within two years prior to the patent expiration. SFDA it will be in charge of reviewing the applications according to the actual regulations and, after the patent has expired, approve import or production for an application that meets the requirement. (SFDA 2002)
Specialized courts
In 2014, China set up courts specialized in IP rights, to adjudicate all the matters related. The specialized IP courts are established in Beijing, Guangzhou, and Shanghai. These courts are in charge of the administrative and civil cases involving IP rights, the criminal cases are a matter for another type of courts. If the case involves complex technology, there are technology investigators who can be appointed, they don’t have any judicial power but provide technical capability and support. Also, to guarantee an effective process, the cases are heard by judges specialized in IP laws. (H y B. 2012)
3.1.1 INTELLECTUAL PROPERTY REFORMS
As it was stated above, the pharmaceutical firms are playing an important role in the Chinese healthcare industry, through the development of new drugs; meanwhile the government policies focus on the control of the different actors participating, imposing the participation of domestic companies in the process and increasing the standards and regulations for the production and marketing of the products.
Table 1. Patent applications by top fields of technology (2001 – 2015)
| Field of Technology | Share |
| Digital communication | 6.68 |
| Electrical machinery, apparatus, energy | 6.67 |
| Computer technology | 6.31 |
| Measurement | 5.75 |
| Pharmaceuticals | 5.37 |
| Materials, metallurgy | 4.29 |
| Basic materials chemistry | 4.23 |
| Machine tools | 4.09 |
| Food chemistry | 4.06 |
| Civil engineering | 3.84 |
| Others | 48.71 |
Source: World Intellectual Property Organization (WIPO)
The Table 1, shows the patent applications in China by top fields of technology from 2001 to 2015; the pharmaceutical field it takes the fifth position with a share of 5.37; also, it is important to consider the resources in terms of money, processes and professionals; needed to develop and patentee new products in this area. Finally, considering the importance of the industry in the government healthcare strategies and the companies needs to growth its business, the follow section refers to the reforms that have been discussed the most in different articles and it relates to pharmaceutical field sensitive areas in terms of IP.
Third Amendment
It took 8 years to China to passage the third amendment, after the 2000 or second amendment, which provisions aims to facilitate the patent application process for foreign entities in China and for Chinese abroad, eliminating procedures related to approval from administrative authorities; also to strengthen protection and enforcement of patent rights in China, and further limits conditions for granting compulsory licenses. (J. Chen 2001)
The 2008 amendment it was enacted with the specific purpose to further China`s own IP ambitions since it was passaged in light of China`s new IP strategy. The amendment includes important changes in the IP law, aiming to: encourage patent exploitation, heighten the requirement for patentability, increase patent protection, promote patent application, address compulsory licensing and establish protection of genetic resources.
Encouraging patent exploitation
The Article 15, provides that the exploitation of patent rights between co-owners should be determined by an agreement. In the case, there is not agreement available, any of them may exploit the patent or grant licenses (i.e. non-exclusive licensees) to others, and the licensing fee shall be shared between co-owners. Moreover, in the case of one of the co-owners seeks to grant an exclusive license, he would need to obtain consent from the co-owners of the patent. The article 15, also provides added protection for companies that engage in joint research or joint venture in China. (Patent Law of PRC 2008) (Article 15)
Heightened requirements for patentability
The heightened requirements or standards for patentability aims to guarantee the quality of the patents in China since the market it has been plagued by the so-called “garbage patents”. This kind of patents is the one without sufficient technical and juridical support to warranty patent protections. In those cases, when a “garbage patent” passes through a reexamination procedure it will often be invalidated, as result, not only affects negatively the quality of patents granted in China, also causes waste of judicial and administrative resources.
The previous legislation, in terms of novelty requirement, accepted a “relative novelty”, as sufficient to grant an invention patent, the 2008 amendment raises the novelty requirement to “absolute novelty”; meaning that for example, the previous law only requires that no identical invention or utility model has been disclosed in publications in China or abroad or has been publicly used or made known to the public in China. (Patent Law of PRC 2008) (Article 22) On the other hand, under the 2008 amendment, the novelty requirement can be satisfied only if the invention or utility model disclosed doesn’t constitute a part of “currently available technology”, that it is not a technology publicly known in China or abroad prior to the date of patent application.
Increased patent protection
The 2008 amendment, increased monetary damages against violators of patent law and provides additional administrative and judicial tools. The amendment works under two types of patent law violations: 1) patent infringement; and 2) act of passing off patents.The patent infringement refers to the act of use or exploitation of technology that is not own by the individual or entity, the amendment provides a guideline for calculating the amount of compensation for damages caused by the infringing act. (Patent Law of PRC 2008) (Article 65)
The calculation of the damage it can base on the losses suffered by the patentee, the profit generated by the infringer through the infringing act, or in case it can’t be determinate through the last methods, it can be assessed by reference to the appropriate multiple of the amount of the exploitation fee of that patent under contractual licenses. (Patent Law of PRC 2008)
With Regard to acts of passing off patents, meaning a person making use of a patent which its no its own, the 2008 amendment determinate events when it can occur as follow: passes the patent of another person off as his own; passes a non-patented product or process off as a patented product of the process. The amendment also provides detailed acts that can be taking into consideration at the time as, use the patent number of another person in a commercial or other forms of advertising materials, without permission, to confuse others; falsify or alter another person`s patent certificate, patent documents or patent application; sell or manufacture a non-patented product marked as a patented product; to continue marking a product as a patented one, after the patent was invalidated; a to represent a non-patented technology as a patented technology in a contract.
Promote patent application
The promotion of patent applications is a significant objective of the 2008 amendment, some of the new regulations affect directly to foreign individuals or entities, for example, under this amendment, is required to appoint an established patent agency to represent them for patent matters in China. The result of such changes is expected to increase the number of agencies in China capable of managing intellectual property processes, creating more opportunities for actual law firms, and also can potentially translate in foreign clients more motivated to apply for patents in China. (Patent Law of PRC 2008)
The article 9, from the 2008 amendment, greatly promote the patent application, treating the double patenting issues. Double patenting usually create disputes regarding the patent invalidation proceedings. The article provides that “if prior the grant of an invention patent, the previously granted utility model patent has not expired, the patentee can elect to abandon the utility model patent right to render the granting of an invention patent permissible”. This is only applicable where the same applicant files both application for the identical invention-creation on the same day. (Patent Law of PRC 2008)
Chinese entities and individuals also appear encouraged to apply for patents abroad, the amendment allows Chinese who made an invention in China to submit a foreign application prior to applying for a Chinese patent, removing the requirement that Chinese investing in the country, must apply first in China. However, the applicant must first request a security examination by the Patent Administrative Department to ensure that the foreign application does not contain state secrets. (Patent Law of PRC 2008)
Finally, in order to promote the patent application, the 2008 amendment requires the Patent Administrative Department to periodically publish a Patent Gazette. The gazette would provide transparency during the application process. (Patent Law of PRC 2008)
Addressing compulsory licenses
The 2008 amendment address compulsory licenses, the articles 48 to 51, focus on the conditions under which a grant of compulsory license is allowed. The article 48 called scenarios when the patentee, after the expiration of three years from the date of the patent and after the expiration of four years from the filing date of the patent application, has not exploited the patent or has not sufficiently exploited the patent without justified reasons.
Moreover, when the act of the patentee, is confirmed through judicial proceedings as a monopoly act, it can be also considered for a compulsory license. The article 49, provides for a grant of compulsory license to exploit a patent under a national emergency, this article it was not amended. The amendment instead, introduces a new article 50, to address grating for purposes related to public health. 81 This article affected and focused directly on the pharmaceutical industry; also It was enacted according to paragraph six of the Doha Declaration on the TRIPS Agreement and public health. The discussion around the topic introduced by this article and its effects in the Chinese Patent Law, started even before the amendment, in an order issued by the State Intellectual Property Office in 2005. (SIPO 2005)
If China decided to grant a compulsory license for public health purpose, it would be a decision that likely has a negative impact on pharmaceutical companies holding patents in China. Considering the image that the country has in terms of Intellectual Property, it could discourage pharmaceutical from obtaining patents in the country, or future investments that can affect their business in terms of IP protection.
The article 51 provides another condition for granting compulsory licenses, allowing the patentee to request the Patent Administrative Department to grant a compulsory license if the technology developed involves an important technical advance of considerable economic significance and it requires the use of the earlier patented invention. (Patent Law of PRC 2008)
Protection of genetic resources
The Convention on Biological Diversity (CBD), it was the source for two of the articles, 5 and 26, for the protection of genetic resources. The bio piracy, it has been a public concern, since the country Is losing its genetic resources. One of the most public and called into a discussion over protection of genetic resources is the case of the “Peking duck”, when a British company, cherry valley, patented its product in the Chinese market, growing significantly. (Chen y Weina 2007)
The 2008 Amendment, it had a significant impact on pharmaceutical companies seeking to apply for patents in China. The pharmaceuticals who entered the Chinese market, seeking and engaging in research and development using genetic materials from China, passed through a new process, where the administration studied the cases of use of the resources and determining if those violated the law. (Patent Law of PRC 2008)
The companies that managed the genetic materials are now forced to keep tracking the records for their acquired genetic resources, as failure to disclose such information may hinder future applications for patents in China.
Reform 2012 - compulsory licenses
The 2012 reform referred in this document is the Order of the Director of the State Intellectual Property Office No. 64. (SIPO 2012) The general reaction of the new order, coming within the same month as the Indian Nexavar decision, focused mostly in the pharmaceutical community. “China Changes patent law in fight for cheaper drugs” it was the headline from Reuters, as a reaction for the possible reform`s effects. The 2012 measures basically clarify how the mechanism for compulsory licensing must be administered in the country, from a procedural perspective.
The reform clarifies the procedural timelines for application and response periods and the circumstances under which a compulsory license may be terminated; at the same time, require the applicant to specify the requested term for the compulsory license; the parties are expected and required to attempt to reach agreement between themselves before requesting SIPO to issue a ruling on the amount of royalty; and finally brings the compulsory
3.2 PHARMACEUTICAL INDUSTRY
The new era of the pharmaceutical industry is related to the 19th century since it is the time when the chemical industry evolved to the pharmaceutical industry. Some of the most recognized companies were Merck and Schering (Germany), Hoffmann-La Roche (Switzerland), Burroughs Wellcome (England), Etienne Poulenc (France), Eli Lilly, Upjohn and Smith Kline (U.S.). (Chemical & Engineering News s.f.)
The pharmaceutical industry is recognized as a high-tech industry with also a high R&D intensity, the industry is significantly based on R&D, demanding high-qualified professionals, collaboration with academia and specialized research. Nowadays the pharmaceutical industry is presenting a considerable growth in terms of revenue, according to the portal Statista, the revenue of the worldwide pharmaceutical market from 2001 to 2015, move from 390,2 billion U.S. dollars to 1.072 billion U.S. dollars. (Statista 2016)
3.2.1 Pharmaceutical Industry in China
The pharmaceutical Industry in China is still in an early stage, it has progressed rapidly in the last decade, mainly regarding improvements in factors that are determinants for the processes in the industry as a regulatory system, patent protection, research, investment, talent, and incentives. China worked intensively in the creation of an environment that reach first-class and allows high standards innovation in drug R&D. (Qi, y otros 2011)
In conclusion, although the innovative pharmaceutical industry in China is still in the early stages of development, it has progressed rapidly in the past decade. Moreover, China has made considerable improvements and continues to improve the key factors — regulatory systems, patent protection, basic research, investment, talent and market incentives — for the creation of a first-class environment for innovative drug R&D. (Frew, y otros 2008)
China have presented a good time in terms of opportunities to the industry, taking as an example the healthcare reform, from March 2009, where the country committed around RMB850 billion to develop the health care system, establishing a national essential drug system, expanding infrastructure for grassroots medical networks, implementing pilot reforms of public hospitals, etc. basically sweeping health care overhaul. (Kahler 2011)
The recent reforms presented by Ministry of Health aimed to equalize international standards, had a direct impact on the pharmaceutical industry. Such reforms regulate the activities in the industry, imposing strict parameters for an improved quality, access to the masses and increased investment; these changes also affected the business strategy. These reforms have moved China`s pharmaceutical market – in terms of industry value – from 9th to 2nd place in the period between 2005 to 2016, as the Figure 2 bellow shows.
$Billion – all figures in U$ billion
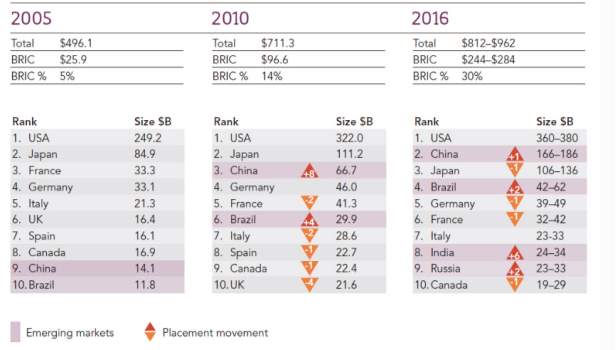
Source: IMS Health report, May 2012 Spending in US$ with variable exchange rates
Figure 2. Top 10 Total pharmaceutical Markets in the World, 2005-2016
The 13th Five-Year Plan
The 13th Five-year plan of China is taking the period between 2016 and 2020; targets as a real GDP growth (%) over 6.5%, introducing a target for labor productivity from 87.000 Yuan per person in 2015 to 120.000 in 2020 and also an expecting an increased average life expectancy of 77,34 years. The plan shows China`s aim to become an innovation-driven nation with industrial transformation concentrate on the development of the “ten priority strategic industries” that includes “medicine and medical devices”.
The plan also introduces the aim to build a “healthy China”, an opportunity for the pharmaceutical industry to reach the expanded urban and rural areas covered by public hospitals, the plan shows an intention to appropriately price medication, increase the free supply of HIV treatment medication and encourage the private sector to provide healthcare services. (pwccn 2015)
Unit: RMB bn
Source: China pharmaceutical Distribution Industry Report, 2013 by ResearchInChina
Figure 3. Revenue of Major pharmaceutical circulation Enterprises in China,2013
Drug registration
Drug registration in China involves a number of regulatory bodies at various regional levels, as well as governmental levels. The applications must be sent to provincial and municipal authorities to be reviewed in an initial stage, passing to the SFDA for approval. The total time of the procedure to be approved is generally close to 18 to 26 months. (Espicom Business Intelligence 2011)
China demands domestic clinical trials for all the drugs that are new to the Chinese market, according to the Good Clinical Practice (GCP) guidelines. The trials are granted by the SFDA and the Ministry of Health, normally taking around 12 months for trial process. The product, after the trials have been completed, must undergo a quality test. Samples must be provided to conduct three complete tests. The quality test takes around three months, and also counts with questions from the authorities to manufacturers. (Espicom Business Intelligence 2011)
The foreign drug manufacturers who want to enter the Chinese market are expected to partner or acquire Chinese companies that are qualified for a drug license. The SFDA issued a final notice on Mandatory Good Manufacturing Practice (GMP) inspections, on 25 April 2011, where all pharmaceutical companies doing business in China. (SFDA Notice and proposed regulation) For imported products, documents must pass through customs as an initial stage and then pass it to the central SFDA office. Customs inspection authorities will determinate if the drugs infringe any national legislation.
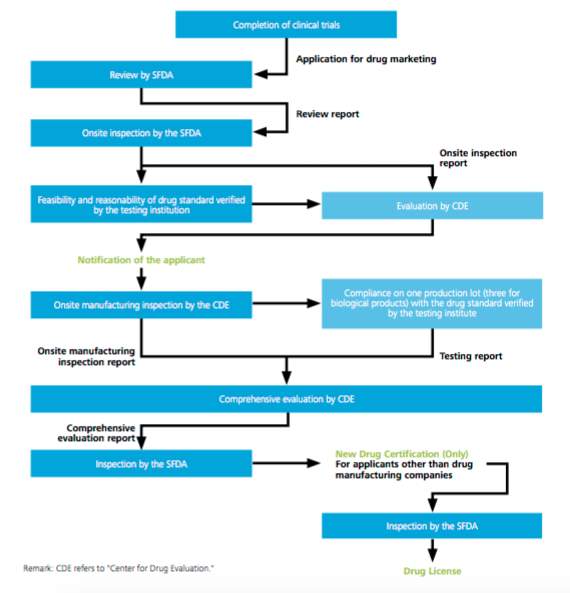
Source: CMS, Life sciences: a legal guide to China
Figure 4. Diagram of China`s approval procedures for new drugs
3.2.2 Patented drug market
The improved IP protection from the third amendment it was expected to draw in more global pharmaceutical companies aiming to enter and participating in the Chinese market. The domestic generic brands then started competing with the high confidence that Chinese consumers present to foreign brands, which entered the market with patented drugs shifting the market shares accordingly. According to a Deloitte report, sales of patented drugs, which rocketed upward at a Compound Annual Growth Rate (CAGR) of 35.7 percent from 2007 through 2010, are forecast to continue growing at just over 25 percent from 2010 through 2015.
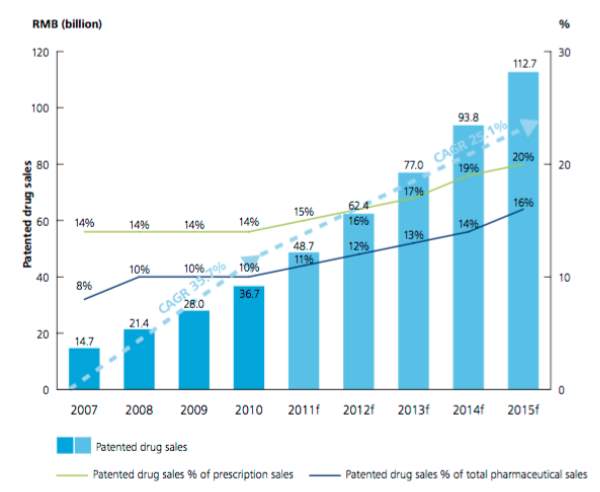
Source: SMEI, AESGP, BMI
Figure 5. Patented drug sales in China, 2007- 2015
3.2.3 Diseases profiles
China`s estimate population reach 1.38 billion, with a population over 65 years old of 131 million (9.6%). At the time that population is increasing, it is also the income levels and chronic diseases burden. The changes in diets, lifestyles, air and water pollution, are contributing to an increased incidence of cancer, heart disease, diabetes and other chronic conditions. China has 110 million diabetics, with many more undiagnosed. (Ward y Waldmeir 2015), (Sjolin 2016) three times more than the US. Also, China reported in 2015, 4.3 million new cancer cases, 20 percent of the worldwide total. (Chen, y otros 2015). Finally, the population over 65 is expected to reach 170 million by 2020, which it will generate a huge demand for treatments and services in years to come.
3.2.4 Domestic market and competitive environment
China`s domestic manufacturers are dominating the generic market, with a large number of low-cost products, the drug manufacturers account around 5,000 companies, with the top 100 having one-third of the market. The government policies aim to reduce industry fragmentation. Last decades the government imposed regulatory requirements, shutting down small manufacturers for non-compliance issues. CFDA estimates as many as 500 small drug producers could be shut down for non-compliance issues. (Shih 2015)
The biopharmaceuticals have been the target by the Chinese government since it is intending to generate a competitive edge from this part of the industry. The reforms appear to improve the conditions for domestic companies to invest and play in the market, while the foreign companies appear to be forced to collaborate with the domestic ones in order to enter. for example, see the Made in China 2025 Plan and 13 Five Year Plan. (Shobert 2015) (Sabow 2015)
3.2.5 The SFDA
The State Food and Drug Administration (SFDA) is one of the most important institutions allies for the protection of exclusivity rights in China, (SFDA 2007) According to the “Regulations on administrative protection for pharmaceuticals”, In order to ensure drug safety for the public, aiming at the current characteristics of the production and distribution of counterfeit drugs, The State Food and Drug Administration (SFDA) cracks down on selling counterfeit drugs over the Internet and through postal or express delivery from the origin, and combats making and selling counterfeit drugs along with the relevant departments. Notably, since the establishment of SFDA’s leading group of the special operation against IPR infringement and counterfeiting in November 2010, the SFDA has organized the nationwide food and drug regulatory departments involved in this special operation and has made stage achievements with the support and cooperation from the departments of public security, departments of industry and information technology and other relevant departments. (SFDA 2011)
The State Food and Drug Administration (SFDA) sponsored Pharmaceutical IPR Summit Forum 2005 on July 19, 2005. Zhang Jingli, Deputy Commissioner of SFDA addressed the forum, saying that China will continue to improve its pharmaceutical intellectual property rights (IPR) protection system.
While strengthening drug administration and ensuring drug safety and effectiveness, SFDA is active and shoulders responsibility in the facilitation of pharmaceutical IPR protection in four aspects: 1) conduct administrative protection for pharmaceuticals and has authorized administrative protection to 155 applications submitted by pharmaceutical exclusive rights owners from 12 countries; 2) abide by the promises made by the Chinese government regarding the accession to the World Trade Organization (WTO) and has added data protection requirements to the regulations of drug administration while abolishing the administrative protection for new drugs; SFDA has started the revision of Decree Governing the Protection of Traditional Chinese Medicines, as required by the legislative plan of the State Council; 3) engage in active collaboration with the IPR authorities, strengthen international exchange and cooperation, introduce, apply and reference internationally accepted rules and advanced experience overseas and implement pharmaceutical IPR protection; and 4) conduct early intervention in innovative drug research and development (R&D), guide and standardize the R&D of innovative drugs with self-owned IPR. (SFDA 2005) licensing of medicines for public health interest into line with TRIPS requirements.
Chapter 4. Hypothesis and Methodology
4.1 Hypothesis
The hypothesis of this research come from the literature review and the revision of the last reforms in IP Law, also from the information collected during the study of the industry.
H -1 The third amendment and the 2012 reform affected the number of patent applications from the industry.
Hypothesis 1 refers to the aim of the third amendment to promote patent application, the establishment of protection of genetic resources, the increased patent protection, and patent exploitation; those areas are usually discussed in the industry, as it can be seen in chapter 2; since affects the operations of the companies in the country; in this case the third amendment it was well received by the pharmaceutical companies and general media, since permitted China to get in line with international Law. Meanwhile, the 2012 reform, addressed directly the compulsory licenses, without mentioning benefits for the inventors, but clarifying the way to manufacturers to take technology, considering the situation in countries as India, the publications alarmed the industry in China. The hypothesis 1 of the study, aims to test whether the pharmaceutical companies operating in China responded to the new government policies in terms of applications in the country or not.
H-2 There is no significant impact from the third amendment and 2012 reforms on the IP strategies of pharmaceutical companies.
Hypothesis 2 refers to the effects of legislation on companies IP strategies, specifically the pharmaceutical companies operating in China and the reforms that have been introduced in chapter 3. As it was presented in chapter 2, scholars have studied the impact on sales, revenue and investment activities of companies after legislative reforms; in this study, the main focus is their IP strategy. The third amendment provides more security and extends Chinese legislation to an international standard. The effect of a renewed legislation would generate a confidence of investment on the part of companies interested in the market. In addition to the great growth that China has presented in the last years, a reform that establishes clear procedures, prepares the companies to continue operating in the country, without needing significant changes to its current strategy.
H – 3 There are significant changes in the factors highlighted for the pharmaceutical companies during the study in the recent reforms and government IP strategies.
Hypothesis 3 refers to the future results of the factors that companies have considered important or very important for patent application and IP strategies. This hypothesis is based on the negative general comments and international media publications that refer to China as a country with a weak IP legislation. Chapter 2 cited scholars who studied the IP system in China and its implications in the economic field, also referring to the bad image of the system.
4.2 Methodology
To investigate the influence and effect of the last reforms and governmental strategies over the IP Law (focused in Patent Law). This study will first rely on previous literature (Periodicals, journals, surveys, case studies, other research materials). A questionnaire that is expected to measure the influence of the reforms over the actors related to the application process. Also, data from patents in the pharmaceutical field it will be used to analyze the effect of the reforms. The detailed methodology used in the study is given below.
4.2.1 Research Design
The study focus on various factors associated with the patent application for pharmaceutical inventions in China and the efforts of pharmaceutical companies in China towards R&D and IP strategies, with particular emphasize in last major reforms effects. The first stage of the research involve a patent search emphasize in the applications from last years and the second stage involves qualitative research to explore the chosen topic of study in order to achieve the stated research objective. It was intended that, through questioning and dialogue, insights from divergent thinking and inquiry are collected.
4.2.2 Research Area
Chinese and foreign pharmaceutical/biotech companies engaged in drug research and development, located in China, with patent applications, and activities in the country from at least 2 years’ prior the first reform selected.
4.2.3 Research Instrument
The major emphasis is laid on primary sources since it gives the first-hand information, as well as insights and new perspectives to the study. Moreover, the data has been collected from primary and secondary sources, since the research is inferential, analytical and descriptive in nature.
Primary Data: The primary data were collected with a structured questionnaire, as well as conducting interviews with strategist in IP area, innovation directors and people in charge of IP strategy for pharmaceutical organizations, to get a deep view of the respondents and cross-examine responses regarding issues.
Secondary Data: The secondary research, involves the collation, synthesis, and analysis of data from the public domain, as books, journals, newspapers, internet, and associations that have referred to the objectives and hypothesis of the study. On the other hand, the study analyzes the application of the patent through a determined search formula for the patent application in the industry.
4.2.3.1 Patent Search
The patent search took into consideration the IPC (International Patent classification) marks associated with pharmaceutical products, ingredients, and others, which commonly are used in the industry. The International Patent Classification established by Strasbourg Agreement, 1971, provides a hierarchical system of language independent symbols for classification of patents according to the different areas of technology to which they pertain. The IPC is the widely used for patent search since it allows more detailed and filtered results. Additionally, the IPC marks were based on the study “A patent analytics study on the Australian Pharmaceutical Industry” from de Department of Industry, Innovation, and Science of Australian Government, which also focus on the pharmaceutical industry. Finally, the marks selected for the search were validated through the revision of the patent results.
The search incorporates applications with China as a country to apply, without excluding the PTC (Patent Cooperation Treaty) applications; meaning that probably most of the applicants also considered or apply to other countries with a unique application. The patent search it was realized with Thomson innovation, one of the complete tools for IP related search.
4.2.3.2 Questionnaire
The questionnaire is the main tool for collecting data for the research. Questions have been based on various parameters depending on the information required to meet the research objectives. Also, included general questions regarding the IP system, environment, and industry that were included for further information to get a more profound comprehension of the issues and problems faced by the industry, but are not necessarily analyzed for testing the hypothesis.
The questions 1 to 5, deal with the Intellectual Property system in China and examines whether the respondents consider the system useful and friendly for the industry, as well as the patent application process.
The questions 6 to 14, reflects the factors that influence the companies to apply for a patent in the country while proposing factors that can be controlled or supported by the government and factors that are merely a concern for the company or industry.
The questions 15, 16, 17 and 20 scrutinizes information related to the company commitment and focus on IP strategy. Additionally, refers to the reaction after the reform in terms of financial investment.
The questions 18, 19 enquires whether the companies were affected in terms of expenditure, after the reforms and the IP strategy. Also, probes about the initiatives taken by pharmaceutical companies after the reforms.
The questions 21 to 26, deals with the factors that the companies consider important for the IP strategy, following the books “Essentials of Licensing Intellectual Property” by Alexander I. Poltorak and Paul J. Lerner and “Guide to Intellectual Property: What it is, how to protect it, how to exploit it” by The Economist and Stephen Johnson.
4.2.4 Sampling
The study is based on companies with a specific context and activities, a non-probability sampling which is non-random and subjective is applied since there are a limited number of individuals of interest. The judgmental sampling technique is used, selecting units to be sampled based on the activities, experience and a professional judgment. To avoid sampling error, the companies used in the study are on the top of most experienced authority in the field of interest.
4.3 Data analysis and findings
This chapter deals with the data analysis and findings from information collected from 60.000 patents applications in China, from companies operating in the country, filtered by the IPC marks referred in Appendix 1. and from questionnaires data from 18 companies, operating in China. The first section describes the patents application number from 2004 to 2014, and its trend during the time of the reforms selected in this study. The second section initially deals with the perception on IP system, environment, factors that influence the application process, changes in the company strategy and budget after the reforms, and finally discuss factors that are considered important in the companies’ IP strategy.
The patent search encompasses Chinese-originating applications. The applications had as one of the countries China, pertaining to pharmaceuticals, commonly be indexed with a classification associated with the active pharmaceutical ingredients, detailed in Appendix 1.
The search it was made with Thomson innovation, with a maximum amount of 60.000 results to be counted in a unique search, the formula used, which detailed description of acronyms it can be seen in the Glossary. it was the follow;
IC= (A61K OR A61P OR C07D OR C07C OR C12N OR (C07K AND A*)) AND DS=(CN) AND AC=(CN) AND (AY>=(2004) AND AY
The international classification in the formula as explained above is required to be related to documents with China as the Designated States and Application Country, which at the same time is required to find applications during the years 2004 to 2014. In resume, the formula will have as result a list of patent applications related to pharmaceutical products, with China as a country of application and designated state from 2004 to 2014.
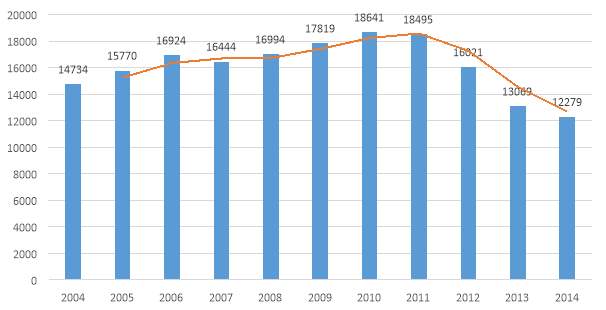
Source: Thomson Reuters innovation
Figure 6. Patent application in pharmaceutical industry 2004-2014 in China
The Figure 6, shows an increasing trend in the number of application from the industry during the period 2004 to 2011, and a decreasing trend from the year 2011 to 2014. The results clearly support the hypothesis of a positive impact in the applications after the reform of 2008 (third amendment), where the government focused on getting in line with the international legislation, and a negative impact in the application trend after the reform of 2012, where the possibility to obtain a compulsory license were clarified and more structured.
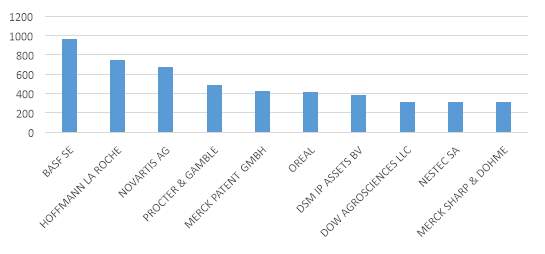
Source: Thomson Reuters innovation
Figure 7. Top Assignee in pharmaceutical Industry patent application period 2004-2014
The graphic above shows the main applicants for patents in the industry during the period from 2004 to 2014. The graphic it shows a clear dominance of companies which activities are mainly focused on chemical industry and pharmaceutical ingredients.
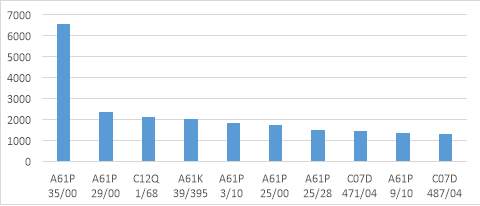
Source: Thomson Reuters innovation
Figure 8. Top IPC marks in pharmaceutical Industry patent application period 2004-2014
The graphic above shows the main fields of technology for the industry patent applications. As it can be seen the IPC mark A61P, appears in most of the application, being the most common in the industry following the patent search mentioned.
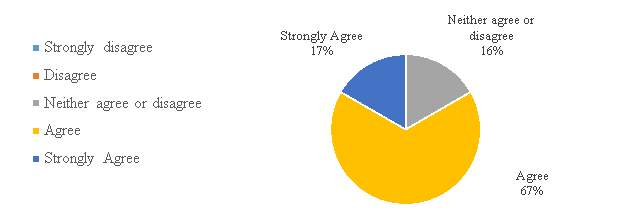
Figure 9. Chinese IPR system to Chinese pharmaceutical companies.
In figure 9, the response is overwhelming as 84% of the respondents have either agree (67%) or strongly agree (17%) to the statement that the Chinese Intellectual Property Rights system is useful to pharmaceutical companies. Results clearly show that the system has reached a stage where the companies value and find the system useful to the industry.
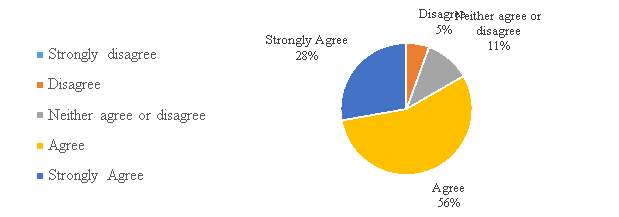
Figure 10. The Chinese IPR system and its help in growth to pharmaceutical Companies.
In figure 10, a large number of respondents (84%) have either agree or strongly agree with the statement that the Chinese Intellectual Property Rights system helped in growth to pharmaceutical Companies. Results Indicate that the companies find the system supportive in the development of the sector, meaning an understanding of the system as a part of the industry growth.
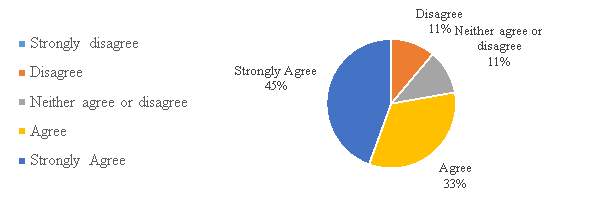
Figure 11. The Chinese IPR system is user-friendly
The figure 11, shows that a majority of the respondents 78%, have either agree or strongly agree with the statement that the Chinese Intellectual Property Rights system is user-friendly. Result says that the system is well accepted for the industry and companies find it easy to use.
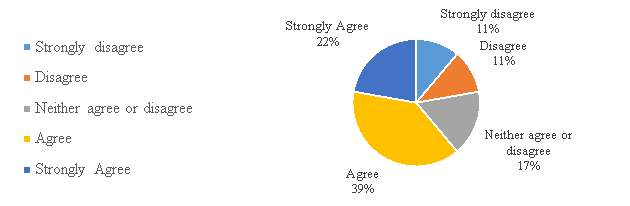
Figure 12. The process of filling patent application in China is user-friendly
The figure 12, shows that a majority of the respondents 61%, have either agree or strongly agree with the process of filing the patent application in China is user-friendly. The respondents in this group consist of a large group of companies with in-house counsel. Results clearly indicate that companies with IP activities and in-house counsel find the filling patent application process user-friendly, these group or companies are more familiar with the area, while the other group of companies disagrees with the statement, finding the process, no user-friendly.
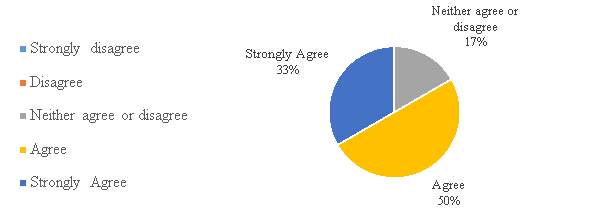
Figure 13. The period required to register a patent in China is adequate.
In figure 13, a large number of the respondents 83% agree with the statement that period required to register a patent in China is adequate. Result says that the companies in the industry are satisfied with the period required. Considering that China according to the WIPO IP facts and figures 2016 report, it was the country with the major number of patent applications in a single year (2015). It received almost as many applications as Japan, the republic of Korea and the U.S. combined.
Table 2. Determinant factors in company decision to apply for a patent in China
| Not important at all | Somewhat unimportant | Neutral | Somewhat important | Very important | |
| Documentation needed for application | 3 | 5 | 0 | 7 | 3 |
| 17% | 28% | 0% | 39% | 17% | |
| Financial support | 0 | 2 | 3 | 2 | 11 |
| 0% | 11% | 17% | 11% | 61% | |
| Actual local partners | 0 | 0 | 6 | 5 | 7 |
| 0% | 0% | 33% | 28% | 39% | |
| Possible future partners | 1 | 2 | 3 | 7 | 5 |
| 6% | 11% | 17% | 39% | 28% | |
| Market Size | 1 | 1 | 4 | 8 | 4 |
| 6% | 6% | 22% | 44% | 22% | |
| Licensing opportunities in the country | 0 | 1 | 2 | 9 | 6 |
| 0% | 6% | 11% | 50% | 33% | |
| Support to the industry | 0 | 0 | 2 | 10 | 6 |
| 0% | 0% | 11% | 56% | 33% | |
| Inventive capability in the country | 0 | 1 | 4 | 6 | 7 |
| 0% | 6% | 22% | 33% | 39% | |
| IP protection in the country | 1 | 3 | 4 | 8 | 2 |
| 6% | 17% | 22% | 44% | 11% |
The table 2, shows the results over 9 factors that impact the patent application decision, as the documentation needed to apply, financial support, actual local partners, market size, licensing opportunities in the country, support to the industry, inventive capability in the country and IP protection in the country. After analyzing the results, around 72% of the respondents either consider somewhat important or very important the financial support and Inventive capability in the country for the patent application decision. For licensing opportunities in the country, 83% of the respondents considered this as somewhat important or very important factor, and finally, 89% of the respondents considered the support to the industry either somewhat important or very important.
The pharmaceutical industry is constantly undergoing a change. The practices to develop new drugs and protect the information involve in the process have changed dramatically. The results underline the importance of the country develop for R&D resources, as financial support and inventive capability in the country, but also, it was considered important to look at a more specific and specialized support to the industry.
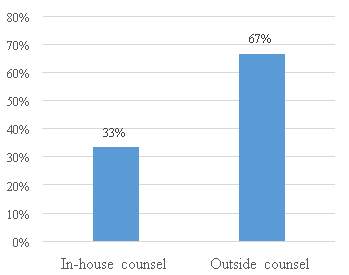
Figure 14. Preparation of patent application in the companies
As figure 14, above shows, the majority of the respondents usually prepared the applications with outside counsel. Some of the respondents stated that they prefer to rely on outside counsel because of the expertise of those company`s employees in the Chinese market.
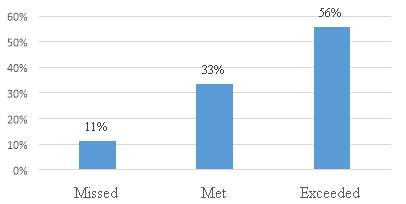
Figure 15. The company met the goal/target for patent applications in the last year?
The figure 15 shows that a large number of the respondents 89%, exceeded or met the company goal/target for patent applications the last year. The results indicate a good performance of the companies in terms of inventions in China.
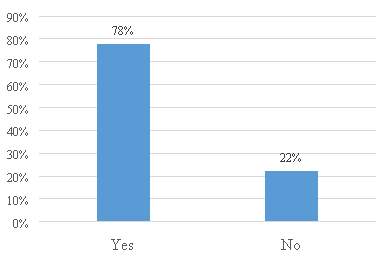
Figure 16. Are all patent application functions centralized in the IP department?
The figure 16 shows that a large number of the respondents 78% centralize the patent application functions in the IP department. The results indicate that the companies selected to fill the questionnaire have an intended and clear strategy related to the protection of their inventions in the country.
Third amendment (2009) Compulsory licenses (2012)
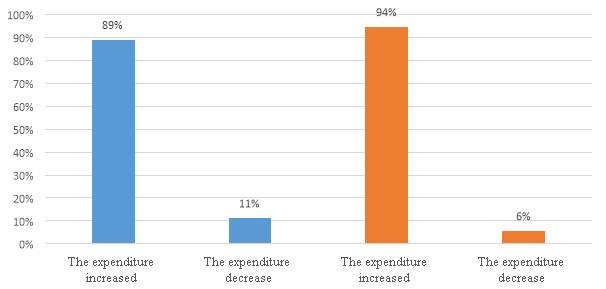
Figure 17. Expenditure in IP services or operation during reforms timeline
The figure 17, shows that 89% of the companies presented an increased expenditure in IP services or operations during the period of the third amendment, and a large amount of the companies 94%, presented an increased expenditure during the period of the second reform (2012). The results clearly support the hypothesis of the impact of the reforms in those periods. During the questionnaire, the companies were asked about the main factors for such decision, and the preparation of new patents and a clearer legislation in terms of IP for the industry it was a constant answer.
Third amendment (2009) Compulsory licenses (2012)
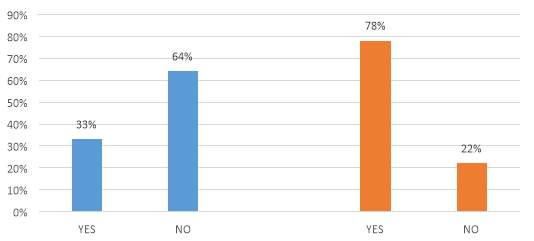
Figure 18. IP strategy in the reforms timeline
The figure 18, related to the companies IP strategy during the timeline of the reforms, shows an effect from 2012 one, with 78% of the companies reconsidering their strategy. On the other hand, only 33% of the companies changed or modified their IP strategy after the third amendment. When the companies were asked about their decision, they remain discreet, but the general comments focused more in the trade secrets and the implementation of practices to use the technology already protected in the country.
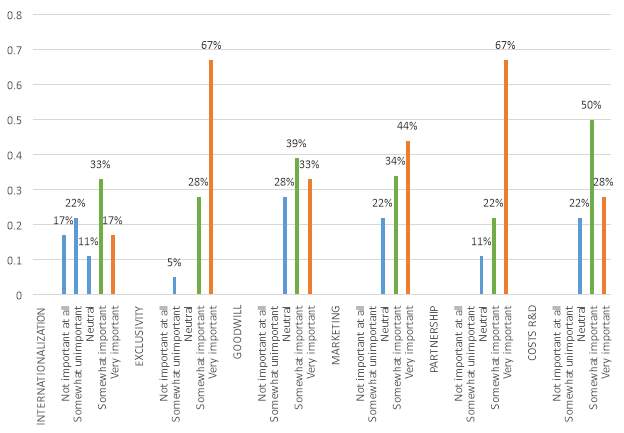
Figure 19 Determinant factors in IP strategy
The figure 19, refers to the respondent’s importance levels for the determinant factors in IP strategy for pharmaceutical companies operating in China, the factors were taken from the strategies proposed and remarked in the books “Essentials of Licensing Intellectual Property” by Alexander I. Poltorak and Paul J. Lerner and “Guide to Intellectual Property: What it is, how to protect it, how to exploit it” by The Economist and Stephen Johnson. The results show exclusivity and partnership as the factors with the highest perceived importance, those factors are directly related to licensing, the companies major source of income in terms of patent ownership. The factors marketing and goodwill share similar results; those factors are connected by the tendency of using the patents as publicity to advertise companies’ leadership in the industry. Finally, the factor Internationalization, which is widely discussed by Poltorak and Lerner, in terms of the need of companies to develop a business capable of sustaining a competitive advantage in terms of cost of production, it doesn’t appear very important for companies operating in China; this result it can be related to the fact that the companies are already established in the country.
Chapter 5. CONCLUSIONS AND DISCUSSION
5.1 Research finding discussion
The findings of the research are as follows:
- The patent application activity is the future of China pharmaceutical companies. Taking into consideration the domestic and foreign companies. It can be seen from the results in Figure 6. a clear increasing trend in the patent application from the industry. China, as it was stated above, is a huge drugs market, bringing many opportunities for the research & development of new products and the treatment of new diseases. Additionally, the country aims to create a safe and better environment in terms of Patent Law, is also seen and discussed by the international community, what brings more pressure, awareness, and support. The majority of the respondents in the study, over 84%, says IPR system in China is useful and helps in the growth of pharmaceutical industry. The implication which comes out from this analysis is that these firms have realized and recognized the efforts of the government in this field, and such appreciation have been increasing the R&D activities.
- China`s growth in terms of patent filing and application, becoming the leader receiving over a million patent applications in a single year, has challenged the country to enforce the system to be operational sufficient, facing the incoming demand. Most of the respondents agree with the statements that called the process, user-friendly and also refers to an adequate timing. Again, the implications which come out from this analysis is that the companies from the industry recognize the Chinese IPR system and procedures as competitive.
- Regarding IP strategy and factors related to the patent application process, the companies remain focused in licensing opportunities. China is recognized by its advanced industries and manufacturers; this is a meaningful opportunity for companies with patents in the country to licensing their technology. The factors exclusivity and partnership were highlighted from companies in terms of IP strategy, factors that refer to licensing, and referring to determinant factors for a patent application, most of the companies agree with the fact that “licensing opportunities in the country” is either important or very important factor.
- Finally, referring to the impact of the reforms in the industry; it has implications for both sides, companies, and government. First, the companies were more satisfied with the first reform studied (third amendment), not changing significantly their strategy, expenditure or patent application trend, in comparison with the another reform studied (2012); the third amendment was more structural and responded to the country`s need to get in line with international laws. Meanwhile, the second reform, it was seen as a threat to the companies, since opened possibilities for the country to make more pressure on international corporations to negotiate licenses with domestic companies.
5.2 CONCLUSIONS
Regardless of whether the expected changes in the Chinese IP system will fundamentally affect the quantity of patent filings in China remains to be seen. The study undertaken to ascertain the impact of patents recent reforms on pharmaceutical industry is able to establish to a great extent the fact that the patent system contemplated in the Chinese Law is able to promote the applications and impact on company’s strategies and IP activities.
The patent law in China has undergone many changes to incorporate TRIPs provisions, assuring an acceptance from the International community. The third amendment it was well received by the companies as it can be seen in the above chapters, meanwhile, the reform of 2012 referring to compulsory licenses created an uncertainty environment for the companies in the industry. Overall the Chinese government has reached a point where the Law is acceptable for companies to invest in the country.
The licensing opportunity remains as a factor that influences the decision of companies in the pharmaceutical industry to file patents in China, even when the market is constantly growing and the government continues to generate strategies for the promotion of the industry through development plans; the industry continues to value the industrial and production capacities of the country. The trend in this market and efforts to strengthen the law show that the opportunities are focused on creating new licenses and pass on the companies that are currently producing illegally to a legal production through clear conditions for its operation.
5.3 Significance of the study
This study focuses on the impact that new reforms or changes in the patent legislation can have in pharmaceutical industry, as well as the perceptions and opinions that can be generated through them. It also contributes to the understanding of the factors that impact the industry in terms of IP strategies and the determinants of patent application in the country. The results have shown that companies focus more on future opportunities of the invention than on previous processes. The results presented allow a better understanding of what government entities can offer to attract the industry.
5.4 Study Limitation
Patents are a highly sensitive topic to discuss the information which companies may share, in most of the cases is considered an extremely private and confidential. The following limitations were faced in the study:
- Patents are a sensitive area and companies, especially in private sector consider most of the data as confidential.
- Even though the study had aimed collecting as must responses possible, the data confined to only 18 companies.
- Lack of literature in English of pharmaceutical industry activities in China, related to IP.
- Selection of the respondents and contact information of people working for a specific area in the organizations is another big challenge.
5.5 Direction for future research
Most of the respondents in the study were foreign pharmaceutical companies that have R&D centers or invested in China with research purposes. It would be valuable to extend the study to more Chinese companies, since are the one expected to do a major use of the IP system in the country. The study has a further scope with respects to:
- The present study has only 18 companies, extending it to more companies with dedicated R&D activities in a specific area or molecules.
- An exclusive research can be conducted for companies who prefer or use only in-house counsel for a patent application, since the results of the study showed a difference in the respondent’s trend, in the in-house counsel and outside counsel groups.
REFERENCES
Alexander I. Poltorak, Paul J. Lerner “Essentials of Intellectual Property: Law, Economics, and Strategy”. Wiley; 2 Edition, 2011.
Alford, William P. To Steal a Book is an Elegant Offense: Intellectual Property Law in Chinese Civilization. Vol. VIII. 1995.
Andrew Ward, Patti Waldmeir. China healthcare: Missing a beat. Financial Times, 2015.
Awokuse, and Titus O. and Hong Yin. “Intellectual Property Rights Protection and the Surge in FDI in China.” 216-224. 2010.
BSA Business Software Alliance. “Shadow Market, 2011 BSA Global software piracy study.” The study, 2011.
Chemical & Engineering News. Chemical & Engineering News. http://pubs.acs.org/cen/coverstory/83/8325/8325emergence.html (accessed April 14, 2017).
Chen, Jin. “Better Patent Law for International Commitment – The Amendment of Chinese Patent Law.” (2 RICH. J. GLOBAL L. & BUS) 2001: 61.
Chen, Wanqing, et al. “Cancer Statistics in China, 2015.” 2015.
Chen, Zhang, and Zhao Weina. December 19, 2007. http://www.eeo.com.cn/ens/Industry/2007/12/19/89565.html.
Christopher P. Adams, Van V. Brantner. “Estimating The Cost Of New Drug Development: Is It Really $802 Million?” Health Affairs, March 2006.
D. Bosworth, D. Yang. World Trade organization and patents in China. World Trade Organization, 2002.
D. Yang, D. Bosworth. IP law, technology flow and licensing opportunities in China. Vol. 9. International Business Review, 2000.
Espicom Business Intelligence. “World Pharmaceutical Market- China.” 2011.
Frew, Sarah E, et al. “Chinese health biotech and the billion-patient market.” 2008.
H, Wengang, and Li B. 2012. http://www.managingip.com/Article/3421120/China-Patents-Latest- developments-on-IP-specialised-courts.html (accessed January 12, 2017).
Kahler, Christine. China Business Review. January 11, 2011. http://www.chinabusinessreview.com/chinas-healthcare-reform-how-far-has-it-come/ .
Keupp, Angela Beckenbauer, and Marcus Matthias and Oliver Gassmann. “How Managers Protect Intellectual Property Rights in China Using De Facto Strategies.” 214-224. 2009.
Kwang, M. China: compelling reasons to join WTO. The Sunday Times, 1999.
Lei, Zhen. Penn State University. 05 24, 2012. http://is.jrc.ec.europa.eu/pages/ISG/patents/documents/LeiChinaPatentSystem.pdf .
Lei, Zhen, Zhen Sunt, and Brian Wright. http://funginstitute.berkeley.edu/wp-content/uploads/2013/12/patent_subsidy_Zhen.pdf.
Liu, M. “Economic analysis of Intellectual Property rights Law.” (Law publishing) 1996.
Paul S. Ropp, Timothy Hugh Barrett. Heritage of China: Contemporary Perspectives on Chinese Civilization. University of California Press, 1990.
pwccn. “Prosperity for the masses by 2020.” Price Waterhouse Coopers, 2015.
Qi, Jingzong, Qingli Wang, Zhenhang Yu, Xin Chen, and Fengshan Wang. Innovative drug R&D in China. News & Analysis, nature, Macmillan Publishers Limited, 2011.
Richard, Wilder. “Market Segmentation: techniques, actors, and incentives – The use of intellectual property rights.” 2011.
Sabow, David. Forbes. December 2015.
SFDA. State Food and Drug Administration. October 15, 2002. (accessed February 18, 2017).
—. Provisions for Drug Registration. 2007. http://eng.sfda.gov.cn/WS03/CL0768/61645.html.
SFDA. SFDA facilitates pharmaceutical IPR protection. 2005.
SFDA. SFDA Makes stage achievements in the special operation against IPR infringement and counterfeiting. 2011.
Shih, Jerry C. China Sets its sights on life sciences. August 2015.
Shobert, Bejamin. Forbes. January 2015.
SIPO. Approach to Implementing Compulsory License Involving Issues Relating to Public Health. 2005. http://big5.sipo.gov.cn/www/sipo2008/zwgs/ling/200804/t20080402_366969.html.
—. Measures of March 15, 2012, for Compulsory Licensing of Patent Implementation. 2012. http://www.wipo.int/wipolex/en/text.jsp?file_id=297934.
Sjolin, Sara. Market Watch. March 21, 2016. http://www.marketwatch.com/story/chinas-diabetes-boom-promises-23-billion-put-for-drug-makers-2016-01-15 (accessed February 15, 2017).
State Intellectual Property Office of the P.R.C. 2008. http://english.sipo.gov.cn/laws/lawsregulations/201101/t20110119_566244.html (accessed 04 15, 2017).
Statista. 2016. https://www.statista.com/statistics/263102/pharmaceutical-market-worldwide-revenue-since-2001/ (accessed March 15, 2017).
The Economist, Stephen Johnson; “Guide to Intellectual Property: What it is, How to protect it, how to exploit it (Economist Books)”; Paperback, July 14 2015
Wang Yan-yun, “Reflections on the IP strategy in Enterprises”; Journal of Hebei University of Economics and Trade”, 2004
Ward, Andrew, and Patti Waldmeir. Financial Times. December 2015. http://www.ft.com/intl/cms/s/0/f1f8c034-9a80-11e5-be4f-0abd1978acaa.html#axzz494hchPhy (accessed March 14, 2017).
WIPO. Wolrd Intellectual Property Organization. 1979. http://www.wipo.int/treaties/en/ip/paris/summary_paris.html (accessed April 15, 2017).
Yang, Deli. “The development of Intellectual Property in China.” Bradford University School of Management, 2003.
Zheng, C. “Intellectual Property.” (Law publishing House) 1999.
—. Textbook for Intellectual Property Law. Beijing: Law publishing House, 1997.
Appendices
Appendix 1: IPC Marks description
| Subclass | Main group | Description |
| A61K | PREPARATIONS FOR MEDICAL, DENTAL, OR TOILET PURPOSES | |
| 31 | Medicinal preparations containing organic active ingredients | |
| 35 | Medicinal preparations containing materials in the indeterminate constitution: materials from mammals, other animals, bacteria etc. | |
| 38 | Medicinal preparations containing peptides | |
| 39 | Medicinal preparations containing antibodies or antigens | |
| A61P | SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS | |
| A61Q | SPECIFIC USE OF COSMETICS OR SIMILAR TOILET PREPARATIONS | |
| C07C | ACYCLIC OR CARBOCYCLIC COMPOUNDS | |
| C07D | HETEROCYCLIC COMPOUNDS | |
| C07F | ACYCLIC, CARBOCYCLIC, OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM | |
| C07H | SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS | |
| 21 | Compounds containing two or more mononucleotide units having separate phosphate or polyphosphate groups linked by saccharide radicals of nucleoside groups, e.g. nucleic acids |
|
| C07J | STEROIDS | |
| C07K | PEPTIDES | |
| C08* | ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON | |
| C12N | MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF | |
| 5 | Undifferentiated human, animal or plant cells | |
| C12Q | MEASURING OR TESTING PROCESSES INVOLVING ENZYMES OR MICROORGANISMS (immunoassay G01N 33/53); COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES | |
| G01N | INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES (measuring or testing processes other than immunoassay, involving enzymes or microorganisms C12M, C12Q) |
Appendix 2: Questionnaire in Chinese
制药行业专利在我国的应用的决定性因素
亲爱的答辩人,我想请你花点时间来填写一下我的硕士论文调查问卷。 本研究的目的是分析中国知识产权系统用户的看法和期望值,专注于医药行业以及近几年专利法影响了医药公司支出和策略的改革。
公司名称: _________________________________
你会同意或不同意以下阐述?
1.中国知识产权制度有益于中国的制药公司。
强烈反对 _
不同意 _
既不同意也不反对 _
同意 _
强烈同意 _
- 中国知识产权系统对中国制药公司的成长有帮助。
强烈反对 _
不同意 _
既不同意也不反对 _
同意 _
强烈同意 _
- 中国知识产权系统方便实用
强烈反对 _
不同意 _
既不同意也不反对 _
同意 _
强烈同意 _
- 填充在中国申请专利的过程是用户友好 中国专利申请的填写过程也非常的方便
强烈反对 _
不同意 _
既不同意也不反对 _
同意 _
强烈同意 _
- 在中国注册专利所需的时间是足够的。在中国注册专利的所需的时间也很宽裕
强烈反对 _
不同意 _
既不同意也不反对 _
同意 _
强烈同意 _
- 以下各项因素在公司决定在中国申请专利的如何重要。以下各因素对于公司决定在中国申请专利非常重要
申请所需的文件。
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 财务支持。
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 实际的当地合作伙伴。
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 未来可能的合作伙伴。
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 市场规模
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 国家的许可
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 对该行业的支持
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 国家的创造力
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 我国的知识产权保护
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 公司的服务比较完善
公司法律顾问 _
外部法律顾问 _
- 该公司去年实现了专利申请的目标吗?
没有 _
完成 _
额 _
- 所有专利申请职能集中在知识产权部门吗?
在 _
不在 _
- 在下次改革最后期限期间公司对于知识产权服务或营运支出方面有所改动
- 中国 2009年-2010 年期间专利法律第三次修订 (2009 年)
增加的开支 _
开支减少 _
多少 (百分比)?
- 政府 2012年-2013 年期间发出布强制许可 (2012 年)
增加的开支 _
开支减少 _
多少 (百分比)?
- 在接下来改革后,公司对于知识产权策略方面有所改动
- 中国专利法律第三次修订 (2009 年)
有 _
没有 _
- 政府颁发的强制许可 (2012 年)
有 _
没有 _
- 在接下来改革后,公司对于知识产权策略或部门预算方面有所改动
- 中国专利法律第三次修订 (2009 年)
增加 _
下降 _
- 政府颁发的强制许可 (2012 年)
增加 _
下降 _
以下因素对公司知识产权战略有多重要?
- 参与新兴市场 (国际化)
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 产品专营权
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 商誉
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 市场营销
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 伙伴关系
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
- 在发展新技术的成本
根本不重要 _
不太重要 _
中性 _
有些重要 _
非常重要 _
非常谢谢你的宝贵时间。
Appendix 3 Questionnaire in English
The impact of recent reforms and determinants of pharmaceutical Industry patent application in China
Dear respondent, I would like you to take your time to fill the following survey for my Master thesis research. The purpose of this study is to analyze the perception and expectations of Chinese Intellectual Property Rights system users, focused on the pharmaceutical industry and the effect of patent law last few years’ reforms in companies’ expenditure and strategies.
Company name: ______________
How much you agree or disagree with the following statements?
- The Chinese Intellectual Property Rights system is useful to Chinese pharmaceutical Companies.
Strongly disagree _
Disagree _
Neither agree or disagree _
Agree _
Strongly Agree _
- The Chinese Intellectual Property Rights system helped in growth to Chinese pharmaceutical Companies.
Strongly disagree _
Disagree _
Neither agree or disagree _
Agree _
Strongly Agree _
- The Chinese Intellectual Property Rights system is user-friendly
Strongly disagree _
Disagree _
Neither agree or disagree _
Agree _
Strongly Agree _
- The process of filling patent application in China is user-friendly
Strongly disagree _
Disagree _
Neither agree or disagree _
Agree _
Strongly Agree _
- The period required to register a patent in China is adequate.
Strongly disagree _
Disagree _
Neither agree or disagree _
Agree _
Strongly Agree _
How important were each of the following factors in the company decision to apply for a patent in China?
- Documentation needed for the application.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Financial support.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Actual local partners.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Possible future partners.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Market size.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Licensing opportunities in the country.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Support to the industry.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Inventive capability in the country.
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- IP protection in the country
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Applications in the company are usually prepared
In-house counsel _
Outside counsel _
- The company met the goal/target for patent applications in the last year?
Missed _
Met _
Exceeded _
- Are all patent application functions centralized in the IP department?
Yes _
No _
- In the company there was a change in the IP services or operations expenditure during the next reforms timeline:
- Chinese patent law third amendment (2009) – period between 2009-2010
The expenditure increased __
The expenditure decrease __
How much (percentage)?
- Government issued compulsory licenses (2012) – period between 2012- 2013
The expenditure increased __
The expenditure decrease __
How much (percentage)?
- In the company there was a change in the IP strategy after the follow reforms;
- Chinese patent law third amendment (2009)
Yes _
No _
- Government issued compulsory licenses (2012)
Yes _
No _
The company changes the strategy almost every year.
- In the company there was a change in the budget for IP strategy or department after the follow reforms;
- Chinese patent law third amendment (2009)
Increased _
Decreased _
- Government issued compulsory licenses (2012)
Increased _
Decreased _
How important are the following factors for the company IP strategy?
- Participation in new markets – internationalization
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Product exclusivity
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Goodwill
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Marketing
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Partnerships
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
- Costs in development of new technologies
Not important at all _
Somewhat unimportant _
Neutral _
Somewhat important _
Very important _
Thank you so much for your time.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Intellectual Property Law"
Intellectual Property (IP) law is a specialist field of law dealing with the ownership and protection of creative works such as inventions, designs, brands, music etc. Protection methods include patents, copyrights, and trademarks.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




